Comparison of the Properties of Natural Sorbents for the Calcium Looping Process
Abstract
1. Introduction
2. Materials and Methods
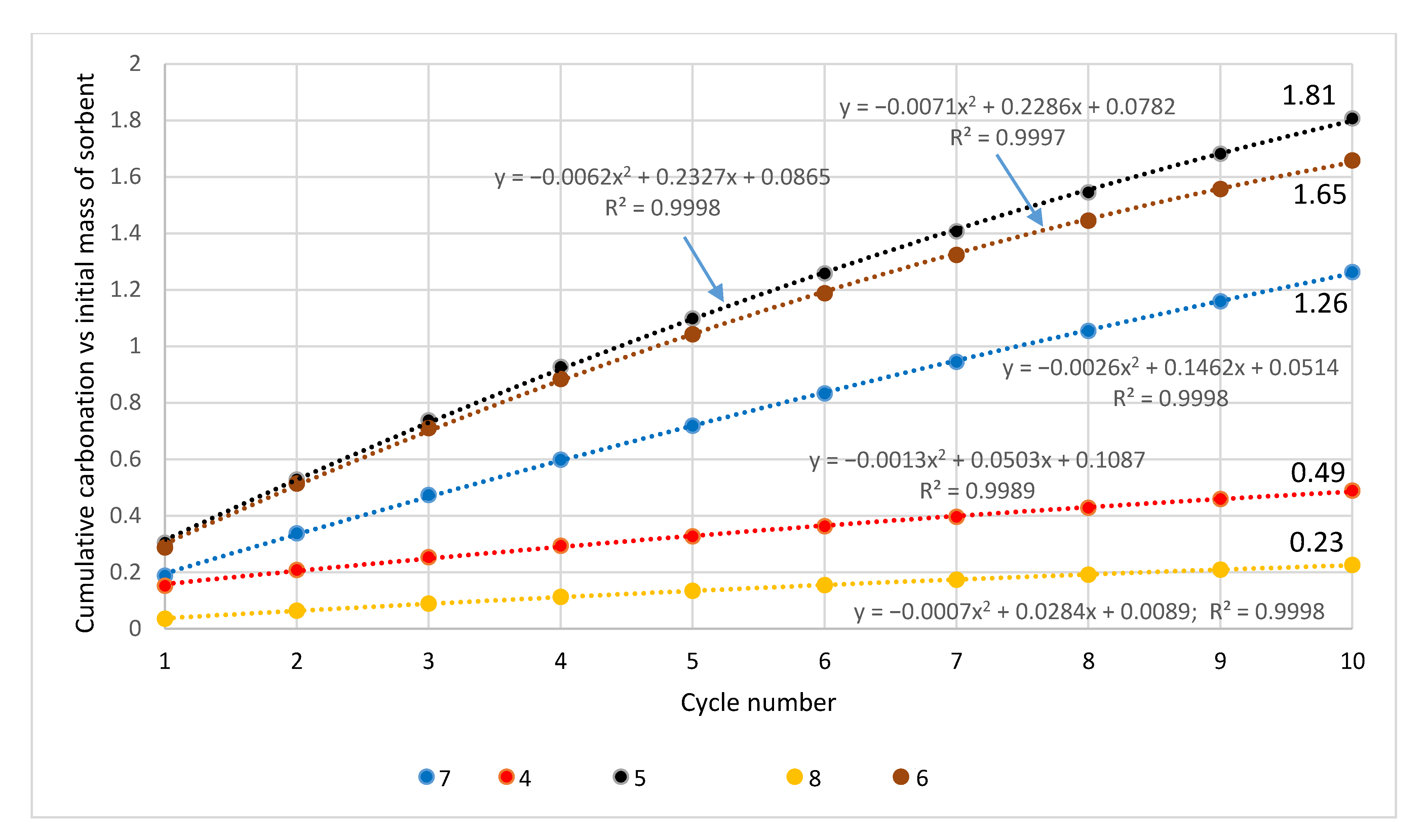
3. Results
3.1. Raw Sorbents
3.1.1. Dolomite
3.1.2. Saint Anne Mountain Limestone
3.1.3. Marl
3.1.4. Nephelinite
3.1.5. Magnesite
3.1.6. Serpentinite
3.2. Thermal Pretreatment of Sorbents
- Doping—aimed at postponing or avoiding sintering of sorbent in order to moderate sintering and abrasion of the sorbent (e.g., [18,19]). The effectiveness of doping depends on the concentration of the substrate used. Too low of a concentration will have no effect, while too high of a concentration may block the pores [12,20];
4. Discussion
5. Summary and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- International Energy Agency. Energy and Climate Change—World Energy Outlook—Special Briefing for COP21; International Energy Agency: Paris, France, 2015. [Google Scholar]
- Olivier, J.G.J.; Janssens-Maenhout, G.; Muntean, M.; Peters, J.A.H.W. Trends in Global CO2 Emissions-2015 Report; No: JRC98184; PBL Netherlands Environmental Assessment Agency: The Hague, The Netherlands, 2015. [Google Scholar]
- Sanna, A.; Uibu, M.; Caramanna, G.; Kuusik, R.; Maroto-Valer, M.M. A review of mineral carbonation technologies to sequester CO2. Chem. Soc. Rev. 2014, 43, 8049. [Google Scholar] [CrossRef] [PubMed]
- Gislason, S.R.; Wolff-Boenisch, D.; Stefansson, A.; Oelkers, E.H.; Gunnlaugsson, E.; Sigurdardottir, H.; Sigfusson, B.; Broecker, W.S.; Matter, J.M.; Stute, M.; et al. Mineral sequestration of carbon dioxide in basalt: A pre-injection overview of the carbfix project. Int. J. Greenh. Gas Contr. 2010, 4, 537–545. [Google Scholar] [CrossRef]
- McKelvy, M.J.; Chizmeshya, A.V.G.; Diefenbacher, J.; Béarat, H.; Wolf, G. Exploration of the role of heat activation in enhancing serpentine carbon sequestration reactions. Environ. Sci. Technol. 2004, 38, 6897–6903. [Google Scholar] [CrossRef] [PubMed]
- Olajire, A.A. A review of mineral carbonation technology in sequestration of CO2. J. Pet. Sci. Eng. 2013, 109, 364–392. [Google Scholar] [CrossRef]
- Werner, M.; Verduyn, M.; van Mossel, G.; Mazzotti, M. Direct flue gas CO2 mineralization using activated serpentine: Exploring the reaction kinetics by experiments and population balance modelling. Energy Procedia 2014, 4, 2043–2049. [Google Scholar] [CrossRef][Green Version]
- Huijgen, W.J.J.; Comans, R.N.J. Carbon Dioxide Sequestration by Mineral Carbonation: Literature Review; ECN—Clean Fossil Fuels Environmental Risk Assessment: Petten, The Netherlands, 2003; p. 112. [Google Scholar]
- Kirsch, K. CO2-Induced Metal Release from Sandstones: Implications for Geologic Carbon Sequestration. Master’s Thesis, Hydrologic Science and Engineering Faculty, School of Mines, Golden, CO, USA, 2013. [Google Scholar]
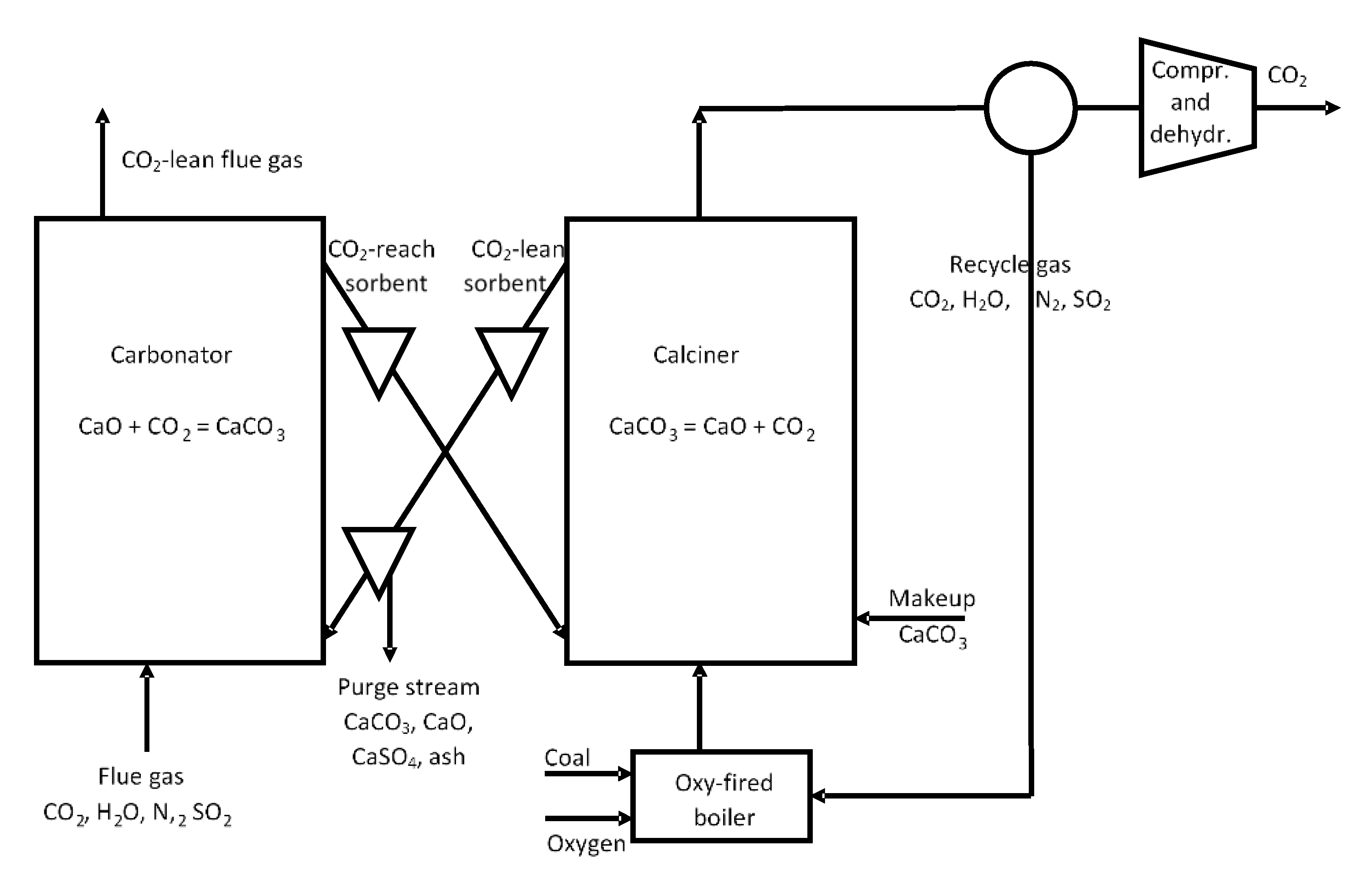
- Blamey, J.; Anthony, E.J.; Wang, J.; Fennell, P.S. The calcium looping cycle for large-scale CO2 capture. Prog. Energy Combust. Sci. 2010, 36, 260–279. [Google Scholar] [CrossRef]
- Laursen, K.; Duo, W.; Grace, J.R.; Lim, J. Sulfation and reactivation characteristics of nine limestones. Fuel 2000, 79, 153–163. [Google Scholar] [CrossRef]
- Dean, C.C.; Blamey, J.; Florin, N.H.; Al-Jeboori, M.J.; Fennell, P.S. The calcium looping cycle for CO2 capture from power generation, cement manufacture and hydrogen production. Chem. Eng. Res. Des. 2011, 89, 836–855. [Google Scholar] [CrossRef]
- Mantripragadaa, H.C.; Rubin, E.S. Calcium looping cycle for CO2 capture: Performance, cost and feasibility analysis. Energy Procedia 2014, 63, 2199–2206. [Google Scholar] [CrossRef]
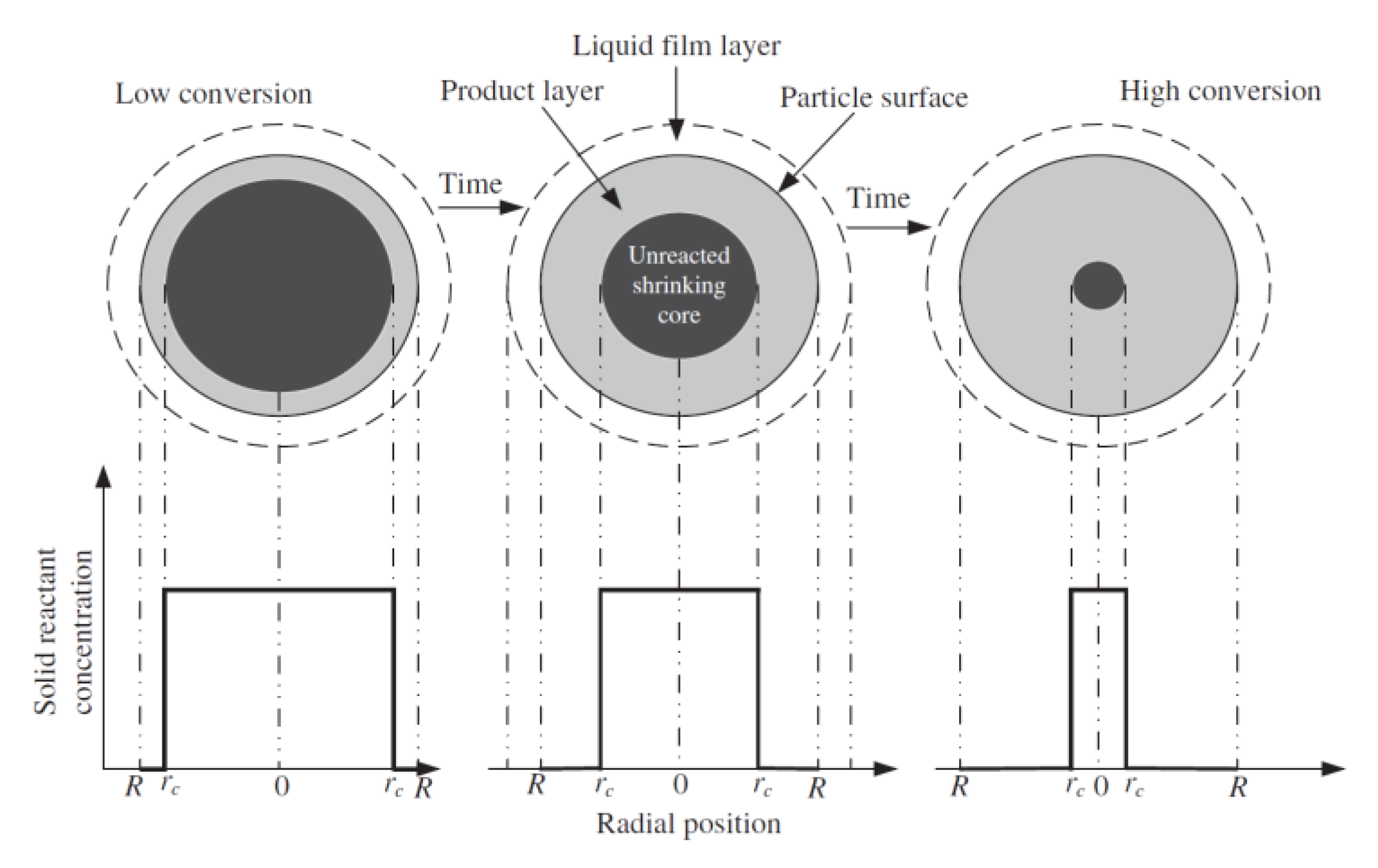
- Lee, D. An apparent kinetic model for the carbonation of calcium oxide by carbon dioxide. Chem. Eng. J. 2004, 100, 71–77. [Google Scholar] [CrossRef]
- Oakeson, W.G.; Cutler, I.B. Effect of CO2 Pressure on the Reaction with CaO. J. Am. Ceram. Soc. 2006, 62, 556–558. [Google Scholar] [CrossRef]
- Butler, J.; Lim, J.; Grace, J. Kinetics of CO2 absorption by CaO through pressure swing cycling. Fuel 2014, 127, 78–87. [Google Scholar] [CrossRef]
- Levenspiel, O. Chemical Reaction Engineering, 2nd ed.; John Wiley and Sons: New York, NY, USA, 1972. [Google Scholar]
- Salvador, C.; Lu, D.; Anthony, E.J.; Abanades, J.C. Enhancement of CaO for CO2 capture in an FBC environment. Chem. Eng. J. 2003, 96, 187–195. [Google Scholar] [CrossRef]
- González, B.; Blamey, J.; McBride-Wright, M.; Carter, N.; Dugwell, D.; Fennell, P. Calcium looping for CO2 capture: Sorbent enhancement through doping. Energy Procedia 2011, 4, 402–409. [Google Scholar] [CrossRef]
- Manovic, V.; Anthony, E.J. Lime-Based sorbents for high-temperature CO2 capture—A review of sorbent modification methods. Int. J. Environ. Res. Public Health 2010, 7, 3129–3140. [Google Scholar] [CrossRef]
- Li, Y.J.; Zhao, C.S.; Duan, L.B.; Liang, C.; Li, Q.Z.; Zhou, W. Cyclic calcination/carbonation looping of dolomite modified with acetic acid for CO2 capture. Fuel Process. Technol. 2008, 89, 1461–1469. [Google Scholar] [CrossRef]
- Ridha, F.N.; Manovic, V.; Macchi, A.; Anthony, E.J. The effect of SO2 on CO2 capture by CaO-based pellets prepared with a kaolin derived Al(OH)3 binder. Appl. Energy 2012, 92, 415–520. [Google Scholar] [CrossRef]
- Erans, M.; Manovic, V.; Anthony, E.J. Calcium looping sorbents for CO2 capture. Appl. Energy 2016, 180, 722–742. [Google Scholar] [CrossRef]
- Albrecht, K.O.; Wagenbach, K.S.; Satrio, J.A.; Shanks, B.H.; Wheelock, T.D. Development of a CaO-based sorbent with improved cyclic stability. Ind. Eng. Chem. Res. 2008, 47, 7841–7848. [Google Scholar] [CrossRef]
- Manovic, V.; Anthony, E.J. Thermal Activation of CaO-Based Sorbent and Self-Reactivation during CO2 Capture Looping Cycles. Environ. Sci. Technol. 2008, 42, 4170–4174. [Google Scholar] [CrossRef]
- Manovic, V.; Anthony, E.J.; Grasa, G.; Abanades, J.C. CO2 Looping Cycle Performance of a High-Purity Limestone after Thermal Activation/Doping. Energy Fuels 2008, 22, 3258–3264. [Google Scholar] [CrossRef]
- Lysikov, A.I.; Salanov, A.N.; Okunev, A.G. Change of CO2 carrying capacity of CaO in isothermal recarbonation-decomposition cycles. Ind. Eng. Chem. Res. 2007, 46, 4633–4638. [Google Scholar] [CrossRef]
- Arias, B.; Grasa, G.S.; Alonso, M.; Abanades, J.C. Post-combustion calcium looping process with a highly stable sorbent activity by recarbonation. Energy Environ. Sci 2012, 5, 7353–7359. [Google Scholar] [CrossRef]
- Chen, Z.; Song, H.S.; Portillo, M.; Lim, C.J.; Grace, J.R.; Anthony, E.J. Long-term calcination/carbonation cycling and thermal pretreatment for CO2 capture by limestone and dolomite. Energy Fuels 2009, 23, 1437–1444. [Google Scholar] [CrossRef]
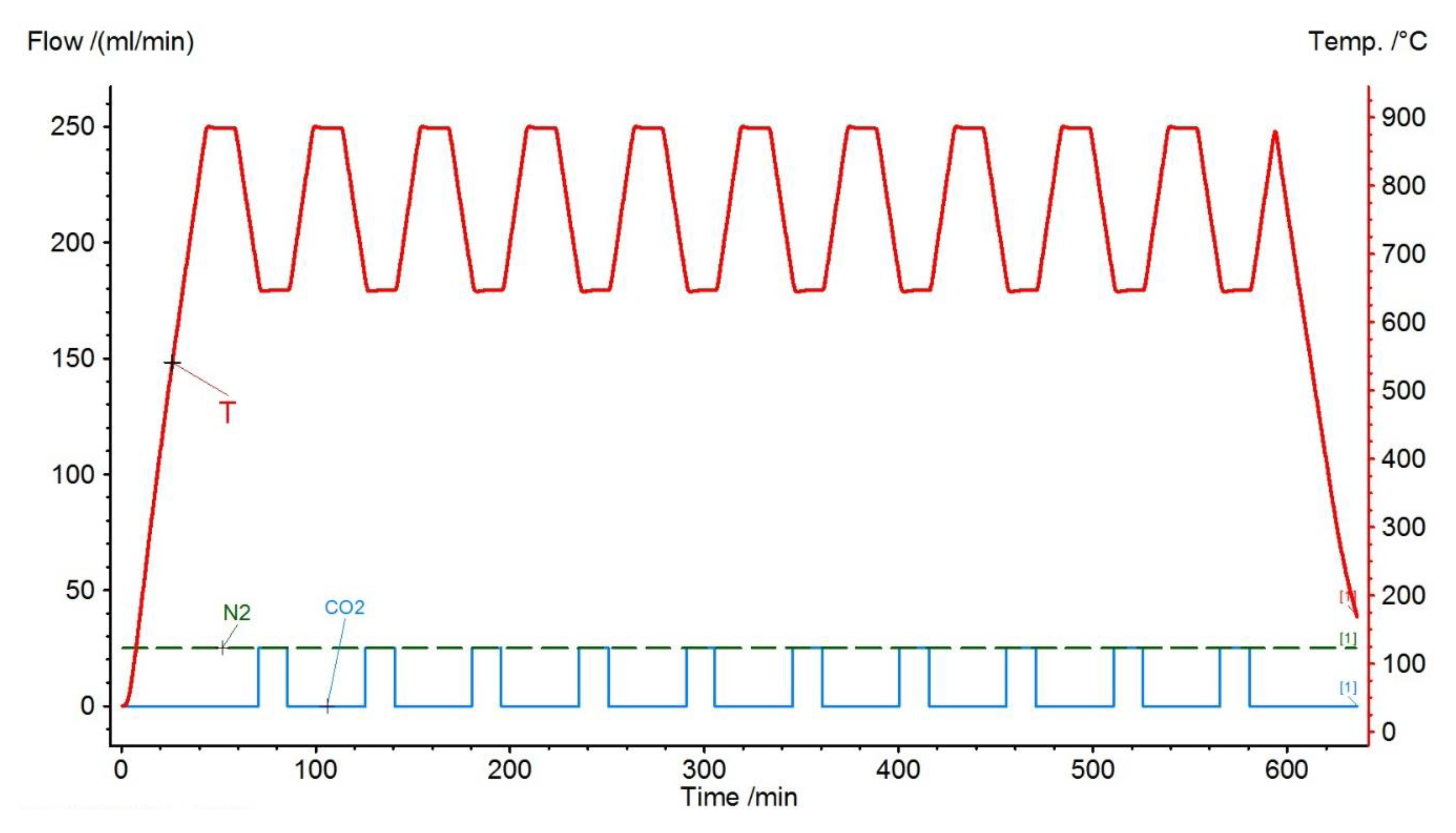



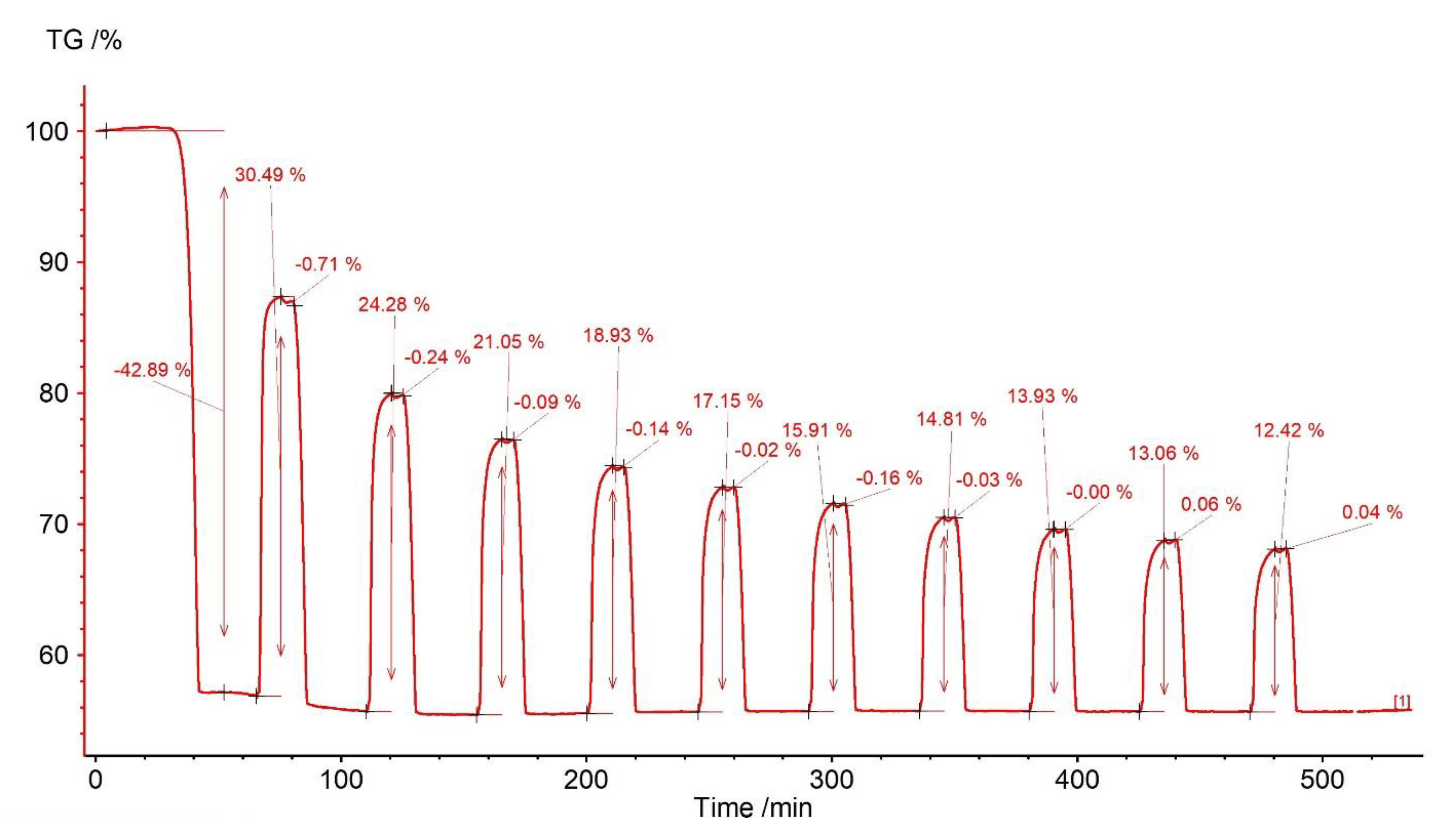
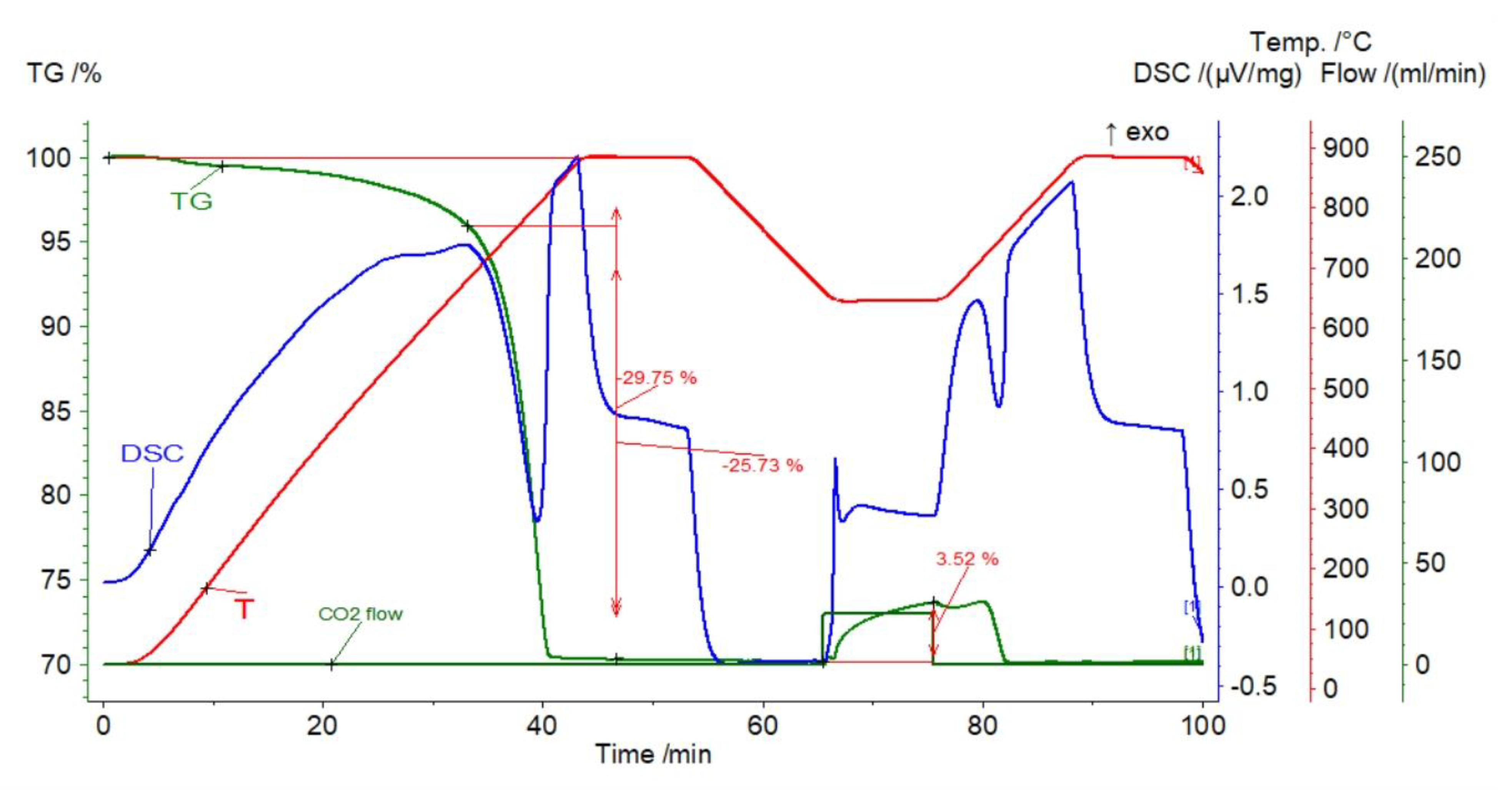


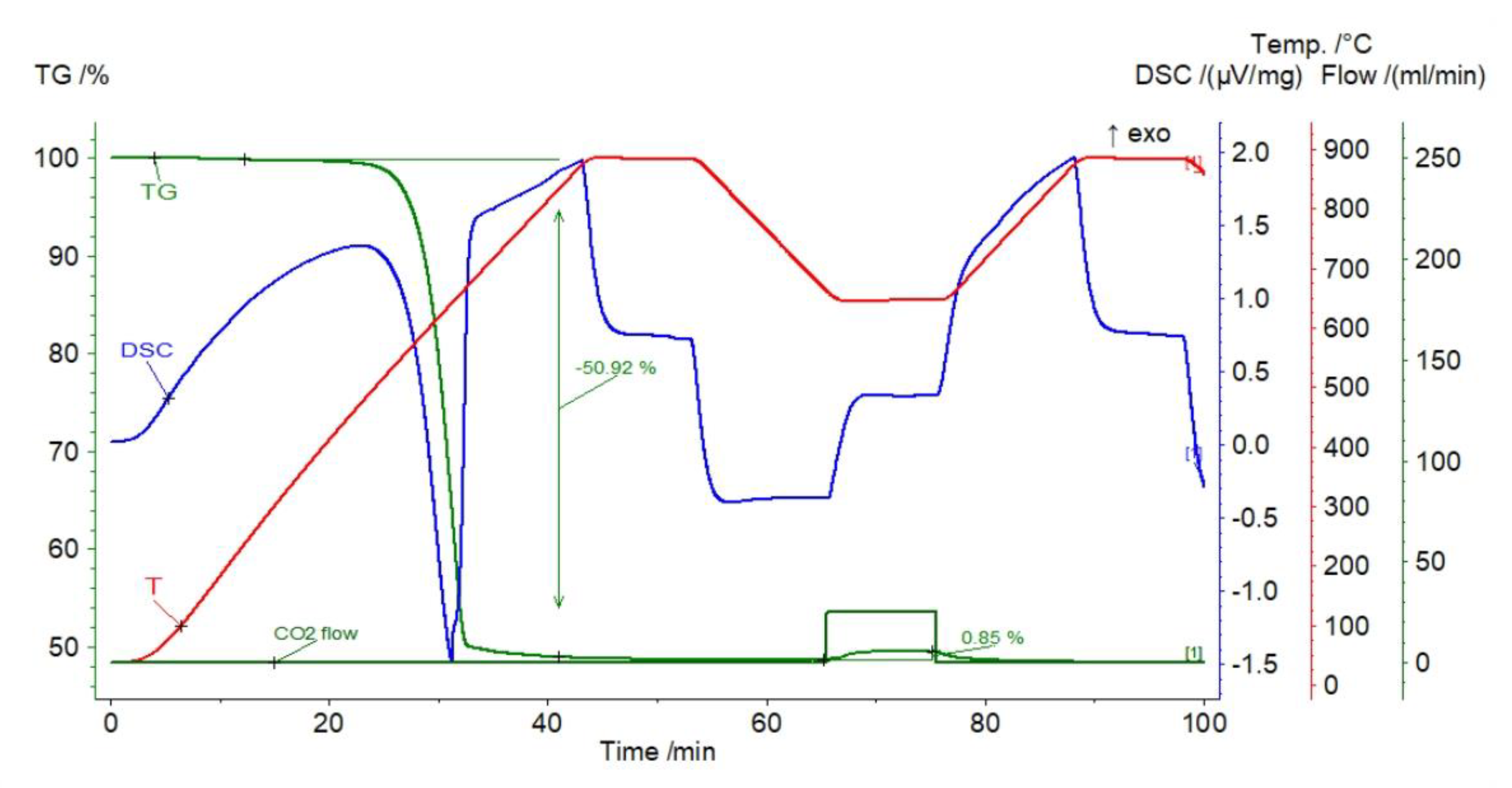
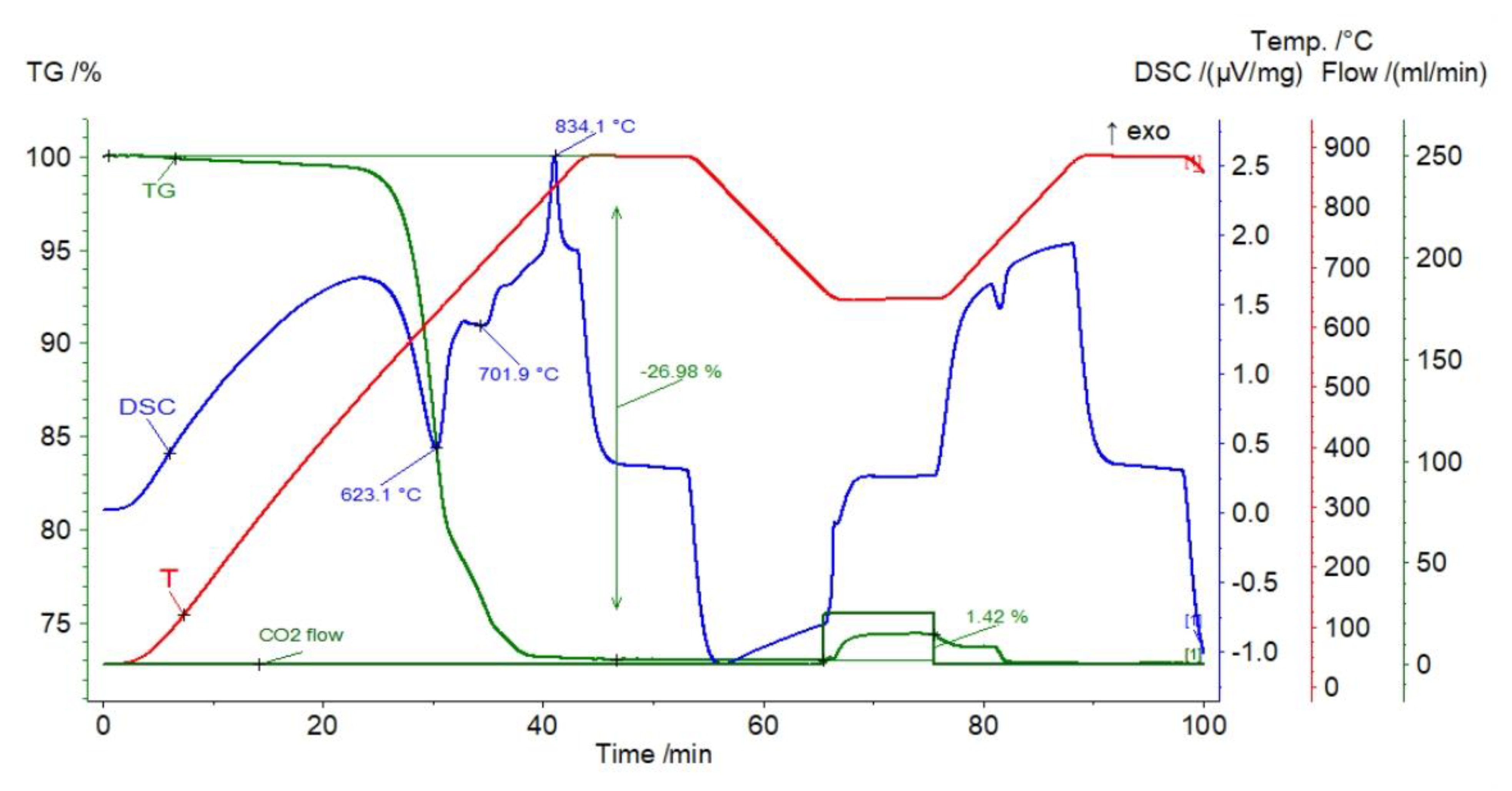
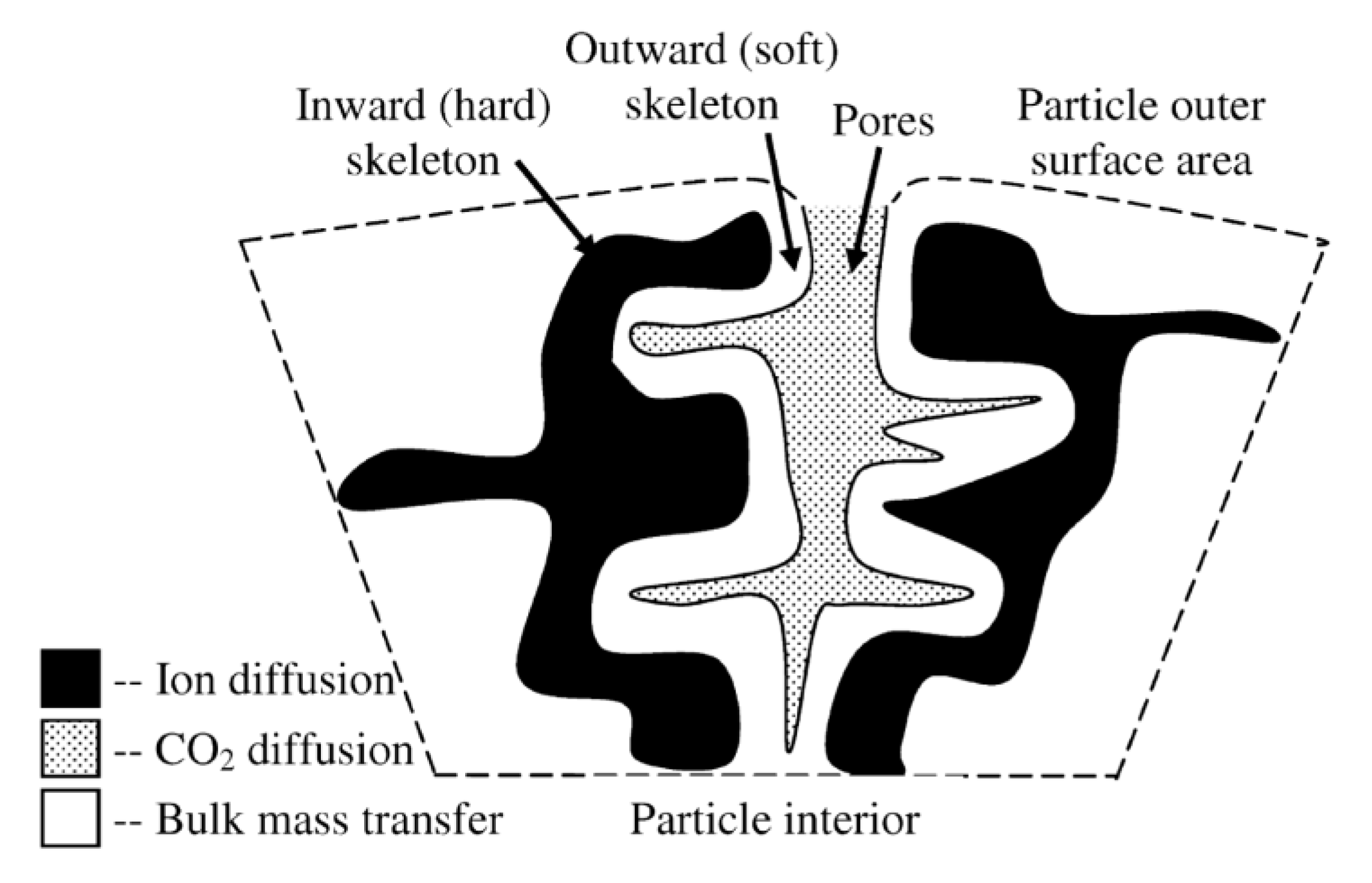
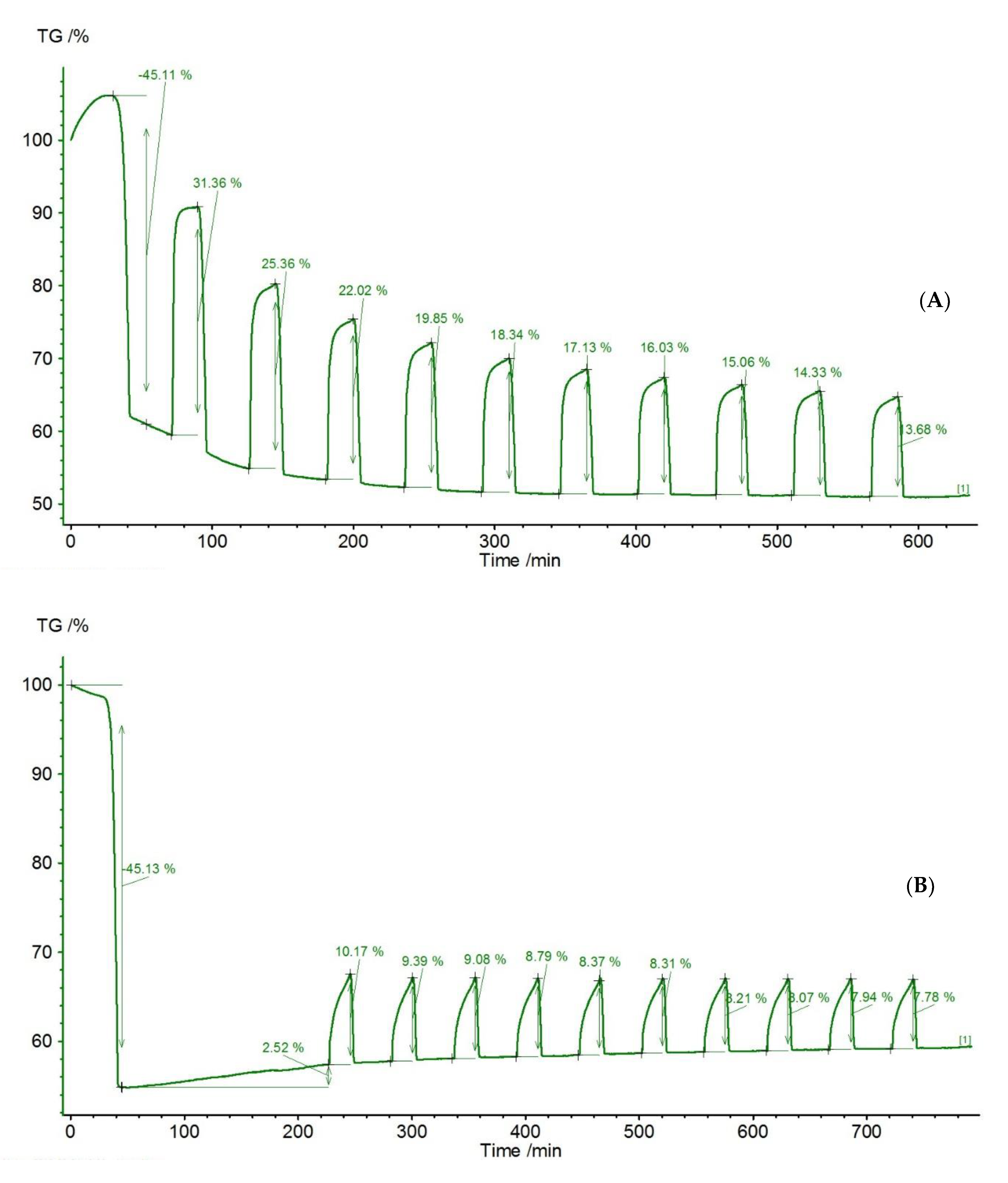
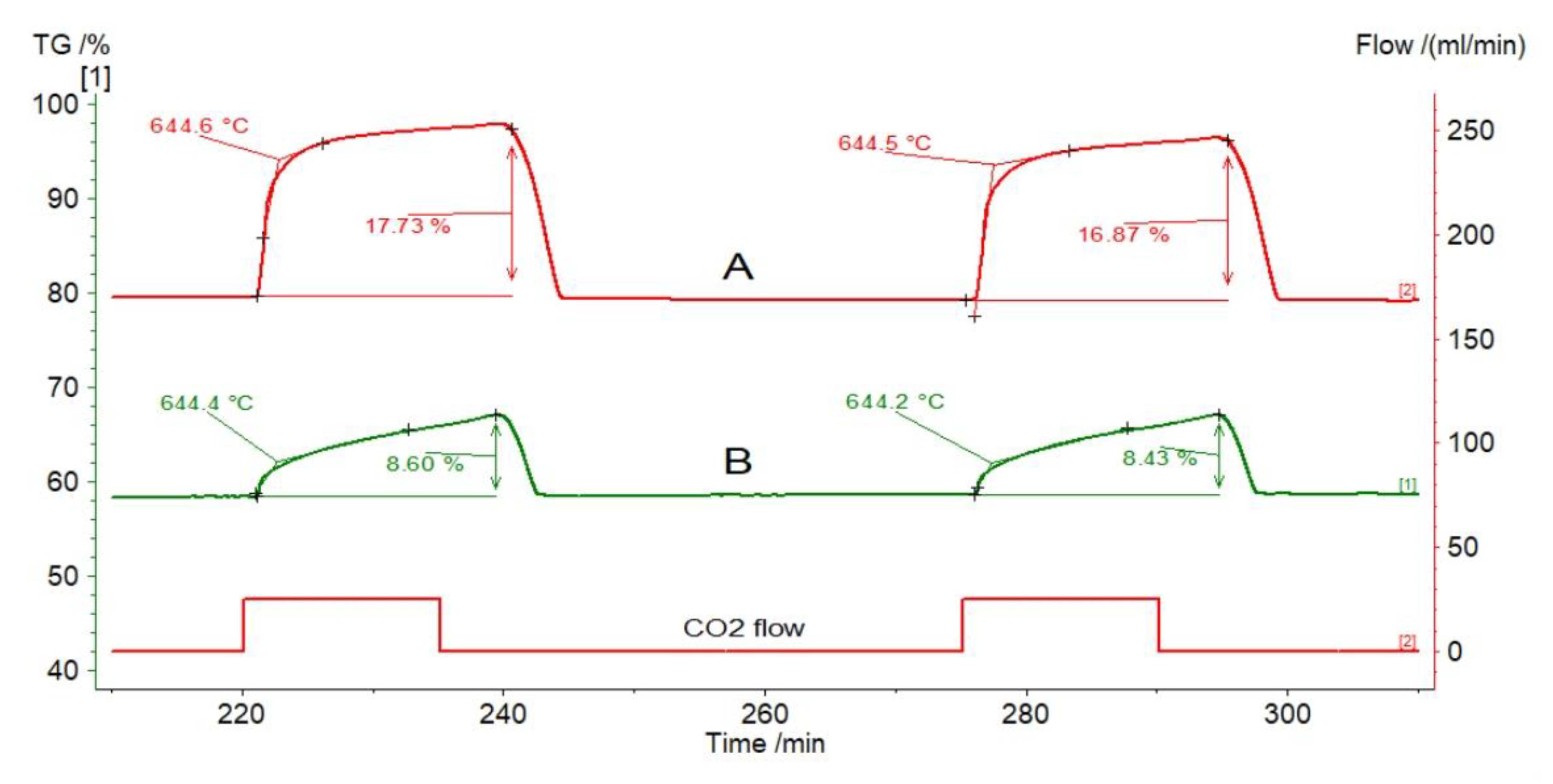
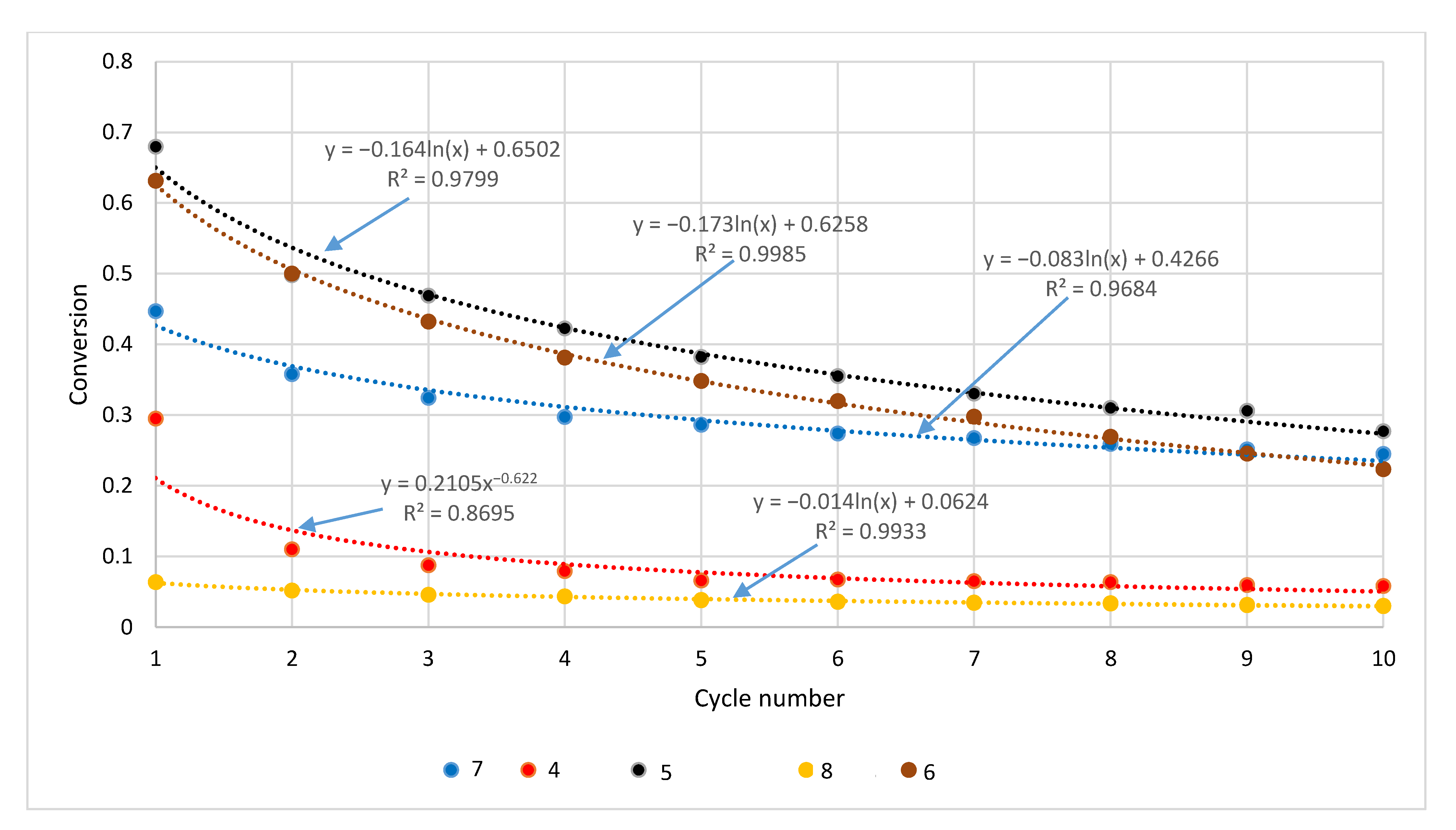
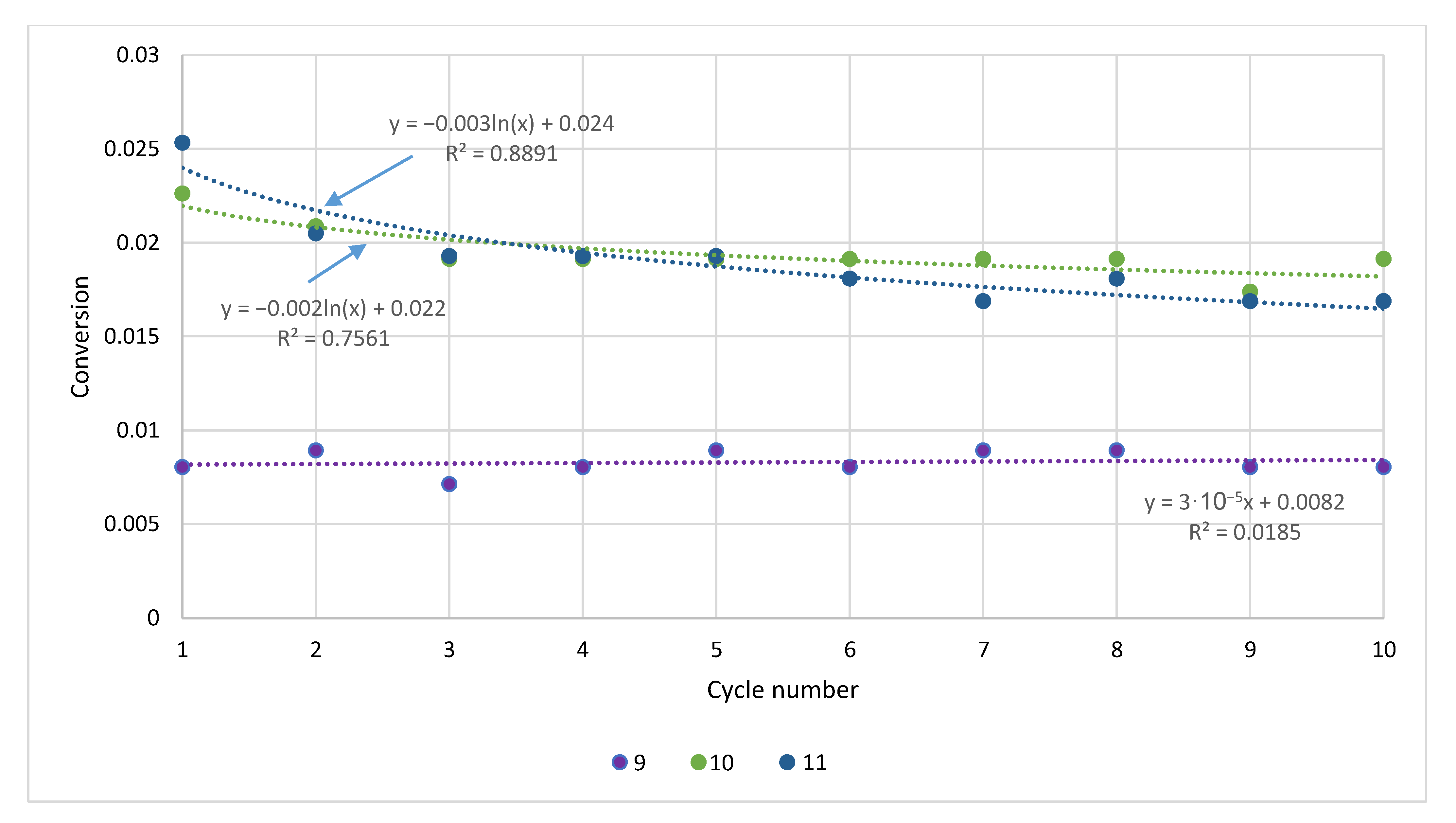

| Sample | Rock Type | Site/Age | Sample Mass [mg] | Mass Loss [%] | CaCO3 [%] |
|---|---|---|---|---|---|
| 1 | limestone | Stramberk(Czechia)/Jurassic | 20.02 | 41.57 | 90.1 |
| 2 | limestone | Podlesie (Poland)/Devonian | 19.58 | 39.00 | 84.5 |
| 3 | limestone | Butkov (Slovakia)/Cretaceous | 20.32 | 31.81 | 68.9 |
| 4 | bituminous limestone | Dębnik (Poland)/Devonian | 14.72 | 34.51 | 78.5 |
| 5 | limestone | Saint Anne Mountain (Poland)/Triassic | 15.46 | 42.88 | 97.5 |
| 6 | limestone | Gorazdze (Poland)/Triassic | 13.9 | 42.09 | 95.7 |
| 7 | dolomite | Olkusz (Poland)/Triassic | 14.99 | 46.06 | 94.4 1 |
| 8 | marl | Cisownica (Poland)/Cretaceous | 15.09 | 29.75 | 67.7 |
| 9 | basalt (nefelinite) | Saint Anne Mountain (Poland)/Tertiary | 14.55 | 1.92 | - |
| 10 | magnesite | Braszowice (Poland)/Tertiary | 14.91 | 50.91 | 97.5 2 |
| 11 | serpentinite | Jordanów (Poland)/older than UpperDevonian | 14.46 | 26.97 | - |
| Cycle | Sample | |||||
|---|---|---|---|---|---|---|
| Štramberk | Podlesie | Butkov | ||||
| 90.1% CaCO3 | 84.5% CaCO3 | 68.9% CaCO3 | ||||
| Untreated | Pretreated | Untreated | Pretreated | Untreated | Pretreated | |
| 1 | 0.73 | 0.24 | 0.61 | 0.14 | 0.28 | 0.09 |
| 2 | 0.59 | 0.22 | 0.49 | 0.12 | 0.17 | 0.08 |
| 3 | 0.51 | 0.21 | 0.42 | 0.12 | 0.13 | 0.08 |
| 4 | 0.46 | 0.20 | 0.38 | 0.11 | 0.11 | 0.07 |
| 5 | 0.43 | 0.19 | 0.34 | 0.11 | 0.10 | 0.07 |
| 6 | 0.40 | 0.19 | 0.31 | 0.11 | 0.09 | 0.07 |
| 7 | 0.37 | 0.19 | 0.28 | 0.11 | 0.09 | 0.06 |
| 8 | 0.35 | 0.19 | 0.25 | 0.10 | 0.09 | 0.06 |
| 8 | 0.33 | 0.18 | 0.23 | 0.10 | 0.09 | 0.06 |
| 10 | 0.32 | 0.18 | 0.21 | 0.10 | 0.08 | 0.06 |
| Cycle | Sample | |||||
|---|---|---|---|---|---|---|
| Štramberk | Podlesie | Butkov | ||||
| Untreated | Pretreated | Untreated | Pretreated | Untreated | Pretreated | |
| 1 | 0.31 | 0.10 | 0.28 | 0.06 | 0.14 | 0.05 |
| 2 | 0.57 | 0.20 | 0.50 | 0.12 | 0.23 | 0.09 |
| 3 | 0.79 | 0.29 | 0.70 | 0.17 | 0.29 | 0.13 |
| 4 | 0.99 | 0.37 | 0.87 | 0.22 | 0.35 | 0.16 |
| 5 | 1.17 | 0.46 | 1.03 | 0.27 | 0.40 | 0.20 |
| 6 | 1.34 | 0.54 | 1.17 | 0.32 | 0.45 | 0.23 |
| 7 | 1.50 | 0.62 | 1.30 | 0.37 | 0.50 | 0.26 |
| 8 | 1.65 | 0.70 | 1.41 | 0.41 | 0.54 | 0.29 |
| 8 | 1.80 | 0.78 | 1.52 | 0.46 | 0.58 | 0.32 |
| 10 | 1.93 | 0.86 | 1.62 | 0.50 | 0.62 | 0.35 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Labus, K. Comparison of the Properties of Natural Sorbents for the Calcium Looping Process. Materials 2021, 14, 548. https://doi.org/10.3390/ma14030548
Labus K. Comparison of the Properties of Natural Sorbents for the Calcium Looping Process. Materials. 2021; 14(3):548. https://doi.org/10.3390/ma14030548
Chicago/Turabian StyleLabus, Krzysztof. 2021. "Comparison of the Properties of Natural Sorbents for the Calcium Looping Process" Materials 14, no. 3: 548. https://doi.org/10.3390/ma14030548
APA StyleLabus, K. (2021). Comparison of the Properties of Natural Sorbents for the Calcium Looping Process. Materials, 14(3), 548. https://doi.org/10.3390/ma14030548






