Effect of a Nitrite/Nitrate-Based Accelerator on the Strength Development and Hydrate Formation in Cold-Weather Cementitious Materials
Abstract
1. Introduction
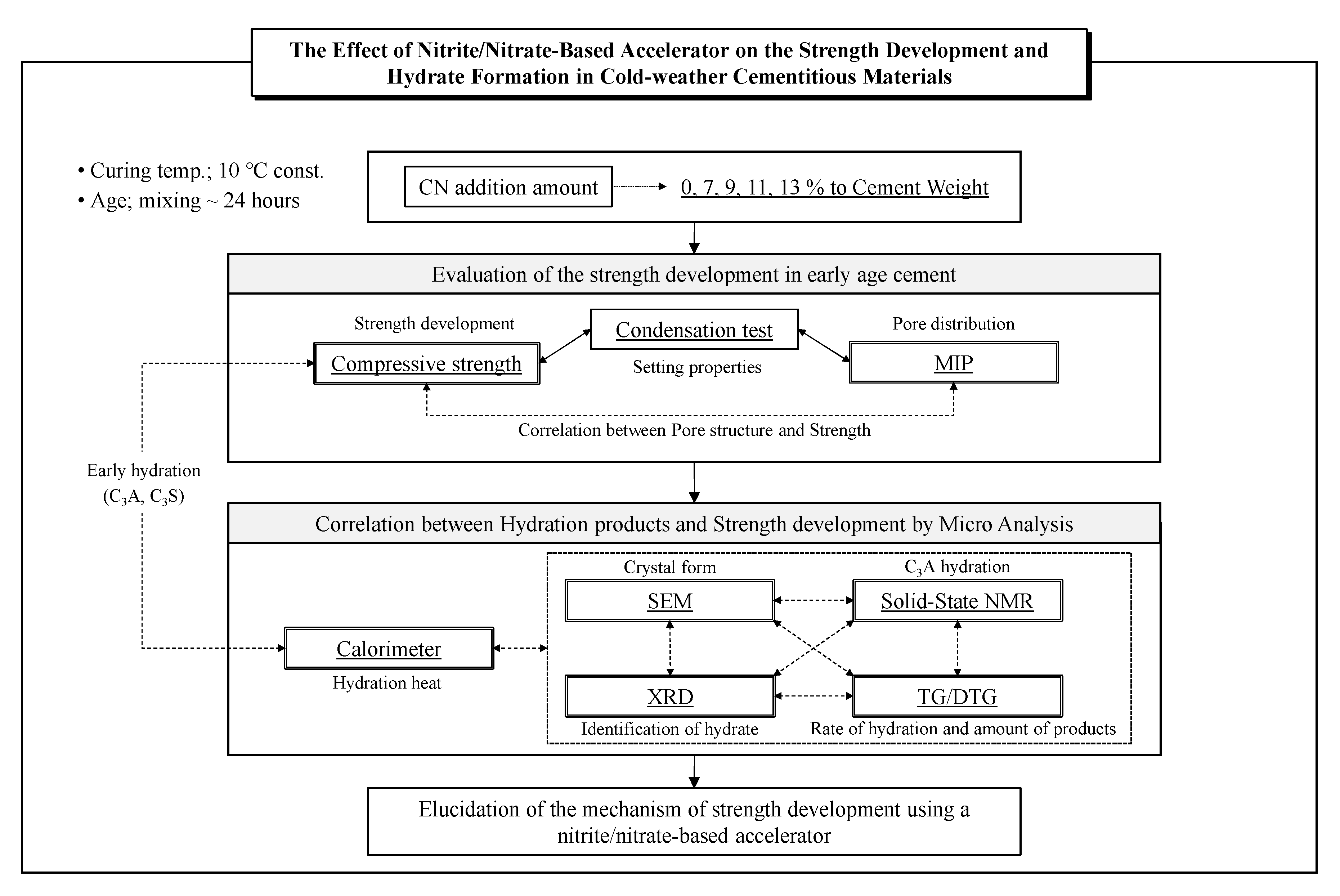
2. Experimental Overview
2.1. Materials and Procedures
2.2. Compressive Strength
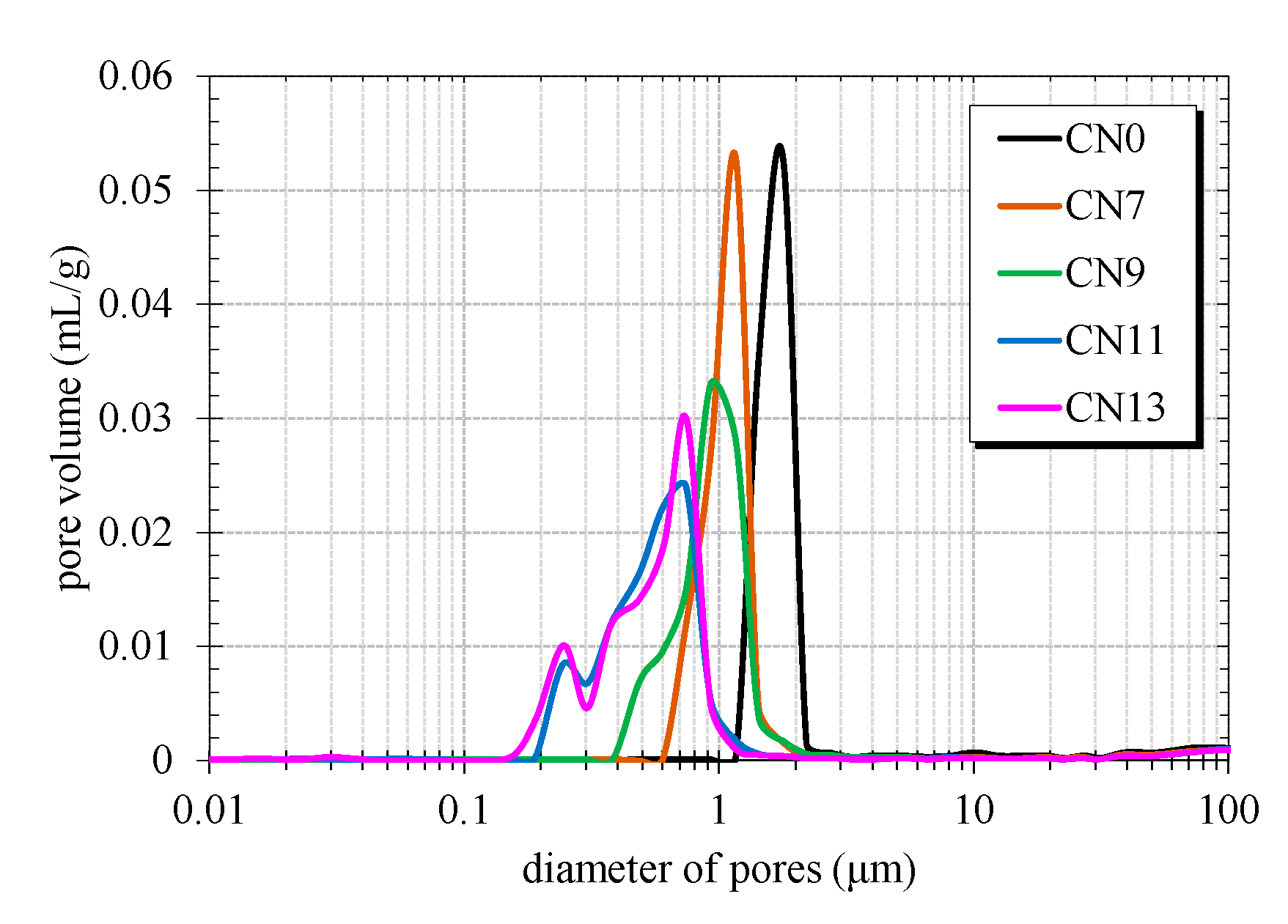
2.3. Mercury Intrusion Porosimetry (MIP)
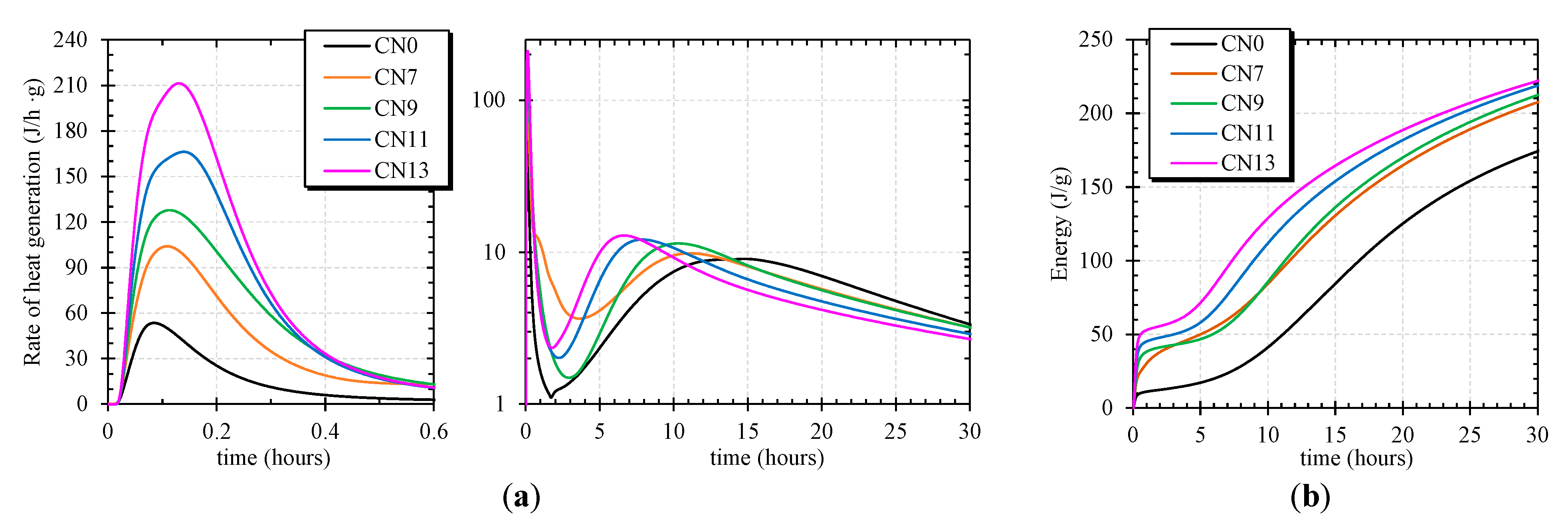
2.4. Calorimeter
2.5. Condensation Test
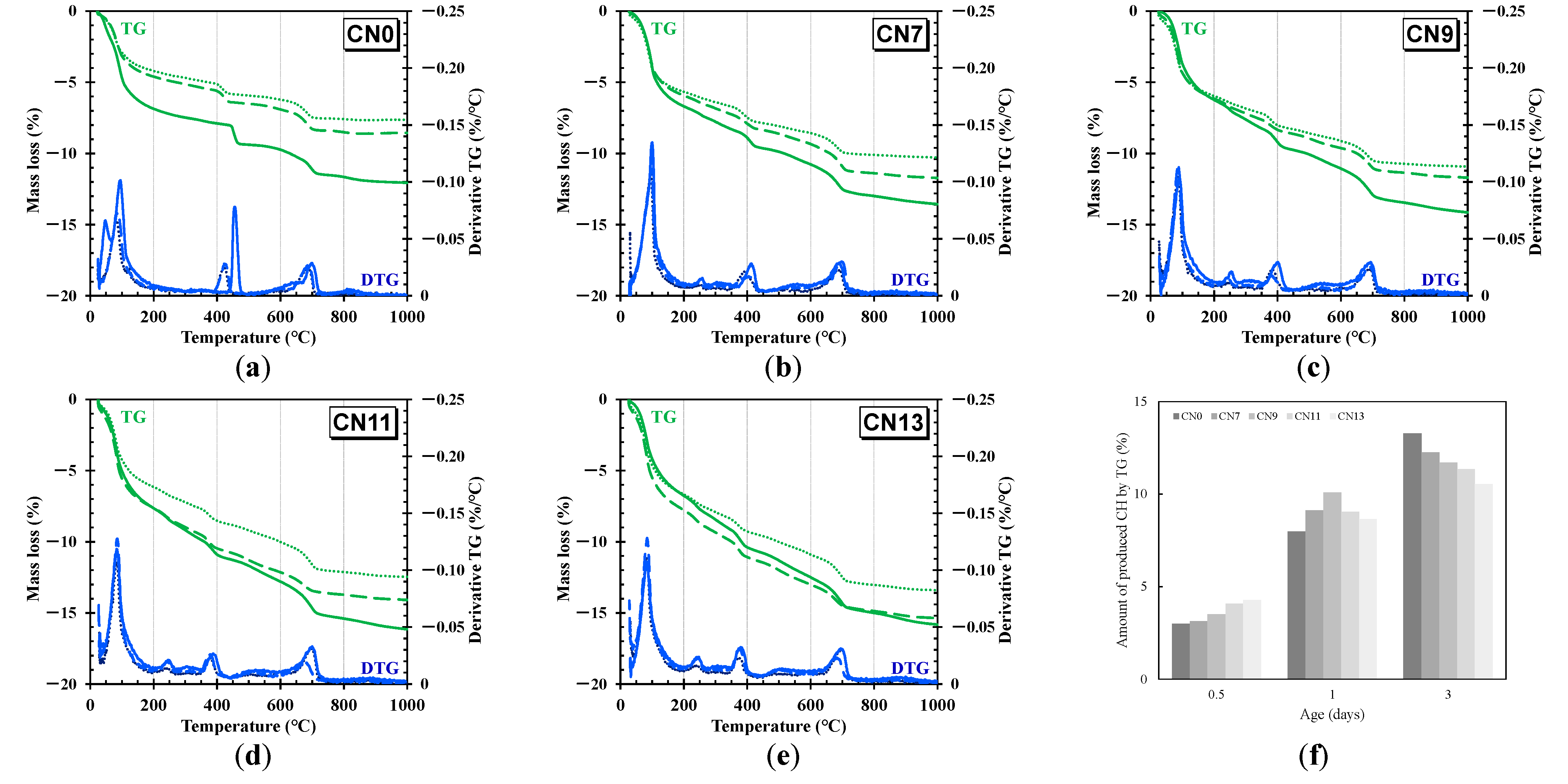
2.6. Thermogravimetric Differential Thermal Analysis
2.7. X-ray Diffraction
2.8. Solid-State Nuclear Magnetic Resonance
2.9. Scanning Electron Microscope (SEM)
3. Results and Discussion
3.1. Evaluation of the Strength Development in the Early Age Group
3.2. Effect of Calcium Nitrite on Setting Characteristics
3.3. Effect of CN Addition on the Hydration of Portland Cement
3.4. Thermogravimetric Differential Thermal analysis
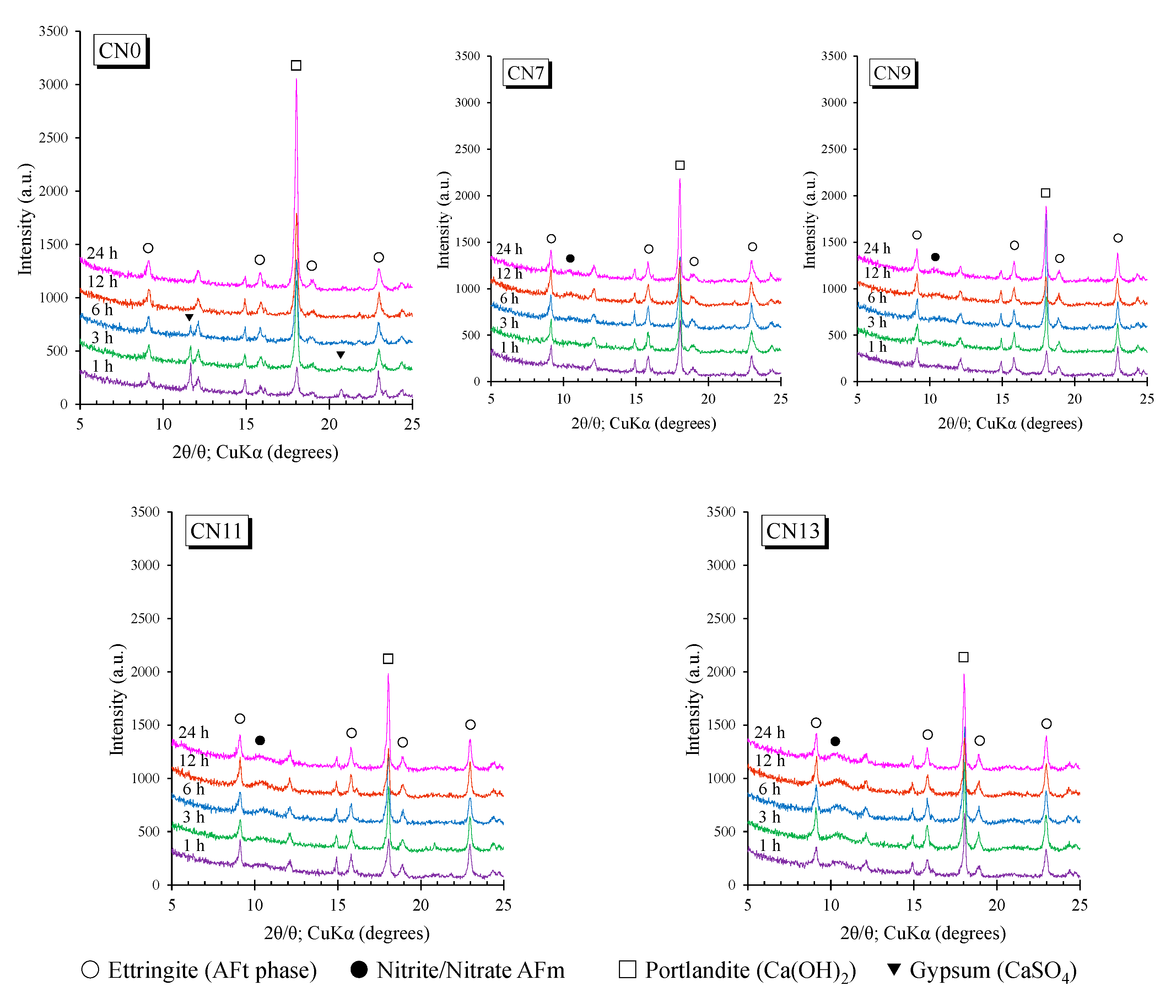
3.5. X-ray Diffraction
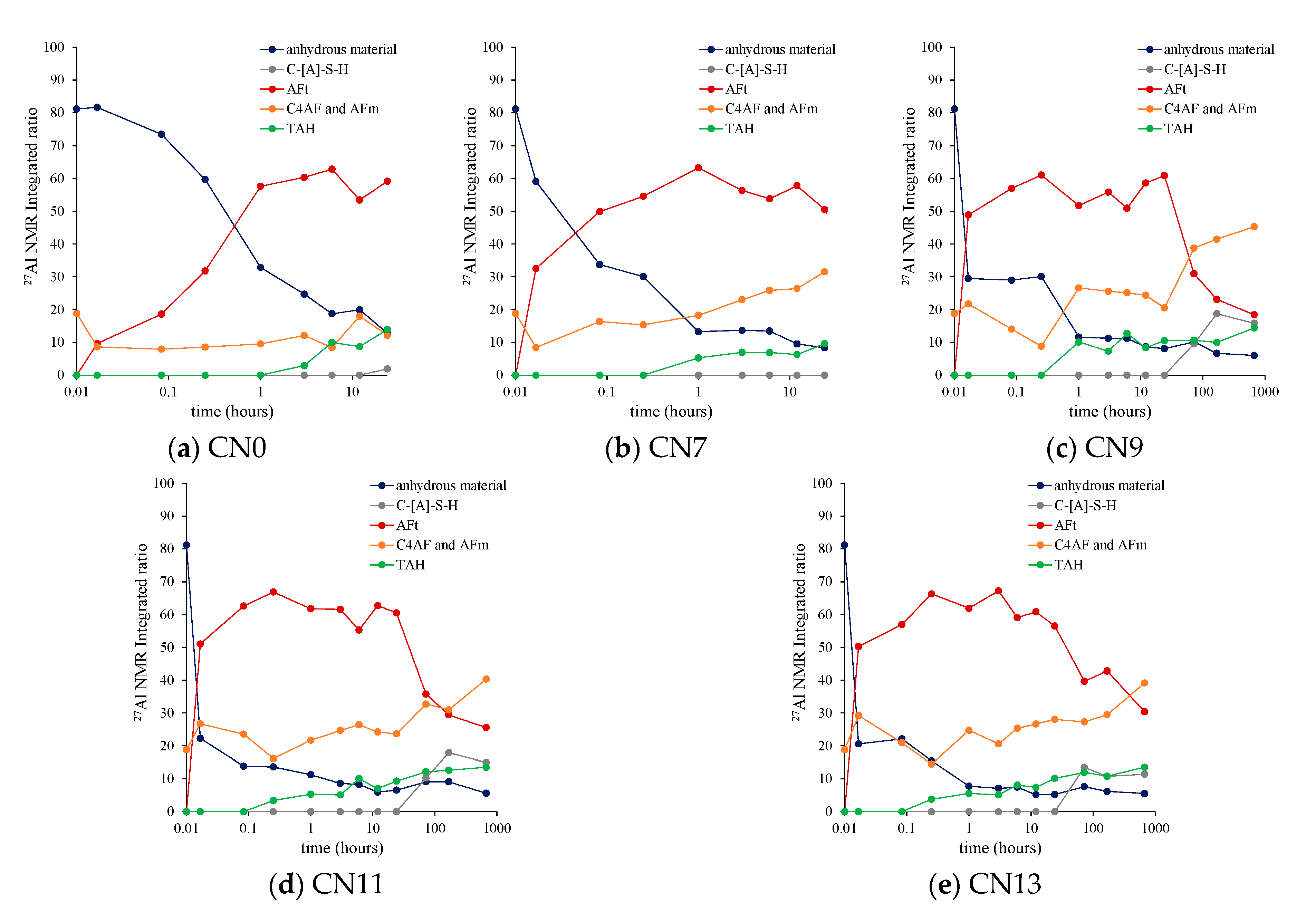
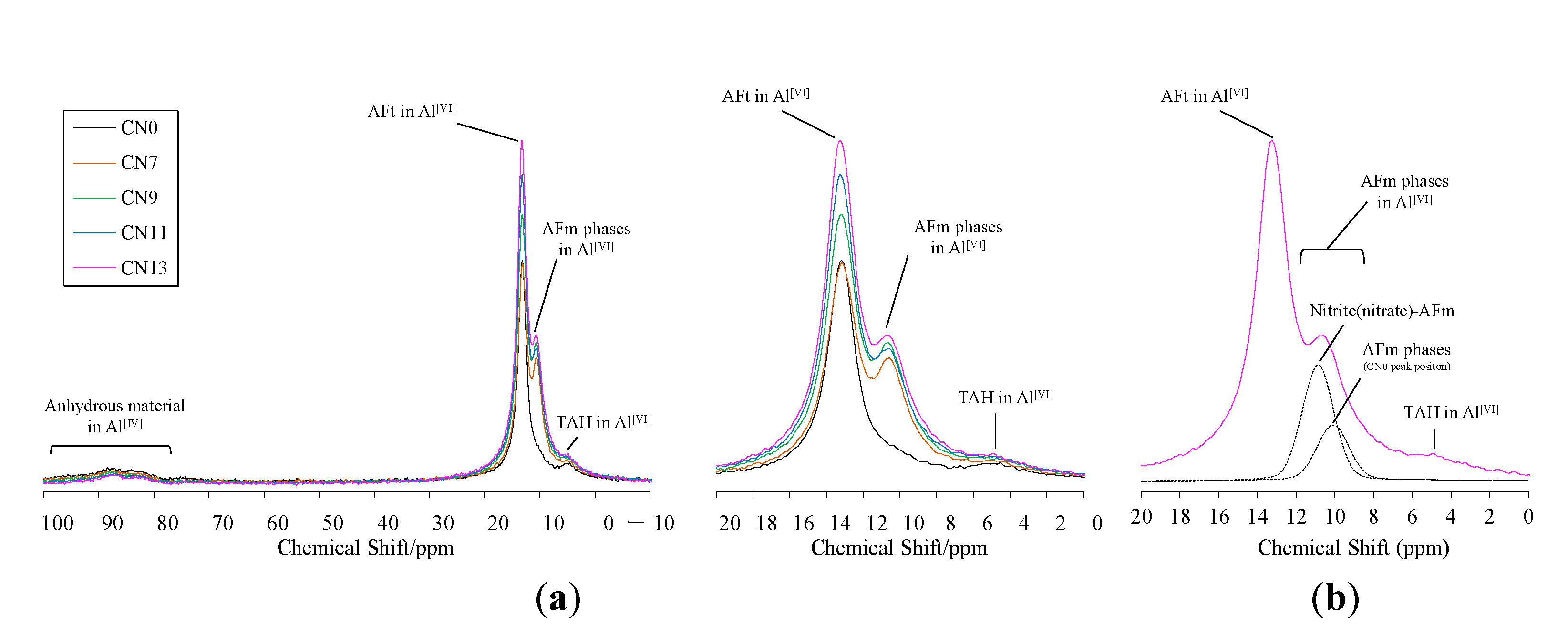
3.6. 27Al MAS NMR
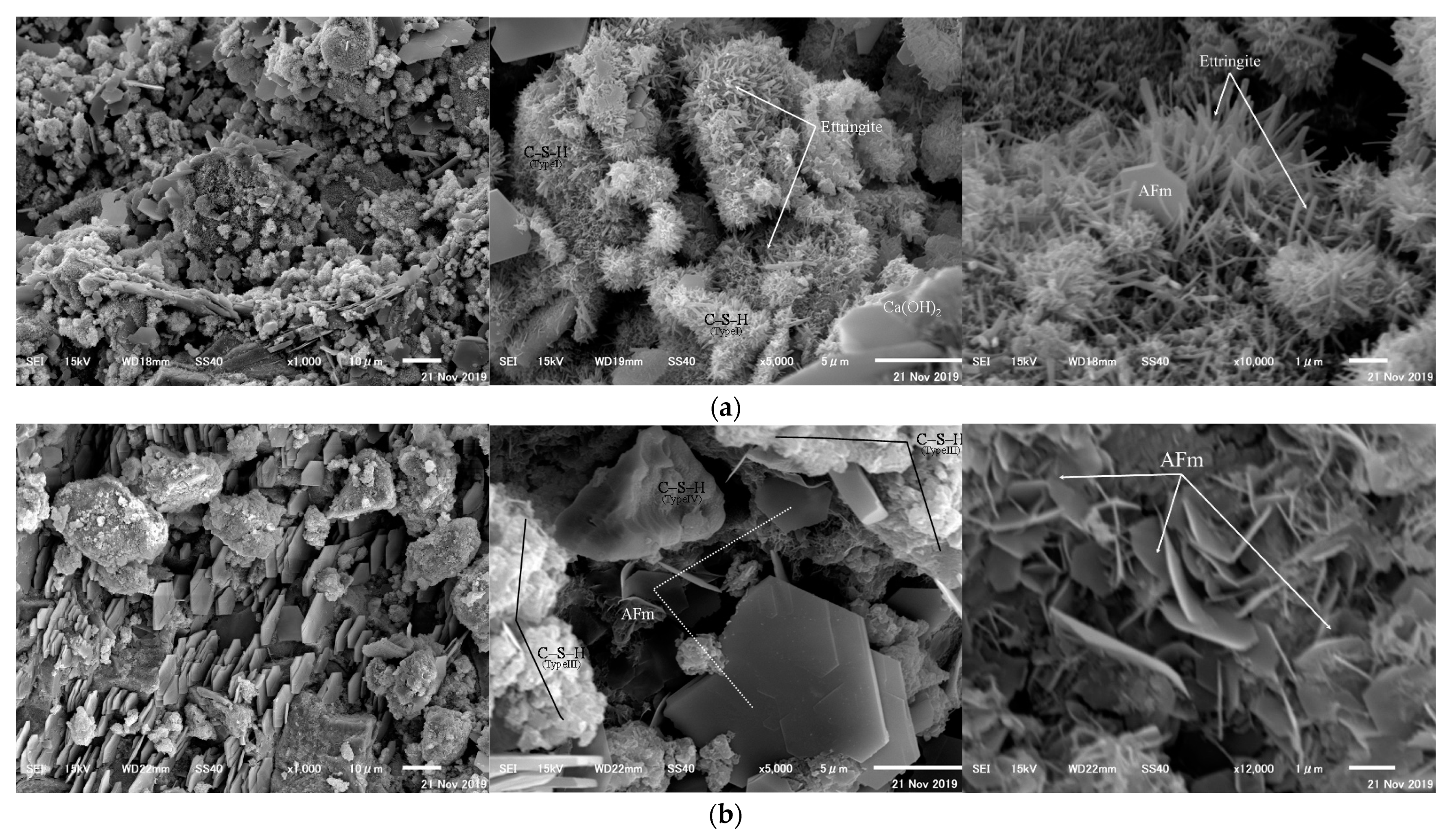
3.7. Crystal Form
4. Conclusions
- (1)
- When CN was added, the rate of ion elution from the cement clinker increased owing to the effect of NO2− and NO3− ions, and the rate of hydrate formation increased (especially within 12 h).
- (2)
- The addition of CN accelerates the usual hydration reactions (C3A, C3S) that occur in the cement matrix, while additionally forming nitrite/nitrate-AFm through the reaction between C3A and NO2−, NO3− ions. The hydrate generated effectively contributes to strength development by filling the pores.
- (3)
- Nitrite/nitrate-AFm was rapidly deposited as hexagonal-plate crystals immediately after contact with water, and the production amount tended to increase as the amount of added CN increased.
- (4)
- By adding CN, hexagonal plate-like crystals, which are presumed to be nitrite/nitrate-AFm, were confirmed in a wide range, and these are considered to contribute significantly to the strength development at an early age.
- (5)
- Furthermore, the calorimeter, TG/DTG, and SEM results reveal that the hydration acceleration of C3S also contributes to the filling of pores and strength development.
- (6)
- When nitrite/nitrate-based accelerator is added, it accelerates the hydration reaction of the initial cement and greatly contributes to the development of strength, so that problems such as delay in setting and initial frost damage in cold-weather concrete works can be improved. Furthermore, by enabling early demolding, the overall construction period can be shortened. However, there is a concern that the amount of expansion and contraction will increase due to the promotion of hydration, and there may be problems such as cracking due to heat of hydration, so it is necessary to adjust the amount used.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Standard Specifications for Concrete; Japan Society of Civil Engineers: Tokyo, Japan, 1991.
- Japanese Architectural Standard Specifications for Reinforced Concrete Work JASS5; Architectural Institute of Japan: Tokyo, Japan, 1997.
- Akama, T.; Inoue, M.; Sudoh, U.; Mikami, S. Fresh properties and early strength development of concrete using calcium nitrite and water-reducing agents. Proc. Jpn. Concr. Inst. 2012, 34, 155–159. [Google Scholar]
- Taniguchi, M.; Nakamura, T.; Koike, S.; Nishi, H. Study on effect and mechanism of accelerator for freeze protection. Res. Rep. Hokkaido Res. Organ. North. Reg. Build. Res. Ins. 2015, 358, 1–11. [Google Scholar]
- Hama, Y.; Kamada, E. The properties of concrete containing a frost-resistant accelerator. Concr. J. 1999, 37, 3–8. [Google Scholar] [CrossRef]
- Hama, Y.; Kamada, E. Strength development under freezing conditions and freezing behavior of water in concrete with accelerators for freeze protection. Concr. Res. Technol. 1997, 8, 73–80. [Google Scholar] [CrossRef][Green Version]
- Nmai, C.K. Cold weather concreting admixtures. Cem. Concr. Compos. 1998, 20, 121–128. [Google Scholar] [CrossRef]
- Neville, A.M. Properties Concrete; Longman London: Harlow, UK, 1995. [Google Scholar]
- Ramachanran, V.S. Concrete Admixture Handbook; Noyes Publications: Park Ridge, NJ, USA, 1995; pp. 741–799. [Google Scholar]
- Sassani, A.; Ceylan, H.; Kim, S.; Gopalakrishnan, K.; Arabzadeh, A.; Taylor, P.C. Influence of mix design variables on engineering properties of carbon fiber-modified electrically conductive concrete. Constr. Build. Mater. 2017, 152, 168–181. [Google Scholar] [CrossRef]
- Cheung, J.; Jeknavorian, A.; Roberts, L.; Silva, D. Impact of admixtures on the hydration kinetics of Portland cement. Cem. Concr. Res. 2011, 41, 1289–1309. [Google Scholar] [CrossRef]
- Edwards, G.C.; Angstadt, R.L. The effect of some soluble inorganic admixtures on the early hydration of portland cement. Int. J. Appl. Chem. 1966, 16, 166–168. [Google Scholar] [CrossRef]
- Balonis, M.; Medala, M.; Glasser, F.P. Influence of calcium nitrate and nitrite on the constitution of AFm and AFt cement hydrates. Adv. Cem. Res. 2011, 23, 129–143. [Google Scholar] [CrossRef]
- Choi, H.; Inoue, M.; Choi, H.; Kim, J.; Sudoh, Y.; Kwon, S.; Lee, B.; Yoneyama, A. Physicochemical study on the strength development characteristics of cold weather concrete using a nitrite–nitrate based accelerator. Materials 2019, 12, 2706. [Google Scholar] [CrossRef] [PubMed]
- Balonis, M. The Influence of Inorganic Chemical Accelerators and Corrosion Inhibitors on the Mineralogy of Hydrated Portland Cement Systems. Ph.D. Thesis, University of Aberdeen, Hong Kong, China, 2010. [Google Scholar]
- Japanese Industrial Standards. Portland Cement, JIS R 5210; Japanese Standards Association: Tokyo, Japan, 2009. [Google Scholar]
- Construction Promotion Council. Operation Manual of Anti-Freezing Agent; Ministry of Land, Infrastructure, Transport and Tourism: Tokyo, Japan, 2005.
- Practical Guideline for Investigation. In Recommendation for Practice of Cold Weather Concreting; Architectural Institute of Japan: Tokyo, Japan, 2010; p. 57.
- Kim, J.; Honda, D.; Choi, H.; Hama, Y. Investigation of the Relationship between Compressive Strength and Hydrate Formation Behavior of Low-Temperature Cured Cement upon Addition of a Nitrite-Based Accelerator. Materials 2019, 12, 3936. [Google Scholar] [CrossRef] [PubMed]
- Japanese Industrial Standards. Method of Test. for Compressive Strength of Concrete, JIS A 1108; Japanese Standards Association: Tokyo, Japan, 2018. [Google Scholar]
- Japanese Industrial Standards. Method of Test. for Compressive Strength of Concrete, JIS A 1147; Japanese Standards Association: Tokyo, Japan, 2019. [Google Scholar]
- Mehta, P.K.; Monteiro, P.J. Concrete, Microstructure, Properties, and Materials, 2nd ed.; Englewood Cliffs, N.J., Ed.; Prentice Hall: Upper Saddle River, NJ, USA, 1993. [Google Scholar]
- Skalny, J.P.; Mindess, S. Materials Science of Concrete II, v.2; 600 North Cleveland Avenue, Suite 210; American Ceramic Society: Westerville, OH, USA, 1991. [Google Scholar]
- American Mineralogist Crystal Structure Database Record, RRUFF; RRUFF Project, Department of Geosciences, University of Arizona, 1040 E 4th, Tucson, AZ, USA Mineral Group: [Ettringite (5)], RRUFF ID: R070208.9, Powder Diffraction.
- American Mineralogist Crystal Structure Database Record, RRUFF; RRUFF Project, Department of Geosciences, University of Arizona, 1040 E 4th Mineral Group: [gypsum (12)], RRUFF ID: R040029.19, Powder Diffraction.
- American Mineralogist Crystal Structure Database Record, RRUFF; RRUFF Project, Department of Geosciences, University of Arizona, 1040 E 4th Mineral Group: [Portlandite], RRUFF ID: R070210.2, Powder Diffraction.
- Chudek, J.A.; Hunter, G.; Jones, M.R.; Scrimgeour, S.N.; Hewlett, P.C.; Kudryavtsev, A.B. Aluminium-27 solid state NMR spectroscopic studies of chloride binding in Portland cement and blends. J. Mater. Sci. 2000, 35, 4275–4288. [Google Scholar] [CrossRef]
- Skibsted, J.; Henderson, E.; Jakobsen, H.J. Characterization of calcium aluminate phases in cements by 27Al MAS NMR spectroscopy. Inorg. Chem. 1993, 32, 1013–1027. [Google Scholar] [CrossRef]
- L’Hopital, E.; Lothenbach, B.; Le Saout, G.; Kulik, D.A.; Scrivener, K.L. Incorporation of aluminium in calcium-silicate-hydrates. Cem. Concr. Res. 2015, 75, 91–103. [Google Scholar] [CrossRef]
- Richardson, I.G.; Brough, A.R.; Brydson, R.; Groves, G.W.; Dobson, C.M. Location of Aluminum in Substituted Calcium Silicate Hydrate (C-S-H) Gels as Determined by 29Si and 27Al NMR and EELS. J. Am. Ceram. Soc. 1993, 76, 2285–2288. [Google Scholar] [CrossRef]
- Andersen, M.D.; Jakobsen, H.J.; Skibsted, J. A new aluminium-hydrate species in hydrated Portland cements characterized by 27Al and 29Si MAS NMR spectroscopy. Cem. Concr. Res. 2006, 36, 3–17. [Google Scholar] [CrossRef]
- Dumm, J.Q.; Brown, P.W. Phase assemblages in the system CH-Al2O3-Ca(NO3)2-H2O. Adv. Cem Res. 1996, 8, 143–153. [Google Scholar] [CrossRef]
- Diamond, S. Hydraulic Cement Pastes: Their Structure and Properties. Cem. Concr. Assoc. 1976, 2–30. [Google Scholar]


| Materials (Code) | Properties |
|---|---|
| Cement (C) | Ordinary Portland Cement, Density; 3.16 g/cm3 |
| Anti-freezing agent (CN) | Main component; calcium nitrite, calcium nitrate (45% water solution), Density; 1.43 g/cm3 |
| Component | Component Ratio | Density of Aquarius Solution (g/cm3) | pH Aquarius Solution |
|---|---|---|---|
| Ca(NO2)2 | 23.02% | 1.43 | 9.3 |
| Ca(NO3)2 | 22.81% |
| Chemical Composition (%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Ordinary Portland Cement | SiO2 | Al2O3 | Fe2O3 | CaO | MgO | SO3 | CaSO4 | Ig.loss | Alkali Content |
| 21.4 | 5.5 | 2.8 | 64.3 | 2.1 | 1.9 | - | 0.56 | 0.25 | |
| Type | W/C (%) | Unit Content (kg/m3) | Anti-Freezing Agent (C × %) | |
|---|---|---|---|---|
| W | C | CN | ||
| CN0 | 50 | 612 | 1225 | 0 |
| CN7 | 7 | |||
| CN9 | 9 | |||
| CN11 | 11 | |||
| CN13 | 13 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoneyama, A.; Choi, H.; Inoue, M.; Kim, J.; Lim, M.; Sudoh, Y. Effect of a Nitrite/Nitrate-Based Accelerator on the Strength Development and Hydrate Formation in Cold-Weather Cementitious Materials. Materials 2021, 14, 1006. https://doi.org/10.3390/ma14041006
Yoneyama A, Choi H, Inoue M, Kim J, Lim M, Sudoh Y. Effect of a Nitrite/Nitrate-Based Accelerator on the Strength Development and Hydrate Formation in Cold-Weather Cementitious Materials. Materials. 2021; 14(4):1006. https://doi.org/10.3390/ma14041006
Chicago/Turabian StyleYoneyama, Akira, Heesup Choi, Masumi Inoue, Jihoon Kim, Myungkwan Lim, and Yuhji Sudoh. 2021. "Effect of a Nitrite/Nitrate-Based Accelerator on the Strength Development and Hydrate Formation in Cold-Weather Cementitious Materials" Materials 14, no. 4: 1006. https://doi.org/10.3390/ma14041006
APA StyleYoneyama, A., Choi, H., Inoue, M., Kim, J., Lim, M., & Sudoh, Y. (2021). Effect of a Nitrite/Nitrate-Based Accelerator on the Strength Development and Hydrate Formation in Cold-Weather Cementitious Materials. Materials, 14(4), 1006. https://doi.org/10.3390/ma14041006







