Characterization of Glass Insulating Thick Films with Ag Conductors for Multilayer Packages
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Insulating Thick Films
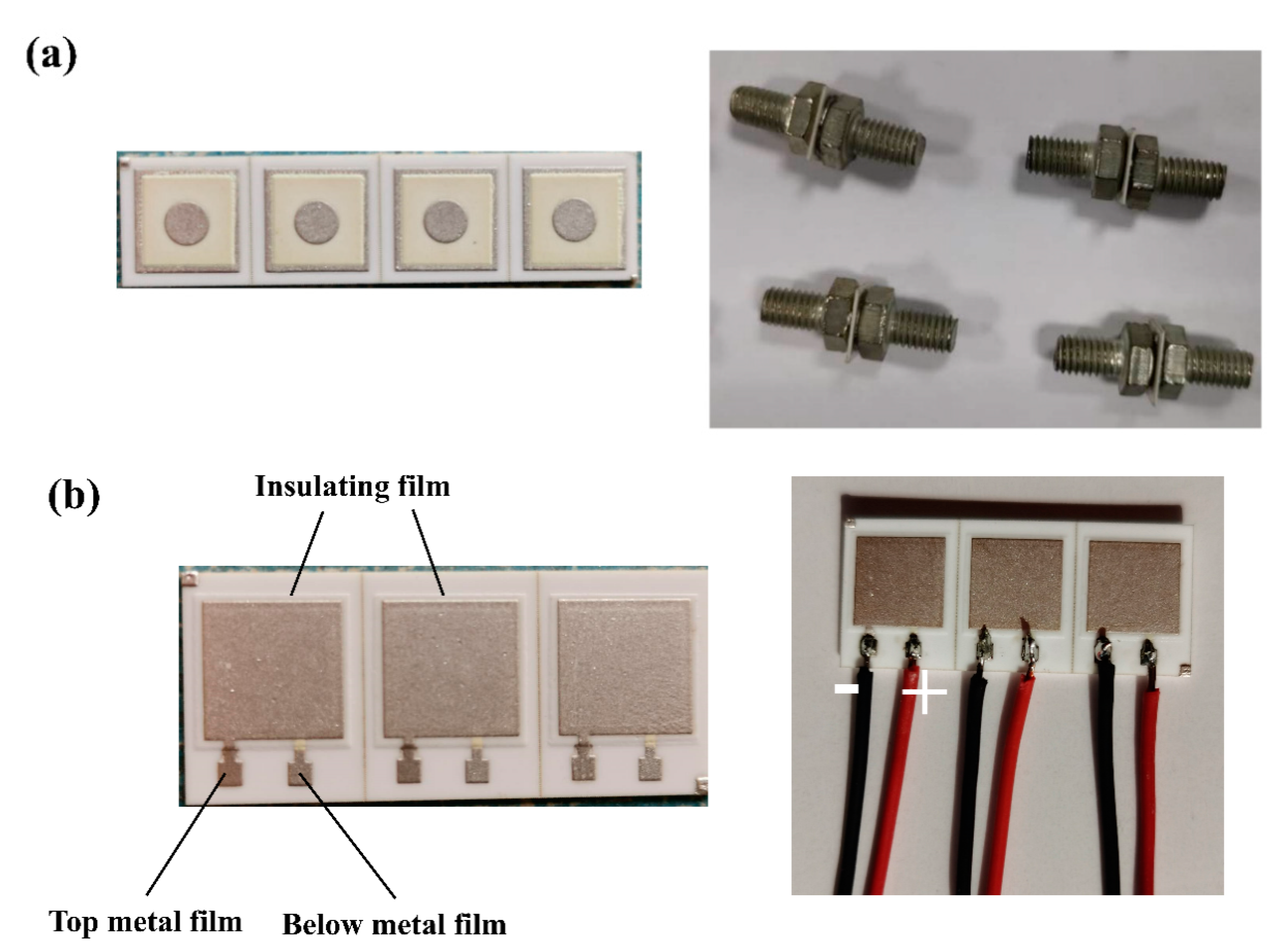
2.2. Fabrication of Insulating Thick Films
3. Results and Discussion
3.1. Glass Selection for Insulating Thick Films
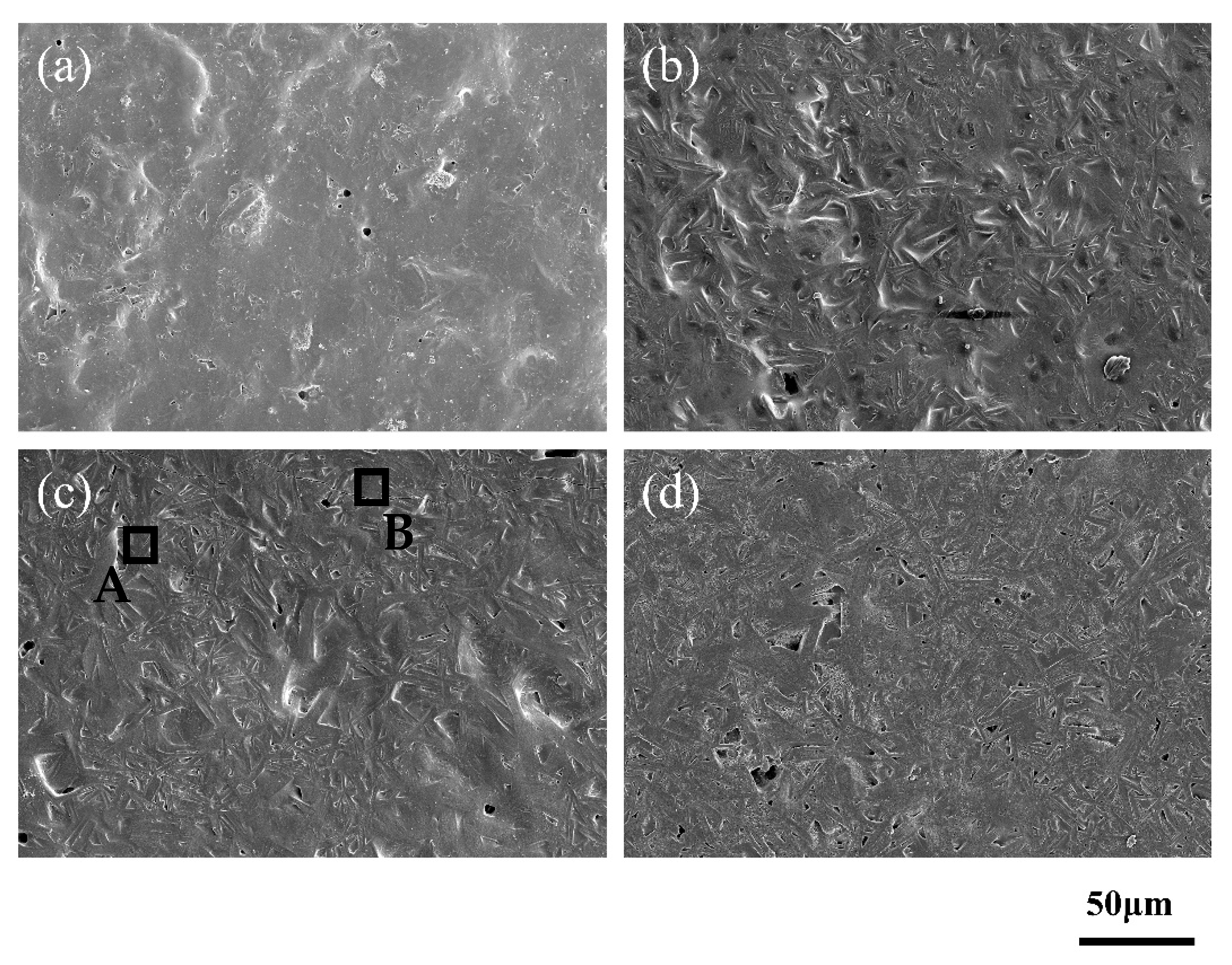
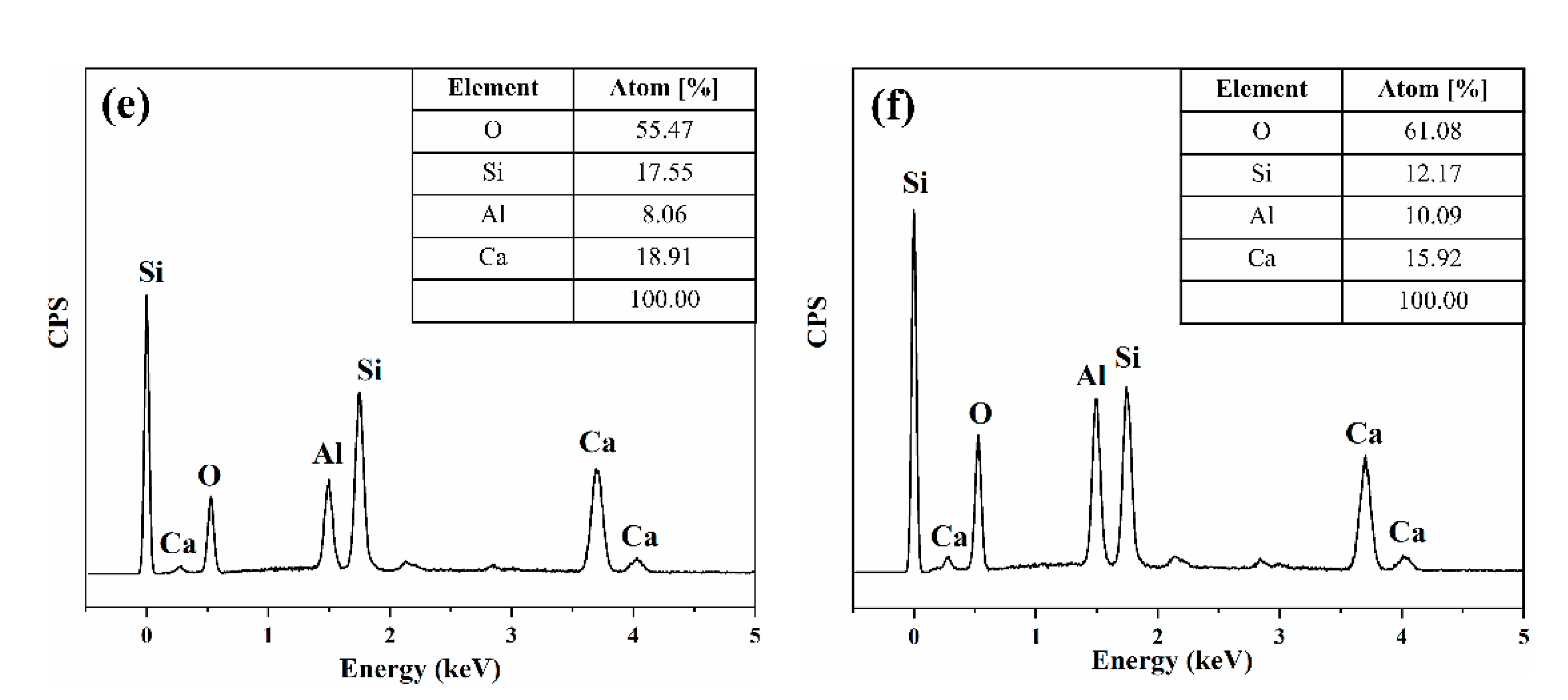
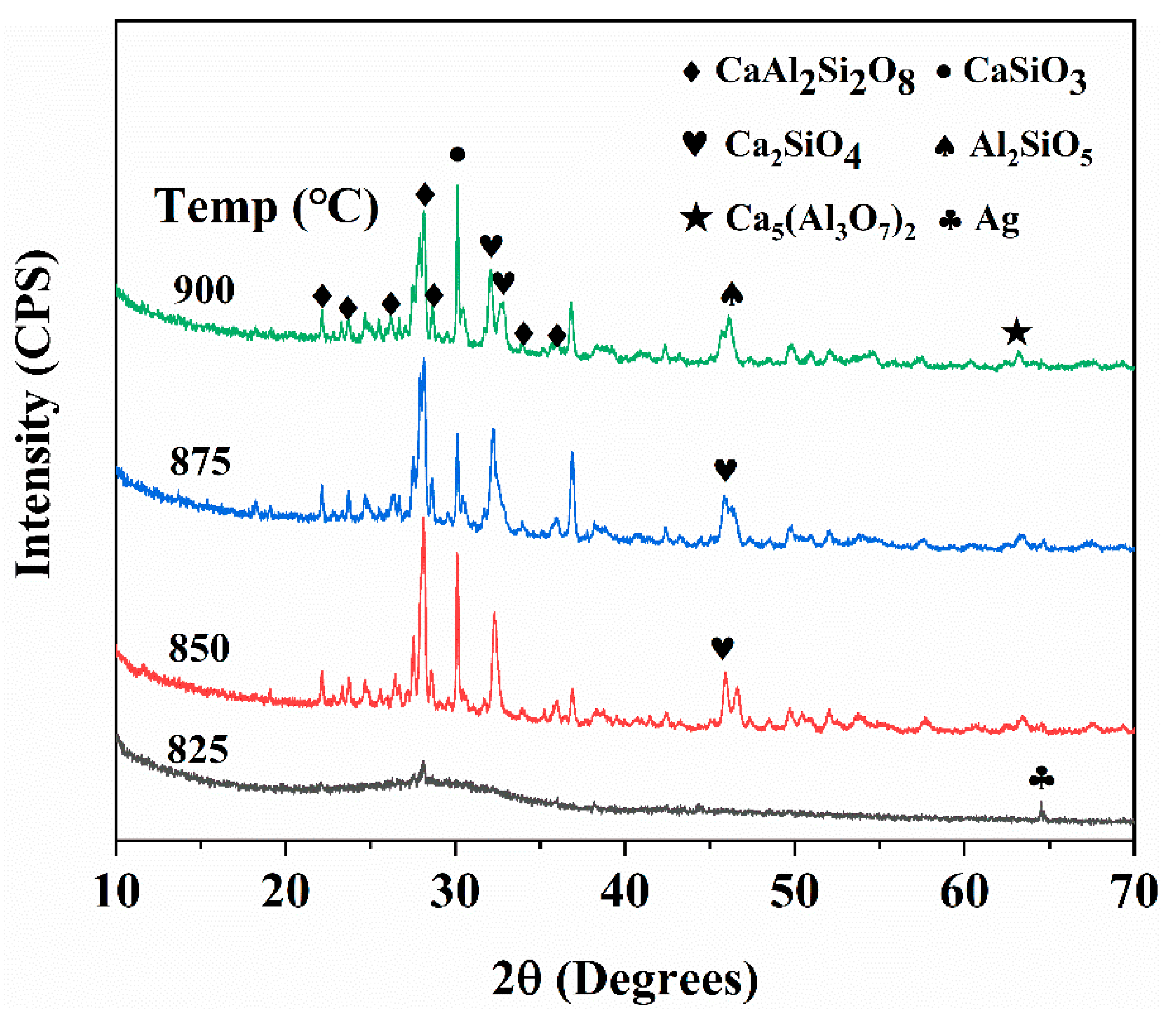
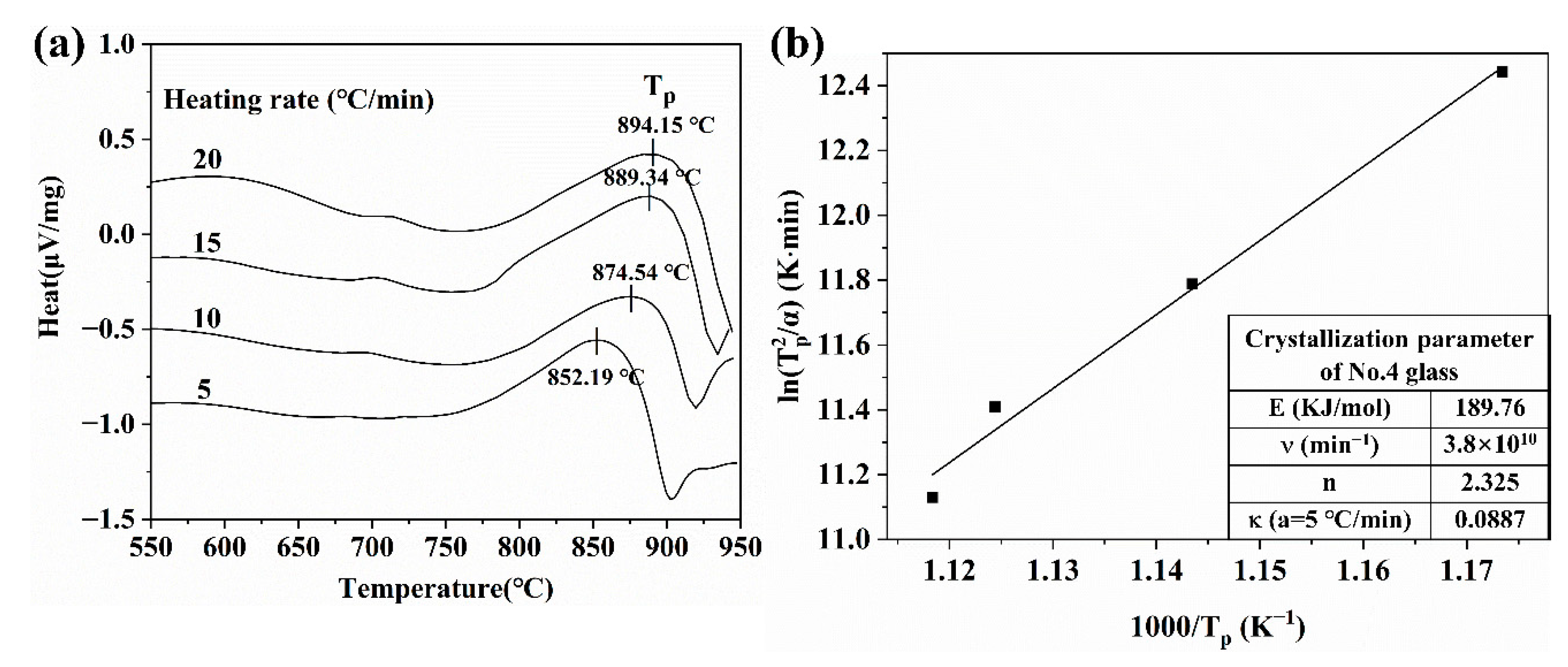
3.2. Phase Analysis and Composition of Glass for Insulating Thick Films
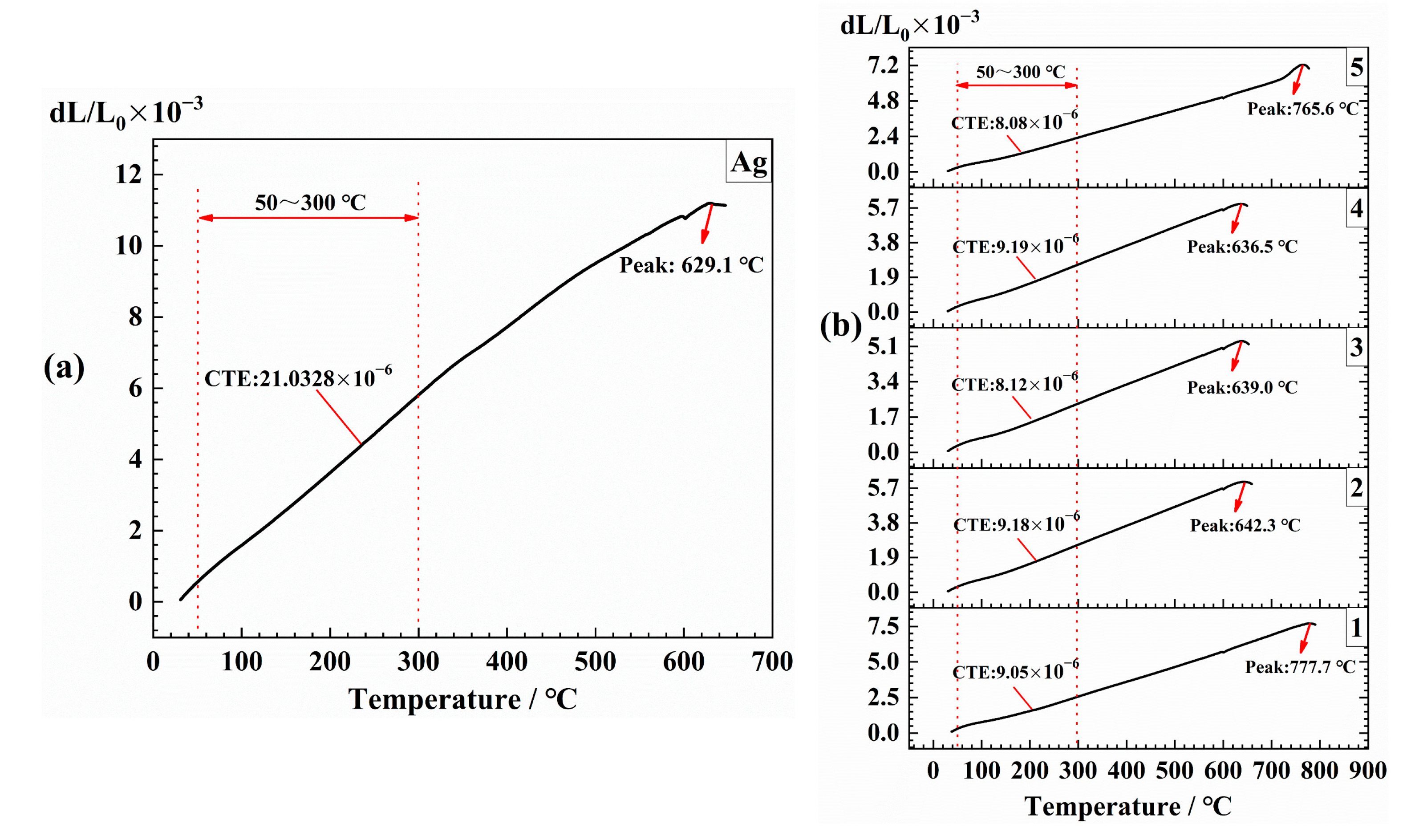
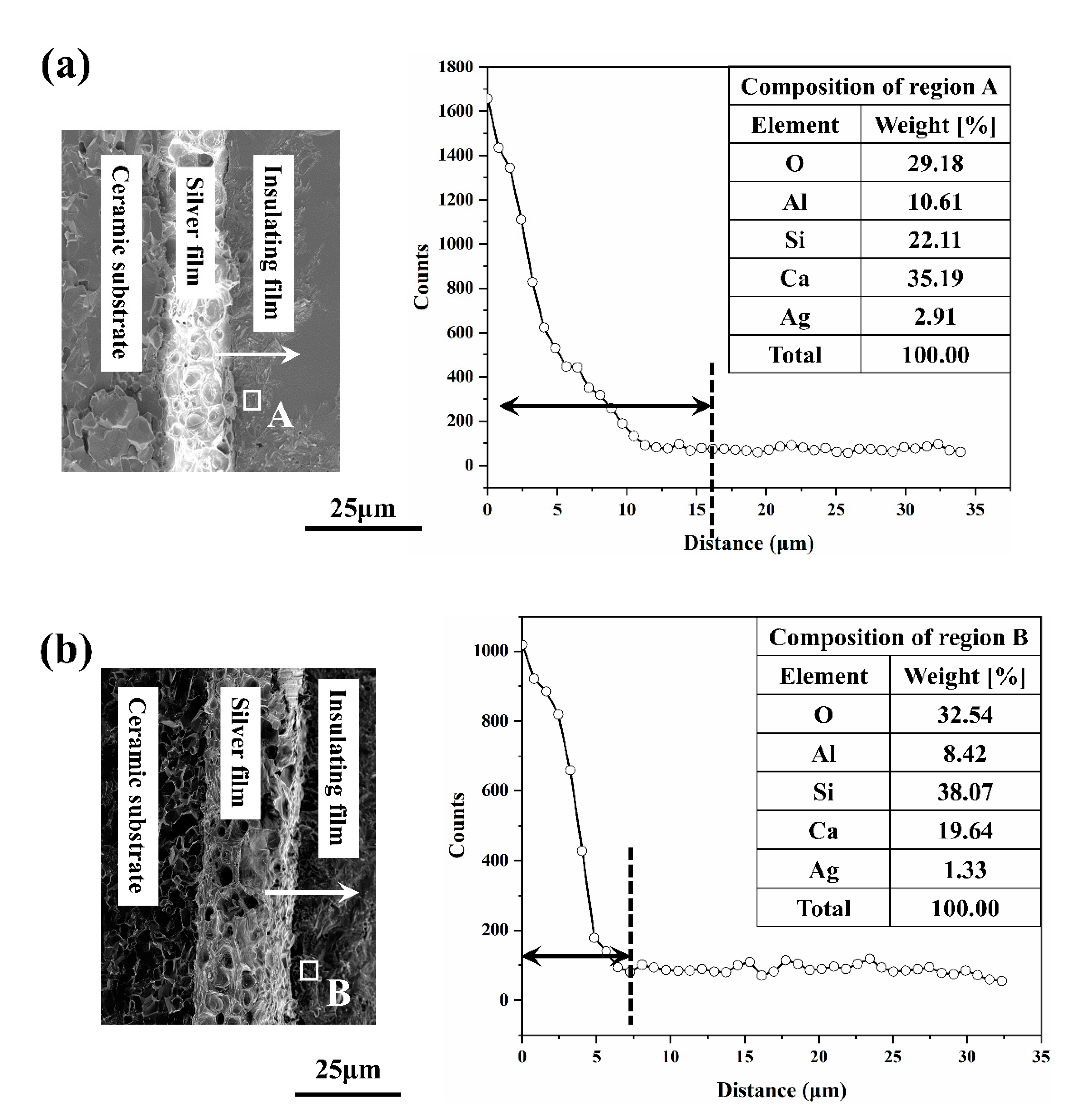
3.3. Property Variations of Insulating Thick Films
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yee, C.F.; Jambek, A.B.; Al-Hadi, A.A. Advantages and Challenges of 10-Gbps Transmission on High-Density Interconnect Boards. J. Electron. Mater. 2016, 45, 3134–3141. [Google Scholar] [CrossRef]
- Ortolino, D.; Engelbrecht, A.; Lauterbach, H.; Braeu, M.; Kita, J.; Moos, R. Effect of Repeated Firing on the Resistance of Screen-Printed Thick-Film Conductors. J. Ceram. Sci. Technol. 2014, 5, 317–326. [Google Scholar] [CrossRef]
- Fang, J.; Fu, R.; Gu, X.; Li, G.; Zhang, X. The Synthesis of Multi-Layer Silver, Copper and Aluminum Thick Films on Alumina Substrates using a Screen-Printing Method. J. Ceram. Sci. Technol. 2020, 11, 103–110. [Google Scholar] [CrossRef]
- Cho, Y.S.; Schulze, W.A.; Amarakoon, V.R.W. Crystallization Kinetics and Properties of Nonstoichiometric Cordierite-Based Thick-Film Dielectrics. J. Am. Ceram. Soc. 1999, 82, 3186–3192. [Google Scholar] [CrossRef]
- Cho, Y.; Koyuncu, M.; Schulze, W.; Amarakoon, V. Characterization of Glass-Ceramic Dielectric Thick Films With Cu Conductors for Multilayer Packages. MRS Proc. 2011, 445. [Google Scholar] [CrossRef]
- Hlina, J.; Reboun, J.; Hamacek, A. Multilayer Thick Printed Copper Structures. In Proceedings of the 2018 41st International Spring Seminar on Electronics Technology (ISSE), Zlatibor, Serbia, 16–20 May 2018. [Google Scholar]
- Hlina, J.; Reboun, J.; Hermansky, V.; Simonovsky, M.; Johan, J.; Hamacek, A. Study of co-fired multilayer structures based on Thick Printed Copper technology. Mater. Lett. 2019, 238, 313–316. [Google Scholar] [CrossRef]
- Cai, X.; Sun, X.; Pang, L. Interface diffusion of silver electrode into bismuth-based ceramics and its effects on the dielectric properties. In Proceedings of the 2017 Joint IEEE International Symposium on the Applications of Ferroelectric (ISAF)/International Workshop on Acoustic Transduction Materials and Devices (IWATMD)/Piezoresponse Force Microscopy (PFM), Atlanta, GA, USA, 7–11 May 2017. [Google Scholar]
- Chou, C.C.; Chang, C.Y.; Chen, G.Y.; Liao, W.J.; Feng, K.C. Control of Silver Diffusion in Low-Temperature Co-Fired Diopside Glass-Ceramic Microwave Dielectrics. Materials 2018, 11, 55. [Google Scholar] [CrossRef]
- Feng, K.-C.; Chu, M.-W.; Lu, C.-A.; Iizuka, Y.; Chen, P.; Tu, C.-S.; Chen, C.-S.; Chien, R.R.; Tsao, C.-Y. Silver diffusion and mechanism of CaO-MgO-SiO2 glass ceramics in millimeter-wave properties. Ceram. Int. 2019, 46. [Google Scholar] [CrossRef]
- Chen, G.H.; Tang, L.J.; Cheng, J.; Jiang, M.H. Synthesis and characterization of CBS glass/ceramic composites for LTCC application. J. Alloy. Compd. 2009, 478, 858–862. [Google Scholar] [CrossRef]
- Chiang, C.-C.; Wang, S.-F.; Wang, Y.-R.; Hsu, Y.-F. Characterizations of CaO–B2O3–SiO2 glass–ceramics: Thermal and electrical properties. J. Alloy. Compd. 2008, 461, 612–616. [Google Scholar] [CrossRef]
- Hsiang, H.-I.; Chen, C.-C.; Yang, S.-Y. Structure, crystallization, and dielectric properties of the Al2O3 filled CaO–B2O3–SiO2–Al2O3 glass composites for LTCC applications. Jpn. J. Appl. Phys. 2019, 58, 091010. [Google Scholar] [CrossRef]
- Lee, T.H.; Cho, S.H.; Lee, T.G.; Kim, H.T.; You, I.K.; Nahm, S. Carbon nanotube/graphene oxide-added CaO-B2O3-SiO2 glass/Al2O3 composite as substrate for chip-type supercapacitor. J. Am. Ceram. Soc. 2018, 101, 3156–3167. [Google Scholar] [CrossRef]
- Zhu, X.; Kong, F.; Ma, X. Sintering behavior and properties of MgTiO3/CaO-B2O3-SiO2 ceramic composites for LTCC applications. Ceram. Int. 2019, 45, 1940–1945. [Google Scholar] [CrossRef]
- Ma, M.; Liu, Z.; Zhang, F.; Liu, F.; Li, Y.; Bordia, R. Suppression of Silver Diffusion in Borosilicate Glass-Based Low-Temperature Cofired Ceramics by Copper Oxide Addition. J. Am. Ceram. Soc. 2016, 99, 2402–2407. [Google Scholar] [CrossRef]
- Yilmaz, S.; Özkan, O.T.; Günay, V. Crystallization kinetics of basalt glass. Ceram. Int. 1996, 22, 477–481. [Google Scholar] [CrossRef]
- Guo, X.; Yang, H.; Han, C.; Song, F. Crystallization and microstructure of Li2O–Al2O3–SiO2 glass containing complex nucleating agent. Thermochim. Acta 2006, 444, 201–205. [Google Scholar] [CrossRef]
- Chang, C.-R.; Jean, J.-H. Crystallization Kinetics and Mechanism of Low-Dielectric, Low-Temperature, Cofirable CaO-B2O3-SiO2 Glass-Ceramics. J. Am. Ceram. Soc. 1999, 82, 1725–1732. [Google Scholar] [CrossRef]
- Hoflund, G.; Hazos, Z.; Salaita, G. Surface Characterization Study of Ag, AgO, and Ag2O Using X-ray Photoelectron Spectroscopy and Electron Energy-Loss Spectroscopy. Phys. Rev. B 2000, 62. [Google Scholar] [CrossRef]
- Hsi, C.S.; Chen, Y.R.; Hsiang, H.I. Diffusivity of silver ions in the low temperature co-fired ceramic (LTCC) substrates. J. Mater. Sci. 2011, 46, 4695–4700. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, H.; Bian, W.; Dong, Y.; Fu, Z.; Ta, S.; Chen, T.; Wang, L.; Zhang, Q. Inhibition of silver diffusion in LTCC by MgTiO3 addition. Ceram. Int. 2020, 46, 10729–10733. [Google Scholar] [CrossRef]
- Hidekazu, T.; Toshihiko, M.; Masatoshi, Y.; Teruo, A.; Ryuichi, T. Inhibition of silver-yellowish coloration on leucite glass-ceramics using cerium oxide. J. Ceram. Soc. Jpn. 2017, 125, 79–84. [Google Scholar]


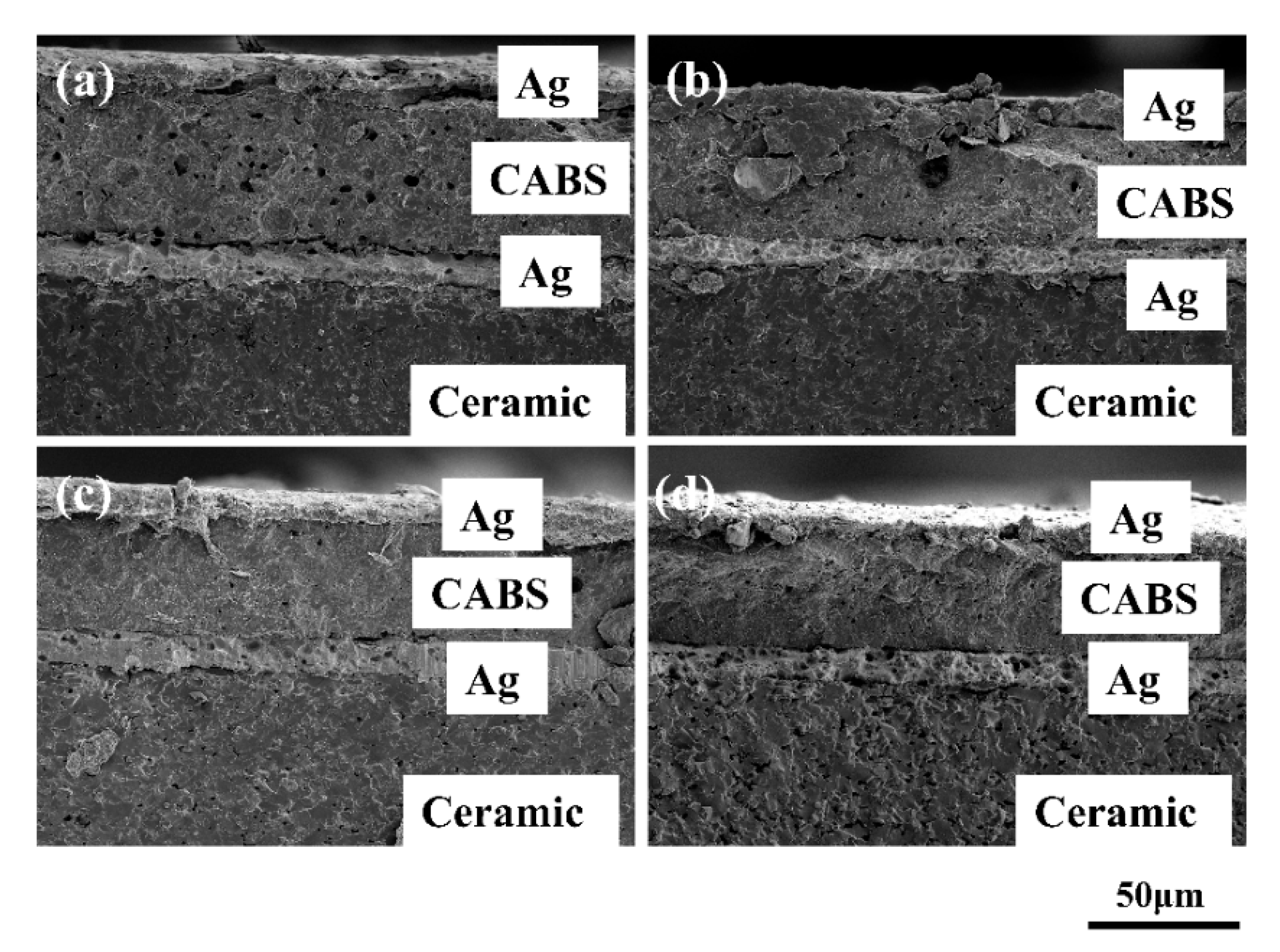
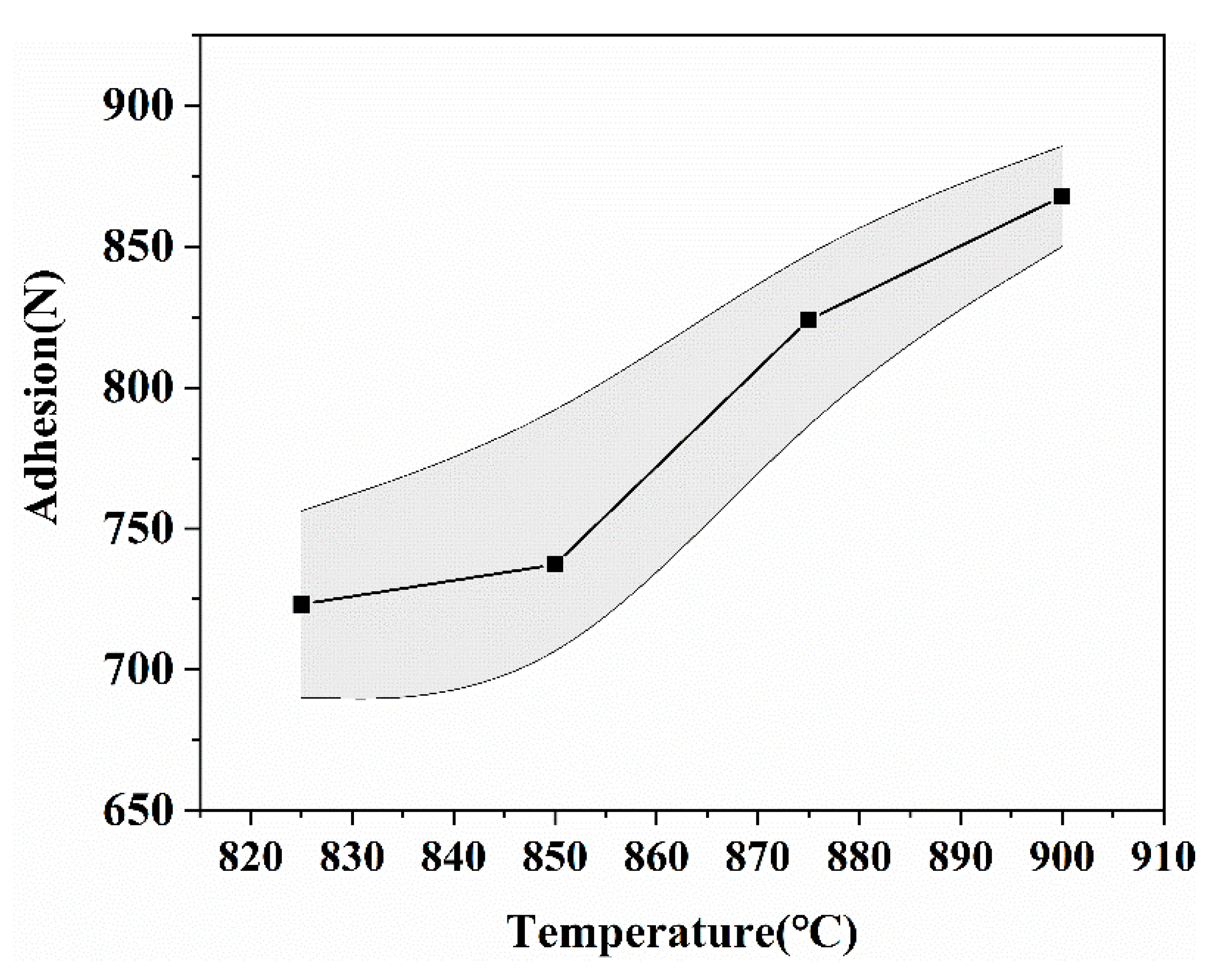
| Raw Materials | CaCO3 (wt %) | Al2O3 (wt %) | SiO2 (wt %) | H3BO3 (wt %) | |
|---|---|---|---|---|---|
| Sample | |||||
| 1 | 52 | 18 | 20 | 10 | |
| 2 | 45 | 20 | 25 | 10 | |
| 3 | 38 | 22 | 30 | 10 | |
| 4 | 35 | 15 | 40 | 10 | |
| 5 | 30 | 15 | 45 | 10 | |
| Film | Paste | Thickness (μm) | Dimensions | |
|---|---|---|---|---|
| Electrical Measurement | Adhesion Measurement | |||
| Below Ag film | 3Focus F14015 | 41 | Square, 8 × 8 mm2 | Square, 7 × 7 mm2 |
| Insulating film | Our own insulating paste | measurement | Square, 10 × 10 mm2 | Square, 6 × 6 mm2 |
| Top Ag film | 3Focus F14015 | 29 | Square, 9 × 9 mm2 | Circle, Φ 3 mm |
| Electrical Parameter | Sintering Temperature (°C) | |||
|---|---|---|---|---|
| 825 | 850 | 875 | 900 | |
| Capacity at 100 kHz (pF) | 62.95 | 87.48 | 94.68 | 96.5 |
| Dielectric constant at 100 kHz | 3.25 | 4.52 | 4.87 | 4.98 |
| Dielectric loss at 100 kHz | 0.0018 | 0.0012 | 0.0011 | 0.0013 |
| Breakdown voltage (V) | 361 | 388 | 485 | 472 |
| Dielectric strength (kV/mm) | 9.76 | 10.49 | 13.11 | 12.76 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fang, J.; Fu, R.; Gu, X.; Zhang, X.; Li, G. Characterization of Glass Insulating Thick Films with Ag Conductors for Multilayer Packages. Materials 2021, 14, 494. https://doi.org/10.3390/ma14030494
Fang J, Fu R, Gu X, Zhang X, Li G. Characterization of Glass Insulating Thick Films with Ag Conductors for Multilayer Packages. Materials. 2021; 14(3):494. https://doi.org/10.3390/ma14030494
Chicago/Turabian StyleFang, Jun, Renli Fu, Xiguang Gu, Xinyao Zhang, and Guojun Li. 2021. "Characterization of Glass Insulating Thick Films with Ag Conductors for Multilayer Packages" Materials 14, no. 3: 494. https://doi.org/10.3390/ma14030494
APA StyleFang, J., Fu, R., Gu, X., Zhang, X., & Li, G. (2021). Characterization of Glass Insulating Thick Films with Ag Conductors for Multilayer Packages. Materials, 14(3), 494. https://doi.org/10.3390/ma14030494