Upconversion Luminescence of Silica–Calcia Nanoparticles Co-doped with Tm3+ and Yb3+ Ions
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis of SiO2–CaO Particles Doped with Rare Earth Ions
2.2. Characterization
3. Results and Discussion
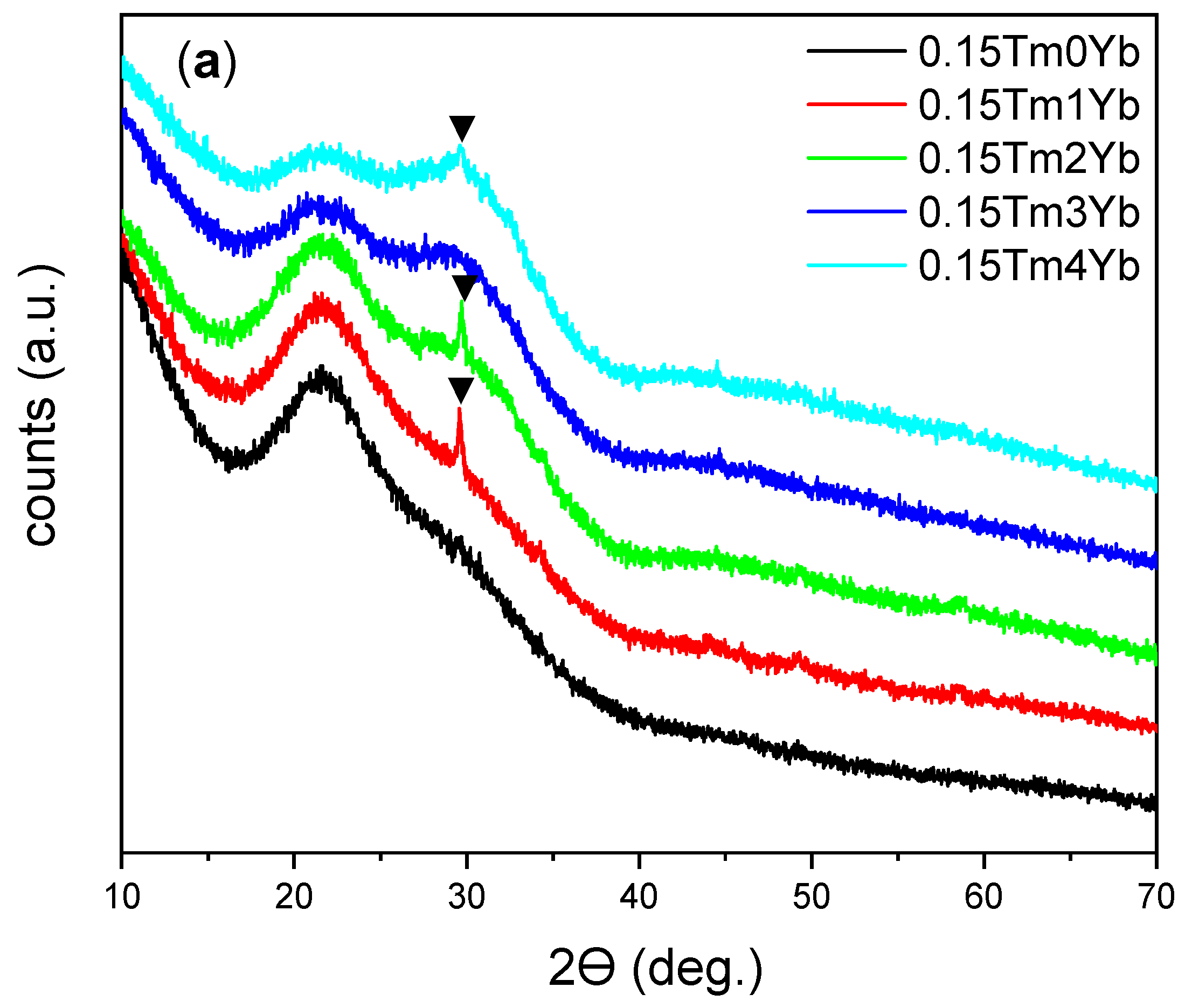
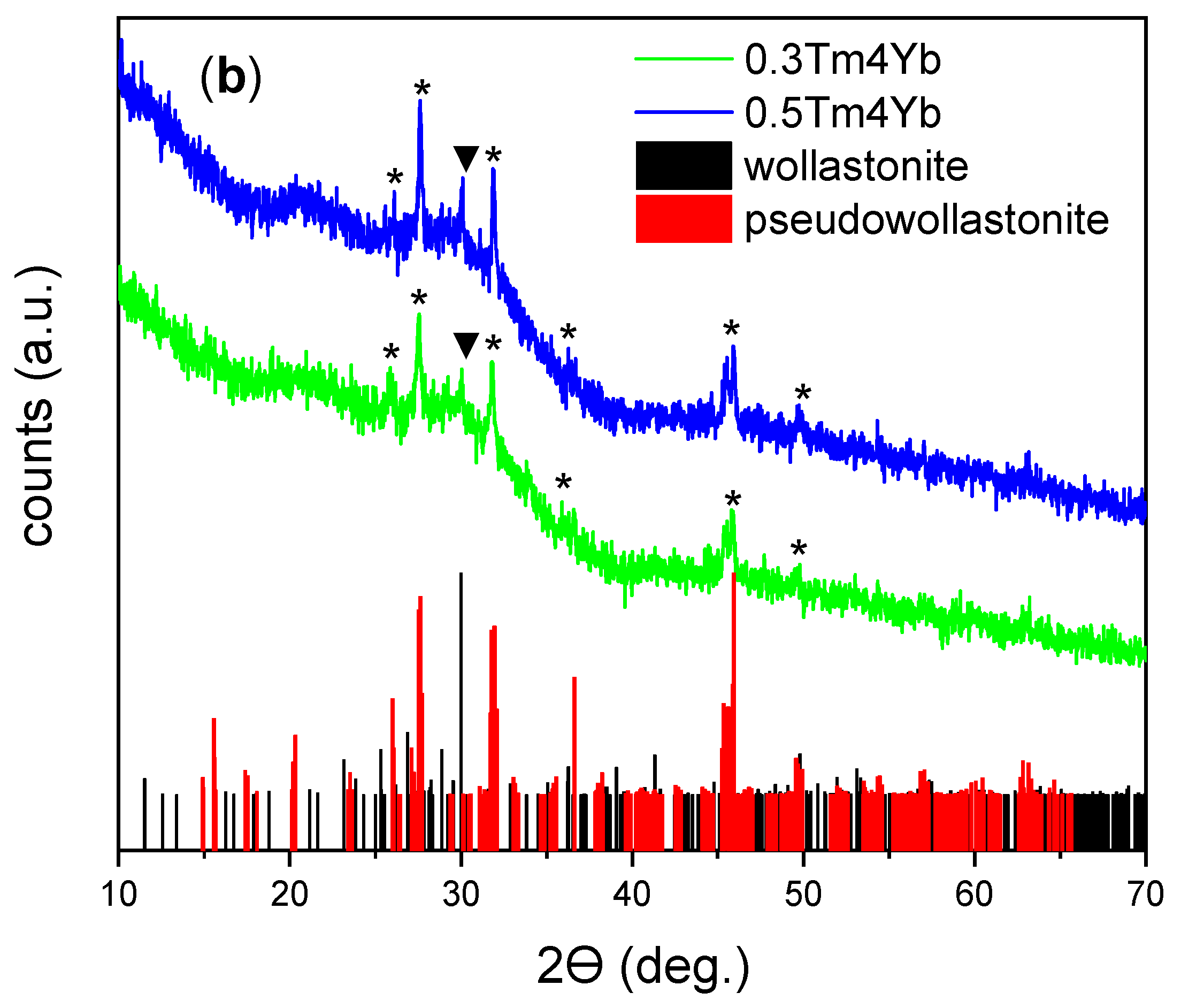
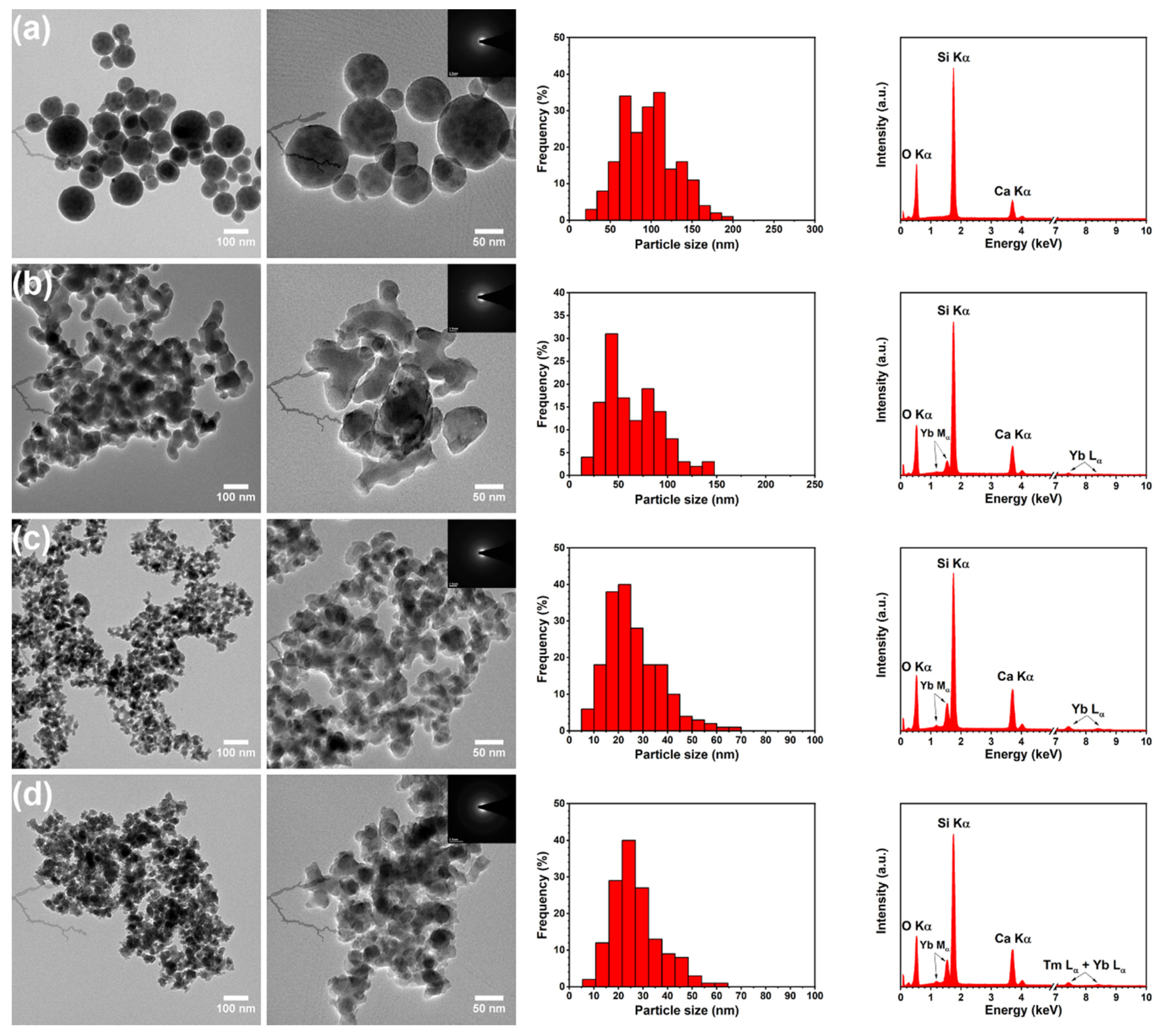
3.1. Structural and Morphological Characterization
3.2. Spectroscopic Properties
3.2.1. Absorption Spectra
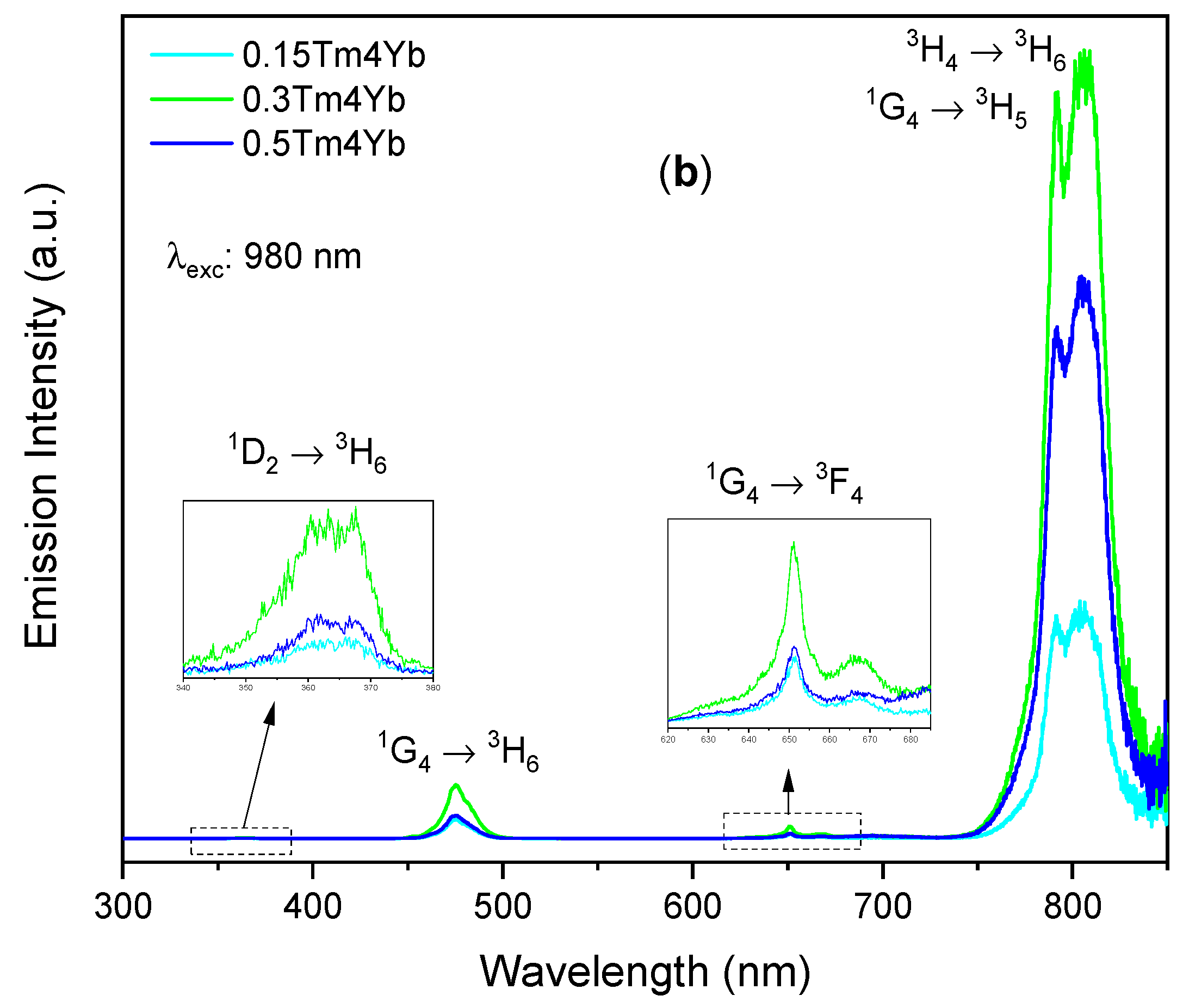
3.2.2. Emission Spectra
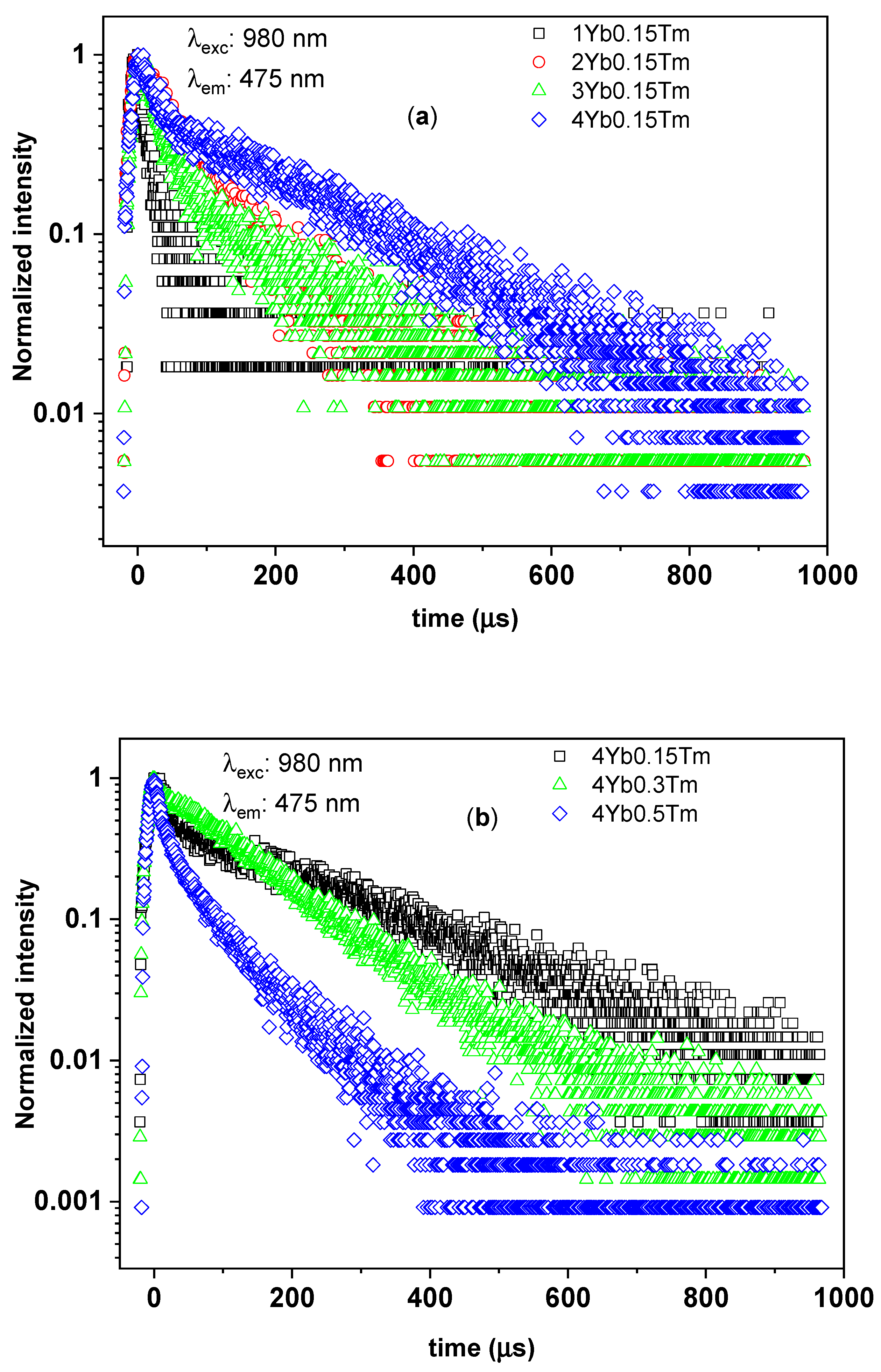
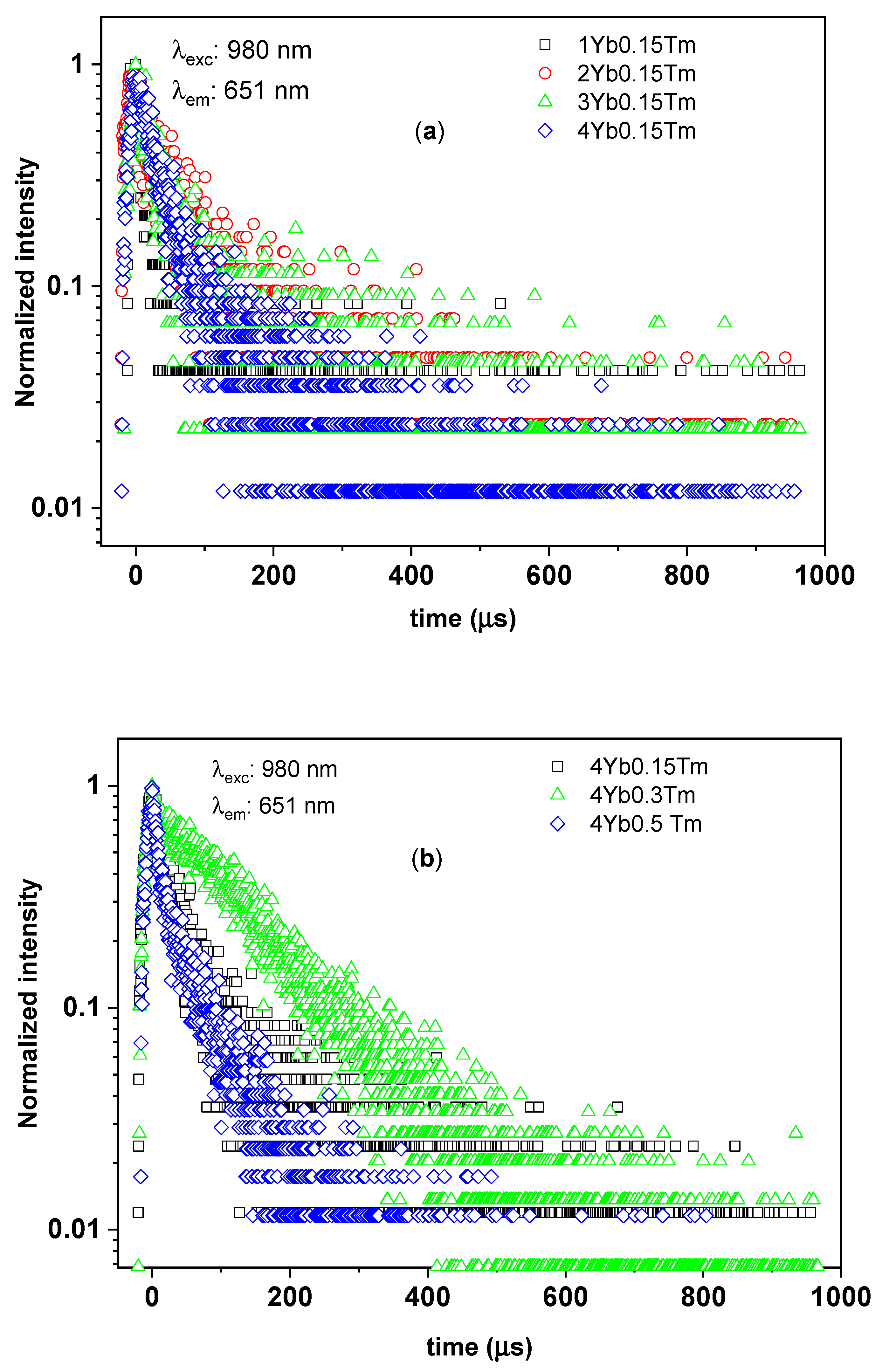
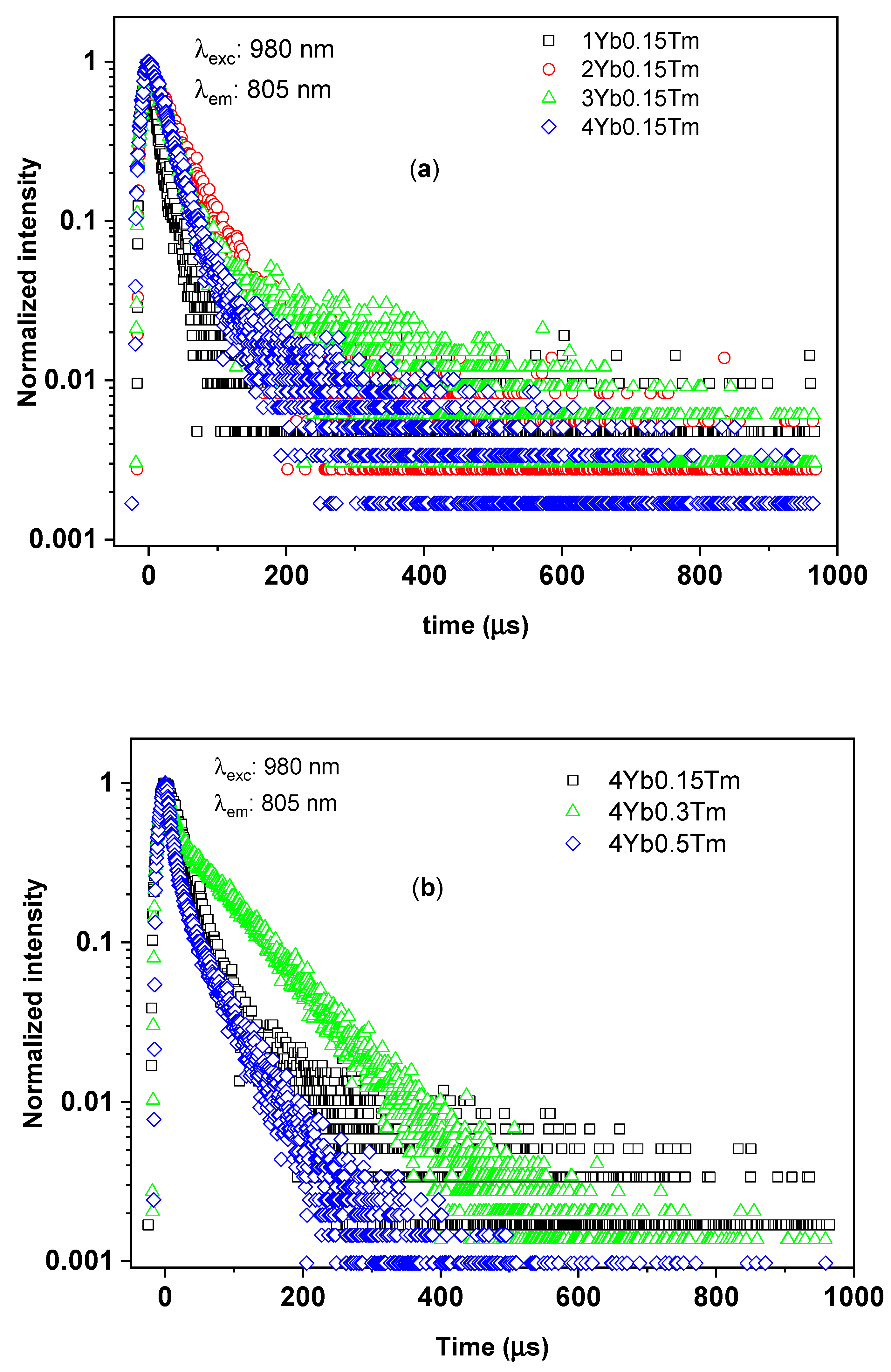
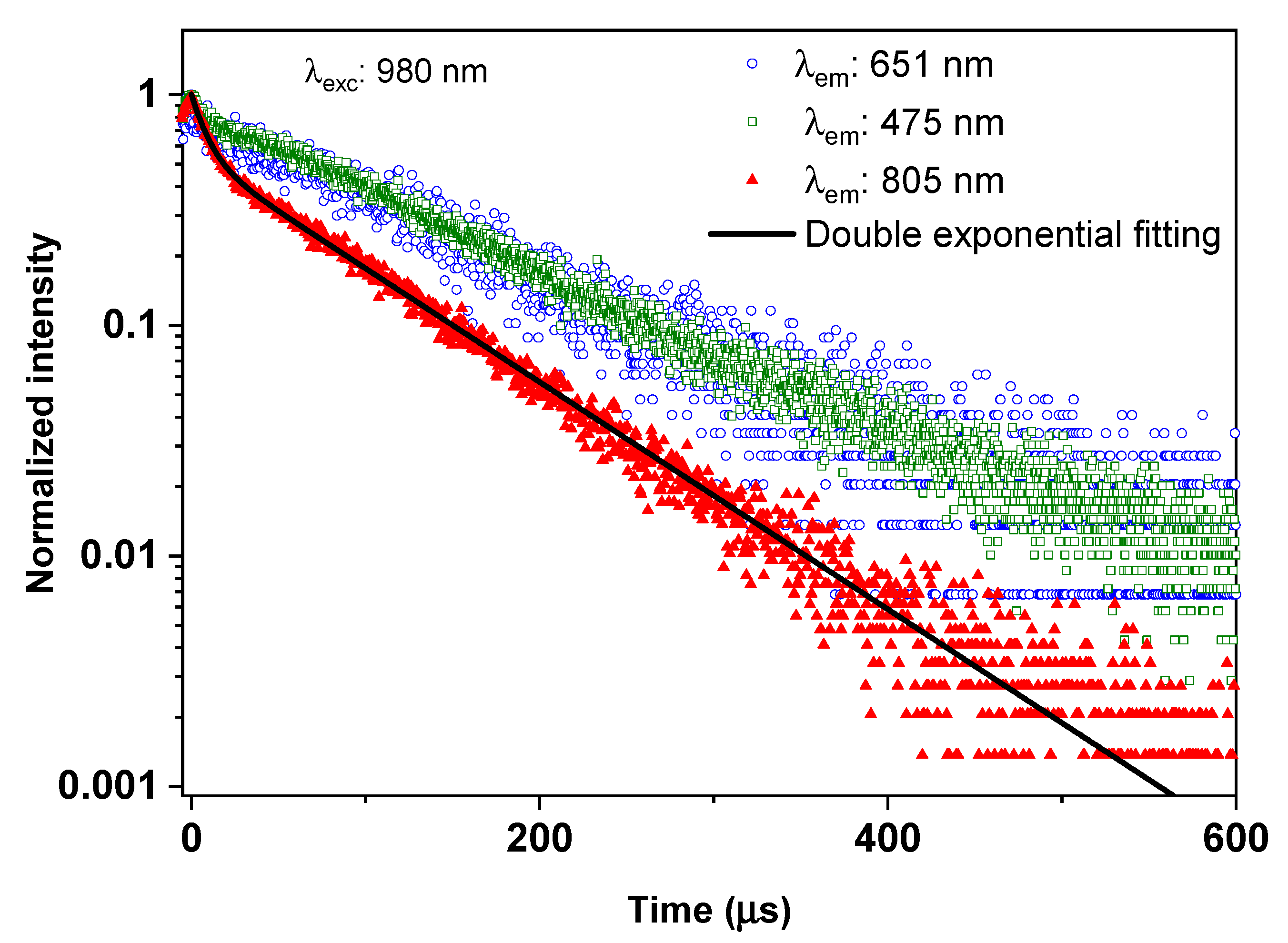
3.2.3. Time-Resolved UC Photoluminescence
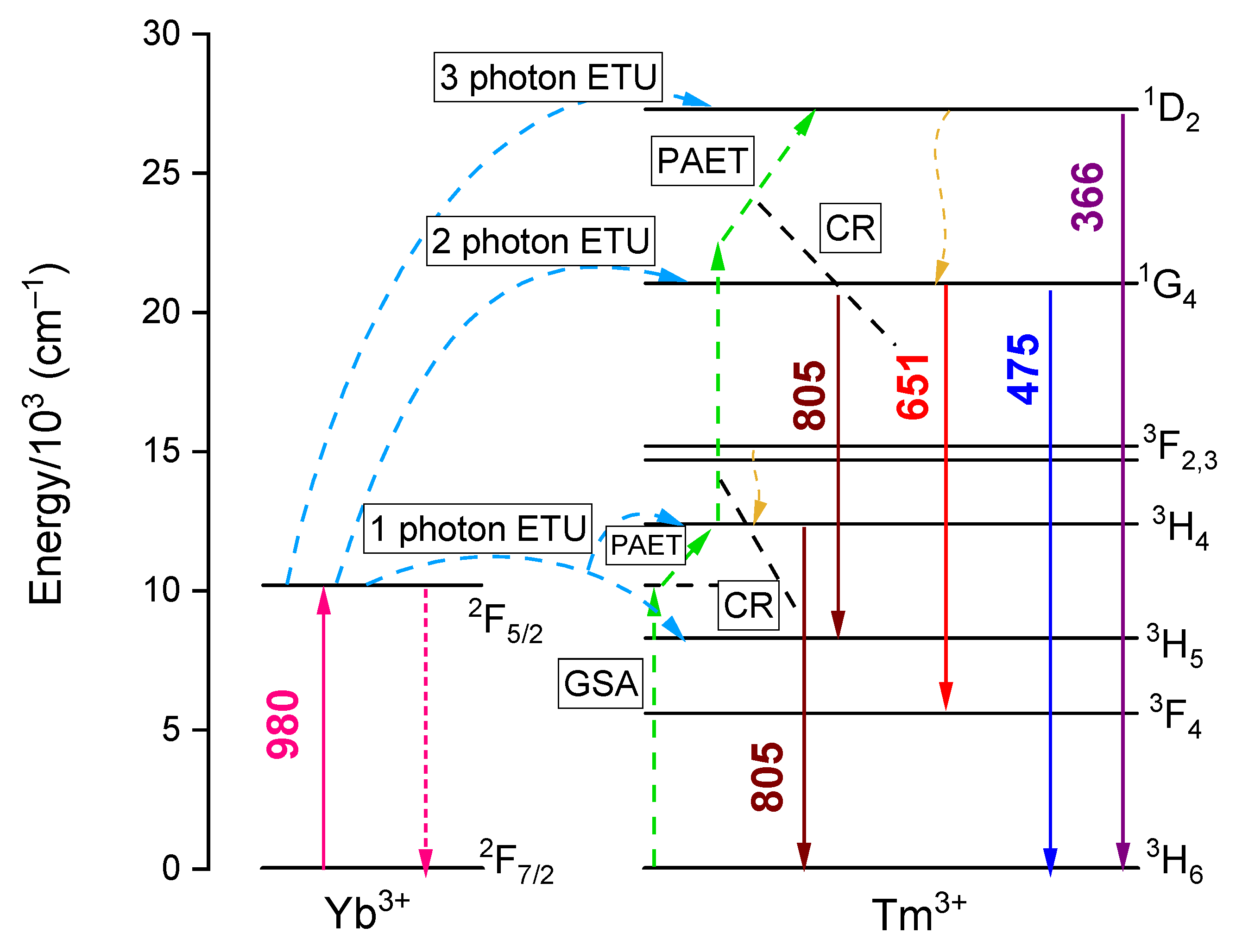
3.2.4. UC Excitation Power Dependence and Yb3+-Tm3+ UC Energy Transfer
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lukowiak, A.; Zur, L.; Tomala, R.; LamTran, T.N.; Bouajaj, A.; Strek, W.; Righini, G.C.; Wickleder, M.; Ferrari, M. Rare earth elements and urban mines: Critical strategies for sustainable development. Ceram. Int. 2020, 46, 26247–26250. [Google Scholar] [CrossRef]
- Lukowiak, A.; Chiasera, A.; Chiappini, A.; Righini, G.C.; Ferrari, M. Active sol-gel materials, fluorescence spectra, and lifetimes. In Handbook of Sol-Gel Science and Technology; Klein, L., Aparicio, M., Jitianu, A., Eds.; Springer International Publishing: Berlin/Heidelberg, Germany, 2018; pp. 1–43. ISBN 978-3-319-32099-1. [Google Scholar]
- Quandt, A.; Ferrari, M.; Righini, G.C. Advancement of glass-ceramic materials for photonic applications. In Sol-Gel Based Nanoceramic Materials: Preparation, Properties and Applications; Mishra, A.K., Ed.; Springer International Publishing: Berlin/Heidelberg, Germany, 2016; pp. 133–155. ISBN 978-3-319-49512-5. [Google Scholar]
- Ferrari, M.; Righini, G.C. Glass-ceramic materials for guided-wave optics. Int. J. Appl. Glass Sci. 2015, 6, 240–248. [Google Scholar] [CrossRef]
- Ferrari, M.; Righini, G.C. Physics and Chemistry of Rare-Earth Ions Doped Glasses; Trans Tech Publications: Bäch, Switzerland, 2008; pp. 71–120. [Google Scholar]
- Chen, Y.; Chen, G.; Liu, X.; Xu, J.; Zhou, X.; Yang, T.; Yuan, C.; Zhou, C. Upconversion luminescence, optical thermometric properties and energy transfer in Yb3+/Tm3+ co-doped phosphate glass. Opt. Mater. (Amst.) 2018, 81, 78–83. [Google Scholar] [CrossRef]
- Xia, H.; Lei, L.; Xia, J.; Hua, Y.; Deng, D.; Xu, S. Yb/Er/Tm tri-doped Na3ZrF7 upconversion nanocrystals for high performance temperature sensing. J. Lumin. 2019, 209, 8–13. [Google Scholar] [CrossRef]
- Deng, Y.; Niu, C. Up-conversion luminescence properties of Er3+/Yb3+ co-doped oxyfluoride glass ceramic. J. Lumin. 2019, 209, 39–44. [Google Scholar] [CrossRef]
- Kasprowicz, D.; Brik, M.G.; Majchrowski, A.; Michalski, E.; Głuchowski, P. Up-conversion emission in triply-doped Ho3+/Yb3+/Tm3+ KGd(WO4)2 single crystals. Opt. Commun. 2011, 284, 2895–2899. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, C.; Han, Q.; Wang, G.; Zhang, M.; Zhang, J.; Gao, W.; Zheng, H. Metal-enhanced upconversion luminescence of NaYF4:Yb/Er with Ag nanoparticles. Mater. Res. Bull. 2017, 88, 182–187. [Google Scholar] [CrossRef]
- Pokhrel, M.; Valdes, C.; Mao, Y. Ultraviolet upconversion enhancement in triply doped NaYF4:Tm3+,Yb3+ particles: The role of Nd3+ or Gd3+ Co-doping. Opt. Mater. (Amst.) 2016, 58, 67–75. [Google Scholar] [CrossRef]
- Hassairi, M.A.; Dammak, M.; Zambon, D.; Chadeyron, G.; Mahiou, R. Red–green–blue upconversion luminescence and energy transfer in Yb3+/Er3+/Tm3+ doped YP5O14 ultraphosphates. J. Lumin. 2017, 181, 393–399. [Google Scholar] [CrossRef]
- Xu, S.; Huang, S.; He, Q.; Wang, L. Upconversion nanophosphores for bioimaging. TrAC Trends Anal. Chem. 2015, 66, 72–79. [Google Scholar] [CrossRef]
- Zmojda, J.; Kochanowicz, M.; Miluski, P.; Righini, G.C.; Ferrari, M.; Dorosz, D. Investigation of upconversion luminescence in Yb3+/Tm3+/Ho3+ triply doped antimony-germanate glass and double-clad optical fiber. Opt. Mater. (Amst.) 2016, 58, 279–284. [Google Scholar] [CrossRef]
- Rafique, R.; Kailasa, S.K.; Park, T.J. Recent advances of upconversion nanoparticles in theranostics and bioimaging applications. TrAC Trends Anal. Chem. 2019, 120, 115646. [Google Scholar] [CrossRef]
- Auzel, F. Upconversion and anti-Stokes processes with f and d ions in solids. Chem. Rev. 2004, 104, 139–173. [Google Scholar] [CrossRef]
- Lukowiak, A.; Stefanski, M.; Ferrari, M.; Strek, W. Nanocrystalline lanthanide tetraphosphates: Energy transfer processes in samples co-doped with Pr3+/Yb3+ and Tm3+/Yb3+. Opt. Mater. (Amst.) 2017, 74, 159–165. [Google Scholar] [CrossRef]
- Sudarshanam, V.; Abedin, K.S.; Nicholson, J.W.; Headley, C.E.; DiGiovanni, D.J. Kilo-Watt high-power Yb fiber laser at 1117 nm. In Proceedings of the Fiber Lasers XV: Technology and Systems, San Francisco, CA, USA, 29 January–1 February 2018; Carter, A.L., Hartl, I., Eds.; SPIE: Bellingham, WA, USA, 2018; Volume 10512, p. 12. [Google Scholar]
- Chiappini, A.; Zur, L.; Enrichi, F.; Boulard, B.; Lukowiak, A.; Righini, G.C.; Ferrari, M. Glass ceramics for frequency conversion. In Solar Cells and Light Management: Materials, Strategies and Sustainability; Enrichi, F., Righini, G., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 391–414. ISBN 9780081027622. [Google Scholar]
- Boulard, B.; Dieudonné, B.; Gao, Y.; Chiasera, A.; Ferrari, M. Up-conversion visible emission in rare-earth doped fluoride glass waveguides. Opt. Eng. 2014, 53, 071814. [Google Scholar] [CrossRef]
- Lahoz, F.; Martín, I.R.; Méndez-Ramos, J.; Núñez, P. Dopant distribution in a Tm3+-Yb3+ codoped silica based glass ceramic: An infrared-laser induced upconversion study. J. Chem. Phys. 2004, 120, 6180–6190. [Google Scholar] [CrossRef] [PubMed]
- Watekar, P.R.; Ju, S.; Boo, S.; Han, W.T. Linear and non-linear optical properties of Yb3+/Tm3+ co-doped alumino-silicate glass prepared by sol-gel method. J. Non-Cryst. Solids 2005, 351, 2446–2452. [Google Scholar] [CrossRef]
- Simpson, D.A.; Gibbs, W.E.; Collins, S.F.; Blanc, W.; Dussardier, B.; Monnom, G.; Peterka, P.; Baxter, G.W. Visible and near infra-red up-conversion in Tm3+/Yb3+ co-doped silica fibers under 980 nm excitation. Opt. Express 2008, 16, 13781. [Google Scholar] [CrossRef]
- Maalej, O.; Lukowiak, A.; Bouajaj, A.; Chiasera, A.; Righini, G.C.; Ferrari, M.; Boulard, B. Blue to NIR down-conversion in Tm3+/Yb3+-codoped fluorozirconate glasses compared to Pr3+/Yb3+ ion-pair. J. Lumin. 2018, 193, 22–28. [Google Scholar] [CrossRef]
- Li, Q.; Xing, M.; Chen, Z.; Wang, X.; Zhao, C.; Qiu, J.; Yu, J.; Chang, J. Er3+/Yb3+ co-doped bioactive glasses with up-conversion luminescence prepared by containerless processing. Ceram. Int. 2016, 42, 13168–13175. [Google Scholar] [CrossRef]
- Kalaivani, S.; Srividiya, S.; Vijayalakshmi, U.; Kannan, S. Bioactivity and up-conversion luminescence characteristics of Yb3+/Tb3+ co-doped bioglass system. Ceram. Int. 2019, 45, 18640–18647. [Google Scholar] [CrossRef]
- Zambanini, T.; Borges, R.; Faria, P.C.; Delpino, G.P.; Pereira, I.S.; Marques, M.M.; Marchi, J. Dissolution, bioactivity behavior, and cytotoxicity of rare earth-containing bioactive glasses (RE = Gd, Yb). Int. J. Appl. Ceram. Technol. 2019, 16, 2028–2039. [Google Scholar] [CrossRef]
- Borges, R.; Schneider, J.F.; Marchi, J. Structural characterization of bioactive glasses containing rare earth elements (Gd and/or Yb). J. Mater. Sci. 2019, 54, 11390–11399. [Google Scholar] [CrossRef]
- Lukowiak, A.; Lao, J.; Lacroix, J.; Marie Nedelec, J. Bioactive glass nanoparticles obtained through sol–gel chemistry. Chem. Commun. 2013, 49, 6620–6622. [Google Scholar] [CrossRef] [PubMed]
- Righini, G.C.; Ferrari, M. Photoluminescence of rare-earth-doped glasses. La Rivista del Nuovo Cimento 2005, 28, 1–53. [Google Scholar] [CrossRef]
- Tsuboi, T. Luminescence of Tm3+ Ion in LiNbO3 Crystal. J. Electrochem. Soc. 2000, 147, 1997. [Google Scholar] [CrossRef]
- Serra, O.A.; Nassar, E.J.; Calefi, P.S.; Rosa, I.L.V. Luminescence of a new Tm3+ β-diketonate compound. J. Alloys Compd. 1998, 275–277, 838–840. [Google Scholar] [CrossRef]
- Gomes, A.S.L.; Carvalho, M.T.; Sundheimer, M.L.; Bastos-Filho, C.J.A.; Martins-Filho, J.F.; Von der Weid, J.P.; Margulis, W. Low-pump-power, short-fiber copropagating dual-pumped (800 and 1050 nm) thulium-doped fiber amplifier. Opt. Lett. 2003, 28, 334. [Google Scholar] [CrossRef]
- Davydov, A.S. The Radiationless transfer of energy of electronic excitation between impurity molecules in crystals. Phys. Status Solidi 1968, 30, 357–366. [Google Scholar] [CrossRef]
- Yu, D.; Yu, T.; Bunningen, A.J.; Zhang, Q.; Meijerink, A.; Rabouw, F.T. Understanding and tuning blue-to-near-infrared photon cutting by the Tm3+/Yb3+ couple. Light Sci. Appl. 2020, 9, 107. [Google Scholar] [CrossRef]
- Marconi da Silva MD Linhares, H.; Felipe Henriques Librantz, A.; Gomes, L.; Coronato Courrol, L.; Lícia Baldochi, S.; Marcia Ranieri, I. Energy transfer rates and population inversion investigation of 1G4 and 1D2 excited states of Tm3+ in Yb:Tm:Nd:KY3F10 crystals. J. Appl. Phys. 2011, 109, 083533. [Google Scholar] [CrossRef]
- Dwaraka Viswanath, C.S.; Babu, P.; Martín, I.R.; Venkatramu, V.; Lavín, V.; Jayasankar, C.K. Near-infrared and upconversion luminescence of Tm3+ and Tm3+/Yb3+-doped oxyfluorosilicate glasses. J. Non-Cryst. Solids 2019, 507, 1–10. [Google Scholar] [CrossRef]
- Grzyb, T.; Balabhadra, S.; Przybylska, D.; Węcławiak, M. Upconversion luminescence in BaYF5, BaGdF5 and BaLuF5 nanocrystals doped with Yb3+/Ho3+, Yb3+/Er3+ or Yb3+/Tm3+ ions. J. Alloys Compd. 2015, 649, 606–616. [Google Scholar] [CrossRef]
- Chen, G.; Ohulchanskyy, T.Y.; Kumar, R.; Ågren, H.; Prasad, P.N. Ultrasmall monodisperse NaYF4:Yb3+/Tm3+ nanocrystals with enhanced near-infrared to near-infrared upconversion photoluminescence. ACS Nano 2010, 4, 3163–3168. [Google Scholar] [CrossRef]
- Qiu, H.; Yang, C.; Shao, W.; Damasco, J.; Wang, X.; Ågren, H.; Prasad, P.; Chen, G. Enhanced Upconversion Luminescence in Yb3+/Tm3+-Codoped Fluoride Active Core/Active Shell/Inert Shell Nanoparticles through Directed Energy Migration. Nanomaterials 2014, 4, 55–68. [Google Scholar] [CrossRef] [PubMed]
- Pollnau, M.; Gamelin, D.R.; Lüthi, S.R.; Güdel, H.U. Power dependence of upconversion luminescence in lanthanide and transition-metal-ion systems. Phys. Rev. B 2000, 61, 3337–3346. [Google Scholar] [CrossRef]
- Chen, Q.-Z.; Rezwan, K.; Françon, V.; Armitage, D.; Nazhat, S.N.; Jones, F.H.; Boccaccini, A.R. Surface functionalization of Bioglass®-derived porous scaffolds. Acta Biomater. 2007, 3, 551–562. [Google Scholar] [CrossRef] [PubMed]


| Sample Name | Lanthanides Concentration (mol%) | Chemical Composition (wt.%) (a) | ||||
|---|---|---|---|---|---|---|
| Tm3+ | Yb3+ | SiO2 | CaO | Tm2O3 (b) | Yb2O3 | |
| 0Tm0Yb | 0 | 0 | 86.8 | 13.2 | - | - |
| 0.15Tm0Yb | 0.15 | 0 | 86.4 | 13.6 | - | - |
| 0.15Tm1Yb | 0.15 | 1 | 86.5 | 9.6 | - | 3.9 |
| 0.15Tm2Yb | 0.15 | 2 | 76.5 | 16.3 | - | 7.2 |
| 0.15Tm3Yb | 0.15 | 3 | 73.4 | 15.8 | - | 10.8 |
| 0.15Tm4Yb | 0.15 | 4 | 70,7 | 16.9 | - | 12.4 |
| 0.3Tm4Yb | 0.3 | 4 | 69.4 | 18.0 | - | 12.6 |
| 0.5Tm4Yb | 0.5 | 4 | 69.5 | 16.7 | 1.2 | 12.6 |
| Tm3+ (mol%) | Yb3+ (mol%) | Yb3+:Tm3+ Concentration Ratio | 1/e Decay Time (µs) | ||
|---|---|---|---|---|---|
| 1G4–3H6 Transition (@475 nm) | 1G4–3F4 Transition (@651 nm) | 3H4–3H6, 1G4–3H5 Transitions (@805 nm) | |||
| 0.15 | 1 | 7 | 13 ± 7 | 15 ± 8 | 14 ± 2 |
| 0.15 | 2 | 13 | 63 ± 7 | 27 ± 14 | 36 ± 5 |
| 0.15 | 3 | 20 | 41 ± 14 | 23 ± 6 | 31 ± 2 |
| 0.15 | 4 | 27 | 63 ± 14 | 34 ± 4 | 31 ± 2 |
| 0.3 | 4 | 13 | 104 ± 28 | 109 ± 9 | 39 ± 5 |
| 0.5 | 4 | 8 | 19 ± 2 | 15 ± 4 | 13 ± 1 |
| Double Exponential Function Fitting of 805 nm Decay Curve (1G4 + 3H4) | 1G4 Decay Time (µs) | |||||
|---|---|---|---|---|---|---|
| Long decay component | Short decay component | R-square | Derived from 1G4–3H6 Transition (@475 nm) | Derived from 1G4–3F4 Transition (@651 nm) | ||
| A | τ1 (µs) | 1-A | τ2 (µs) | |||
| 0.55 ± 0.01 | 88 ± 2 | 0.45 ± 0.01 | 9 ± 1 | 0.998 | 104 ± 28 | 109 ± 9 |
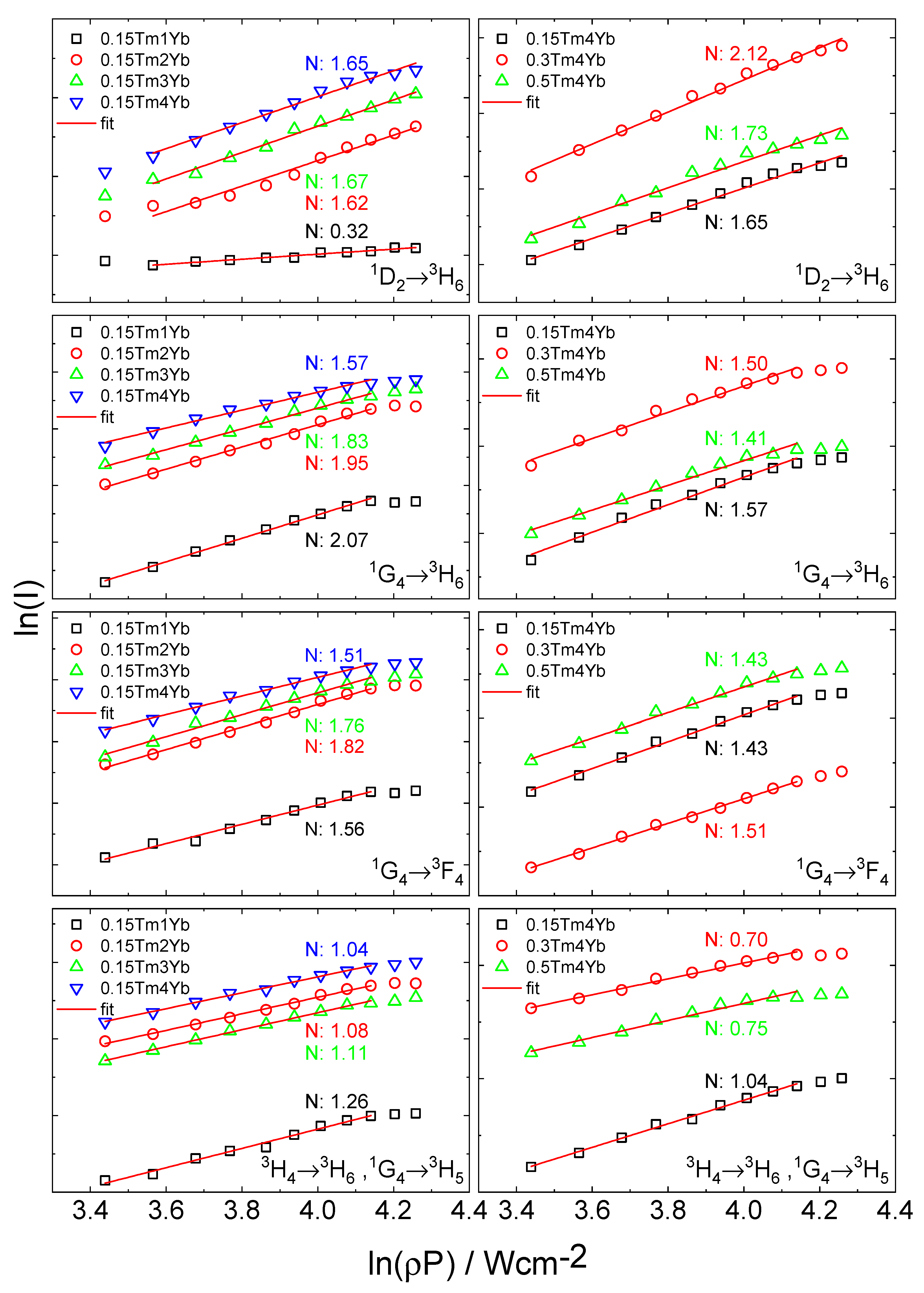
| Tm3+ (mol%) | Yb3+ (mol%) | N Number | |||
|---|---|---|---|---|---|
| 366 nm | 475 nm | 651 nm | 805 nm | ||
| 0.15 | 1 | (0.32) | 2.07 | 1.56 | 1.26 |
| 0.15 | 2 | 1.62 | 1.95 | 1.82 | 1.08 |
| 0.15 | 3 | 1.67 | 1.83 | 1.76 | 1.04 |
| 0.15 | 4 | 1.65 | 1.57 | 1.51 | 1.11 |
| 0.3 | 4 | 2.12 | 1.50 | 1.43 | 0.70 |
| 0.5 | 4 | 1.73 | 1.41 | 1.43 | 0.75 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Halubek-Gluchowska, K.; Szymański, D.; Tran, T.N.L.; Ferrari, M.; Lukowiak, A. Upconversion Luminescence of Silica–Calcia Nanoparticles Co-doped with Tm3+ and Yb3+ Ions. Materials 2021, 14, 937. https://doi.org/10.3390/ma14040937
Halubek-Gluchowska K, Szymański D, Tran TNL, Ferrari M, Lukowiak A. Upconversion Luminescence of Silica–Calcia Nanoparticles Co-doped with Tm3+ and Yb3+ Ions. Materials. 2021; 14(4):937. https://doi.org/10.3390/ma14040937
Chicago/Turabian StyleHalubek-Gluchowska, Katarzyna, Damian Szymański, Thi Ngoc Lam Tran, Maurizio Ferrari, and Anna Lukowiak. 2021. "Upconversion Luminescence of Silica–Calcia Nanoparticles Co-doped with Tm3+ and Yb3+ Ions" Materials 14, no. 4: 937. https://doi.org/10.3390/ma14040937
APA StyleHalubek-Gluchowska, K., Szymański, D., Tran, T. N. L., Ferrari, M., & Lukowiak, A. (2021). Upconversion Luminescence of Silica–Calcia Nanoparticles Co-doped with Tm3+ and Yb3+ Ions. Materials, 14(4), 937. https://doi.org/10.3390/ma14040937








