Investigation of the Structural Heterogeneity and Corrosion Performance of the Annealed Fe-Based Metallic Glasses
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation and Characterization of MGs
2.2. Nanoindentation Tests
2.3. Electrochemical Measurements
2.4. XPS Analysis
3. Results and Discussion
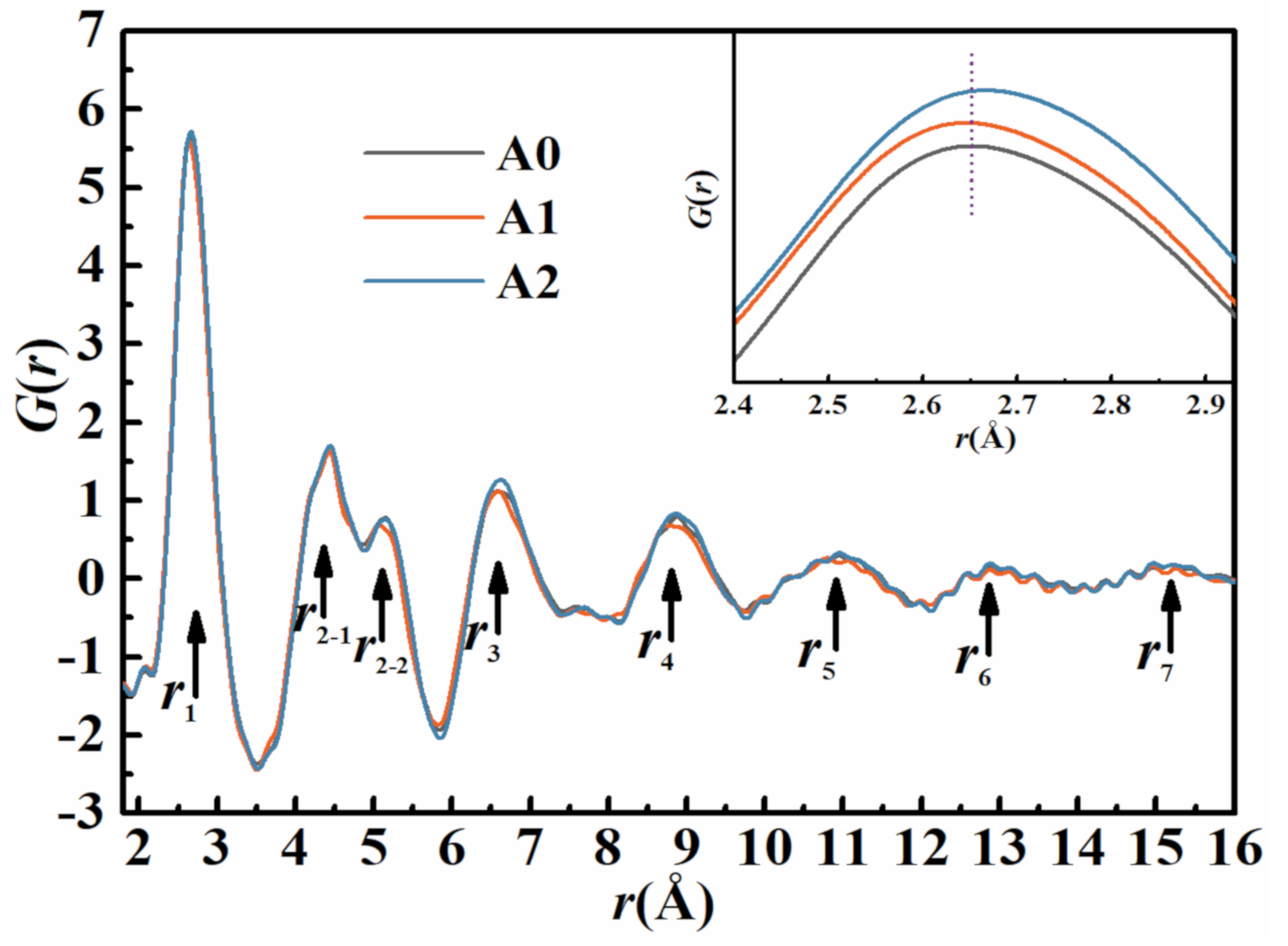
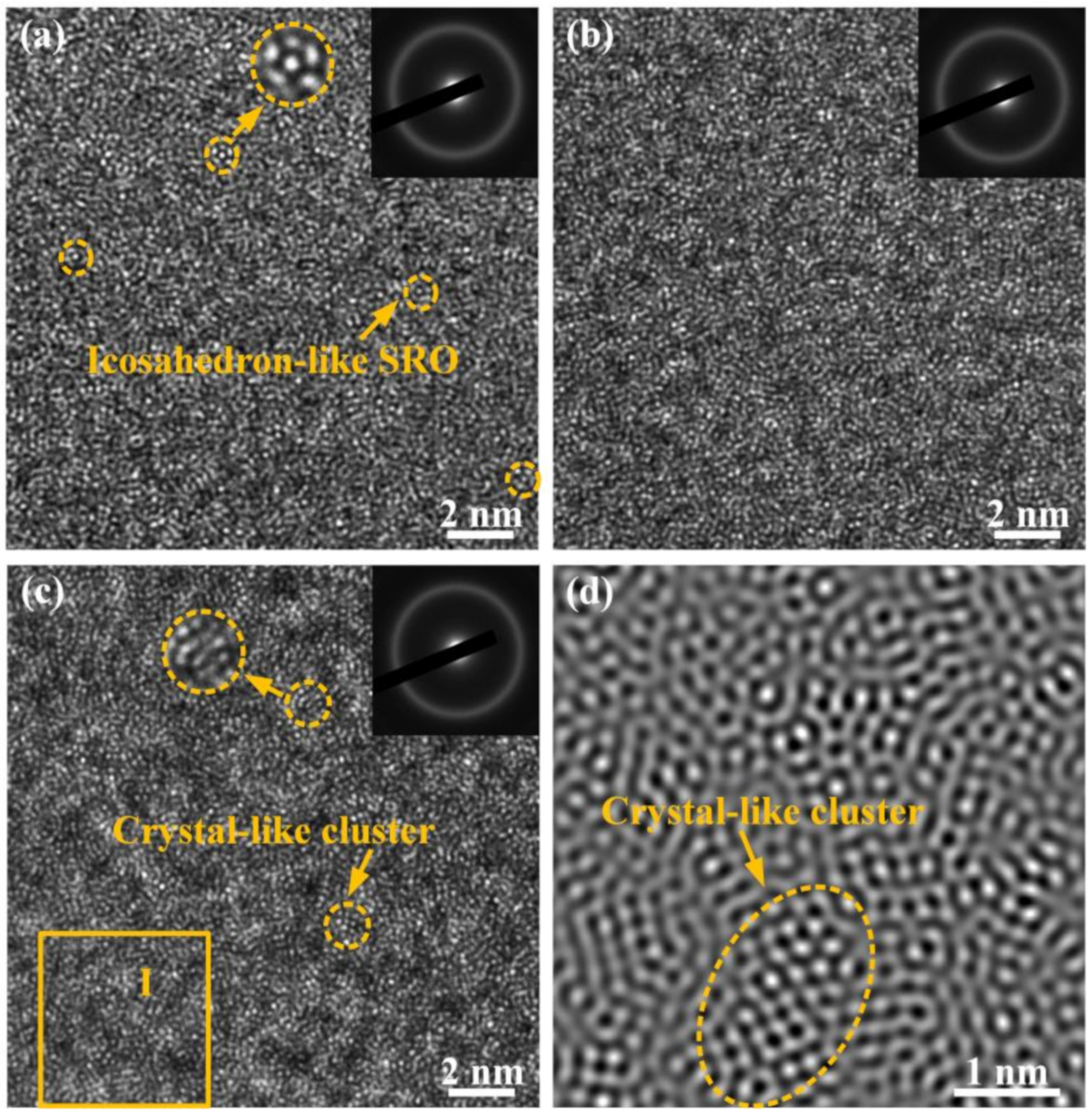
3.1. Characteristics of Fe-Based MGs
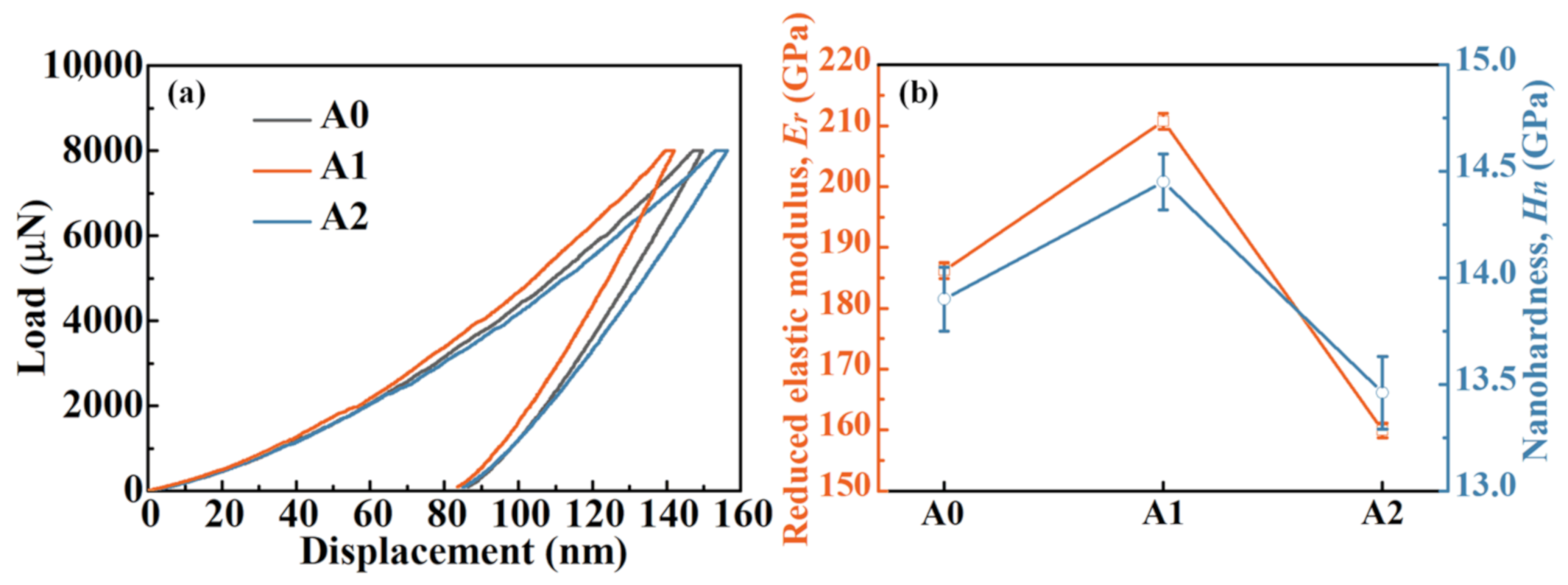
3.2. Mechanical Property of Fe-Based MGs
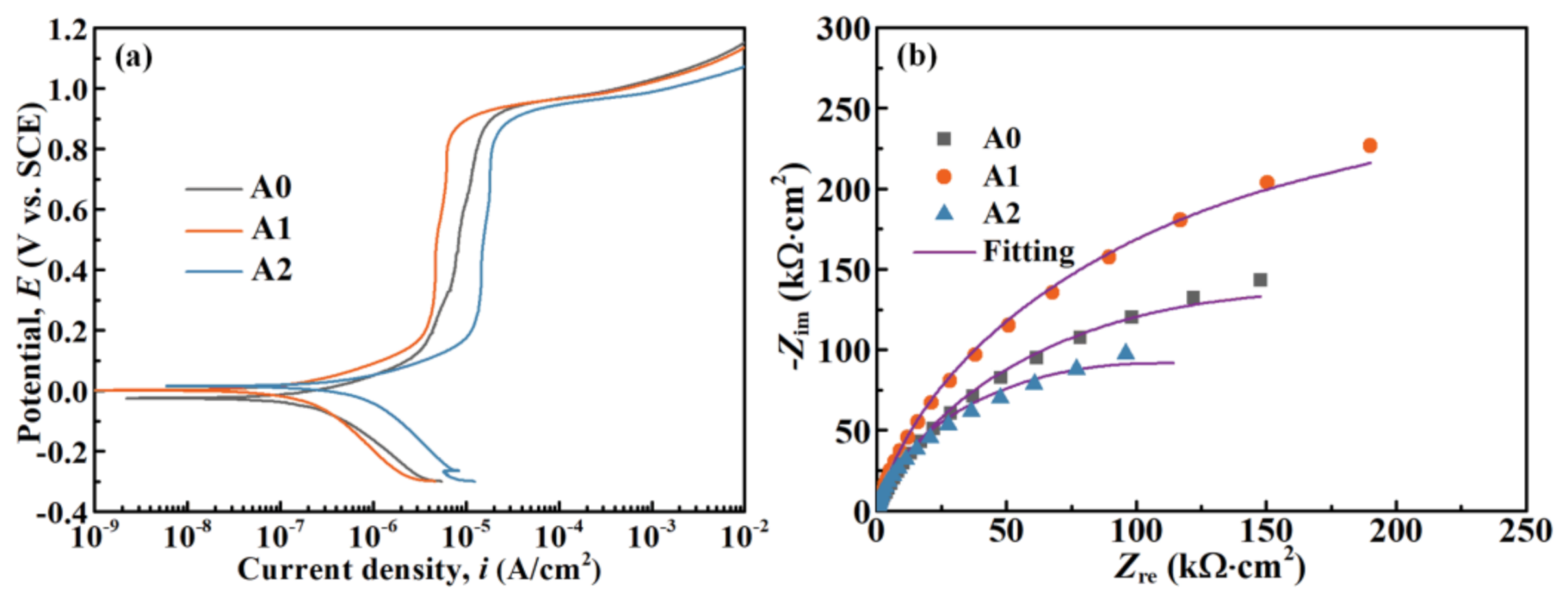
3.3. Electrochemical Measurements of MGs
3.4. Semiconducting Properties of Passive Films
3.5. XPS Depth Profiling Analysis
3.6. Effect of Annealing Temperature on the Structural Heterogeneity of MGs
3.7. Correlation Between the Structural Heterogeneity and Corrosion Performance of MGs
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rezaei-Shahreza, P.; Seifoddini, A.; Hasani, S.; Jaafari, Z.; Sliwa, A.; Nabialek, M. Isokinetic Analysis of Fe(41)Co(7)Cr(15)Mo(14)Y(2)C(15)B(6)Bulk Metallic Glass: Effect of Minor Copper Addition. Materials 2020, 13, 3704. [Google Scholar] [CrossRef]
- Sagasti, A.; Palomares, V.; Porro, J.M.; Orúe, I.; Sánchez-Ilárduya, M.B.; Lopes, A.C.; Gutiérrez, J. Magnetic, Magnetoelastic and Corrosion Resistant Properties of (Fe–Ni)-Based Metallic Glasses for Structural Health Monitoring Applications. Materials 2019, 13, 57. [Google Scholar] [CrossRef]
- Hirata, A.; Guan, P.; Fujita, T.; Hirotsu, Y.; Inoue, A.; Yavari, A.R.; Sakurai, T.; Chen, M. Direct observation of local atomic order in a metallic glass. Nat. Mater. 2010, 10, 28–33. [Google Scholar] [CrossRef]
- Zhu, F.; Hirata, A.; Liu, P.; Song, S.; Tian, Y.; Han, J.; Fujita, T.; Chen, M. Correlation between Local Structure Order and Spatial Heterogeneity in a Metallic Glass. Phys. Rev. Lett. 2017, 119, 215501. [Google Scholar] [CrossRef]
- Wagner, H.; Bedorf, D.; Kuechemann, S.; Schwabe, M.; Zhang, B.; Arnold, W.; Samwer, K. Local elastic properties of a metallic glass. Nat. Mater. 2011, 10, 439–442. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.H.; Wang, D.; Nakajima, K.; Zhang, W.; Hirata, A.; Nishi, T.; Inoue, A.; Chen, M.W. Characterization of Nanoscale Mechanical Heterogeneity in a Metallic Glass by Dynamic Force Microscopy. Phys. Rev. Lett. 2011, 106, 125504. [Google Scholar] [CrossRef] [PubMed]
- Lan, S.; Ren, Y.; Wei, X.Y.; Wang, B.; Gilbert, E.P.; Shibayama, T.; Watanabe, S.; Ohnuma, M.; Wang, X.-L. Hidden amorphous phase and reentrant supercooled liquid in Pd-Ni-P metallic glasses. Nat. Commun. 2017, 8, 14679. [Google Scholar] [CrossRef] [PubMed]
- Giordano, V.M.; Ruta, B. Unveiling the structural arrangements responsible for the atomic dynamics in metallic glasses during physical aging. Nat. Commun. 2016, 7, 10344. [Google Scholar] [CrossRef] [PubMed]
- Şopu, D.; Yuan, X.; Moitzi, F.; Stoica, M.; Eckert, J. Structure⁻Property Relationships in Shape Memory Metallic Glass Composites. Materials 2019, 12, 1419. [Google Scholar] [CrossRef]
- Liu, Y.H.; Wang, G.; Wang, R.J.; Zhao, D.Q.; Pan, M.X.; Wang, W.H. Super Plastic Bulk Metallic Glasses at Room Temperature. Science 2007, 315, 1385–1388. [Google Scholar] [CrossRef]
- Sarac, B.; Ivanov, Y.P.; Chuvilin, A.; Schöberl, T.; Stoica, M.; Zhang, Z.; Eckert, J. Origin of large plasticity and multiscale effects in iron-based metallic glasses. Nat. Commun. 2018, 9, 1333. [Google Scholar] [CrossRef]
- Bednarcik, J.; Michalik, S.; Kolesar, V.; Rütt, U.; Franz, H. In situ XRD studies of nanocrystallization of Fe-based metallic glass: A comparative study by reciprocal and direct space methods. Phys. Chem. Chem. Phys. 2013, 15, 8470. [Google Scholar] [CrossRef]
- Ouyang, S.; Song, L.J.; Liu, Y.H.; Huo, J.T.; Wang, J.Q.; Xu, W.; Li, J.L.; Wang, C.T.; Wang, X.M.; Li, R.W. Correlation between the viscoelastic heterogeneity and the domain wall motion of Fe-based metallic glass. Phys. Rev. Mater. 2018, 2, 063601. [Google Scholar] [CrossRef]
- Wang, X.; Pan, Y.; Zhu, Z.; Wu, J. Efficient degradation of rhodamine B using Fe-based metallic glass catalyst by Fenton-like process. Chemosphere 2014, 117, 638–643. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Liu, X.; Wang, H.; Zhou, D.; Wu, Y.; Lu, Z. Formation mechanism and characterization of nanoporous silver with tunable porosity and promising capacitive performance by chemical dealloying of glassy precursor. Acta Mater. 2016, 105, 367–377. [Google Scholar] [CrossRef]
- Hu, Y.C.; Wang, Y.Z.; Su, R.; Cao, C.R.; Lin, W.Z.; Sun, C.W.; Yang, Y.; Guan, P.F.; Ding, D.W.; Wang, Z.L.; et al. A Highly Efficient and Self-Stabilizing Metallic-Glass Catalyst for Electrochemical Hydrogen Generation. Adv. Mater. 2016, 28, 10293–10297. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.H.; Jiang, F.; Green, B.A.; Liu, F.X.; Liaw, P.K.; Choo, H.; Qiu, K.Q. Electrochemical corrosion behavior of a Zr-based bulk-metallic glass. Appl. Phys. Lett. 2007, 91, 041904. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, S.; Ma, A.; Hu, H.; Zheng, Y.; Yang, B.; Wang, J. Thermally induced structure evolution on the corrosion behavior of Al-Ni-Y amorphous alloys. Corros. Sci. 2018, 144, 172–183. [Google Scholar] [CrossRef]
- Tailleart, N.; Huang, R.; Aburada, T.; Horton, D.; Scully, J. Effect of thermally induced relaxation on passivity and corrosion of an amorphous Al–Co–Ce alloy. Corros. Sci. 2012, 59, 238–248. [Google Scholar] [CrossRef]
- Duarte, M.J.; Klemm, J.; Klemm, S.O.; Mayrhofer, K.J.J.; Stratmann, M.; Borodin, S.; Romero, A.H.; Madinehei, M.; Crespo, D.; Serrano, J.; et al. Element-Resolved Corrosion Analysis of Stainless-Type Glass-Forming Steels. Science 2013, 341, 372–376. [Google Scholar] [CrossRef]
- Jindal, R.; Raja, V.; Gibson, M.; Styles, M.; Bastow, T.; Hutchinson, C. Effect of annealing below the crystallization temperature on the corrosion behavior of Al–Ni–Y metallic glasses. Corros. Sci. 2014, 84, 54–65. [Google Scholar] [CrossRef]
- Tang, J.; Yu, L.; Qiao, J.; Wang, Y.; Wang, H.; Duan, M.; Chamas, M. Effect of atomic mobility on the electrochemical properties of a Zr58Nb3Cu16Ni13Al10 bulk metallic glass. Electrochim. Acta 2018, 267, 222–233. [Google Scholar] [CrossRef]
- Filik, J.; Ashton, A.W.; Chang PC, Y.; Chater, P.A.; Day, S.J.; Drakopoulos, M.; Gerring, M.W.; Hart, M.L.; Magdysyuk, O.V.; Michalik, S.; et al. Processing two-dimensional X-ray diffraction and small-angle scattering data in DAWN 2. J. Appl. Crystallogr. 2017, 50, 959–966. [Google Scholar] [CrossRef]
- Basham, M.; Filik, J.; Wharmby, M.T.; Chang, P.C.; El Kassaby, B.; Gerring, M.; Aishima, J.; Levik, K.; Pulford, B.C.A.; Sikharulidze, I.; et al. Data Analysis WorkbeNch (DAWN). J. Synchrotron Radiat. 2015, 22, 853–858. [Google Scholar] [CrossRef] [PubMed]
- Qiu, X.; Thompson, J.W.; Billinge, S.J.L. PDFgetX2: A GUI-driven program to obtain the pair distribution function from X-ray powder diffraction data. J. Appl. Crystallogr. 2004, 37, 678. [Google Scholar] [CrossRef]
- Liang, D.; Ma, J.; Cai, Y.; Liu, X.; Xie, S.; Wei, X.; Xu, G.; Shen, J. Characterization and elevated-temperature tribological performance of AC–HVAF-sprayed Fe-based amorphous coating. Surf. Coat. Technol. 2020, 387, 125535. [Google Scholar] [CrossRef]
- Lou, Y.; Xv, S.; Liu, Z.; Ma, J. Rejuvenation of Zr-Based Bulk Metallic Glasses by Ultrasonic Vibration-Assisted Elastic Deformation. Materials 2020, 13, 4397. [Google Scholar] [CrossRef]
- Murali, P.; Ramamurty, U. Embrittlement of a bulk metallic glass due to sub-Tg annealing. Acta Mater. 2005, 53, 1467–1478. [Google Scholar] [CrossRef]
- Shin, S.S.; Kim, H.K.; Lee, J.C.; Park, I.M. Effect of Sub-Tg Annealing on the Corrosion Resistance of the Cu–Zr Amorphous Alloys. Acta Metall. Sin. (Engl. Lett.) 2017, 31, 273–280. [Google Scholar] [CrossRef]
- Wang, X.; Gong, P.; Deng, L.; Jin, J.; Wang, S.; Li, F. Sub-Tg annealing effect on the kinetics of glass transition and crystallization for a Ti-Zr-Be-Fe bulk metallic glass. J. Non-Cryst. Solids 2017, 473, 132–140. [Google Scholar] [CrossRef]
- An, Y.; Hou, G.; Chen, J.; Zhao, X.; Liu, G.; Zhou, H.; Chen, J. Microstructure and tribological properties of iron-based metallic glass coatings prepared by atmospheric plasma spraying. Vacuum 2014, 107, 132–140. [Google Scholar] [CrossRef]
- Stillinger, F.H. A Topographic View of Supercooled Liquids and Glass Formation. Science 1995, 267, 1935–1939. [Google Scholar] [CrossRef] [PubMed]
- Sastry, S.; DeBenedetti, P.G.; Stillinger, F.H. Signatures of distinct dynamical regimes in the energy landscape of a glass-forming liquid. Nat. Cell Biol. 1998, 393, 554–557. [Google Scholar] [CrossRef]
- DeBenedetti, P.G.; Stillinger, F.H. Supercooled liquids and the glass transition. Nat. Cell Biol. 2001, 410, 259–267. [Google Scholar] [CrossRef] [PubMed]
- Evenson, Z.; Koschine, T.; Wei, S.; Gross, O.; Bednarcik, J.; Gallino, I.; Kruzic, J.J.; Rätzke, K.; Faupel, F.; Busch, R. The effect of low-temperature structural relaxation on free volume and chemical short-range ordering in a Au49Cu26.9Si16.3Ag5.5Pd2.3 bulk metallic glass. Scr. Mater. 2015, 103, 14–17. [Google Scholar] [CrossRef]
- Lelito, J. Crystallization Kinetics Analysis of the Amorphouse Mg(72)Zn(24)Ca(4)Alloy at the Isothermal Annealing Temperature of 507 K. Materials 2020, 13, 2815. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Ma, E. Atomic-level structure and structure–property relationship in metallic glasses. Prog. Mater. Sci. 2011, 56, 379–473. [Google Scholar] [CrossRef]
- Pan, S.P.; Qin, J.Y.; Wang, W.M.; Gu, T.K. Origin of splitting of the second peak in the pair-distribution function for metallic glasses. Phys. Rev. B 2011, 84, 092201. [Google Scholar] [CrossRef]
- Jiang, H.-R.; Bochtler, B.; Frey, M.; Liu, Q.; Wei, X.-S.; Min, Y.; Riegler, S.S.; Liang, D.-D.; Busch, R.; Shen, J. Equilibrium viscosity and structural change in the Cu47.5Zr45.1Al7.4 bulk glass-forming liquid. Acta Mater. 2020, 184, 69–78. [Google Scholar] [CrossRef]
- Yang, Y.; Zeng, J.; Volland, A.; Blandin, J.; Gravier, S.; Liu, C. Fractal growth of the dense-packing phase in annealed metallic glass imaged by high-resolution atomic force microscopy. Acta Mater. 2012, 60, 5260–5272. [Google Scholar] [CrossRef]
- Oliver, W.; Pharr, G. An improved technique for determining hardness and elastic modulus using load and displacement sensing indentation experiments. J. Mater. Res. 1992, 7, 1564–1583. [Google Scholar] [CrossRef]
- Liu, X.; Li, F.; Yang, Y. “Softness” as the structural origin of plasticity in disordered solids: A quantitative insight from machine learning. Sci. China Mater. 2018, 62, 154–160. [Google Scholar] [CrossRef]
- Stolpe, M.; Kruzic, J.J.; Busch, R. Evolution of shear bands, free volume and hardness during cold rolling of a Zr-based bulk metallic glass. Acta Mater. 2014, 64, 231–240. [Google Scholar] [CrossRef]
- Louzguineluzgin, D.V.; Bazlov, A.; Ketov, S.; Greer, A.; Inoue, A. Crystal growth limitation as a critical factor for formation of Fe-based bulk metallic glasses. Acta Mater. 2015, 82, 396–402. [Google Scholar] [CrossRef]
- Louzguine-Luzgin, D.; Bazlov, A.; Ketov, S.; Inoue, A. Crystallization behavior of Fe- and Co-based bulk metallic glasses and their glass-forming ability. Mater. Chem. Phys. 2015, 162, 197–206. [Google Scholar] [CrossRef]
- Khan, M.M.; Shabib, I.; Haider, W. A combinatorially developed Zr-Ti-Fe-Al metallic glass with outstanding corrosion resistance for implantable medical devices. Scr. Mater. 2019, 162, 223–229. [Google Scholar] [CrossRef]
- Kocijan, A.; Merl, D.K.; Jenko, M. The corrosion behaviour of austenitic and duplex stainless steels in artificial saliva with the addition of fluoride. Corros. Sci. 2011, 53, 776–783. [Google Scholar] [CrossRef]
- Qiao, Y.; Zheng, Y.; Ke, W.; Okafor, P. Electrochemical behaviour of high nitrogen stainless steel in acidic solutions. Corros. Sci. 2009, 51, 979–986. [Google Scholar] [CrossRef]
- Liang, D.-D.; Wei, X.-S.; Chang, C.-T.; Li, J.-W.; Wang, Y.; Wang, X.-M.; Shen, J. Effects of W Addition on the Electrochemical Behaviour and Passive Film Properties of Fe-Based Amorphous Alloys in Acetic Acid Solution. Acta Met. Sin. (English Lett.) 2018, 31, 1098–1108. [Google Scholar] [CrossRef]
- Wang, Z.; Ma, Y.; Zhang, J.; Hou, W.; Chang, X.; Wang, J. Influence of yttrium as a minority alloying element on the corrosion behavior in Fe-based bulk metallic glasses. Electrochim. Acta 2008, 54, 261–269. [Google Scholar] [CrossRef]
- Liang, D.; Wei, X.; Wang, Y.; Jiang, H.; Shen, J. Electrochemical behaviors and passive film properties of Fe-based bulk metallic glass in Cl−-containing acetic acid solutions under high temperature. J. Alloys Compd. 2018, 766, 964–972. [Google Scholar] [CrossRef]
- Ningshen, S.; Mudali, U.K.; Mittal, V.; Khatak, H. Semiconducting and passive film properties of nitrogen-containing type 316LN stainless steels. Corros. Sci. 2007, 49, 481–496. [Google Scholar] [CrossRef]
- Fernández-Domene, R.M.; Blasco-Tamarit, E.; García-García, D.M.; Garcia-Anton, J. Passivity Breakdown of Titanium in LiBr Solutions. J. Electrochem. Soc. 2013, 161, C25–C35. [Google Scholar] [CrossRef]
- Xu, D.; Zhou, B.; Wang, Q.; Zhou, J.; Yang, W.; Yuan, C.; Xue, L.; Fan, X.; Ma, L.; Shen, B. Effects of Cr addition on thermal stability, soft magnetic properties and corrosion resistance of FeSiB amorphous alloys. Corros. Sci. 2018, 138, 20–27. [Google Scholar] [CrossRef]
- Man, C.; Dong, C.; Cui, Z.; Xiao, K.; Yu, Q.; Li, X. A comparative study of primary and secondary passive films formed on AM355 stainless steel in 0.1 M NaOH. Appl. Surf. Sci. 2018, 427, 763–773. [Google Scholar] [CrossRef]
- Zhu, F.; Nguyen, H.K.; Song, S.X.; Aji, D.P.B.; Hirata, A.; Wang, H.; Nakajima, K.; Chen, M.W. Intrinsic correlation between β-relaxation and spatial heterogeneity in a metallic glass. Nat. Commun. 2016, 7, 11516. [Google Scholar] [CrossRef]
- Gu, B.; Liu, F. Characterization of structural inhomogeneity in Al88Ce8Co4 metallic glass. Acta Mater. 2016, 112, 94–104. [Google Scholar] [CrossRef]
- Huang, Y.; Fan, H.; Zhou, X.; Xue, P.; Ning, Z.; Daisenberger, D.; Sun, J.; Shen, J. Structure and mechanical property modification of a Ti-based metallic glass by ion irradiation. Scr. Mater. 2015, 103, 41–44. [Google Scholar] [CrossRef]
- Evertz, S.; Schneider, J.M. Effect of the Free Volume on the Electronic Structure of Cu70Zr30 Metallic Glasses. Materials 2020, 13, 4911. [Google Scholar]
- Liu, W.; Ruan, H.; Zhang, L. Atomic rearrangements in metallic glass: Their nucleation and self-organization. Acta Mater. 2013, 61, 6050–6060. [Google Scholar] [CrossRef]
- Wang, Y.-J.; Du, J.-P.; Shinzato, S.; Dai, L.-H.; Ogata, S. A free energy landscape perspective on the nature of collective diffusion in amorphous solids. Acta Mater. 2018, 157, 165–173. [Google Scholar] [CrossRef]
- González, S.; Pellicer, E.; Suriñach, S.; Baró, M.D.; Sort, J. Mechanical and corrosion behaviour of as-cast and annealed Zr60Cu20Al10Fe5Ti5 bulk metallic glass. Intermetallics 2012, 28, 149–155. [Google Scholar] [CrossRef]
- Zhao, G.; Zou, Y.; Zhang, H.; Zou, Z. Correlation between corrosion resistance and the local atomic structure of electroless, annealed Ni–P amorphous alloys. Mater. Lett. 2014, 132, 221–223. [Google Scholar] [CrossRef]
- Zhang, B.; Wang, J.; Wu, B.; Guo, X.W.; Wang, Y.J.; Chen, D.; Zhang, Y.C.; Du, K.; Oguzie, E.E.; Ma, X.L. Unmasking chloride attack on the passive film of metals. Nat. Commun. 2018, 9, 1–9. [Google Scholar] [CrossRef]
- Gebert, A.; Gostin, P.; Uhlemann, M.; Eckert, J.; Schultz, L. Interactions between mechanically generated defects and corrosion phenomena of Zr-based bulk metallic glasses. Acta Mater. 2012, 60, 2300–2309. [Google Scholar] [CrossRef]

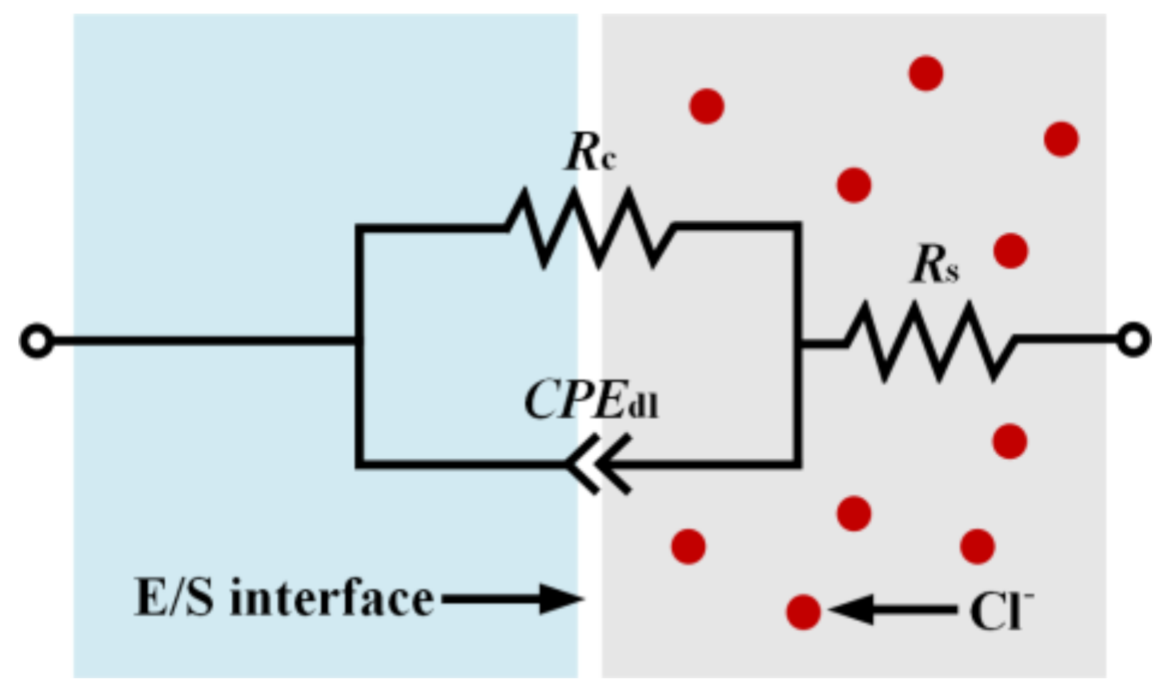
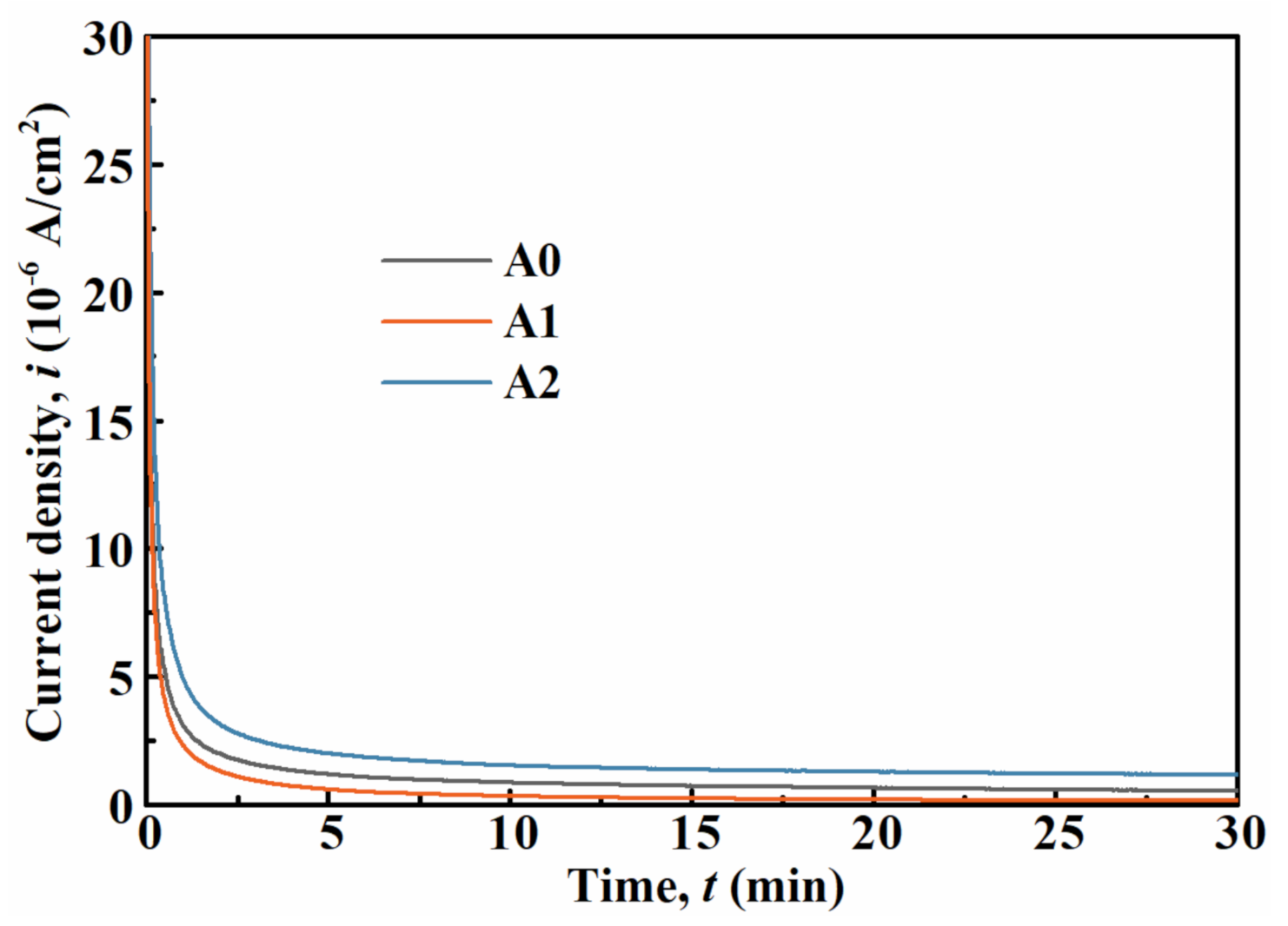

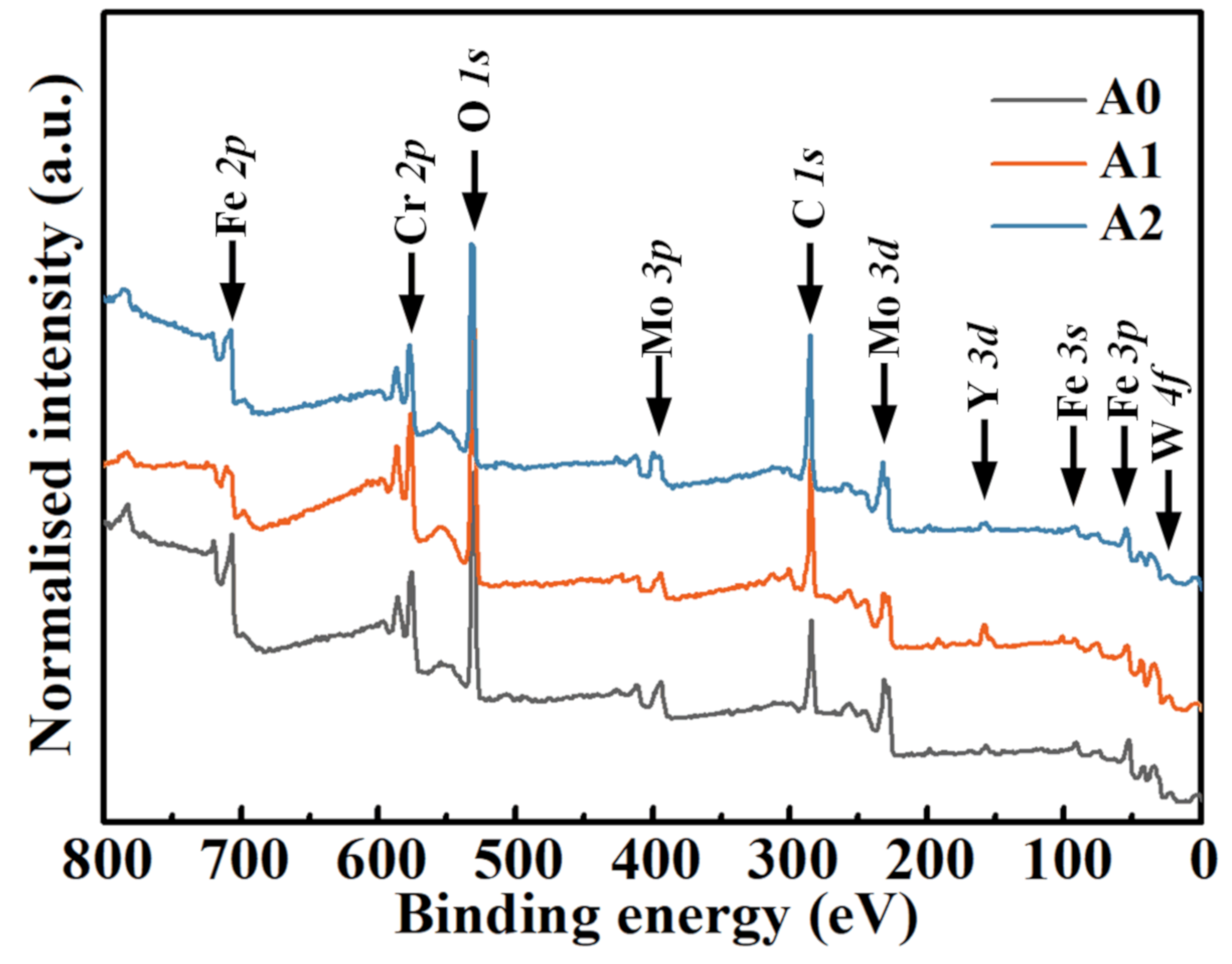
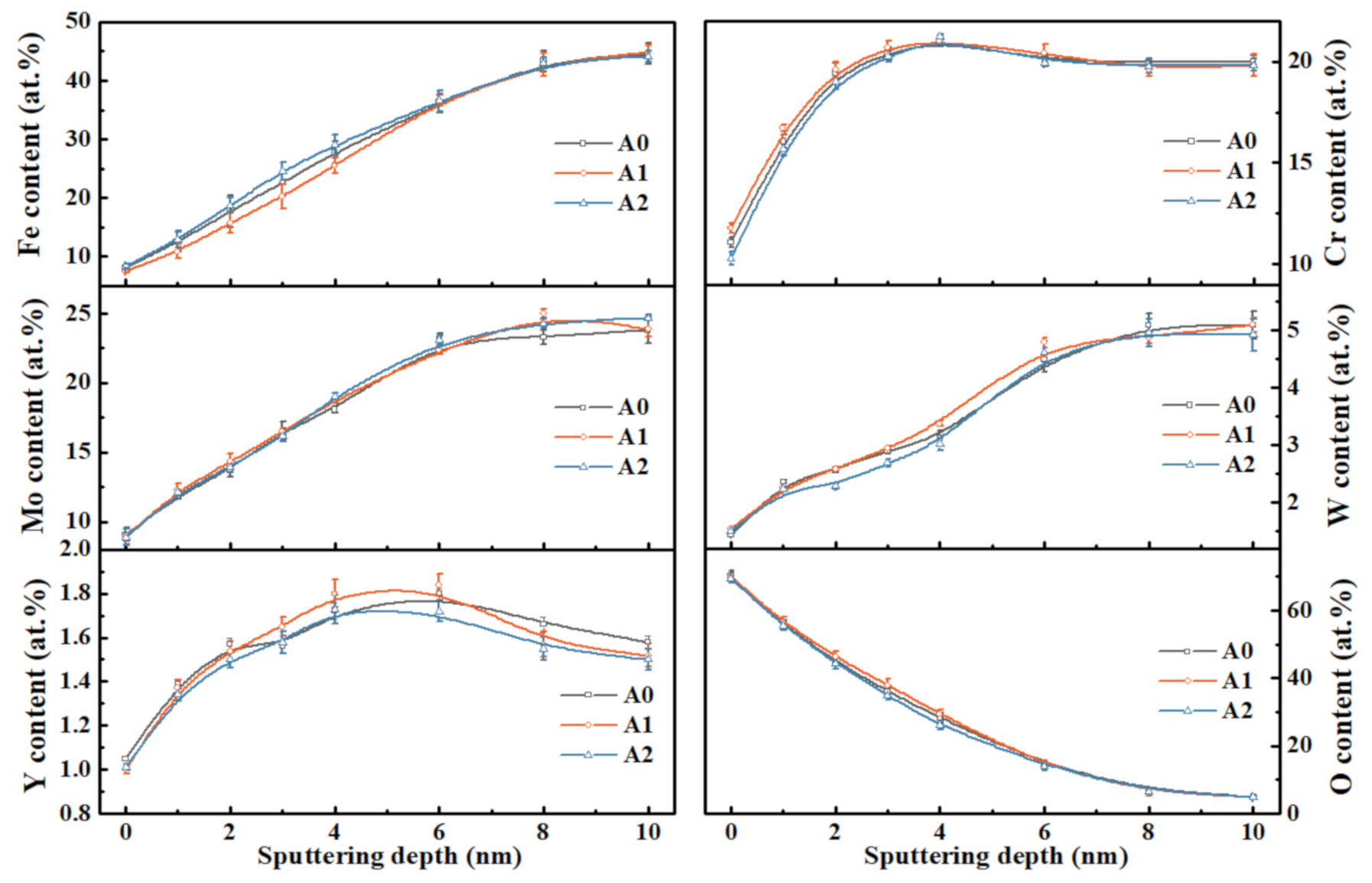
| Sample | icorr (µA/cm2) | Ecorr (mV) | ipass (µA/cm2) | Epit (mV) | Rc (kΩ·cm2) |
|---|---|---|---|---|---|
| A0 | 0.21 ± 0.12 | −25.64 ± 17.62 | 8.45 ± 0.31 | 923.01 ± 3.32 | 300.32 ± 12.74 |
| A1 | 0.34 ± 0.15 | −1.82 ± 2.56 | 4.62 ± 0.73 | 938.87 ± 0.16 | 458.75 ± 14.78 |
| A2 | 0.54 ± 0.11 | 30.62 ± 14.57 | 13.67 ± 2.94 | 886.00 ± 15.24 | 202.46 ± 10.87 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, D.; Tseng, J.-C.; Liu, X.; Cai, Y.; Xu, G.; Shen, J. Investigation of the Structural Heterogeneity and Corrosion Performance of the Annealed Fe-Based Metallic Glasses. Materials 2021, 14, 929. https://doi.org/10.3390/ma14040929
Liang D, Tseng J-C, Liu X, Cai Y, Xu G, Shen J. Investigation of the Structural Heterogeneity and Corrosion Performance of the Annealed Fe-Based Metallic Glasses. Materials. 2021; 14(4):929. https://doi.org/10.3390/ma14040929
Chicago/Turabian StyleLiang, Dandan, Jo-Chi Tseng, Xiaodi Liu, Yuanfei Cai, Gang Xu, and Jun Shen. 2021. "Investigation of the Structural Heterogeneity and Corrosion Performance of the Annealed Fe-Based Metallic Glasses" Materials 14, no. 4: 929. https://doi.org/10.3390/ma14040929
APA StyleLiang, D., Tseng, J.-C., Liu, X., Cai, Y., Xu, G., & Shen, J. (2021). Investigation of the Structural Heterogeneity and Corrosion Performance of the Annealed Fe-Based Metallic Glasses. Materials, 14(4), 929. https://doi.org/10.3390/ma14040929





