Physico-Chemical Investigation of Endodontic Sealers Exposed to Simulated Intracanal Heat Application: Hydraulic Calcium Silicate-Based Sealers
Abstract
:1. Introduction
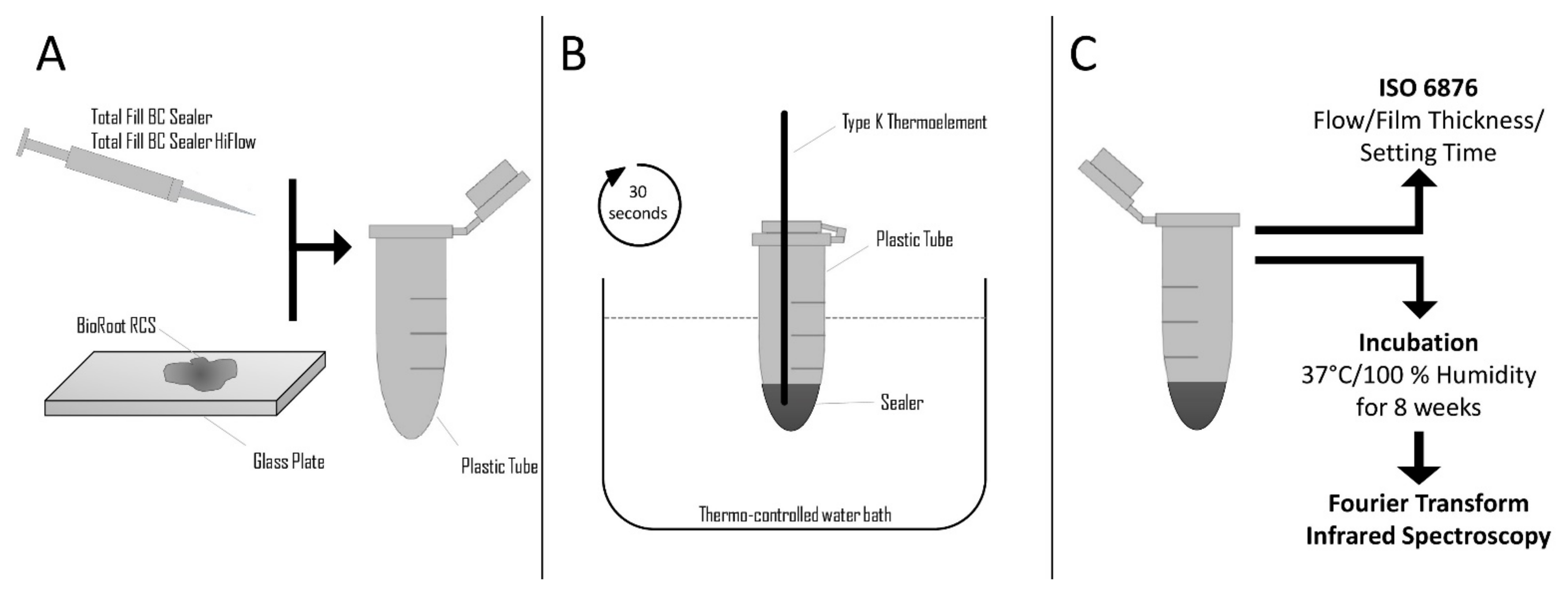
2. Materials and Methods
2.1. Heat Application on the Sealers
2.2. Evaluation of Physical Characteristics
2.2.1. Setting Time
2.2.2. Film Thickness
2.2.3. Flow
2.3. Evaluation of Chemical Characteristics
2.4. Statistical Analysis
3. Results
3.1. Impact of Heat Application on Physical Characteristics
3.1.1. Setting Time
3.1.2. Film Thickness
3.1.3. Flow
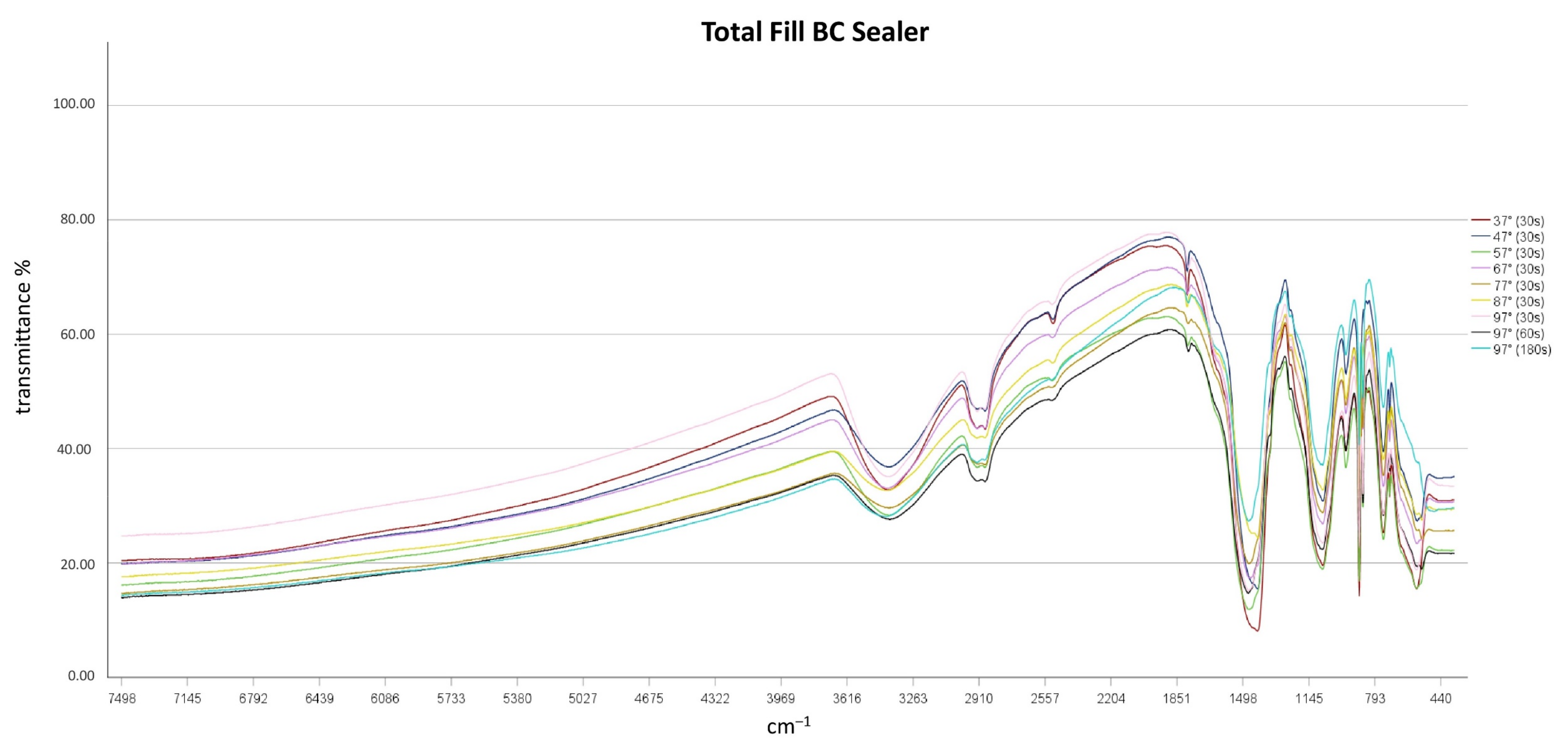
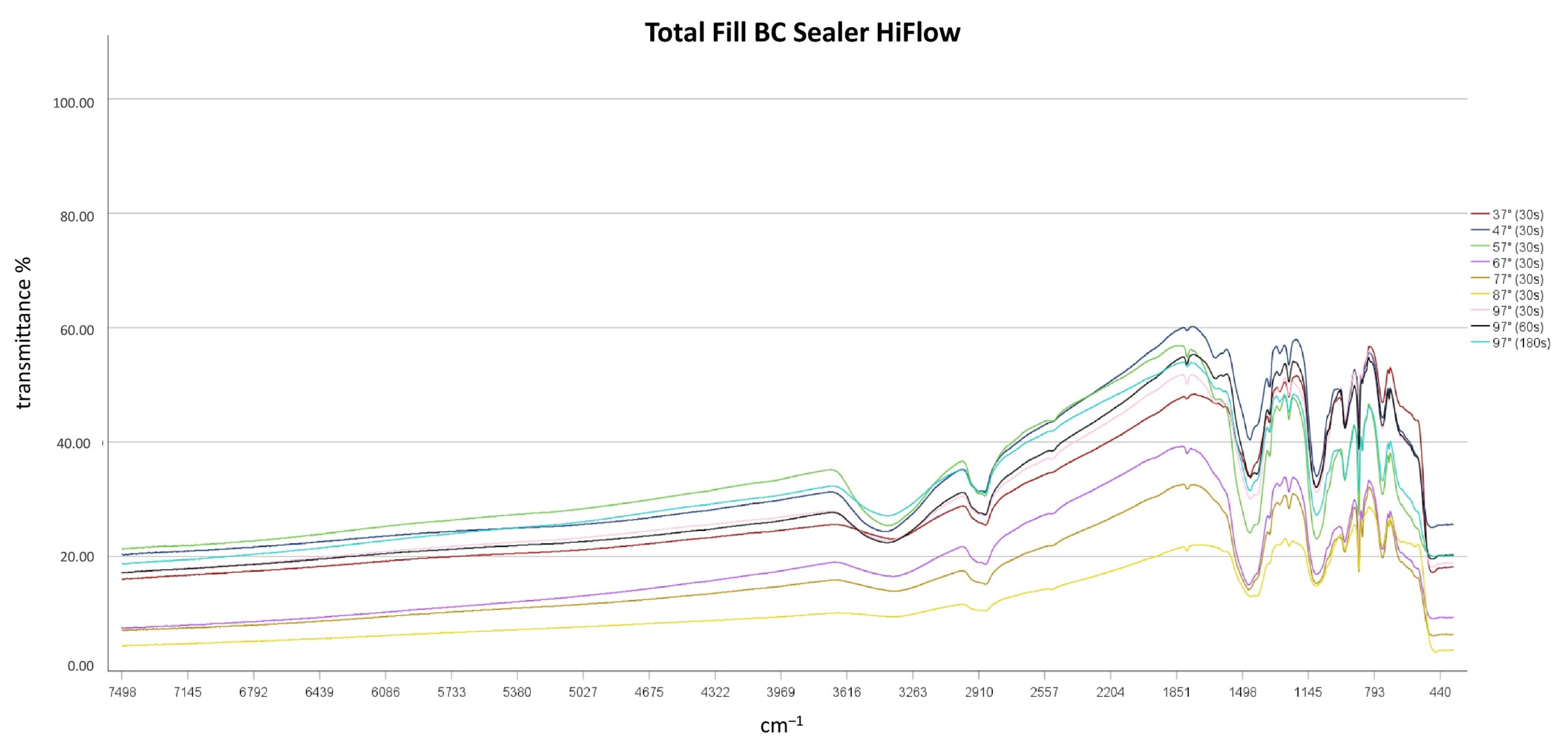
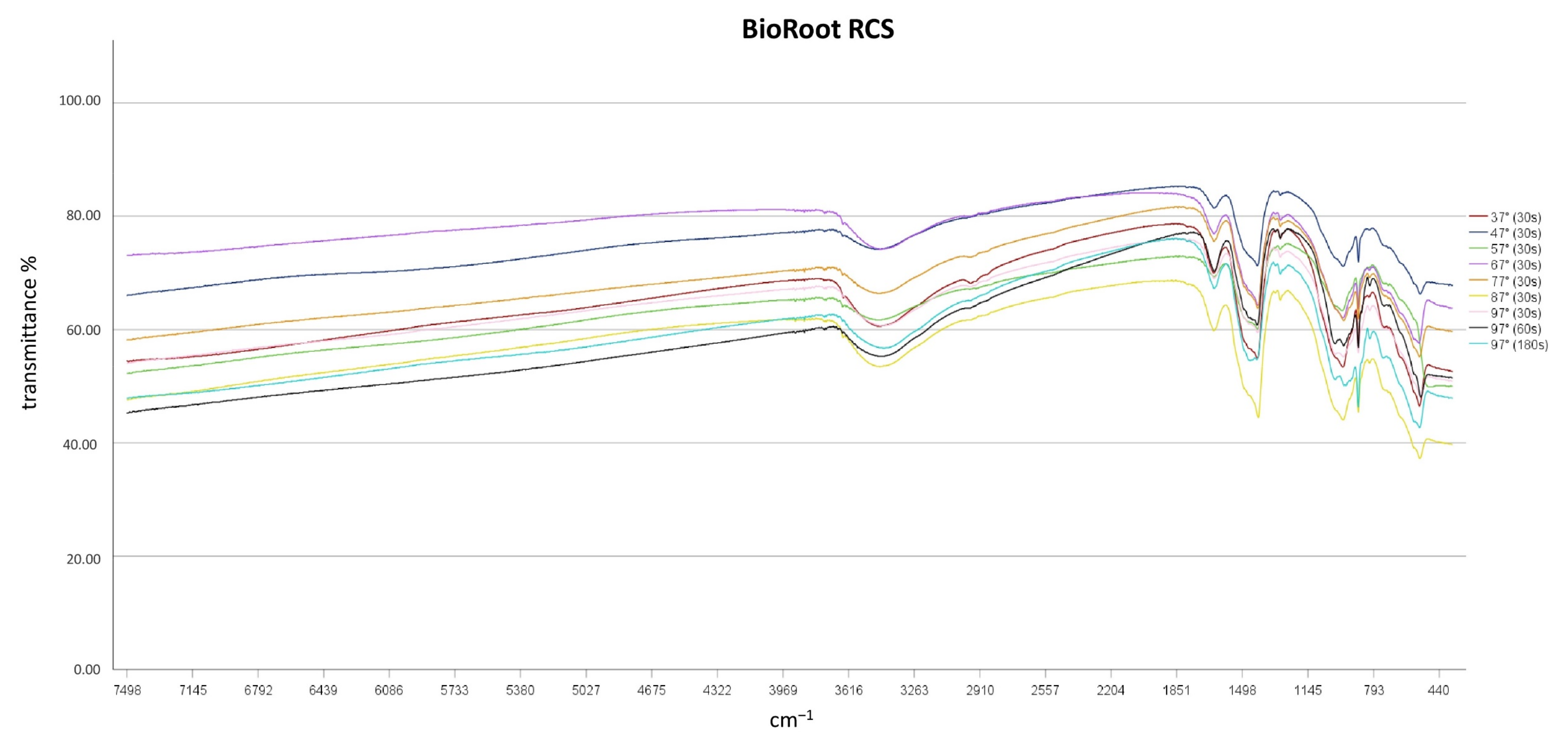
3.2. Impact of Heat Application on Chemical Characteristics
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Donnermeyer, D.; Bürklein, S.; Dammaschke, T.; Schäfer, E. Endodontic sealers based on calcium silicates: A systematic review. Odontology 2019, 107, 421–436. [Google Scholar] [CrossRef]
- Harms, C.S.; Schäfer, E.; Dammaschke, T. Clinical evaluation of direct pulp capping using a calcium silicate cement-treatment outcomes over an average period of 2.3 years. Clin. Oral. Investig. 2019, 23, 3491–3499. [Google Scholar] [CrossRef] [PubMed]
- Donfrancesco, O.; Seracchiani, M.; Morese, A.; Ferri, V.; Nottola, S.A.; Relucenti, M.; Gambarini, G.; Testarelli, L. Analysis of Stability in Time of Marginal Adaptation of Endosequence Root Repair Material on Biological Samples. Dent. Hypotheses 2020, 11, 11–15. [Google Scholar]
- Donnermeyer, D.; Dammaschke, T.; Schäfer, E. Endodontics in the era of hydraulic materials. Hydraulic calcium silicate-based sealers—A game changer in root canal obturation? ENDO Endod. Pract. Today 2020, 4, 197–203. [Google Scholar]
- Schilder, H. Filling root canals in three dimensions. J. Endod. 1967, 32, 281–290. [Google Scholar] [CrossRef]
- Schäfer, E.; Köster, M.; Bürklein, S. Percentage of gutta-percha-filled areas in canals instrumented with nickel-titanium systems and obturated with matching single cones. J. Endod. 2013, 39, 924–928. [Google Scholar] [CrossRef] [PubMed]
- Buchanan, L.S. Filling root canal systems with centered condensation: Concepts, instruments, and techniques. Dent. Today 2004, 23, 102–106. [Google Scholar]
- Ørstavik, D. Materials used for root canal obturation: Technical, biological and clinical testing. Endod. Top. 2005, 12, 25–38. [Google Scholar] [CrossRef]
- Rodríguez-Lozano, F.J.; López-García, S.; García-Bernal, D.; Tomás-Catalá, C.J.; Santos, J.M.; Llena, C.; Lozano, A.; Murcia, L.; Forner, L. Chemical composition and bioactivity potential of the new Endosequence BC sealer formulation HiFlow. Int. Endod. J. 2020, 53, 1216–1228. [Google Scholar] [CrossRef] [PubMed]
- Donnermeyer, D.; Urban, K.; Bürklein, S.; Schäfer, E. Physico-chemical investigation of endodontic sealers exposed to simulated intracanal heat application: Epoxy resins and zinc oxide-eugenols. Int. Endod. J. 2020, 53, 690–697. [Google Scholar] [CrossRef]
- Aksel, H.; Makowka, S.; Bosaid, F.; Guardian, M.G.; Sarkar, D.; Azim, A.A. Effect of heat application on the physical properties and chemical structure of calcium silicate-based sealers. Clin. Oral Investig. 2020. [Google Scholar] [CrossRef]
- Atmeh, A.R.; Hadis, M.; Camilleri, J. Real-time chemical analysis of root filling materials with heating: Guideline for safe temperature levels. Int. Endod. J. 2020, 53, 698–708. [Google Scholar] [CrossRef]
- Chen, B.; Haapasalo, M.; Mobuchon, C.; Li, X.; Ma, J.; Shen, Y. Cytotoxicity and the Effect of Temperature on Physical Properties and Chemical Composition of a New Calcium Silicate-based Root Canal Sealer. J. Endod. 2020, 46, 531–538. [Google Scholar] [CrossRef] [PubMed]
- Hadis, M.; Camilleri, J. Characterization of heat resistant hydraulic sealer for warm vertical obturation. Dent. Mater. 2020, 36, 1183–1189. [Google Scholar] [CrossRef] [PubMed]
- Yamauchi, S.; Watanabe, S.; Okiji, T. Effects of heating on the physical properties of premixed calcium silicate-based root canal sealers. J. Oral Sci. 2020, 63, 65–69. [Google Scholar] [CrossRef]
- Chedella, S.C.V.; Berzins, D.W. A differential scanning calorimetry study of the setting reaction of MTA. Int. Endod. J. 2020, 43, 509–518. [Google Scholar] [CrossRef]
- Donnermeyer, D. ‚ Schäfer, E.; Bürklein, S. Real-time intracanal temperature measurement during different obturation techniques. J. Endod. 2018, 44, 1832–1836. [Google Scholar] [CrossRef]
- Atmeh, A.; AlShwaimi, E. The effect of heating time and temperature on epoxy resin and calcium silicate-based endodontic sealers. J. Endod. 2017, 43, 2112–2118. [Google Scholar] [CrossRef] [PubMed]
- International Organization for Standardization. International Standard ISO 6876: Dentistry—Root Canal Sealing Materials; International Organization for Standardization: Geneva, Switzerland, 2012. [Google Scholar]
- Mollah, M.Y.A.; Yu, W.; Schennach, R.; Cocke, D.L. A Fourier transform infrared spectroscopic investigation of the early hydration of Portland cement and the influence of sodium lignosulfonate. Cem. Concr. Res. 2000, 30, 267–273. [Google Scholar] [CrossRef]
- Ylmén, R.; Jäglid, U.; Steenari, B.; Panas, I. Early hydration and setting of Portland cement monitored by IR, SEM and Vicat techniques. Cem. Concr. Res. 2009, 39, 433–439. [Google Scholar] [CrossRef]
- Wang, P.; Li, C.; Gong, H.; Jiang, X.; Wang, H.; Li, K. Effects of synthesis conditions on the morphology of hydroxyapatite nanoparticles produced by wet chemical process. Powder Technol. 2010, 203, 315–321. [Google Scholar] [CrossRef]
- Maeda, Y.; Nakamura, T.; Ikeda, I. Changes in the hydration starts of Poly(N-n-propylmethacrylamide) and Poly(N-isopropylmethacrylamide) during their phase transition in water observed by FTIR spectroscopy. Macromolecules 2001, 34, 8246–8251. [Google Scholar] [CrossRef]
- Chérgoles Asensio, R.; San Andrés Moya, M.; de la Roja, J.M.; Gómez, M. Analytiacal characterization of polymers used in conservation and restoration by ATR-FTIR sprectroscopy. Anal. Bioanal. Chem. 2009, 395, 2081–2096. [Google Scholar] [CrossRef]
- Zhou, H.M.; Shen, Y.; Zheng, W.; Li, L.; Zheng, Y.F.; Haapasalo, M. Physical properties of 5 root canal sealers. J. Endod. 2013, 39, 1281–1286. [Google Scholar] [CrossRef] [PubMed]
- Kebudi Benezra, M.; Schembri Wismayer, P.; Camilleri, J. Influence of environment on testing of hydraulic sealers. Sci. Rep. 2017, 7, 17927. [Google Scholar] [CrossRef]
- Viapiana, R.; Baluci, C.A.; Tanomaru-Filho, M.; Camilleri, J. Investigation of chemical changes in sealers during application of the warm vertical compaction technique. Int. Endod. J. 2015, 48, 16–27. [Google Scholar] [CrossRef] [PubMed]
- Zhan, B.J.; Xuan, D.X.; Poon, C.S.; Shi, S.J.; Kou, S.C. Characterization of C-S-H formed in coupled CO2-water cured Portland cement pastes. Mater. Struct. 2018, 51, 92. [Google Scholar] [CrossRef]
| Component 1 | Component 2 | |
|---|---|---|
| BioRoot RCS | Powder: Tricalcium silicate, zirconium oxide, povidone | Liquid: Aqueous solution of calcium chloride and polycarboxylate |
| Total Fill BC Sealer | Zirconium oxide, dicalcium silicate, tricalcium silicate, calcium phosphate monobasic, calcium hydroxide, filler, thickening agents | |
| Total Fill BC Sealer HiFlow | Zirconium oxide, dicalcium silicate, tricalcium silicate, calcium hydroxide, filler |
| Group Number | Setting Time (min) | Film Thickness (µm) | Flow (mm) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Different from Group Number | Mean | SD | Different from Group Number | Mean | SD | Different from Group Number | ||
| 37° (30 s) | 1 | 63.3 | 4.2 | 3 | 7.7 | 0.00 | - | 20.9 | 2.1 | - |
| 47° (30 s) | 2 | 67.3 | 2.1 | 3,4,5 | 7.8 | 0.04 | - | 20.7 | 3.1 | - |
| 57° (30 s) | 3 | 52.3 | 1.5 | 1,2,4,5 | 7.7 | 0.01 | - | 21.7 | 1.4 | - |
| 67° (30 s) | 4 | 60.0 | 1.7 | 2,3 | 7.7 | 0.01 | - | 21.8 | 1.9 | - |
| 77° (30 s) | 5 | 58.3 | 4.0 | 2,3 | 7.7 | 0.01 | - | 23.0 | 2.0 | - |
| 87° (30 s) | 6 | * | * | * | * | * | * | |||
| 97° (30 s) | 7 | * | * | * | * | * | * | |||
| 97° (60 s) | 8 | * | * | * | * | * | * | |||
| 97° (180 s) | 9 | * | * | * | * | * | * | |||
| P-value | P = 0.025057 | P = 0.973880 | P = 0.609215 | |||||||
| Group Number | Setting Time (h) | Film Thickness (µm) | Flow (mm) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Different from Group Number | Mean | SD | Different from Group Number | Mean | SD | Different from Group Number | ||
| 37° (30 s) | 1 | Mean | SD | 2,4,5,9 | 7.7 | 0.00 | 4,6,4,9 | 24.9 | 0.7 | - |
| 47° (30 s) | 2 | 19.3 | 1.4 | 1,6,7 | 7.7 | 0.00 | 6,7,9 | 25.0 | 0.7 | - |
| 57° (30 s) | 3 | 17.5 | 0.5 | 4 | 7.7 | 0.00 | 7,9 | 24.6 | 0.3 | - |
| 67° (30 s) | 4 | 18.1 | 0.1 | 1,3,6,7,8 | 7.7 | 0.01 | 1,8 | 24.7 | 0.8 | - |
| 77° (30 s) | 5 | 17.1 | 0.4 | 1,6,7 | 7.7 | 0.00 | 6,7,9 | 25.4 | 1.1 | - |
| 87° (30 s) | 6 | 17.5 | 0.3 | 2,4,5,8,9 | 7.7 | 0.01 | 1,2,5,8 | 23.9 | 0.5 | - |
| 97° (30 s) | 7 | 19.2 | 0.4 | 2,4,5,9 | 7.7 | 0.01 | 1,2,3,5,8 | 28.0 | 2.0 | - |
| 97° (60 s) | 8 | 19.1 | 0.6 | 4,6 | 7.7 | 0.00 | 4,6,7,9 | 24.8 | 1.5 | - |
| 97° (180 s) | 9 | 18.1 | 0.4 | 1,6,7 | 7.7 | 0.00 | 1,2,3,5,8 | 26.2 | 2.8 | - |
| P-value | P = 0.020231 | P = 0.0187664 | P = 0.213943 | |||||||
| Group Number | Setting Time (h) | Film Thickness (µm) | Flow (mm) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Different from Group Number: | Mean | SD | Different from Group Number: | Mean | SD | Different from Group Number: | ||
| 37° (30 s) | 1 | 23.0 | 0.0 | 6,7,8,9 | 7.8 | 0.07 | - | 29.0 | 1.1 | 5,8 |
| 47° (30 s) | 2 | 23.2 | 0.2 | 4,5,6,7,8,9 | 7.7 | 0.01 | - | 30.7 | 1.7 | 4,5,6,8,9 |
| 57° (30 s) | 3 | 23.0 | 0.9 | 6,7,8,9 | 7.7 | 0.01 | - | 30.7 | 0.8 | 4,5,6,8,9 |
| 67° (30 s) | 4 | 22.5 | 0.7 | 2,8,9 | 7.7 | 0.00 | - | 28.4 | 1.0 | 2,3,8 |
| 77° (30 s) | 5 | 22.3 | 0.5 | 2,8 | 7.7 | 0.01 | - | 27.7 | 0.2 | 1,2,3,7 |
| 87° (30 s) | 6 | 22.2 | 0.1 | 1,2,3,8 | 7.7 | 0.00 | - | 28.5 | 0.3 | 2,3,8 |
| 97° (30 s) | 7 | 22.0 | 0.4 | 1,2,38 | 7.7 | 0.01 | - | 29.1 | 1.3 | 5,8 |
| 97° (60 s) | 8 | 20.0 | 0.6 | 1,2,3,4,5,6,7 | 7.7 | 0.01 | - | 26.7 | 0.2 | 1,2,3,4,6,7 |
| 97° (180 s) | 9 | 21.6 | 0.1 | 1,2,3,4 | 7.7 | 0.00 | - | 28.1 | 1.7 | 2,3 |
| P-value | P = 0.0111865 | P = 0.204195 | P = 0.021105 | |||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Donnermeyer, D.; Ibing, M.; Bürklein, S.; Weber, I.; Reitze, M.P.; Schäfer, E. Physico-Chemical Investigation of Endodontic Sealers Exposed to Simulated Intracanal Heat Application: Hydraulic Calcium Silicate-Based Sealers. Materials 2021, 14, 728. https://doi.org/10.3390/ma14040728
Donnermeyer D, Ibing M, Bürklein S, Weber I, Reitze MP, Schäfer E. Physico-Chemical Investigation of Endodontic Sealers Exposed to Simulated Intracanal Heat Application: Hydraulic Calcium Silicate-Based Sealers. Materials. 2021; 14(4):728. https://doi.org/10.3390/ma14040728
Chicago/Turabian StyleDonnermeyer, David, Magdalena Ibing, Sebastian Bürklein, Iris Weber, Maximilian P. Reitze, and Edgar Schäfer. 2021. "Physico-Chemical Investigation of Endodontic Sealers Exposed to Simulated Intracanal Heat Application: Hydraulic Calcium Silicate-Based Sealers" Materials 14, no. 4: 728. https://doi.org/10.3390/ma14040728






