Effect of Aluminium Powder on Kaolin-Based Geopolymer Characteristic and Removal of Cu2+
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Material
2.2. Synthesis of Geopolymer
2.3. Testing
3. Results and Discussion
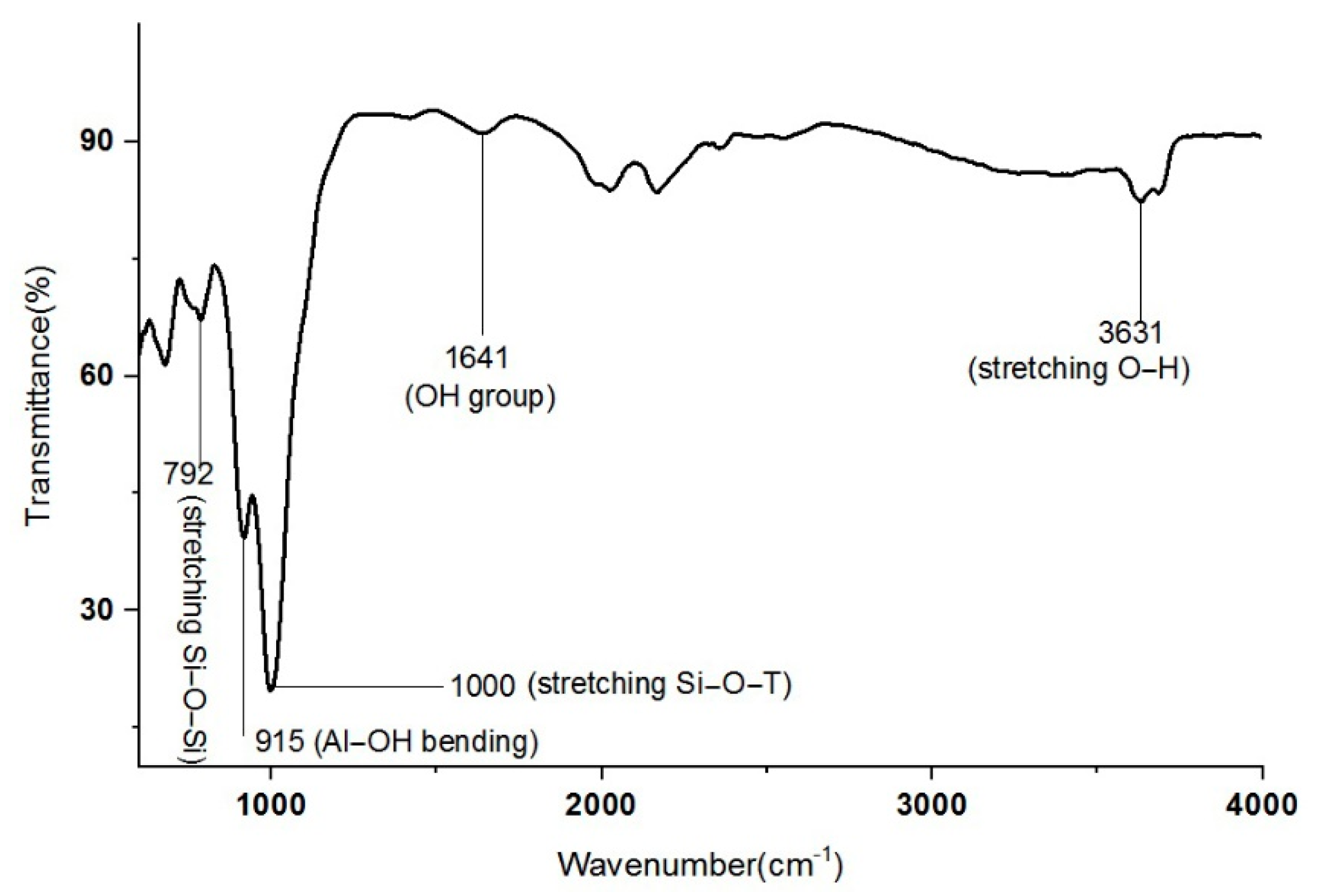
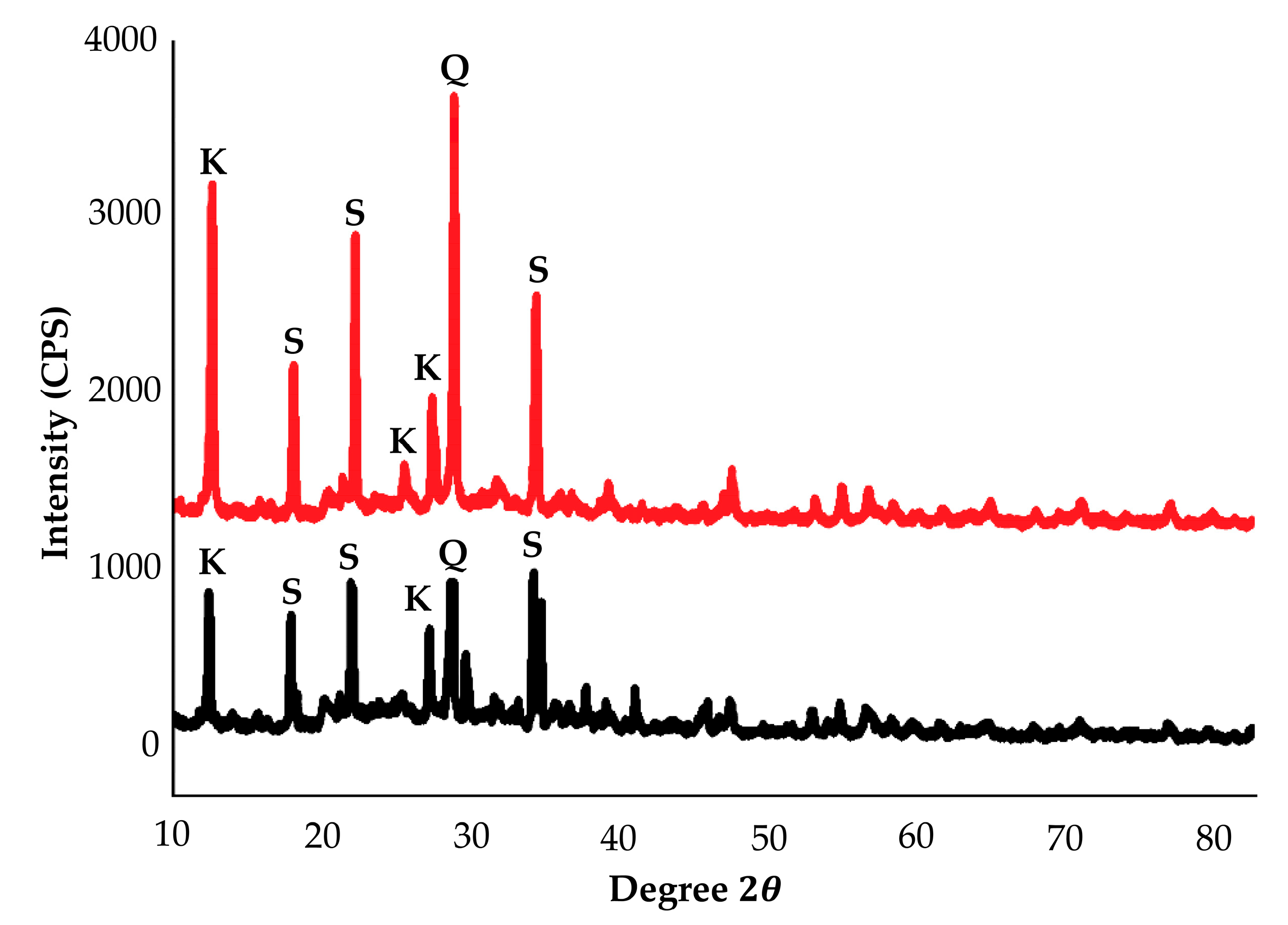
3.1. Characterization of Raw Material
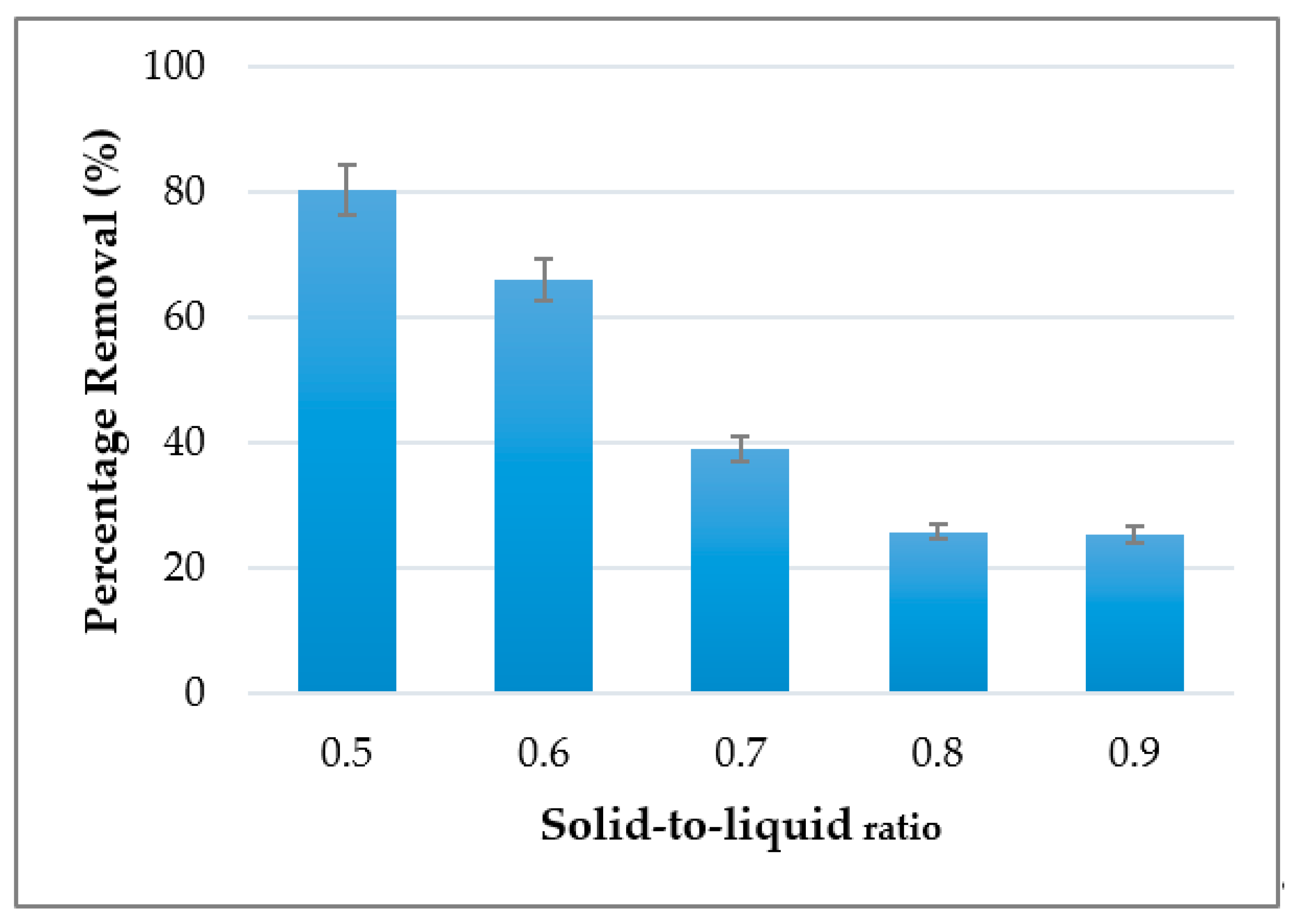
3.2. Effect of Solid-to-Liquid on Adsorption of Cu2+
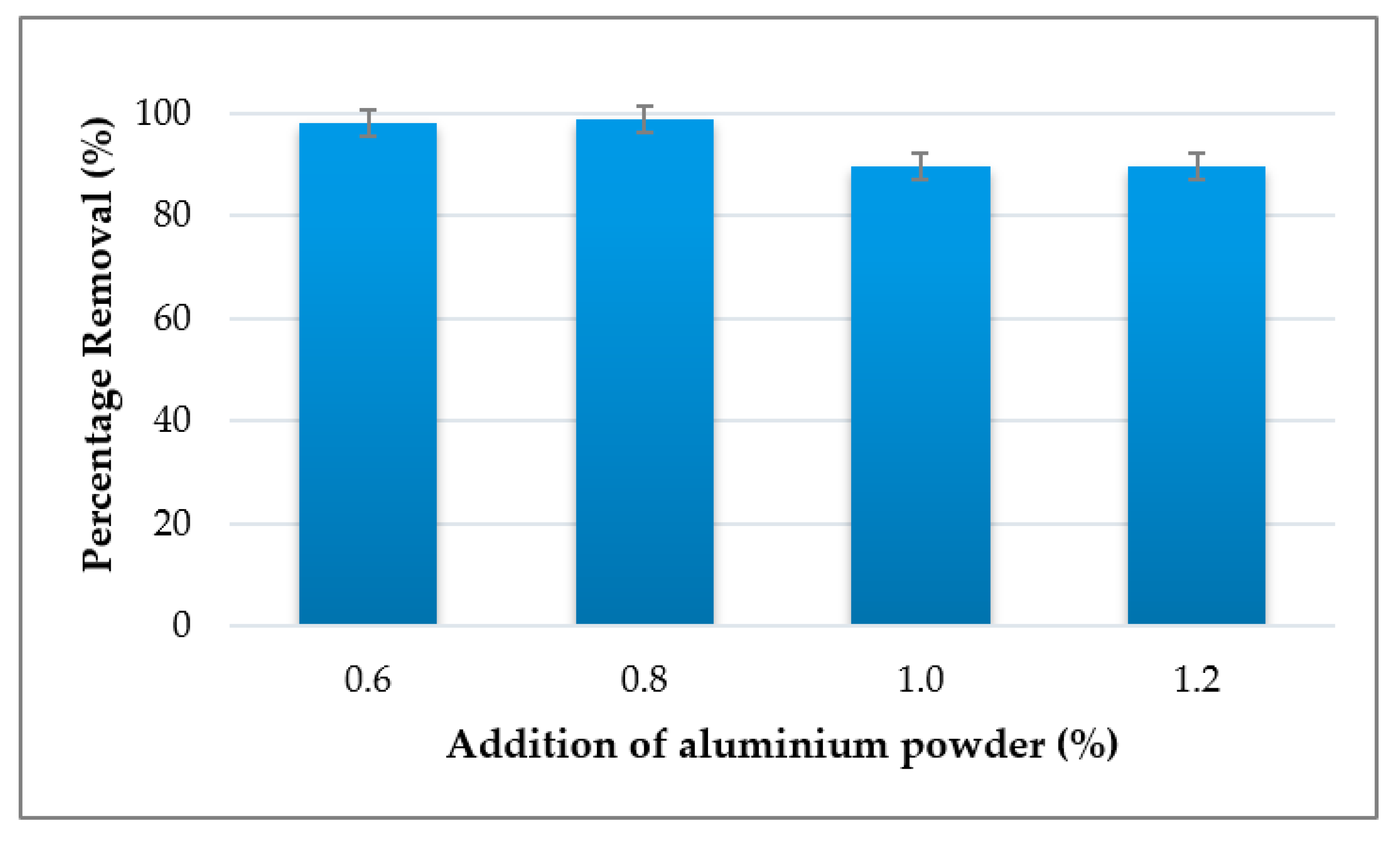
3.3. Effect of Foaming Agent on Adsorption of Cu2+
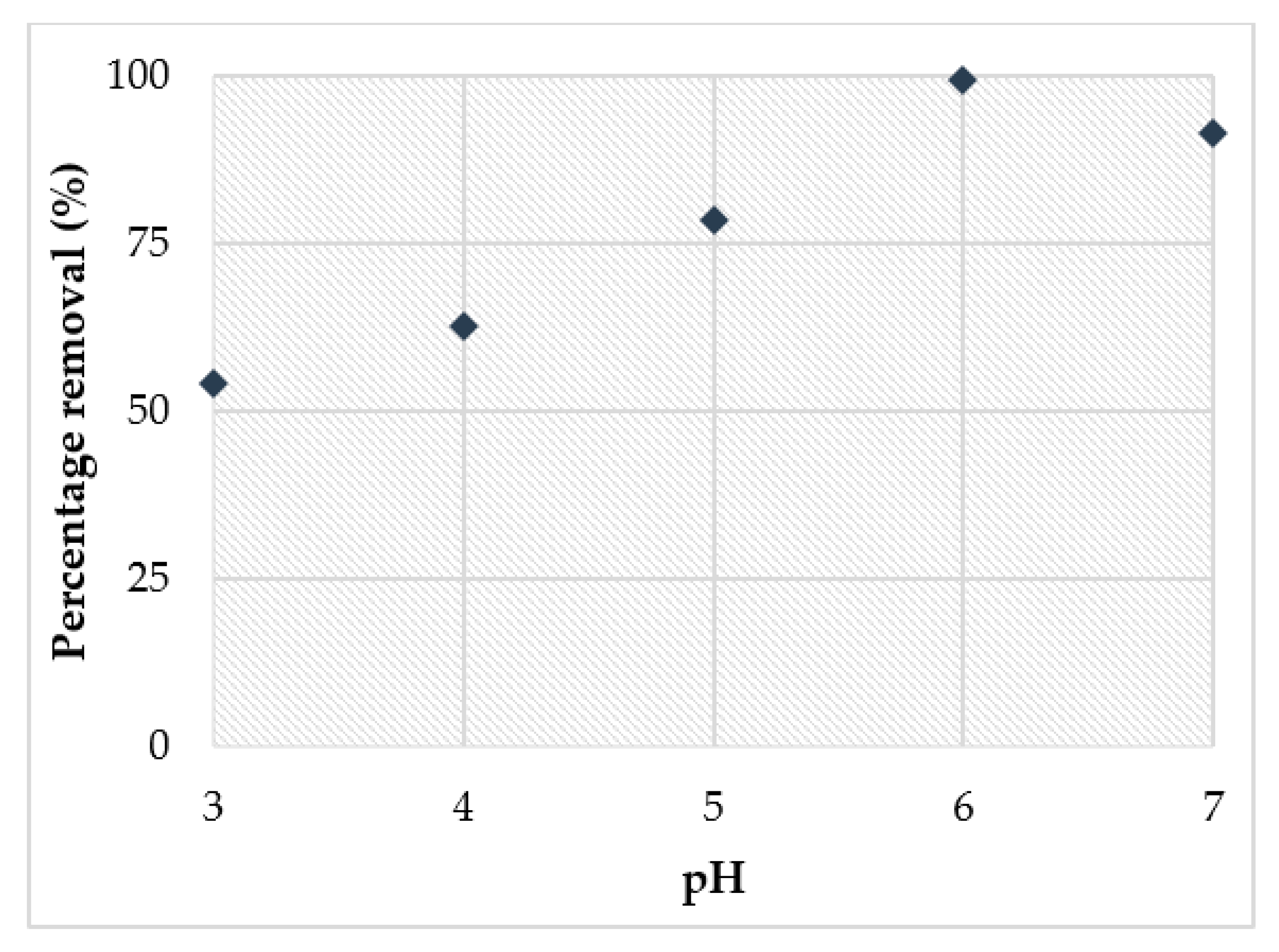
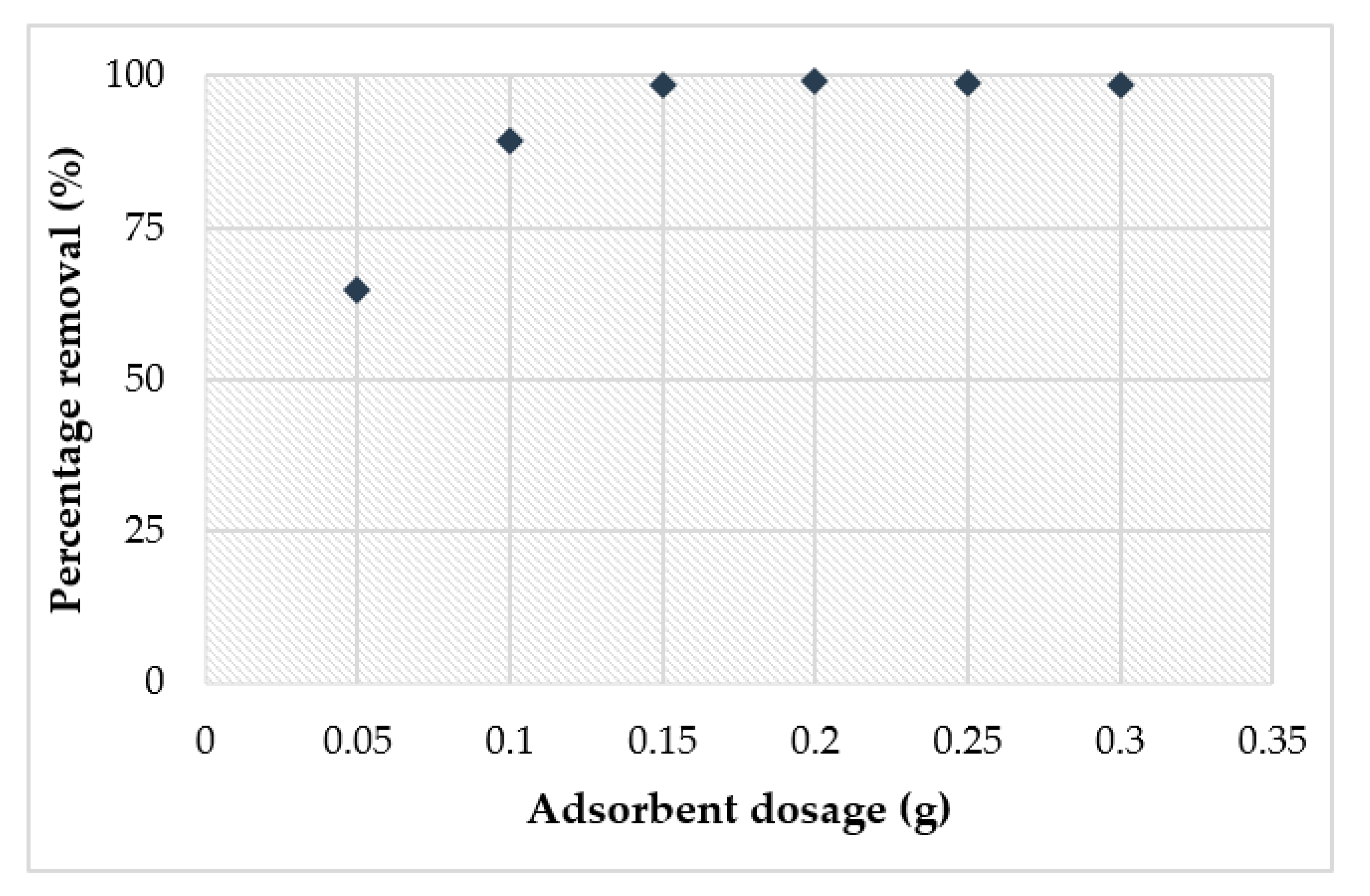
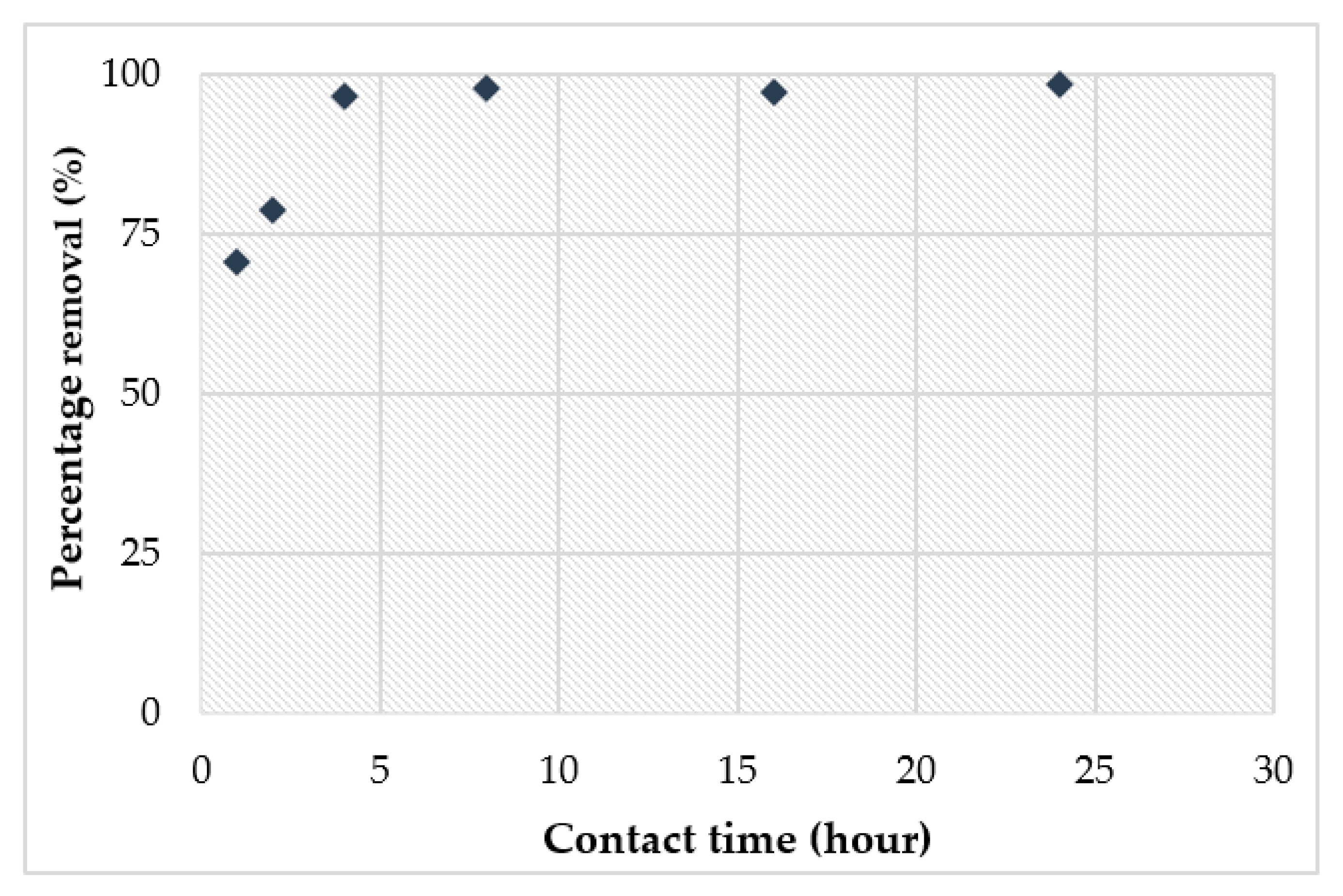
3.4. Adsorption Study of Cu2+
4. Conclusions
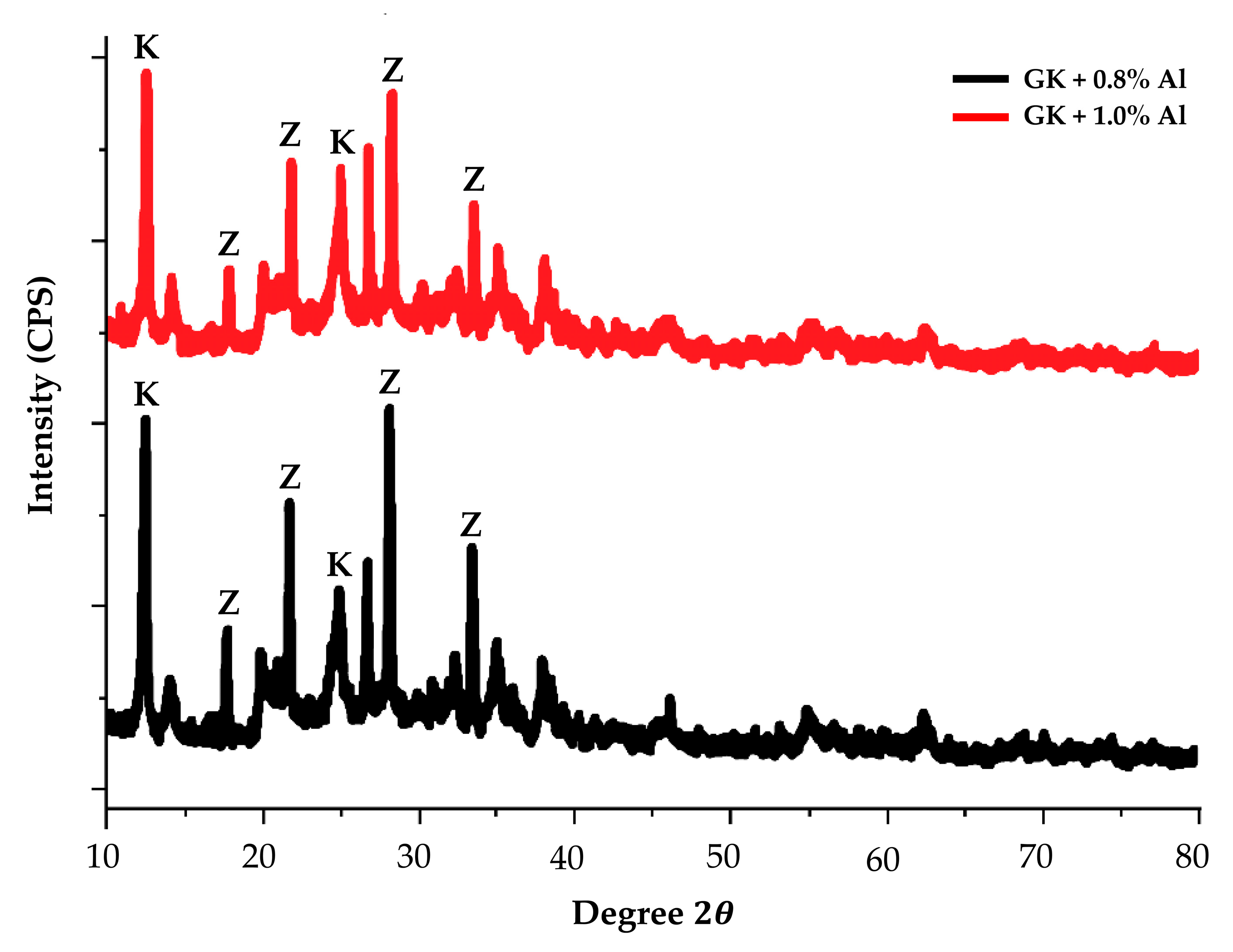
- The XRD diffractogram indicates the presence of zeolite peaks of kaolin-based geopolymer which were obtained at 80 °C, which is lower than the sintering temperature for conventional zeolite.
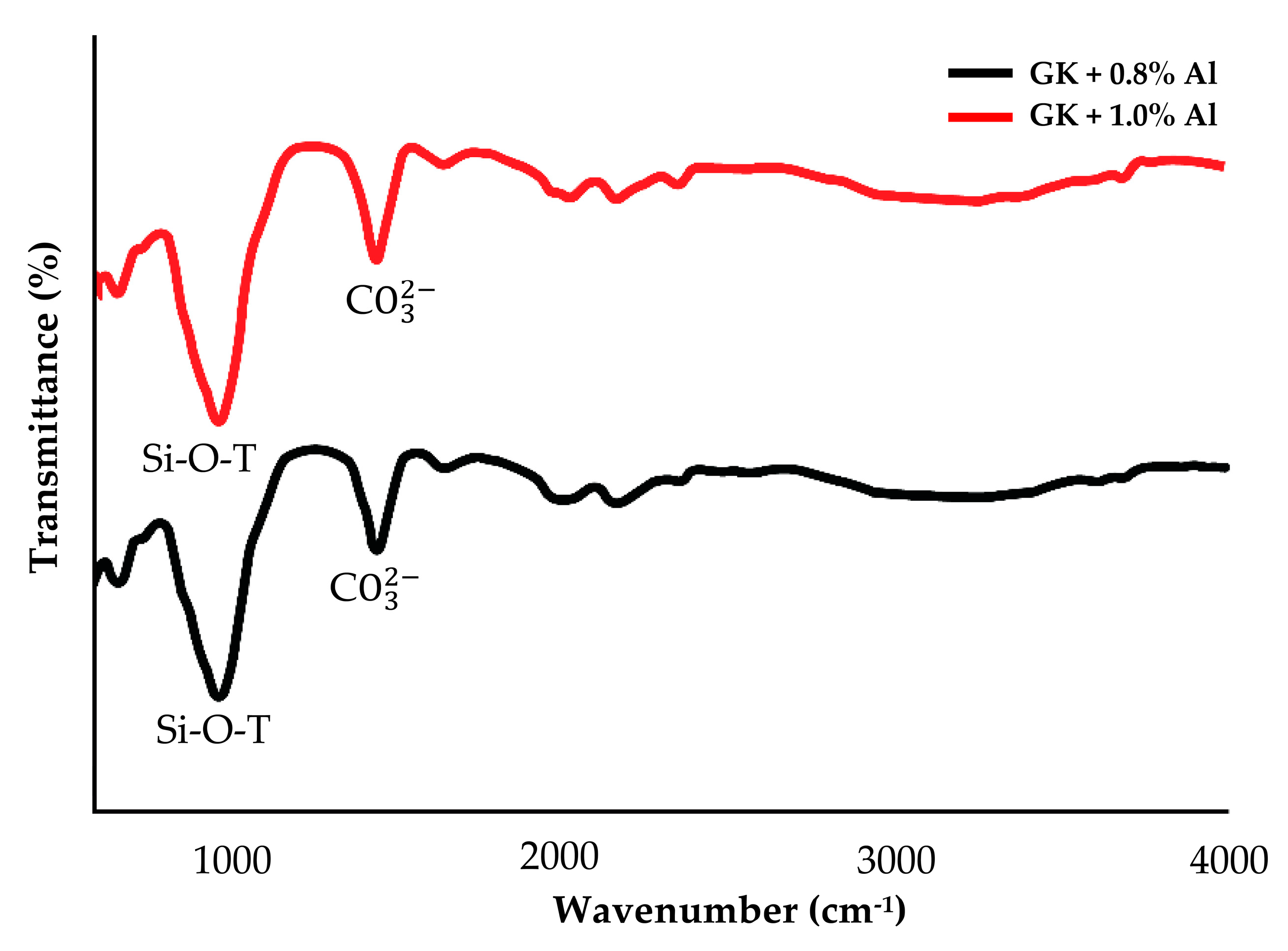
- IR spectra indicate that kaolin-based geopolymer dehydroxyled the OH group completely, consequently increasing the active surface area to adsorb Cu2+.
- The porous structure in the geopolymer adsorbent is attributed by increase in surface area from 23.58 m2/g to 54.81 m2/g.
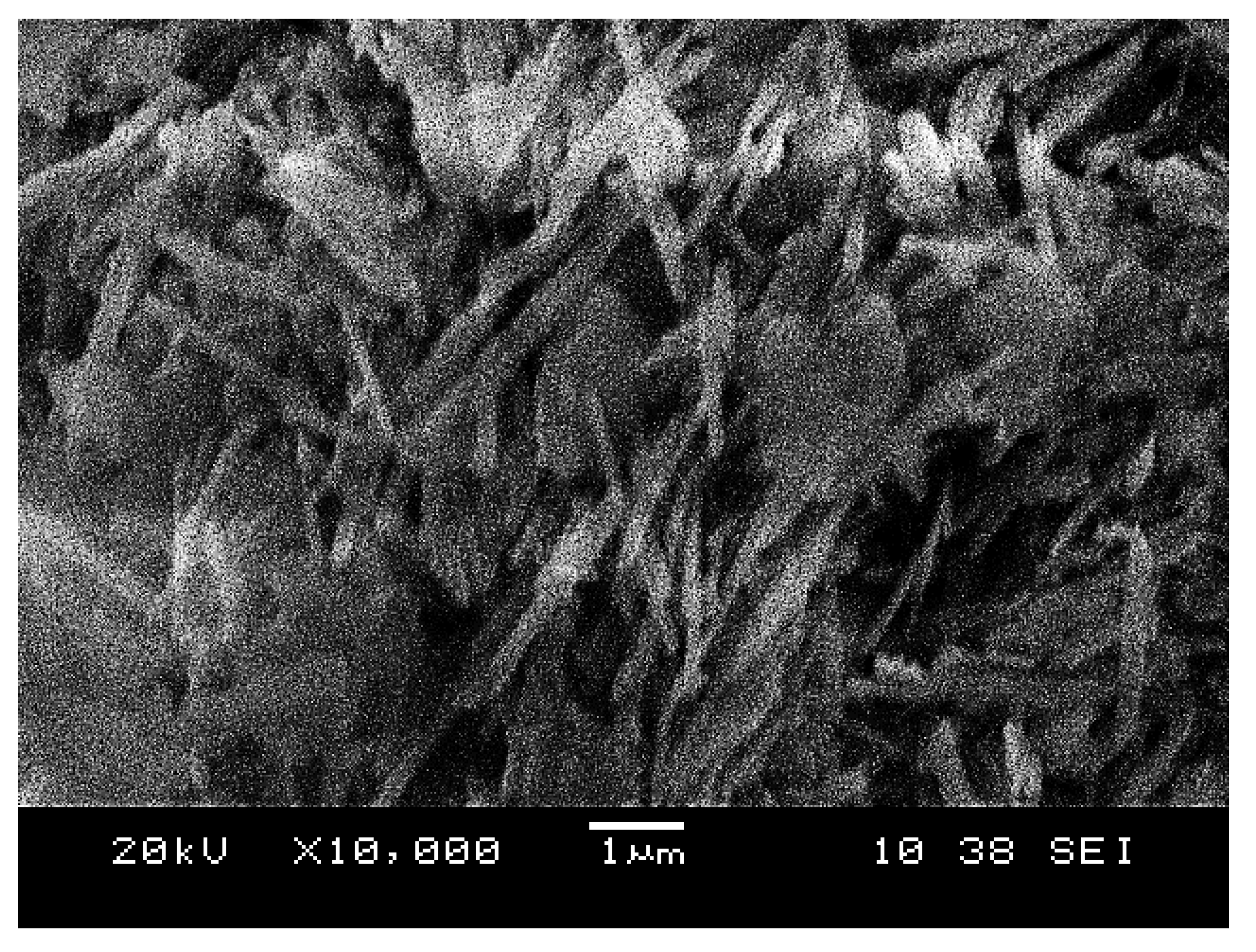
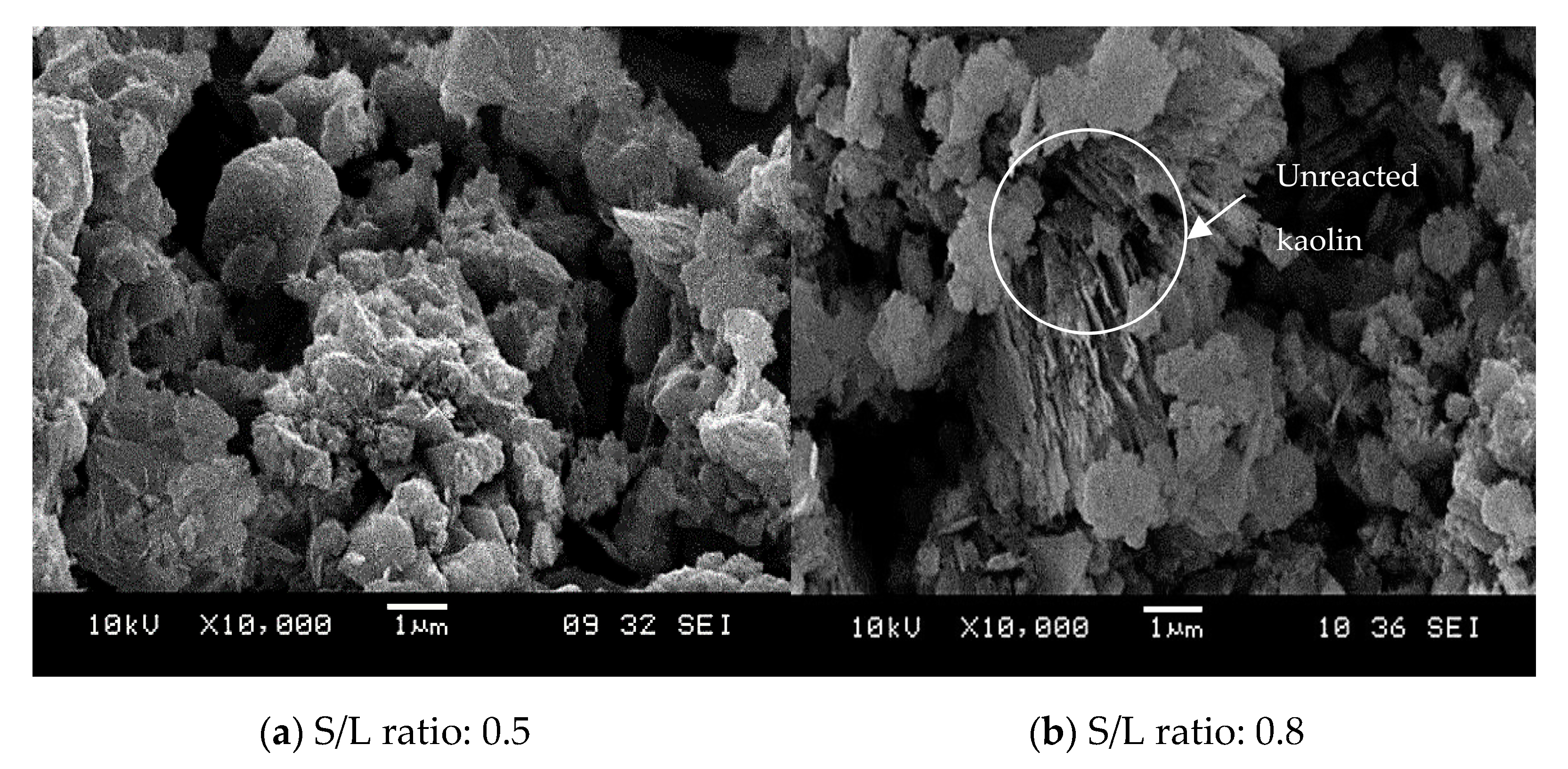
- The morphology showed that the geopolymer adsorbent contains a well-developed porous surface area. Metallic reaction from aluminium powder contributes to better interaction of unreacted particles and increased microstructural growth. Thus, increased geopolymerization reaction will homogeneously produce geopolymer paste, consequently increasing the rate of copper adsorption.
- The adsorption study showed that the highest removal of Cu2+ (98%) obtained at pH 6 achieved the optimum adsorbent dosage at 0.15 g within 4 h.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tchounwou, P.B.; Yedjou, C.G.; Patlolla, A.K.; Sutton, D.J. Heavy metal toxicity and the environment. Molec. Clinical. Environ. Toxic. 2012, 101. [Google Scholar] [CrossRef]
- Al-Saydeh, S.A.; El-Naas, M.H.; Zaidi, S.J. Copper removal from industrial wastewater: A comprehensive review. J. Ind. Eng. Chem. 2017, 56, 35–44. [Google Scholar] [CrossRef]
- Bashir, A.; Ahmad, L.; Sozia, M.; Taniya, A.; Mudasir, M.; Bhat, A. Removal of heavy metal ions from aqueous system by ion-exchange and biosorption methods. Environ. Chem. Lett. 2019, 17, 729–754. [Google Scholar] [CrossRef]
- Gautam, R.K.; Sharma, S.K.; Mahiya, S.; Chattopadhyaya, M.C. Contamination of heavy metals in aquatic media: Transport, toxicity and technologies for remediation. In Heavy Metals in Water: Presence, Removal and Safety; Royal Society of Chemistry: Dublin, Ireland, 2014. [Google Scholar] [CrossRef]
- Rajeswari, T.R. Impact of heavy metals on environmental pollution. J. Chem. Pharm. Sci. 2014, 3, 175–181. [Google Scholar]
- Yu, B.; Wang, X.; Fei, K.; Xiao, G.; Ma, D. Heavy metal concentrations in aquatic organisms (fishes, shrimp and crabs) and health risk assessment in China. Mar. Pollut. Bull. 2020, 159, 111505. [Google Scholar] [CrossRef]
- Singh, J.; Kalamdhad, A.S. Effects of heavy metals on soil, plants, human health and aquatic life. Int. J. Res. Chem. Environ. 2011, 1, 15–21. [Google Scholar]
- Masindi, V.; Muedi, L. Environmental Contamination By Heavy Metals. Heavy Met. 2018, 10, 115–132. [Google Scholar] [CrossRef]
- Liu, H.; Wang, C. Chitosan scaffolds for recyclable adsorption of Cu(II) ions. RSC Adv. 2014, 3864–3872. [Google Scholar] [CrossRef]
- Raouf, M.S.; Raheim, A.R.M. Removal of heavy metals from industrial waste water by biomass-based materials: A review. J. Pollut. Eff. Control. 2016, 5, 1–13. [Google Scholar] [CrossRef]
- Ge, Y.; Cui, X.; Kong, Y.; Li, Z.; He, Y.; Zhou, Q. Porous geopolymeric spheres for removal of Cu(II) from aqueous solution: Synthesis and evaluation. J. Hazard. Mater. 2015, 283, 244–251. [Google Scholar] [CrossRef]
- Aziz, H.A.; Adlan, M.N.; Ariffin, K.S. Heavy metals (Cd, Pb, Zn, Ni, Cu and Cr (III)) removal from water in Malaysia: Post treatment by high quality limestone. Bioresour. Technol. 2008, 99, 1578–1583. [Google Scholar] [CrossRef] [PubMed]
- Goswami, A.K.; Kulkarni, S.J.; Dharmadhikari, S.K.; Phutke, M. Adsorption of Copper (II) ions from synthetic waste water by fly ash. Int. J. Sci. Eng. Technol. Res. 2013, 2, 1353–1355. [Google Scholar]
- Yan, Y.; Liang, X.; Ma, J.; Shen, J. Rapid removal of copper from wastewater by Fe-based amorphous alloy. Intermetallics 2020, 124, 106849. [Google Scholar] [CrossRef]
- Yuna, Z. Review of the natural, modified, and synthetic zeolites for heavy metals removal from wastewater. Environ. Eng. Sci. 2015, 33, 443–454. [Google Scholar] [CrossRef]
- Hong, M.; Yu, L.; Wang, Y.; Zhang, J.; Chen, Z.; Dong, L.; Zan, Q.; Li, R. Heavy metal adsorption with zeolites: The role of hierarchical pore architecture. Chem. Eng. J. 2019, 359, 363–372. [Google Scholar] [CrossRef]
- Keane, M.A. The removal of copper and nickel from aqueous solution using Y zeolite ion exchangers. Colloid Surf. A-Physicochem. Eng. Asp. 1998, 138, 11–20. [Google Scholar] [CrossRef]
- Panayotova, M.I. Kinetics and thermodynamics of copper ions removal from wastewater by use of zeolite. J. Waste Manag. 2001, 21, 671–676. [Google Scholar] [CrossRef]
- Liu, Y.; Yan, C.; Zhao, J.; Zhang, Z.; Wang, H.; Zhou, S. Synthesis of zeolite P1 from fly ash under solvent-free conditions for ammonium removal from water. J. Clean. Prod. 2018, 202, 11–22. [Google Scholar] [CrossRef]
- Cheng, T.W.; Lee, M.L.; Ko, M.S.; Ueng, T.H.; Yang, S.F. The heavy metal adsorption characteristics on metakaolin-based geopolymer. Appl. Clay Sci. 2012, 56, 90–96. [Google Scholar] [CrossRef]
- Al-Harahsheh, M.S.; Al Zboon, K.; Al-Makhadmeh, L.; Hararah, M.; Mahasneh, M. Fly ash based geopolymer for heavy metal removal: A case study on copper removal. J. Environ. Chem. Eng. 2015, 3, 1669–1677. [Google Scholar] [CrossRef]
- Kozub, B.; Bazan, P.; Mierzwinski, D.; Korniejenko, K. Fly ash-based geopolymers reinforced by melamine fibers. Materials 2020, 14, 400. [Google Scholar] [CrossRef]
- Wang, S.; Li, L.; Zhu, Z.H. Solid-state conversion of fly ash to effective adsorbents for Cu removal from wastewater. J. Hazard. Mater. 2007, 139, 254–259. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, M.; Singh, K. Heavy metal removal from wastewater using various adsorbent: A review. J. Water Reuse Des. 2017, 387–419. [Google Scholar] [CrossRef]
- López Guzmán, F.J. Study of Geopolymer Adsorbents Prepared from Metakaolin and Rice Husk Silica for Targeting to Heavy Metal Capture. Ph.D Thesis, Nagaoka University of Technology, Nagaoka, Japan, 2014. [Google Scholar]
- Petrillo, A.; Cioffi, R.; Ferone, C.; Colangelo, F. Eco-sustainable geopolymer concrete blocks production process. Agric. Agric. Sci. Procedia. 2016, 8, 408–418. [Google Scholar] [CrossRef]
- Komnitsas, K.; Zaharaki, D. Geopolymerisation: A review and prospects for the minerals industry. Miner. Eng. 2007, 20, 1261–1277. [Google Scholar] [CrossRef]
- Youssef, N.; Lafhaj, Z.; Chapiseau, C. Economic analysis of geopolymer brick manufacturing: A french case study. Sustainability 2020, 12, 7403. [Google Scholar] [CrossRef]
- Taye, E.A.; Roether, J.A.; Schubert, D.W.; Redda, D.T.; Boccaccini, A.R. Hemp fiber reinforced red mud/fly ash fire-resistant geopolymer composite materials: Effect of fiber content on mechanical strength. Materials 2021, 14, 511. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.H.; Zhu, H.J.; Zhou, C.H.; Wang, H. Geopolymer from kaolin in China: An overview. Appl. Clay Sci. 2016, 119, 31–41. [Google Scholar] [CrossRef]
- Heah, C.H.; Kamarudin, H.; Al Bakri, A.M.M.; Luqman, M.; Nizar, I.K. Potential application of kaolin without calcine as greener concrete: A review. Aust. J. Basic Appl. Sci. 2011, 5, 1026–1035. [Google Scholar]
- Ahmaruzzaman, M. A review on the utilization of fly ash. Prog. Energy Combust. Sci. 2010, 36, 327–363. [Google Scholar] [CrossRef]
- Swenson, H.; Stadie, N.P. Langmuir’s Theory of adsorption: A centennial review. Langmuir 2019, 35, 5409–5426. [Google Scholar] [CrossRef]
- Yakout, S.M.; Biochar, K.; Rice, A. Effect of porosity and surface chemistry on the adsorption- desorption of uranium (VI) from aqueous solution and groundwater. J. Radioanal. Nucl. Chem. 2016, 308, 555–565. [Google Scholar] [CrossRef]
- Feng, J.; Zhang, R.; Gong, L.; Li, Y.; Cao, W.; Cheng, X. Development of porous fly ash-based geopolymer with low thermal conductivity. Mater. Des. 2015, 65, 529–533. [Google Scholar] [CrossRef]
- Bai, C.; Colombo, P. Processing, properties and applications of highly porous geopolymers: A review. Ceram. Int. 2018, 44, 16103–16118. [Google Scholar] [CrossRef]
- Duan, P.; Yan, C.; Zhou, W.; Ren, D. Development of fly ash and iron ore tailing based porous geopolymer for removal of Cu(II) from wastewater. Ceram. Int. 2016, 42, 13507–13518. [Google Scholar] [CrossRef]
- Nyale, S.M.; Babajide, O.O.; Birch, G.D.; Böke, N.; Petrik, L.F. The influence of NaOH and NaOCl on the characteristics of fly ash-based foamed geopolymer. Proceeding of the 14th International Conference on Environmental Science and Technology, Rhodes, Greece, 3–5 September 2015. [Google Scholar]
- Beghoura, I.; Castro-gomes, J. Design of alkali-activated aluminium powder foamed materials for precursors with different particle sizes. Constr. Build. Mater. 2019, 224, 682–690. [Google Scholar] [CrossRef]
- Ibrahim, W.M.W.; Hussin, K.; Abdullah, M.M.A. Influence of foaming agent/water ratio and foam/ geopolymer paste ratio to the properties of fly ash-based lightweight geopolymer for brick application. Revistadechimie 2017, 68, 1978–1982. [Google Scholar] [CrossRef]
- Bachaga, T.; Daly, R.; Escoda, L.; Suñol, J.J.; Khitouni, M. Amorphization of Al50(Fe2B)30Nb 20 mixture by mechanical alloying. Metall. Mater. Trans. A 2013, 44, 4718–4724. [Google Scholar] [CrossRef]
- Kränzlein, E.; Pöllmann, H.; Krcmar, W. Metal powders as foaming agents in fly ash based geopolymer synthesis and their impact on the structure depending on the Na /Al ratio. Cem. Concr. Compos. 2018, 90, 161–168. [Google Scholar] [CrossRef]
- Strozi Cilla, M.; Colombo, P.; Raymundo Morelli, M. Geopolymer foams by gelcasting. Ceram. Int. 2014, 40, 5723–5730. [Google Scholar] [CrossRef]
- Olivares-Ramírez, J.M.; De Jesús, Á.M.; Jimenez-Sandoval, O.; Pless, R.C. Hydrogen Generation by Treatment of Aluminium Metal with Aqueous Solutions: Procedures and Uses; Intechopen: London, UK, 2012; pp. 55–76. [Google Scholar] [CrossRef]
- Hajimohammadi, A.; Ngo, T.; Mendis, P. How does aluminium foaming agent impact the geopolymer formation mechanism? Cem. Concr. Compos. 2017, 80, 277–286. [Google Scholar] [CrossRef]
- Masi, G.; Rickard, W.D.A.; Vickers, L.; Chiara, M.; Van Riessen, A. A comparison between different foaming methods for the synthesis of light weight geopolymers. Ceram. Int. 2014, 40, 13891–13902. [Google Scholar] [CrossRef]
- Novais, R.M.; Pullar, R.C.; Labrincha, J.A. Geopolymer foams: An overview of recent advancements. Prog. Mater. Sci. 2019, 109, 100621. [Google Scholar] [CrossRef]
- Tang, Q.; Wang, K.; Yaseen, M.; Tong, Z.; Cui, X. Synthesis of highly efficient porous inorganic polymer microspheres for the adsorptive removal of Pb(II) from wastewater. J. Clean. Prod. 2018, 193, 351–362. [Google Scholar] [CrossRef]
- Phair, J.W.; Van Deventer, J.S.J.; Smith, J.D. Effect of Al source and alkali activation on Pb and Cu immobilisation in fly-ash based geopolymers. Appl. Geochem. 2004, 19, 423–434. [Google Scholar] [CrossRef]
- He, K.; Chen, Y.; Tang, Z.; Hu, Y. Removal of heavy metal ions from aqueous solution by zeolite synthesized from fly ash. Environ. Sci. Pollut. Res. 2015, 23, 2778–2788. [Google Scholar] [CrossRef] [PubMed]
- Rasaki, Z.; Guarecuco, R.; Thomas, T.; Minghui, Y. Geopolymer for use in heavy metals adsorption, and advanced oxidative processes: A critical review. J. Clean. Prod. 2019, 213, 42–58. [Google Scholar] [CrossRef]
- Sarkar, J.; Basu, J.K.; Samanta, A.N. Removal of Ni 2 + ion from waste water by Geopolymeric Adsorbent derived from LD Slag. J. Water Process Eng. 2017, 17, 237–244. [Google Scholar] [CrossRef]
- Van Sindhunata, J.S.J.; Deventer, G.; Lukey, C.; Xu, H. Effect of curing temperature and silicate concentration on fly-ash-based geopolymerization. Ind. Eng. Chem. Res. 2006, 45, 3559–3568. [Google Scholar] [CrossRef]
- Keawpapasson, P.; Tippayasam, C.; Ruangjan, S. Metakaolin-based porous geopolymer with aluminium powder metakaolin-based porous geopolymer with aluminium powder. Key. Eng. Mater. 2014, 608, 132–138. [Google Scholar] [CrossRef]
- Wu, Y.; Lu, B.; Wang, H.; Du, F.; Zhang, Y.; Cai, L.; Jiang, C.; Wang, W. Geopolymer, green alkali activated cementitious material: Synthesis, applications and challenges. Constr. Build. Mater. 2019, 224, 930–949. [Google Scholar] [CrossRef]
- Nergis, D.D.B.; Abdullah, M.M.A.; Vizureanu, P.; Tahir, M.F.M. Geopolymers and their uses: Review. IOP Conf. Ser.: Mater. Sci. Eng. 2018, 374. [Google Scholar] [CrossRef]
- El-Eswed, B.; Alshaaer, M.; Ibrahim Yousef, R.; Hamadneh, I.; Khalili, F. Adsorption of Cu(II), Ni(II), Zn(II), Cd(II) and Pb(II) onto kaolin/zeolite based-Geopolymers. Adv. Mater. Phys. Chem. 2012, 2, 119–125. [Google Scholar] [CrossRef]
- Worasith, N.; Goodman, B.A.; Neampan, J.; Jeyachoke, N.; Thiravetyan, P. Characterization of modified kaolin from the Ranong deposit Thailand by XRD, XRF, SEM, FTIR and EPR techniques. Clay Miner. 2011, 46, 539–559. [Google Scholar] [CrossRef]
- Naghsh, M.; Shams, K. Synthesis of a kaolin-based geopolymer using a novel fusion method and its application in effective water softening. Appl. Clay Sci. 2017, 146, 238–245. [Google Scholar] [CrossRef]
- Saeed, K.A.; Kassim, K.A.; Nur, H. Physicochemical characterization of cement treated kaolin clay. Gradjevinar 2014, 66, 513–521. [Google Scholar] [CrossRef]
- Liew, Y.M.; Cheng-yong, H.; Abdullah, M.M.A.; Hussin, K. Structure and properties of clay-based geopolymer cements: A review. Prog. Mater. Sci. 2016, 83, 595–629. [Google Scholar] [CrossRef]
- Kara, I.; Yilmazer, D.; Akar, S.T. Metakaolin based geopolymer as an effective adsorbent for adsorption of zinc(II) and nickel(II) ions from aqueous solutions. Appl. Clay Sci. 2017, 139, 54–63. [Google Scholar] [CrossRef]
- El Alouani, M.; Alehyen, S.; El Achouri, M.; Taibi, M. Preparation, characterization, and application of metakaolin-based geopolymer for removal of methylene blue from aqueous solution. J. Chem. 2019, 2019. [Google Scholar] [CrossRef]
- Vickers, L.; Van Riessen, A.; Rickard, W.D. Fire-Resistant Geopolymers Role of Fibres and Fillers to Enhance Thermal Properties; SpringerBriefs in Materials: Perth, WA, Australia, 2015; ISBN 978-981-287-311-8. [Google Scholar]
- Hidayu, N.; Abdullah, M.M.A.; Pa, F.C.; Mohamad, H.; Ibrahim, W.M.A.; Chaiprapa, J. Influences of SiO2, Al2O3, CaO and MgO in phase transformation of sintered kaolin-ground granulated blast furnace slag geopolymer. J. Mater. Res. Technol. 2020, 9, 14922–14932. [Google Scholar] [CrossRef]
- Morsy, M.S.; Alsayed, S.H.; Al-Salloum, Y.; Almusallam, T. Effect of sodium silicate to sodium hydroxide ratios on strength and microstructure of fly ash geopolymer binder. Arab. J. Sci. Eng. 2014, 39, 4333–4339. [Google Scholar] [CrossRef]
- Xu, H.; Van Deventer, J.S.J. The geopolymerisation of alumino-silicate minerals. Int. J. Miner. Process. 2000, 59, 247–266. [Google Scholar] [CrossRef]
- Kenne Diffo, B.B.; Elimbi, A.; Cyr, M.; Dika Manga, J.; Tchakoute Kouamo, H. Effect of the rate of calcination of kaolin on the properties of metakaolin-based geopolymers. J. Asian Ceram. Soc. 2015, 3, 130–138. [Google Scholar] [CrossRef]
- Siyal, A.A.; Shamsuddin, M.R.; Khan, M.I.; Rabat, N.E.; Zulfiqar, M.; Man, Z.; Siame, J.; Azizli, K.A. A review on geopolymers as emerging materials for the adsorption of heavy metals and dyes. J. Environ. Manag. 2018. [Google Scholar] [CrossRef] [PubMed]
- Abbar, B.; Pantet, A.; Ahfir, N.; Bizet, L. Experimental investigation on removal of heavy metals (Cu2+, Pb2+, and Zn2+) from aqueous solution by flax fibres. Process Saf. Environ. Prot. 2017, 109, 639–647. [Google Scholar] [CrossRef]
- Kobielska, P.A.; Howarth, A.J.; Farha, O.K.; Nayak, S. Metal–organic frameworks for heavy metal removal from water. Coord. Chem. Rev. 2018, 358, 92–107. [Google Scholar] [CrossRef]



| Parameter | Kaolin |
|---|---|
| Solid-to-liquid ratio | 0.5, 0.6, 0.7, 0.8, 0.9 |
| NaOH-to-Na2SiO3 ratio | 1.0 |
| Curing Temperature | 80 |
| Molarity of NaOH | 10 |
| Sieve size | <150 µm |
| Parameter | Kaolin-Based Geopolymer |
|---|---|
| Foaming agent (wt%) | 0.6, 0.8, 1.0, 1.2 |
| Alkali activator | S/L ratio 0.5, Na2SiO3/NaOH ratio 1.5 |
| Sieve size | <150 µm |
| Element | Kaolin (%) |
|---|---|
| SiO2 | 56.4 |
| Al2O3 | 33.6 |
| Fe2O3 | 4.06 |
| K2O | 3.48 |
| TiO2 | 0.76 |
| RuO2 | 0.17 |
| ZrO2 | 0.08 |
| LOI | 1.45 |
| Properties | Kaolin Geopolymer | |
|---|---|---|
| S/L ratio | 0.5 | 1.0 |
| Surface area (m2/g) | 23.58 | 20.32 |
| Pore volume (cm3/g) | 0.05 | 0.051 |
| Properties | GK + 0.8% Al | GK + 1.0% Al |
|---|---|---|
| Surface area (m2/g) | 54.81 | 52.08 |
| Pore Volume (cm3/g) | 0.049 | 00.32 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ariffin, N.; Abdullah, M.M.A.B.; Postawa, P.; Zamree Abd Rahim, S.; Mohd Arif Zainol, M.R.R.; Putra Jaya, R.; Śliwa, A.; Omar, M.F.; Wysłocki, J.J.; Błoch, K.; et al. Effect of Aluminium Powder on Kaolin-Based Geopolymer Characteristic and Removal of Cu2+. Materials 2021, 14, 814. https://doi.org/10.3390/ma14040814
Ariffin N, Abdullah MMAB, Postawa P, Zamree Abd Rahim S, Mohd Arif Zainol MRR, Putra Jaya R, Śliwa A, Omar MF, Wysłocki JJ, Błoch K, et al. Effect of Aluminium Powder on Kaolin-Based Geopolymer Characteristic and Removal of Cu2+. Materials. 2021; 14(4):814. https://doi.org/10.3390/ma14040814
Chicago/Turabian StyleAriffin, Nurliyana, Mohd Mustafa Al Bakri Abdullah, Przemysław Postawa, Shayfull Zamree Abd Rahim, Mohd Remy Rozainy Mohd Arif Zainol, Ramadhansyah Putra Jaya, Agata Śliwa, Mohd Firdaus Omar, Jerzy J. Wysłocki, Katarzyna Błoch, and et al. 2021. "Effect of Aluminium Powder on Kaolin-Based Geopolymer Characteristic and Removal of Cu2+" Materials 14, no. 4: 814. https://doi.org/10.3390/ma14040814
APA StyleAriffin, N., Abdullah, M. M. A. B., Postawa, P., Zamree Abd Rahim, S., Mohd Arif Zainol, M. R. R., Putra Jaya, R., Śliwa, A., Omar, M. F., Wysłocki, J. J., Błoch, K., & Nabiałek, M. (2021). Effect of Aluminium Powder on Kaolin-Based Geopolymer Characteristic and Removal of Cu2+. Materials, 14(4), 814. https://doi.org/10.3390/ma14040814











