Thermal Expansion of a Multiphase Intermetallic Ti-Al-Nb-Mo Alloy Studied by High-Energy X-ray Diffraction
Abstract
:1. Introduction
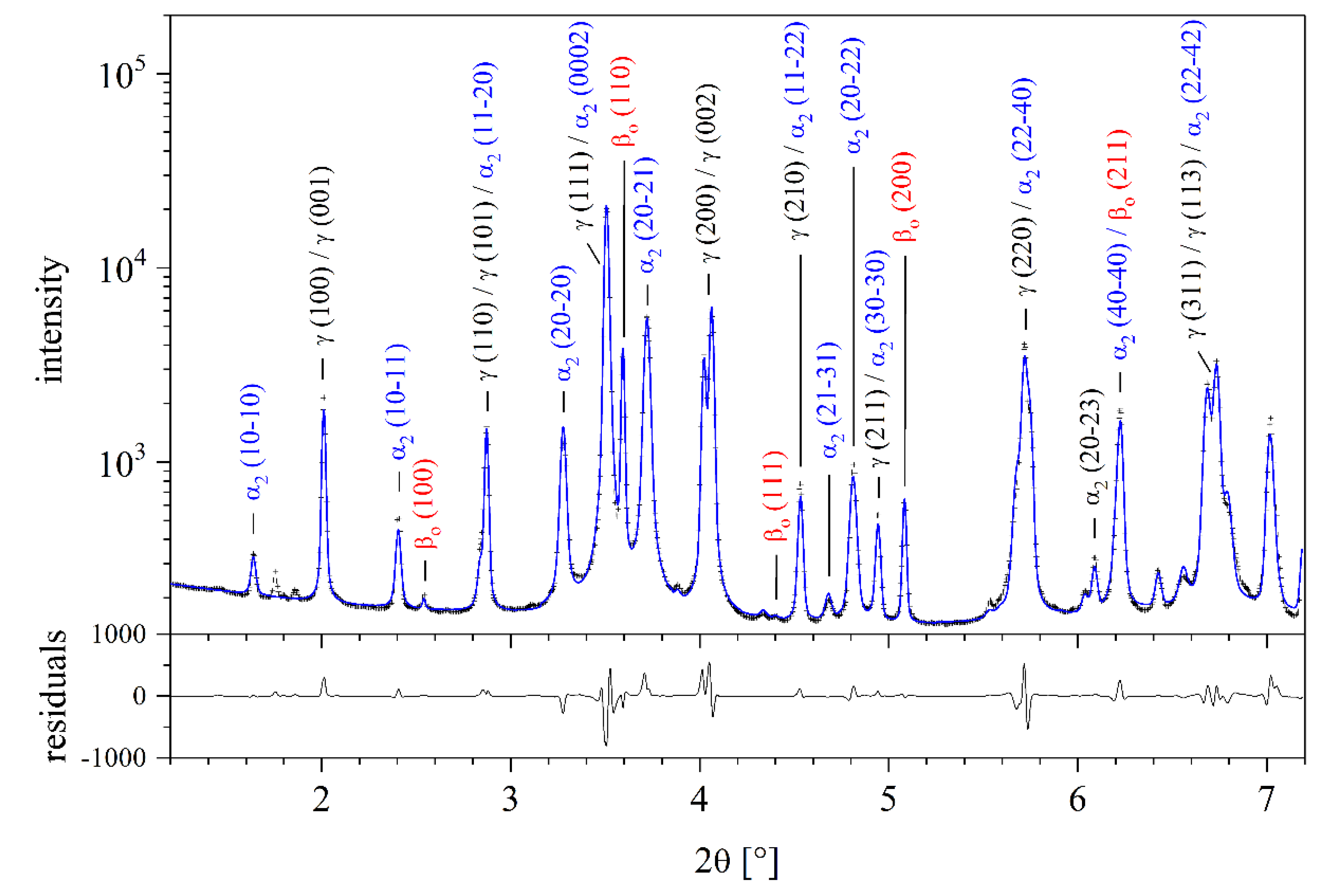
2. Materials and Methods
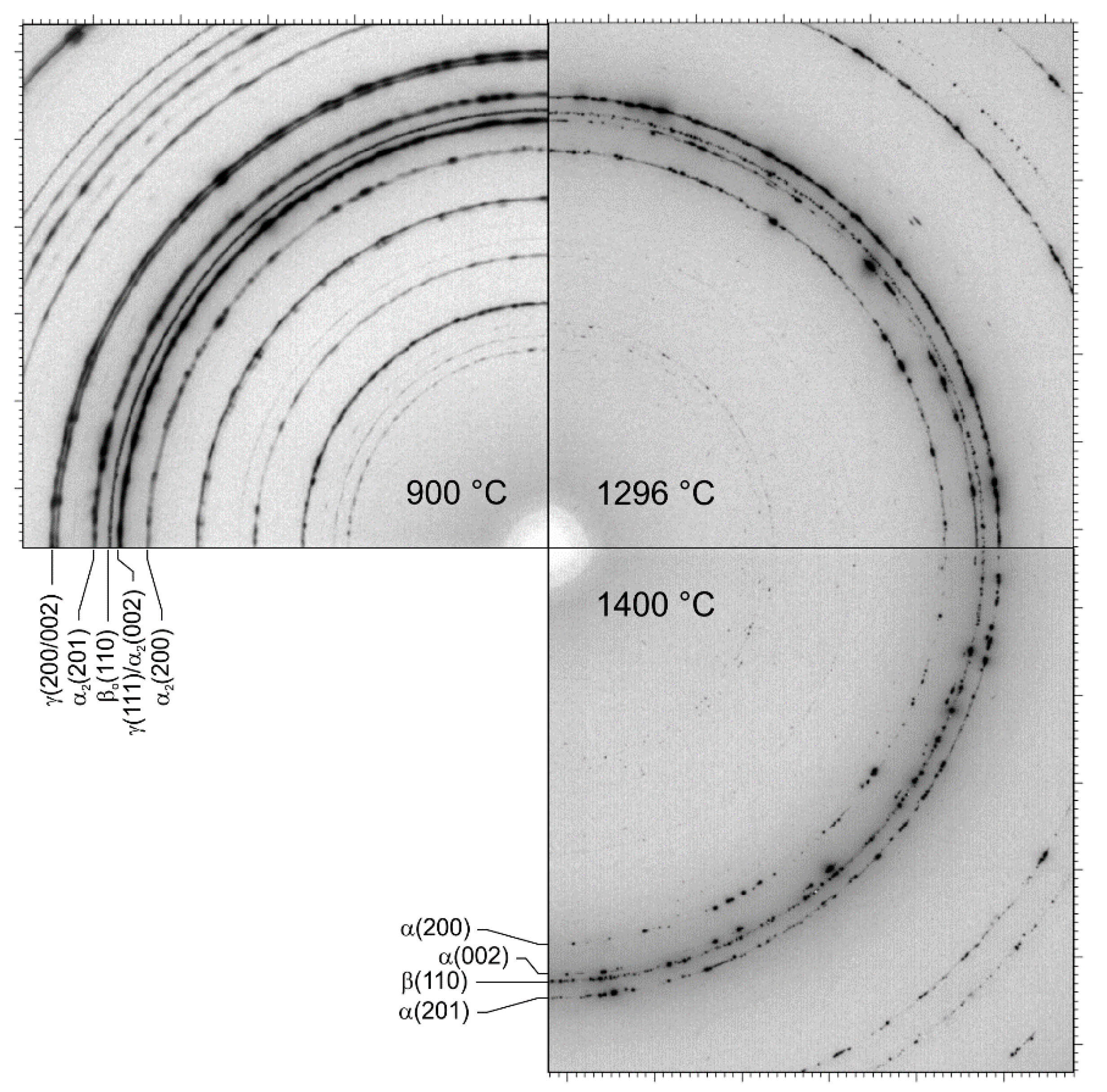
3. Results and Discussion
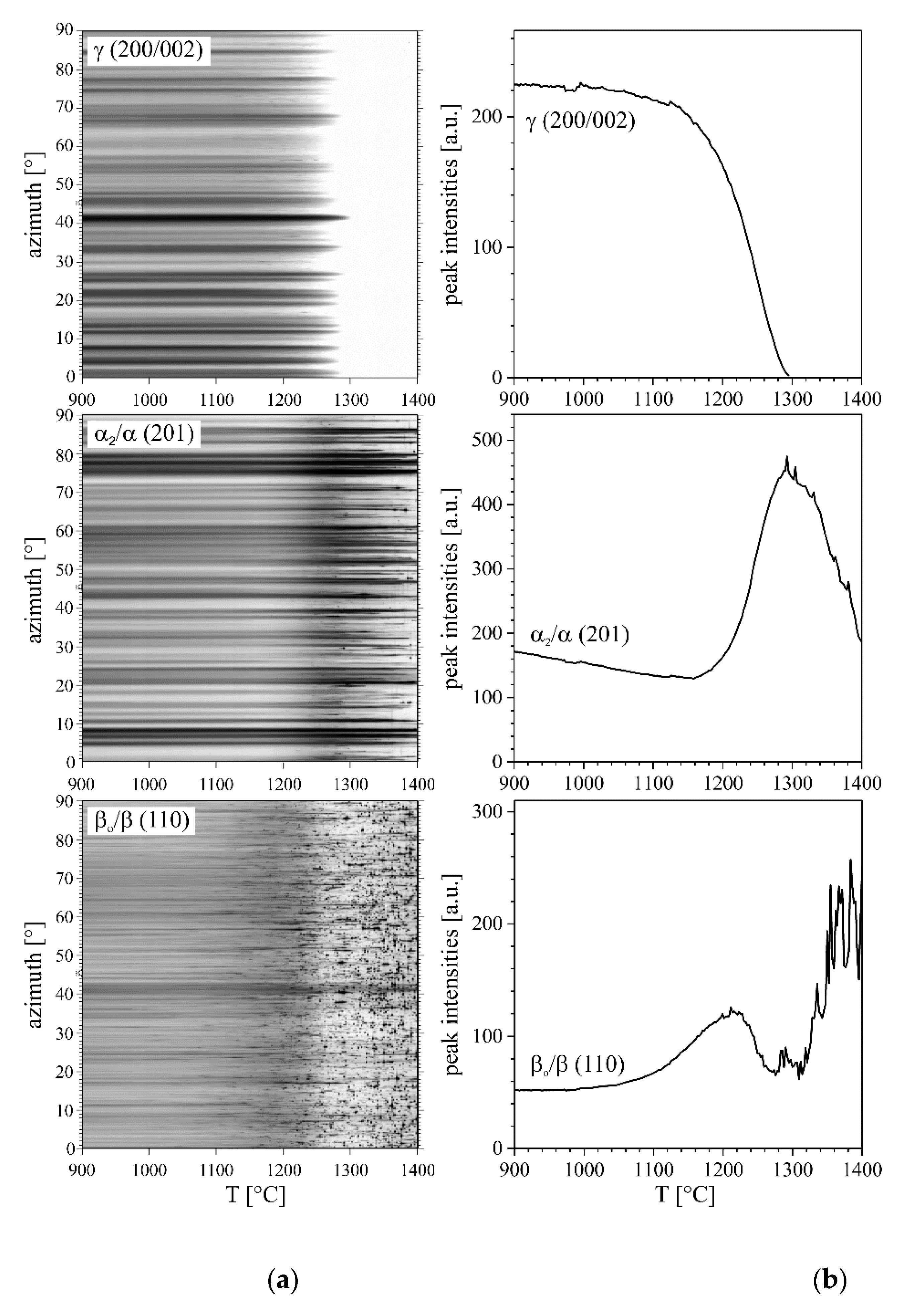
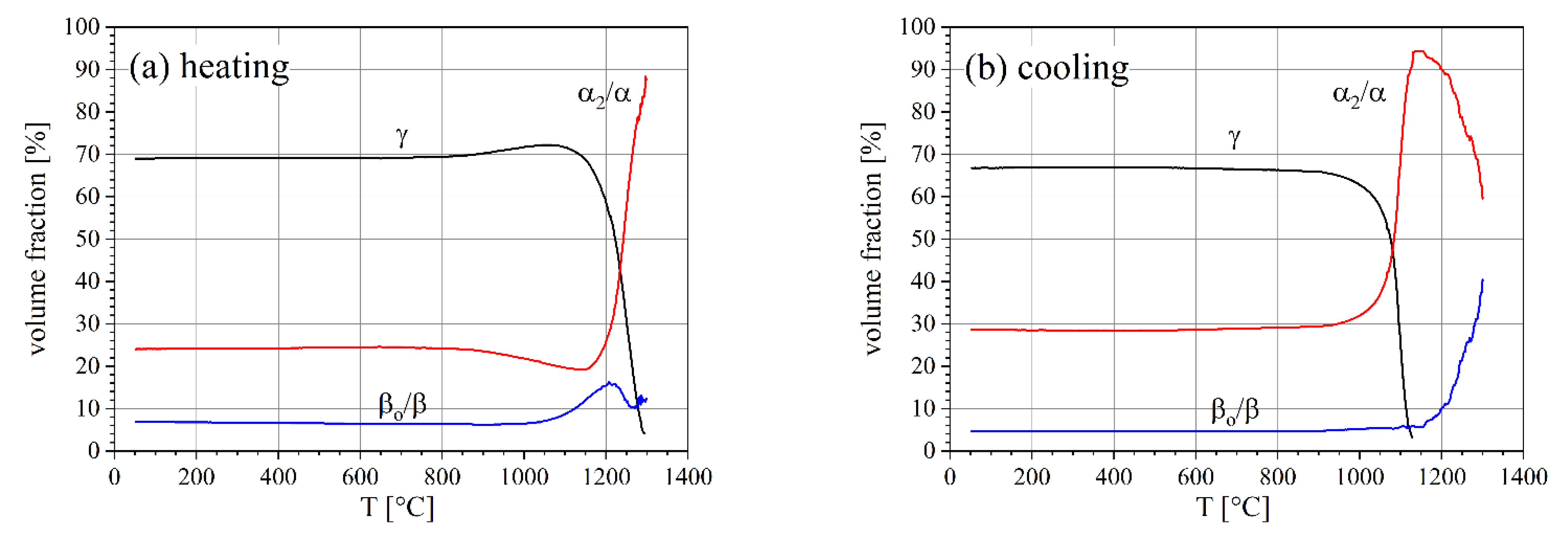
3.1. Phase Fractions
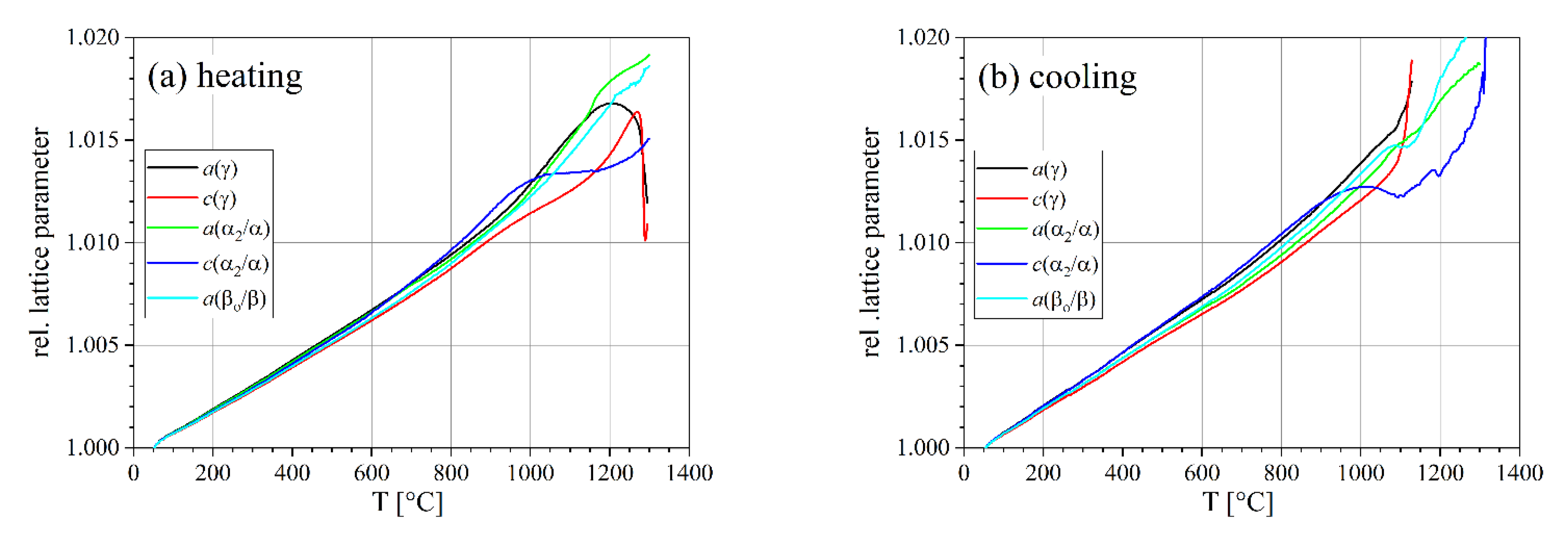
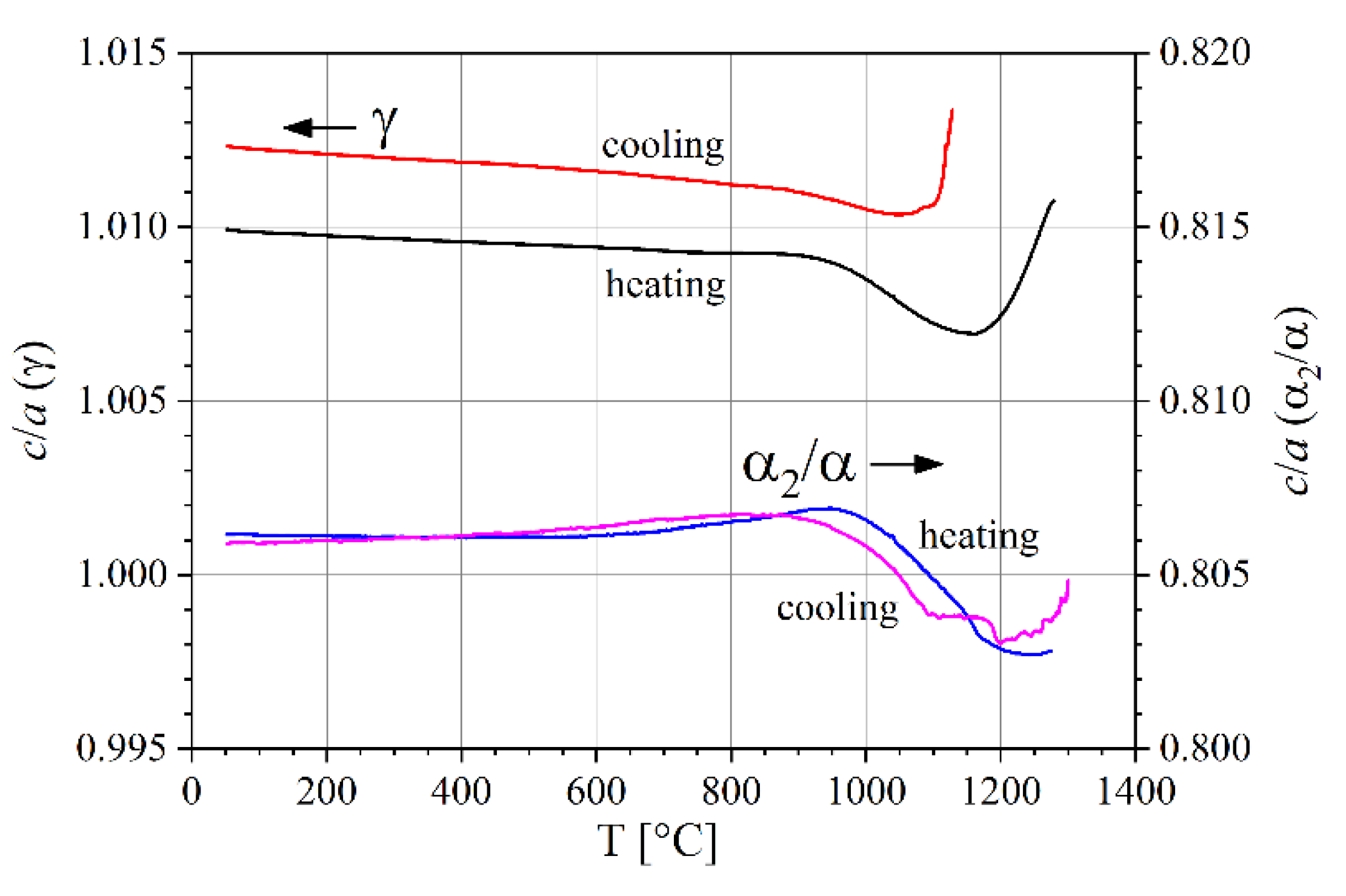
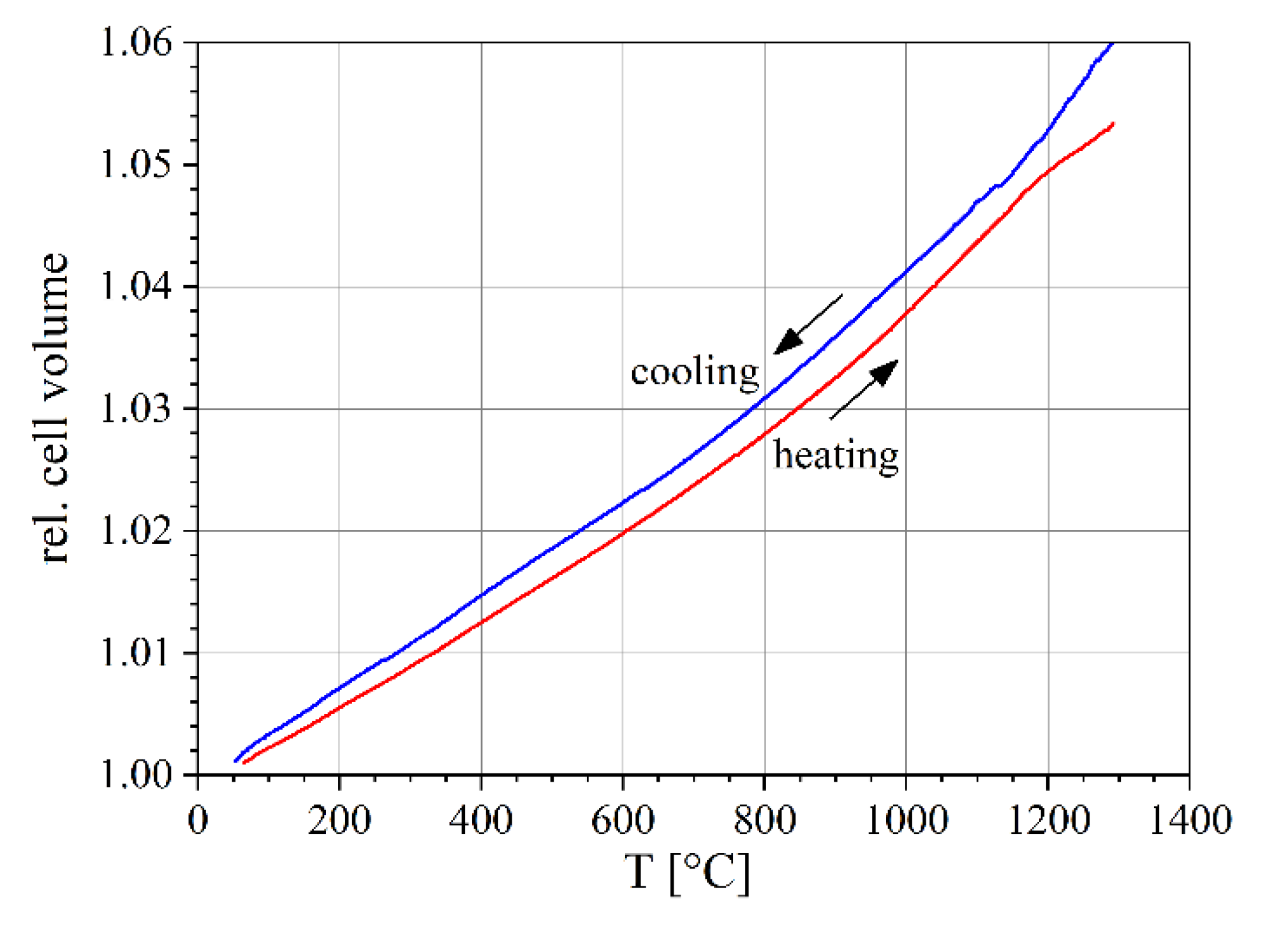
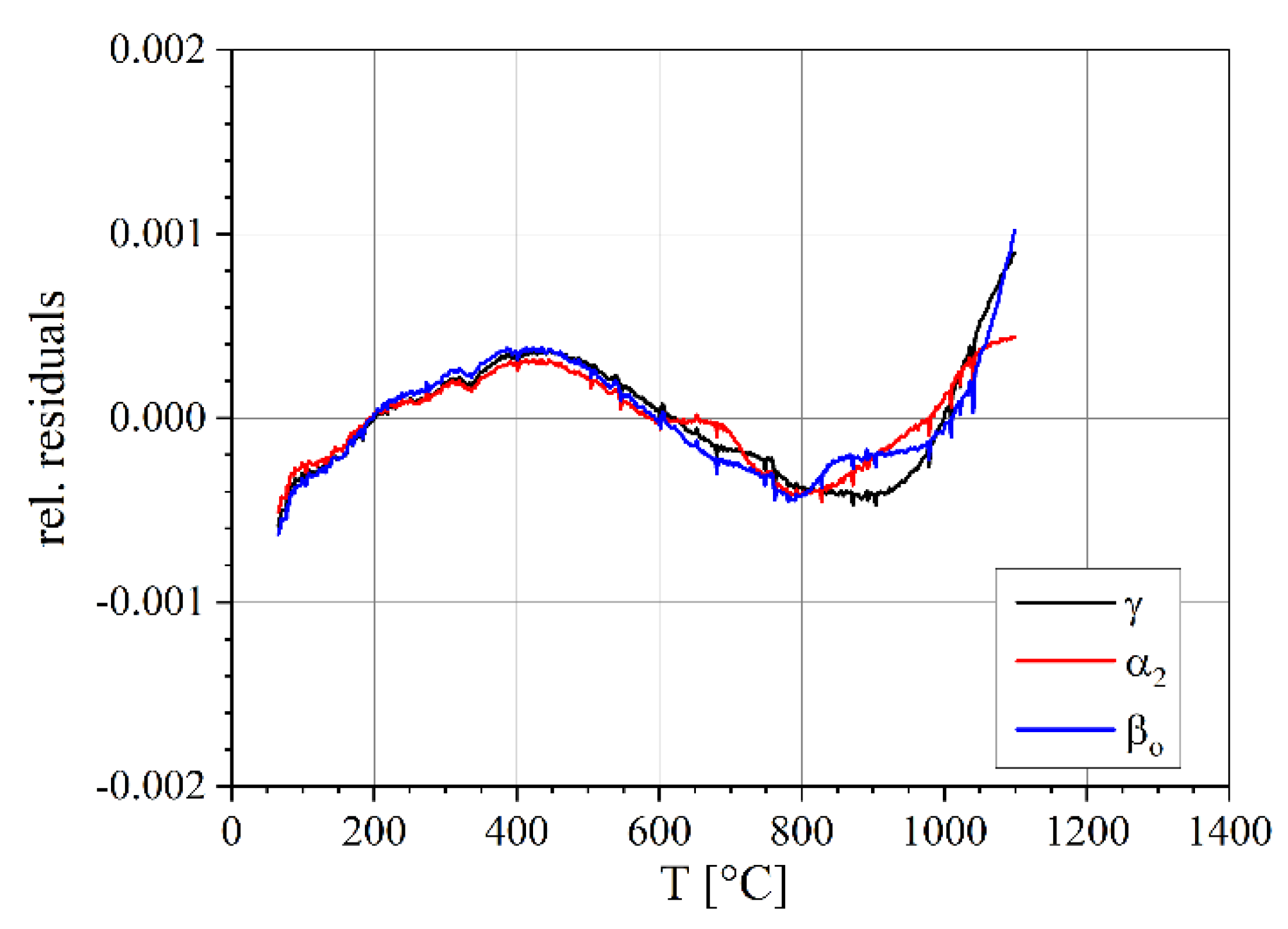
3.2. Lattice Parameters
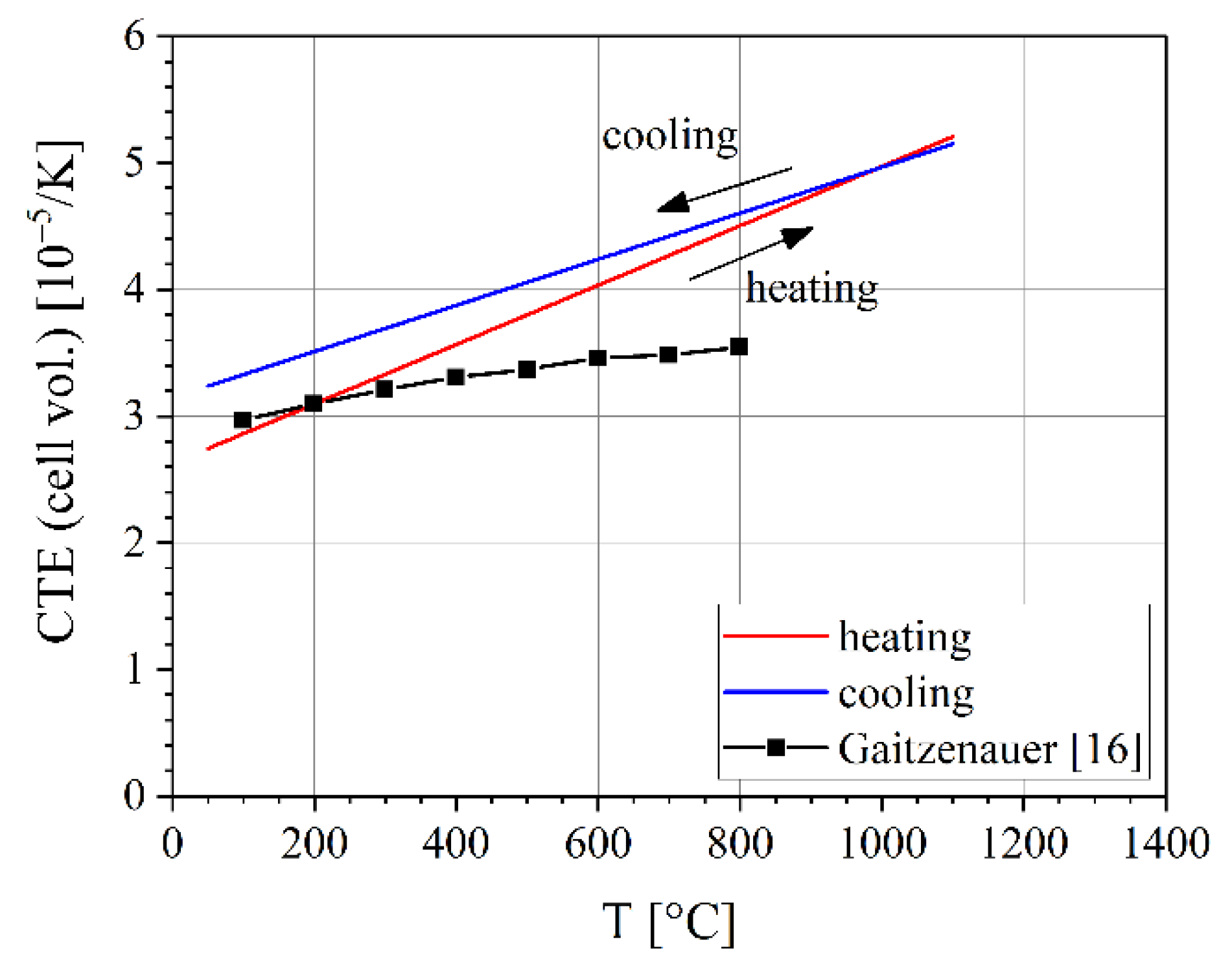
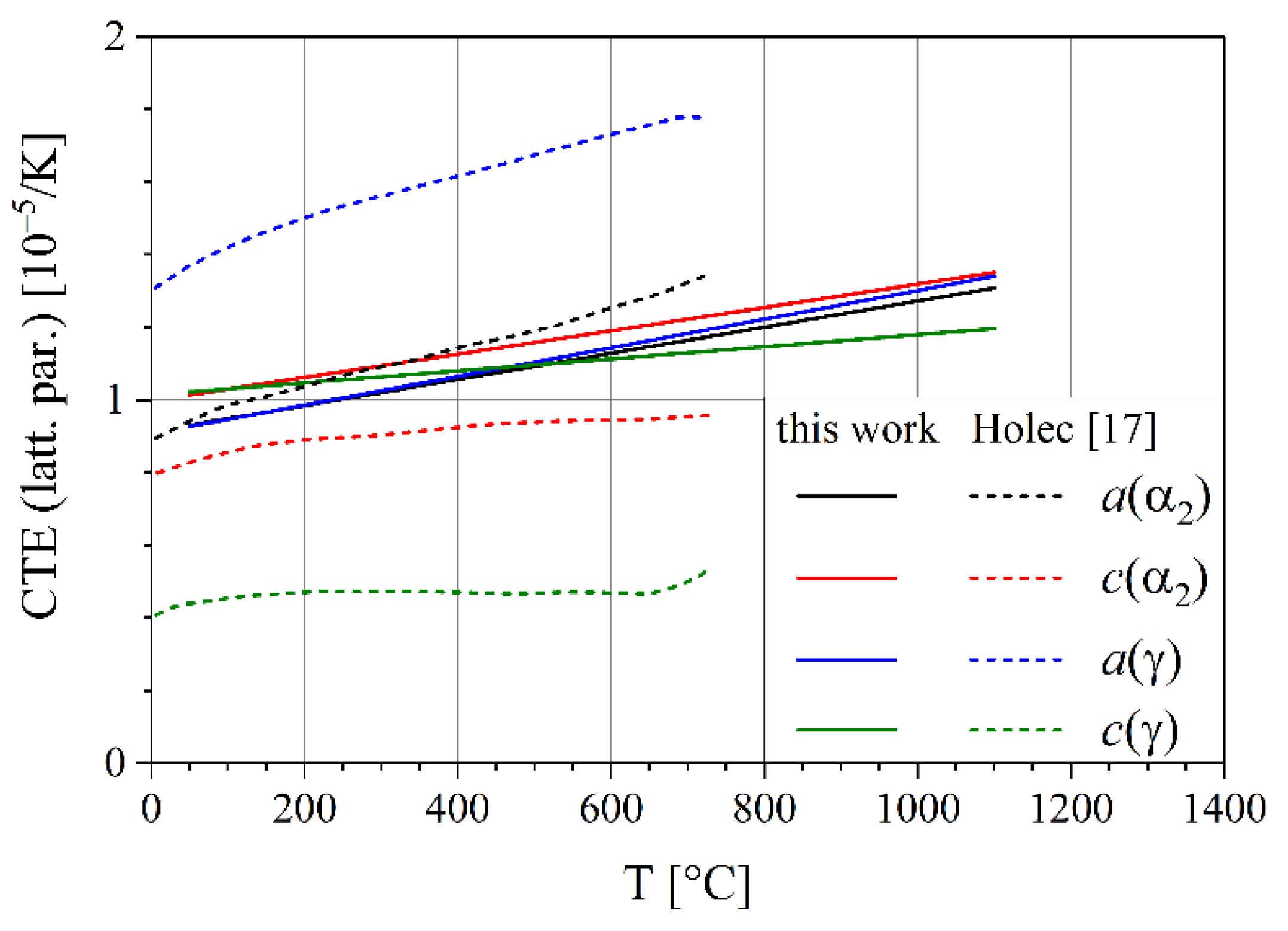
3.3. Thermal Expansion Coefficients
3.4. Importance of the CTE for Internal Stresses and Cracking
4. Summary
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bewlay, B.P.; Nag, S.; Suzuki, A.; Weimer, M.J. TiAl alloys in commercial aircraft engines. Mater. High Temp. 2016, 33, 549–559. [Google Scholar] [CrossRef]
- Habel, U.; Heutling, F.; Helm, D.; Kunze, C.; Smarsly, W.; Das, G.; Clemens, H. Forged Intermetallic γ-TiAl Based Alloy Low Pressure Turbine Blade in the Geared Turbofan. In Proceedings of the 13th World Conference on Titanium, San Diego, CA, USA, 16–20 August 2015; Venkatesh, V., Pilchak, A.L., Allison, J.E., Sreeramamurthy, A., Boyer, R., Christodoulou, J., Fraser, H.L., Imam, M.A., Kosaka, Y., Rack, H.J., et al., Eds.; Wiley-TMS: San Diego, CA, USA, 2016; pp. 1223–1227. [Google Scholar]
- Appel, F.; Paul, J.D.H.; Oehring, M. Gamma Titanium Aluminide Alloys; Wiley-VCH: Weinheim, Germany, 2011. [Google Scholar]
- Clemens, H.; Mayer, S. Intermetallic titanium aluminides in aerospace applications—Processing, microstructure and properties. Mater. High. Temp. 2016, 33, 560–570. [Google Scholar] [CrossRef]
- Mayer, S.; Erdely, P.; Fischer, F.D.; Holec, D.; Kastenhuber, M.; Klein, T.; Clemens, H. Intermetallic β-Solidifying γ-TiAl Based Alloys—From Fundamental Research to Application. Adv. Eng. Mat. 2017, 19, 1600735. [Google Scholar] [CrossRef]
- Huber, D.; Werner, R.; Clemens, H.; Stockinger, M. Influence of process parameter variation during thermo-mechanical processing of an intermetallic β-stabilized γ-TiAl based alloy. Mater. Charact. 2015, 109, 116–121. [Google Scholar] [CrossRef]
- Schmölzer, T.; Liss, K.-D.; Zickler, G.A.; Watson, I.J.; Droessler, L.M.; Wallgram, W.; Buslaps, T.; Studer, A.; Clemens, H. Phase fractions, transition and ordering temperatures in TiAl–Nb–Mo alloys: An in- and ex-situ study. Intermetallics 2010, 18, 1544–1552. [Google Scholar] [CrossRef]
- Liu, J.; Ventzke, V.; Staron, P.; Schell, N.; Kashaev, N.; Huber, N. Investigation of In Situ and Conventional Post-Weld Heat Treatments on Dual-Laser-Beam-Welded γ-TiAl-Based Alloy. Adv. Eng. Mat. 2012, 14, 923–927. [Google Scholar] [CrossRef]
- Kirby, R.K. Methods of Measuring Thermal Expansion. In Compendium of Thermophysical Property Measurement Methods; Maglić, K.D., Cezairliyan, A., Peletsky, V.E., Eds.; Springer: Boston, MA, USA, 1992. [Google Scholar]
- James, J.D.; Spittle, J.E.; Brown, S.G.R.; Evans, R.W. A Review of Measurement Techniques for the Thermal Expansion Coefficient of Metals and Alloys at Elevated Temperatures. Meas. Sci. Technol. 2001, 12, R1–R15. [Google Scholar] [CrossRef]
- He, Y.; Schwarz, R.B.; Darling, T.; Hundley, M.; Whang, S.H.; Wang, Z.M. Elastic constants and thermal expansion of single crystal γ-TiAl from 300 to 750 K. Mater. Sci. Eng. A 1997, 239–240, 157–163. [Google Scholar] [CrossRef]
- Zhang, W.J.; Reddy, B.V.; Deevi, S.C. Physical Properties of TiAl-Base Alloys. Scripta Mater. 2001, 45, 645–651. [Google Scholar] [CrossRef]
- Warlimont, H.; Martienssen, W. (Eds.) Springer Handbook of Materials Data; Springer: Berlin/Heidelberg, Germany, 2018. [Google Scholar]
- Shull, R.D.; Cline, J.P. High Temperature X-ray Diffractometry of Ti-Al Alloys. In Materials Chemistry at High Temperatures; Springer: Berlin/Heidelberg, Germany, 1990; pp. 95–117. [Google Scholar]
- Yeoh, L.A.; Liss, K.-D.; Bartels, A.; Chladil, H.; Avdeev, M.; Clemens, H.; Gerling, R.; Buslaps, T. In situ high-energy X-ray diffraction study and quantitative phase analysis in the α+ γ phase field of titanium aluminides. Scripta Mater. 2007, 57, 1145–1148. [Google Scholar] [CrossRef] [Green Version]
- Liss, K.-D.; Funakoshi, K.-I.; Dippenaar, R.; Higo, Y.; Shiro, A.; Reid, M.; Suzuki, H.; Shobu, T.; Akita, K. Hydrostatic compression behavior and high-pressure stabilized β-phase in γ-based titanium aluminide intermetallics. Metals 2016, 6, 165. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Dippenaar, R.; Shiro, A.; Shobu, T.; Higo, Y.; Reid, M.; Suzuki, H.; Akita, K.; Funakoshi, K.I.; Liss, K.-D. Lattice parameter evolution during heating of Ti-45Al-7.5Nb-0.25/0.5C alloys under atmospheric and high pressures. Intermetallics 2018, 102, 120–131. [Google Scholar] [CrossRef]
- Gaitzenauer, A.; Schenk, M.; Kuchling, W.; Clemens, H.; Voigt, P.; Hempel, R.; Mayer, S. Gefüge und Eigenschaften einer mehrphasigen intermetallischen Titanaluminidlegierung für innovative Leichtbauanwendungen. BHM 2013, 158, 113–117. [Google Scholar] [CrossRef]
- Holec, D.; Abdoshahi, N.; Mayer, S.; Clemens, H. Thermal Expansion and Other Thermodynamic Properties of α2-Ti3Al and γ-TiAl Intermetallic Phases from First Principles Methods. Materials 2019, 12, 1292. [Google Scholar] [CrossRef] [Green Version]
- Clemens, H.; Wallgram, W.; Kremmer, S.; Güther, V.; Otto, A.; Bartels, A. Design of Novel β-Solidifying TiAl Alloys with Adjustable β/B2-Phase Fraction and Excellent Hot-Workability. Adv. Eng. Mater. 2008, 10, 707–713. [Google Scholar] [CrossRef]
- Güther, V.; Allen, M.; Klose, J.; Clemens, H. Metallurgical processing of titanium aluminides on industrial scale. Intermetallics 2018, 103, 12–22. [Google Scholar] [CrossRef]
- Gaitzenauer, A.; Müller, M.; Clemens, H.; Voigt, P.; Hempel, R.; Mayer, S. Optimized Hot-forming of an Intermetallic Multi-phase γ-TiAl Based Alloy. Mater. Res. Soc. Symp. Proc. 2013, 1516, 29–34. [Google Scholar] [CrossRef]
- Clemens, H.; Mayer, S. Intermetallic Titanium Aluminides as Innovative High Temperature Lightweight Structural Materials—How Materialographic Methods Have Contributed to Their Development. Pract. Metallogr. 2015, 52, 691–720. [Google Scholar] [CrossRef]
- Schell, N.; King, A.; Beckmann, F.; Fischer, T.; Müller, M.; Schreyer, A. The High Energy Materials Science Beamline (HEMS) at PETRA III. Mater. Sci. Forum 2014, 772, 57–61. [Google Scholar] [CrossRef] [Green Version]
- Stark, A.; Oehring, M.; Pyczak, F.; Schreyer, A. In Situ Observation of Various Phase Transformation Paths in Nb-Rich TiAl Alloys during Quenching with Different Rates. Adv. Eng. Mater. 2011, 13, 700–704. [Google Scholar] [CrossRef]
- Hammersley, A.P. FIT2D: An Introduction and Overview. In ESRF Internal Report ESRF97HA02T; Addison-Wesley Publishing Company: Grenoble, France, 1997. [Google Scholar]
- Hammersley, A.P.; Svensson, S.O.; Hanfland, M.; Fitch, A.N.; Häusermann, D. Two-Dimensional Detector Software: From Real Detector to Idealised Image or Two-Theta Scan. High Press. Res. 1996, 14, 235–248. [Google Scholar] [CrossRef]
- Lutterotti, L.; Bortolotti, M.; Ischia, G.; Lonardelli, I.; Wenk, H.-R. Rietveld Texture Analysis from Diffraction Images. Z. Kristallogr. Suppl. 2007, 26, 125–130. [Google Scholar] [CrossRef]
- Clemens, H.; Glatz, W.; Schretter, P.; Klassen, M.; Schubert, E.; Sepold, G.; Fleischer, T.; Schrock, H.W.; Franke, R. Intermetallic Gamma Titanium Aluminides—Potential Candidates for Spacecraft Structures. In Proceedings of the Spacecraft Structures Materials and Mechanical Testing, ESA, SP-386, Noordwijk, The Netherlands, 27–29 March 1996; ESA Publications Division: Noordwijk, The Netherlands, 1996; Volume 3, pp. 1297–1304, ISBN 92-9092-227-3. [Google Scholar]


| Phase | a [Å] | c [Å] | V [Å3] |
|---|---|---|---|
| γ | 4.0160(7) | 4.0560(1) | 65.416 |
| α2 | 5.7473(3) | 4.6333(5) | 132.540 |
| βo | 3.2103(1) | - | 33.085 |
| Author | Ref. | Alloy, Phase | T (°C) | CTE (10–5 K–1) |
|---|---|---|---|---|
| Zhang et al. | [12] | Ti-50Al | RT | 3.3 |
| “ | 600 | 4.2 | ||
| Ti-47Al-1.5(Nb,Cr,Si) | 200 | 2.9 | ||
| “ | 800 | 3.5 | ||
| Ti-47Al-4(Nb,W,B) (“CTI-8”) | 100 | 2.6 | ||
| “ | 900 | 3.5 | ||
| Handbook of materials data | [13] | Ti-50Al | 27 | 3.3 |
| “ | 850 | 3.9 | ||
| Ti-48Al-2Cr | 100 | 2.7 | ||
| “ | 700 | 3.5 | ||
| Li et al. | [17] | Ti-45Al-7.5Nb-(0.25,0.5)C, γ phase | 200–780 | 3.67 |
| Ti-45Al-7.5Nb-(0.25,0.5)C, αα phase | 200–780 | 3.62 | ||
| Gaitzenauer et al. | [18] | Ti-43.5Al-4Nb-1Mo-0.1B (“TNM”) | 100–800 | 3.0–3.5 |
| this work | Ti-43.5Al-4Nb-1Mo-0.1B (“TNM”) | 50–1100 | 3.0–5.2 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Staron, P.; Stark, A.; Schell, N.; Spoerk-Erdely, P.; Clemens, H. Thermal Expansion of a Multiphase Intermetallic Ti-Al-Nb-Mo Alloy Studied by High-Energy X-ray Diffraction. Materials 2021, 14, 727. https://doi.org/10.3390/ma14040727
Staron P, Stark A, Schell N, Spoerk-Erdely P, Clemens H. Thermal Expansion of a Multiphase Intermetallic Ti-Al-Nb-Mo Alloy Studied by High-Energy X-ray Diffraction. Materials. 2021; 14(4):727. https://doi.org/10.3390/ma14040727
Chicago/Turabian StyleStaron, Peter, Andreas Stark, Norbert Schell, Petra Spoerk-Erdely, and Helmut Clemens. 2021. "Thermal Expansion of a Multiphase Intermetallic Ti-Al-Nb-Mo Alloy Studied by High-Energy X-ray Diffraction" Materials 14, no. 4: 727. https://doi.org/10.3390/ma14040727
APA StyleStaron, P., Stark, A., Schell, N., Spoerk-Erdely, P., & Clemens, H. (2021). Thermal Expansion of a Multiphase Intermetallic Ti-Al-Nb-Mo Alloy Studied by High-Energy X-ray Diffraction. Materials, 14(4), 727. https://doi.org/10.3390/ma14040727






