Textile Materials Modified with Stimuli-Responsive Drug Carrier for Skin Topical and Transdermal Delivery
Abstract
1. Introduction
2. Comparative Analysis of Peroral and Transdermal Medication
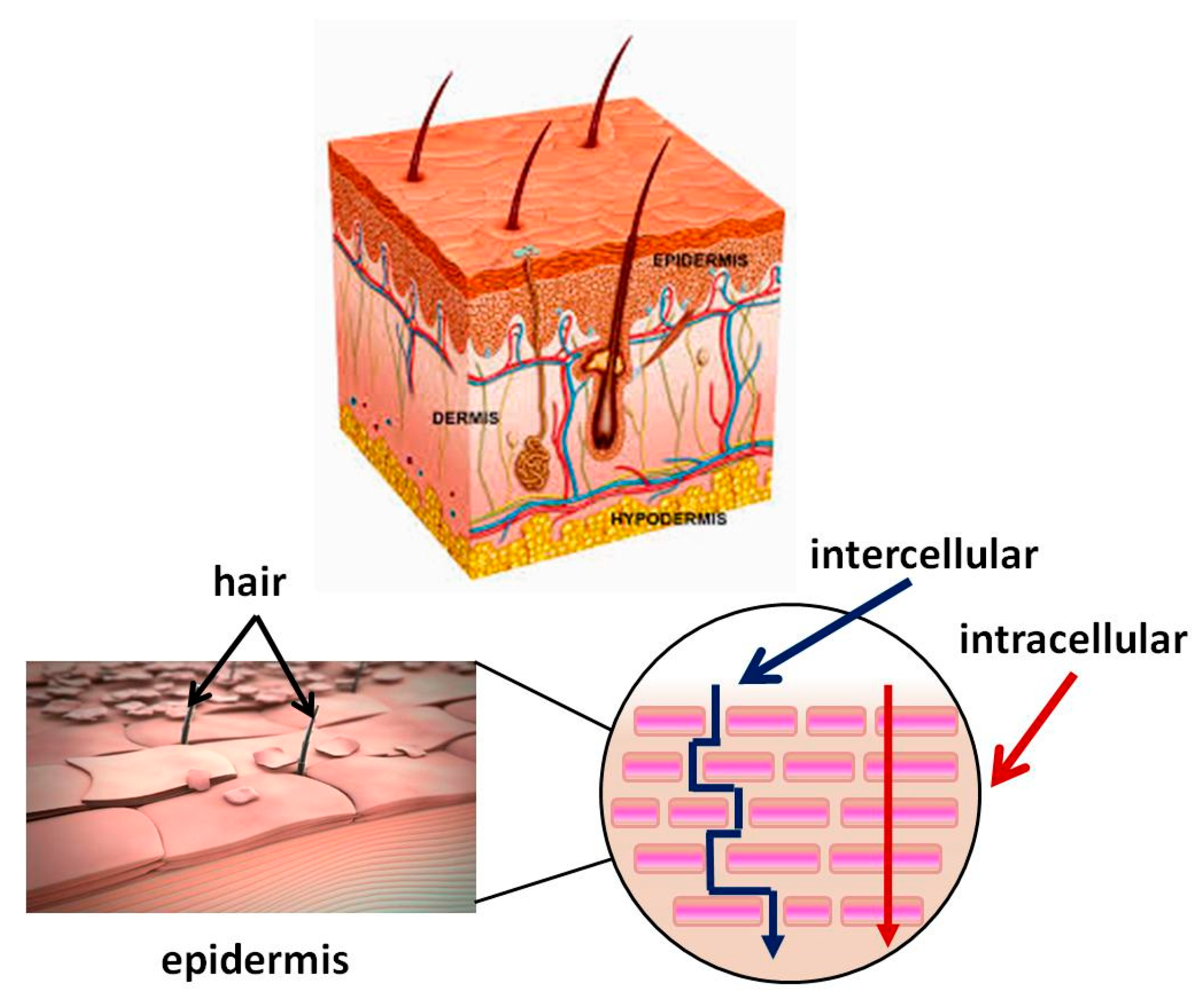
3. Skin Structure. Mechanism of the Transdermal System for BAS Delivery. Factors Determining Its Effectiveness
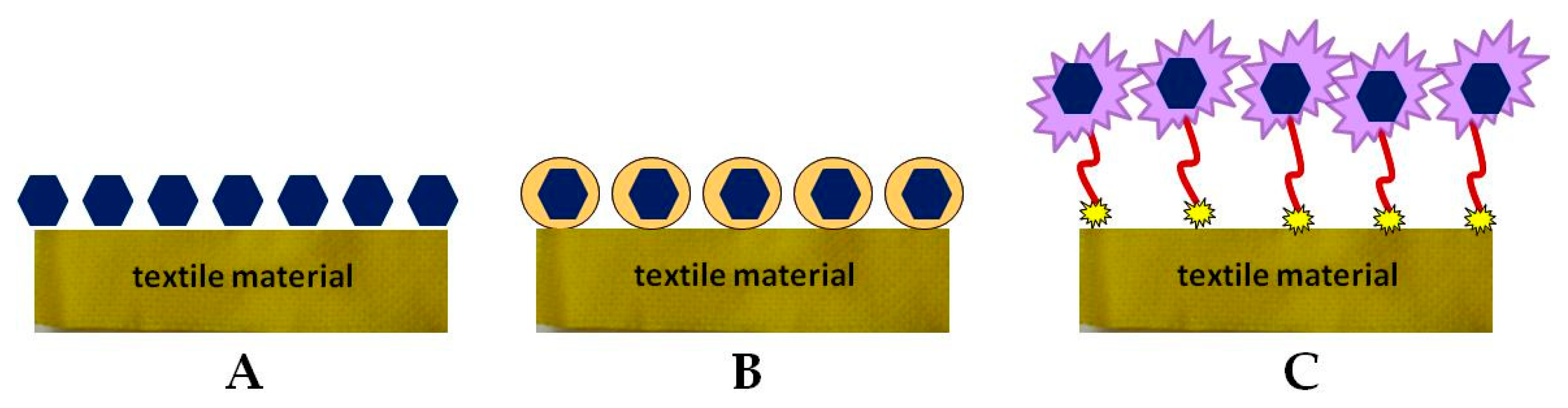
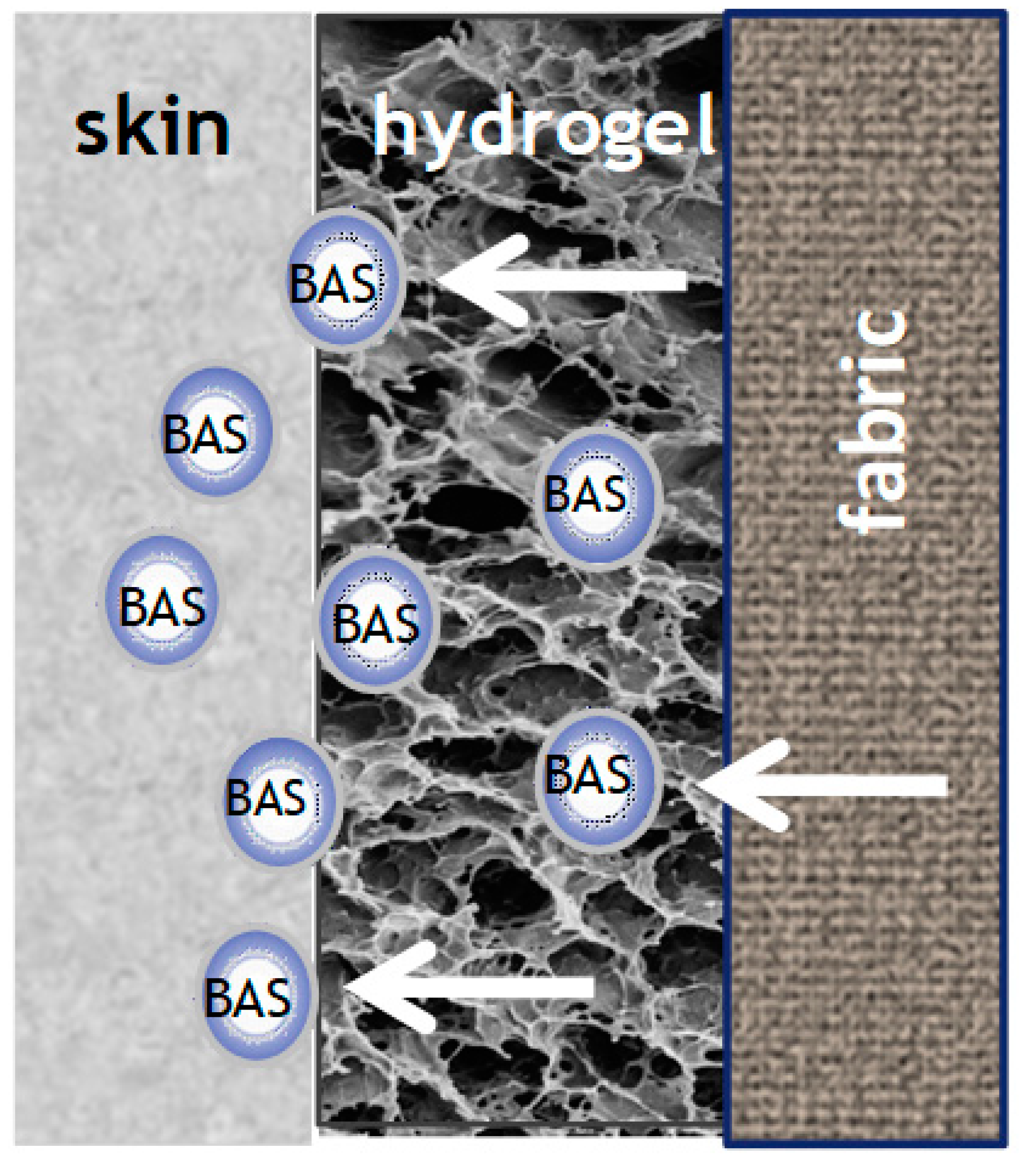
4. Biofunctional Textile Materials and Their Preparative Methods
5. Requirements to Be Met by BAS Delivering Systems and Biofunctional Textiles
6. Instrumental/Analytical Techniques Used for Characterization of Biofunctional Textiles
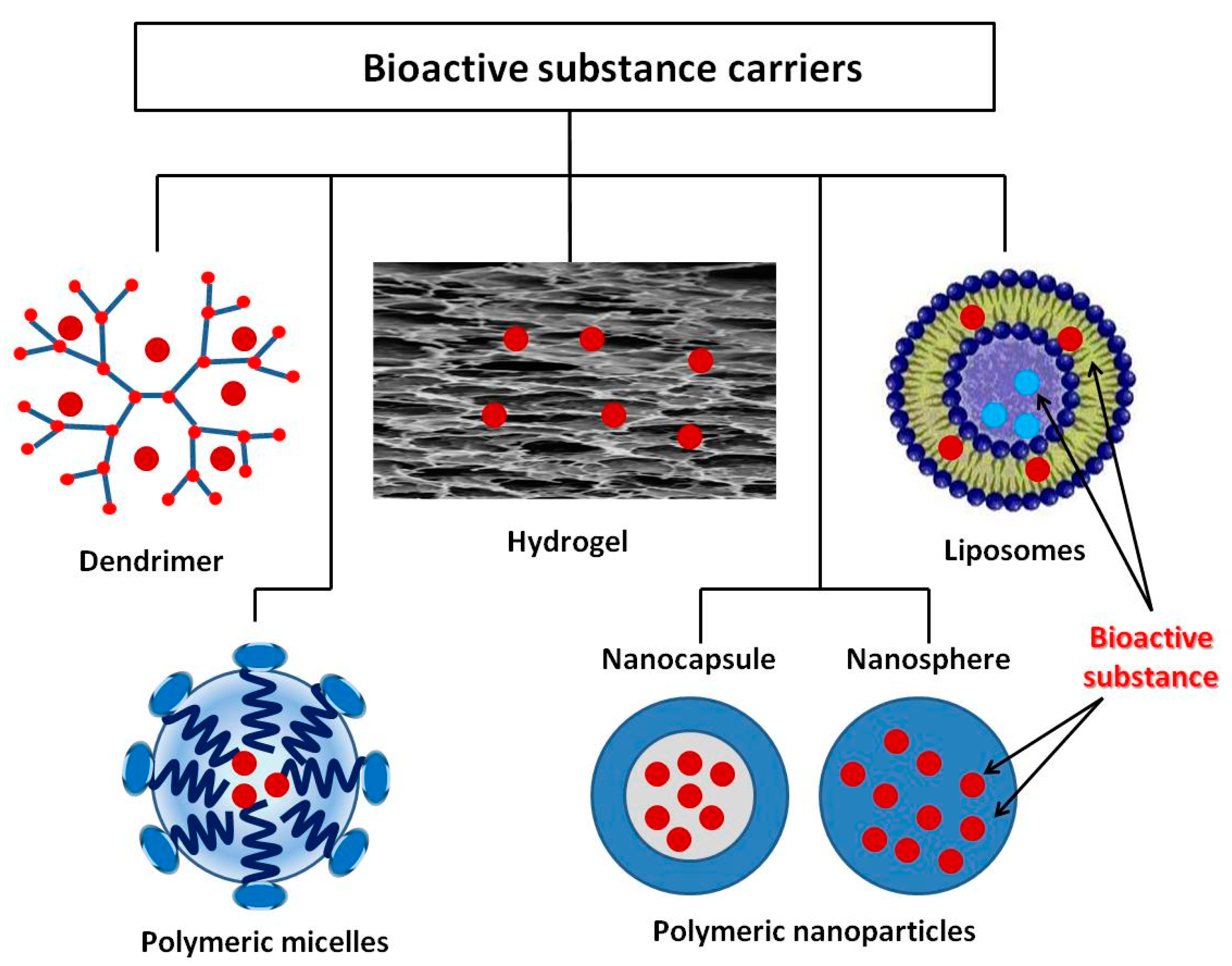
7. Stimuli-Responsive Drug Carrier for Delivering Bioactive Substances
7.1. Dendrimers
7.2. Polymeric Micelles
7.3. Liposomes
7.4. Polymeric Nanoparticles
7.5. Hydrogels
8. Multifunctional and Intelligent Textiles—Perspectives and Expectations
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mečņika, V.; Hoerr, M.; Krieviņš, I.; Schwarz, A. Smart textiles for healthcare: Applications and technologies. Rural Environ. Educ. Personal. REEP 2014, 7, 150–161. [Google Scholar]
- Boesel, L.F.; Furundžić, D.P.; Furundžić, N.Z.; Gedanken, A.; Grabchev, I.; Haj Taieb, A.; Ivanoska-Dacik, A.; Malionowski, S.; Marković, D.; Mohr, G.; et al. Smart Textiles for Healthcare and Medicine Applications (WG1): State-of-the Art Report, CONTEXT Project; University College Cork: Cork, Ireland, 2020. [Google Scholar]
- Zhong, W. An Introduction to Healthcare and Medical Textiles; DEStech Publications Inc.: Lancaster, PA, USA, 2013. [Google Scholar]
- Grabchev, I.; Staneva, D.; Vasileva-Tonkova, E.; Alexandrova, R. Surface functionalization of cotton fabric with fluorescent dendrimers, spectral characterization, cytotoxicity, antimicrobial and antitumor activity. Chemosensors 2019, 7, 17. [Google Scholar] [CrossRef]
- Malis, D.; Jeršek, B.; Tomšič, B.; Štular, D.; Golja, B.; Kapun, G.; Simončič, B. Antibacterial activity and biodegradation of cellulose fiber blends with incorporated ZnO. Materials 2019, 12, 3399. [Google Scholar] [CrossRef] [PubMed]
- Staneva, D.; Vasileva-Tonkova, E.; Bosch, P.; Grozdanov, P.; Grabchev, I. Synthesis and characterization of a new PAMAM metallodendrimer for antimicrobial modification of cotton fabric. Macromol. Res. 2018, 26, 332–340. [Google Scholar] [CrossRef]
- Dhivya, S.; Padma, V.V.; Santhini, E. Wound dressings—A review. BioMedicine 2015, 5, 22. [Google Scholar] [CrossRef] [PubMed]
- Van Langenhove, L. Smart Textiles for Medicine and Healthcare: Materials, Systems and Applications; Woodhead Publishing: Cambridge, UK, 2007. [Google Scholar]
- Jerkovic, I.; Grancaric, A.M.; Koncar, V. Structural health monitoring of composites with newly developed textile sensors in situ. IOP Conf. Ser. Mater. Sci. Eng. 2018, 460, 012046. [Google Scholar] [CrossRef]
- Wagner, M. Multidisciplinary Know-How for Smart-Textiles Developers; Woodhead Publishing: Cambridge, UK, 2013; pp. 444–467. [Google Scholar]
- Shishoo, R. Textiles in Sportswear, 1st ed.; Woodhead Publishing: Cambridge, UK, 2015. [Google Scholar]
- Chittenden, T. Skin in the game: The use of sensing smart fabrics in tennis costume as a means of analyzing performance. Fash. Text. 2017, 4, 22. [Google Scholar] [CrossRef]
- Dolez, P.I.; Mlynarek, J. Smart materials for personal protective equipment. In Smart Textiles and Their Applications; Koncar, V., Ed.; Woodhead Publishing: Cambridge, UK, 2016. [Google Scholar]
- Oliveira, A. Smart textile for architecture: Living with technology. In Proceedings of the 2nd International Conference on Human Interaction and Emerging Technologies: Future Applications (IHIET—AI 2020), Lausanne, Switzerland, 23–25 April 2020. [Google Scholar]
- Qureshi, A.K. Utilizing smart textiles in interior design to replace conventional architectural finishes. TEXTEH Proc. 2019, 105–109. [Google Scholar] [CrossRef]
- Youssef, M.M. Smart textiles as hybrid interactive materials A responsive behaviour towards transformable surfaces. Int. Des. J. 2017, 7, 215–226. [Google Scholar] [CrossRef]
- Thakur, S. Shape memory polymers for smart textile applications. In Textiles for Advanced Applications; Kumar, B., Ed.; IntechOpen: London, UK, 2017. [Google Scholar]
- Staneva, D.; Grabchev, I. Heterogeneous sensors for ammonia, amines and metal ions based on a dendrimer modified fluorescent viscose fabric. Dyes Pigment. 2018, 155, 164–170. [Google Scholar] [CrossRef]
- Micus, S.; Haupt, M.; Gresser, G.T. Automatic joining of electrical components to smart textiles by ultrasonic soldering. Sensors 2021, 21, 545. [Google Scholar] [CrossRef] [PubMed]
- Keum, K.; Heo, J.S.; Eom, J.; Lee, K.W.; Park, S.K.; Kim, Y.-H. Highly sensitive textile-based capacitive pressure sensors using PVDF-HFP/ionic liquid composite films. Sensors 2021, 21, 442. [Google Scholar] [CrossRef]
- Hadjiev, H.; Rrahnev, I.; Philippov, P. Hybrid microelectronic technology in textile techniques with the aim of realizing smart textiles. In Proceedings of the 38th International Spring Seminar on Electronics Technology (ISSE), Eger, Hungary, 6–10 May 2015; pp. 75–79. [Google Scholar]
- Qin, Y. Medical Textile Materials; Woodhead Publishing: Cambridge, UK, 2016. [Google Scholar]
- Jiao, Y.; Li, C.; Liu, L.; Wang, F.; Liu, X.; Mao, J.; Wang, L. Construction and application of the textile-based tissue engineering scaffold: A review. Biomater. Sci. 2020, 8, 3574–3600. [Google Scholar] [CrossRef] [PubMed]
- van Langenhove, L. Advances in Smart Medical Textiles, Treatments and Healt Monitoring; Woodhead Publishing: Cambridge, UK, 2016. [Google Scholar]
- West, A.J.; Annett-Hitchcock, K.E. A critical review of aroma therapeutic applications for textiles. JTATM 2014, 9, 1–13. [Google Scholar]
- Hashemikia, S.; Hemmatinejad, N.; Ahmadi, E.; Montazer, M. Antibacterial and anti-inflammatory drug delivery properties on cotton fabric using betamethasoneloaded mesoporous silica particles stabilized with chitosan and silicone softener. Drug Deliv. 2016, 23, 2946–2955. [Google Scholar] [CrossRef] [PubMed]
- Zurita, R.; Puiggalí, J.; Rodríguez-Galán, A. Loading and release of ibuprofen in multiand monofilament surgical sutures. Macromol. Biosci. 2006, 6, 767–775. [Google Scholar] [CrossRef] [PubMed]
- Kopper, N.W.; Gudeman, J.; Thompson, D.J. Transdermal hormone therapy in postmenopausal women: A review of metabolic effects and drug delivery technologies. Drug Des. Dev. Ther. 2008, 2, 193–202. [Google Scholar] [CrossRef]
- Atabay, N.; Sariişik, A.M.; Karavana, S.Y.; Rençber, S. A novel plaster containing benzoyl peroxide microsponges: Formulation, development and evaluation. J. Ind. Text. 2020. [Google Scholar] [CrossRef]
- Radu, C.D.; Cerempei, A.; Salariu, M.; Parteni, O.; Ulea, E.; Campagne, C. The potential of improving medical textile for cutaneous diseases. IOP Conf. Ser. Mater. Sci. Eng. 2017, 254, 062010. [Google Scholar] [CrossRef]
- Palmer, B.C.; DeLouise, L.A. Nanoparticle-enabled transdermal drug delivery systems for enhanced dose control and tissue targeting. Molecules 2016, 21, 1719. [Google Scholar] [CrossRef]
- Wang, W.; Hui, P.C.L.; Kan, C.-W. Functionalized textile based therapy for the treatment of atopic dermatitis. Coatings 2017, 7, 82. [Google Scholar] [CrossRef]
- Naves, L.B.; Dhand, C.; Venugopal, J.R.; Rajamani, L.; Ramakrishna, S.; Almeida, L. Nanotechnology for the treatment of melanoma skin cancer. Prog. Biomater. 2017, 6, 13–26. [Google Scholar] [CrossRef]
- Volkmar, T.B. Biofunctional Textiles and the Skin, 1st ed.; Hipler, U.C., Elsner, P., Burg, G., Itin, P.H., Eds.; Kanger Publishers: Basel, Switzerland, 2006; pp. 51–66. [Google Scholar]
- Schoe llhammer, C.; Blankschtein, D.; Langer, R. Skin permeabilization for transdermal drug delivery: Recent advances and future prospects. Expert. Opin. Drug Deliv. 2014, 11, 393–407. [Google Scholar] [CrossRef]
- Massella, D.; Argenziano, M.; Ferri, A.; Guan, J.; Giraud, S.; Cavalli, R.; Barresi, A.A.; Salaün, F. Bio-functional textiles: Combining pharmaceutical nanocarriers with fibrous materials for innovative dermatological therapies. Pharmaceutics 2019, 11, 403. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, S.; Hui, C.L. Review of stimuli-responsive polymers in drug delivery and textile application. Molecules 2019, 24, 2547. [Google Scholar] [CrossRef] [PubMed]
- Saghazadeh, S.; Rinoldi, C.; Schot, M.; Kashaf, S.S.; Sharifi, F.; Jalilian, E.; Nuutila, K.; Giatsidis, G.; Mostafalu, P.; Derakhshandeh, H.; et al. Drug Delivery Systems and Materials for Wound Healing Applications. Adv. Drug Deliv. Rev. 2018, 127, 138–166. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, C.; Mallow, J.; Narsavage, G.L. Delivering telemedicine interventions in chronic respiratory disease. Breathe 2014, 10, 199–212. [Google Scholar] [CrossRef]
- Yetisen, A.K.; Martinez-Hurtado, J.L.; Ünal, B.; Khademhosseini, A.; Butt, H. Wearables in medicine. Adv. Mater. 2018, 30, 1706910. [Google Scholar] [CrossRef]
- Lis, M.J.; Carmona, Ó.G.; Carmona, C.G.; Bezerra, F.M. Inclusion complexes of citronella oil with β-cyclodextrin for controlled release in biofunctional textiles. Polymers 2018, 10, 1324. [Google Scholar] [CrossRef] [PubMed]
- Pastore, M.N.; Kalia, Y.N.; Horstmann, M.; Roberts, M.S. Transdermal patches: History, development and pharmacology. Br. J. Pharmacol. 2015, 172, 2179–2209. [Google Scholar] [CrossRef] [PubMed]
- Margetts, L.; Sawyer, R. Transdermal drug delivery: Principles and opioid therapy. Contin. Educ. Anaesth. Crit. Care Pain 2007, 7, 171–176. [Google Scholar] [CrossRef]
- Hu, J.; Liu, W.; Liu, B. Fabric-Supported Chitosan Modified Temperature Responsive PNIPAAm/PU Hydrogel and the Use Thereof in Preparation of Facial Mask. U.S. Patent 7,780,979, 24 August 2010. [Google Scholar]
- Elias, P. Epidermal lipids, barrier function, and desquamation. J. Investig. Dermatol. 1983, 80, 44. [Google Scholar] [CrossRef]
- Pilgram, G.; Engelsma-van Pelt, A.; Bouwstra, J.; Koerten, H. Electron diffraction provides new information on human stratum corneum lipid organization studied in relation to depth and temperature. J. Investig. Dermatol. 1999, 113, 403–409. [Google Scholar] [CrossRef]
- Bouwstra, J.; Gooris, G.; Ponec, M. Skin lipid organization, composition and barrier function. IFSCC Mag. 2007, 10, 297–307. [Google Scholar] [CrossRef]
- Barry, B. Drug delivery routes in skin: A novel approach. Adv. Drug Deliv. Rev. 2002, 54, 31–40. [Google Scholar] [CrossRef]
- Cannon, J.B. Lipids in transdermal and topical drug delivery. Am. Pharm. Rev. 2014, 17, 7. [Google Scholar]
- Boateng, J.S.; Matthews, K.H.; Stevens, H.N.E.; Eccleston, G.M. Wound healing dressings and drug delivery systems: A review. J. Pharm. Sci. 2008, 97, 2892–2923. [Google Scholar] [CrossRef] [PubMed]
- Tanwar, H.; Sachdeva, R. Transdermal drug delivery system: A review. IJPSR 2016, 7, 2274–2290. [Google Scholar]
- Wiedersberg, S.; Guy, R.H. Transdermal drug delivery: 30 years of war and still fighting! J. Control. Release 2014, 190, 150–156. [Google Scholar] [CrossRef]
- Alkilani, A.Z.; McCrudden, M.T.C.; Donnelly, R.F. Transdermal drug delivery: Innovative pharmaceutical developments based on disruption of the barrier properties of the stratum corneum. Pharmaceutics 2015, 7, 438–470. [Google Scholar] [CrossRef]
- Hipler, U.; Elsner, P. Biofunctional textiles and the skin. In Current Problems in Dermatology, 1st ed.; Burg, G., Ed.; S. Karger AG: Basel, Switzerland, 2006; Volume 33, pp. 1–204. [Google Scholar]
- Hu, J. Active Coatings for Smart Textiles; Woodhead Publishing: Cambridge, UK, 2016. [Google Scholar]
- Mihailiasa, M.; Caldera, F.; Li, J.; Peila, R.; Ferri, A.; Trotta, F. Preparation of functionalized cotton fabrics by means of melatonin loaded β-cyclodextrin nanosponges. Carbohydr. Polym. 2016, 142, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Ruela, A.; Perissinato, A.; de Sousa Lino, M.; Mudrik, P.; Pereira, G. Evaluation of skin absorption of drugs from topical and transdermal formulations. Braz. J. Pharm. Sci. 2016, 52, 527–544. [Google Scholar] [CrossRef]
- Roberts, M.; Mohammed, Y.; Pastore, M.; Namjoshi, S.; Yousef, S.; Alinaghi, A.; Haridass, I.; Abd, E.; Leite-Silva, V.; Benson, H.; et al. Topical and cutaneous delivery using nanosystems. J. Control. Release 2017, 247, 86–105. [Google Scholar] [CrossRef] [PubMed]
- Joshi, S.; Jalalpure, S.; Kempwade, A.; Peram, M. Fabrication and in-vivo evaluation of lipid nanocarriers based transdermal patch of colchicine. J. Drug Deliv. Sci. Technol. 2017, 41, 444–453. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, Z.; Lu, W.W.; Zhen, W.; Yang, D.; Peng, S. Novel biomaterial strategies for controlled growth factor delivery for biomedical applications. NPG Asia Mater. 2017, 9, 435. [Google Scholar] [CrossRef]
- Maitz, M.F. Applications of synthetic polymers in clinical medicine. Biosurface Biotribol. 2015, 1, 161–176. [Google Scholar] [CrossRef]
- El Ghoul, Y.; Salah, F.; Majdoub, H.; Sakli, F. Synthesis and study of drug delivery system obtained via b-cyclodextrin functionalization of viscose/polyester dressings. J. Ind. Text. 2017, 47, 489–504. [Google Scholar] [CrossRef]
- Mostafalu, P.; Kiaee, G.; Giatsidis, G.; Khalilpour, A.; Nabavinia, M.; Dokmeci, M.R.; Sonkusale, S.; Orgill, D.P.; Tamayol, A.; Khademhosseini, A. A textile dressing for temporal and dosage controlled drug delivery. Adv. Funct. Mater. 2017, 27, 1702399. [Google Scholar] [CrossRef]
- Singh, J. Nonwoven: A versatile fabric. Text. Sci. Eng. 2014, 4, 5. [Google Scholar]
- Marcincin, A. Modification of fiber-forming polymers by additives. Prog. Polym. Sci. 2002, 27, 853–913. [Google Scholar] [CrossRef]
- Massella, D.; Ancona, A.; Garino, N.; Cauda, V.; Guan, J.; Salaun, F.; Barresi, A.A.; Ferri, A. Preparation of bio-functional textiles by surface functionalization of cellulose fabrics with caffeine loaded nanoparticles. IOP Conf. Ser. Mater. Sci. Eng. 2018, 460, 012044. [Google Scholar] [CrossRef]
- Nemani, S.K.; Annavarapu, R.K.; Mohammadian, B.; Raiyan, A.; Heil, J.; Haque, M.A.; Abdelaal, A.; Sojoudi, H. Surface modification of polymers: Methods and applications. Adv. Mater. Interfaces 2018, 5, 1801247. [Google Scholar] [CrossRef]
- El-Domyati, M.; Attia, S.; Saleh, F.; Brown, D.; Birk, D.E.; Gasparro, F.; Ahmad, H.; Uitto, J. Intrinsec aging vs. photoaging: A comparative histological, immunohistolochemical, and ultrastructural study of skin. Exp. Dermatol. 2002, 11, 398–405. [Google Scholar] [CrossRef] [PubMed]
- Raza, A.; Rasheed, T.; Nabeel, F.; Hayat, U.; Bilal, M.; Iqbal, H.M.N. Endogenous and exogenous stimuli-responsive drug delivery systems for programmed site-specific release. Molecules 2019, 24, 1117. [Google Scholar] [CrossRef] [PubMed]
- Bartels, V.T. Handbook of Medical Textiles; Woodhead Publishing: Cambridge, UK, 2011. [Google Scholar]
- Ten Breteler, M.; Nierstrasz, V.; Warmoeskerken, M. Textile slow-release systems with medical applications. AUTEX Res. J. 2002, 2, 175–189. [Google Scholar]
- Korting, H.; Schäfer-Korting, M. Carriers in the topical treatment of skin disease. In Handbook of Experimental Pharmacology; Springer: New York, NY, USA, 2010; Volume 197, pp. 435–468. [Google Scholar]
- Wolinsky, J.; Grinstaff, M. Therapeutic and diagnostic applications of dendrimers for cancer treatment. Adv. Drug Deliv. Rev. 2008, 9, 1037–1055. [Google Scholar] [CrossRef]
- Roy, I.; Gupta, M.N. Smart polymeric materials: Emerging biochemical applications. Chem. Biol. 2003, 10, 1161–1171. [Google Scholar] [CrossRef]
- Stuart, M.A.C.; Huck, W.T.S.; Minko, S. Emerging applications of stimuli-responsive polymer materials. Nat. Mater. 2010, 9, 101–111. [Google Scholar] [CrossRef]
- Newkome, G.R.; Shreiner, C.D. Poly(amidoamine), polypropylenimine, and related dendrimers and dendrons possessing different 1 → 2 branching motifs: An overview of the divergent procedures. Polymer 2008, 49, 1–173. [Google Scholar] [CrossRef]
- Pedziwiatr-Werbicka, E.; Milowska, K.; Dzmitruk, V.; Ionov, M.; Shcharbin, D.; Bryszewska, M. Dendrimers and hyperbranched structures for biomedical applications. Eur. Polym. J. 2019, 119, 61–73. [Google Scholar] [CrossRef]
- Saluja, V.; Mankoo, A.; Saraogi, G.K.; Tambuwala, M.M.; Mishra, V. Smart dendrimers: Synergizing the targeting of anticancer bioactives. J. Drug Deliv. Sci. Technol. 2019, 52, 15–26. [Google Scholar] [CrossRef]
- Kataoka, K.; Harada, A.; Nagasaki, Y. Block copolymer micelles for drug delivery–design, characterization and biological significance. Adv. Drug Deliv. Rev. 2001, 47, 113–131. [Google Scholar] [CrossRef]
- Akbari, S. The application of dendritic material in textile engineering. Sci. Bull. Escorena 2013, 7, 11–26. [Google Scholar]
- Santos, A.; Veiga, F.; Figueiras, A. Dendrimers as pharmaceutical excipients: Synthesis, properties, toxicity and biomedical applications. Materials 2020, 13, 65. [Google Scholar] [CrossRef] [PubMed]
- Sikwal, D.R.; Kalhapure, R.S.; Govender, T. An emerging class of amphiphilic dendrimers for pharmaceutical and biomedical applications: Janus amphiphilic dendrimers. Eur. J. Pharm. Sci. 2016, 97, 113–134. [Google Scholar] [CrossRef]
- Sanz del Olmo, N.; Carloni, R.; Ortega, P.; García-Gallego, S.; de la Mata, F.J. Metallodendrimers as a promising tool in the biomedical field: An overview. Adv. Organomet. Chem. 2020, 74, 1–52. [Google Scholar]
- Cheng, Y.; Man, N.; Xu, T.; Fu, R.; Wang, X.; Wang, X.; Wen, L.-P. Transdermal delivery of nonsteroidal anti-inflammatory drugs mediated by polyamidoamine (PAMAM) dendrimers. J. Pharm. Sci. 2007, 96, 595–602. [Google Scholar]
- Chauhan, A.S.; Sridevi, S.; Chalasani, K.B.; Jain, A.K.; Jain, S.K.; Jain, N.K.; Diwan, P.V. Dendrimer-mediated transdermal delivery: Enhanced bioavailability of indomethacin. J. Control. Release 2003, 90, 335–343. [Google Scholar] [CrossRef]
- Le, N.T.T.; Nguyen, T.N.Q.; Cao, V.D.; Hoang, D.T.; Ngo, V.C.; Thi, T.T.H. Recent progress and advances of multi-stimuli-responsive dendrimers in drug delivery for cancer treatment. Pharmaceutics 2019, 11, 591. [Google Scholar] [CrossRef]
- Yang, Y.; Sunoqrot, S.; Stowell, C.; Ji, J.; Lee, C.-W.; Kim, J.W.; Khan, S.A.; Hong, S. Effect of size, surface charge, and hydrophobicity of poly(amidoamine) dendrimers on their skin penetration. Biomacromolecules 2012, 13, 2154–2162. [Google Scholar] [CrossRef]
- Wang, H.; Huang, Q.; Chang, H.; Xiao, J.; Cheng, Y. Stimuli-responsive dendrimers in drug delivery. Biomater. Sci. 2016, 4, 375–390. [Google Scholar] [CrossRef] [PubMed]
- Papahadjopoulos, D.; Kimelberg, H. Phospholipid vesicles (liposomes) as models for biological membranes: Their properties and interactions with cholesterol and proteins. Prog. Surf. Sci. 1974, 4, 141–144. [Google Scholar] [CrossRef]
- Ahmad, Z.; Shah, A.; Siddiqa, M.; Kraatz, H.-B. Polymeric micelles as drug delivery vehicles. RSC Adv. 2014, 4, 17028–17038. [Google Scholar] [CrossRef]
- Yu, G.; Ning, Q.; Mo, Z.; Tang, S. Intelligent polymeric micelles for multidrug co-delivery and cancer therapy. Artif. Cells Nanomed. Biotechnol. 2019, 47, 1476–1487. [Google Scholar] [CrossRef] [PubMed]
- Barani, H.; Montazer, M. A review on applications of liposomes in textile processing. J. Liposome Res. 2008, 18, 249–262. [Google Scholar] [CrossRef]
- Mahrhauser, D.; Reznicek, G.; Gehrig, S.; Geyer, A.; Ogris, M.; Kieweler, R.; Valenta, C. Simultaneous determination of active component and vehicle penetration from F-DPPC liposomes into porcine skin layers. Eur. J. Pharm. Biopharm. 2015, 97, 90–95. [Google Scholar] [CrossRef]
- Zangabad, P.S.; Mirkiani, S.; Shahsavari, S.; Masoudi, B.; Masroor, M.; Hamed, H.; Jafari, Z.; Taghipour, Y.D.; Hashemi, H.; Karimi, M.; et al. Stimulus-responsive liposomes as smart nanoplatforms for drug delivery applications. Nanotechnol. Rev. 2018, 7, 95–122. [Google Scholar] [CrossRef] [PubMed]
- Martí, M.; Martínez, V.; Rubio, L.; Coderch, L.; Parra, J. Biofunctional textiles prepared with liposomes: In vivo and in vitro assessment. J. Microencapsul. 2011, 28, 799–806. [Google Scholar] [CrossRef]
- Martina, M.; Hutmacher, D. Biodegradable polymers applied in tissue engineering research: A review. Polym. Int. 2007, 57, 145–157. [Google Scholar] [CrossRef]
- Benson, H.A.E.; Grice, J.E.; Mohammed, Y.; Namjoshi, S.; Roberts, M.S. Topical and transdermal drug delivery: From simple potions to smart technologies. Curr. Drug Deliv. 2019, 16, 444–460. [Google Scholar] [CrossRef]
- Jelezova, I.; Drakalska, E.; Momekova, D.; Shalimova, N.; Momekov, G.; Konstantinov, S.; Rangelov, S.; Pispas, S. Curcumin loaded pH-sensitive hybrid lipid/block copolymer nanosized drug delivery systems. Eur. J. Pharm. Sci. 2015, 78, 67–78. [Google Scholar] [CrossRef]
- Yamazaki, N.; Sugimoto, T.; Fukushima, M.; Teranishi, R.; Kotaka, A.; Shinde, C.; Kumei, T.; Sumida, Y.; Munekata, Y.; Maruyama, K.; et al. Dual-stimuli responsive liposomes using pH- and temperature-sensitive polymers for controlled transdermal delivery. Polym. Chem. 2017, 8, 1507. [Google Scholar] [CrossRef]
- Jijie, R.; Barras, A.; Boukherroub, R.; Szunerits, S. Nanomaterials for transdermal drug delivery: Beyond the state of the art of liposomal structures. J. Mater. Chem. B R. Soc. Chem. 2017, 5, 8653–8675. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Gong, C.; Huang, M.; Wang, J.; Pan, Y.; Zhang, Y.; Li, G.; Gou, M.; Wang, K.; Tu, M.; et al. Thermoreversible gel-sol behavior of biodegradable pcl-peg-pcl triblock copolymer in aqueous solutions. J. Biomed. Mater. Res. B Appl. Biomater. 2008, 84B, 165–175. [Google Scholar] [CrossRef]
- Pulkkinena, M.; Malin, M.; Böhmd, J.; Tarvainen, T.; Wirth, T.; Seppälä, J.; Järvinen, K. In vivo implantation of 2,2′-bis(oxazoline)-linked poly-epsilon–caprolactone: Proof for enzyme sensitive surface erosion and biocompatibility. Eur. J. Pharm. Sci. 2009, 36, 310–319. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Guo, S.; Wu, W. Characterization and in vitro release of praziquantel from poly(epsilon–caprolactone) implants. Int. J. Pharm. 2009, 377, 112–119. [Google Scholar] [CrossRef]
- Wang, S.; Su, R.; Nie, S.; Sun, M.; Zhang, J.; Wu, D.; Moustaid-Moussa, N. Application of nanotechnology in improving bioavailability and bioactivity of diet-derived phytochemicals. J. Nutr. Biochem. 2013, 25, 363–376. [Google Scholar] [CrossRef] [PubMed]
- Haladjova, E.; Toncheva-Moncheva, N.; Apostolova, M.; Trzebicka, B.; Dworak, A.; Petrov, P.; Dimitrov, I.; Rangelov, S.; Tsvetanov, C. Polymeric nanoparticle engineering: From temperature-responsive polymer mesoglobules to gene delivery systems. Biomacromolecules 2014, 15, 4377–4395. [Google Scholar] [CrossRef] [PubMed]
- Peppas, N.; Bures, P.; Leobandung, W.; Ichikawa, H. Hydrogels in pharmaceutical formulations. Eur. J. Pharm. Biopharm. 2000, 50, 27–46. [Google Scholar] [CrossRef]
- Nguyen, K.; West, J. Photopolymerizable hidrogels for tissue engineering applications. Biomaterials 2002, 23, 4307–4314. [Google Scholar] [CrossRef]
- Massella, D.; Leone, F.; Peila, R.; Barresi, A.; Ferri, A. Functionalization of cotton fabrics with polycaprolactone nanoparticles for transdermal release of melatonin. J. Funct. Biomater. 2017, 9, 1. [Google Scholar] [CrossRef] [PubMed]
- Bahney, C.; Lujan, T.; Hsu, C.; Bottlang, M.; West, J.; Johnstone, B. Visible light photoinitiation of mesenchymal stem cell-laden bioresponsive hydrogels. Eur. Cell. Mater. 2011, 22, 43–55. [Google Scholar] [CrossRef]
- Hwang, M.-R.; Kim, J.O.; Lee, J.; Kim, Y.I.; Kim, J.H.; Chang, S.; Jin, S.; Kim, J.A.; Lyoo, W.; Han, S.; et al. Gentamicin-loaded wound dressing with polyvinyl alcohol/dextran hydrogel:gel characterization and in vivo healing evaluation. AAPS Pharm. Sci. Tech. 2010, 11, 1092–1103. [Google Scholar] [CrossRef]
- Cevc, G. Self-regulating smart carriers for non-invasive and targeted drug delivery. Cell. Mol. Biol. 2002, 7, 224–225. [Google Scholar]
- Rodríguez, R.; Alvarez-Lorenzo, C.; Concheir, A. Interactions of ibuprofen with cationic polysaccharides in aqueous dispersions and hydrogels. Rheological and diffusional implications. Eur. J. Pharm. Sci. 2003, 20, 429–438. [Google Scholar] [CrossRef]
- Shishu; Aggarwal, N. Preparation of hydrogels of griseofulvin for dermal application. Int. J. Pharm. 2006, 326, 20–24. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.; Kim, J.; Hwang, S. Hydrogel patches containing triclosan for acne treatment. Eur. J. Pharm. Biopharm. 2003, 56, 407–412. [Google Scholar] [CrossRef]
- Wang, T.; Zhu, X.; Xue, X.; Wu, D. Hydrogel sheets of chitosan, honey and gelatin as burn wound dressings. Carbohydr. Polym. 2012, 88, 75–83. [Google Scholar] [CrossRef]
- Yusof, N.; Hafiza, A.; Zohdi, R.; Bakar, A. Development of honey hydrogel dressing for enhanced wound healing. Radiat. Phys. Chem. 2007, 76, 1767–1770. [Google Scholar] [CrossRef]
- Vogt, P.; Reimer, K.; Hauser, J.; Rossbach, O.; Steinau, H.; Bosse, B.; Muller, S.; Schmidt, T.; Fleischer, W. PVP-iodine in hydrosomes and hydrogel-a novel concept in wound therapy leads to enhanced epithelialization and reduced loss of skin grafts. Burns 2006, 32, 698–705. [Google Scholar] [CrossRef]
- Du, L.; Tong, L.; Jin, Y.; Jia, J.; Liu, Y.; Su, C.; Yu, S.; Li, X. A multifunctional in situ-forming hydro-gel for wound healing. Wound Repair Regen. 2012, 20, 904–910. [Google Scholar] [CrossRef] [PubMed]
- Heilmann, S.; Küchler, S.; Wischke, C.; Lendlein, A.; Stein, C.; Schäfer-Korting, M. A thermo-sensitive morphine-containing hydrogel for the treatment of large-scale skin wounds. Int. J. Pharm. 2013, 444, 96–102. [Google Scholar] [CrossRef]
- Wang, W.; Wat, E.; Hui, P.C.L.; Chan, B.; Ng, F.S.F.; Kan, C.-W.; Wang, X.; Hu, H.; Wong, E.C.W.; Lau, C.B.S.; et al. Dual-functional transdermal drug delivery system with controllable drug loading based on thermosensitive poloxamer hydrogel for atopic dermatitis treatment. Sci. Rep. 2016, 6, 24112. [Google Scholar] [CrossRef]
- Mocanu, G.; Nichifor, M.; Mihai, D.; Oproiu, L.C. Bioactive cotton fabrics containing chitosan and biologically active substances extracted from plants. Mater. Sci. Eng. C 2013, 33, 72–77. [Google Scholar] [CrossRef]
- Wang, X.; Hu, H.; Yang, Z.; He, L.; Kong, Y.; Fei, B.; Xin, J.H. Smart hydrogel-functionalized textile system with moisture management property for skin application. Smart Mater. Struct. 2014, 23, 125027. [Google Scholar] [CrossRef]
- Sun, X.-Z.; Wang, X.; Wu, J.-Z.; Li, S.-D. Development of thermosensitive microgel-loaded cotton fabric for controlled drug release. Appl. Surf. Sci. 2017, 403, 509–518. [Google Scholar] [CrossRef]
- Das, S.S.; Bharadwaj, P.; Bilal, M.; Barani, M.; Rahdar, A.; Taboada, P.; Bungau, S.; Kyzas, G.Z. Stimuli-responsive polymeric nanocarriers for drug delivery, imaging, and theragnosis. Polymers 2020, 12, 1397. [Google Scholar] [CrossRef]
- Morey, M.; Pandit, A. Responsive triggering systems for delivery in chronic wound healing. Adv. Drug Deliv. Rev. 2018, 129, 169–193. [Google Scholar] [CrossRef]
| Textile Chemical Composition and Structure | Drug Carrier | Textile Functionalization | Application | Ref. |
|---|---|---|---|---|
| nonwoven cotton fabric | Chitosan modified temperature responsive poly(N-isopropylacrylamide)polyurethane (PNIPA Am/PU) hydrogel | copolymerization on the surface of fabric | loading a variety of nutrients (or other functional components), which can release at body temperature | [44] |
| knitted cotton fabrics single jersey made of 100% cotton and a blend of viscose/micromodal 70/30% | Nanoparticles from poly-ε-caprolactone with encapsulated hydrophilic BAS caffeine | imbibition | antioxidant properties of the caffeine in transdermal delivery | [66] |
| cotton and polyamide fabrics | Liposomes made of two kinds of lipids (internal wool lipids or phosphatidylcholine) | exhaustion | transdermal delivery | [95] |
| cotton fabrics | Nanoparticles from poly-ε-caprolactone with encapsulated hydrophilic BAS melatonin | imbibition | melatonin in transdermal delivery | [108] |
| cotton fabric or chemically modified cotton (having aldehyde or carboxymethyl functional groups) | chitosan either upon crosslinking with glutaraldehyde or as fine particles (after crosslinking with natrium tripolyphosphate). | coating | clothes for people with allergies or other skin problems, due to the specific biological activity | [121] |
| nonwoven fabric pad, made of rayon/polyester | thermoresponsive hydrogel poly (ethylene glycol) and poly (ε-caprolactone) with hexamethylene diisocyanate | coating | skincare, wound healing, drug release control of aloin and curcumin | [122] |
| cotton fabric | thermosensitive microgels copolymer of poly(N-vinylcaprolactam) and chitosan oligosaccharide | pad-dry-cure and attachment by citric acid | textile-based drug delivery system for treating sunburn or skin care | [123] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Atanasova, D.; Staneva, D.; Grabchev, I. Textile Materials Modified with Stimuli-Responsive Drug Carrier for Skin Topical and Transdermal Delivery. Materials 2021, 14, 930. https://doi.org/10.3390/ma14040930
Atanasova D, Staneva D, Grabchev I. Textile Materials Modified with Stimuli-Responsive Drug Carrier for Skin Topical and Transdermal Delivery. Materials. 2021; 14(4):930. https://doi.org/10.3390/ma14040930
Chicago/Turabian StyleAtanasova, Daniela, Desislava Staneva, and Ivo Grabchev. 2021. "Textile Materials Modified with Stimuli-Responsive Drug Carrier for Skin Topical and Transdermal Delivery" Materials 14, no. 4: 930. https://doi.org/10.3390/ma14040930
APA StyleAtanasova, D., Staneva, D., & Grabchev, I. (2021). Textile Materials Modified with Stimuli-Responsive Drug Carrier for Skin Topical and Transdermal Delivery. Materials, 14(4), 930. https://doi.org/10.3390/ma14040930








