Facile Synthesis of Spherical TiO2 Hollow Nanospheres with a Diameter of 150 nm for High-Performance Mesoporous Perovskite Solar Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis of Carbonaceous Nanospheres (CNs)
2.2. Synthesis of the TiO2 Hollow Nanospheres
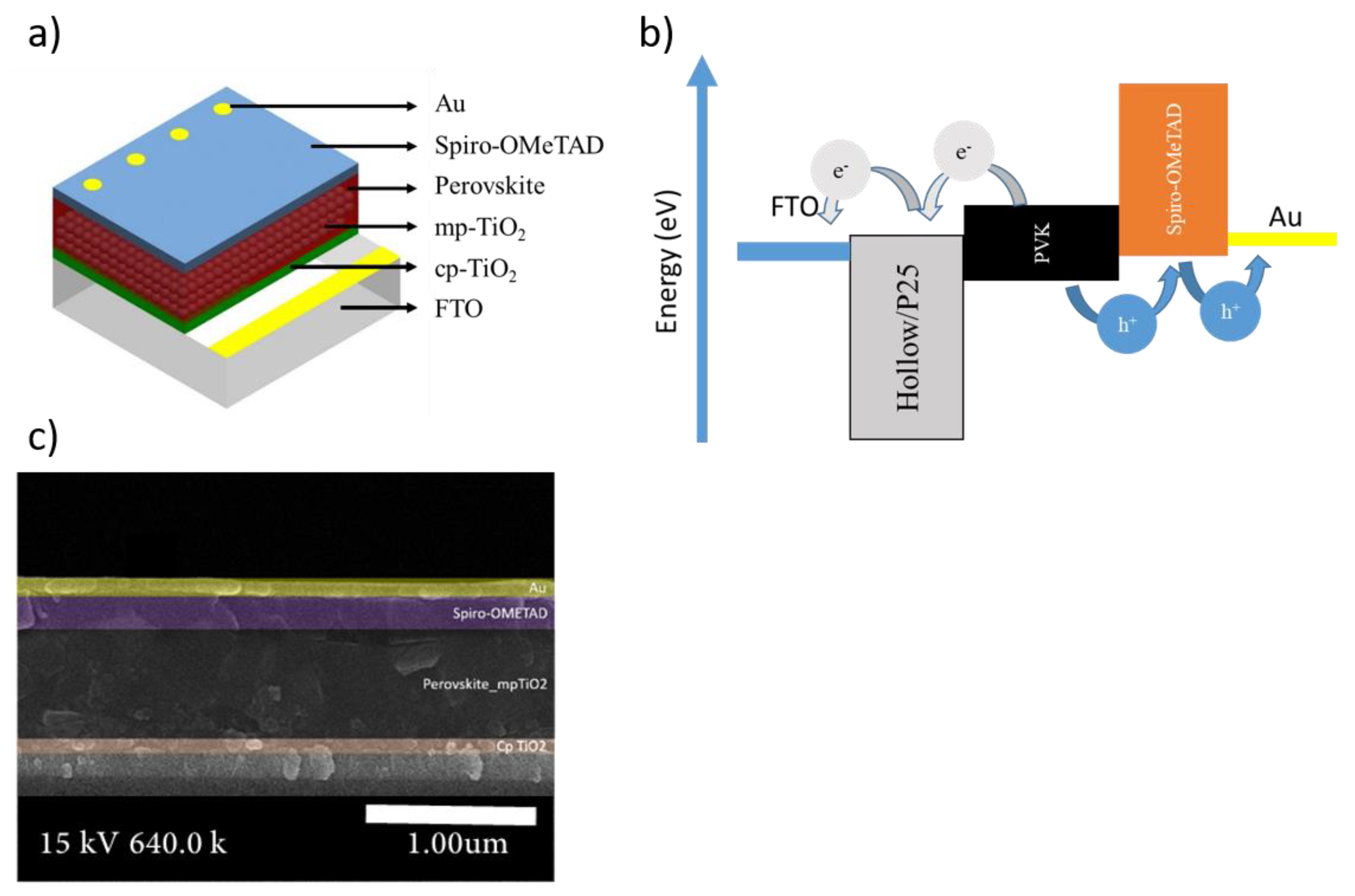
2.3. Fabrication of the Perovskite Solar Cells
2.4. Device Characterization
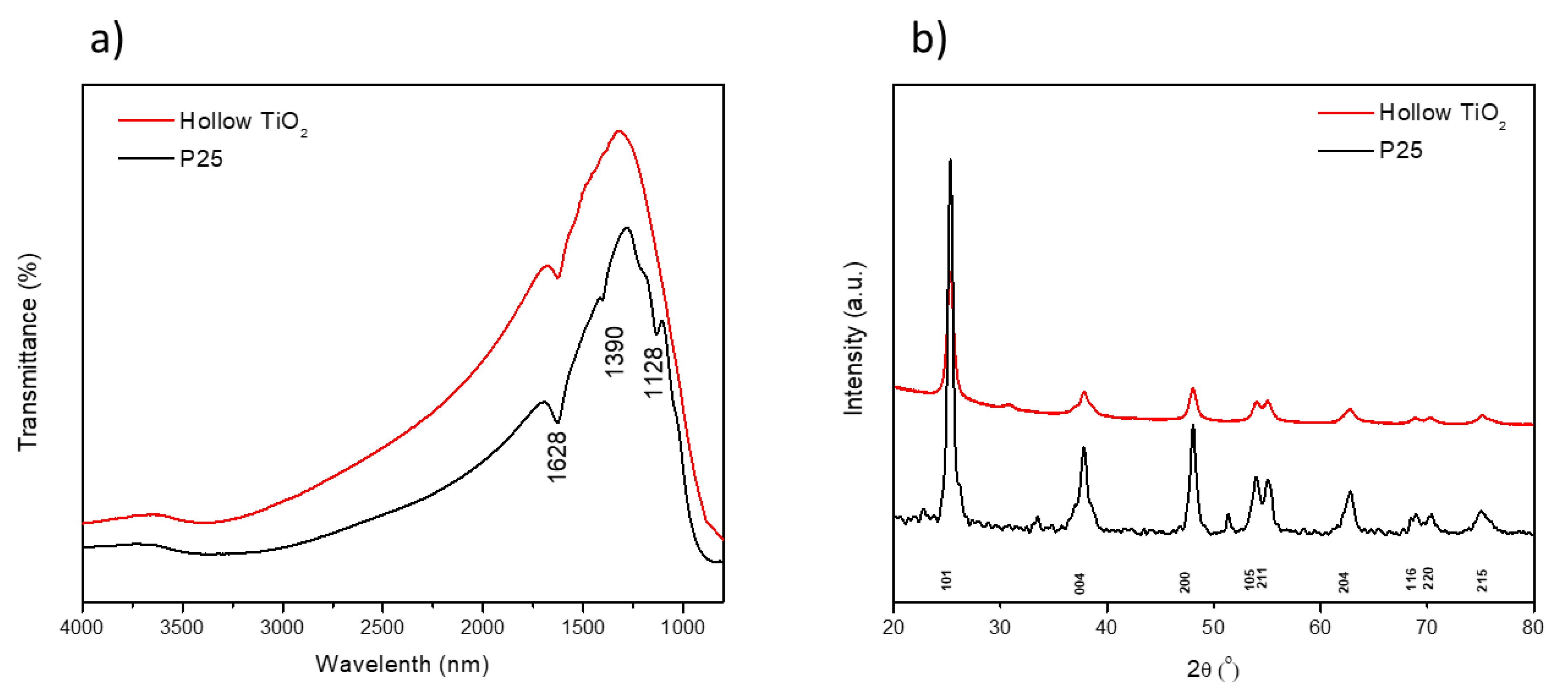
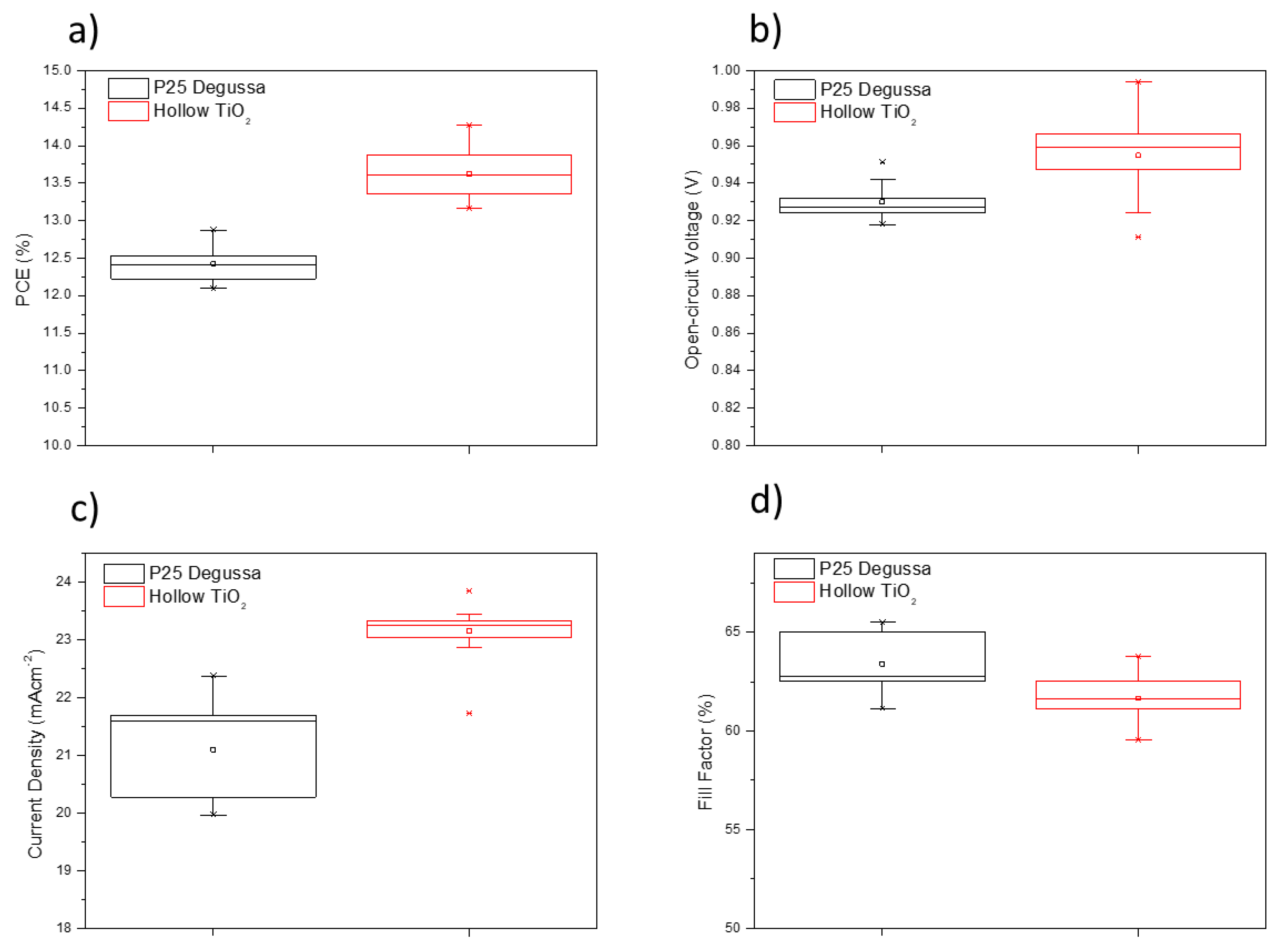
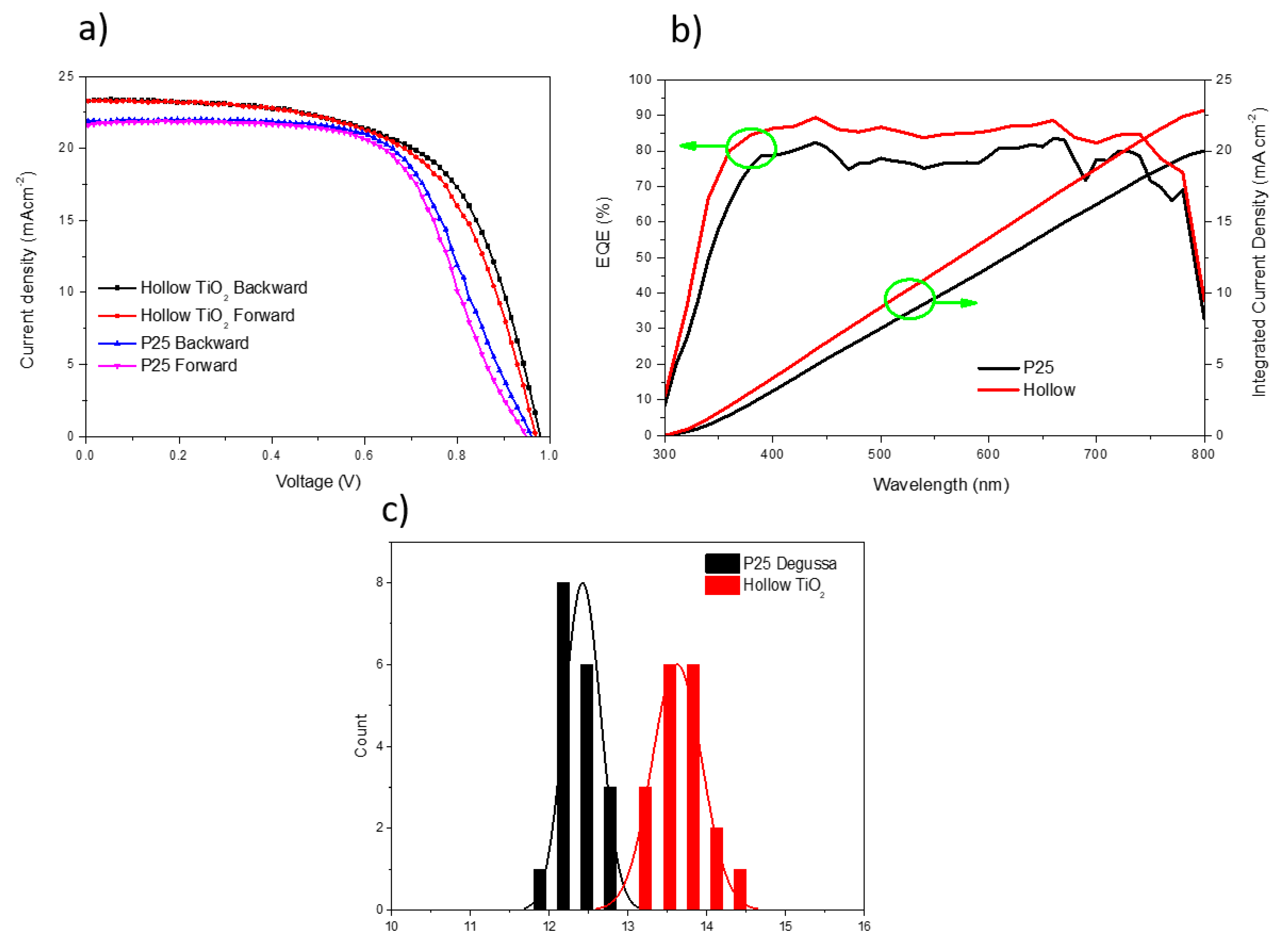
3. Results
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yang, W.S.; Park, B.-W.; Jung, E.H.; Jeon, N.J.; Kim, Y.C.; Lee, D.U.; Shin, S.S.; Seo, J.; Kim, E.K.; Noh, J.H. Iodide management in formamidinium-lead-halide-based perovskite layers for efficient solar cells. Science 2017, 356, 1376–1379. [Google Scholar] [CrossRef] [PubMed]
- Choi, K.; Lee, J.; Kim, H.I.; Park, C.W.; Kim, G.W.; Choi, H.; Park, S.; Park, S.A.; Park, T. Thermally stable, planar hybrid perovskite solar cells with high efficiency. Energy Environ. Sci. 2018, 11, 3238–3247. [Google Scholar] [CrossRef]
- Yang, D.; Yang, R.; Wang, K.; Wu, C.; Zhu, X.; Feng, J.; Ren, X.; Fang, G.; Priya, S.; Liu, S.F. High efficiency planar-type perovskite solar cells with negligible hysteresis using EDTA-complexed SnO2. Nat. Commun. 2018, 9, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.; Zhang, L.; Wang, H.; Yang, X.; Meng, J.; Liu, H.; Yin, Z.; Wu, J.; Zhang, X.; You, J. Enhanced electron extraction using SnO2 for high-efficiency planar-structure HC(NH2)2PbI3-based perovskite solar cells. Nat. Energy 2016, 2, 1–7. [Google Scholar] [CrossRef]
- Eperon, G.E.; Stranks, S.D.; Menelaou, C.; Johnston, M.B.; Herz, L.M.; Snaith, H.J. Formamidinium lead trihalide: A broadly tunable perovskite for efficient planar heterojunction solar cells. Energy Environ. Sci. 2014, 7, 982–988. [Google Scholar]
- Jeon, N.J.; Noh, J.H.; Yang, W.S.; Kim, Y.C.; Ryu, S.; Seo, J.; Seok, S.I. Compositional engineering of perovskite materials for high-performance solar cells. Nature 2015, 517, 476–480. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.; Min, H.; Lee, K.S.; Yoon, S.M.; Seok, S.I. Impact of strain relaxation on performance of α-formamidinium lead iodide perovskite solar cells. Science 2020, 370, 108–112. [Google Scholar]
- NREL. Best Research-Cell Efficiencies; National Renewable Energy Laboratory: Golden, Colorado, 2019. Available online: https://www.nrel.gov/pv/cell-efficiency.html (accessed on 25 April 2020).
- Kim, H.-S.; Park, N.-G. Parameters affecting I–V hysteresis of CH3NH3PbI3 perovskite solar cells: Effects of perovskite crystal size and mesoporous TiO2 layer. J. Phys. Chem. Lett. 2014, 5, 2927–2934. [Google Scholar]
- Chen, H.-W.; Sakai, N.; Ikegami, M.; Miyasaka, T. Emergence of hysteresis and transient ferroelectric response in organo-lead halide perovskite solar cells. J. Phys. Chem. Lett. 2015, 6, 164–169. [Google Scholar] [CrossRef]
- Heo, J.H.; Im, S.H.; Noh, J.H.; Mandal, T.N.; Lim, C.S.; Chang, J.A.; Lee, Y.H.; Kim, H.J.; Sarkar, A.; Nazeeruddin, M.K. Efficient inorganic-organic hybrid heterojunction solar cells containing perovskite compound and polymeric hole conductors. Nat. Photonics 2013, 7, 486–491. [Google Scholar] [CrossRef]
- Burschka, J.; Pellet, N.; Moon, S.J.; Humphry-Baker, R.; Gao, P.; Nazeeruddin, M.K.; Grätzel, M. Sequential deposition as a route to high-performance perovskite-sensitized solar cells. Nature 2013, 499, 316–319. [Google Scholar] [CrossRef] [PubMed]
- Tress, W.; Marinova, N.; Moehl, T.; Zakeeruddin, S.M.; Nazeeruddin, M.K.; Grätzel, M. Understanding the rate-dependent J–V hysteresis, slow time component, and aging in CH3NH3PbI3 perovskite solar cells: The role of a compensated electric field. Energy Environ. Sci. 2015, 8, 995–1004. [Google Scholar] [CrossRef]
- Tsai, H.; Nie, W.; Blancon, J.C.; Stoumpos, C.C.; Asadpour, R.; Harutyunyan, B.; Neukirch, A.J.; Verduzco, R.; Crochet, J.J.; Tretiak, S. High-efficiency two-dimensional Ruddlesden-Popper perovskite solar cells. Nature 2016, 536, 312–316. [Google Scholar] [PubMed]
- Grancini, G.; Roldán-Carmona, C.; Zimmermann, I.; Mosconi, E.; Lee, X.; Martineau, D.; Narbey, S.; Oswald, F.; De Angelis, F.; Graetzel, M. One-Year stable perovskite solar cells by 2D/3D interface engineering. Nat. Commun. 2017, 8, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Yang, I.S.; You, J.S.; Do Sung, S.; Chung, C.W.; Kim, J.; Lee, W.I. Novel spherical TiO2 aggregates with diameter of 100 nm for efficient mesoscopic perovskite solar cells. Nano Energy 2016, 20, 272–282. [Google Scholar] [CrossRef]
- Zhou, Y.; Antonietti, M. Synthesis of very small TiO2 nanocrystals in a room-temperature ionic liquid and their self-assembly toward mesoporous spherical aggregates. J. Am. Chem. Soc. 2003, 125, 14960–14961. [Google Scholar] [CrossRef]
- Luo, P.; Niu, H.; Zheng, G.; Bai, X.; Zhang, M.; Wang, W. Enhancement of photoelectric conversion by high-voltage electric field assisted crystallization of a novel ternary-encapsulated spherical TiO2 aggregate for solar cells. Electrochim. Acta 2010, 55, 2697–2705. [Google Scholar]
- Liao, H.C.; Guo, P.; Hsu, C.P.; Lin, M.; Wang, B.; Zeng, L.; Huang, W.; Soe, C.M.M.; Su, W.F.; Bedzyk, M.J. Enhanced efficiency of hot-cast large-area planar perovskite solar cells/modules having controlled chloride incorporation. Adv. Energy Mater. 2017, 7, 1601660. [Google Scholar] [CrossRef]
- Mei, A.; Li, X.; Liu, L.; Ku, Z.; Liu, T.; Rong, Y.; Xu, M.; Hu, M.; Chen, J.; Yang, Y. A hole-conductor-free, fully printable mesoscopic perovskite solar cell with high stability. Science 2014, 345, 295–298. [Google Scholar] [CrossRef]
- Nie, W.; Tsai, H.; Asadpour, R.; Blancon, J.-C.; Neukirch, A.J.; Gupta, G.; Crochet, J.J.; Chhowalla, M.; Tretiak, S.; Alam, M.A. High-efficiency solution-processed perovskite solar cells with millimeter-scale grains. Science 2015, 347, 522–525. [Google Scholar] [CrossRef]
- Wang, H.; Jiang, R.; Sun, M.; Yin, X.; Guo, Y.; He, M.; Wang, L. Titanate hollow nanospheres as electron-transport layer in mesoscopic perovskite solar cell with enhanced performance. J. Mater. Chem. C 2019, 7, 1948–1954. [Google Scholar] [CrossRef]
- Ma, S.; Ye, T.; Wu, T.; Wang, Z.; Wang, Z.; Ramakrishna, S.; Vijila, C.; Wei, L. Hollow rice grain-shaped TiO2 nanostructures for high-efficiency and large-area perovskite solar cells. Sol. Energy Mater. Sol. Cells 2019, 191, 389–398. [Google Scholar] [CrossRef]
- Sun, X.; Li, Y. Colloidal carbon spheres and their core/shell structures with noble-metal nanoparticles. Angew. Chem. 2004, 116, 607–611. [Google Scholar] [CrossRef]
- Cui, J.; Hu, C.; Yang, Y.; Wu, Y.; Yang, L.; Wang, Y.; Liu, Y.; Jiang, Z. Facile fabrication of carbonaceous nanospheres loaded with silver nanoparticles as antibacterial materials. J. Mater. Chem. 2012, 22, 8121–8126. [Google Scholar] [CrossRef]
- Du, Q.-S.; Tang, P.-D.; Huang, H.-L.; Du, F.-L.; Huang, K.; Xie, N.-Z.; Long, S.-Y.; Li, Y.-M.; Qiu, J.-S.; Huang, R.B. A new type of two-dimensional carbon crystal prepared from 1, 3, 5-trihydroxybenzene. Sci. Rep. 2017, 7, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Sun, D.D.; Guo, P.; Leckie, J.O. One-step fabrication and high photocatalytic activity of porous TiO2 hollow aggregates by using a low-temperature hydrothermal method without templates. Chem. Eur. J. 2007, 13, 1851–1855. [Google Scholar] [CrossRef] [PubMed]
- Ye, M.; Chen, Z.; Wang, W.; Shen, J.; Ma, J. Hydrothermal synthesis of TiO2 hollow microspheres for the photocatalytic degradation of 4-chloronitrobenzene. J. Hazard. Mater. 2010, 184, 612–619. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Wu, F.; Chi, D.; Shi, K.; Huang, S. High-efficiency perovskite solar cells with poly (vinylpyrrolidone)-doped SnO2 as an electron transport layer. Mater. Adv. 2020, 1, 617–624. [Google Scholar] [CrossRef]
- Jiang, Q.; Chu, Z.; Wang, P.; Yang, X.; Liu, H.; Wang, Y.; Yin, Z.; Wu, J.; Zhang, X.; You, J. Planar-structure perovskite solar cells with efficiency beyond 21%. Adv. Mater. 2017, 29, 1703852. [Google Scholar] [CrossRef]
- Yang, W.S.; Noh, J.H.; Jeon, N.J.; Kim, Y.C.; Ryu, S.; Seo, J.; Seok, S.I. High-performance photovoltaic perovskite layers fabricated through intramolecular exchange. Science 2015, 348, 1234–1237. [Google Scholar] [CrossRef]
- Jeon, N.J.; Noh, J.H.; Kim, Y.C.; Yang, W.S.; Ryu, S.; Seok, S.I. Solvent engineering for high-performance inorganic-organic hybrid perovskite solar cells. Nat. Mater. 2014, 13, 897–903. [Google Scholar] [CrossRef] [PubMed]
- Anaya, M.; Zhang, W.; Hames, B.C.; Li, Y.; Fabregat-Santiago, F.; Calvo, M.E.; Snaith, H.J.; Míguez, H.; Mora-Seró, I. Electron injection and scaffold effects in perovskite solar cells. J. Mater. Chem. C 2017, 5, 634–644. [Google Scholar] [CrossRef] [PubMed]
- Mei, Y.; Liu, H.; Li, X.; Wang, S. Hollow TiO2 spheres as mesoporous layer for better efficiency and stability of perovskite solar cells. J. Alloys Compd. 2020, 158079. [Google Scholar] [CrossRef]
- Huang, Y.; Zhu, J.; Ding, Y.; Chen, S.; Zhang, C.; Dai, S. TiO2 sub-microsphere film as scaffold layer for efficient perovskite solar cells. ACS Appl. Mater. Interfaces 2016, 8, 8162–8167. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Yu, Z.; Cheng, N.; Wang, C.; Gong, Y.; Bai, S.; Zhao, X.-Z. Low-cost and Efficient Hole-Transport-Material-free perovskite solar cells employing controllable electron-transport layer based on P25 nanoparticles. Electrochim. Acta 2016, 213, 83–88. [Google Scholar] [CrossRef]
- Huang, H.; Shi, J.; Lv, S.; Li, D.; Luo, Y.; Meng, Q. Sprayed P25 scaffolds for high-efficiency mesoscopic perovskite solar cells. Chem. Commun. 2015, 51, 10306–10309. [Google Scholar] [CrossRef]


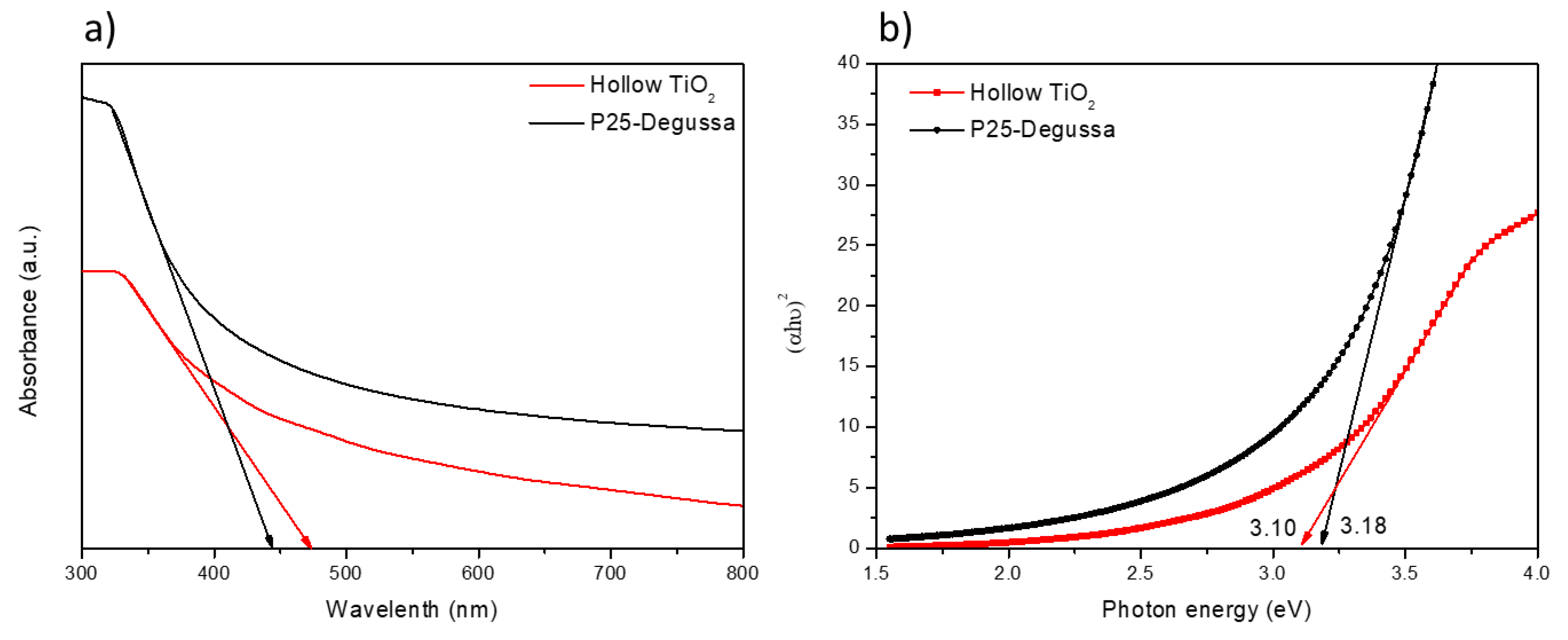


| TiO2 | Diameter (nm) | Bandgap (eV) | Surface Area (m2g−1) | Average Pore Size (nm) |
|---|---|---|---|---|
| P25 | <25 | 3.18 | 54.32 | 15.81 |
| TiO2 hollow | ~150 | 3.10 | 85.23 | 11.24 |
| PSCs | JSC (mAcm−2) | VOC (V) | FF | PCE (%) | |
|---|---|---|---|---|---|
| P25 | Champion | 22.369 | 0.951 | 0.65 | 12.87 |
| Average | 21.097 ± 0.068 | 0.930 ± 0.021 | 0.63 ± 0.021 | 12.42 ± 0.45 | |
| TiO2 hollow | Champion | 23.842 | 0.994 | 0.64 | 14.27 |
| Average | 23.149 ± 0.693 | 0.955 ± 0.039 | 0.61 ± 0.021 | 13.62 ± 0.646 | |
| Particle Size (nm) | Structural Type | Preparation Method | JSC (mAcm−2) | VOC (V) | FF | PCE (%) | Ref. |
|---|---|---|---|---|---|---|---|
| 150 nm | Hollow nanospheres | Sol-gel reaction | 23.84 | 0.99 | 0.64 | 14.27 | This study |
| 200 nm | Hollow nanospheres | Sol-gel reaction | 22.23 | 1.07 | 0.74 | 17.60 | [34] |
| 250 nm | Hollow nanospheres | Sol-gel reaction | 23.92 | 1.01 | 0.65 | 15.87 | [22] |
| 300 nm | Hollow rice grain-shaped | Electro-spinning | 21.60 | 1.07 | 0.61 | 4.20 | [23] |
| 100 nm | Spherical aggregates | Hydrothermal reaction | 22.91 | 1.04 | 0.75 | 18.41 | [16] |
| 250 nm | Spherical aggregates | Sol-gel reaction | 19.41 | 1.05 | 0.73 | 15.01 | [35] |
| <25 nm | Nanoparticles | Commercial | 20.83 | 0.89 | 0.67 | 12.48 | [36] |
| <25 nm | Nanoparticles | Commercial | 20.3 | 974.1 | 0.71 | 14.1 | [37] |
| <25 nm | Nanoparticles | Commercial | 22.369 | 0.951 | 0.65 | 12.87 | This study |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quy, H.V.; Truyen, D.H.; Kim, S.; Bark, C.W. Facile Synthesis of Spherical TiO2 Hollow Nanospheres with a Diameter of 150 nm for High-Performance Mesoporous Perovskite Solar Cells. Materials 2021, 14, 629. https://doi.org/10.3390/ma14030629
Quy HV, Truyen DH, Kim S, Bark CW. Facile Synthesis of Spherical TiO2 Hollow Nanospheres with a Diameter of 150 nm for High-Performance Mesoporous Perovskite Solar Cells. Materials. 2021; 14(3):629. https://doi.org/10.3390/ma14030629
Chicago/Turabian StyleQuy, Hoang Van, Dang Hai Truyen, Sangmo Kim, and Chung Wung Bark. 2021. "Facile Synthesis of Spherical TiO2 Hollow Nanospheres with a Diameter of 150 nm for High-Performance Mesoporous Perovskite Solar Cells" Materials 14, no. 3: 629. https://doi.org/10.3390/ma14030629
APA StyleQuy, H. V., Truyen, D. H., Kim, S., & Bark, C. W. (2021). Facile Synthesis of Spherical TiO2 Hollow Nanospheres with a Diameter of 150 nm for High-Performance Mesoporous Perovskite Solar Cells. Materials, 14(3), 629. https://doi.org/10.3390/ma14030629







