Structure and Properties of Cast Ti-Al-Si Alloys
Abstract
1. Introduction
2. Materials and Methods
3. Results
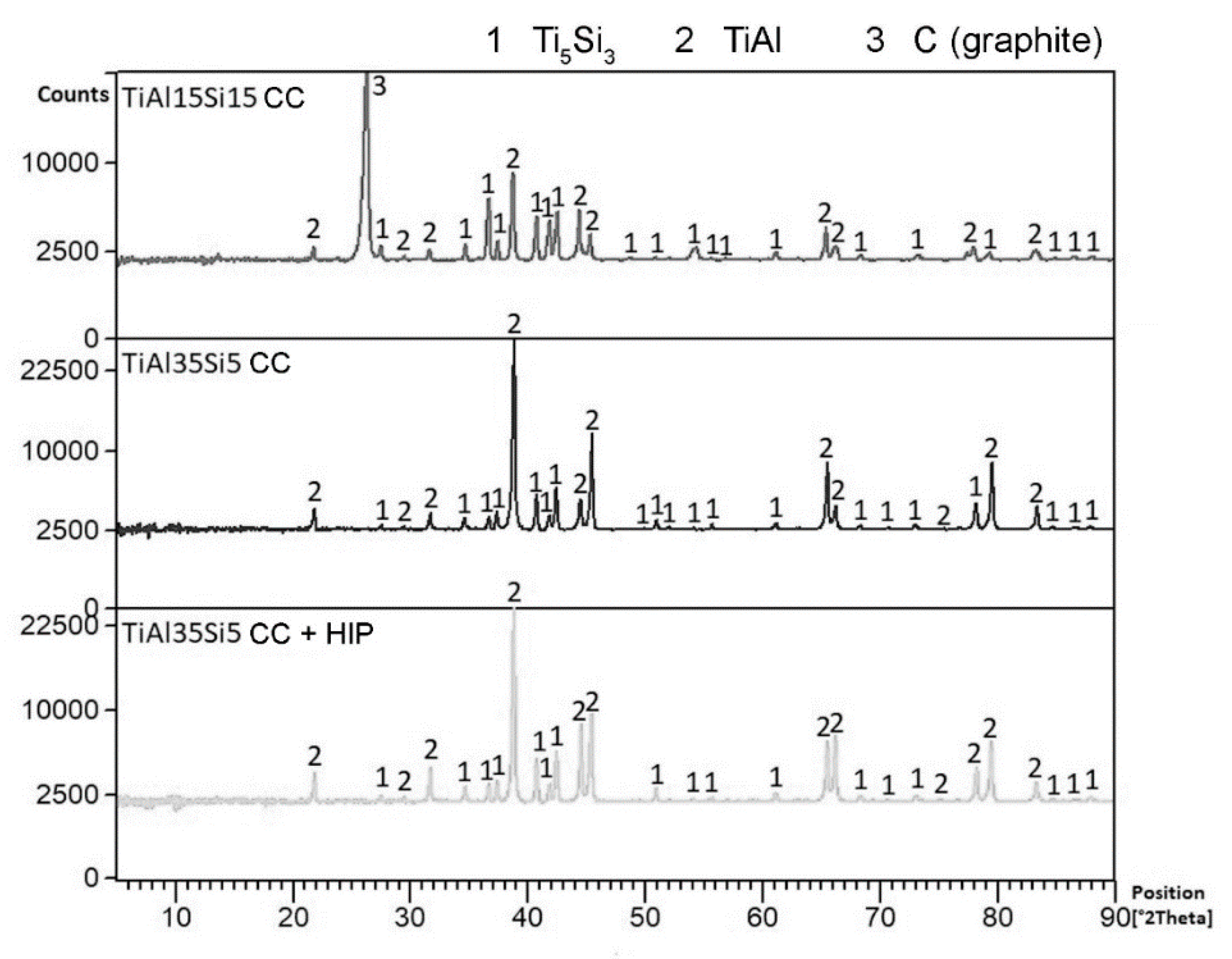
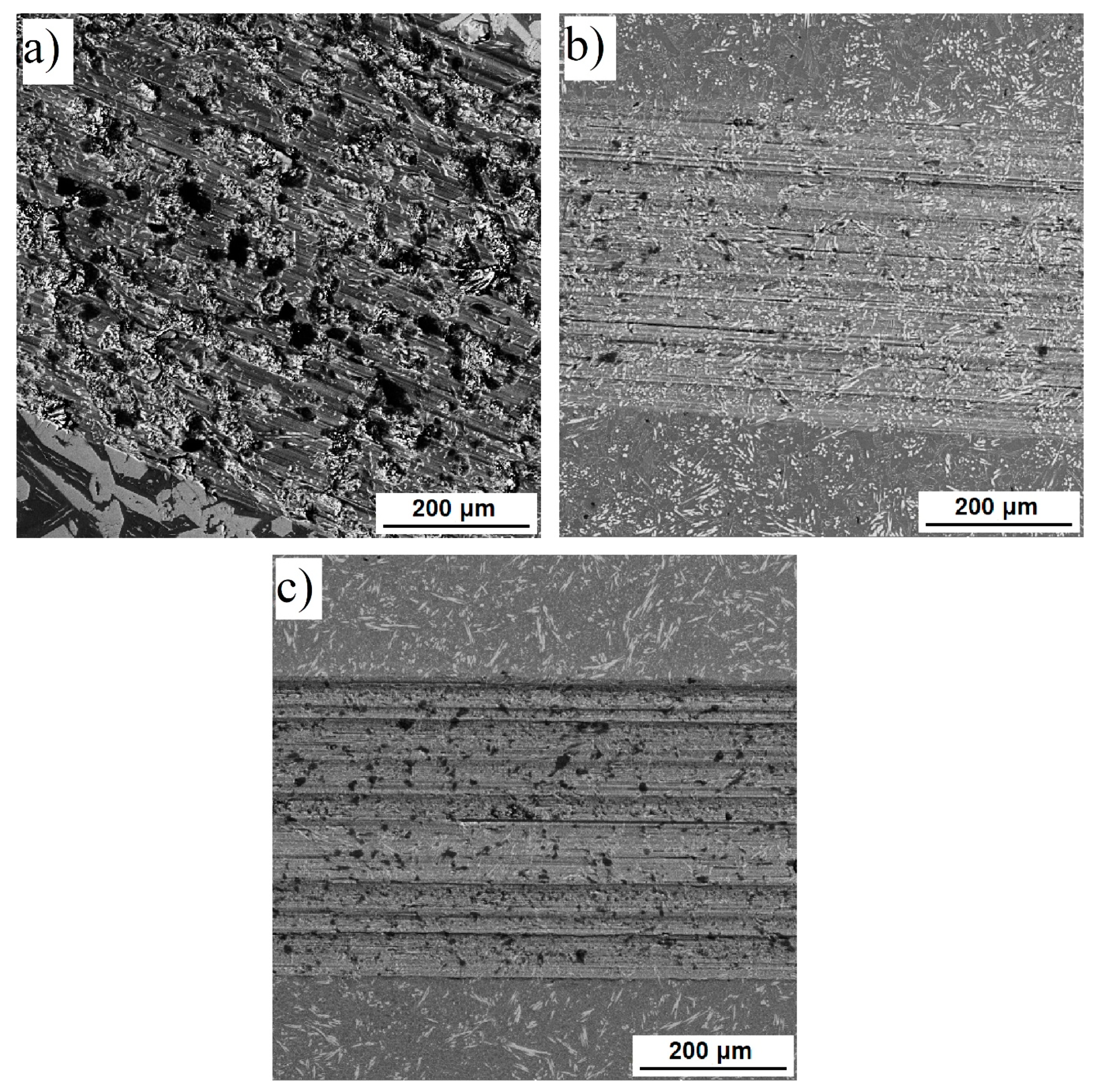
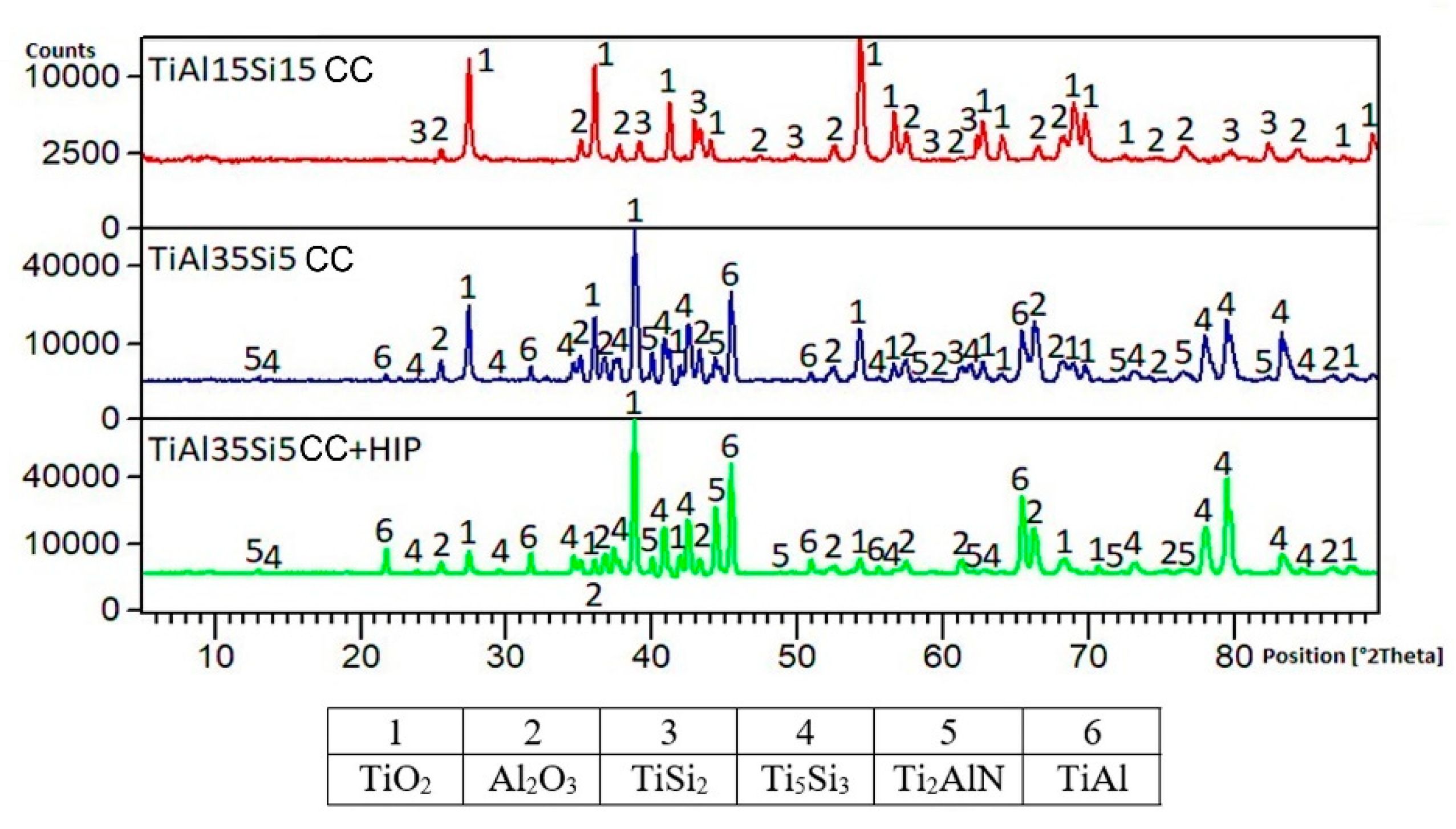
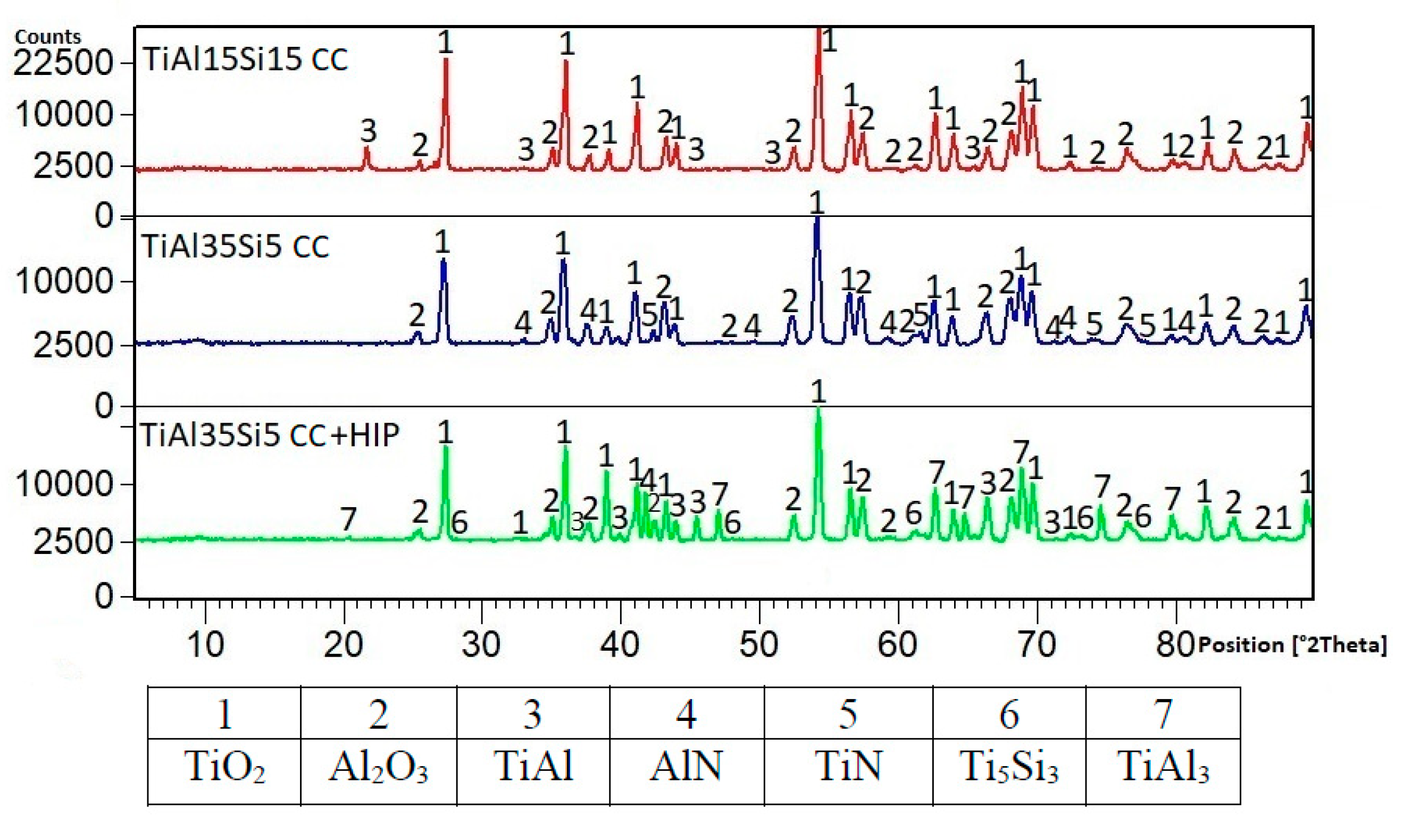
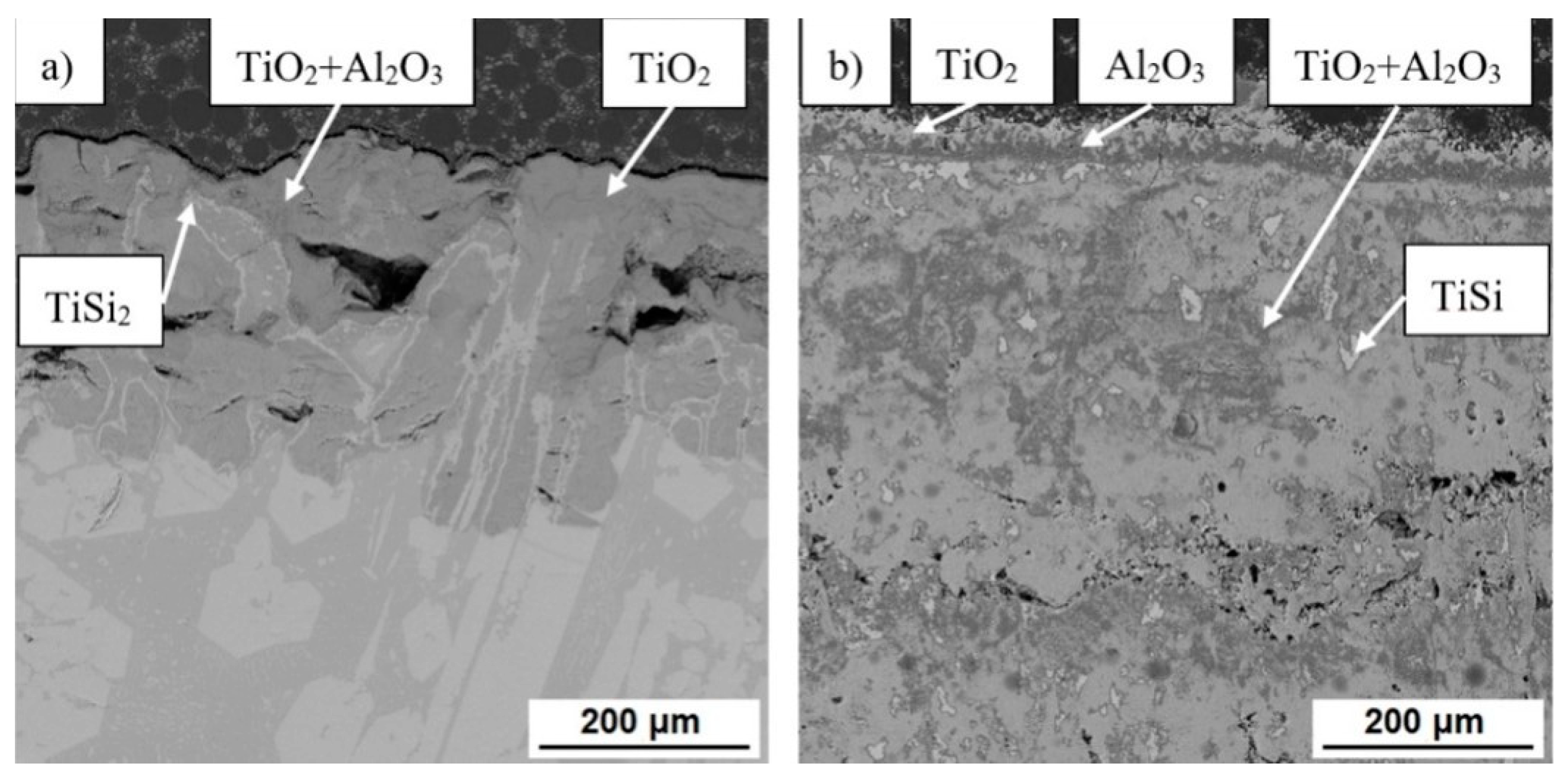
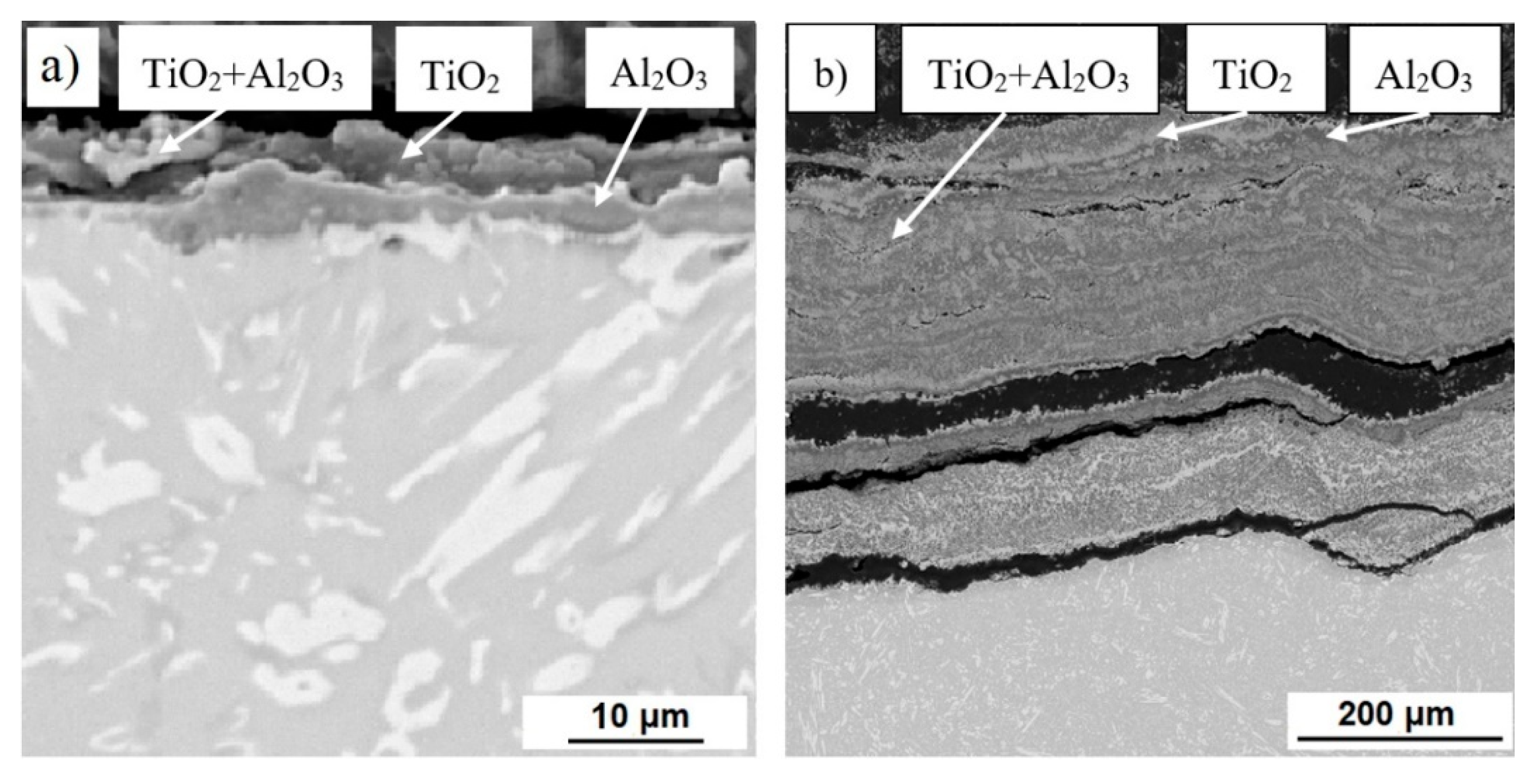
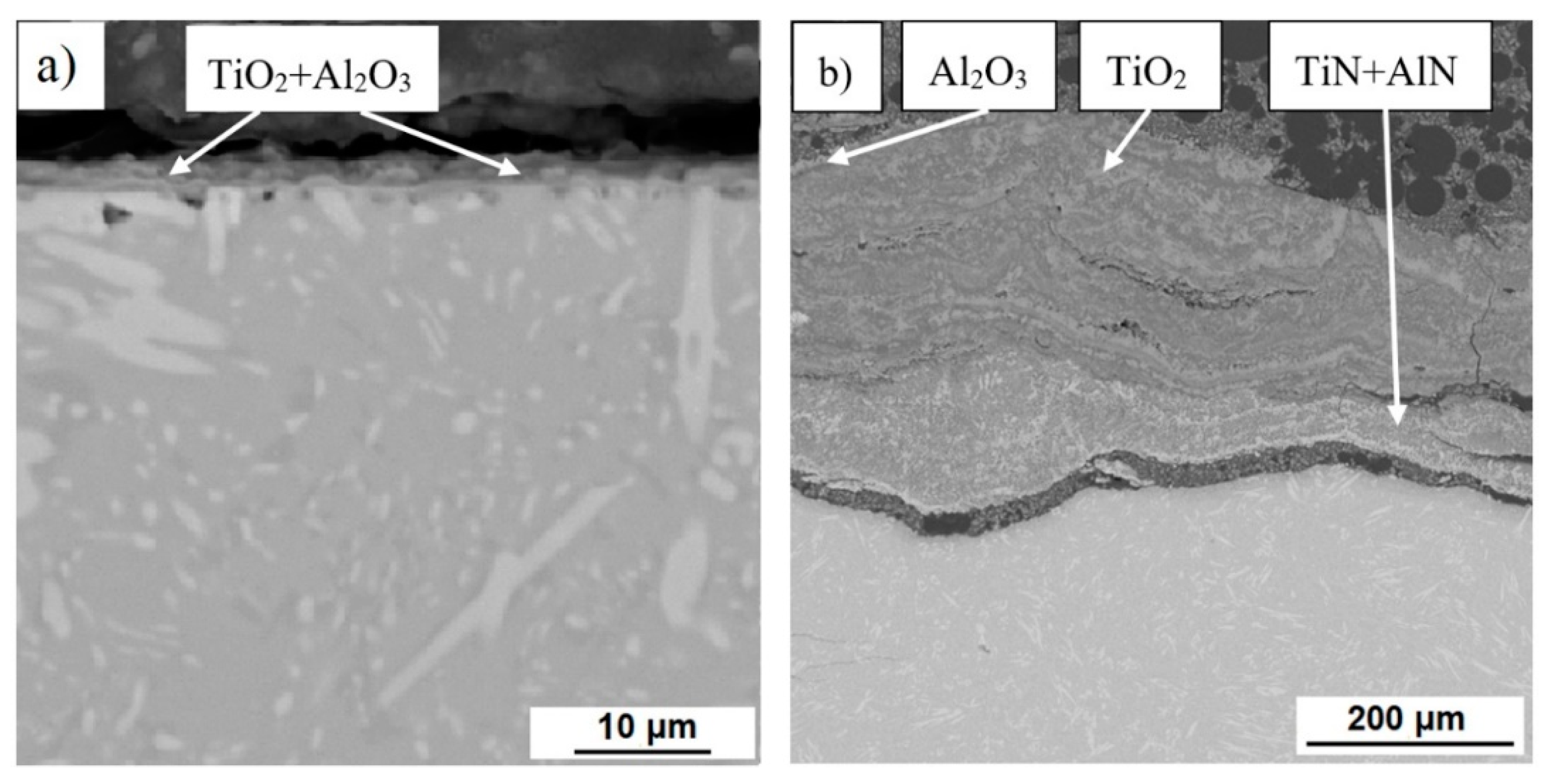
3.1. Microstructure and Phase Composition
3.2. Mechanical and Tribological Properties
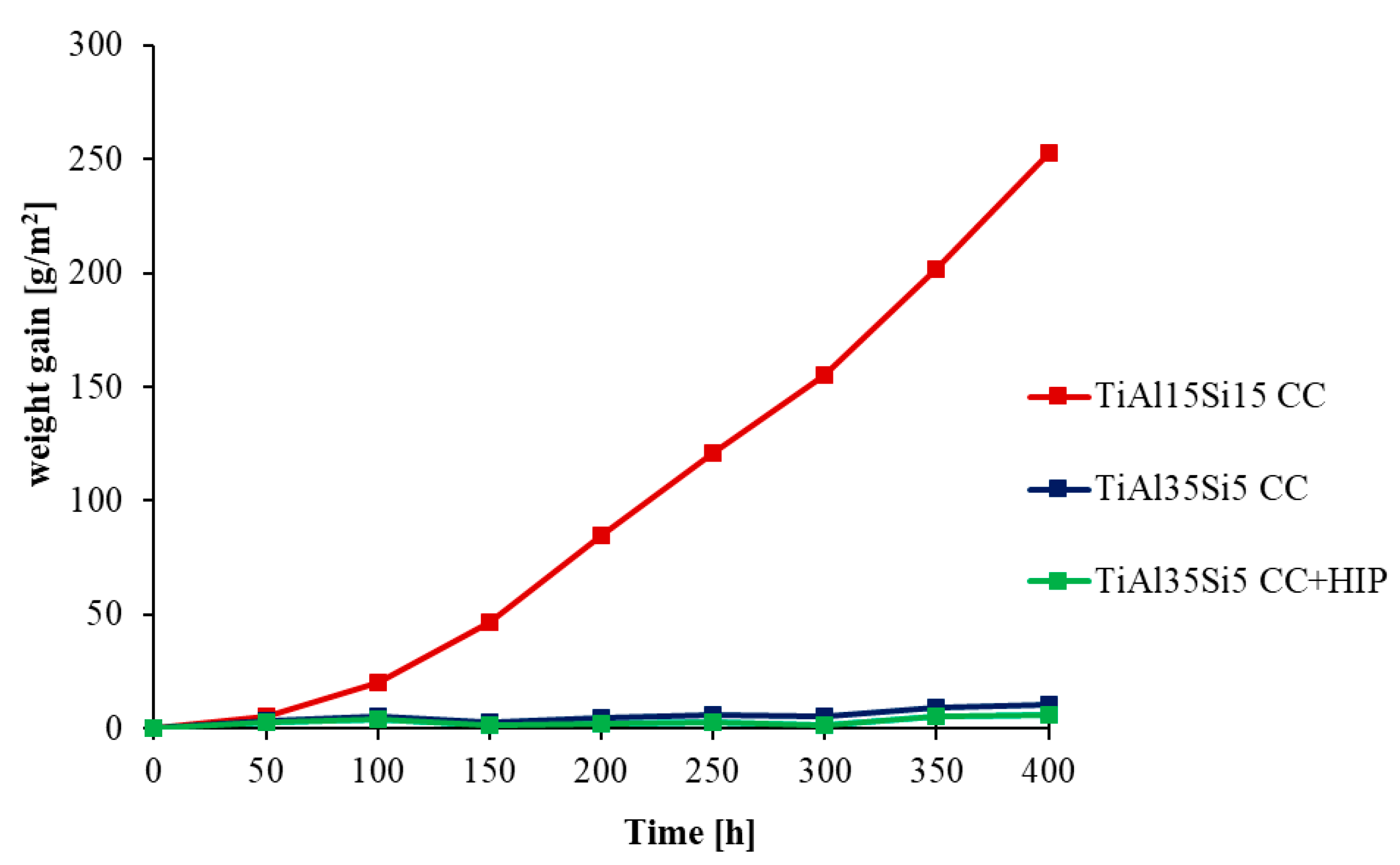
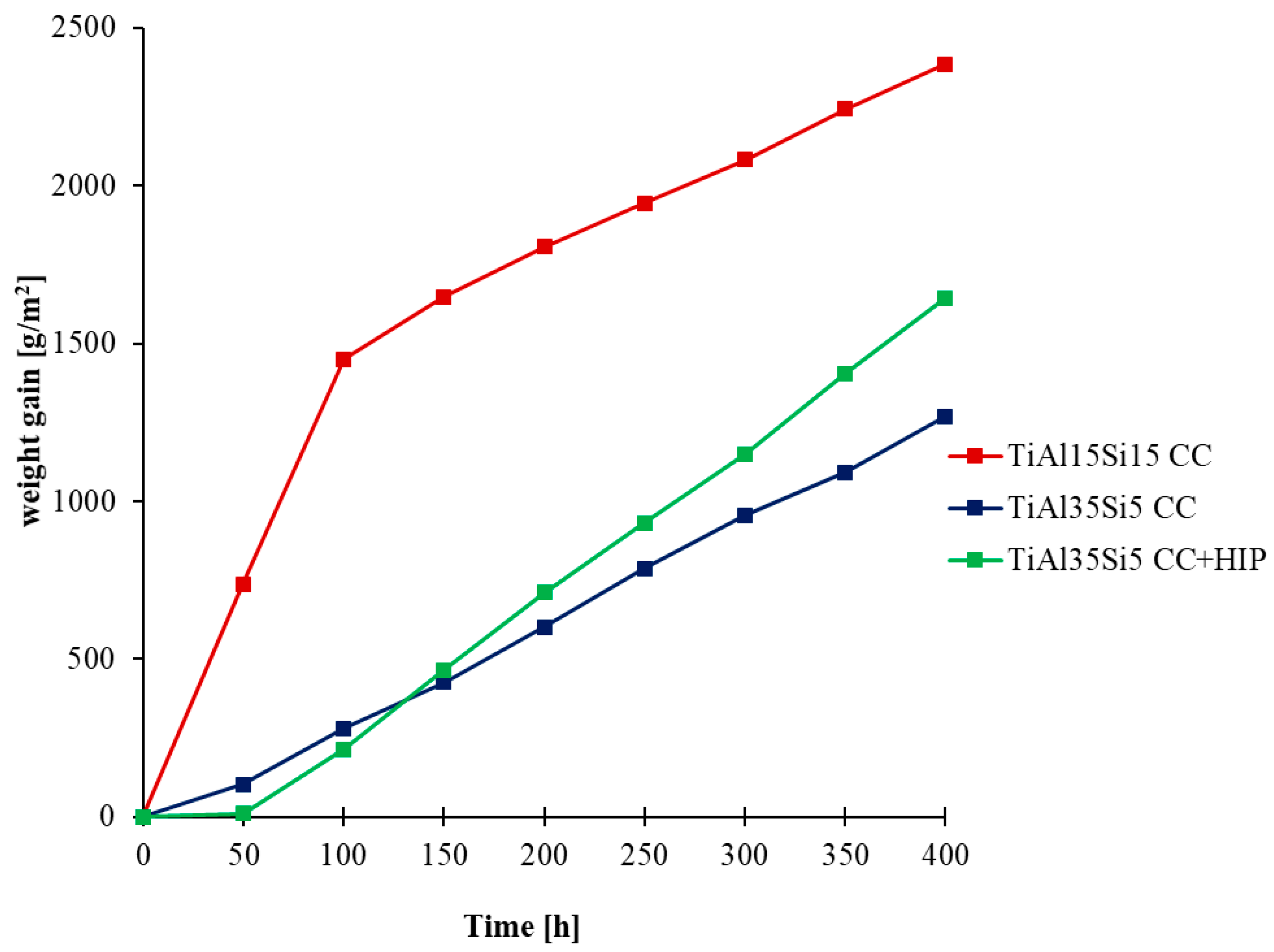
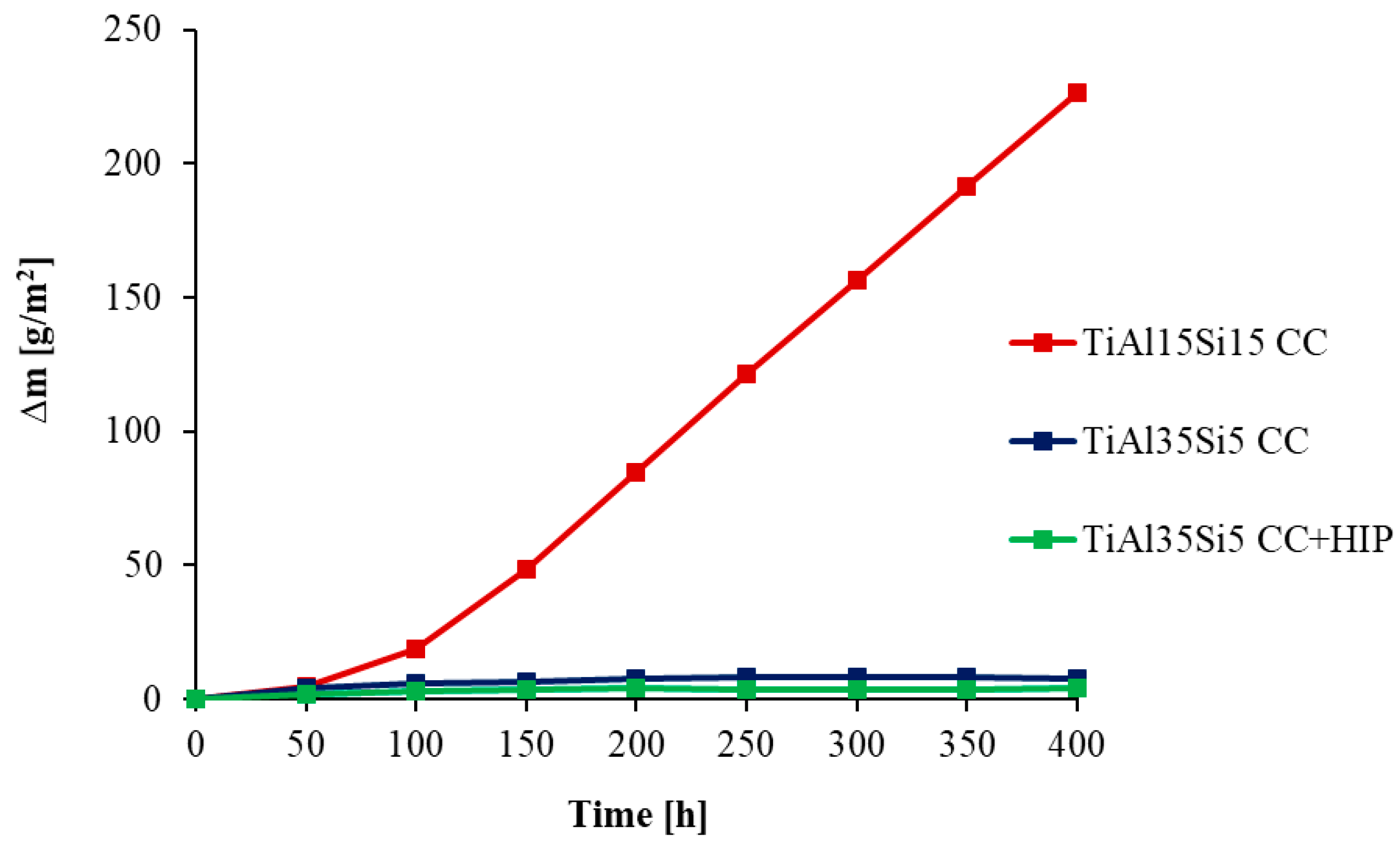
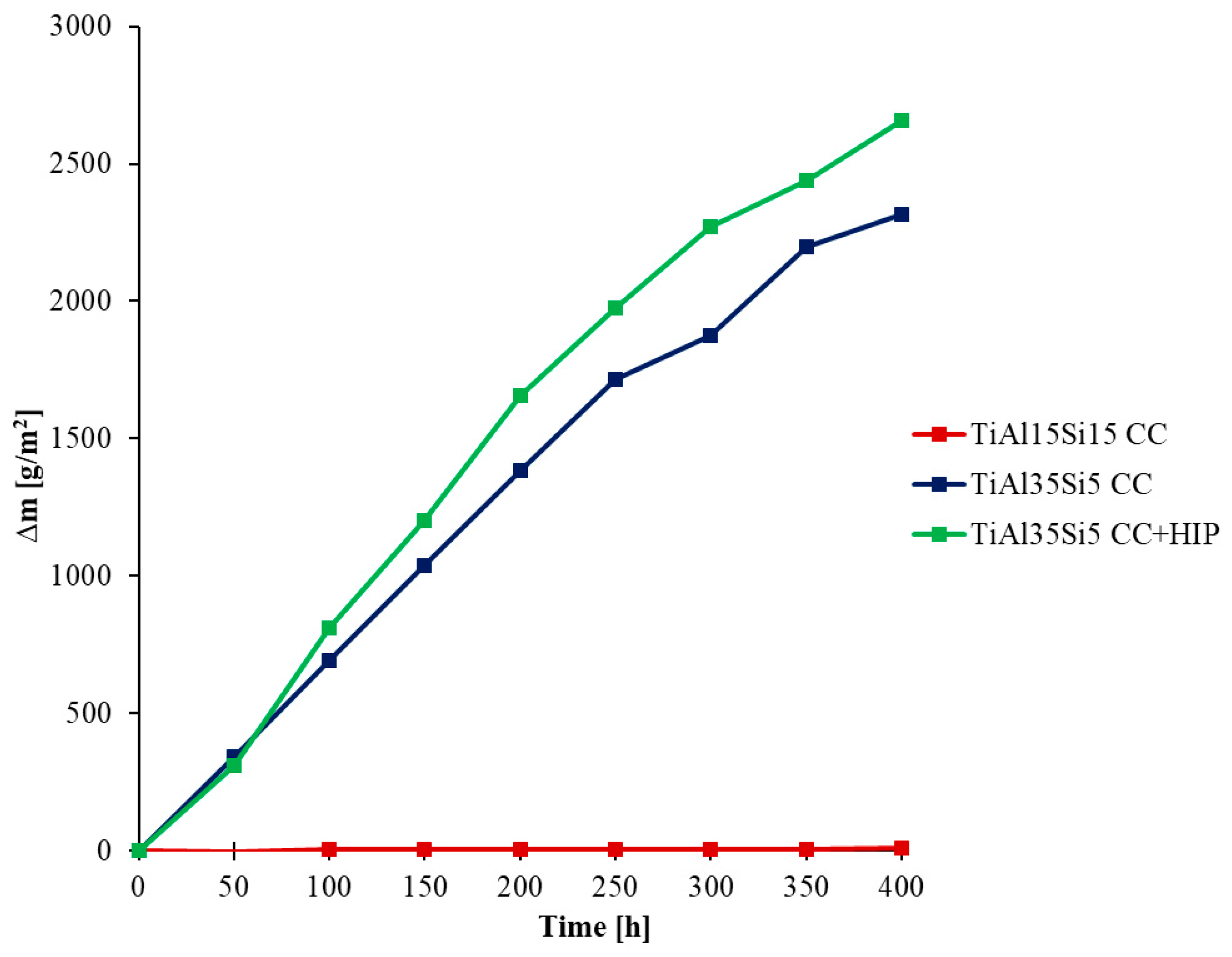
3.3. High-Temperatute Properties
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bewlay, B.P.; Nag, S.; Suzuki, A.; Weimer, M.J. TiAl alloys in commercial aircraft engines. Mater. High Temp. 2016, 33, 549–559. [Google Scholar] [CrossRef]
- Fang, H.; Chen, R.; Yang, Y.; Su, Y.; Ding, H.; Guo, J.; Fu, H. Role of graphite on microstructural evolution and mechanical properties of ternary TiAl alloy prepared by arc melting method. Mater. Design 2018, 156, 300–310. [Google Scholar] [CrossRef]
- Bauer, V.; Christ, H.J.I. Thermomechanical fatigue behaviour of a third generation#³-TiAl intermetallic alloy. Intermetallics 2009, 17, 370–377. [Google Scholar]
- Lapin, J. TiAl-based alloys: Present status and future perspectives. In Proceedings of the Metal; Tanger: Ostrava, Czech Republic, 2009; Volume 19, No. 21.5; p. 2019. [Google Scholar]
- Noda, T. Application of cast gamma TiAl for automobiles. Intermetallics 1998, 6, 709–713. [Google Scholar] [CrossRef]
- Fu, P.X.; Kang, X.H.; Ma, Y.C.; Liu, K.; Li, D.Z.; Li, Y.Y. Centrifugal casting of TiAl exhaust valves. Intermetallics 2008, 16, 130–138. [Google Scholar] [CrossRef]
- Ye, X.-C.; Xiao, K.-Q.; Cao, R.-X.; Wu, H.; Zhao, G.-w.; Li, B. Microstructure evolution and microhardness of TiAl based alloy blade by vacuum suction casting. Vacuum 2019, 163, 186–193. [Google Scholar] [CrossRef]
- Deevi, S.C.; Sikk, V.K. Exo-MeltTM process for melting and casting intermetallics. Intermetallics 1997, 5, 17–27. [Google Scholar] [CrossRef]
- Čegan, T.; Szurman, I. Thermal stability and precipitation strengthening of fully lamellar Ti-45Al-5Nb-0.2B-0.75C alloy. Kov. Mater. 2017, 55, 421–430. [Google Scholar] [CrossRef]
- Clemens, H.; Mayer, S. Design, Processing, Microstructure, Properties, and Applications of Advanced Intermetallic TiAl Alloys. Adv. Eng. Mater. 2013, 15, 191–215. [Google Scholar] [CrossRef]
- Lapin, J.; Pelachová, T. Microstructural stability of a cast Ti–45.2Al–2W–0.6Si–0.7B alloy at temperatures 973–1073K. Intermetallics 2006, 14, 1175–1180. [Google Scholar] [CrossRef]
- Fashu, S.; Lototskyy, M.; Davids, M.W.; Pickering, L.; Linkov, V.; Tai, S.; Renheng, T.; Fangming, X.; Fursikov, P.V.; Tarasov, B.P. A review on crucibles for induction melting of titanium alloys. Mater. Design 2020, 186, 108295. [Google Scholar] [CrossRef]
- Barbosa, J.; Ribeiro, C.S.; Monteiro, A.C. Influence of superheating on casting of γ-TiAl. Intermetallics 2007, 15, 945–955. [Google Scholar] [CrossRef]
- Wu, X. Review of alloy and process development of TiAl alloys. Intermetallics 2006, 14, 1114–1122. [Google Scholar] [CrossRef]
- Kostov, A.; Friedrich, B. Selection of crucible oxides in molten titanium and titanium aluminum alloys by thermo-chemistry calculations. J. Min. Metall. Sect. BMetall. 2005, 41, 113–125. [Google Scholar] [CrossRef]
- Gomes, F.; Puga, H.; Barbosa, J.; Ribeiro, C.S. Effect of melting pressure and superheating on chemical composition and contamination of yttria-coated ceramic crucible induction melted titanium alloys. J. Mater. Sci. 2011, 46, 4922–4936. [Google Scholar] [CrossRef]
- Schafföner, S.; Aneziris, C.G.; Berek, H.; Hubálková, J.; Rotmann, B.; Friedrich, B. Corrosion behavior of calcium zirconate refractories in contact with titanium aluminide melts. J. Eur. Ceram. Soc. 2015, 35, 1097–1106. [Google Scholar] [CrossRef]
- Čegan, T.; Szurman, I.; Kursa, M.; Holesinsky, J.; Vontorova, J. Preparation of TiAl-based alloys by induction melting in graphite crucibles. Kov. Mater. 2015, 53, 69–78. [Google Scholar] [CrossRef]
- Zhang, Z.; Frenzel, J.; Neuking, K.; Eggeler, G. On the reaction between NiTi melts and crucible graphite during vacuum induction melting of NiTi shape memory alloys. Acta Mater. 2005, 53, 3971–3985. [Google Scholar] [CrossRef]
- Kulakov, B.; Dubrovin, V.; Karpinskiy, A. Computing Simulation of Casting Using Titanium Aluminide Intermetallic Alloys. In Materials Science Forum; Trans Tech Publications Ltd.: Bäch, Switzerland, 2016; pp. 213–216. [Google Scholar]
- Vacuum Casting a Turbocharger in Titanium Aluminide. Available online: https://www.topcast.it/en/news/vacuum-casting-a-turbocharger-in-titanium-aluminide_7.html (accessed on 13 January 2021).
- Aguilar, J.; Hecht, U.; Schievenbusch, A. Qualification of an Investment Casting Process for Production of Titanium Aluminide Components for Aerospace and Automotive Applications. Mater. Sci. Forum 2010, 638–642, 1275–1280. [Google Scholar] [CrossRef]
- Duarte, A.; Viana, F.; Santos, H.M. As-cast titanium aluminides microstructure modification. Mater. Res. 1999, 2, 191–195. [Google Scholar] [CrossRef]
- Saqib, M.; Apgar, L.S.; Eylon, D.; Weiss, I. The effects of HIP processing on microstructure and phase relations in α2-base titanium aluminides. Mater. Sci. Eng. A 1992, 153, 726–735. [Google Scholar] [CrossRef]
- Novák, P.; Průša, F.; Šerák, J.; Vojtěch, D.; Michalcová, A. Oxidation resistance and thermal stability of Ti-Al-Si alloys produced by reactive sintering. In Proceedings of the Metal; Tanger: Ostrava, Czech Republic, 2009. [Google Scholar]
- Knaislová, A.; Novák, P.; Cabibbo, M.; Průša, F.; Paoletti, C.; Jaworska, L.; Vojtěch, D. Combination of reaction synthesis and Spark Plasma Sintering in production of Ti-Al-Si alloys. J. Alloys Compd. 2018, 752, 317–326. [Google Scholar] [CrossRef]
- Novák, P.; Kříž, J.; Průša, F.; Kubásek, J.; Marek, I.; Michalcová, A.; Voděrová, M.; Vojtěch, D. Structure and properties of Ti–Al–Si-X alloys produced by SHS method. Intermetallics 2013, 39, 11–19. [Google Scholar] [CrossRef]
- Knaislová, A.; Novák, P.; Průša, F.; Cabibbo, M.; Jaworska, L.; Vojtěch, D. High-temperature oxidation of Ti–Al–Si alloys prepared by powder metallurgy. J. Alloys Compd. 2019, 810, 151895. [Google Scholar] [CrossRef]
- Novák, P.; Vojtěch, D.; Šerák, J.; Kubásek, J.; Průša, F.; Knotek, V.; Michalcová, A.; Novák, M. Synthesis of Intermediary Phases in Ti-Al-Si System by Reactive Sintering. Chem. Listy 2009, 103, 1022–1026. [Google Scholar]
- Knaislová, A.; Linhart, J.; Novák, P.; Průša, F.; Kopeček, J.; Laufek, F.; Vojtěch, D. Preparation of TiAl15Si15 intermetallic alloy by mechanical alloying and the spark plasma sintering method. Powder Metall. 2019, 62, 56–60. [Google Scholar] [CrossRef]
- Knaislová, A.; Novák, P.; Kopeček, J.; Průša, F. Properties Comparison of Ti-Al-Si Alloys Produced by Various Metallurgy Methods. Materials 2019, 12, 3084. [Google Scholar] [CrossRef]
- Hlaváčová, I.M.; Sadílek, M.; Váňová, P.; Szumilo, Š.; Tyč, M. Influence of Steel Structure on Machinability by Abrasive Water Jet. Materials 2020, 13, 4424. [Google Scholar] [CrossRef] [PubMed]
- Vojtech, D.; Kubatík, T.; Čížová, H. Application of Silicon for a Protection of Titanium against High-Temperature Oxidation. Mater. Sci. Forum 2005, 482, 243–246. [Google Scholar] [CrossRef]
- Novák, P.; Kříž, J.; Michalcová, A.; Vojtech, D. Microstructure Evolution of Fe-Al-Si and Ti-Al-Si Alloys during High-Temperature Oxidation. Mater. Sci. Forum 2014, 782, 353–358. [Google Scholar] [CrossRef]

| Mechanical Properties | TiAl15Si15 (CC) | TiAl35Si5 (CC) | TiAl35Si5 (CC + HIP) |
|---|---|---|---|
| Hardness HV 5 | 459 ± 15 | 375 ± 16 | 374 ± 7 |
| Ultimate compressive strength [MPa] | 1205 ± 80 | 1867 ± 75 | 1801 ± 47 |
| Tribological Properties | TiAl15Si15 (CC) | TiAl35Si5 (CC) | TiAl35Si5 (CC + HIP) |
|---|---|---|---|
| friction coefficient [–] | 0.555 ± 0.006 | 0.453 ± 0.004 | 0.462 ± 0.003 |
| wear rate [mm3m−1N−1] | (4.17 ± 0.09) × 10 −4 | (1.25 ± 0.02) × 10 −4 | (1.21 ± 0.04) × 10 −4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Knaislová, A.; Novák, P.; Linhart, J.; Szurman, I.; Skotnicová, K.; Juřica, J.; Čegan, T. Structure and Properties of Cast Ti-Al-Si Alloys. Materials 2021, 14, 813. https://doi.org/10.3390/ma14040813
Knaislová A, Novák P, Linhart J, Szurman I, Skotnicová K, Juřica J, Čegan T. Structure and Properties of Cast Ti-Al-Si Alloys. Materials. 2021; 14(4):813. https://doi.org/10.3390/ma14040813
Chicago/Turabian StyleKnaislová, Anna, Pavel Novák, Jiří Linhart, Ivo Szurman, Kateřina Skotnicová, Jan Juřica, and Tomáš Čegan. 2021. "Structure and Properties of Cast Ti-Al-Si Alloys" Materials 14, no. 4: 813. https://doi.org/10.3390/ma14040813
APA StyleKnaislová, A., Novák, P., Linhart, J., Szurman, I., Skotnicová, K., Juřica, J., & Čegan, T. (2021). Structure and Properties of Cast Ti-Al-Si Alloys. Materials, 14(4), 813. https://doi.org/10.3390/ma14040813









