On the Response of a Micro Non-Destructive Testing X-ray Detector
Abstract
1. Introduction
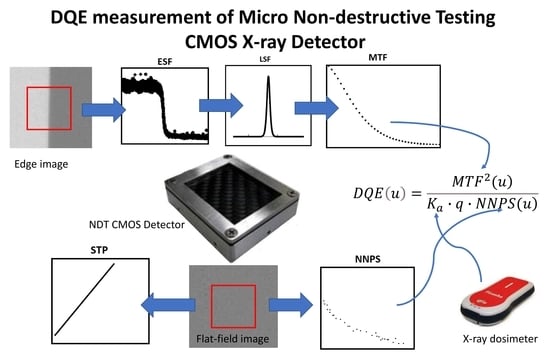
2. Materials and Methods
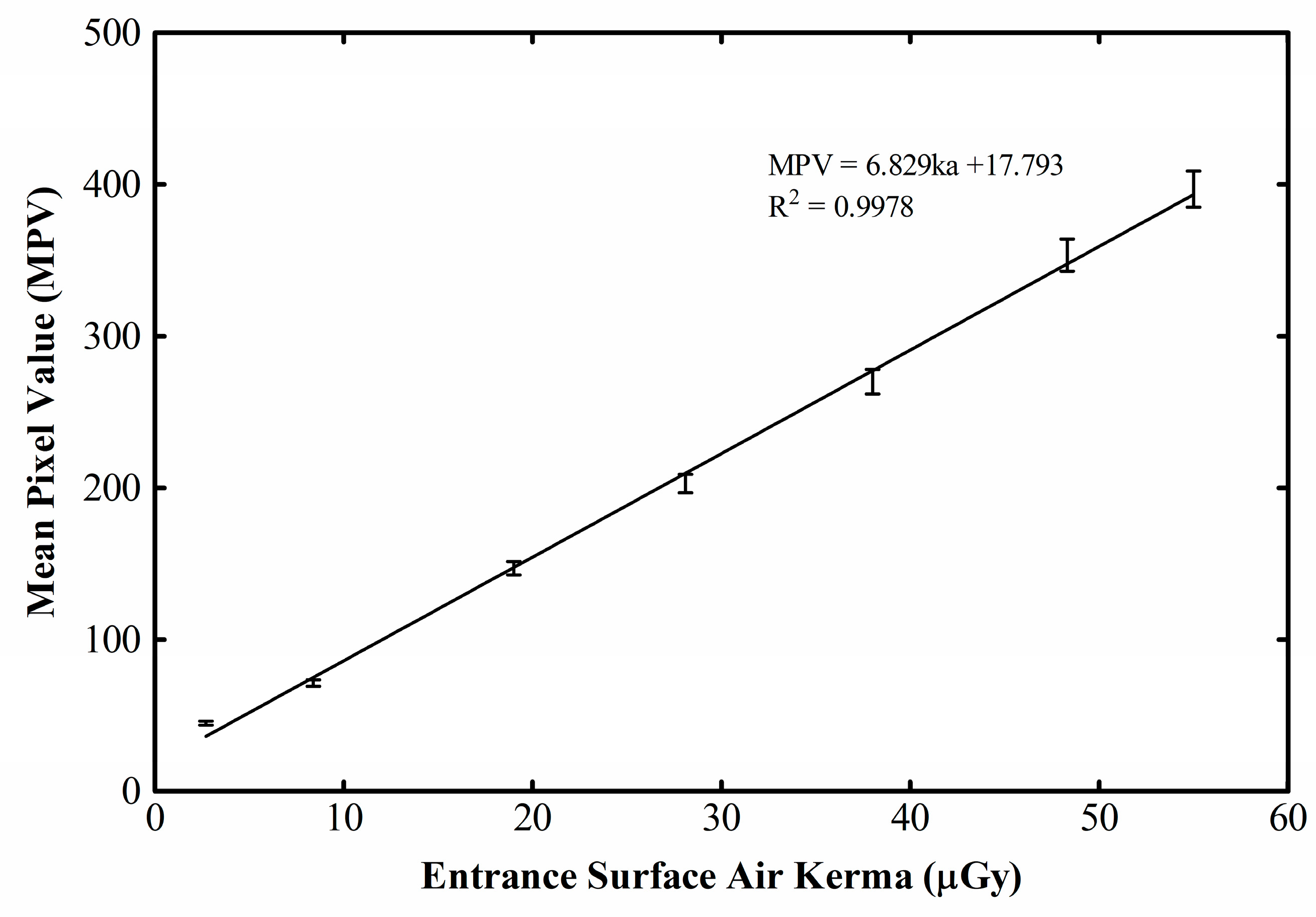
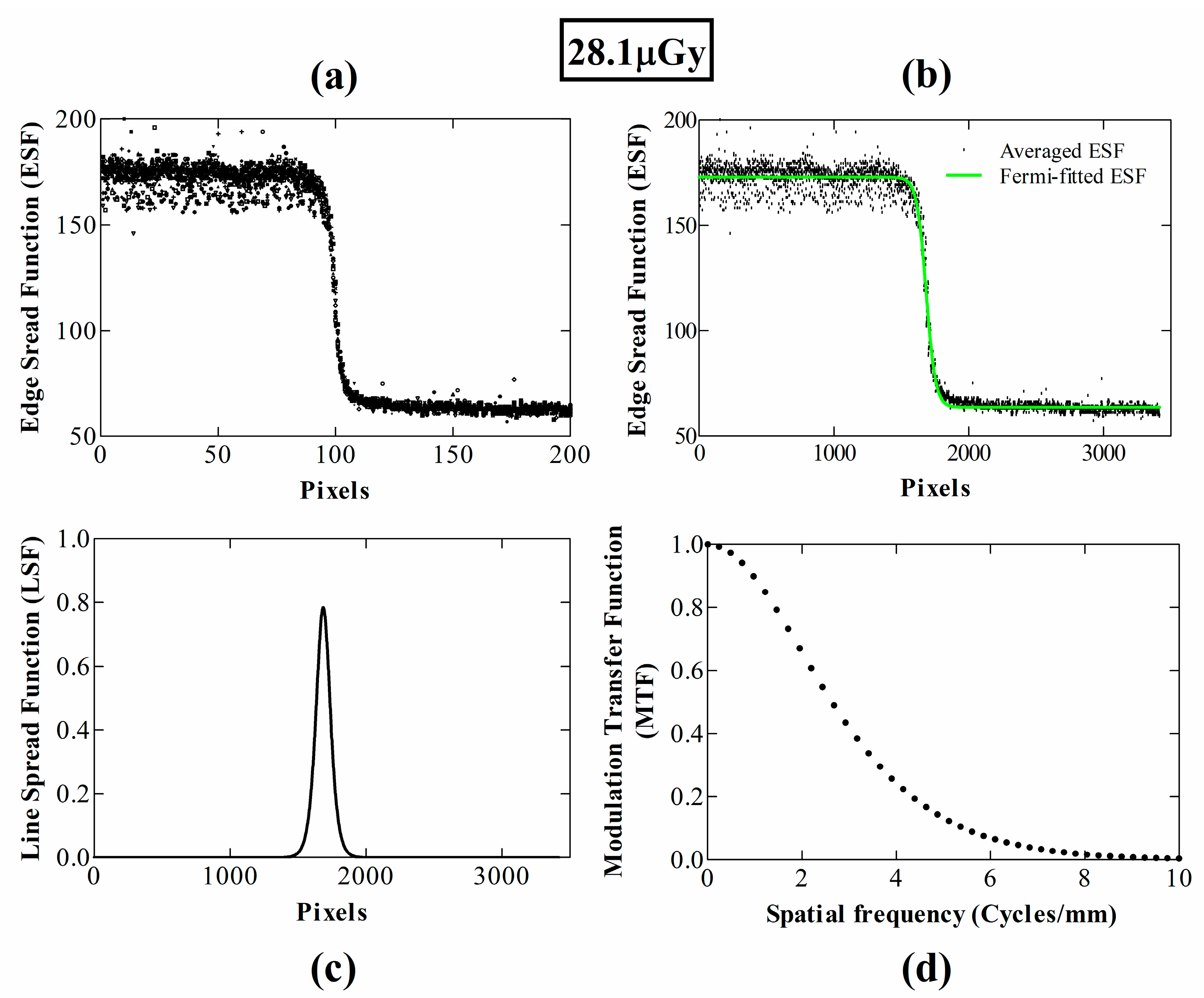
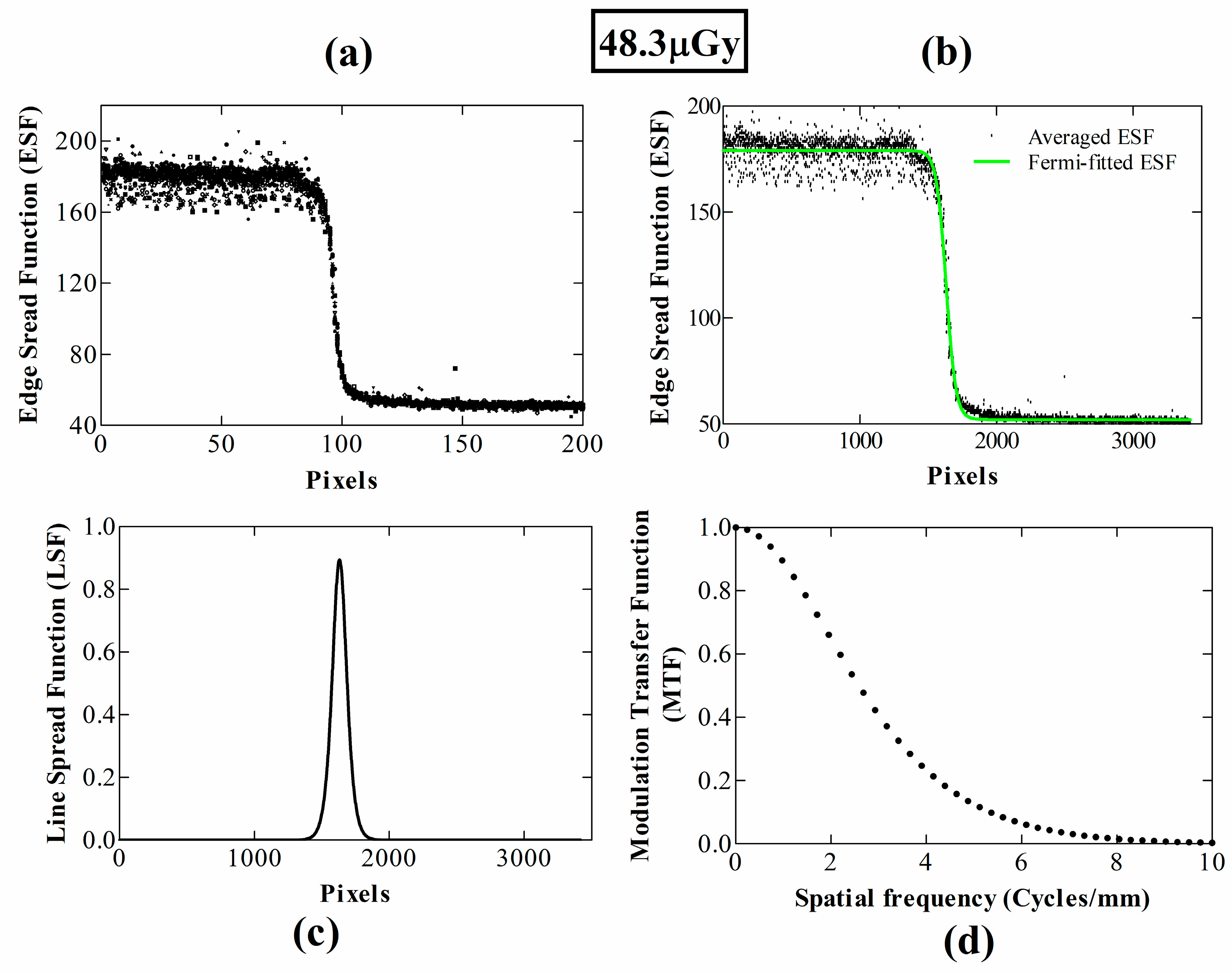
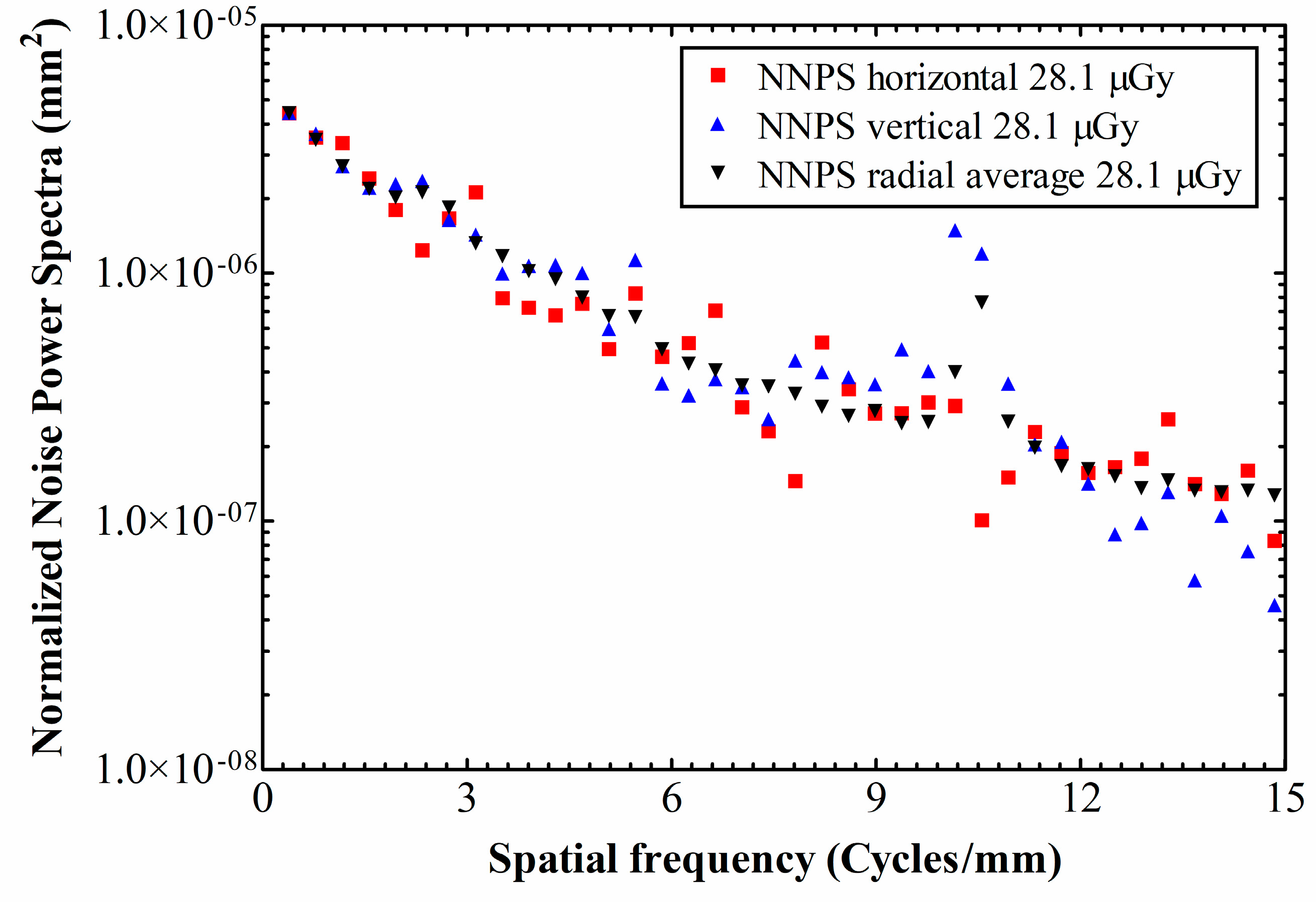
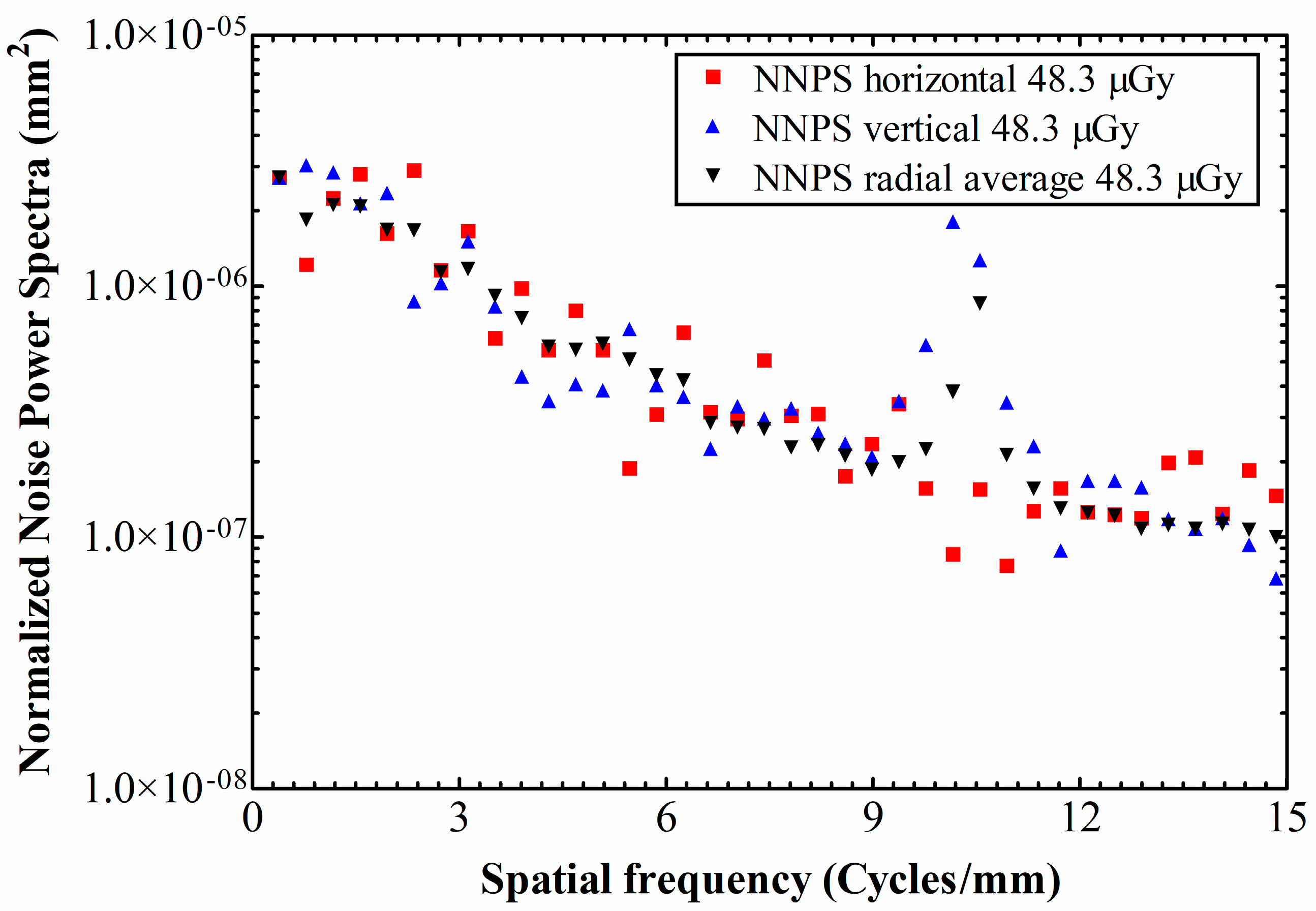
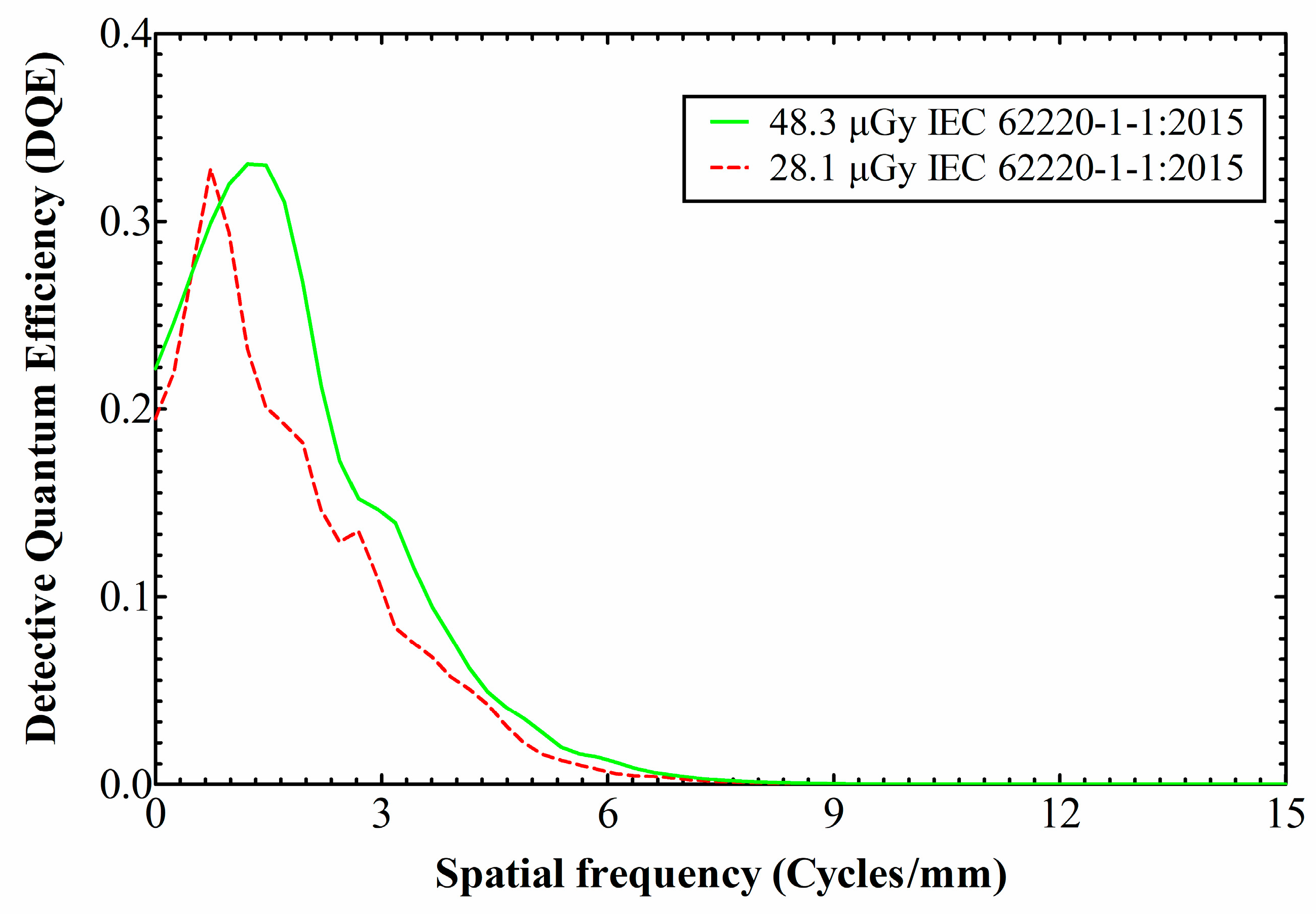
3. Results and Discussion
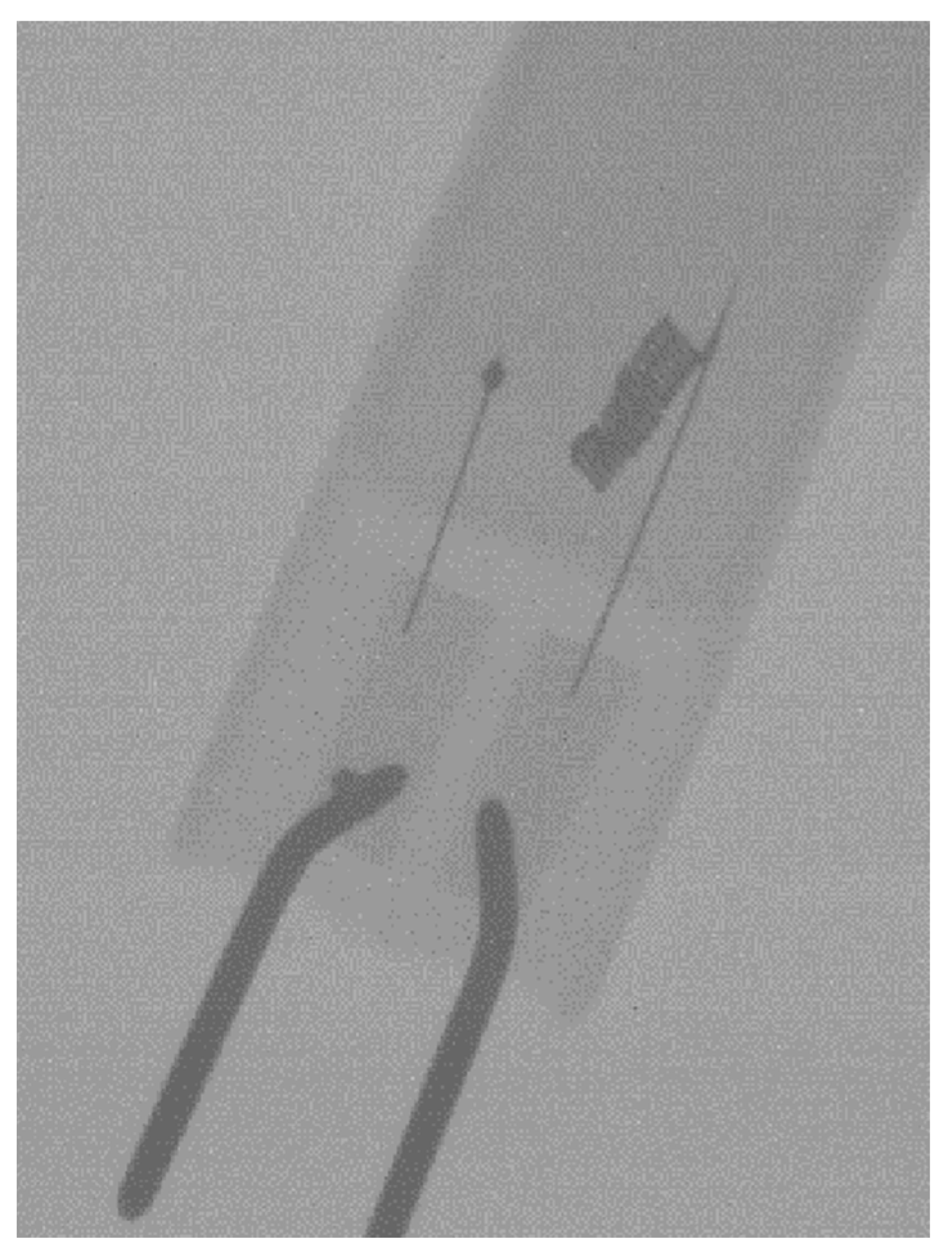
Potential Application for Dual-Energy Imaging
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhao, C.; Kanicki, J.; Konstantinidis, A.C.A.C.; Patel, T. Large area CMOS active pixel sensor X-ray imager for digital breast tomosynthesis: Analysis, modeling, and characterization. Med. Phys. 2015, 42, 6294–6308. [Google Scholar] [CrossRef]
- Konstantinidis, A.C.; Szafraniec, M.B.; Speller, R.D.; Olivo, A. The Dexela 2923 CMOS X-ray detector: A flat panel detector based on CMOS active pixel sensors for medical imaging applications. Nucl. Instrum. Methods Phys. Res. A 2012, 689, 12–21. [Google Scholar] [CrossRef]
- Zhao, C.; Konstantinidis, A.C.; Zheng, Y.; Anaxagoras, T.; Speller, R.D.; Kanicki, J. 50 μm pixel pitch wafer-scale CMOS active pixel sensor X-ray detector for digital breast tomosynthesis. Phys. Med. Biol. 2015, 60, 8977–9001. [Google Scholar] [CrossRef]
- Zhao, C.; Kanicki, J. Amorphous In-Ga-Zn-O thin-film transistor active pixel sensor X-ray imager for digital breast tomosynthesis. Med. Phys. 2014, 41, 091902. [Google Scholar] [CrossRef]
- Cabello, J.; Bailey, A.; Kitchen, I.; Clark, A.; Crooks, J.; Halsall, R.; Key-Charriere, M.; Martin, S.; Prydderch, M.; Turchetta, R.; et al. Digital autoradiography using CCD and CMOS imaging technology. In Proceedings of the 2006 IEEE Nuclear Science Symposium Conference Record (NSS/MIC 2006), San Diego, CA, USA, 29 October–4 November 2006; Volume 4, pp. 2607–2612. [Google Scholar]
- Pham, T.N.; Finck, C.; Marchand, P.; Brasse, D.; Boisson, F.; Laquerriere, P. 18F autoradiography using pixelated CMOS technology. In Proceedings of the 2017 15th IEEE International New Circuits and Systems Conference (NEWCAS 2017), Strasbourg, France, 25–28 June 2017; pp. 41–44. [Google Scholar]
- O’Connell, D.W.; Morgan, K.S.; Ruben, G.; Schaff, F.; Croton, L.C.P.; Buckley, G.A.; Paganin, D.M.; Uesugi, K.; Kitchen, M.J. Photon-counting, energy-resolving and super-resolution phase contrast X-ray imaging using an integrating detector. Opt. Express 2020, 28, 7080–7094. [Google Scholar] [CrossRef]
- Kupsch, A.; Hentschel, M.; Lange, A.; Bruno, G.; Müller, B. Direct X-ray Refraction of Micro Structures. In Proceedings of the 11th European Conference on Non-Destructive Testing (ECNDT 2014), Prague, Czech Republic, 6–10 October 2014. [Google Scholar]
- Mishurova, T.; Artzt, K.; Haubrich, J.; Requena, G.; Bruno, G. New aspects about the search for the most relevant parameters optimizing SLM materials. Addit. Manuf. 2019, 25, 325–334. [Google Scholar] [CrossRef]
- Prosch, A.; Larson, B. Real Time Radiography NDT Course Booklet. Available online: https://www.nde-ed.org/TeachingResources/Downloadable/Real-Time%20Radiography/Real-time%20Radiography%20Booklet.htm (accessed on 12 February 2021).
- Nikl, M. Scintillation detectors for X-rays. Meas. Sci. Technol. 2006, 17, R37–R54. [Google Scholar] [CrossRef]
- A Elbakri, I.; McIntosh, B.J.; Rickey, D.W. Physical characterization and performance comparison of active- and passive-pixel CMOS detectors for mammography. Phys. Med. Biol. 2009, 54, 1743–1755. [Google Scholar] [CrossRef] [PubMed]
- Michail, C.M.; Spyropoulou, V.A.; Fountos, G.P.; Kalyvas, N.I.; Valais, I.G.; Kandarakis, I.S.; Panayiotakis, G.S. Experimental and Theoretical Evaluation of a High Resolution CMOS Based Detector Under X-ray Imaging Conditions. IEEE Trans. Nucl. Sci. 2010, 58, 314–322. [Google Scholar] [CrossRef]
- Konstantinidis, A.; Anaxagoras, T.; Esposito, M.; Allinson, N.; Speller, R. DynAMITe: A prototype large area CMOS APS for breast cancer diagnosis using X-ray diffraction measurements. SPIE Med Imaging 2012, 8313, 83135. [Google Scholar] [CrossRef]
- Teledyne CCD290-99 Product Datasheet. Available online: https://www.teledyne-e2v.com/markets/space/astronomy-imaging/ccd290-99/ (accessed on 12 February 2021).
- E Bohndiek, S.; Cook, E.J.; Arvanitis, C.D.; Olivo, A.; Royle, G.J.; Clark, A.T.; Prydderch, M.L.; Turchetta, R.; Speller, R.D. A CMOS active pixel sensor system for laboratory- based X-ray diffraction studies of biological tissue. Phys. Med. Biol. 2008, 53, 655–672. [Google Scholar] [CrossRef]
- Rowlands, J.A.; Yorkston, J. Flat Panel Detectors for Digital Radiography (Part I, Chapter 4). In Handbook of Medical Imaging. Volume 1: Physics and Psychophysics; Beutel, J., Kundel, H.L., Van Metter, R.L., Eds.; SPIE Press: Bellingham, WA, USA, 2000; Volume 1, pp. 223–327. ISBN 978-0-8194-7772-9. [Google Scholar]
- Konstantinidis, A.C.; Szafraniec, M.B.; Rigon, L.; Tromba, G.; Dreossi, D.; Sodini, N.; Liaparinos, P.F.; Naday, S.; Gunn, S.; McArthur, A.; et al. X-ray Performance Evaluation of the Dexela CMOS APS X-ray Detector Using Monochromatic Synchrotron Radiation in the Mammographic Energy Range. IEEE Trans. Nucl. Sci. 2013, 60, 3969–3980. [Google Scholar] [CrossRef]
- Gopinath, D.; Soman, M.; Holland, A.; Keelan, J.; Hall, D.; Holland, K.; Colebrook, D. Soft X-ray radiation damage in EM-CCDs used for Resonant Inelastic X-ray Scattering. J. Instrum. 2018, 13, C02027. [Google Scholar] [CrossRef]
- Adams, R.; Zboray, R. Gamma radiography and tomography with a CCD camera and Co-60 source. Appl. Radiat. Isot. 2017, 127, 82–86. [Google Scholar] [CrossRef]
- Arvanitis, C.D.; Bohndiek, S.E.; Royle, G.; Blue, A.; Liang, H.X.; Clark, A.; Prydderch, M.; Turchetta, R.; Speller, R. Empirical electro-optical and X-ray performance evaluation of CMOS active pixels sensor for low dose, high resolution X-ray medical imaging. Med. Phys. 2007, 34, 4612–4625. [Google Scholar] [CrossRef]
- Ahn, J.; Moon, C.-R.; Kim, B.; Lee, K.; Kim, Y.; Lim, M.; Lee, W.; Park, H.; Moon, K.; Yoo, J.; et al. Advanced Image Sensor Technology for Pixel Scaling down toward 1.0 μm (Invited). In Proceedings of the 2008 IEEE International Electron Devices Meeting, San Francisco, CA, USA, 15–17 December 2008; pp. 1–4. [Google Scholar]
- Asakura, K.; Hayashida, K.; Hanasaka, T.; Kawabata, T.; Yoneyama, T.; Okazaki, K.; Ide, S.; Noda, H.; Matsumoto, H. X-ray imaging polarimetry with a 2.5-μm pixel CMOS sensor for visible light at room temperature. J. Astron. Telesc. Instrum. Syst. 2019, 5, 035002. [Google Scholar] [CrossRef]
- Zhang, L.; Jin, Y.; Lin, L.; Li, J.; Du, Y. The Comparison of CCD and CMOS Image Sensors. In Proceedings of the SPIE, Proceedings of 2008 International Conference on Optical Instruments and Technology: Advanced Sensor Technologies and Applications, Beijing, China, 16–19 November 2008; Wang, A., Liao, Y., Song, A., Ishii, Y., Fan, X., Eds.; Curran Associates, Inc.: Red Hook, NY, USA, 2009. [Google Scholar]
- Olbinado, M.P.; Just, X.; Gelet, J.-L.; Lhuissier, P.; Scheel, M.; Vagovic, P.; Sato, T.; Graceffa, R.; Schulz, J.; Mancuso, A.; et al. MHz frame rate hard X-ray phase-contrast imaging using synchrotron radiation. Opt. Express 2017, 25, 13857–13871. [Google Scholar] [CrossRef]
- Teledyne Lince11M CMOS Sensor. Available online: https://imaging.teledyne-e2v.com/products/2d-cmos-image-sensors/lince/lince-11m/ (accessed on 23 November 2020).
- Kuroda, R.; Sugawa, S. Cameras with On-chip Memory CMOS Image Sensors. In The Micro-World Observed by Ultra High-Speed Cameras; Springer International Publishing: New York, NY, USA, 2018; pp. 103–124. [Google Scholar]
- Koukou, V.; Martini, N.; Fountos, G.; Michail, C.; Bakas, A.; Oikonomou, G.; Kandarakis, I.; Nikiforidis, G. Application of a dual energy X-ray imaging method on breast specimen. Results Phys. 2017, 7, 1634–1636. [Google Scholar] [CrossRef]
- Martini, N.; Koukou, V.; Fountos, G.; Michail, C.; Bakas, A.; Kandarakis, I.; Speller, R.; Nikiforidis, G. Characterization of breast calcification types using dual energy X-ray method. Phys. Med. Biol. 2017, 62, 7741–7764. [Google Scholar] [CrossRef] [PubMed]
- Teledyne Remote RadEye HR. Available online: https://www.teledynedalsa.com/en/products/imaging/industrial-X-ray/remote-radeye-hr/ (accessed on 31 January 2021).
- Cho, M.K.; Kim, H.K.; Graeve, T.; Yun, S.M.; Lim, C.H.; Cho, H.; Kim, J.-M. Measurements of X-ray Imaging Performance of Granular Phosphors With Direct-Coupled CMOS Sensors. IEEE Trans. Nucl. Sci. 2008, 55, 1338–1343. [Google Scholar] [CrossRef]
- Konstantinidis, A. Evaluation of Digital X-ray Detectors for Medical Imaging Applications. PhD Thesis, University College London, London, UK, 2011. [Google Scholar]
- Michail, C.; Valais, I.; Martini, N.; Koukou, V.; Kalyvas, N.; Bakas, A.; Kandarakis, I.; Fountos, G. Determination of the detective quantum efficiency (DQE) of CMOS/CsI imaging detectors following the novel IEC 62220-1-1:2015 International Standard. Radiat. Meas. 2016, 94, 8–17. [Google Scholar] [CrossRef]
- International Electrotechnical Commission IEC 62220-1:2003 Medical Electrical Equipment-Characteristics of Digital X-ray Imaging Devices-Part 1: Determination of the Detective Quantum Efficiency; IEC: Geneva, Switzerland, 2003.
- International Electrotechnical Commission IEC 62220-1-1:2015 Medical Electrical Equipment: Characteristics of Digital X-ray Imaging Devices. Part 1-1; IEC: Geneva, Switzerland, 2015; ISBN 978-2-8322-2389-5.
- Donini, B.; Rivetti, S.; Lanconelli, N.; Bertolini, M. Free software for performing physical analysis of systems for digital radiography and mammography. Med. Phys. 2014, 41, 051903. [Google Scholar] [CrossRef]
- ImageJ, An Open Platform for Scientific Image Analysis. Available online: https://imagej.net/Welcome (accessed on 12 February 2021).
- Neitzel, U.; Günther-Kohfahl, S.; Borasi, G.; Samei, E. Determination of the detective quantum efficiency of a digital X-ray detector: Comparison of three evaluations using a common image data set. Med. Phys. 2004, 31, 2205–2211. [Google Scholar] [CrossRef]
- Samei, E.; Flynn, M.J.; Reimann, D.A. A method for measuring the presampled MTF of digital radiographic systems using an edge test device. Med. Phys. 1998, 25, 102–113. [Google Scholar] [CrossRef]
- Kandarakis, I.; Cavouras, D.; Sianoudis, I.; Nikolopoulos, D.; Episkopakis, A.; Linardatos, D.; Margetis, D.; Nirgianaki, E.; Roussou, M.; Melissaropoulos, P.; et al. On the response of Y3Al5O12:Ce (YAG:Ce) powder scintillating screens to medical imaging X-rays. Nucl. Instrum. Methods Phys. Res. A 2005, 538, 615–630. [Google Scholar] [CrossRef]
- Dobbins III, J.T. Image Quality Metrics for Digital Systems (Part I, Chapter 3). In Handbook of Medical Imaging. Volume 1: Physics and Psychophysics; Beutel, J., Kundel, H.L., Van Metter, R.L., Eds.; SPIE Press: Bellingham, WA, USA, 2000; Volume 1, pp. 161–222. ISBN 978-0-8194-7772-9. [Google Scholar]
- Michail, C.; Valais, I.; Seferis, I.; Kalyvas, N.; Fountos, G.; Kandarakis, I. Experimental measurement of a high resolution CMOS detector coupled to CsI scintillators under X-ray radiation. Radiat. Meas. 2015, 74, 39–46. [Google Scholar] [CrossRef]
- Fountos, G.P.; Michail, C.M.; Zanglis, A.; Samartzis, A.; Martini, N.; Koukou, V.; Kalatzis, I.; Kandarakis, I.S. A novel easy-to-use phantom for the determination of MTF in SPECT scanners. Med. Phys. 2012, 39, 1561. [Google Scholar] [CrossRef] [PubMed]
- Michail, C. Image Quality Assessment of a CMOS/Gd2O2S:Pr,Ce,F X-ray Sensor. J. Sens. 2015, 2015, 1–6. [Google Scholar] [CrossRef]
- Seferis, I.; Michail, C.; Valais, I.; Fountos, G.; Kalyvas, N.; Stromatia, F.; Oikonomou, G.; Kandarakis, I.; Panayiotakis, G. On the response of a europium doped phosphor-coated CMOS digital imaging detector. Nucl. Instrum. Methods Phys. Res. A 2013, 729, 307–315. [Google Scholar] [CrossRef]
- Chan, H.-P.; Doi, K. Radiation dose in diagnostic radiology: Monte Carlo simulation studies. Med Phys. 1984, 11, 480–490. [Google Scholar] [CrossRef]
- Marshall, N.W. Detective quantum efficiency measured as a function of energy for two full-field digital mammography systems. Phys. Med. Biol. 2009, 54, 2845–2861. [Google Scholar] [CrossRef]
- Howansky, A.; Mishchenko, A.; Lubinsky, A.R.; Zhao, W. Comparison of CsI:Tl and Gd2O2S:Tb indirect flat panel detector X-ray imaging performance in front- and back-irradiation geometries. Med. Phys. 2019, 46, 4857–4868. [Google Scholar] [CrossRef]
- Choi, S.; Seo, C.-W.; Cha, B.K. Effect of Filtered Back-Projection Filters to Low-Contrast Object Imaging in Ultra-High-Resolution (UHR) Cone-Beam Computed Tomography (CBCT). Sensors 2020, 20, 6416. [Google Scholar] [CrossRef]
- Schulze, R.K.W.; I Doering, C. Simple computation of the approximated modulation transfer function (MTF) using spreadsheet-software: Method and evaluation in five maxillofacial CBCT-devices. Dentomaxillofacial Radiol. 2019, 48, 20180350. [Google Scholar] [CrossRef]
- Hamamatsu Flat Panel Sensor C9732DK-11. Available online: https://www.hamamatsu.com/eu/en/product/type/C9732DK-11/index.html (accessed on 31 January 2021).
- Bohndiek, S.E.; Blue, A.; Cabello, J.; Clark, A.T.; Guerrini, N.; Evans, P.M.; Harris, E.J.; Konstantinidis, A.; Maneuski, D.; Osmond, J.; et al. Characterization and Testing of LAS: A Prototype ‘Large Area Sensor’ With Performance Characteristics Suitable for Medical Imaging Applications. IEEE Trans. Nucl. Sci. 2009, 56, 2938–2946. [Google Scholar] [CrossRef]
- Monnin, P.; Gutierrez, D.; Bulling, S.; Guntern, D.; Verdun, F.R. A comparison of the performance of digital mammography systems. Med. Phys. 2007, 34, 906–914. [Google Scholar] [CrossRef]
- Litichevskyi, V.; Galkin, S.; Lalayants, O.; Voronkin, E.; Breslavskiy, I.; Tretiak, S.; Kosinov, N. Scintillation Panels Based on Zinc Selenide and Oxide Scintillators. Funct. Mater. 2011, 18, 391–397. [Google Scholar]
- Koukou, V.; Martini, N.; Fountos, G.; Michail, C.; Sotiropoulou, P.; Bakas, A.; Kalyvas, N.; Kandarakis, I.; Speller, R.; Nikiforidis, G. Dual energy subtraction method for breast calcification imaging. Nucl. Instrum. Methods Phys. Res. A 2017, 848, 31–38. [Google Scholar] [CrossRef]
- Martini, N.; Koukou, V.; Michail, C.; Fountos, G. Dual Energy X-ray Methods for the Characterization, Quantification and Imaging of Calcification Minerals and Masses in Breast. Cryst. 2020, 10, 198. [Google Scholar] [CrossRef]
- Lecoq, P.; Gektin, A.; Korzhik, M. Inorganic Scintillators for Detector Systems: Physical Principles and Crystal Engineering; Springer: Berlin, Germany, 2006; ISBN 978-3-540-27766-8. [Google Scholar]
- Linardatos, D.; Konstantinidis, A.; Valais, I.; Ninos, K.; Kalyvas, N.; Bakas, A.; Kandarakis, I.; Fountos, G.; Michail, C. On the Optical Response of Tellurium Activated Zinc Selenide ZnSe:Te Single Crystal. Crystals 2020, 10, 961. [Google Scholar] [CrossRef]
- Seferis, I.E.; Michail, C.; Zeler, J.; Kalyvas, N.; Valais, I.; Fountos, G.; Bakas, A.; Kandarakis, I.; Zych, E.; Panayiotakis, G.S. Detective quantum efficiency (DQE) of high X-ray absorption Lu2O3:Eu thin screens: The role of shape and size of nano- and micro-grains. Appl. Phys. A 2018, 124, 604. [Google Scholar] [CrossRef]
- Altman, A.; Shapiro, O.; Levene, S.; Wainer, N. Double Decker Detector for Spectral CT. US Patent US7968853B2, 28 June 2011. [Google Scholar]
- Bick, U.; Diekmann, F. Digital Mammography; Springer: Heidelberg, Germany, 2010; ISBN 978-3-540-78449-4. [Google Scholar]
- Maier, D.S.; Schock, J.; Pfeiffer, F. Dual-energy micro-CT with a dual-layer, dual-color, single-crystal scintillator. Opt. Express 2017, 25, 6924–6935. [Google Scholar] [CrossRef] [PubMed]
- Han, J.C.; Kim, H.K.; Kim, N.W.; Yun, S.; Youn, H.; Kam, S.; Tanguay, J.; Cunningham, I.A. Single-shot dual-energy X-ray imaging with a flat-panel sandwich detector for preclinical imaging. Curr. Appl. Phys. 2014, 14, 1734–1742. [Google Scholar] [CrossRef]

| Detector | RadEye HR 20.0 μm Pitch | RadEye HR 22.5 μm Pitch | Dexela 150 μm CsI | Dexela 600 μm CsI | Hamamatsu C9732DK | LAS |
|---|---|---|---|---|---|---|
| Pixel Pitch (μm) | 20.0 | 22.5 | 74.8 | 74.8 | 50 | 40 |
| Dimensions (pixels) | 1650 × 1246 | 1200 × 1600 | 1944 × 1536 | 1944 × 1536 | 2400 × 2400 | 1350 × 1350 |
| Dimensions (cm2) | 2.49 × 3.3 | 2.7 × 3.6 | 14.5 × 11.5 | 14.5 × 11.5 | 12 × 12 | 5.4 × 5.4 |
| MTF@10% (cycles/mm) | 10.4 | 10.9 | 7.7 | 4.4 | 9 | 4.1 |
| MTF@50% (cycles/mm) | 3.6 | 3.8 | 2.7 | 1.3 | 2.7 | 1.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Linardatos, D.; Koukou, V.; Martini, N.; Konstantinidis, A.; Bakas, A.; Fountos, G.; Valais, I.; Michail, C. On the Response of a Micro Non-Destructive Testing X-ray Detector. Materials 2021, 14, 888. https://doi.org/10.3390/ma14040888
Linardatos D, Koukou V, Martini N, Konstantinidis A, Bakas A, Fountos G, Valais I, Michail C. On the Response of a Micro Non-Destructive Testing X-ray Detector. Materials. 2021; 14(4):888. https://doi.org/10.3390/ma14040888
Chicago/Turabian StyleLinardatos, Dionysios, Vaia Koukou, Niki Martini, Anastasios Konstantinidis, Athanasios Bakas, George Fountos, Ioannis Valais, and Christos Michail. 2021. "On the Response of a Micro Non-Destructive Testing X-ray Detector" Materials 14, no. 4: 888. https://doi.org/10.3390/ma14040888
APA StyleLinardatos, D., Koukou, V., Martini, N., Konstantinidis, A., Bakas, A., Fountos, G., Valais, I., & Michail, C. (2021). On the Response of a Micro Non-Destructive Testing X-ray Detector. Materials, 14(4), 888. https://doi.org/10.3390/ma14040888