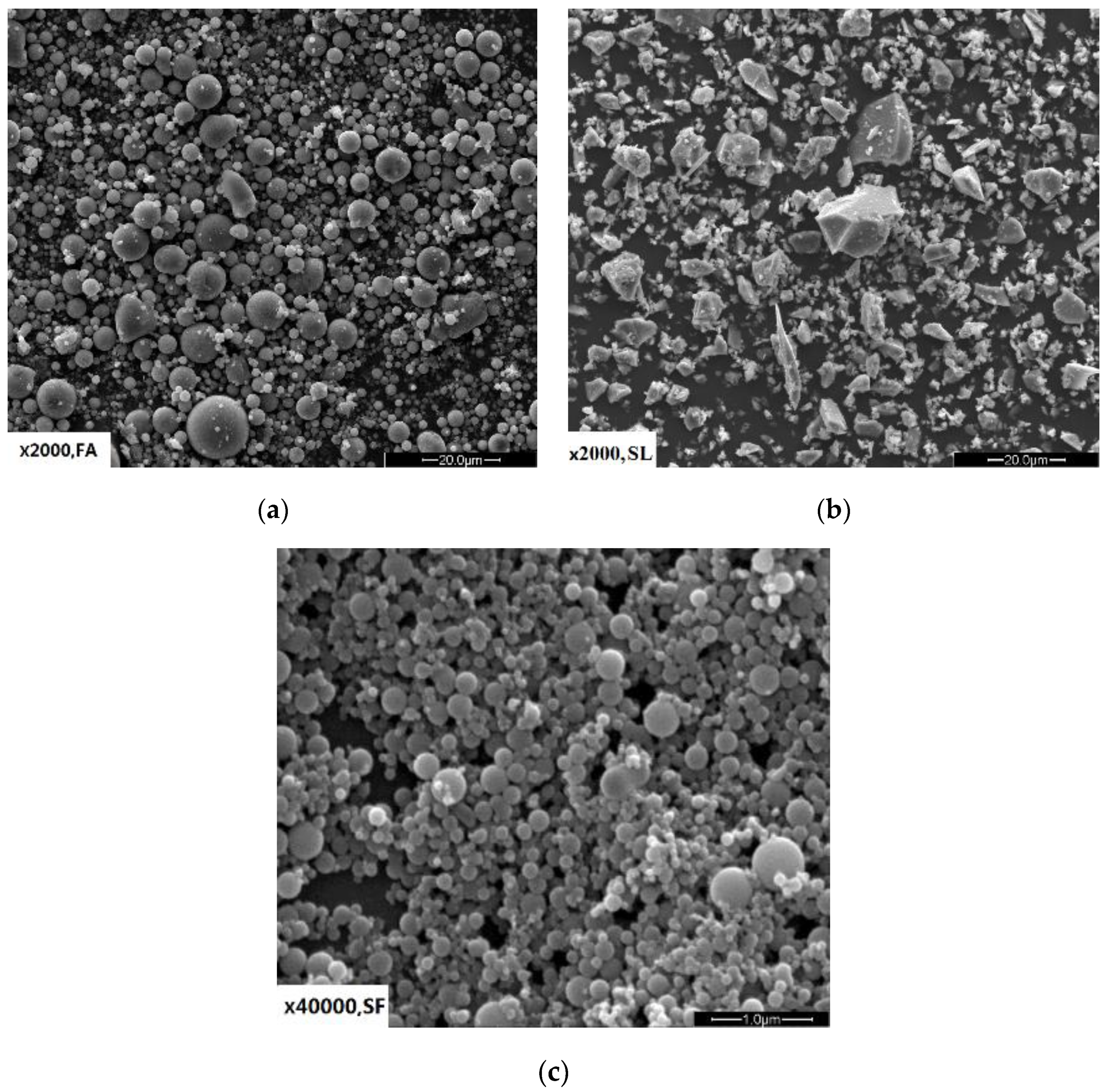
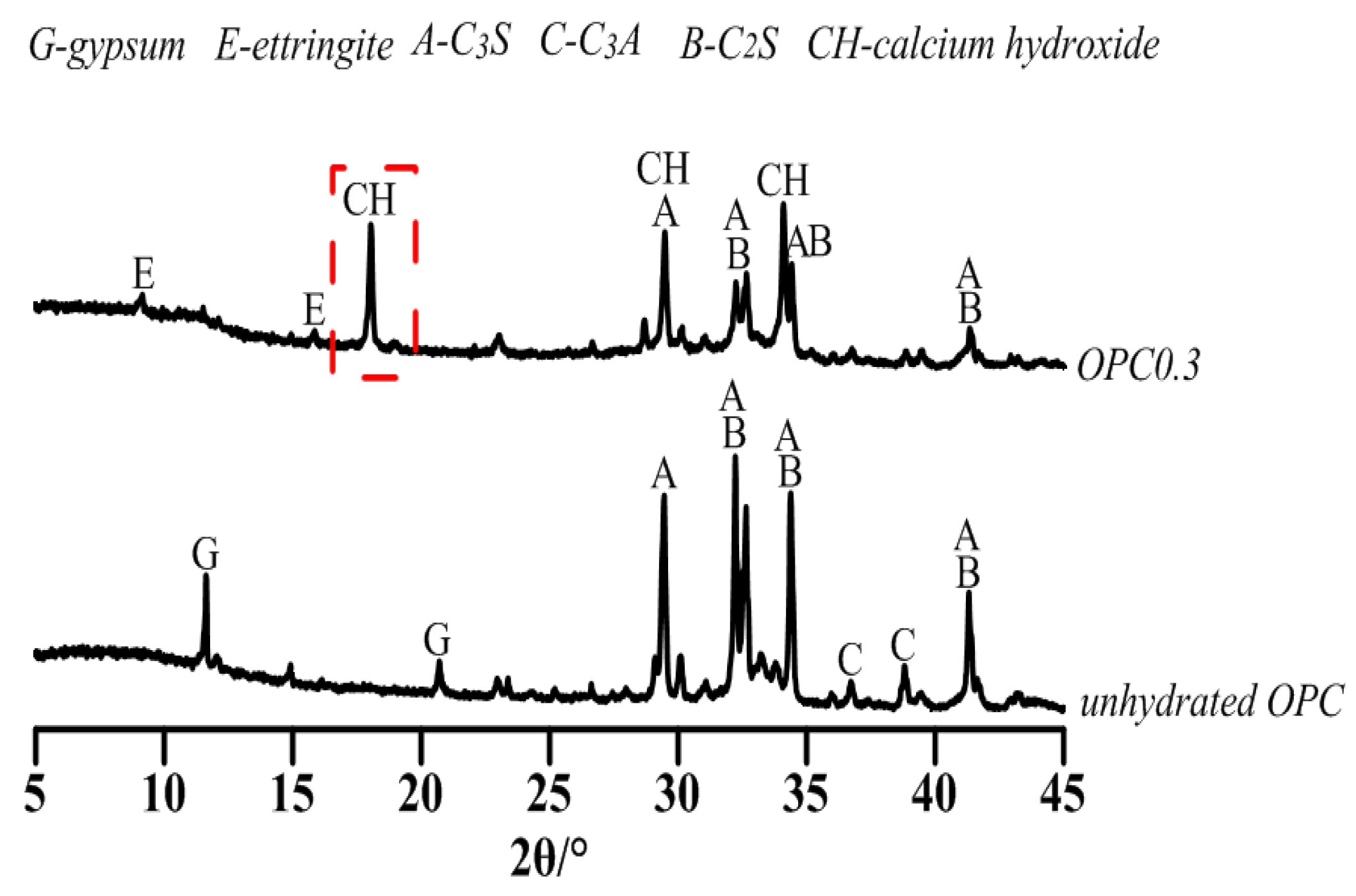
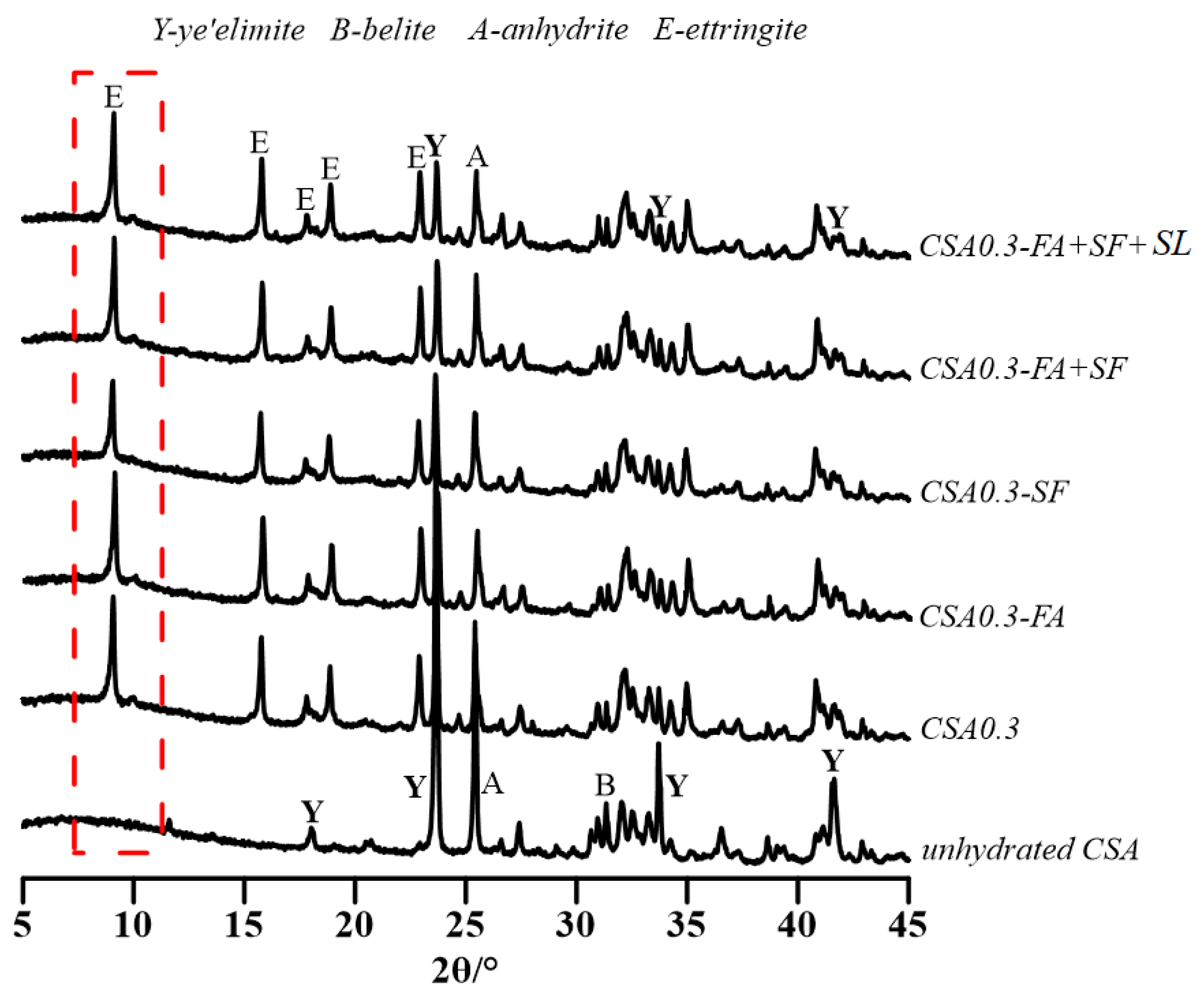
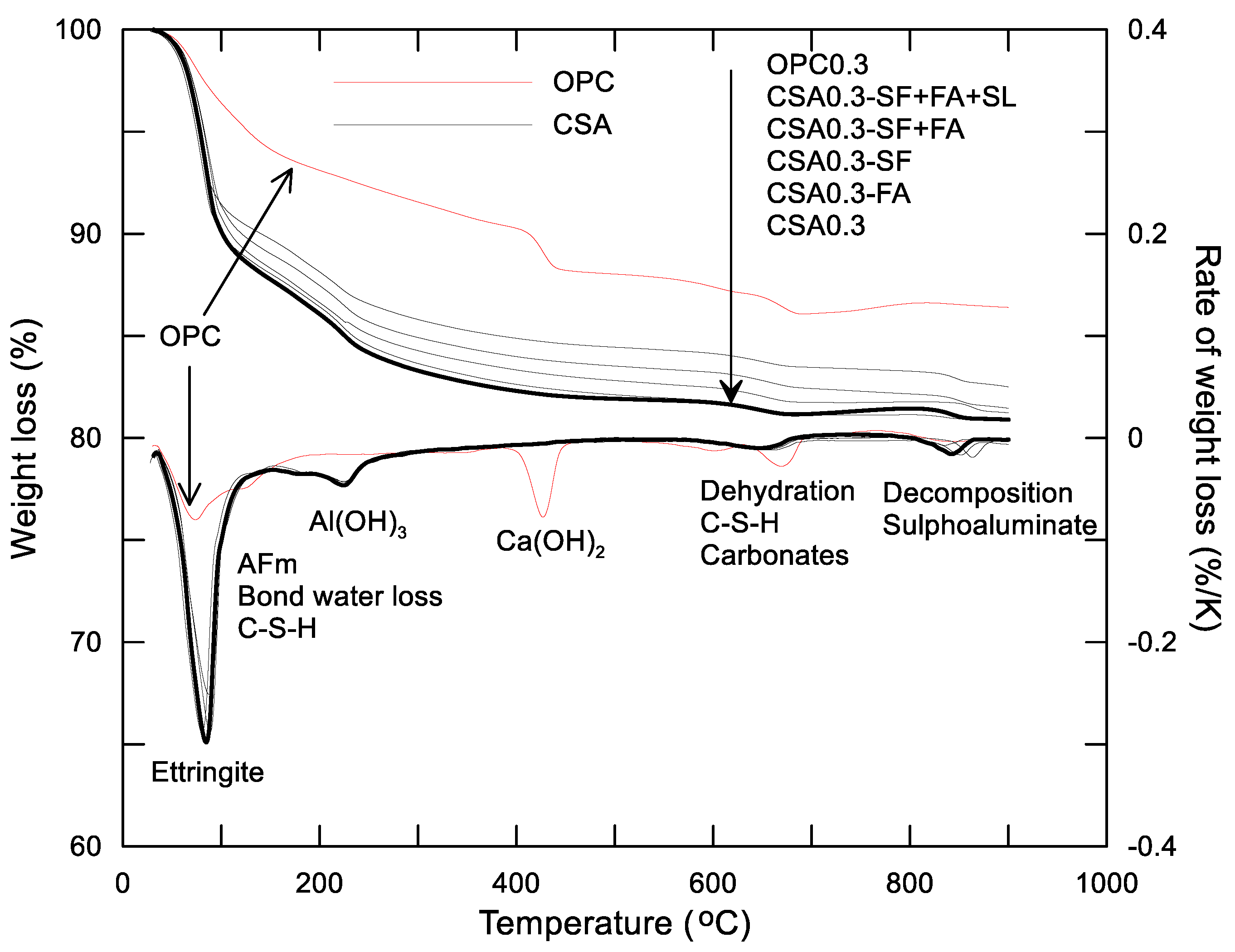
3.2.1. Early Hydration Heat of CSA and OPC Pastes with w/c = 0.3
The test measuring the relationship between heat-releasing rate and mixing time within 40 h since water added of CSA and OPC paste with w/c of 0.3 is displayed in
Figure 6a. The corresponding diagram of accumulative heat and age of the two pastes is presented in
Figure 6b.
From
Figure 6a, three exothermic peaks are observed within the 40 h since the mixing of both the OPC and CSA pastes, although the individual peak strength and occurring time are obviously different between the two pastes. The first peak (see the small inserted figure in
Figure 6a) is similar in peak strength and in ascending-descending performance for the two pastes. The second and the third peaks of the CSA paste are much stronger and occur much earlier than that of the OPC paste. As discussed in existing studies on this topic [
24,
27], the first peak corresponds to the dissolution heat of solid grains that happens immediately after mixing the binder and water. The peak of the releasing rate of the dissolution heat of the OPC paste is a little higher than that of the CSA paste (0.032 and 0.028 W/g, respectively, for OPC and CSA paste), while CSA paste displays a slow decaying rate after the peak. This small difference may be principally due to the differences in the composition of the two cements, especially the dissolution of C
3A and the possible precipitation of ettringite in OPC paste. The listing period of the dissolution heat releasing of the two pastes is very short (≈0.25 h). This is the so-called the initial hydration period of cement hydration. In the initial hydration period, the total releasing heat of the two pastes is almost the same, 18–20 J/g (see
Figure 6b). After that, both of them enter the induction period of cement hydration. The induction period of the OPC paste is relatively longer than that of the CSA paste. Until about 5 h after mixing, the heat flow of the OPC paste starts to grow again, and the hydration process enters the acceleration period. In this period, two heat flow peaks of the OPC paste are observed. One appears at 12.6 h, with a value of 2.34 mW/g, and the other appears at 17.7 h, with a value of 2.53 mW/g. The study by Jansen et al. [
27,
28] revealed that the accelerative hydration of the OPC paste in the acceleration period is primarily due to the hydration of C
3S (silicate reaction) and C
3A (aluminate reaction). The first peak of heat flow in this period is mainly attributed to the silicate reaction, including the dissolution of the alite and precipitation of portlandite and C-S-H gel. The second heat flow peak is attributed to the dissolution of C
3A, the precipitation of ettringite, and the silicate reaction. For the CSA paste, the induction period is considerably shorter than that of the OPC paste. At about 50 min after mixing, the heat flow of the CSA paste starts to grow up, and the hydration process enters the acceleration period. In this period, two much stronger heat flow peaks than that of the OPC paste are observed. One appears at 1.5 h, with a value of 44.9 mW/g, and the other appears at 3 h, with a value of 10.3 mW/g. The maximum heat flow of the CSA paste is 17.8 times that of the OPC paste. The first heat flow peak of the CSA paste in this period corresponds to the reaction heat of calcium sulfoaluminate, anhydrite, and water to form ettringite, including the dissolution of the sulfates and the precipitation of ettringite. The second peak corresponds to the reaction heat generated from the local formation of ettringite and monosulfate around the cement grains under the ion diffusion and/or migration controlled state, that happens after the formation of a solid frame linking the solid particles. A more detailed test observation and discussion regarding the hydration process of the CSA paste can be found in the literature [
23,
29,
30]. After the second peak, the heat flow decreases with age and the hydration process enters the retardation period.
It can be seen that for the same w/c, the second and third heat flow peaks (both reaction peaks) between the CSA and OPC pastes is obviously different both in the peak strength and in the occurring time. The CSA paste displays a stronger and earlier heat-releasing peak of cement hydration (the second and third peaks) compared to that of the OPC paste. These great differences in the hydration heat-releasing result are obvious different performances of accumulative heat development, as displayed in
Figure 6b. From the figure, it can be seen, first, that the dissolution heat resulting from the initial hydration period of the two pastes is almost the same. After that, the CSA paste displays a shorter induction period (≈0.8 h), while the OPC paste presents a longer induction period (≈5 h). In the accelerative period, the CSA paste presents a much faster development of accumulative heat compared with that of the OPC paste. The inflection point of accumulative heat of the CSA paste is reached about 6 h after mixing, while the accumulative heat continuously increases with age at the end of the test, at 40 h. At 36.8 h, the accumulative heat of the OPC paste exceeds that of the CSA paste. The fast hydration and high early-age heat-releasing characteristics of CSA are clearly presented compared with OPC.
3.2.2. Early Hydration Heat of the CSA Paste with Different SCMs
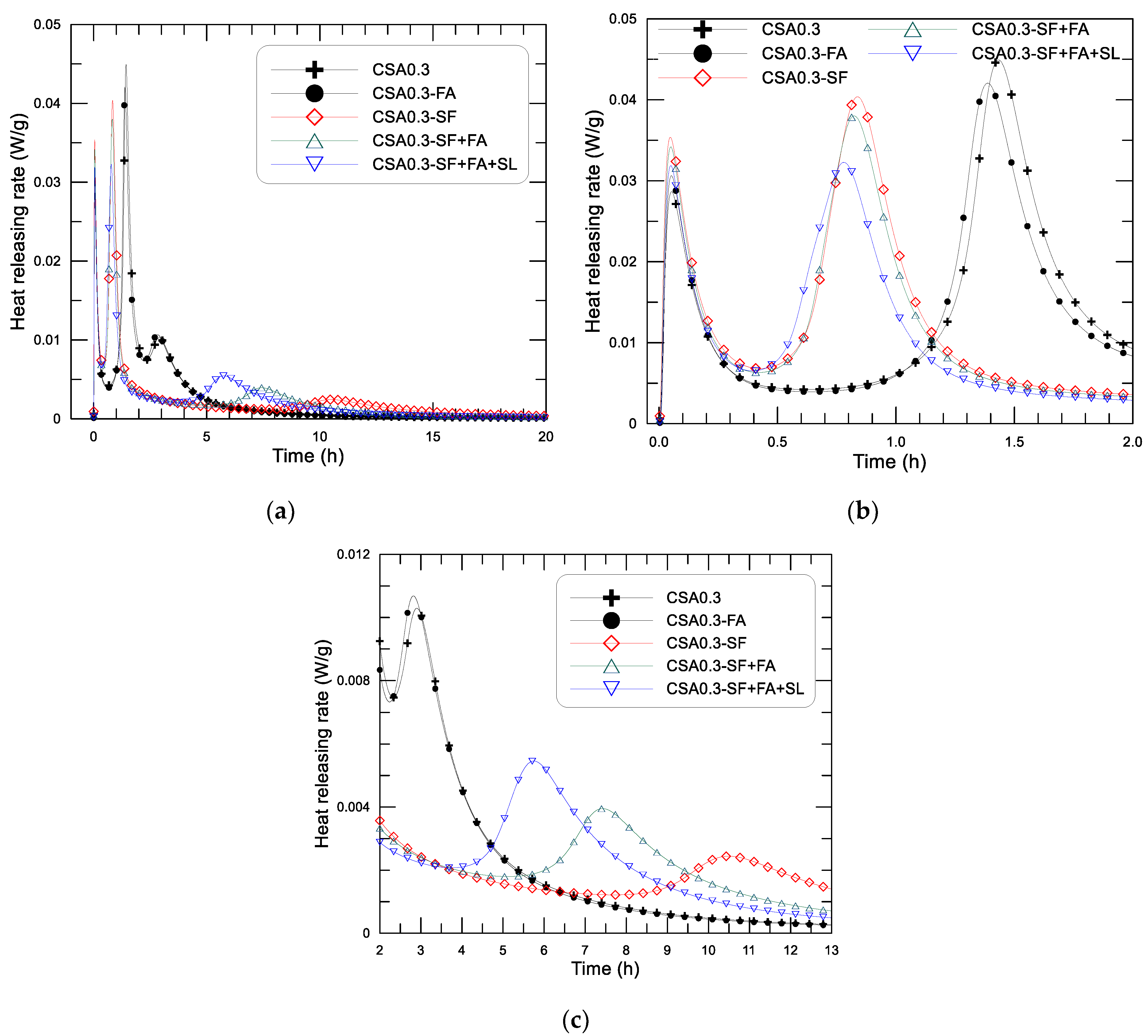
Figure 7 displays the test-determined diagram of hydration-heat-releasing rate and age since the mixing of the CSA pastes (w/c = 0.3) with different addition of SCMs, in which (a) shows the results within 20 h, (b) and (c) are the results within the initial 2 h and the results between 2–13 h to show the variation of the rate peaks more clearly. The corresponding accumulative heat and age diagrams of the pastes are presented in
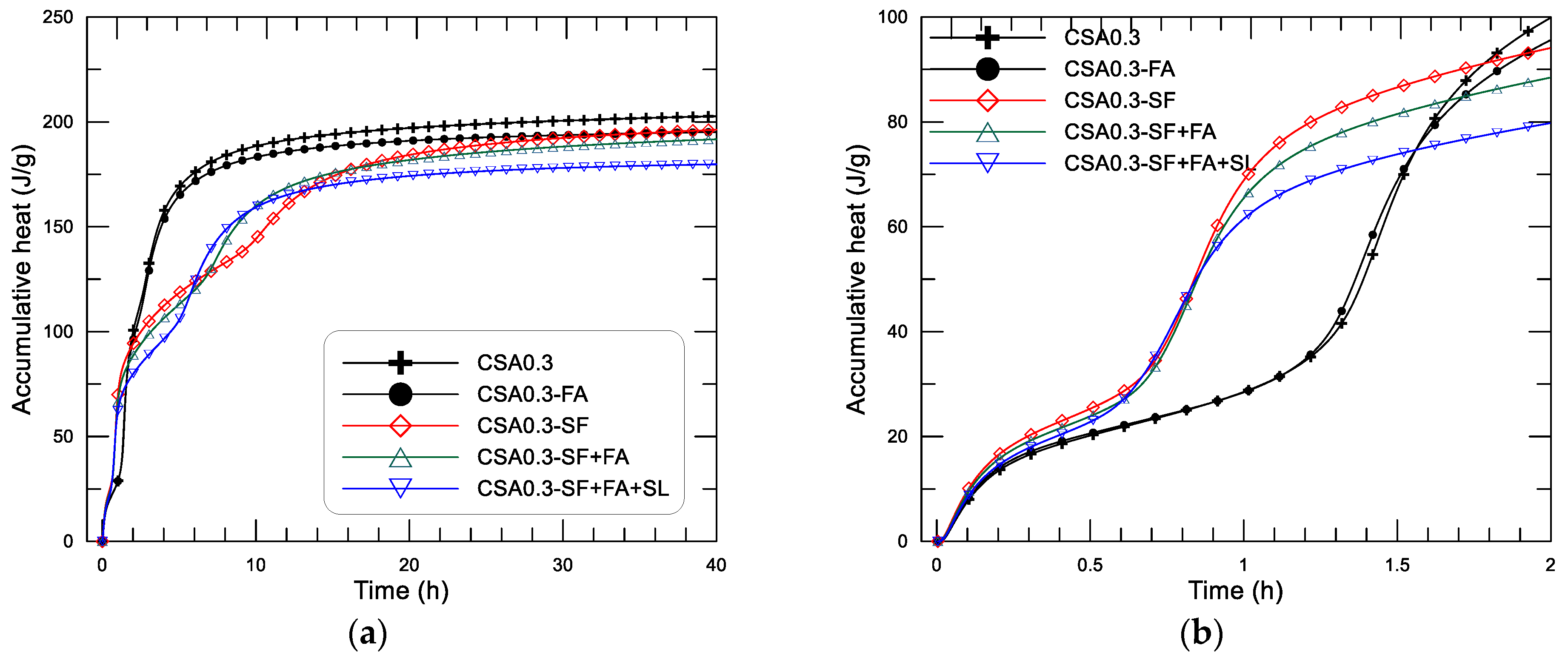
Figure 8, in which (a) shows the results within 40 h and (b) shows the results within the initial 2 h.
From
Figure 7, the hydration heat-releasing characteristics presented as three heat flow peaks in the early age can still be observed on the CSA pastes with different use of SCMs. The heat-releasing performance of the CSA pastes (w/c = 0.3) with different addition of SCMs can obviously be divided into two kinds according to whether the mixture contains silica fume. For the mixtures with silica fume addition, such as CSA0.3-SF, CSA0.3-SF+FA, and CSA0.3-SF+FA+SL, the second heat flow peak clearly occurs earlier (at around 0.8–0.9 h) than that of the rest mixtures without silica fume addition, such as CSA0.3 and CSA0.3-FA (at around 1.4–1.6 h) (see
Figure 7b). In other words, the addition of silica fume can greatly reduce the length of the induction period of the paste. On the first heat flow maxima (first peak), i.e., the dissolution heat resulted peak, the appearing time of the five mixtures is quite similar, immediately after mixing. But the peak strength is different, ranging from 0.028 to 0.035 W/g. The order of the first exothermic peak is as follows: CSA0.3-SF > CSA0.3-SF+FA > CSA0.3-SF+FA+SL > CSA0.3+FA > CSA0.3. Clearly, the addition of finer particles to the mixture, especially silica fume, can increase the releasing strength of the dissolution heat of the paste. Under the same binder weight, the more the finer particles, the higher the releasing rate of the dissolution heat. These results are understandable because the dissolution heat is normally proportional to the specific area of the solid particles in the system. In addition, the dilution effect of SCMs, especially the ultra-fine silica fume in the binder system, may also improve the dispersion of CSA in water, which will increase the dissolution heat as well [
22,
23].
Now looking at the second exothermic peak (first reaction peak, see
Figure 7b), the nucleation effect of SF on the formation of Aft in the paste significantly shifts the peak to an earlier date, from 1.5 h (CSA0.3, CSA0.3-FA) to 0.8–0.9 h (CSA0.3-SF, CSA0.3-SF+FA, CSA0.3-SF+FA+SL). By contrast, the effect of FA and SL on the formation age of AFt is minor, although FA and SL are finer than the cement grains and certain nucleation action can still be observed—for example, the order of occurring time of the second peak of the pastes with SF and the combination use of SF+FA and SF+FA+SL are as follows: CSA0.3-SF+FA+SL < CSA0.3-SF+FA < CSA0.3-SF. The peak value, on the other hand, decreases with the increase of SCMs, from 10% to 30% of the total binder weight. The value of the second peak is 0.045, 0.042, 0.041, 0.038, and 0.032 W/g, respectively, for CSA0.3, CSA0.3-FA, CSA0.3-SF, CSA0.3-SF+FA, and CSA0.3-SF+FA+SL. It seems that the effect of SF on hydration heat evolution is purely physical, rather than chemical, because no new hydration products are founded after adding it to the mixture at 3 days after mixing, as described in the previous section. However, the addition of SF significantly advances the formation of AFt in the paste, which may influence the setting and strength development of the paste.
The third exothermic peak (the second reaction peak), as displayed in
Figure 7c, is much weaker than the second exothermic peak of the corresponding paste, that is, about 1/4 for CSA0.3 and CSA0.3-FA, and about 1/8 for the rest mixtures of the second peak. The magnitude of the third peak is 0.0105 and 0.0102 W/g for CSA0.3 and CSA0.3-FA (group I), and 0.0055, 0.0040, and 0.0030 W/g for CSA0.3-SF+FA+SL, CSA0.3-SF+FA, and CSA0.3-SF (group II), respectively. The appearing time is contrary to that of the second peak, i.e., the appearing time of CSA0.3 and CSA0.3-FA (2.8, 3.0 h) is earlier than that of CSA0.3-SF+FA+SL (5.8 h) CSA0.3-SF+FA (7.5 h) and CSA0.3-SF (10.5 h). The second and third peaks are closer for CSA0.3 and CSA0.3-FA than those of the rest mixtures. Among the mixtures with the addition of SF, the mixture for the latest and the earliest appearance of the third peak is CSA0.3-SF and CSA0.3-SF+FA+SL, respectively. Referencing the hydration analyses on CSA paste carried out by Zhang et al. [
29], the third exothermic peak corresponds to the local formation of AFt and/or AFm inside of the hydration product layer around cement grains formed at the second peak under an ion diffusion controlled environment. Owing to the addition of very fine particles, such as SF (average particle size less than 1 μm), in the mixtures, the capillary pore of the paste becomes smaller, and the diffusion rate of ion becomes slower. The third peak of the pastes with SF addition becomes weaker and occurs later than that of the paste without SF. It is interesting to note that SF can advance the second exothermic peak (first reaction peak) of the CSA paste. On the other hand, it can delay the third peak (second reaction peak).
The effect of SCMs on the heat-releasing performance of the CSA paste may be summarized as follows. The three heat-releasing peaks can still be observed after adding the different SCMs to the paste. The effect of SCMs on the dissolution heat peak (first peak) is minor, displaying a slight increase on the exothermic peak as finer SCMs are added. The significant influence of SCMs is reflected on the two reaction exothermic peaks, i.e., the second and the third heat-releasing peaks. The addition of silica fume can greatly reduce the length of the induction period of the paste, thus making the second peak appear earlier. The value of the second peak decreases with the increase of SCMs from 10% to 30% of the total binder weight. The effect of SCMs on the third heat peak (second reaction peak) is mainly reflected by the effect of SF. The addition of SF can delay the appearing time of the third peak and reduce the heat flow strength as well.
These effects of SCMs on the hydration heat-releasing performance result in different behaviors of accumulative heat development of the paste, as displayed in
Figure 8. From
Figure 8, it can be seen that the dissolution heat produced in the initial hydration period of the pastes varied between 20.2 J/g and 25.3 J/g for the pastes with different addition of SCMs. As expected, CSA0.3-SF has the highest dissolution heat, and CSA0.3 has the lowest (see
Figure 8b). After that, all the pastes undergo an induction period, while the mixtures with SF addition—CSA0.3-SF, CSA0.3-SF+FA, and CSA0.3-SF+FA+SL—are the first to enter the acceleration period (second heat flow peak), at about 0.7 h, with a fast increase of accumulative heat. The rest mixtures (without use of SF)—CSA0.3 and CSA0.3-FA—enter the acceleration stage at about 1.3 h after mixing. After that, mixtures of CSA0.3 and CSA0.3-FA generate the third exothermic peak at an age closer to their second peak, and the accumulative heat continues to increase until the heat-releasing rate is close to zero. The mixtures with SF addition generate the third exothermic peak with a lower rate and a later age compared to their second peak, as displayed in
Figure 7c, in which the mixture with only SF (CSA0.3-SF) becomes the weakest and farthest one of the third exothermic peak among the mixtures. Therefore, after the second peak, the accumulative heat of CSA0.3-SF starts to increase again at about 10.5 h, when the third peak appears. For the two rest mixtures with SF addition, CSA0.3-SF+FA and CSA0.3-SF+FA+SL, the accumulative heat starts to increase after the second peak at an earlier age compared to that of CSA0.3-SF. To summarize, the addition of SF can obviously increase the hydration heat of the CSA paste at an age around 0.7 to 1.3 h. The heat evolution at the end of the tests (40 h after mixing) decreases with the increase of SCMs in an approximately proportional manner. Based on the present tests, the hydration heat at 40 h after mixing of the CSA pastes (w/c = 0.3) with different use of SCMs is 202.7, 195.2 and 196.2, 191.7, 179.8 J/g, respectively, for CSA0.3 (0% SCMs), CSA0.3-FA and CSA0.3-SF (10% SCMs), CSA0.3-SF+FA (20% SCMs), CSA0.3-SF+FA+SL (30% SCMs).
3.2.3. Early Hydration Heat of the CSA Paste with Different w/c
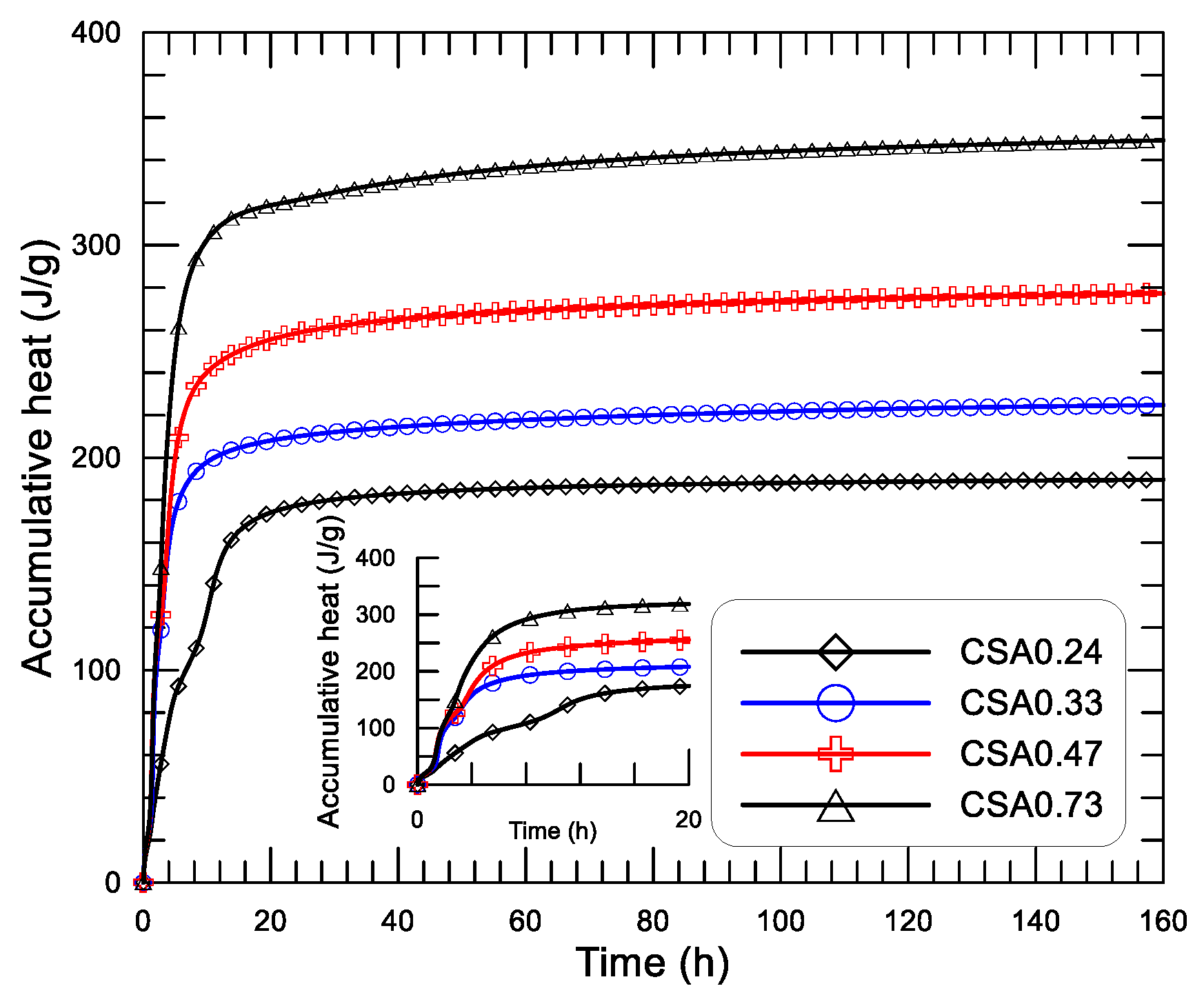
Figure 9 displays the diagrams of the releasing rate of hydration heat and age since mixing of the four CSA pastes with water to cement ratio (w/c) of 0.24, 0.33, 0.47, and 0.73.
Figure 9a shows the results within 20 h,
Figure 7b presents the results within the initial 2.5 h, and
Figure 9c shows the results of 2 to 13 h. The diagram of accumulative heat and age (up to 160 h) of the pastes with different w/c is presented in
Figure 10, in which the inserted small figure displays the results at the initial 20 h.
From
Figure 9a, first, the three exothermic peaks that are noted in both the OPC and CSA pastes with w/c of 0.3 can still be observed on the pastes with w/c varying from 0.24 to 0.73. The analyses of the formation mechanism of each exothermic peak were already discussed in the previous section. Second,
Figure 9b shows that the dissolution heat peak of the pastes can be divided into two groups, 0.0166 W/g and 0.0269, 0.0231, and 0.0239 W/g, respectively, for CSA0.24 and CSA0.33, CSA0.47, and CSA0.73. For a given particle composition, the dissolution heat should be a constant as long as water can be well adsorbed on the surface of the particles. For the pastes with w/c of 0.33, 0.47, and 0.73, the water supplied may already be sufficient for the adsorption of the cement particles, while the water in the paste with w/c of 0.24 may not be sufficient for cement adsorption and may partially be adsorbed by the superplasticizer used in the paste. Therefore, the dissolution heat peak of CSA0.24 is a little lower than that of the rest of the pastes. No effect of w/c on the appearing time of the dissolution heat peak is observed. Third, a similar duration of the induction period is noted in the pastes with different w/c, ended at around 1 h after mixing. The magnitude of the second exothermic peak (first reaction peak) in the acceleration period is significantly influenced by w/c. The value of the second peak is 0.0064, 0.0434, 0.0386, and 0.0352 W/g, respectively, for CSA0.24, CSA0.33, CSA0.47, and CSA0.73. Obviously, a much lower value of the second heat flow peak is noted for the paste with w/c of 0.24, while a comparable value of the second peak is noted for the rest pastes with w/c of 0.33, 0.47, and 0.73. These findings indicate, for the CSA paste within the initial 2 h after mixing, that the hydration heat is greatly influenced as w/c changes from 0.33 to 0.24, while it may not be influenced significantly as w/c varies from 0.33 to 0.73. The third exothermic peak (second reaction peak) in the deceleration period is also greatly influenced by w/c (see
Figure 9c). According to the appearing time of the third peak, the mixtures can also be divided into two groups: group one consists of CSA0.33, CSA0.47, and CSA0.73, and the occurring time of the third peak is around 3–4 h, and group two consists of CSA0.24, and the occurring time of the third peak is 10.5 h. The value of the third maxima of heat flow is 0.0037, 0.0114, 0.0126, and 0.0197 W/g, respectively, for the pastes with w/c of 0.24, 0.33, 0.47, and 0.73. Apparently, the third exothermic peak gradually decreased with the decrease of w/c from 0.73 to 0.33, and dramatically decreases as w/c changes from 0.33 to 0.24, apart from the obvious difference on the appearing time of the third heat flow peak. This may indicate that the ion diffusion in the paste is significantly influenced as w/c is changed from 0.33 to 0.24, while the influence is more gradual as w/c is changed from 0.33 to 0.73. The effect of w/c on the microstructure of the paste should be responsible for the above changes either on the peak strength or on the appearing time of the third heat flow peak. Certainly, the critical value of w/c for the above changes of the CSA paste may not be exactly equal to 0.24 (they should be between 0.33 to 0.24), but it is guaranteed as w/c is less than 0.24.
From
Figure 10, the accumulative heat and age diagrams of the CSA pastes with different w/c can also be divided into two groups within 2 h (first and second exothermic peak controlled period). Group one consist of the low heat-releasing paste of CSA0.24 (low second peak) only, and the other group consists of CSA0.33, CSA0.47, and CSA0.73 (relative higher second peak, which presents a relative higher accumulative heat. After 2 h, the accumulative heat starts to develop under different rates of CSA0.33, CSA0.47, and CSA0.73 (third exothermic peak controlled period). In this stage, for a given age, the larger the w/c, the higher the accumulative heat. The accumulative heat of CSA0.24 starts to grow at a later age due to the delayed appearing of the third peak (see
Figure 9c). At about 20 h after mixing, the accumulative heat-age diagram steps into a stage under a low and gradually slower rate. Clearly, the accumulative heat decreases with the decrease of w/c in a later age (after 2 h). At the end of the tests, 160 h later, the accumulative heat is 189.6, 224.7, 277.4, and 349.3 J/g, respectively, for CSA0.24, CSA0.33, CSA0.47, and CSA0.73. It should be noted that although the higher hydration heat means a higher amount of hydration products or a higher cement hydration degree, the strength or the mechanical properties of the cement paste is not simply governed by the amount of hydration products or the cement’s hydration degree. The strength or other mechanical properties of cement paste or concrete is proportional to the volume of capillary pores in the paste, which is normally in reverse proportion to w/c. Therefore, the compressive strength of the CSA pastes with different w/c increases significantly with the decrease of w/c (see the test results listed in
Table 2).
3.2.4. Combination Effect of w/c and SCMs on Early Hydration Heat
As described in the section on mixture design, the fourth series was intended to study the combined effect of water to binder ratio and SCMs on the hydration heat of the CSA paste. In this series, four pairs of mixtures were formed according to the water to binder ratio, and each pair of mixtures may be used in concrete to form different strength grades in practice. The four pairs of pastes are (CSA0.24, CSA0.19-SF+FA+SL), (CSA0.33, CSA0.30-SF+FA), (CSA0.47, CSA0.42-FA), and (CSA0.73, CSA0.62-1.5FA).
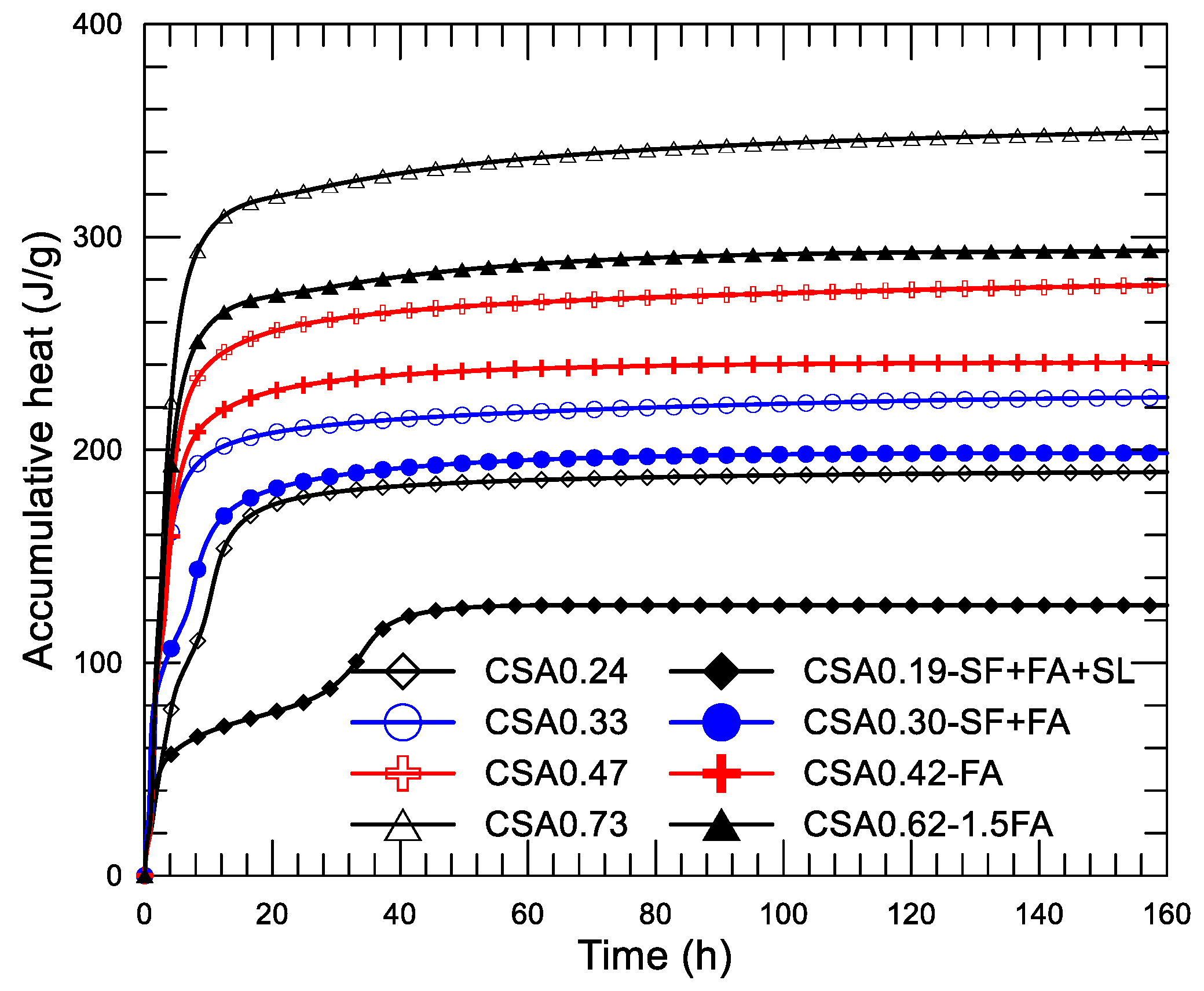
Figure 11a–d display the diagram of the heat-releasing rate and age since the mixing of the four pairs of the CSA pastes, respectively. The inserted small figure in each shows the result of the initial 2 h to present the results more clearly. Corresponding accumulative heat-age diagrams (up to 160 h) of the pastes are presented in
Figure 12.
First,
Figure 9a,b offer a double confirmation of the action of SF, i.e., advancing the first reaction peak (from 1.5 h of CSA0.24 and CSA0.33 to about 0.8 h of CSA0.19-SF+FA+SL and CSA0.30-SF+FA) and delaying the second reaction peak (from 10.5 h of CSA0.24 and 3.5 h of CSA0.33 to about 35 h of CSA0.19-SF+FA+SL and 7.5 h of CSA0.30-SF+FA). The action of nucleation and consolidation of SF in the paste is obvious. Second, the effect of w/c on the heat-releasing characteristic of the CSA paste is confirmed again. The first reaction peak of CSA0.19-SF+FA+SL is comparable to that of CSA0.24. A similar observation can also be found on CSA0.30-SF+FA and CSA0.33. The second reaction peak is significantly delayed for CSA0.19-SF+FA+SL compared to CSA0.30-SF+FA due to the lower w/c, leading to slow ion diffusion.
Figure 11c,d show that the addition of FA hardly influences the releasing heat of the paste. A few advances and a little reduction on the second (first reaction peak) and third (second reaction peak) exothermic peaks, compared with the reference mixture, are observed. They may be simply due to the weak nucleation, dilution, and filler actions. It should be noted that the difference on w/c in each pair of mixtures plays a role in the process of heat releasing.
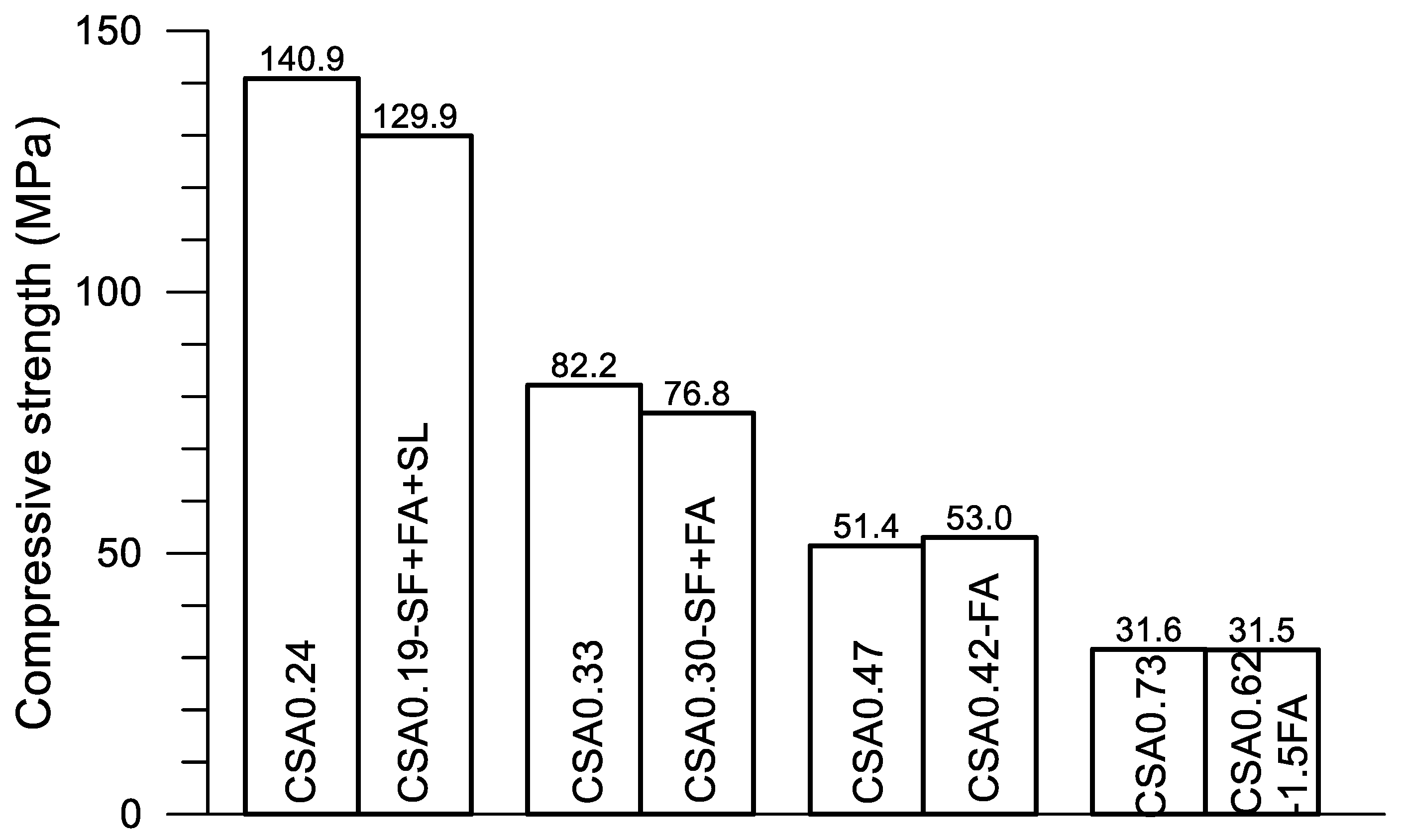
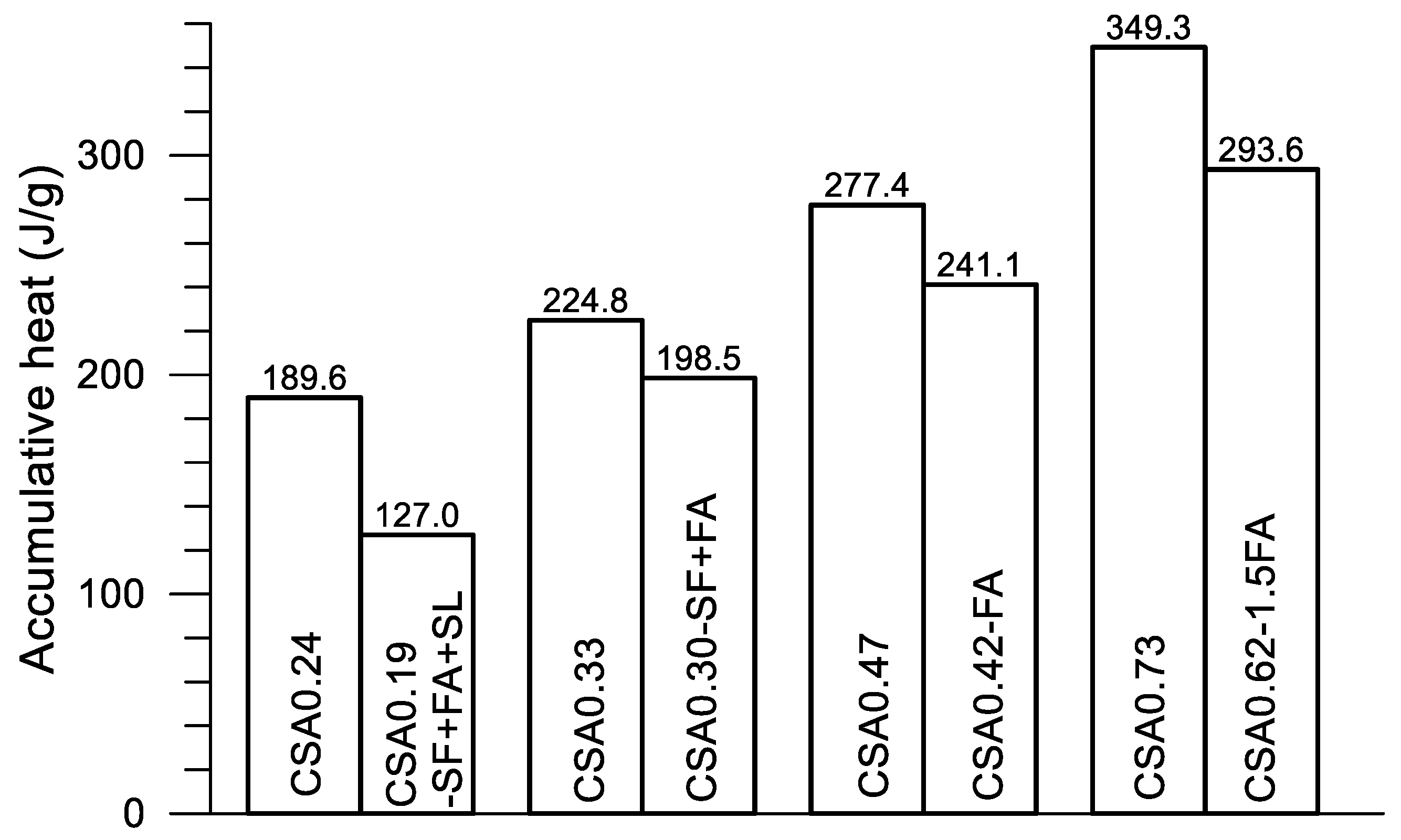
In
Figure 12, great differences on the progress of accumulative heat can be observed among the four pairs of mixtures. Accumulative heat is reduced with the decrease of w/c and the addition of SCMs under a given age. As described previously, each pair of mixtures is designed for a given strength grade of concrete. Using the compressive strength data given in
Table 2,
Figure 13 presents the strength at 28 days of the four pairs of pastes. The accumulative heat at 160 h after the mixing of the corresponding paste is displayed in
Figure 14. Clearly, four strength grades—130–140, 80–75, 50–53 and 30–32 MPa—are obtained using the present four pairs of paste mixtures, although a certain amount of SCMs are used in the mixtures. On the other hand, the accumulative heat is significantly reduced after using SCMs in the mixture. For CSA0.24 and CSA0.19-SF+FA+SL, the accumulative heat at 160 h is reduced by 33.0% after the use of SCMs (30% in weight), while the compressive strength is reduced only 7.8%. Certainly, saving cement in CSA0.19-SF+FA+SL is also an advantage of the mixture. Similarly, for CSA0.33 and CSA0.30-SF+FA, the accumulative heat is reduced by 11.7% after the use of SCMs (20% in weight), while the compressive strength is reduced by 6.6%. For CSA0.47 and CSA0.42-FA, the accumulative heat is reduced by 13.1% after the use of SCMs (10% in weight), while the compressive strength is increased by 3.1%. For CSA0.73 and CSA0.62-1.5FA, the accumulative heat is reduced by 15.9% after the use of SCMs (15% in weight), while the compressive strength is reduced only by 0.3%. Through an optimized use of SCMs in the CSA paste, both strength and low hydration heat are guaranteed, apart from the significant improvements on the rheological properties of fresh CSA pastes with SCMs [
10,
26].