Chemical Shrinkage of Low Water to Cement (w/c) Ratio CEM I and CEM III Cement Pastes Incorporating Silica Fume and Filler
Abstract
1. Introduction
2. Materials and Methods
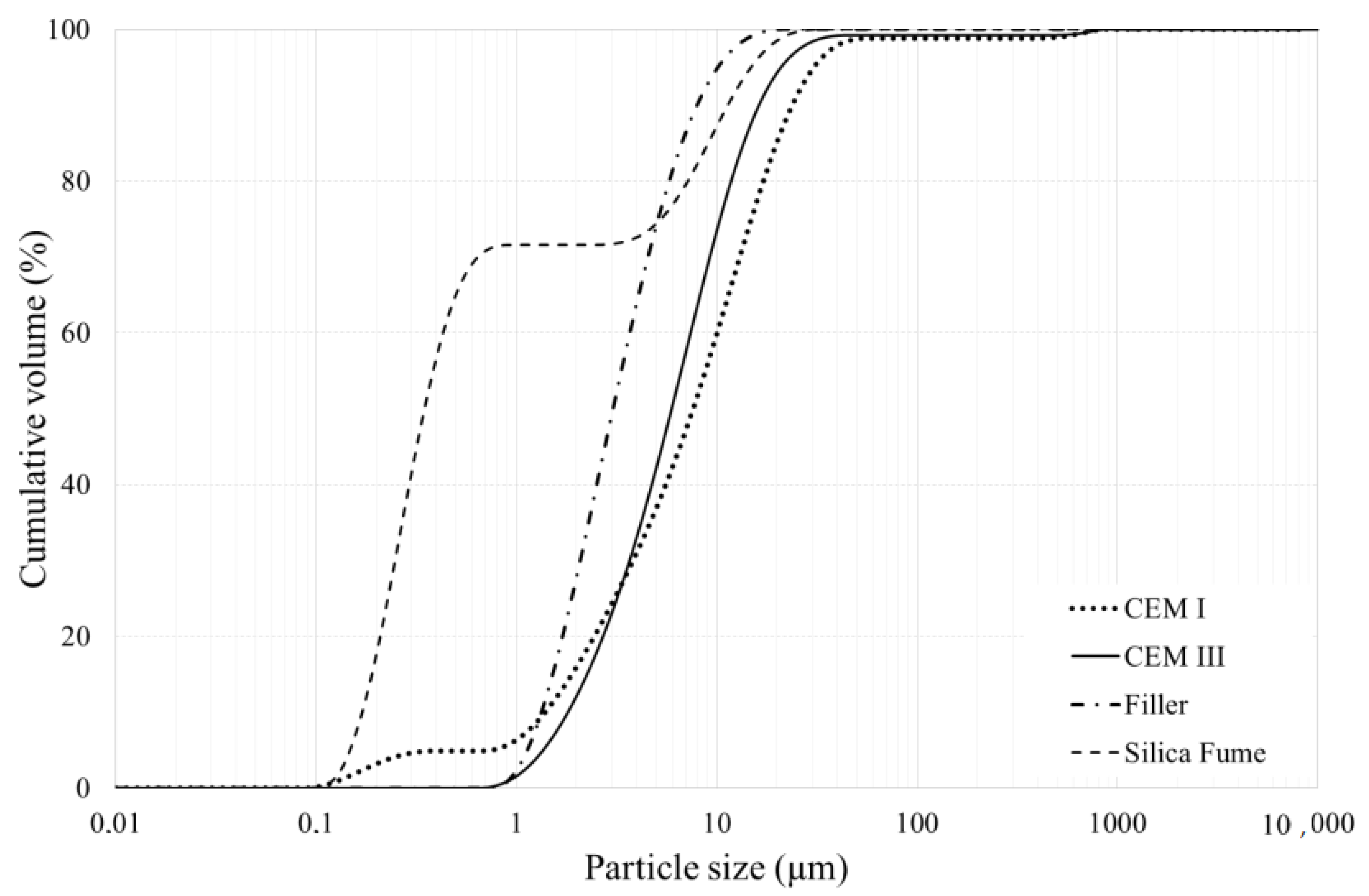
2.1. Materials
2.2. Cement Pastes Composition
2.3. Mixing Protocol
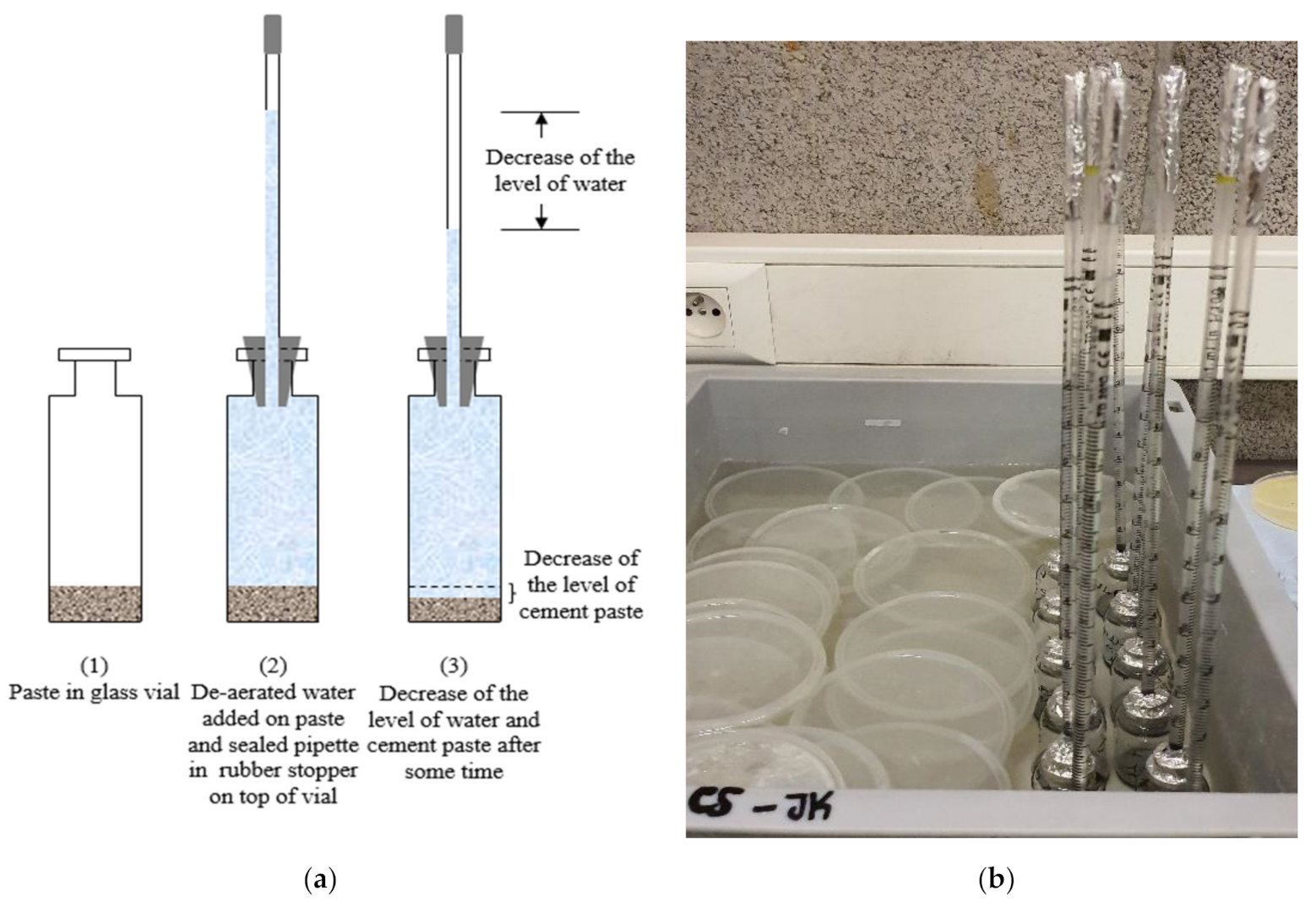
2.4. Chemical Shrinkage
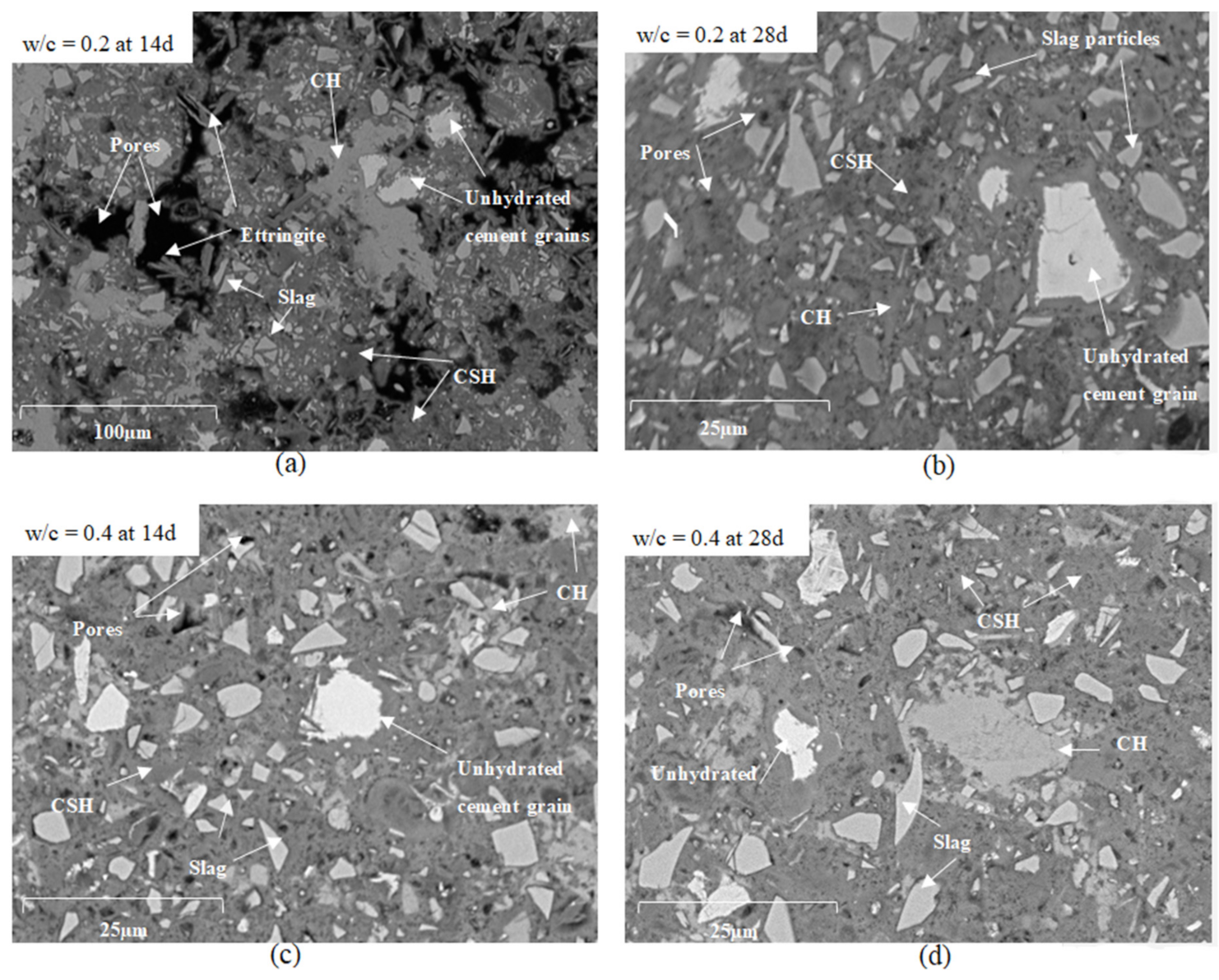
2.5. Scanning Electron Microscopy (SEM)
3. Results and Discussion
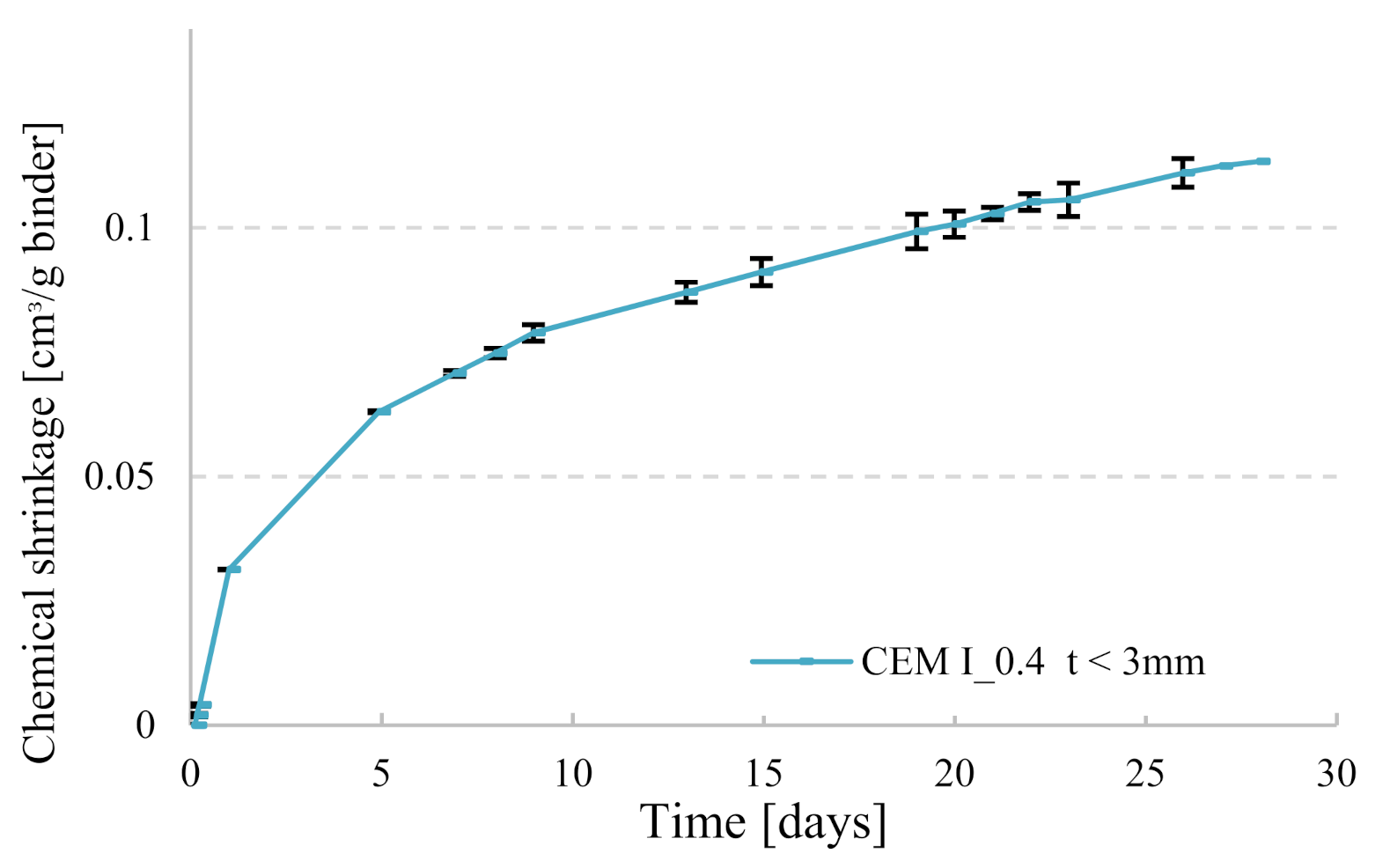
3.1. Duration of Testing Period
3.2. Effect of Superplasticizer on Chemical Shrinkage of Cement Pastes
3.3. Effect of Paste Thickness on Chemical Shrinkage of Cement Pastes
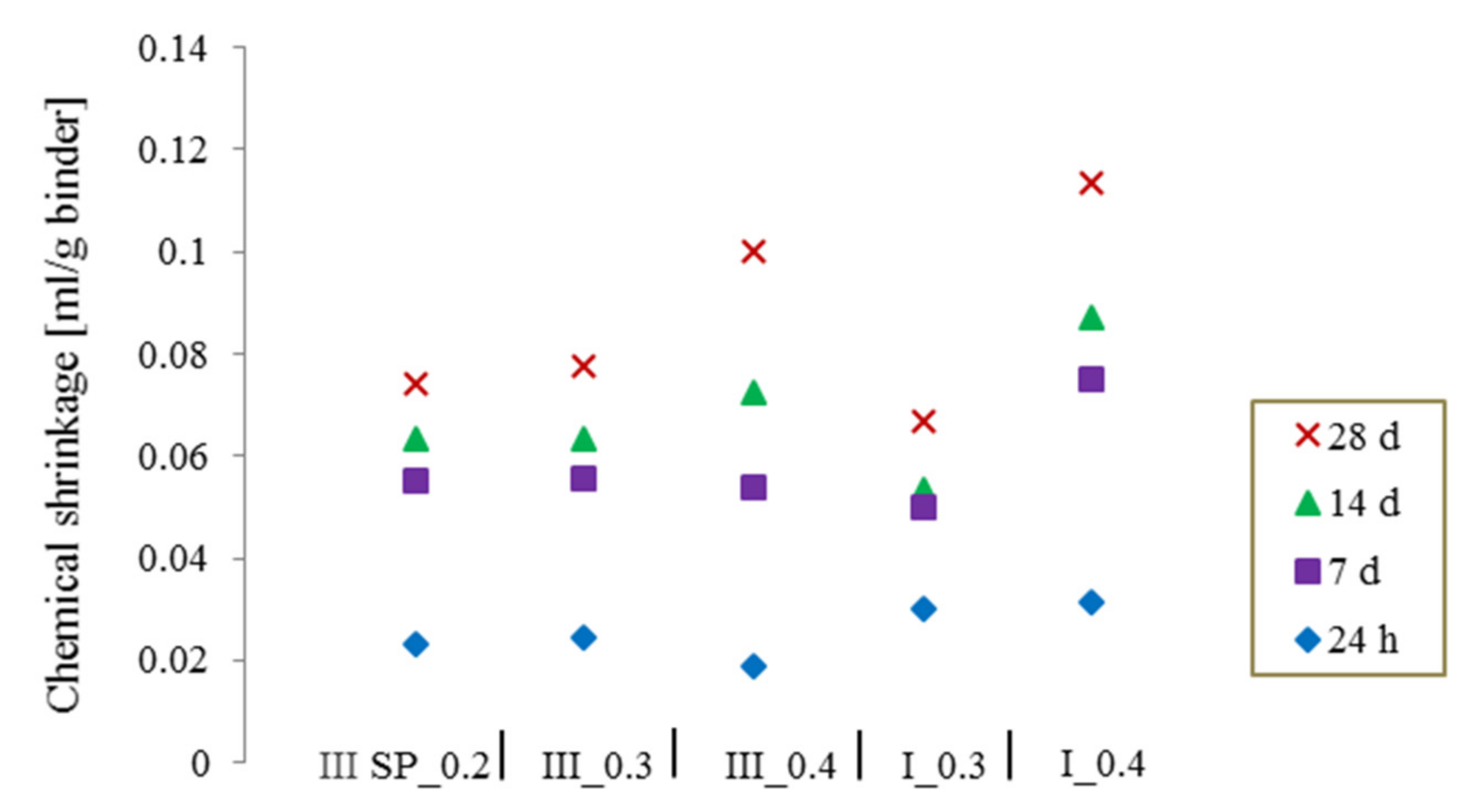
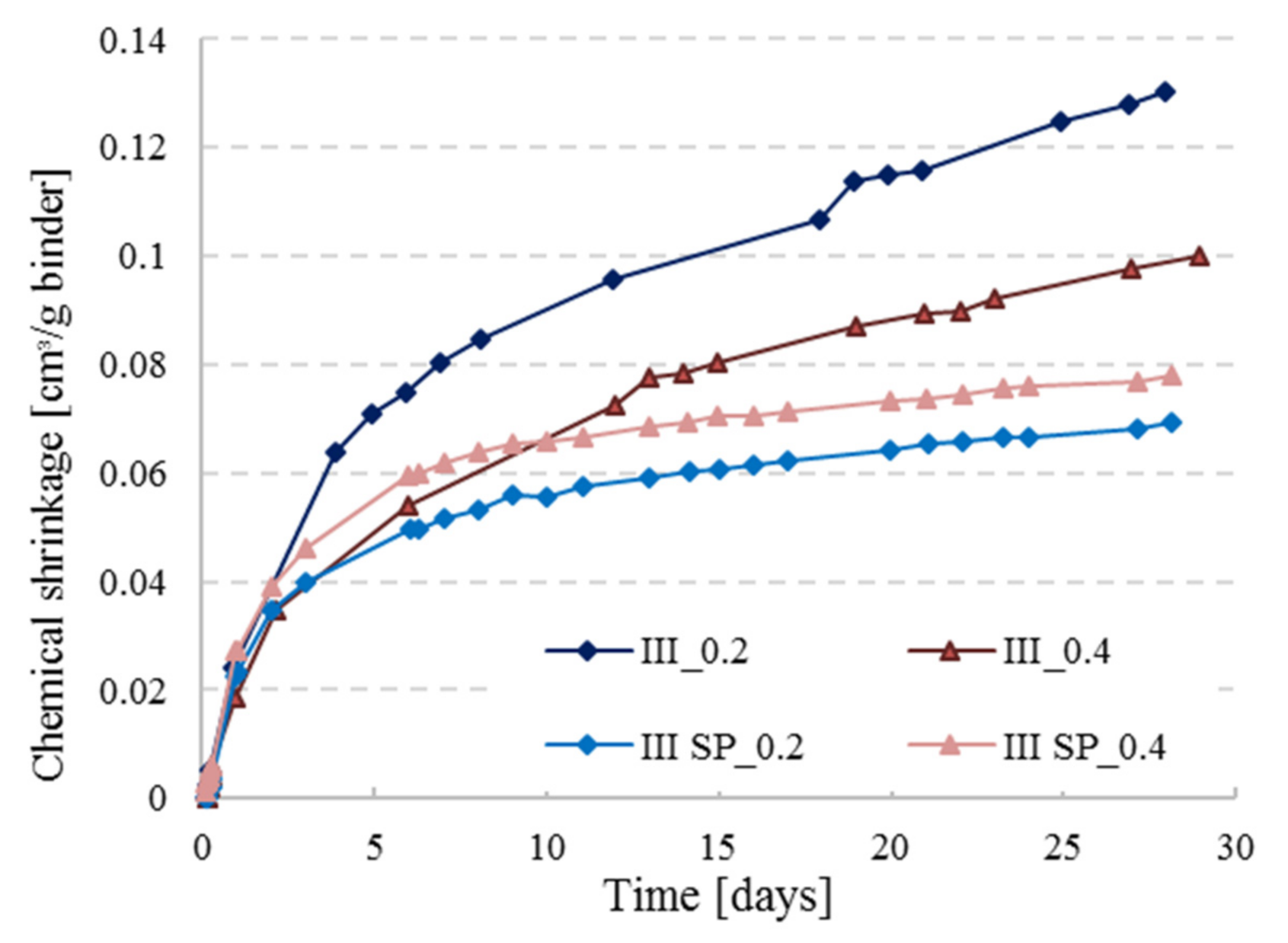
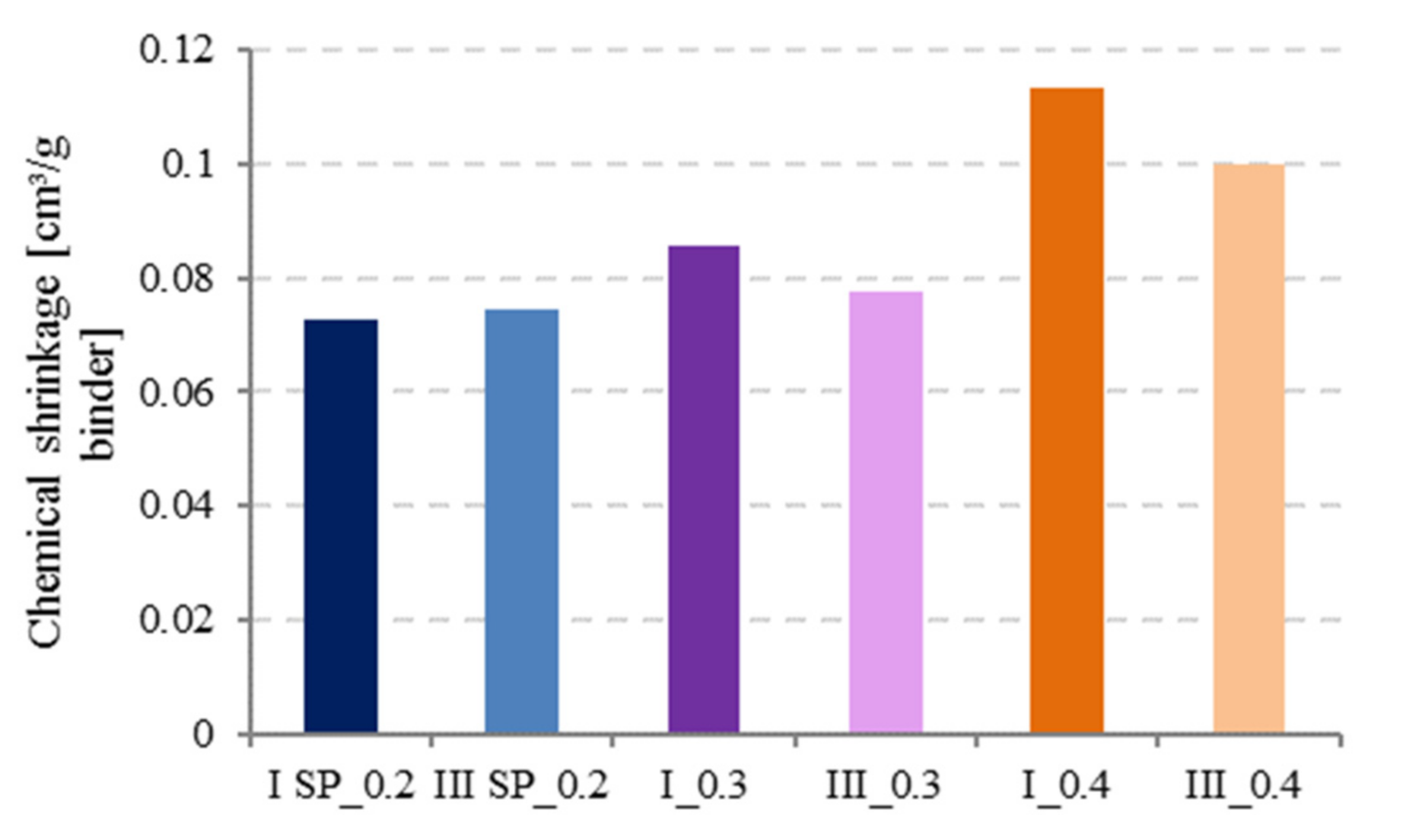
3.4. Effect of Water to Cement (W/C) Ratio and Cement Type on Chemical Shrinkage
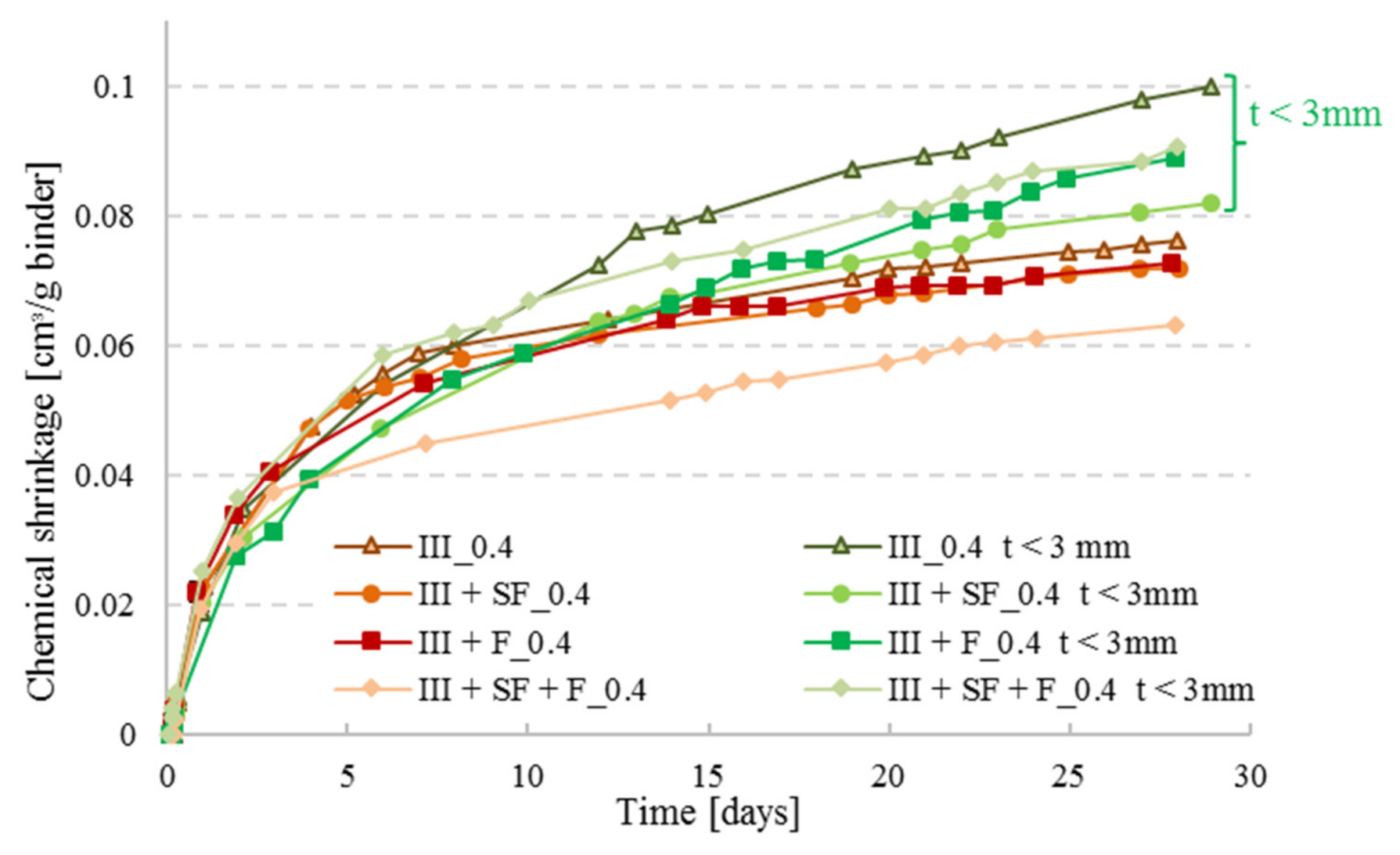
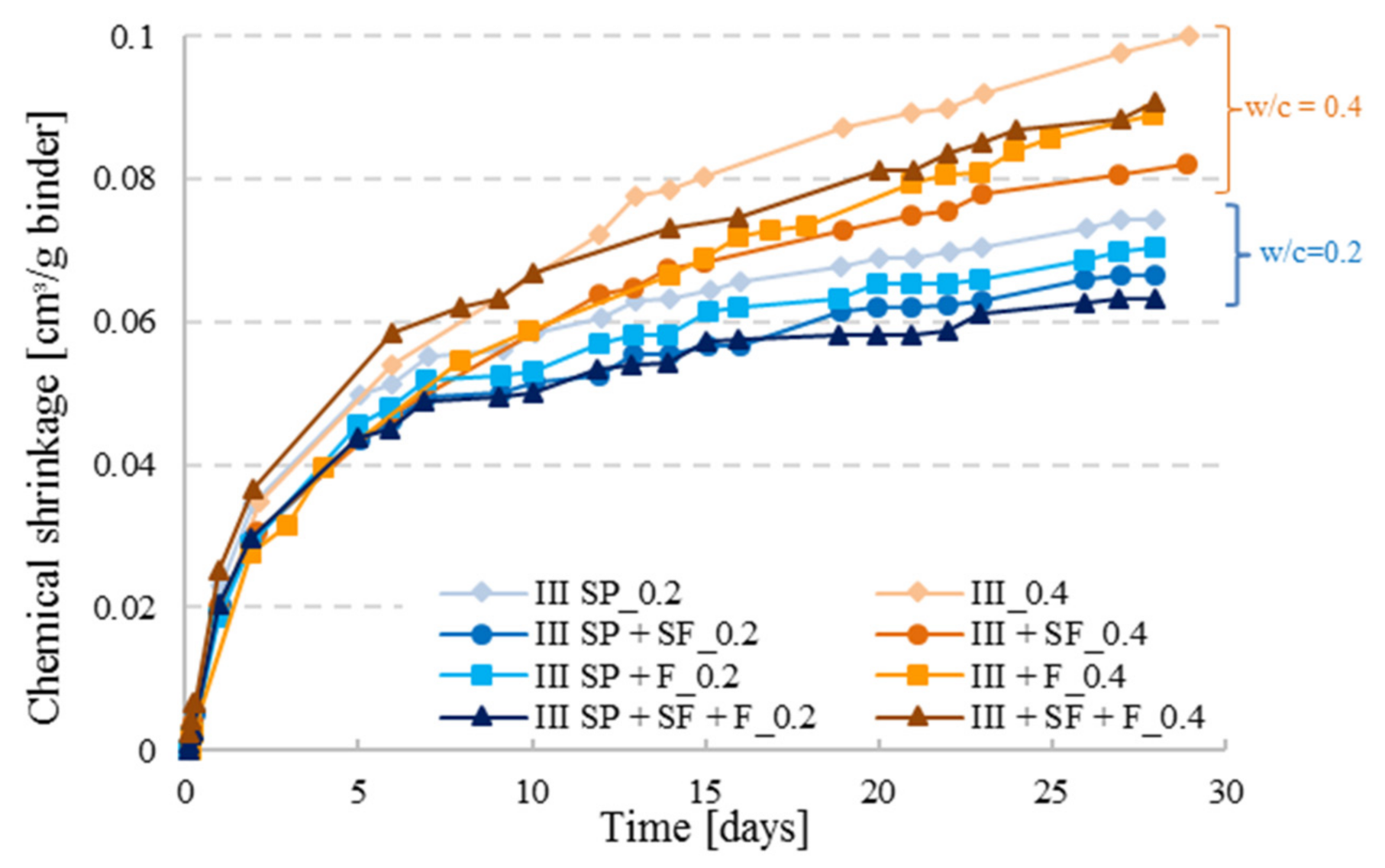
3.5. Effect of Mineral Additions on Chemical Shrinkage of Cement Pastes
4. Conclusions
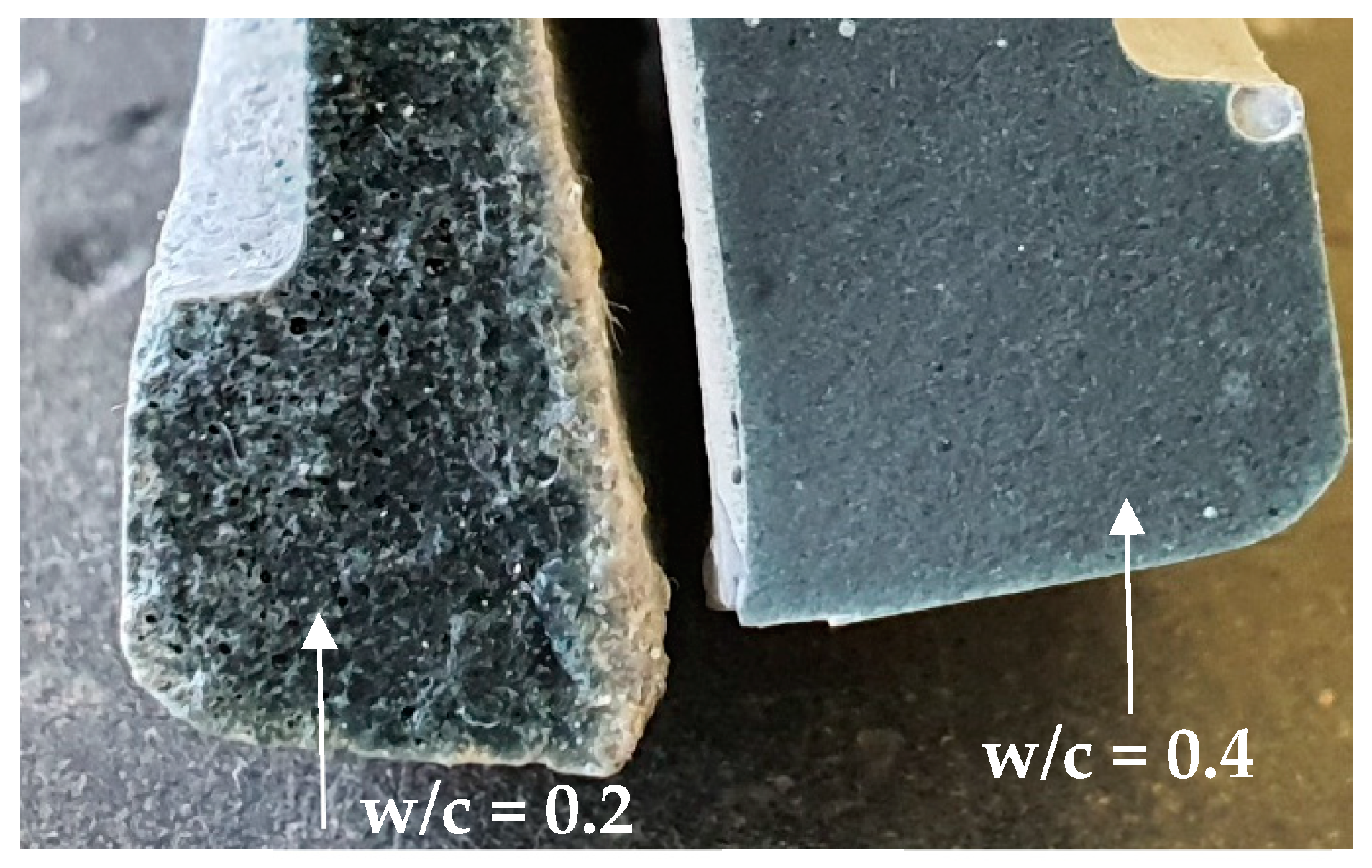
- Adding SP to low w/c cement pastes (≤0.2) and a careful compaction of paste samples by proper vibration must be provided when using the ASTM C1608 procedure. SEM images showed that bad compaction and absence of SP result in large pore connectivity that highly affects the shrinkage results.
- When comparing the method for different w/c ratios, it is important to choose the same paste thickness for all samples. The effect of paste thickness does not have a direct influence on the CS but rather on the water transport inside the material.
- Increase of w/c ratio results in higher CS values because higher w/c ratio pastes have higher degree of hydration, and more water is present in the mixture for hydrating cement grains.
- Cement type does not play a major role in the behavior of CS, but BFS cements (CEM III) tend to have slightly higher CS values than Portland cements at low w/c ratios, while the opposite seems to be true at higher w/c ratios.
- Addition of mineral components reduces the CS of CEM III pastes, as it is the case for silica fume and filler, and a combination of mineral additions (silica fume + filler) sometimes reduces CS further.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Klausen, A.B.E. Early Age Crack Assessment of Concrete Structures Experimental Investigation of Decisive Parameters. Ph.D. Thesis, Norwegian University of Science and Technology (NTNU), Trondheim, Norway, 2016. [Google Scholar]
- Mounanga, P.; Khelidj, A.; Loukili, A. Predicting Ca(OH)2 content and chemical shrinkage of hydrating cement pastes using nalytical approach. Cem. Concr. Res. 2004, 34, 255–265, 2004. [Google Scholar] [CrossRef]
- Whiting, D.A.; Detwiler, R.J.; Lagergren, E.S. Cracking tendency and drying shrinkage of silica fume concrete for bridge deck applications. ACI Struct. J. 2000, 97, 71–77. [Google Scholar] [CrossRef]
- Zhang, M.H.; Tam, C.T.; Leow, M.P. Effect of water-to-cementitious materials ratio and silica fume on the autogenous shrinkage of concrete. Cem. Concr. Res. 2003, 33, 1687–1694. [Google Scholar] [CrossRef]
- Tangtermsirikul, S. Effect of chemical composition and particle size of fly ash on autogenous shrinkage paste. Int. Workshop Autogenous Shrinkage Concr. 1998, 175–186. [Google Scholar]
- Lee, K.M.; Lee, H.K.; Lee, S.H.; Kim, G.Y. Autogenous shrinkage of concrete containing granulated blast-furnace slag. Cem. Concr. Res. 2006, 36, 1279–1285. [Google Scholar] [CrossRef]
- Holt, E.E. Early Age Autogenous Shrinkage of Concrete, 1st ed.; Technical Research Centre of Finland: Espoo, Finland, 2001. [Google Scholar]
- ACI Committee 231. Report on Early-Age Cracking: Causes, Measurement and Mitigation; ACI 231R-10; American Concrete Institute: Indianapolis, IN, USA, 2010. [Google Scholar]
- Yodsudjai, W.; Wang, K. Chemical shrinkage behavior of pastes made with different types of cements. Constr. Build. Mater. 2013, 40, 854–862. [Google Scholar] [CrossRef]
- Wyrzykowski, M.; Pietro, L.; Igarashi, S.; Mechtcherine, V. Recommendation of RILEM TC 260-RSC: Using superabsorbent polymers (SAP) to mitigate autogenous shrinkage. Mater. Struct. 2018, 51, 1–7. [Google Scholar] [CrossRef]
- Jensen, O.M.; Hansen, P.F. Autogenous deformation and RH-change in perspective. Cem. Concr. Res. 2001, 31, 1859–1865. [Google Scholar] [CrossRef]
- Sant, G.; Lura, P.; Weiss, J. Measurement of volume change in cementitious materials at early ages review of testing protocols and interpretation of results. Transp. Res. Rec. 2006, C, 21–29. [Google Scholar] [CrossRef]
- Lu, T.; Li, Z.; Huang, H. Effect of supplementary materials on the autogenous shrinkage of cement paste. Materials 2020, 13, 3367. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.H.; Gu, Y.M.; Kang, Q.B. Effect of Fly Ash, MgO and Curing Solution on the Chemical Shrinkage of Alkali-Activated Slag Cement. Adv. Mater. Res. 2011, 168–170, 2008–2011. [Google Scholar] [CrossRef]
- Parrott, L.J.; Geiker, M.; Gutteridge, W.A.; Killoh, D. Monitoring Portland Cement Hydration: Comparison of Methods. Cem. Concr. Res. 1990, 20, 919–926. [Google Scholar] [CrossRef]
- Powers, T.C.; Brownyard, T.L. Studies of the Physical Properties of Hardened Portland Cement Past in Research Laboratories of the Portland Cement Association; American Concrete Institute: Chicago, IL, USA, 1948. [Google Scholar]
- Geiker, M. Studies of Portland Cement Hydration by Measurements of Chemical Shrinkage and a Systematic Evaluation of Hydration Curves by Means of the Dispersion Model. Ph.D. Thesis, Technical University of Denmark, Copenhagen, Denmark, 1983. [Google Scholar]
- Knudsen, T.; Geiker, M. Chemical Shrinkage as an Indicator of the Stage of Hardening. In International Conference on Concrete of Early Ages; ENPC: Paris, France, 1982; pp. 163–165. [Google Scholar]
- Boivin, S.; Acker, P.; Rigaud, S.; Clavaud, B. Experimental Assessment of Chemical Shrinkage of Hydrating Cement Pastes. In Proceedings of the International Workshop on Autogenous Shrinkage of Concrete, Autoshrink’98, Hiroshima, Japan, 13–14 June 1998. [Google Scholar]
- Standard Test Method for Chemical Shrinkage of Hydraulic Cement Paste. ASTM: West Conshohocken, PA, USA, 2017; pp. 1–5.
- Meyst, L.D.; Kheir, J.; Tenório Filho, J.R.; Van Tittelboom, K.; De Belie, N. The use of superabsorbent Polymers in high performance concrete to mitigate autogenous shrinkage in a large-scale demonstrator. Sustainability 2020, 12, 4741. [Google Scholar] [CrossRef]
- Zhang, T.; Gao, P.; Luo, R.; Guo, Y.; Wei, J.; Yu, Q. Measurement of chemical shrinkage of cement paste: Comparison study of ASTM C1608 and an improved method. Constr. Build. Mater. 2014, 48, 662–669. [Google Scholar] [CrossRef]
- Mehta, P.K. Mechanism of expansion associated with ettringite formation. Cem. Concr. Res. 1973, 3, 1–6. [Google Scholar] [CrossRef]
- Zhang, Z.; Scherer, G.W. Measuring chemical shrinkage of ordinary Portland cement pastes with high water-to-cement ratios by adding cellulose nanofibrils. Cem. Concr. Compos. 2020, 111, 103625. [Google Scholar] [CrossRef]
- Yogendran, V.; Langan, B.W.; Ward, M.A. Hydration of Cement and Silica Fume Paste. Cem. Concr. Res. 1991, 21, 691–708. [Google Scholar] [CrossRef]
- Fu, T. Autogenous Deformation and Chemical Shrinkage of High Performance Cementitious Systems. Master’s Thesis, Oregon State University, Corvallis, OR, USA, 2011. [Google Scholar]
| Samples | CEM I 52.5 R (g) | CEM III/A 52.5 R (g) | Water (g) | Silica Fume (SF) (g) | Filler (F) (g) | Superplasticizer (SP) (g) |
|---|---|---|---|---|---|---|
| III_0.2 | - | 900 | 180 | - | - | - |
| III + SF_0.2 | - | 751.8 | 180 | 148.12 | - | - |
| III + F_0.2 | - | 727 | 180 | - | 173 | - |
| III + SF + F_0.2 | - | 627.05 | 180 | 123.52 | 149.23 | - |
| III SP_0.2 | - | 900 | 174.06 * | - | - | 9.9 |
| III SP + SF_0.2 | - | 751.8 | 175.04 * | 148.12 | - | 8.27 |
| III SP + F_0.2 | - | 727 | 175.20 * | - | 173 | 7.99 |
| III SP + SF + F_0.2 | - | 627.05 | 175.86 * | 123.52 | 149.23 | 6.89 |
| III_0.4 | - | 450 | 180 | - | - | - |
| III + SF_0.4 | - | 375.94 | 180 | 74.29 | - | - |
| III + F_0.4 | - | 363.49 | 180 | - | 86.62 | - |
| III + SF + F_0.4 | - | 313.4 | 180 | 61.93 | 74.68 | - |
| I SP_0.2 | 900 | - | 174.06 * | - | - | 9.9 |
| I_0.4 | 450 | - | 180 | - | - | - |
| I_0.3 | 600 | - | 180 | - | - | - |
| III_0.3 | - | 600 | 180 | - | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kheir, J.; Hilloulin, B.; Loukili, A.; De Belie, N. Chemical Shrinkage of Low Water to Cement (w/c) Ratio CEM I and CEM III Cement Pastes Incorporating Silica Fume and Filler. Materials 2021, 14, 1164. https://doi.org/10.3390/ma14051164
Kheir J, Hilloulin B, Loukili A, De Belie N. Chemical Shrinkage of Low Water to Cement (w/c) Ratio CEM I and CEM III Cement Pastes Incorporating Silica Fume and Filler. Materials. 2021; 14(5):1164. https://doi.org/10.3390/ma14051164
Chicago/Turabian StyleKheir, Judy, Benoît Hilloulin, Ahmed Loukili, and Nele De Belie. 2021. "Chemical Shrinkage of Low Water to Cement (w/c) Ratio CEM I and CEM III Cement Pastes Incorporating Silica Fume and Filler" Materials 14, no. 5: 1164. https://doi.org/10.3390/ma14051164
APA StyleKheir, J., Hilloulin, B., Loukili, A., & De Belie, N. (2021). Chemical Shrinkage of Low Water to Cement (w/c) Ratio CEM I and CEM III Cement Pastes Incorporating Silica Fume and Filler. Materials, 14(5), 1164. https://doi.org/10.3390/ma14051164








