Electrochemical Detection of Endosulfan Using an AONP-PANI-SWCNT Modified Glassy Carbon Electrode
Abstract
1. Introduction
2. Experimental
2.1. Materials and Reagents
2.2. Preparation of AONPs, PANI and fSWCNTs
2.3. Preparation of Nanocomposites and Electrode Modification
2.4. Electrochemical Study
3. Results and Discussion
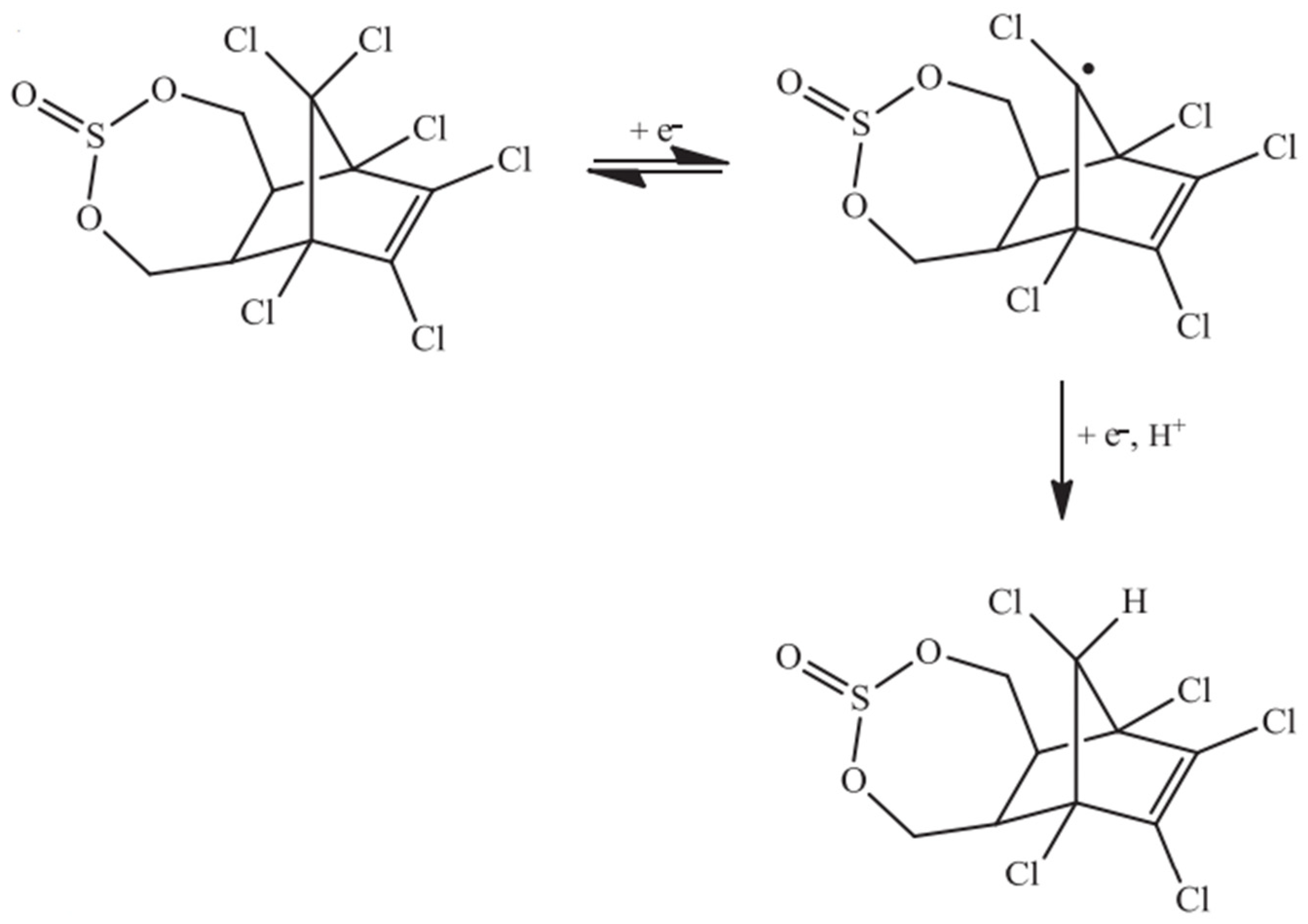
3.1. Electrocatalytic Reduction of Endosulfan
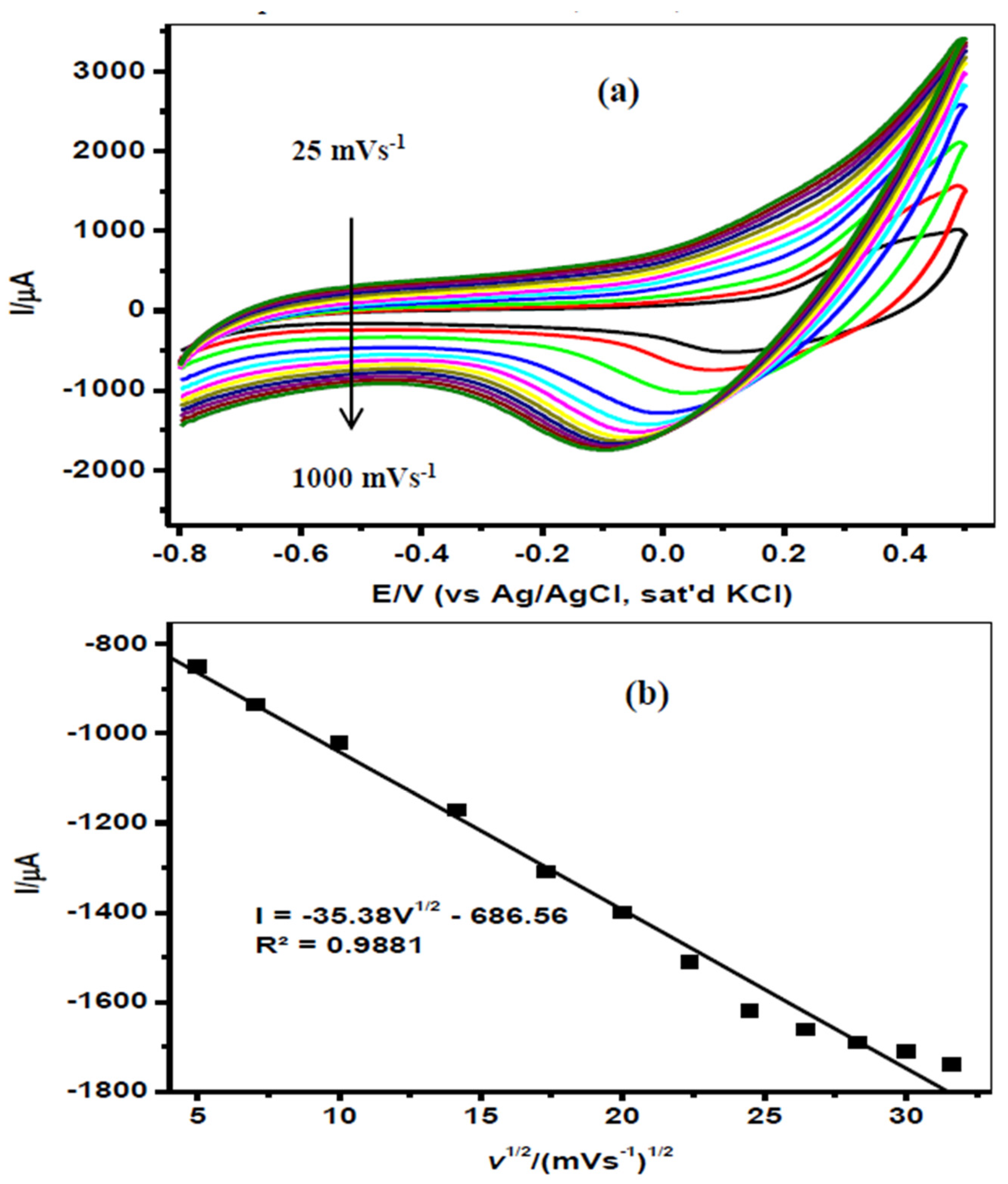
3.2. Effect of Scan Rate on Endosulfan Reduction
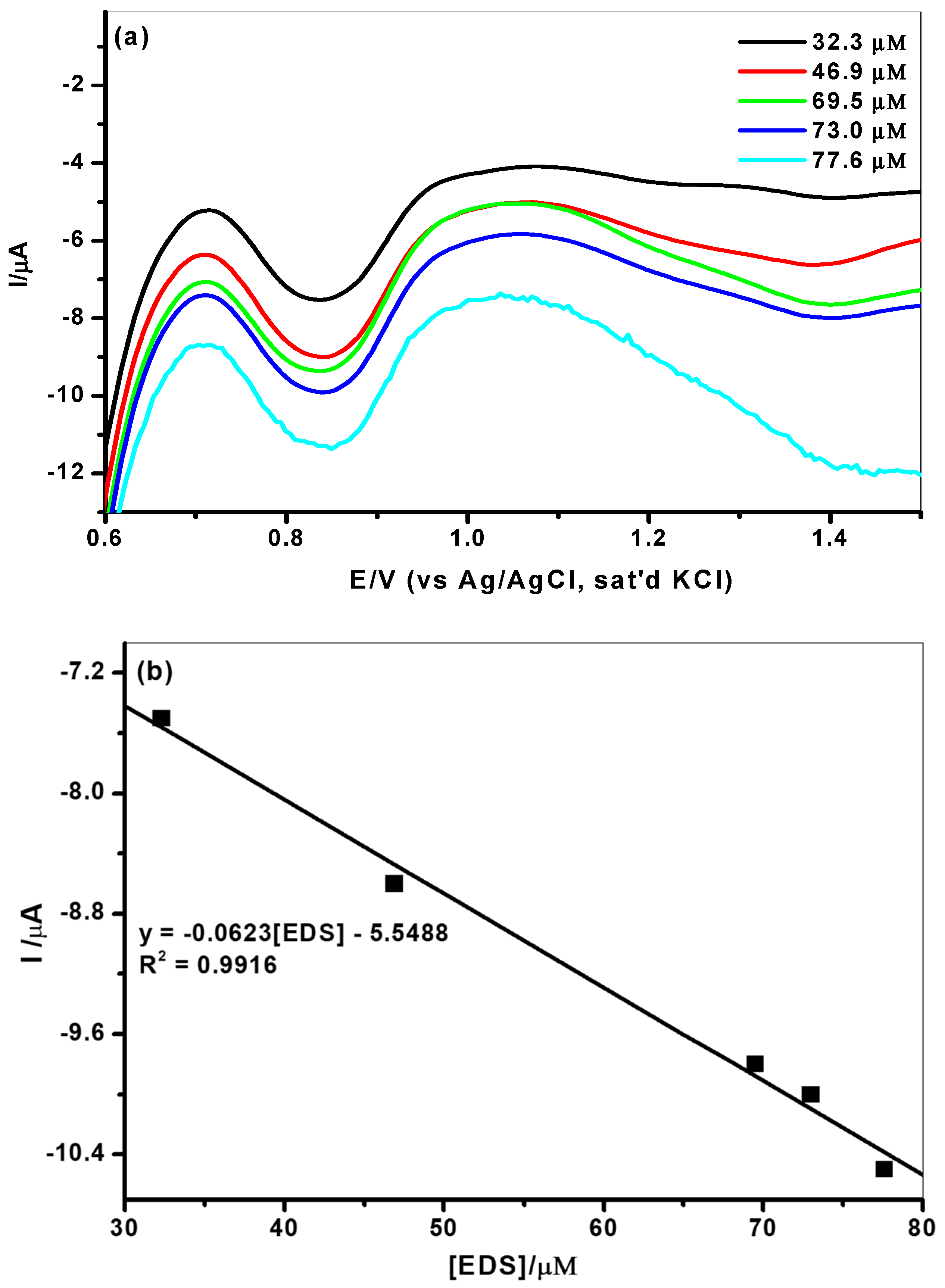
3.3. Electroanalysis of Endosulfan
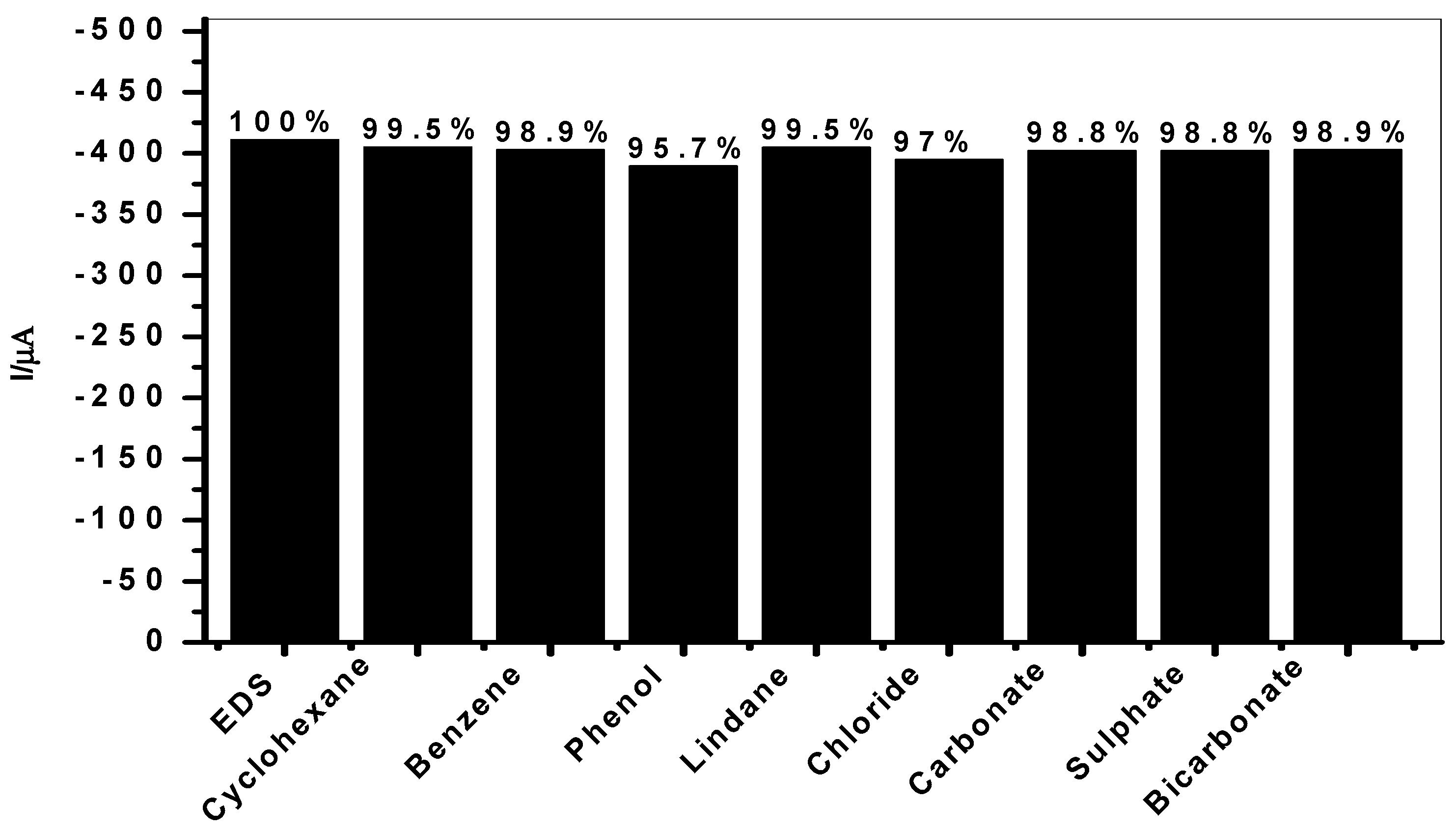
3.4. Endosulfan Interference Studies
3.5. Real Sample Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kumar, M.; Philip, L. Enrichment and Isolation of a Mixed Bacterial Culture for Complete Mineralization of Endosulfan. J. Environ. Sci. Health B 2006, 41, 81–96. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Wang, S.; Liu, J.; Song, D. An Electrochemical Immunosensor Based on Chemical Assembly of Vertically Aligned Carbon Nanotubes on Carbon Substrates for Direct Detection of the Pesticide Endosulfan in Environmental Water. Anal. Chem. 2012, 84, 3921–3928. [Google Scholar] [CrossRef]
- Saiyed, H.; Dewan, A.; Bhatnagar, V.; Shenoy, U.; Shenoy, R.; Rajmohan, H.; Patel, K.; Kashyap, R.; Kulkarni, P.; Rajan, B.; et al. Effect of endosulfan on male reproductive development. Environ. Health Perspect. 2003, 111, 1958–1962. [Google Scholar] [CrossRef]
- Schmidt, W.F.; Hapeman, C.J.; Fettinger, J.C.; Rice, C.P.; Bilboulian, S. Structure and Asymmetry in the Isomeric Conversion of β- to α-Endosulfan. J. Agric. Food Chem. 1997, 45, 1023–1026. [Google Scholar] [CrossRef]
- Deger, A.B.; Gremm, T.J.; Frimmel, F.H.; Mendez, L. Optimization and application of SPME for the gas chromatographic determination of endosulfan and its major metabolites in the ng L−1 range in aqueous solutions. Anal. Bioanal. Chem. 2003, 376, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Han, J.; Liu, Z.; Qu, L.; Gao, Z. Rapid detection of endosulfan by a molecularly imprinted polymer microsphere modified quartz crystal microbalance. Anal. Methods 2013, 5, 4442–4447. [Google Scholar] [CrossRef]
- Desalegn, B.; Takasuga, T.; Harada, K.H.; Hitomi, T.; Fujii, Y.; Yang, H.-R.; Wang, P.; Senevirathna, S.; Koizumi, A. Historical trends in human dietary intakes of endosulfan and toxaphene in China, Korea and Japan. Chemosphere 2011, 83, 1398–1405. [Google Scholar] [CrossRef]
- Ribeiro, F.W.P.; Oliveira, T.M.B.F.; da Silva, F.L.F.; Mendonça, G.L.F.; Homem-de-Mello, P.; Becker, H.; de Lima-Neto, P.; Correia, A.N.; Freire, V.N. Sensitive voltammetric responses and mechanistic insights into the determination of residue levels of endosulfan in fresh foodstuffs and raw natural waters. Microchem. J. 2013, 110, 40–47. [Google Scholar] [CrossRef]
- Albero, B.; Sánchez-Brunete, C.; Tadeo, J.L. Determination of endosulfan isomers and endosulfan sulfate in tomato juice by matrix solid-phase dispersion and gas chromatography. J. Chromatogr. A 2003, 1007, 137–143. [Google Scholar] [CrossRef]
- Sreekumaran Nair, A.; Tom, R.T.; Pradeep, T. Detection and extraction of endosulfan by metal nanoparticles. J. Environ. Monitor. 2003, 5, 363–365. [Google Scholar] [CrossRef]
- Vidal, J.M.; Frias, M.M.; Frenich, A.G.; Olea-Serrano, F.; Olea, N. Trace determination of a-and b-endosulfan and three metabolites in human serum by gas chromatography electron capture detection and gas chromatography tandem mass spectrometry. Rapid Commun. Mass Spectrom. 2000, 14, 939–946. [Google Scholar] [CrossRef]
- Bebe, F.N.; Panemangalore, M. Exposure to Low Doses of Endosulfan and Chlorpyrifos Modifies Endogenous Antioxidants in Tissues of Rats. J. Environ. Sci. Health B 2003, 38, 349–363. [Google Scholar] [CrossRef]
- Lu, Y.; Morimoto, K.; Takeshita, T.; Takeuchi, T.; Saito, T. Genotoxic effects of alpha-endosulfan and beta-endosulfan on human HepG2 cells. Environ. Health Perspect. 2000, 108, 559–561. [Google Scholar] [CrossRef] [PubMed]
- Muthukumar, M.; Sudhakar Reddy, K.; Narendra Reddy, C.; Kondal Reddy, K.; Gopala Reddy, A.; Jagdishwar Reddy, D.; Kondaiah, N. Detection of cyclodiene pesticide residues in buffalo meat and effect of cooking on residual level of endosulfan. J. Food Sci. Technol. 2010, 47, 325–329. [Google Scholar] [CrossRef] [PubMed][Green Version]
- De León-Rodríguez, L.M.; Basuil-Tobias, D.A. Testing the possibility of using UV–vis spectrophotometric techniques to determine non-absorbing analytes by inclusion complex competition in cyclodextrins. Anal. Chim. Acta 2005, 543, 282–290. [Google Scholar] [CrossRef]
- Vagi, M.C.; Petsas, A.S.; Kostopoulou, M.N.; Karamanoli, M.K.; Lekkas, T.D. Determination of organochlorine pesticides in marine sediments samples using ultrasonic solvent extraction followed by GC/ECD. Desalination 2007, 210, 146–156. [Google Scholar] [CrossRef]
- Kalyoncu, L.; Agca, İ.; Aktumsek, A. Some organochlorine pesticide residues in fish species in Konya, Turkey. Chemosphere 2009, 74, 885–889. [Google Scholar] [CrossRef] [PubMed]
- Kafilzadeh, F. Assessment of Organochlorine Pesticide Residues in Water, Sediments and Fish from Lake Tashk, Iran. Achiev. Life Sci. 2015, 9, 107–111. [Google Scholar] [CrossRef]
- Bidari, A.; Ganjali, M.R.; Norouzi, P.; Hosseini, M.R.M.; Assadi, Y. Sample preparation method for the analysis of some organophosphorus pesticides residues in tomato by ultrasound-assisted solvent extraction followed by dispersive liquid–liquid microextraction. Food Chem. 2011, 126, 1840–1844. [Google Scholar] [CrossRef] [PubMed]
- Barnhoorn, I.E.J.; van Dyk, J.C.; Genthe, B.; Harding, W.R.; Wagenaar, G.M.; Bornman, M.S. Organochlorine pesticide levels in Clarias gariepinus from polluted freshwater impoundments in South Africa and associated human health risks. Chemosphere 2015, 120, 391–397. [Google Scholar] [CrossRef]
- Siddique, T.; Zahir, Z.A.; Frankenberger, W.T. Reversed-Phase Liquid Chromatographic Method for Analysis of Endosulfan and Its Major Metabolites. J. Liq. Chromatogr. Relat. Technol. 2003, 26, 1069–1082. [Google Scholar] [CrossRef]
- Rana, S.M.; Asi, M.R.; Niazi, F.; Sultana, S.; Ghazala; Al-Ghanim, K.A. Determination of organochlorine and nitrogen containing pesticide residues in Labeo rohita. Toxicol. Environ. Chem. 2011, 93, 1851–1855. [Google Scholar] [CrossRef]
- Parrilla, P.; Martínez Vidal, J.L. Determination of Pesticide Residues in Water Using LLE or SPE and HPLC/DAD Detection. Anal. Lett. 1997, 30, 1719–1738. [Google Scholar] [CrossRef]
- Lee, N.; Skerritt, J.H.; McAdam, D.P. Hapten synthesis and development of ELISAs for detection of endosulfan in water and soil. J. Agric. Food Chem. 1995, 43, 1730–1739. [Google Scholar] [CrossRef]
- Aragay, G.; Pino, F.; Merkoçi, A. Nanomaterials for Sensing and Destroying Pesticides. Chem. Rev. 2012, 112, 5317–5338. [Google Scholar] [CrossRef]
- Prabu, H.G.; Manisankar, P. Determination of endosulfan by stripping voltammetry. Analyst 1994, 119, 1867–1873. [Google Scholar] [CrossRef]
- Manisankar, P.; Viswanathan, S.; Prabu, H.G. Electroanalysis of Endosulfan and o -Chlorophenol in Polypyrrole Coated Glassy Carbon Electrode. Int. J. Environ. Anal. Chem. 2002, 82, 331–340. [Google Scholar] [CrossRef]
- Priyantha, N.; Thirana, S.M. Comparative Electrochemical Activity of the Insecticide, Endosulfan, at Bare and 5, 10, 15, 20-Tertraphenylporphyrinato-Iron (IH) Chloride-Modified Glassy Carbon Electrodes. Ceylon J. Sci. Biol. Sci. 1999, 6, 38–46. [Google Scholar]
- Rathnakumar, S.S.; Noluthando, K.; Kulandaiswamy, A.J.; Rayappan, B.J.B.; Kasinathan, K.; Kennedy, J.; Maaza, M. Stalling behaviour of chloride ions: A non-enzymatic electrochemical detection of α-Endosulfan using CuO interface. Sens. Actuators B Chem. 2019, 293, 100–106. [Google Scholar] [CrossRef]
- Köksoy, B.; Akyüz, D.; Şenocak, A.; Durmuş, M.; Demirbas, E. Sensitive, Simple and Fast Voltammetric Determination of Pesticides in Juice Samples by Novel BODIPY-Phthalocyanine-SWCNT Hybrid Platform. Food Chem. Toxicol. 2020, 147, 111886. [Google Scholar] [CrossRef]
- Chin, H.S.; Cheong, K.Y.; Razak, K.A. Review on oxides of antimony nanoparticles: Synthesis, properties, and applications. J. Mater. Sci. 2010, 45, 5993–6008. [Google Scholar] [CrossRef]
- Deng, Z.; Tang, F.; Chen, D.; Meng, X.; Cao, L.; Zou, B. A Simple Solution Route to Single-Crystalline Sb2O3 Nanowires with Rectangular Cross Sections. J. Phys. Chem. B 2006, 110, 18225–18230. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Jiang, L.; Liu, Y.; Tang, D.; Liu, F.; Lai, Y. Facile synthesis and photoelectrochemical characterization of Sb2O3 nanoprism arrays. J. Alloy. Compd. 2017, 727, 469–474. [Google Scholar] [CrossRef]
- Jha, A.K.; Prasad, K.; Prasad, K. A green low-cost biosynthesis of Sb2O3 nanoparticles. Biochem. Eng. J. 2009, 43, 303–306. [Google Scholar] [CrossRef]
- Zeng, D.W.; Xie, C.S.; Zhu, B.L.; Song, W.L. Characteristics of Sb2O3 nanoparticles synthesized from antimony by vapor condensation method. Mater. Lett. 2004, 58, 312–315. [Google Scholar] [CrossRef]
- Moraes, F.C.; Silva, T.A.; Cesarino, I.; Machado, S.A.S. Effect of the surface organization with carbon nanotubes on the electrochemical detection of bisphenol A. Sens. Actuators B Chem. 2013, 177, 14–18. [Google Scholar] [CrossRef]
- Notarianni, M.; Liu, J.; Vernon, K.; Motta, N. Synthesis and applications of carbon nanomaterials for energy generation and storage. Beilstein J. Nanotechnol. 2016, 7, 149–196. [Google Scholar] [CrossRef]
- Wei, J.; Yang, D.; Chen, H.; Gao, Y.; Li, H. Stripping voltammetric determination of mercury(II) based on SWCNT-PhSH modified gold electrode. Sens. Actuators B Chem. 2014, 190, 968–974. [Google Scholar] [CrossRef]
- Masibi, K.K.; Fayemi, O.E.; Adekunle, A.S.; Sherif, E.-S.M.; Ebenso, E.E. Electrocatalysis of Lindane Using Antimony Oxide Nanoparticles Based-SWCNT/PANI Nanocomposites. Front. Chem. 2018, 423, 1–16. [Google Scholar] [CrossRef]
- Adekunle, A.S.; Lebogang, S.; Gwala, P.L.; Tsele, T.P.; Olasunkanmi, L.O.; Esther, F.O.; Boikanyo, D.; Mphuthi, N.; Oyekunle, J.A.; Ogunfowokan, A.O. Electrochemical response of nitrite and nitric oxide on graphene oxide nanoparticles doped with Prussian blue (PB) and Fe2O3 nanoparticles. RSC Adv. 2015, 5, 27759–27774. [Google Scholar] [CrossRef]
- Silwana, B.; van der Horst, C.; Iwuoha, E.; Somerset, V. Synthesis, characterisation and electrochemical evaluation of reduced graphene oxide modified antimony nanoparticles. Thin Solid Film. 2015, 592, 124–134. [Google Scholar] [CrossRef]
- Elgrishi, N.; Rountree, K.J.; McCarthy, B.D.; Rountree, E.S.; Eisenhart, T.T.; Dempsey, J.L. A Practical Beginner’s Guide to Cyclic Voltammetry. J. Chem. Educ. 2018, 95, 197–206. [Google Scholar] [CrossRef]
- Hegde, R.N.; Swamy, K.; Sherigara, B.; Nandibewoor, S.T. Electro-oxidation of Atenolol at a Glassy Carbon Electrode. Int. J. Electrochem. Sci. 2008, 3, 302–314. [Google Scholar]
- Singh, D.; Sarat Singh, N. Endosulfan a Cyclodiene Organochlorine Pesticide: Possible Pathways of Its Biodegradation. In Microbe-Induced Degradation of Pesticides; Springer: Cham, Switzerland, 2017; pp. 105–130. [Google Scholar]
- Adekunle, A.S.; Pillay, J.; Ozoemena, K.I. Probing the electrochemical behaviour of SWCNT–cobalt nanoparticles and their electrocatalytic activities towards the detection of nitrite at acidic and physiological pH conditions. Electrochim. Acta 2010, 55, 4319–4327. [Google Scholar] [CrossRef]
- Bow, Y.; Sutriyono, E.; Nasir, S.; Iskandar, I. Molecularly Imprinted Polymers (MIP) Based Electrochemical Sensor for Detection of Endosulfan Pesticide. Int. J. Adv. Sci. Eng. Inf. Technol. 2017, 7, 662–668. [Google Scholar] [CrossRef][Green Version]
- El Bakouri, H.; Jose, M.; Palacios-Santander, J.; Cubillana-Aguilera, L.; Ouassini, A.; Naranjo-Rodríguez, I.; Hidalgo-Hidalgo de Cisneros, J.L. Electrochemical analysis of endosulfan using a C18-modified carbon-paste electrode. Chemosphere 2005, 60, 1565–1571. [Google Scholar] [CrossRef]

| LoD Values Compared with Other Studies | ||||
|---|---|---|---|---|
| Composite Electrode | LoD (µM) | Linear Range (µM) | Sensitivity (µA µM−1) | Reference |
| MIP | 20 | 20–120 | - | [46] |
| HMDE | 0.297 | 0.154–0.157 | 0.0188 | [8] |
| C18/CPE | 9.83 × 10−6 | - | - | [47] |
| GCE-Ph-NH2/SWCNT/PEG/FDMA/endosulfan hapten/antiendosulfan IgG | 0.025 | 0.025–49.2 | - | [2] |
| GCE-AONPs-PANI-SWCNT | 6.8 | 32.3–77.6 | 0.0623 | Present study |
| Sample | Compound | Added (µM) | Found a (µM) | Recovery (%) | RSD (%) |
|---|---|---|---|---|---|
| River water | EDS | 20 | 19.1 | 95.5 | 1.23 |
| 40 | 40.2 | 100.5 | 1.08 | ||
| 80 | 83.3 | 104 | 2.18 | ||
| 100 | 97.5 | 97.5 | 1.06 | ||
| Tap water | EDS | 46 | 42.7 | 92.9 | 1.87 |
| 96 | 96.1 | 100.1 | 4.07 | ||
| 146 | 145.9 | 99.9 | 1.19 | ||
| 196 | 190.0 | 96.9 | 2.52 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Masibi, K.K.; Fayemi, O.E.; Adekunle, A.S.; Al-Mohaimeed, A.M.; Fahim, A.M.; Mamba, B.B.; Ebenso, E.E. Electrochemical Detection of Endosulfan Using an AONP-PANI-SWCNT Modified Glassy Carbon Electrode. Materials 2021, 14, 723. https://doi.org/10.3390/ma14040723
Masibi KK, Fayemi OE, Adekunle AS, Al-Mohaimeed AM, Fahim AM, Mamba BB, Ebenso EE. Electrochemical Detection of Endosulfan Using an AONP-PANI-SWCNT Modified Glassy Carbon Electrode. Materials. 2021; 14(4):723. https://doi.org/10.3390/ma14040723
Chicago/Turabian StyleMasibi, Kgotla K., Omolola E. Fayemi, Abolanle S. Adekunle, Amal M. Al-Mohaimeed, Asmaa M. Fahim, Bhekie B. Mamba, and Eno E. Ebenso. 2021. "Electrochemical Detection of Endosulfan Using an AONP-PANI-SWCNT Modified Glassy Carbon Electrode" Materials 14, no. 4: 723. https://doi.org/10.3390/ma14040723
APA StyleMasibi, K. K., Fayemi, O. E., Adekunle, A. S., Al-Mohaimeed, A. M., Fahim, A. M., Mamba, B. B., & Ebenso, E. E. (2021). Electrochemical Detection of Endosulfan Using an AONP-PANI-SWCNT Modified Glassy Carbon Electrode. Materials, 14(4), 723. https://doi.org/10.3390/ma14040723










