The Effectiveness of Osseodensification Drilling Protocol for Implant Site Osteotomy: A Systematic Review of the Literature and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Inclusion and Exclusion Criteria
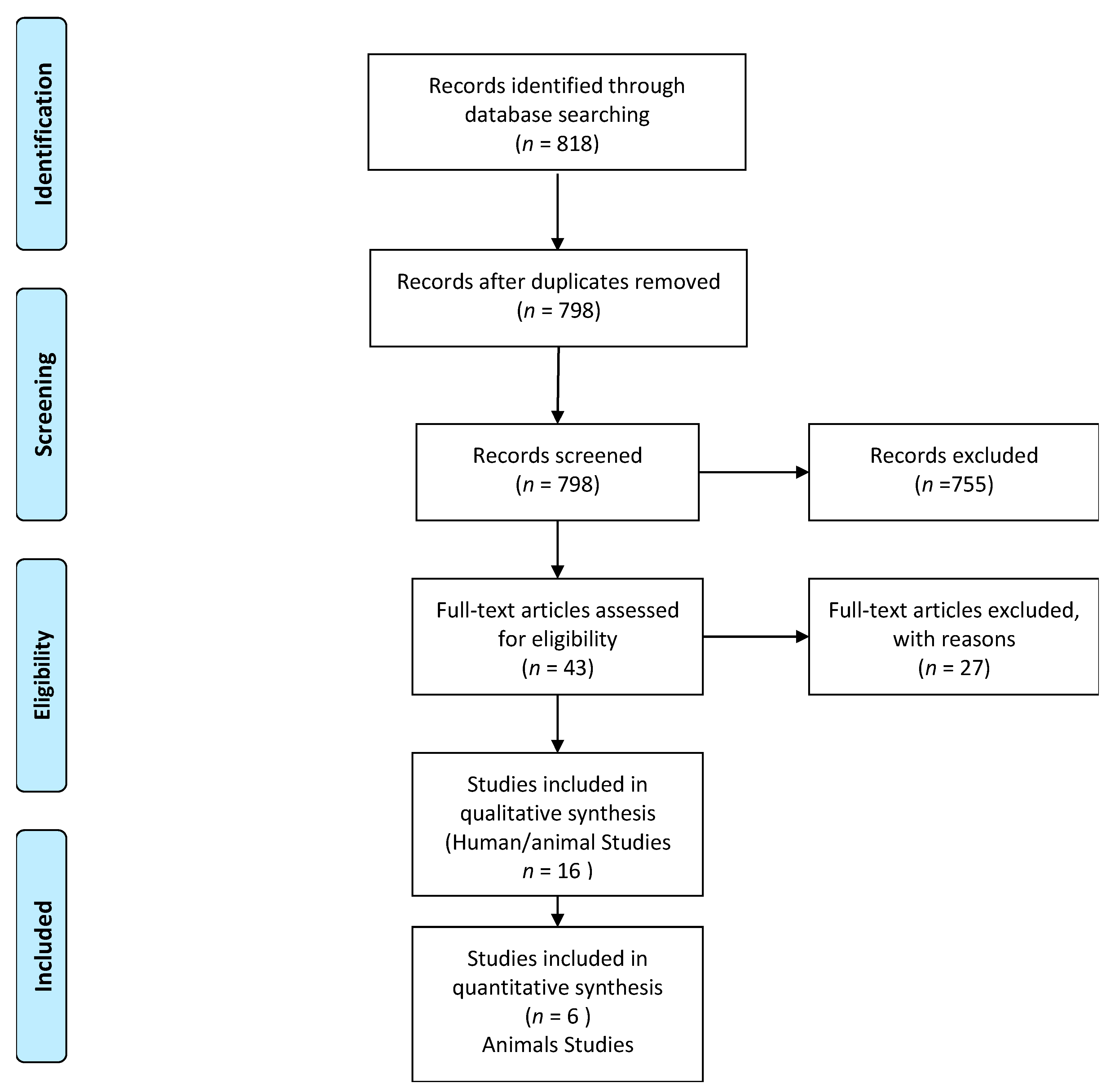
2.3. Study Selection
2.4. Data Extraction
2.5. Critical Appraisals
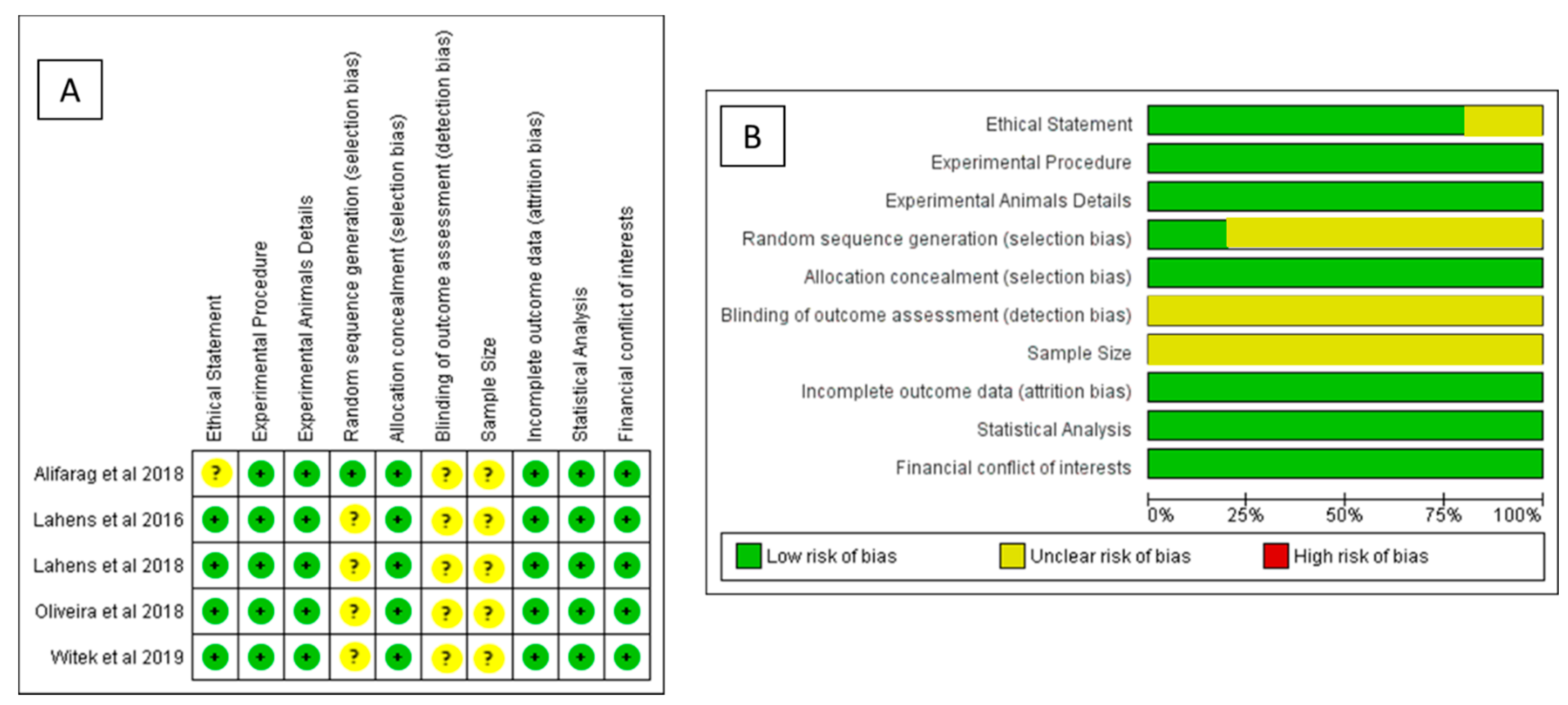
2.6. Meta-Analysis Methodology and Risk of Bias Assessments
3. Results
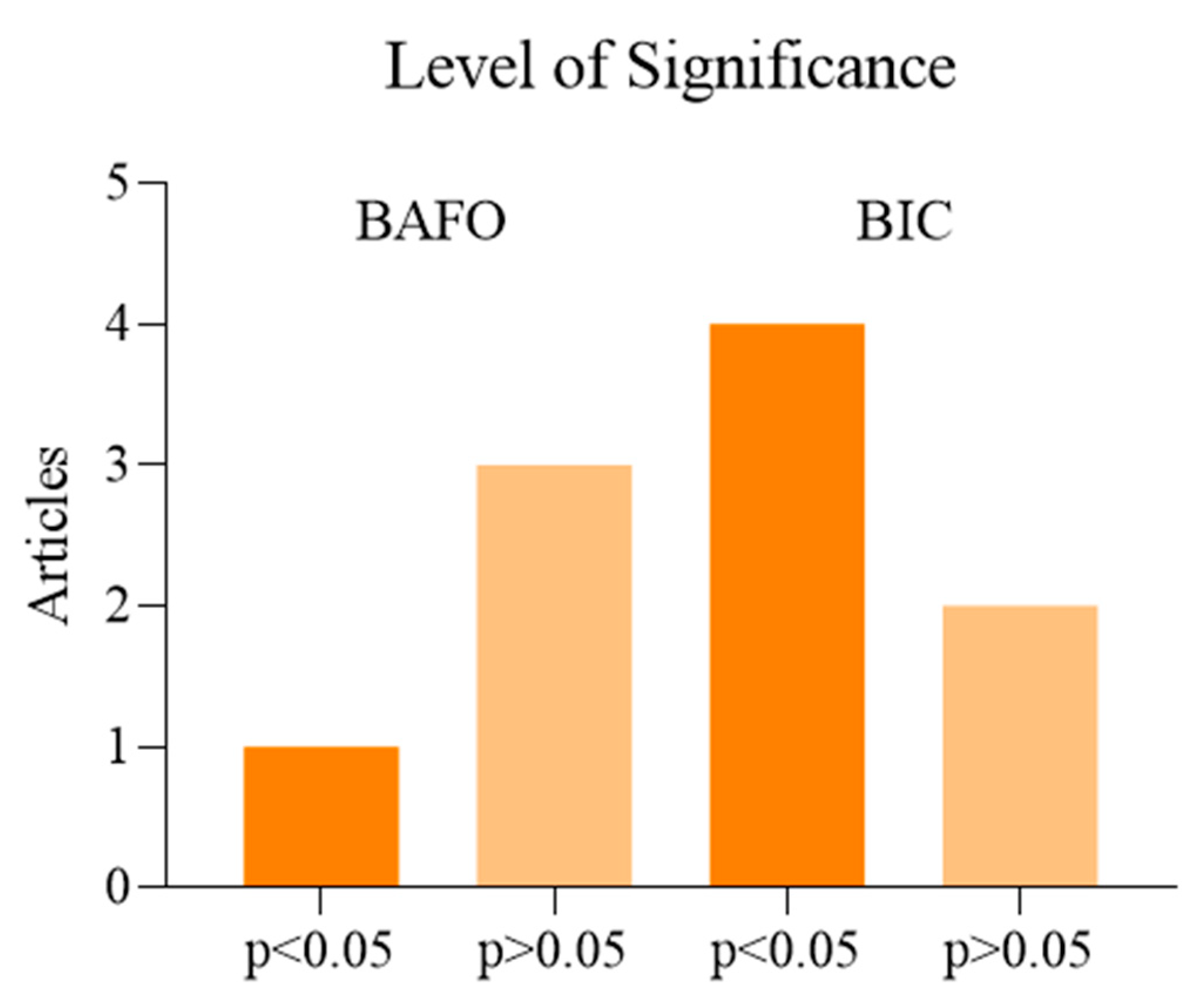
Meta-Analysis and Risk of Bias Measurement
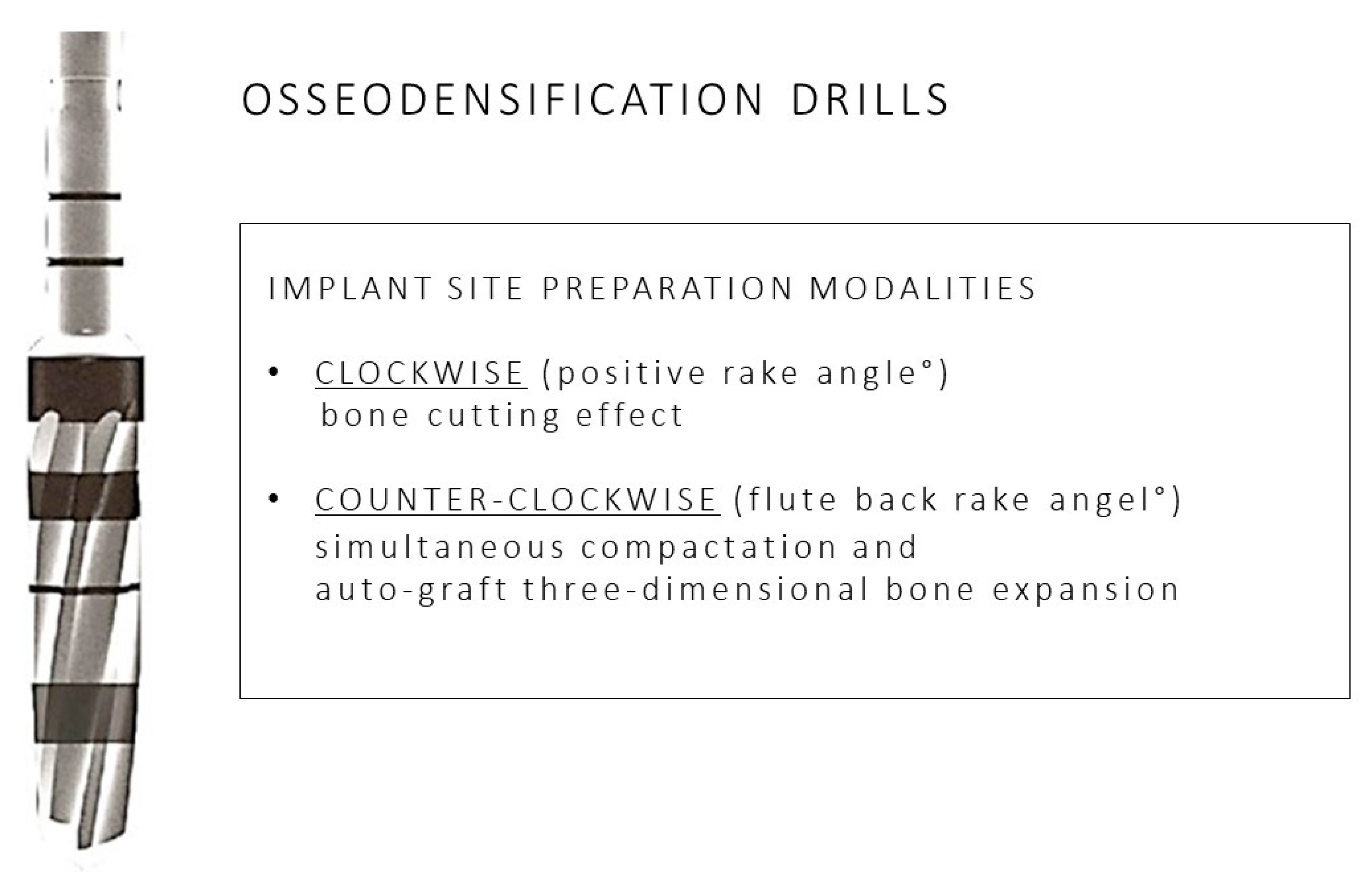
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Albrektsson, T.; Zarb, G.; Worthington, P.; Eriksson, A.R. The Long-Term Efficacy of Currently Used Dental Implants: A Review and Proposed Criteria of Success. Int. J. Oral Maxillofac. Implant. 1986, 1, 11–25. [Google Scholar]
- Albrektsson, T.; Berglundh, T.; Lindhe, J. Osseointegration: Historic Background and Current Concepts. Clin. Periodontol. Implant Dent. 2003, 4, 809–820. [Google Scholar]
- Ballini, A.; Cantore, S.; Farronato, D.; Cirulli, N.; Inchingolo, F.; Papa, F.; Malcangi, G.; Inchingolo, A.D.; Dipalma, G.; Sardaro, N.; et al. Periodontal disease and bone pathogenesis: The crosstalk between cytokines and porphyromonas gingivalis. J. Biol. Regul. Homeost. Agents 2015, 29, 273–281. [Google Scholar] [PubMed]
- Javed, F.; Romanos, G.E. The Role of Primary Stability for Successful Immediate Loading of Dental Implants. A Literature Review. J. Dent. 2010, 38, 612–620. [Google Scholar] [CrossRef] [PubMed]
- Javed, F.; Almas, K.; Crespi, R.; Romanos, G.E. Implant Surface Morphology and Primary Stability: Is There a Connection? Implant Dent. 2011, 20, 40–46. [Google Scholar] [CrossRef]
- Buser, D.; Sennerby, L.; De Bruyn, H. Modern Implant Dentistry Based on Osseointegration: 50 Years of Progress, Current Trends and Open Questions. Periodontology 2000 2017, 73, 7–21. [Google Scholar] [CrossRef] [PubMed]
- Smeets, R.; Stadlinger, B.; Schwarz, F.; Beck-Broichsitter, B.; Jung, O.; Precht, C.; Kloss, F.; Gröbe, A.; Heiland, M.; Ebker, T. Impact of Dental Implant Surface Modifications on Osseointegration. BioMed Res. Int. 2016, 2016, 1–16. [Google Scholar] [CrossRef]
- Fauroux, M.-A.; De Boutray, M.; Malthiéry, E.; Torres, J.-H. New Innovative Method Relating Guided Surgery to Dental Implant Placement. J. Stomatol. Oral Maxillofac. Surg. 2018, 119, 249–253. [Google Scholar] [CrossRef]
- Trindade, R.; Albrektsson, T.; Wennerberg, A. Current Concepts for the Biological Basis of Dental Implants. Oral Maxillofac. Surg. Clin. N. Am. 2015, 27, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Podaropoulos, L. Increasing the Stability of Dental Implants: The Concept of Osseodensification. Balk. J. Dent. Med. 2017, 21, 133–140. [Google Scholar] [CrossRef]
- Fanali, S.; Tumedei, M.; Pignatelli, P.; Inchingolo, F.; Pennacchietti, P.; Pace, G.; Piattelli, A. Implant Primary Stability with an Osteocondensation Drilling Protocol in Different Density Polyurethane Blocks. Comput. Methods Biomech. Biomed. Eng. 2020, 1–7. [Google Scholar] [CrossRef]
- Fujiwara, S.; Kato, S.; Bengazi, F.; Urbizo Velez, J.; Tumedei, M.; Kotsu, M.; Botticelli, D. Healing at Implants Installed in Osteotomies Prepared Either with a Piezoelectric Device or Drills: An Experimental Study in Dogs. Oral Maxillofac. Surg. 2020. [Google Scholar] [CrossRef]
- Kotsu, M.; Urbizo Velez, J.; Bengazi, F.; Tumedei, M.; Fujiwara, S.; Kato, S.; Botticelli, D. Healing at Implants Installed from ~ 70- to <10-Ncm Insertion Torques: An Experimental Study in Dogs. Oral Maxillofac. Surg. 2020. [Google Scholar] [CrossRef]
- Comuzzi, L.; Tumedei, M.; Piattelli, A.; Iezzi, G. Short vs. Standard Length Cone Morse Connection Implants: An In Vitro Pilot Study in Low Density Polyurethane Foam. Symmetry 2019, 11, 1349. [Google Scholar] [CrossRef]
- Comuzzi, L.; Tumedei, M.; Pontes, A.E.; Piattelli, A.; Iezzi, G. Primary Stability of Dental Implants in Low-Density (10 and 20 Pcf) Polyurethane Foam Blocks: Conical vs Cylindrical Implants. Int. J. Environ. Res. Public Health 2020, 17, 2617. [Google Scholar] [CrossRef] [PubMed]
- Pjetursson, B.E.; Thoma, D.; Jung, R.; Zwahlen, M.; Zembic, A. A Systematic Review of the Survival and Complication Rates of Implant-Supported Fixed Dental Prostheses (FDPs) after a Mean Observation Period of at Least 5 Years. Clin. Oral Implant. Res. 2012, 23 (Suppl. 6), 22–38. [Google Scholar] [CrossRef]
- Jung, R.E.; Al-Nawas, B.; Araujo, M.; Avila-Ortiz, G.; Barter, S.; Brodala, N.; Chappuis, V.; Chen, B.; De Souza, A.; Almeida, R.F.; et al. Group 1 ITI Consensus Report: The Influence of Implant Length and Design and Medications on Clinical and Patient-Reported Outcomes. Clin. Oral Implant. Res. 2018, 29 (Suppl. 16), 69–77. [Google Scholar] [CrossRef]
- Chackartchi, T.; Romanos, G.E.; Sculean, A. Soft Tissue-related Complications and Management around Dental Implants. Periodontology 2000 2019, 81, 124–138. [Google Scholar] [CrossRef] [PubMed]
- Sonnenschein, S.K.; Kohnen, R.; Ciardo, A.; Ziegler, P.; Seide, S.; Kim, T. Changes of Clinical Parameters at Implants: A Retrospective Comparison of Implants versus Natural Teeth over 5 Years of Supportive Periodontal Therapy. Clin. Oral Implant. Res. 2020, 31, 646–654. [Google Scholar] [CrossRef]
- Ballini, A.; Santacroce, L.; Cantore, S.; Bottalico, L.; Dipalma, G.; Vito, D.D.; Saini, R.; Inchingolo, F. Probiotics Improve Urogenital Health in Women. Open Access Maced. J. Med. Sci. 2018, 6, 1845–1850. [Google Scholar] [CrossRef]
- Santacroce, L.; Charitos, I.A.; Ballini, A.; Inchingolo, F.; Luperto, P.; De Nitto, E.; Topi, S. The Human Respiratory System and Its Microbiome at a Glimpse. Biology 2020, 9, 318. [Google Scholar] [CrossRef]
- Ballini, A.; Dipalma, G.; Isacco, C.G.; Boccellino, M.; Di Domenico, M.; Santacroce, L.; Nguyễn, K.C.D.; Scacco, S.; Calvani, M.; Boddi, A.; et al. Oral Microbiota and Immune System Crosstalk: A Translational Research. Biology 2020, 9, 131. [Google Scholar] [CrossRef] [PubMed]
- Lorusso, F.; Postiglione, F.; Delvecchio, M.; Rapone, B.; Scarano, A. The impact of diabetes in implant oral rehabilitations: A bibliometric study and literature review. Acta Med. 2020, 36, 3333. [Google Scholar]
- Chappuis, V.; Avila-Ortiz, G.; Araújo, M.G.; Monje, A. Medication-related Dental Implant Failure: Systematic Review and Meta-analysis. Clin. Oral Implant. Res. 2018, 29, 55–68. [Google Scholar] [CrossRef] [PubMed]
- Prasad, D.K.; Shetty, M.; Bansal, N.; Hegde, C. Crestal Bone Preservation: A Review of Different Approaches for Successful Implant Therapy. Indian J. Dent. Res. Off. Publ. Indian Soc. Dent. Res. 2011, 22, 317–323. [Google Scholar] [CrossRef]
- Ekelund, J.-A.; Lindquist, L.W.; Carlsson, G.E.; Jemt, T. Implant Treatment in the Edentulous Mandible: A Prospective Study on Brånemark System Implants over More than 20 Years. Int. J. Prosthodont. 2003, 16, 602–608. [Google Scholar]
- Grassi, F.R.; Ciccolella, F.; D’Apolito, G.; Papa, F.; Iuso, A.; Salzo, A.E.; Trentadue, R.; Nardi, G.M.; Scivetti, M.; De Matteo, M.; et al. Effect of Low-Level Laser Irradiation on Osteoblast Proliferation and Bone Formation. J. Biol. Regul. Homeost. Agents 2011, 25, 603–614. [Google Scholar] [PubMed]
- Dohan Ehrenfest, D.M.; Del Corso, M.; Inchingolo, F.; Charrier, J.-B. Selecting a Relevant in Vitro Cell Model for Testing and Comparing the Effects of a Choukroun’s Platelet-Rich Fibrin (PRF) Membrane and a Platelet-Rich Plasma (PRP) Gel: Tricks and Traps. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2010, 110, 409–411. [Google Scholar] [CrossRef] [PubMed]
- Inchingolo, F.; Martelli, F.S.; Gargiulo Isacco, C.; Borsani, E.; Cantore, S.; Corcioli, F.; Boddi, A.; Nguyễn, K.C.D.; De Vito, D.; Aityan, S.K.; et al. Chronic Periodontitis and Immunity, Towards the Implementation of a Personalized Medicine: A Translational Research on Gene Single Nucleotide Polymorphisms (SNPs) Linked to Chronic Oral Dysbiosis in 96 Caucasian Patients. Biomedicines 2020, 8, 115. [Google Scholar] [CrossRef]
- Cantore, S.; Mirgaldi, R.; Ballini, A.; Coscia, M.F.; Scacco, S.; Papa, F.; Inchingolo, F.; Dipalma, G.; De Vito, D. Cytokine Gene Polymorphisms Associate with Microbiogical Agents in Periodontal Disease: Our Experience. Int. J. Med. Sci. 2014, 11, 674–679. [Google Scholar] [CrossRef]
- Dohan Ehrenfest, D.M.; Del Corso, M.; Inchingolo, F.; Sammartino, G.; Charrier, J.-B. Platelet-Rich Plasma (PRP) and Platelet-Rich Fibrin (PRF) in Human Cell Cultures: Growth Factor Release and Contradictory Results. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2010, 110, 418–421; author reply 421–422. [Google Scholar] [CrossRef]
- Cantore, S.; Ballini, A.; De Vito, D.; Martelli, F.S.; Georgakopoulos, I.; Almasri, M.; Dibello, V.; Altini, V.; Farronato, G.; Dipalma, G.; et al. Characterization of Human Apical Papilla-Derived Stem Cells. J. Biol. Regul. Homeost. Agents 2017, 31, 901–910. [Google Scholar] [PubMed]
- Marei, H.; Abdel-Hady, A.; Al-Khalifa, K.; Al-Mahalawy, H. Influence of Surgeon Experience on the Accuracy of Implant Placement via a Partially Computer-Guided Surgical Protocol. Int. J. Oral Maxillofac. Implant. 2019, 34, 1177–1183. [Google Scholar] [CrossRef]
- Contaldo, M.; Itro, A.; Lajolo, C.; Gioco, G.; Inchingolo, F.; Serpico, R. Overview on Osteoporosis, Periodontitis and Oral Dysbiosis: The Emerging Role of Oral Microbiota. Appl. Sci. 2020, 10, 6000. [Google Scholar] [CrossRef]
- Fuster-Torres, M.Á.; Peñarrocha-Diago, M.; Peñarrocha-Oltra, D.; Peñarrocha-Diago, M. Relationships between Bone Density Values from Cone Beam Computed Tomography, Maximum Insertion Torque, and Resonance Frequency Analysis at Implant Placement: A Pilot Study. Int. J. Oral Maxillofac. Implant. 2011, 26, 1051–1056. [Google Scholar] [PubMed]
- Kola, M.Z.; Shah, A.H.; Khalil, H.S.; Rabah, A.M.; Harby, N.M.H.; Sabra, S.A.; Raghav, D. Surgical Templates for Dental Implant Positioning; Current Knowledge and Clinical Perspectives. Niger. J. Surg. Off. Publ. Niger. Surg. Res. Soc. 2015, 21, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Almutairi, A.S.; Walid, M.A.; Alkhodary, M.A. The Effect of Osseodensification and Different Thread Designs on the Dental Implant Primary Stability. F1000Research 2018, 7, 1898. [Google Scholar] [CrossRef]
- Insua, A.; Monje, A.; Wang, H.-L.; Miron, R.J. Basis of Bone Metabolism around Dental Implants during Osseointegration and Peri-Implant Bone Loss. J. Biomed. Mater. Res. A 2017, 105, 2075–2089. [Google Scholar] [CrossRef]
- Carr, A.B.; Arwani, N.; Lohse, C.M.; Gonzalez, R.L.V.; Muller, O.M.; Salinas, T.J. Early Implant Failure Associated With Patient Factors, Surgical Manipulations, and Systemic Conditions. J. Prosthodont. 2019, 28, 623–633. [Google Scholar] [CrossRef] [PubMed]
- Feher, B.; Lettner, S.; Heinze, G.; Karg, F.; Ulm, C.; Gruber, R.; Kuchler, U. An Advanced Prediction Model for Postoperative Complications and Early Implant Failure. Clin. Oral Implant. Res. 2020, 31, 928–935. [Google Scholar] [CrossRef]
- Lee, K.; Cha, J.; Sanz-Martin, I.; Sanz, M.; Jung, U. A Retrospective Case Series Evaluating the Outcome of Implants with Low Primary Stability. Clin. Oral Implant. Res. 2019, 30, 861–871. [Google Scholar] [CrossRef]
- Norton, M.R. The Influence of Low Insertion Torque on Primary Stability, Implant Survival, and Maintenance of Marginal Bone Levels: A Closed-Cohort Prospective Study. Int. J. Oral Maxillofac. Implant. 2017, 32, 849–857. [Google Scholar] [CrossRef]
- Simonpieri, A.; Del Corso, M.; Vervelle, A.; Jimbo, R.; Inchingolo, F.; Sammartino, G.; M Dohan Ehrenfest, D. Current Knowledge and Perspectives for the Use of Platelet-Rich Plasma (PRP) and Platelet-Rich Fibrin (PRF) in Oral and Maxillofacial Surgery Part 2: Bone Graft, Implant and Reconstructive Surgery. Curr. Pharm. Biotechnol. 2012, 13, 1231–1256. [Google Scholar] [CrossRef]
- Monje, A.; Ravidà, A.; Wang, H.-L.; Helms, J.A.; Brunski, J.B. Relationship between Primary/Mechanical and Secondary/Biological Implant Stability. Int. J. Oral Maxillofac. Implant. 2019, 34, s7–s23. [Google Scholar] [CrossRef]
- Scarano, A.; Lorusso, F.; Arcangelo, M.; D’Arcangelo, C.; Celletti, R.; de Oliveira, P.S. Lateral Sinus Floor Elevation Performed with Trapezoidal and Modified Triangular Flap Designs: A Randomized Pilot Study of Post-Operative Pain Using Thermal Infrared Imaging. Int. J. Environ. Res. Public Health 2018, 15, 1277. [Google Scholar] [CrossRef]
- Scarano, A.; Valbonetti, L.; Marchetti, M.; Lorusso, F.; Ceccarelli, M. Soft Tissue Augmentation of the Face with Autologous Platelet-Derived Growth Factors and Tricalcium Phosphate. Microtomography Evaluation of Mice. J. Craniofac. Surg. 2016, 27, 1212–1214. [Google Scholar] [CrossRef] [PubMed]
- Dohan Ehrenfest, D.M.; Del Corso, M.; Diss, A.; Mouhyi, J.; Charrier, J.-B. Three-Dimensional Architecture and Cell Composition of a Choukroun’s Platelet-Rich Fibrin Clot and Membrane. J. Periodontol. 2010, 81, 546–555. [Google Scholar] [CrossRef]
- Scarano, A.; de Oliveira, P.S.; Traini, T.; Lorusso, F. Sinus Membrane Elevation with Heterologous Cortical Lamina: A Randomized Study of a New Surgical Technique for Maxillary Sinus Floor Augmentation without Bone Graft. Materials 2018, 11, 1457. [Google Scholar] [CrossRef] [PubMed]
- Yoon, H.-G.; Heo, S.-J.; Koak, J.-Y.; Kim, S.-K.; Lee, S.-Y. Effect of Bone Quality and Implant Surgical Technique on Implant Stability Quotient (ISQ) Value. J. Adv. Prosthodont. 2011, 3, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Bezdjian, A.; Klis, S.F.L.; Peters, J.P.M.; Grolman, W.; Stegeman, I. Quality of Reporting of Otorhinolaryngology Articles Using Animal Models with the ARRIVE Statement. Lab. Anim. 2018, 52, 79–87. [Google Scholar] [CrossRef]
- He, Y.; Fok, A.; Aparicio, C.; Teng, W. Contact Analysis of Gap Formation at Dental Implant-abutment Interface under Oblique Loading: A Numerical-experimental Study. Clin. Implant Dent. Relat. Res. 2019. [Google Scholar] [CrossRef]
- Trisi, P.; Berardini, M.; Falco, A.; Podaliri Vulpiani, M. Validation of Value of Actual Micromotion as a Direct Measure of Implant Micromobility after Healing (Secondary Implant Stability). An in Vivo Histologic and Biomechanical Study. Clin. Oral Implant. Res. 2016, 27, 1423–1430. [Google Scholar] [CrossRef]
- Tumedei, M.; Piattelli, A.; Degidi, M.; Mangano, C.; Iezzi, G. A Narrative Review of the Histological and Histomorphometrical Evaluation of the Peri-Implant Bone in Loaded and Unloaded Dental Implants. A 30-Year Experience (1988–2018). Int. J. Environ. Res. Public Health 2020, 17, 2088. [Google Scholar] [CrossRef]
- Tumedei, M.; Piattelli, A.; Degidi, M.; Mangano, C.; Iezzi, G. A 30-Year (1988-2018) Retrospective Microscopical Evaluation of Dental Implants Retrieved for Different Causes: A Narrative Review. Int. J. Periodontics Restor. Dent. 2020, 40, e211–e227. [Google Scholar] [CrossRef]
- Comuzzi, L.; Iezzi, G.; Piattelli, A.; Tumedei, M. An In Vitro Evaluation, on Polyurethane Foam Sheets, of the Insertion Torque (IT) Values, Pull-Out Torque Values, and Resonance Frequency Analysis (RFA) of NanoShort Dental Implants. Polymers 2019, 11, 1020. [Google Scholar] [CrossRef]
- Chauhan, C.; Shah, D.; Sutaria, F. Various Bio-Mechanical Factors Affecting Heat Generation during Osteotomy Preparation: A Systematic Review. Indian J. Dent. Res. 2018, 29, 81. [Google Scholar] [CrossRef]
- Heinemann, F.; Hasan, I.; Bourauel, C.; Biffar, R.; Mundt, T. Bone Stability around Dental Implants: Treatment Related Factors. Ann. Anat. Anat. Anz. Off. Organ Anat. Ges. 2015, 199, 3–8. [Google Scholar] [CrossRef]
- De Benedittis, M.; Petruzzi, M.; Pastore, L.; Inchingolo, F.; Serpico, R. Nd:YAG Laser for Gingivectomy in Sturge-Weber Syndrome. J. Oral Maxillofac. Surg. 2007, 65, 314–316. [Google Scholar] [CrossRef]
- Charitos, I.A.; Ballini, A.; Bottalico, L.; Cantore, S.; Passarelli, P.C.; Inchingolo, F.; D’Addona, A.; Santacroce, L. Special Features of SARS-CoV-2 in Daily Practice. World J. Clin. Cases 2020, 8, 3920–3933. [Google Scholar] [CrossRef]
- Scarano, A.; Inchingolo, F.; Lorusso, F. Facial Skin Temperature and Discomfort When Wearing Protective Face Masks: Thermal Infrared Imaging Evaluation and Hands Moving the Mask. Int. J. Environ. Res. Public Health 2020, 17, 4624. [Google Scholar] [CrossRef]
- Falco, A.; Berardini, M.; Trisi, P. Correlation Between Implant Geometry, Implant Surface, Insertion Torque, and Primary Stability: In Vitro Biomechanical Analysis. Int. J. Oral Maxillofac. Implants 2018, 33, 824–830. [Google Scholar] [CrossRef]
- Slete, F.B.; Olin, P.; Prasad, H. Histomorphometric Comparison of 3 Osteotomy Techniques. Implant Dent. 2018, 27, 424–428. [Google Scholar] [CrossRef] [PubMed]
- Castellanos-Cosano, L.; Rodriguez-Perez, A.; Spinato, S.; Wainwright, M.; Machuca-Portillo, G.; Serrera-Figallo, M.-A.; Torres-Lagares, D. Descriptive Retrospective Study Analyzing Relevant Factors Related to Dental Implant Failure. Med. Oral Patol. Oral Cirugia Bucal 2019, 24, e726–e738. [Google Scholar] [CrossRef]
- Padhye, N.M.; Padhye, A.M.; Bhatavadekar, N.B. Osseodensification—A Systematic Review and Qualitative Analysis of Published Literature. J. Oral Biol. Craniofacial Res. 2020, 10, 375–380. [Google Scholar] [CrossRef]
- Hutton, B.; Salanti, G.; Caldwell, D.M.; Chaimani, A.; Schmid, C.H.; Cameron, C.; Ioannidis, J.P.A.; Straus, S.; Thorlund, K.; Jansen, J.P.; et al. The PRISMA Extension Statement for Reporting of Systematic Reviews Incorporating Network Meta-Analyses of Health Care Interventions: Checklist and Explanations. Ann. Intern. Med. 2015, 162, 777–784. [Google Scholar] [CrossRef] [PubMed]
- Alifarag, A.M.; Lopez, C.D.; Neiva, R.F.; Tovar, N.; Witek, L.; Coelho, P.G. Atemporal Osseointegration: Early Biomechanical Stability through Osseodensification: Early biomechanical stability. J. Orthop. Res. 2018, 36, 2516–2523. [Google Scholar] [CrossRef]
- Hindi, Ar.; Bede, Sy. The Effect of Osseodensification on Implant Stability and Bone Density: A Prospective Observational Study. J. Clin. Exp. Dent. 2020, e474–e478. [Google Scholar] [CrossRef] [PubMed]
- Witek, L.; Alifarag, A.; Tovar, N.; Lopez, C.; Gil, L.; Gorbonosov, M.; Hannan, K.; Neiva, R.; Coelho, P. Osteogenic Parameters Surrounding Trabecular Tantalum Metal Implants in Osteotomies Prepared via Osseodensification Drilling. Med. Oral Patol. Oral Cir. Bucal 2019. [Google Scholar] [CrossRef]
- Koutouzis, T.; Huwais, S.; Hasan, F.; Trahan, W.; Waldrop, T.; Neiva, R. Alveolar Ridge Expansion by Osseodensification-Mediated Plastic Deformation and Compaction Autografting: A Multicenter Retrospective Study. Implant Dent. 2019, 28, 349–355. [Google Scholar] [CrossRef]
- Lahens, B.; Lopez, C.D.; Neiva, R.F.; Bowers, M.M.; Jimbo, R.; Bonfante, E.A.; Morcos, J.; Witek, L.; Tovar, N.; Coelho, P.G. The Effect of Osseodensification Drilling for Endosteal Implants with Different Surface Treatments: A Study in Sheep: OSSEODENSIFICATION OF ENDOSTEAL IMPLANTS. J. Biomed. Mater. Res. B Appl. Biomater. 2019, 107, 615–623. [Google Scholar] [CrossRef]
- Trisi, P.; Berardini, M.; Falco, A.; Podaliri Vulpiani, M. New Osseodensification Implant Site Preparation Method to Increase Bone Density in Low-Density Bone: In Vivo Evaluation in Sheep. Implant Dent. 2016, 25, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Sultana, A.; Makkar, S.; Saxena, D.; Wadhawan, A.; Kusum, C. To Compare the Stability and Crestal Bone Loss of Implants Placed Using Osseodensification and Traditional Drilling Protocol: A Clinicoradiographical Study. J. Indian Prosthodont. Soc. 2020, 20, 45. [Google Scholar] [CrossRef]
- Tian, J.H.; Neiva, R.; Coelho, P.G.; Witek, L.; Tovar, N.M.; Lo, I.C.; Gil, L.F.; Torroni, A. Alveolar Ridge Expansion: Comparison of Osseodensification and Conventional Osteotome Techniques. J. Craniofacial Surg. 2019, 30, 607–610. [Google Scholar] [CrossRef] [PubMed]
- Doi, K.; Kubo, T.; Makihara, Y.; Oue, H.; Morita, K.; Oki, Y.; Kajihara, S.; Tsuga, K. Osseointegration aspects of placed implant in bone reconstruction with newly developed block-type interconnected porous calcium hydroxyapatite. J. Appl. Oral Sci. 2016, 24, 325–331. [Google Scholar] [CrossRef]
- Lahens, B.; Neiva, R.; Tovar, N.; Alifarag, A.M.; Jimbo, R.; Bonfante, E.A.; Bowers, M.M.; Cuppini, M.; Freitas, H.; Witek, L.; et al. Biomechanical and Histologic Basis of Osseodensification Drilling for Endosteal Implant Placement in Low Density Bone. An Experimental Study in Sheep. J. Mech. Behav. Biomed. Mater. 2016, 63, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Lorean, A.; Barer, N.; Barbu, H.; Levin, L. Novel Electrical Conductivity Device for Osteotomy Preparation for Dental Implants Placement: A Cadaver Study. Clin. Implant Dent. Relat. Res. 2018, 20, 569–573. [Google Scholar] [CrossRef]
- Elsayyad, A.A.; Osman, R.B. Osseodensification in Implant Dentistry: A Critical Review of the Literature. Implant Dent. 2019, 28, 306–312. [Google Scholar] [CrossRef]
- Lang, N.P.; Salvi, G.E.; Huynh-Ba, G.; Ivanovski, S.; Donos, N.; Bosshardt, D.D. Early Osseointegration to Hydrophilic and Hydrophobic Implant Surfaces in Humans. Clin. Oral Implant. Res. 2011, 22, 349–356. [Google Scholar] [CrossRef]
- Pai, U. 44. Indirect Sinus Lift of Atrophic Posterior Maxilla Using Osseodensification: A Case Report. J. Indian Prosthodont. Soc. 2018, 18, 108. [Google Scholar] [CrossRef]
- Sakka, S.; Baroudi, K.; Nassani, M.Z. Factors Associated with Early and Late Failure of Dental Implants. J. Investig. Clin. Dent. 2012, 3, 258–261. [Google Scholar] [CrossRef]
- Trisi, P.; Perfetti, G.; Baldoni, E.; Berardi, D.; Colagiovanni, M.; Scogna, G. Implant Micromotion Is Related to Peak Insertion Torque and Bone Density. Clin. Oral Implant. Res. 2009, 20, 467–471. [Google Scholar] [CrossRef] [PubMed]
- Podaropoulos, L.; Veis, A.A.; Trisi, P.; Papadimitriou, S.; Alexandridis, C.; Kalyvas, D. Bone Reactions around Dental Implants Subjected to Progressive Static Load: An Experimental Study in Dogs. Clin. Oral Implant. Res. 2016, 27, 910–917. [Google Scholar] [CrossRef]
- Torroni, A.; Lima Parente, P.E.; Witek, L.; Hacquebord, J.H.; Coelho, P.G. Osseodensification Drilling vs Conventional Manual Instrumentation Technique for Posterior Lumbar Fixation: Ex-Vivo Mechanical and Histomorphological Analysis in an Ovine Model. J. Orthop. Res. Off. Publ. Orthop. Res. Soc. 2020. [Google Scholar] [CrossRef] [PubMed]
- Neugebauer, J.; Traini, T.; Thams, U.; Piattelli, A.; Zöller, J.E. Peri-Implant Bone Organization under Immediate Loading State. Circularly Polarized Light Analyses: A Minipig Study. J. Periodontol. 2006, 77, 152–160. [Google Scholar] [CrossRef] [PubMed]
- Scarano, A.; Assenza, B.; Inchingolo, F.; Mastrangelo, F.; Lorusso, F. New Implant Design with Midcrestal and Apical Wing Thread for Increased Implant Stability in Single Postextraction Maxillary Implants. Case Rep. Dent. 2019, 2019, 9529248. [Google Scholar] [CrossRef]
- Huwais, S.; Mazor, Z.; Ioannou, A.L.; Gluckman, H.; Neiva, R. A Multicenter Retrospective Clinical Study with Up-to-5-Year Follow-up Utilizing a Method That Enhances Bone Density and Allows for Transcrestal Sinus Augmentation Through Compaction Grafting. Int. J. Oral Maxillofac. Implant. 2018, 33, 1305–1311. [Google Scholar] [CrossRef] [PubMed]








| Population\Patients | Intervention | Comparison | Outcomes |
|---|---|---|---|
| Patient group of interest? | What is the main intervention you wish to consider? | Is there an alternative intervention to compare? | What is the clinical outcome? |
| Patients that need oral rehabilitation with dental implant surgery in low-density bone areas | Implant positioning with the bone compaction technique | Conventional implant Site preparation | Can this technique provide optimum primary implant stability? |
| Authors | Study Model | Techniques | Implants Type | N implants | BAFO | BIC | IT |
|---|---|---|---|---|---|---|---|
| Alifarag et coll. 2018 [64] | Ovine iliac crest | Conventional; osseodensification preparation (clockwise and anticlockwise) | Tapered screw vent Trabecular metal (Zimmer) | 36 (18 TSV; 18 TM) | OAO > C p = 0.037 OSO > C p= 0.005 OAO\OSO p > 0.05 | ||
| Hindi et coll. 2020 [65] | Humans | osseodensification preparation | -Diameter 4.1 mm (26;56.2%) 3.5 mm (20;43.8%) -Length 10 mm (21;45.6%) 12 mm (19;41.3%) 8 mm (6;13.1%) | 46 | >35 Ncm 35 implants (76.1%) =35 Ncm 11 implants (23.9%) | ||
| Witek et coll. 2019 [66] | Ovine iliac crest | Conventional; osseodensification preparation (clockwise and anticlockwise) | TM (Zimmer) 3.7 mm diameter 10 mm length | OAO > C p = 0.036 | OD > C p > 0.05 | ||
| Koutouzis et coll. 2019 [67] | Humans | osseodensification preparation | TSV (Zimmer) | 28 | Immediate post-operative +\− 61.3 Ncm, after 3 and 6 weeks respectively +/−56.6 Ncm and +/−59.8 | ||
| Lahens et coll. 2018 [68] | Ovine iliac crest | Conventional; osseodensification preparation (clockwise and anticlockwise) | 72 implants, 36 treated with acid; 36 treated mechanically | OSO > C (p = 0.024) OAO > C (p = 0.006) | OSO + OAO > C (p < 0.001) | ||
| Trisi et coll. 2016 [69] | Ovine iliac crest | Conventional; osseodensification preparation | Dynamic Implant (Cortex) | −10 implants 3.8 mm diameter; 10 mm length −10 implants 5 mm diameter 10 mm length | C = 46.19% +/− 3.98%; OD = 49.58% +/− 3.19% | ||
| Sultana et coll. 2020 [70] | Humans anterior maxilla | Conventional; osseodensification preparation | Tuareg S (Adin) | 20 Several diameters and longitudes | OD = immediate post operation 65.6; after 6 months 66 OD = 57.6 immediate post operation; after 6 months 64.8 OD\C = p > 0.05 | ||
| Tian et coll. 2019 [71] | Swine, mandibular crest | Summers osteotomes; osseodensification preparation | 12 4 mm diameter 13 mm length | OD > C p = 0.198 | C = 31.4% OD = 62.5% OD > C p= 0.018 | ||
| Slete et coll. 2018 [60] | Swine tibia | Conventional + Summers osteotomes; osseodensification preparation | TSV (Zimmer) | 18 4.7 mm diameter 13 mm diameter | OD = 60.3% CS = 40.7% C = 16% | ||
| Oliveira et coll. 2018 [72] | Ovine iliac crest | Conventional; osseodensification preparation (clockwise and anticlockwise) | 60, conical, 4 mm diameters 10 mm length (30 with surface treated with acidifiers, 30 with only mechanic treatment) | OD > C = p = 0.330 | OAO = +/−31% OSO = +/−28% C = +/−24% OD > C = p = 0.148 | C = 10 Ncm OSO = 53 Ncm OAO = 78 Ncm OAO > OSO > C = p < 0.005 | |
| Lahens et coll. 2016 [73] | Ovine | osseodensification preparation (clockwise and anticlockwise) | Axis Tag | 30 4.2 mm diameter 10 mm length | OD > C = p = 0.22 | C = 50% OSO = 60% OAO = 70% OD\C = p < 0.05 | C = 25 Ncm OSO = quasi 100 Ncm OAO = quasi 100 Ncm OD\C = p < 0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Inchingolo, A.D.; Inchingolo, A.M.; Bordea, I.R.; Xhajanka, E.; Romeo, D.M.; Romeo, M.; Zappone, C.M.F.; Malcangi, G.; Scarano, A.; Lorusso, F.; et al. The Effectiveness of Osseodensification Drilling Protocol for Implant Site Osteotomy: A Systematic Review of the Literature and Meta-Analysis. Materials 2021, 14, 1147. https://doi.org/10.3390/ma14051147
Inchingolo AD, Inchingolo AM, Bordea IR, Xhajanka E, Romeo DM, Romeo M, Zappone CMF, Malcangi G, Scarano A, Lorusso F, et al. The Effectiveness of Osseodensification Drilling Protocol for Implant Site Osteotomy: A Systematic Review of the Literature and Meta-Analysis. Materials. 2021; 14(5):1147. https://doi.org/10.3390/ma14051147
Chicago/Turabian StyleInchingolo, Alessio Danilo, Angelo Michele Inchingolo, Ioana Roxana Bordea, Edit Xhajanka, Donato Mario Romeo, Mario Romeo, Carlo Maria Felice Zappone, Giuseppina Malcangi, Antonio Scarano, Felice Lorusso, and et al. 2021. "The Effectiveness of Osseodensification Drilling Protocol for Implant Site Osteotomy: A Systematic Review of the Literature and Meta-Analysis" Materials 14, no. 5: 1147. https://doi.org/10.3390/ma14051147
APA StyleInchingolo, A. D., Inchingolo, A. M., Bordea, I. R., Xhajanka, E., Romeo, D. M., Romeo, M., Zappone, C. M. F., Malcangi, G., Scarano, A., Lorusso, F., Isacco, C. G., Marinelli, G., Contaldo, M., Ballini, A., Inchingolo, F., & Dipalma, G. (2021). The Effectiveness of Osseodensification Drilling Protocol for Implant Site Osteotomy: A Systematic Review of the Literature and Meta-Analysis. Materials, 14(5), 1147. https://doi.org/10.3390/ma14051147

















