Influence of TiO2 Nanoparticles on the Resistance of Cementitious Composite Materials to the Action of Bacteria
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
- No traces of contamination/development of bacterial colonies were observed on the surface of any of the tested cementitious material samples during the entire test period (21 days).
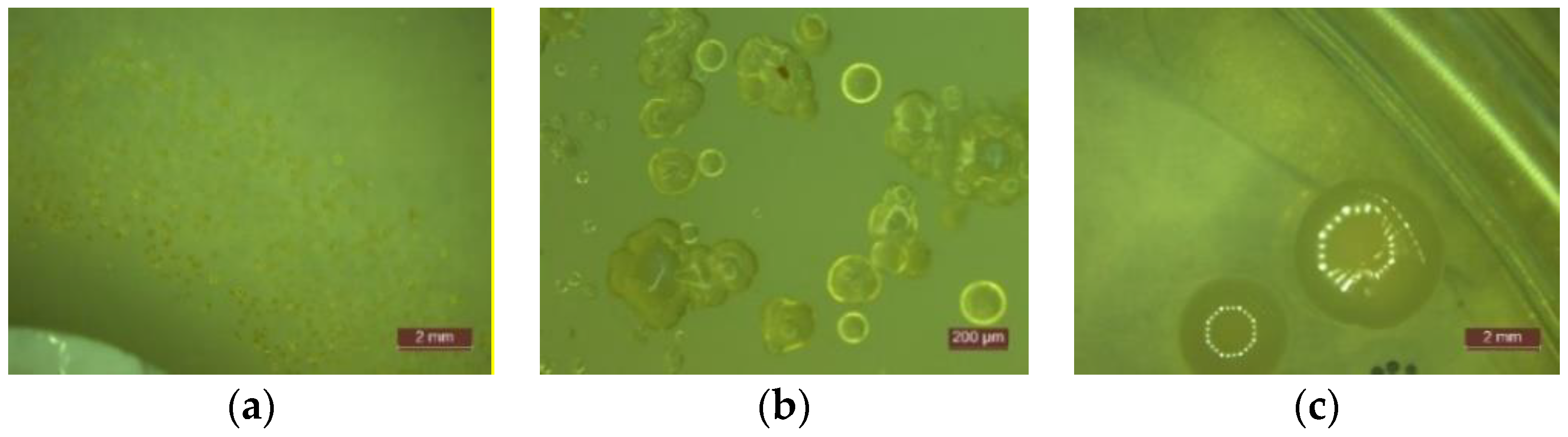
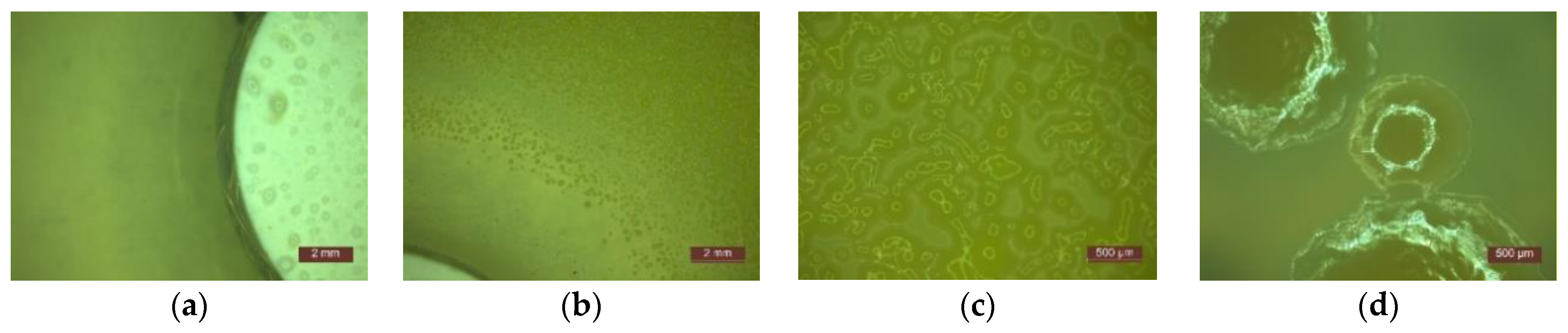
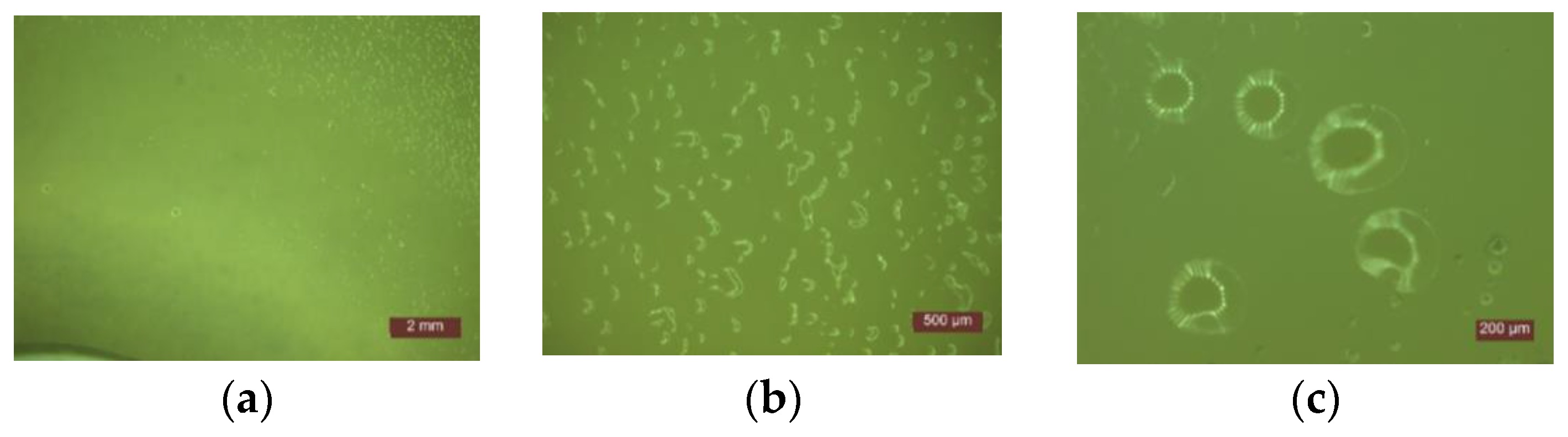
- In the first 48 h after exposure in the contaminated environment, the formation of inhibition haloes was observed, which remained constant in size and shape throughout the test. The systems presented a concentric shape: the cementitious composite sample being surrounded by a circular area with microbiological load, evaluated according to STAS 12718/1989, in Class 0 (-). This was followed by a zone of growth and the development of biological material. This increase was more intense as the distance from the edge of the cementitious composite increased (Figure 2, Figure 3, Figure 4, Figure 5, Figure 6 and Figure 7). The only exception was observed for samples tested with Pseudomonas Aeruginosa, for which no inhibition halo was identified in the cementitious composite control system.
- Sample P10 (12% TiO2) had in general a smaller halo diameter than samples with lower nanoparticle content. This behavior can be attributed to the inhomogeneity and the improper dispersion of nanoparticles in the cementitious matrix, which may tend to agglomerate.
- The P0 system, without the cementitious composite sample, had the most intense and rapid development of colonies of bacteria.
- Microscopic analysis revealed the presence and development of colonies of bacteria in the areas outside the inhibition halo, which again indicate the viability of the suspension used for seeding, the right choice of nutrient substrate, and exposure conditions.
- The formation of the inhibition halo for the control composite system (0% TiO2) also indicated resistance to the development of bacteria. This mainly happened because of the chemical composition of white Portland cement, which usually contains a certain quantity of TiO2.
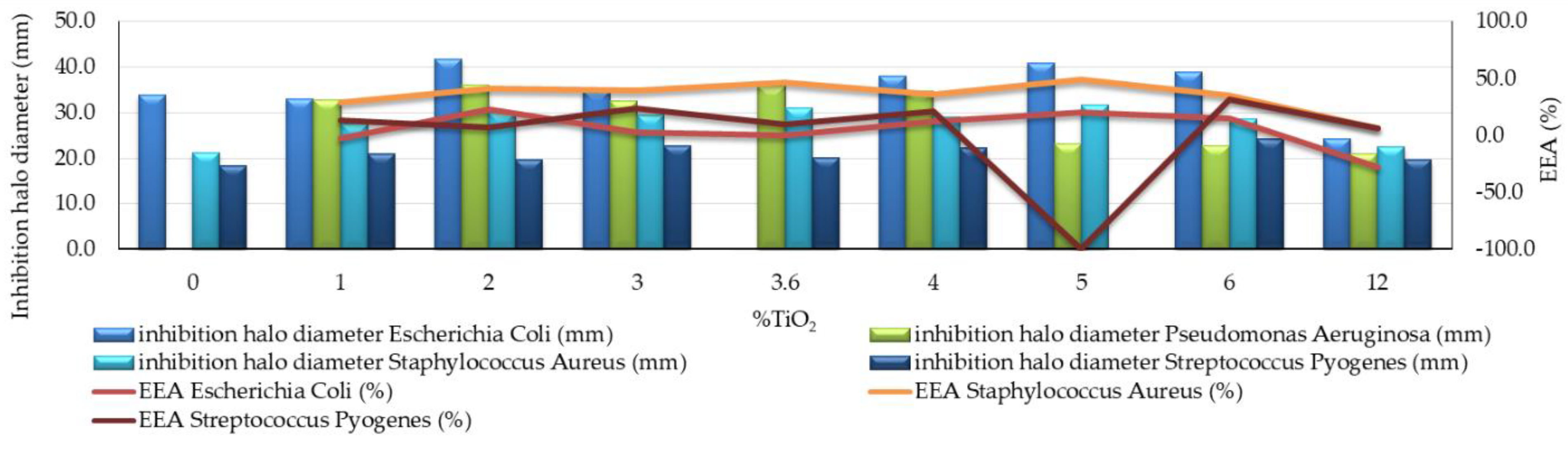
- Cementitious composites with nano-TiO2 content in the range of 2–6% had the most effective behavior, with an efficiency of the antibacterial effect (EEA) of more than 10% (Figure 3). The highest value of this parameter (23%) was reported for the samples with 2% nano-TiO2 addition.
- When evaluating the entire system by quantifying the microbiological load of the system, according to STAS 12718 (Table 3), Classes 0 (-) or 1 (+) were observed/maintained for a longer period. It was also noticed that the samples with 4–12% nano-TiO2—(Class 0 (-))—maintain this sterile behavior longer (even after 7 days of exposure in contaminated environment—sample P6 (4% TiO2)). In addition, the P3 sample (2% TiO2) had a distinguished behavior, by keeping Class 1 (+) constant until the end of the test period.
- In the case of the P0 system, the formation of zones with confluent colonies (Class 3 (+++)) was observed earlier, after only 4 days at exposure in the contaminated environment. This confirmed the viability of the inoculated bacterial material.
- Due to the lack of visible and measurable inhibition halo in the control sample, the effectiveness of the antibacterial effect (EEA) could not be calculated, thus indicating a resistance effect to these bacteria of the cementitious composite matrix (Figure 3). However, for samples with 3.6% and 4% nano-TiO2, large inhibition halos have been observed.
- When evaluating the entire system by quantifying the microbiological load of the system, according to STAS 12718 (Table 4), Class 2 (++) was observed and maintained for a longer period. This happened due to the higher content of nanoparticles in the cementitious composite mass. For composite samples with 3.6–12% nano-TiO2, the framing Class 2 (++) was maintained throughout the 21 days of testing. This also happened for sample P4 (3% TiO2), whose framing class changed from 2 (++) to 3 (+++) only at the last stage of testing (during 14–21 days of exposure in the contaminated environment).
- In the case of the P0 system and the P1 control sample system (0% TiO2), the formation of areas with Class 3 (+++) confluent colonies was rapidly observed after 2 days of exposure in the contaminated environment, which on the one hand indicates the pre-viability of the bacterial inoculum material and on the other hand indicates the lack of antibacterial activity in the case of the P1 control composite matrix (0% TiO2) (Table 4).
- Samples containing nano-TiO2 in the range of 1% to 5% had the most satisfactory behavior, i.e., an efficiency of the antibacterial effect (EEA) of more than 25% (Figure 3). The maximum effectiveness of the antibacterial effect (EEA) was achieved by the samples with 5% nano-TiO2, for which this indicator was 49%.
- The development of Staphylococcus Aureus colonies occurred less readily compared to the other types of bacteria analyzed in the study. The identified colonies were visible to the naked eye after only 2–3 days of exposure in the contaminated environment.
- In the case of the P0 system, colony formation Class 1 (+) was observed after 3 days of exposure, which confirms the viability of the inoculated bacterial material (Table 5).
- Samples with nano-TiO2 content in the range of 3–6% showed better behavior in terms of the ability to inhibit colony growth.
- When evaluating the entire system by quantifying the microbiological load of the system, according to STAS 12718 (Table 6), Classes 1 (+) or 2 (++) were observed and maintained during the first 2–3 days after exposure to the contaminated environment. For samples with 2–6% nano-TiO2, this classification was kept constant for up to 3 days. In these cases, the EEA quantifiable parameter reached the maximum value, i.e., 31%, for the 6% nano-TiO2 composition (Figure 3);
- In the case of the P0 system, the formation of more than 10 colonies, Class 2 (++) was observed after 2 days, Class 3 (+++) confluent colonies were observed after 3 days, and also Class 4 (++++) growth was observed throughout the surface (Table 6). Almost the same behavior, differentiated only by the delay in their development, was observed for P1 (0% TiO2), P2 (1% TiO2), and even the system with the maximum nanoparticle content, P10 (12% TiO2).
4. Conclusions
- The viability of the contaminants, selection of nutrients, and temperature conditions were proven. Therefore, the identification, quantification, and comparison between their action and the results regarding the growth of the biological material, when subjected to the cementitious composites, was demonstrated, based on the retention time of the samples in the contaminated environment and the content of nano-TiO2 in the samples.
- The effect of the development of the inhibition halo, when subjected to Escherichia coli, Pseudomonas Aeruginosa, and Staphylococus Aureus bacteria, namely, Streptococcus Pyogenes has also been confirmed for samples containing nano-TiO2 in the range of 2% to 5%. However, the introduction of large quantities of nanoparticles in the matrix of the composite may be on one hand beneficial in terms of antibacterial effects but, on the other hand, it is harmful as a result of the tendency of agglomeration of the nanoparticles in the matrix of the composite. Therefore, the effect of the antibacterial agent is considerably reduced.
- It was considered that for a good inhibiting activity against the development of contaminants of type Escherichia coli, Pseudomonas Aeruginosa, Staphylococcus Aureus, and Streptococcus Pyogenes, the content of TiO2 nanoparticles in the cementitious composite matrix should be at least 2% and not more than 5% relative to the amount of cement. The possibility remains open that composite samples with more than 5% nano-TiO2 are antibacterial effective if adequate nanoparticle dispersion is ensured. This range of identified nano-TiO2 amount is consistent with reports in the literature [36,37,38,39,40,41,42,51,52,53,54,55,56].
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Haleem Khan, A.A.; Mohan Karuppayil, S. Fungal pollution of indoor environments and its management. Saudi J. Biol. Sci. 2012, 19, 405–426. [Google Scholar] [CrossRef]
- Ebbehoj, N.E.; Hansen, M.O.; Sigsgaard, T.; Larsen, L. Building-related symptoms and molds: A two-step intervention study. Indoor Air 2002, 12, 273–277. [Google Scholar] [CrossRef]
- Zeliger, H.I. Toxic effects of chemical mixtures. Arch. Environ. Health 2003, 58, 23–29. [Google Scholar] [CrossRef]
- Yadav, H.M.; Kim, J.S.; Pawar, S.H. Developments in photocatalytic antibacterial activity of nano TiO2: A review. Korean J. Chem. Eng. 2016, 33, 1989–1998. [Google Scholar] [CrossRef]
- Wang, L.; Hu, C.; Shao, L. The antimicrobial activity of nanoparticles: Present situation and prospects for the future. Int. J. Nanomed. 2017, 12, 1227–1249. [Google Scholar] [CrossRef]
- Kühn, K.P.; Chaberny, I.F.; Massholder, K.; Stickler, M.; Benz, V.W.; Sonntag, H.G.; Erdinger, L. Disinfection of surfaces by photocatalytic oxidation with titanium dioxide and UVA light. Chemosphere 2003, 53, 71–77. [Google Scholar] [CrossRef]
- Drugă, B.; Ukrainczyk, N.; Weise, K.; Koenders, E.; Lackner, S. Interaction between wastewater microorganisms and geopolymer or cementitious materials: Biofilm characterization and deterioration characteristics of mortars. Int. Biodeterior. Biodegrad. 2018, 134, 58–67. [Google Scholar] [CrossRef]
- Machida, M.; Norimoto, K.; Kimura, T. Antibacterial Activity of Photocatalytic Titanium Dioxide Thin Films with Photodeposited Silver on the Surface of Sanitary Ware. J. Am. Ceram. Soc. 2005, 88, 95–100. [Google Scholar] [CrossRef]
- Page, K.; Wilson, M.; Parkin, I.P. Antimicrobial surfaces and their potential in reducing the role of the inanimate environment in the incidence of hospital-acquired infections. J. Mater. Chem. 2009, 19, 3819. [Google Scholar] [CrossRef]
- Watts, R.J.; Kong, S.; Orr, M.P.; Miller, G.C.; Henry, B.E. Photocatalytic inactivation of coliform bacteria and viruses in secondary wastewater effluent. Water Res. 1995, 29, 95–100. [Google Scholar] [CrossRef]
- Vohra, A.; Goswami, D.Y.; Deshpande, D.A.; Block, S.S. Enhanced photocatalytic inactivation of bacterial spores on surfaces in air. J. Ind. Microbiol. Biotechnol. 2005, 32, 364–370. [Google Scholar] [CrossRef]
- Cassar, L. Nanotechnology and photocatalysis in cementitious materials. In NICOM 2: 2nd International Symposium on Nanotechnology in Construction; de Miguel, Y., Porro, A., Bartos, P.J.M., Eds.; RILEM Publications SARL: Paris, France, 2006; pp. 277–284. [Google Scholar]
- Wang, L.; Zhang, H.; Gao, Y. Effect of TiO2 nanoparticles on physical and mechanical properties of cement at low temperatures. Adv. Mater. Sci. Eng. 2018, 2018, 8934689. [Google Scholar] [CrossRef]
- Sadawy, M.M.; Elsharkawy, E.R. Effect of Nano-TiO2 on mechanical properties of concrete and corrosion behavior of reinforcement bars. Int. J. Eng. Res. Appl. 2016, 6, 61–65. [Google Scholar]
- Salemi, N.; Behfarnia, K.; Zaree, S.A. Effect of nanoparticles on frost durability of concrete. Asian J. Civ. Eng. 2014, 15, 411–420. [Google Scholar]
- Shen, W.; Zhang, C.; Li, Q.; Zhang, W.; Cao, L.; Ye, J. Preparation of titanium dioxide nano particle modified photocatalytic self-cleaning concrete. J. Clean. Prod. 2015, 87, 762–765. [Google Scholar] [CrossRef]
- Matsunaga, T.; Tomoda, R.; Nakajima, T.; Nakamura, N.; Komine, T. Continuous-sterilization system that uses photosemiconductor powders. Appl. Environ. Microbiol. 1988, 54, 1330–1333. [Google Scholar] [CrossRef]
- Abdel-Gawwad, H.A.; Mohamed, S.A.; Mohammed, M.S. Recycling of slag and lead-bearing sludge in the cleaner production of alkali activated cement with high performance and microbial resistivity. J. Clean. Prod. 2019, 220, 568–580. [Google Scholar] [CrossRef]
- Guzmán-Aponte, L.; Mejía de Gutiérrez, R.; Maury-Ramírez, A. Metakaolin-Based Geopolymer with Added TiO2 particles: Physicomechanical Characteristics. Coatings 2017, 7, 233. [Google Scholar] [CrossRef]
- Mohd Adnan, M.A.; Muhd Julkapli, N.; Amir, M.N.I.; Maamor, A. Effect on different TiO2 photocatalyst supports on photodecolorization of synthetic dyes: A review. Int. J. Environ. Sci. Technol. 2019, 16, 547–566. [Google Scholar] [CrossRef]
- Haider, A.J.; Anbari, R.; Kadhim, G.R.; Salame, C.T. Exploring potential environmental applications of TiO2 nanoparticles. Energy Procedia 2017, 119, 332–345. [Google Scholar] [CrossRef]
- Hoffmann, M.R.; Martin, S.T.; Choi, W.; Bahnemannt, D.W. Environmental Applications of Semiconductor Photocatalysis. Chem. Rev. 1995, 95, 69–96. [Google Scholar] [CrossRef]
- Mejía, J.M.; Mendoza, J.D.; Yucuma, J.; Mejía de Gutiérrez, R.; Mejía, D.E.; Astudillo, M. Mechanical, in-vitro biological and antimicrobial characterization as an evaluation protocol of a ceramic material based on alkaline activated metakaolin. Appl. Clay Sci. 2019, 178, 105141. [Google Scholar] [CrossRef]
- Syamsidar, D.; Nurfadilla, S. The Properties of Nano TiO2 -Geopolymer Composite as a Material for Functional Surface Application. MATEC Web Conf. 2017, 97, 01013. [Google Scholar] [CrossRef]
- Sikora, P.; Augustyniak, A.; Cendrowski, K.; Horszczaruk, E.; Rucinska, T.; Nawrotek, P.; Mijowska, E. Characterization of mechanical and bactericidal properties of cement mortars containing waste glass aggregate and nanomaterials. Materials 2016, 9, 701. [Google Scholar] [CrossRef] [PubMed]
- Hamidi, F.; Aslani, F. TiO2-based Photocatalytic Cementitious Composites: Materials, Properties, Influential Parameters, and Assessment Techniques. Nanomaterials 2019, 9, 1444. [Google Scholar] [CrossRef] [PubMed]
- Sunada, K.; Watanabe, T.; Hashimoto, K. Bactericidal Activity of Copper-Deposited TiO2 Thin Film under Weak UV Light Illumination. Environ. Sci. Technol. 2003, 37, 4785–4789. [Google Scholar] [CrossRef]
- Saito, T.; Iwase, T.; Horie, J.; Morioka, T. Mode of photocatalytic bactericidal action of powdered semiconductor TiO2 on mutans streptococci. J. Photochem. Photobiol. B 1992, 14, 369–379. [Google Scholar] [CrossRef]
- Oguma, K.; Katayama, H.; Ohgaki, S. Photoreactivation of Escherichia coli after Low- or Medium-Pressure UV Disinfection Determined by an Endonuclease Sensitive Site Assay. Appl. Environ. Microbiol. 2002, 68, 6029–6035. [Google Scholar] [CrossRef]
- Gogniat, G.; Thyssen, M.; Denis, M.; Pulgarin, C.; Dukan, S. The bactericidal effect of TiO2 photocatalysis involves adsorption onto catalyst and the loss of membrane integrity. FEMS Microbiol. Lett. 2006, 258, 18–24. [Google Scholar] [CrossRef]
- Mazurkova, N.A.; Spitsyna, Y.E.; Shikina, N.V.; Ismagilov, Z.R.; Zagrebel’Nyi, S.N.; Ryabchikova, E.I. Interaction of titanium dioxide nanoparticles with influenza virus. Nanotechnol. Russia 2010, 5, 417–420. [Google Scholar] [CrossRef]
- Adams, L.K.; Lyon, D.Y.; McIntosh, A.; Alvarez, P.J.J. Comparative toxicity of nano-scale TiO2, SiO2 and ZnO water suspensions. Water Sci. Technol. 2006, 54, 327–334. [Google Scholar] [CrossRef]
- Armelao, L.; Barreca, D.; Bottaro, G.; Gasparotto, A.; Maccato, C.; Maragno, C.; Tondello, E.; Stangar, U.L.; Bergant, M.; Mahne, D. Photocatalytic and antibacterial activity of TiO2 and Au/TiO2 nanosystems. Nanotechnology 2007, 18, 375709. [Google Scholar] [CrossRef]
- Dědková, K.; Matějová, K.; Lang, J.; Peikertová, P.; Kutláková, K.M.; Neuwirthová, L.; Frydrýšek, K.; Kukutschová, J. Antibacterial activity of kaolinite/nanoTiO2 composites in relation to irradiation time. J. Photochem. Photobiol. B 2014, 135, 17–22. [Google Scholar]
- Gurr, J.R.; Wang, A.S.S.; Chen, C.H. Ultrafine titanium dioxide particles in the absence of photoactivation can induce oxidative damage to human bronchial epithelial cells. Toxicology 2005, 213, 66–73. [Google Scholar] [CrossRef]
- Hamdany, A.H. Photocatalytic Cementitious Material for Self-Cleaning and Anti-Microbial Application. Ph.D. Thesis, Nanyang Technological University, Singapore, 2019. [Google Scholar]
- Davidson, H.; Poon, M.; Saunders, R.; Shapiro, I.M.; Hickok, N.J.; Adams, C.S. Tetracycline tethered to titanium inhibits colonization by Gram-negative bacteria. J. Biomed. Mater. Res. B Appl. Biomater. 2015, 103, 1381–1389. [Google Scholar] [CrossRef]
- Lorenzetti, M.; Dogša, I.; Stošicki, T.; Stopar, D.; Kalin, M.; Kobe, S.; Novak, S. The Influence of Surface Modification on Bacterial Adhesion to Titanium-Based Substrates. ACS Appl. Mater. Interfaces 2015, 7, 1644–1651. [Google Scholar] [CrossRef] [PubMed]
- Peng, Z.; Ni, J.; Zheng, K.; Shen, Y.; Wang, X.; He, G.; Jin, S.; Tang, T. Dual effects and mechanism of TiO2 nanotube arrays in reducing bacterial colonization and enhancing C3H10T1/2 cell adhesion. Int. J. Nanomed. 2013, 8, 3093–3105. [Google Scholar]
- Daly, M.J.; Gaidamakova, E.K.; Matrosova, V.Y.; Vasilenko, A.; Zhai, M.; Leapman, R.D.; Lai, B.; Ravel, B.; Li, S.-M.W.; Kemner, K.M.; et al. Protein Oxidation Implicated as the Primary Determinant of Bacterial Radioresistance. PLoS Biol. 2007, 5, 92. [Google Scholar] [CrossRef]
- Carre, G.; Estner, M.; Gies, J.-P.; Andre, P.; Hamon, E.; Ennahar, S.; Keller, V.; Keller, N.; Lett, M.-C.; Horvatovich, P. TiO2 Photocatalysis Damages Lipids and Proteins in Escherichia coli. Appl. Environ. Microbiol. 2014, 80, 2573–2581. [Google Scholar] [CrossRef] [PubMed]
- Kubacka, A.; Diez, M.S.; Rojo, D.; Bargiela, R.; Ciordia, S.; Zapico, I.; Albar, J.P.; Barbas, C.; Martins dos Santos, V.A.P.; Fernández-garcía, M.; et al. Understanding the antimicrobial mechanism of TiO2-based nanocomposite films in a pathogenic bacterium. Sci. Rep. 2014, 4, 4134. [Google Scholar] [CrossRef] [PubMed]
- Mejía-de Gutiérrez, R.; Villaquirán-Caicedo, M.; Ramírez-Benavides, S.; Astudillo, M.; Mejía, D. Evaluation of the Antibacterial Activity of a Geopolymer Mortar based on Supplemented with TiO2 and CuO Particles Using Glass Waste as Fine Aggregate. Coattings 2020, 10, 157. [Google Scholar] [CrossRef]
- Pacheco-Torgal, F.; Jalali, S. Nanotechnology: Advantages and drawbacks in the field of construction and building materials. Contr. Build. Mater. 2011, 25, 582–590. [Google Scholar] [CrossRef]
- Cho, M.; Chung, H.; Choi, W.; Yoon, J. Linear correlation between inactivation of E. coli and OH radical concentration in TiO2 photocatalytic disinfection. Water Res. 2004, 38, 1069–1077. [Google Scholar] [CrossRef]
- Janus, M.; Zajac, K. Concretes with Photocatalytic Activity. In High Performance Concrete Technology and Applications; Yilmax, S., Baytan, H., Eds.; IntechOpen: London, UK, 2016; Chapter 7; pp. 141–161. [Google Scholar]
- Carmona-Quiroga, P.M.; Martinez-Ramirez, S.; Viles, H.A. Efficiency and durability of a self-cleaning coating on concrete and stones under both natural and artificial ageing trials. App. Surf. Sci. 2018, 433, 312–320. [Google Scholar] [CrossRef]
- Grebenişan, E.; Hegyi, A.; Szilagyi, H.; Lăzărescu, A.V.; Ionescu, B.A. Influence of the Addition of TiO2 Nanoparticles on the Self-Cleaning Performance of Cementitious Composite Surfaces. Proceedings 2020, 1, 42. [Google Scholar] [CrossRef]
- Grebenişan, E.; Hegyi, A.; Lăzărescu, A.V. Research Regarding the Influence of TiO2 Nanoparticles on the Performance of Cementitious Materials. In IOP Conference Series: Materials Science and Engineering, Proceedings of the International Conference on Innovative Research—ICIR EUROINVENT 2020, Iasi, Romania, 21–23 May 2020; IOP Publishing: Bristol, UK, 2020; Volume 877, p. 012004. [Google Scholar]
- Hegyi, A.; Szilagyi, H.; Grebenișan, E.; Sandu, A.V.; Lăzărescu, A.V.; Romila, C. Influence of TiO2 Nanoparticles Addition on the Hydrophilicity of Cementitious Composites Surfaces. Appl. Sci. 2020, 10, 4501. [Google Scholar] [CrossRef]
- Gopalan, A.-I.; Lee, J.-C.; Saianand, G.; Lee, K.-P.; Sonar, P.; Dharmarajan, R.; Hou, Y.; Ann, K.-Y.; Kannan, V.; Kim, W.-J. Recent progress in the abatement of hazardous pollutants using photocatalytic TiO2-based building materials. Nanomaterials 2020, 10, 1854. [Google Scholar] [CrossRef]
- Shaaban, I.G.; El-Sayad, H.; El-Ghaly, A.E.; Moussa, S. Effect of micro TiO2 on cement mortar. EJME 2020, 5, 58–68. [Google Scholar] [CrossRef]
- Daniyal, M.; Azam, A.; Akhtar, S. Application of Nanomaterials in Civil Engineering. In Nanomaterials and Their Applications. Advanced Structured Materials; Khan, Z., Ed.; Springer: Singapore, 2018; Volume 84, pp. 169–189. [Google Scholar]
- Wang, D.; Geng, Z.; Hou, P.; Yang, P.; Cheng, X.; Huang, S. Rhodamine B Removal of TiO2@ SiO2 Core-Shell Nanocomposites Coated to Buildings. Crystals 2020, 10, 80. [Google Scholar] [CrossRef]
- Guo, M.Z.; Maury-Ramirez, A.; Poon, C.S. Self-cleaning ability of titanium dioxide clear paint coated architectural mortar and its potential in field application. J. Clean. Prod. 2016, 112, 3583–3588. [Google Scholar] [CrossRef]
- Sikora, P.; Cendrowski, K.; Markowska-Szczupak, A.; Horszczaruk, E.; Mijowska, E. The effects of silica/titania nanocomposite on the mechanical and bactericidal properties of cement mortars. Constr. Build. Mater. 2017, 150, 738–746. [Google Scholar] [CrossRef]

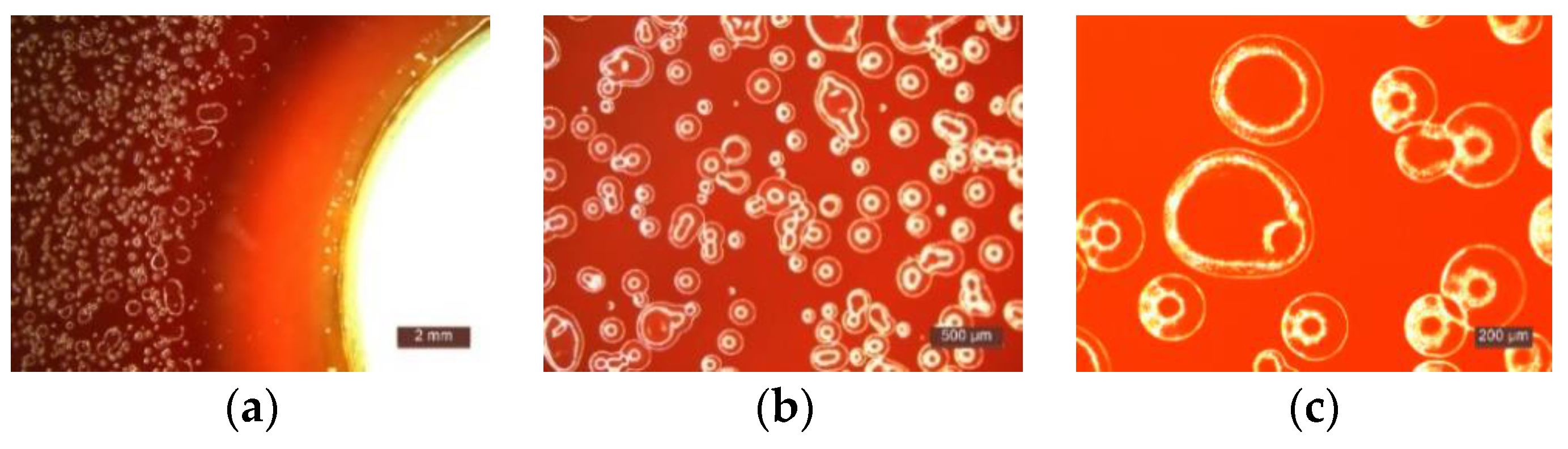
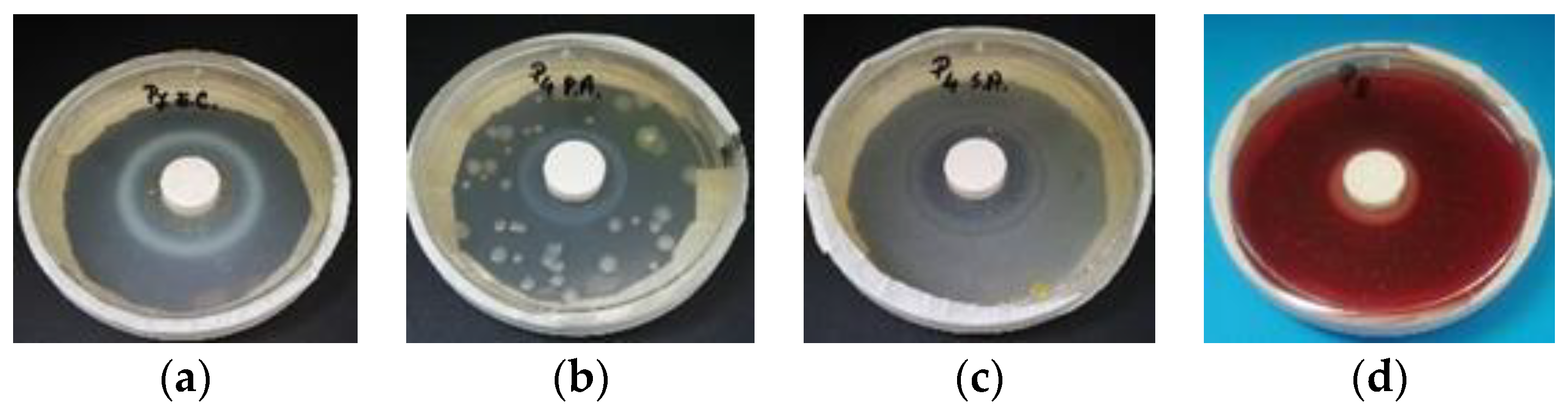
| Mixture Number | P1 | P2 | P3 | P4 | P5 | P6 | P7 | P8 | P9 | P10 |
|---|---|---|---|---|---|---|---|---|---|---|
| Amount of nanoparticles relative to the amount of cement (%) | 0 | 1 | 2 | 3 | 3.6 | 4 | 5 | 6 | 10 | 12 |
| CEM I 52,5R white cement, HOLCIM (g) | 500 | 500 | 500 | 500 | 500 | 500 | 500 | 500 | 500 | 500 |
| Amount of water relative to the amount of total dry mixture (water/(cement + nano-TiO2)) (g) | 0.5 | |||||||||
| Conditioning |
| |||||||||
| 0 (-) | no growth (sterile) |
| 1 (+) | 1–10 colonies of microorganisms |
| 2 (++) | over 10 colonies of microorganisms |
| 3 (+++) | areas with confluent colonies |
| 4 (++++) | growth throughout the surface |
| Exposure Period (Days) | P0 (without Composite Sample) | P1 (0% TiO2) | P2 (1% TiO2) | P3 (2% TiO2) | P4 (3% TiO2) | P5 (3.6% TiO2) | P6 (4% TiO2) | P7 (5% TiO2) | P8 (6% TiO2) | P10 (12% TiO2) |
|---|---|---|---|---|---|---|---|---|---|---|
| 2 | 1 (+) | 1 (+) | 1 (+) | 1 (+) | 1 (+) | 1 (+) | 0 (-) | 0 (-) | 0 (-) | 0 (-) |
| 3 | 1 (+) | 1 (+) | 1 (+) | 1 (+) | 1 (+) | 1 (+) | 0 (-) | 0 (-) | 0 (-) | 0 (-) |
| 4 | 3 (+++) | 1 (+) | 1 (+) | 1 (+) | 1 (+) | 1 (+) | 0 (-) | 0 (-) | 0 (-) | 0 (-) |
| 6 | 3 (+++) | 1 (+) | 1 (+) | 1 (+) | 1 (+) | 1 (+) | 0 (-) | 0 (-) | 1 (+) | 1 (+) |
| 7 | 3 (+++) | 1 (+) | 1 (+) | 1 (+) | 1 (+) | 1 (+) | 0 (-) | 1 (+) | 1 (+) | 1 (+) |
| 14 | 3 (+++) | 1 (+) | 1 (+) | 1 (+) | 1 (+) | 1 (+) | 1 (+) | 1 (+) | 1 (+) | 1 (+) |
| 21 | 3 (+++) | 1 (+) | 3 (+++) | 1 (+) | 3 (+++) | 3 (+++) | 2 (++) | 1 (+) | 1 (+) | 1 (+) |
| Exposure Period (Days) | P0 (without Composite Sample) | P1 (0% TiO2) | P2 (1% TiO2) | P3 (2% TiO2) | P4 (3% TiO2) | P5 (3.6% TiO2) | P6 (4% TiO2) | P7 (5% TiO2) | P8 (6% TiO2) | P10 (12% TiO2) |
|---|---|---|---|---|---|---|---|---|---|---|
| 2 | 3 (+++) | 3 (+++) | 2 (++) | 2 (++) | 2 (++) | 2 (++) | 2 (++) | 2 (++) | 2 (++) | 2 (++) |
| 3 | 3 (+++) | 3 (+++) | 2 (++) | 2 (++) | 2 (++) | 2 (++) | 2 (++) | 2 (++) | 2 (++) | 2 (++) |
| 4 | 3 (+++) | 3 (+++) | 2 (++) | 2 (++) | 2 (++) | 2 (++) | 2 (++) | 2 (++) | 2 (++) | 2 (++) |
| 6 | 3 (+++) | 3 (+++) | 2 (++) | 2 (++) | 2 (++) | 2 (++) | 2 (++) | 2 (++) | 2 (++) | 2 (++) |
| 7 | 3 (+++) | 3 (+++) | 3 (+++) | 2 (++) | 2 (++) | 2 (++) | 2 (++) | 2 (++) | 2 (++) | 2 (++) |
| 14 | 3 (+++) | 3 (+++) | 3 (+++) | 3 (+++) | 2 (++) | 2 (++) | 2 (++) | 2 (++) | 2 (++) | 2 (++) |
| 21 | 3 (+++) | 3 (+++) | 3 (+++) | 3 (+++) | 3 (+++) | 2 (++) | 2 (++) | 2 (++) | 2 (++) | 2 (++) |
| Exposure Period (Days) | P0 (without Composite Sample) | P1 (0% TiO2) | P2 (1% TiO2) | P3 (2% TiO2) | P4 (3% TiO2) | P5 (3.6% TiO2) | P6 (4% TiO2) | P7 (5% TiO2) | P8 (6% TiO2) | P10 (12% TiO2) |
|---|---|---|---|---|---|---|---|---|---|---|
| 2 | 0 (-) | 0 (-) | 0 (-) | 0 (-) | 0 (-) | 0 (-) | 0 (-) | 0 (-) | 0 (-) | 0 (-) |
| 3 | 1 (+) | 1 (+) | 0 (-) | 0 (-) | 0 (-) | 0 (-) | 0 (-) | 1 (+) | 0 (-) | 0 (-) |
| 4 | 1 (+) | 1 (+) | 0 (-) | 0 (-) | 1 (+) | 0 (-) | 0 (-) | 1 (+) | 0 (-) | 1 (+) |
| 6 | 1 (+) | 1 (+) | 1 (+) | 0 (-) | 1 (+) | 0 (-) | 1 (+) | 1 (+) | 0 (-) | 1 (+) |
| 7 | 1 (+) | 1 (+) | 1 (+) | 0 (-) | 1 (+) | 1 (+) | 1 (+) | 3 (+++) | 0 (-) | 1 (+) |
| 14 | 1 (+) | 1 (+) | 1 (+) | 0 (-) | 1 (+) | 1 (+) | 1 (+) | 3 (+++) | 1 (+) | 1 (+) |
| 21 | 1 (+) | 1 (+) | 1 (+) | 1 (+) | 1 (+) | 1 (+) | 1 (+) | 3 (+++) | 1 (+) | 1 (+) |
| Exposure Period (Days) | P0 (without Composite Sample) | P1 (0% TiO2) | P2 (1% TiO2) | P3 (2% TiO2) | P4 (3% TiO2) | P5 (3.6% TiO2) | P6 (4% TiO2) | P7 (5% TiO2) | P8 (6% TiO2) | P10 (12% TiO2) |
|---|---|---|---|---|---|---|---|---|---|---|
| 2 | 2 (++) | 2 (++) | 1 (+) | 1 (+) | 1 (+) | 1 (+) | 1 (+) | 1 (+) | 1 (+) | 2 (++) |
| 3 | 3 (+++) | 2 (++) | 2 (++) | 1 (+) | 1 (+) | 1 (+) | 1 (+) | 1 (+) | 1 (+) | 2 (++) |
| 4 | 3 (+++) | 2 (++) | 2 (++) | 2 (++) | 2 (++) | 2 (++) | 2 (++) | 2 (++) | 2 (++) | 2 (++) |
| 6 | 3 (+++) | 3 (+++) | 3 (+++) | 2 (++) | 2 (++) | 2 (++) | 2 (++) | 2 (++) | 3 (+++) | 3 (+++) |
| 7 | 3 (+++) | 3 (+++) | 3 (+++) | 2 (++) | 2 (++) | 2 (++) | 2 (++) | 3 (+++) | 3 (+++) | 3 (+++) |
| 14 | 4 (++++) | 4 (++++) | 3 (+++) | 3 (+++) | 3 (+++) | 3 (+++) | 3 (+++) | 3 (+++) | 4 (++++) | 4 (++++) |
| 21 | 4 (++++) | 4 (++++) | 4 (++++) | 3 (+++) | 3 (+++) | 3 (+++) | 3 (+++) | 3 (+++) | 4 (++++) | 4 (++++) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hegyi, A.; Lăzărescu, A.-V.; Szilagyi, H.; Grebenişan, E.; Goia, J.; Mircea, A. Influence of TiO2 Nanoparticles on the Resistance of Cementitious Composite Materials to the Action of Bacteria. Materials 2021, 14, 1074. https://doi.org/10.3390/ma14051074
Hegyi A, Lăzărescu A-V, Szilagyi H, Grebenişan E, Goia J, Mircea A. Influence of TiO2 Nanoparticles on the Resistance of Cementitious Composite Materials to the Action of Bacteria. Materials. 2021; 14(5):1074. https://doi.org/10.3390/ma14051074
Chicago/Turabian StyleHegyi, Andreea, Adrian-Victor Lăzărescu, Henriette Szilagyi, Elvira Grebenişan, Jana Goia, and Andreea Mircea. 2021. "Influence of TiO2 Nanoparticles on the Resistance of Cementitious Composite Materials to the Action of Bacteria" Materials 14, no. 5: 1074. https://doi.org/10.3390/ma14051074
APA StyleHegyi, A., Lăzărescu, A.-V., Szilagyi, H., Grebenişan, E., Goia, J., & Mircea, A. (2021). Influence of TiO2 Nanoparticles on the Resistance of Cementitious Composite Materials to the Action of Bacteria. Materials, 14(5), 1074. https://doi.org/10.3390/ma14051074








