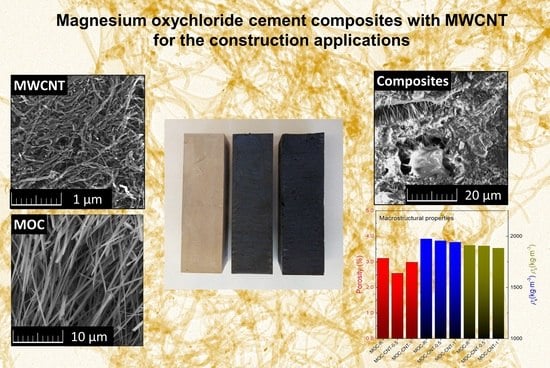
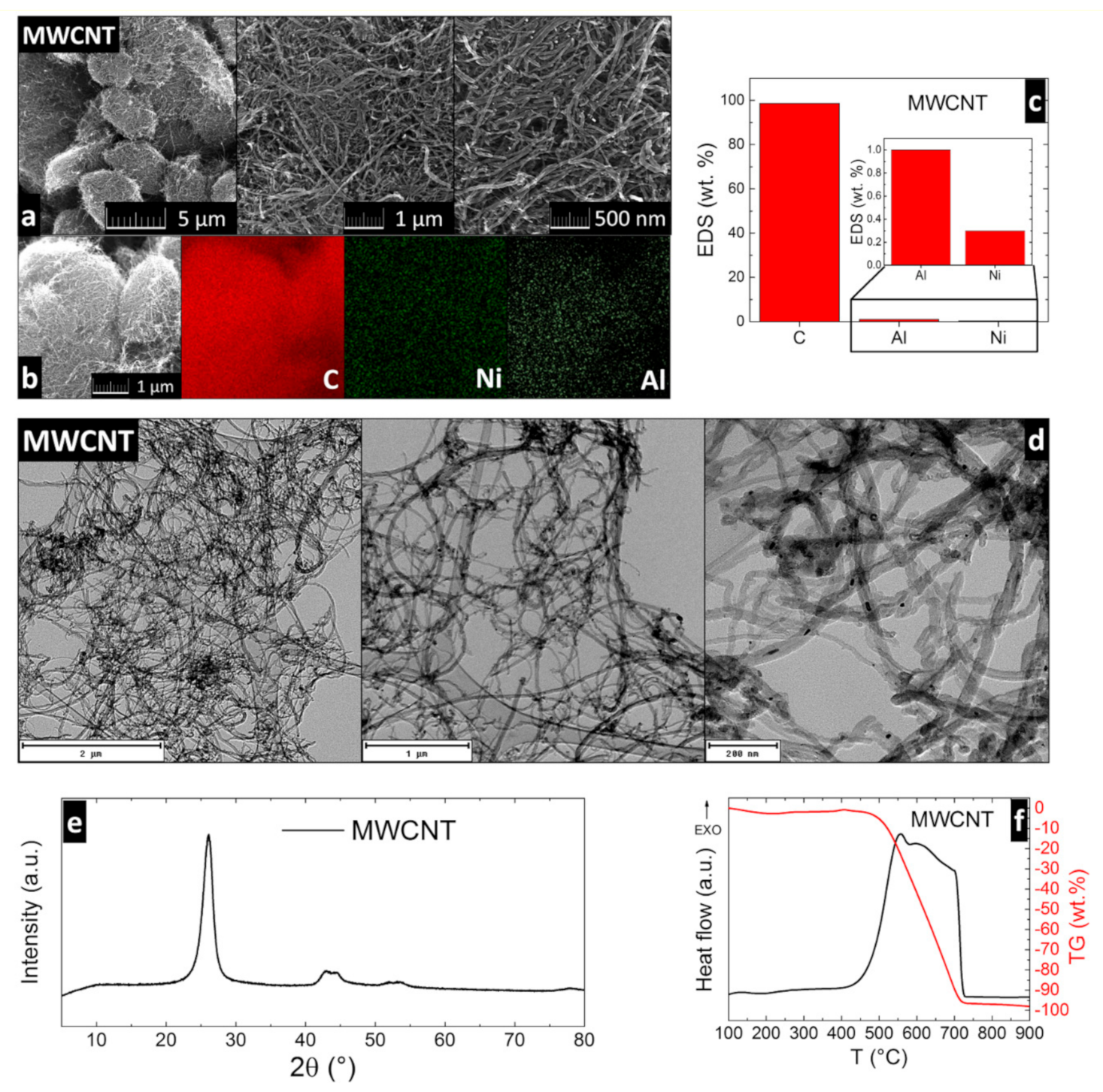
Magnesium Oxychloride Cement Composites with MWCNT for the Construction Applications
Abstract
1. Introduction
2. Experimental Section
2.1. Materials and Synthetic Procedures
2.2. Analytical Techniques
3. Results and Discussion
4. Conclusions
- (i).
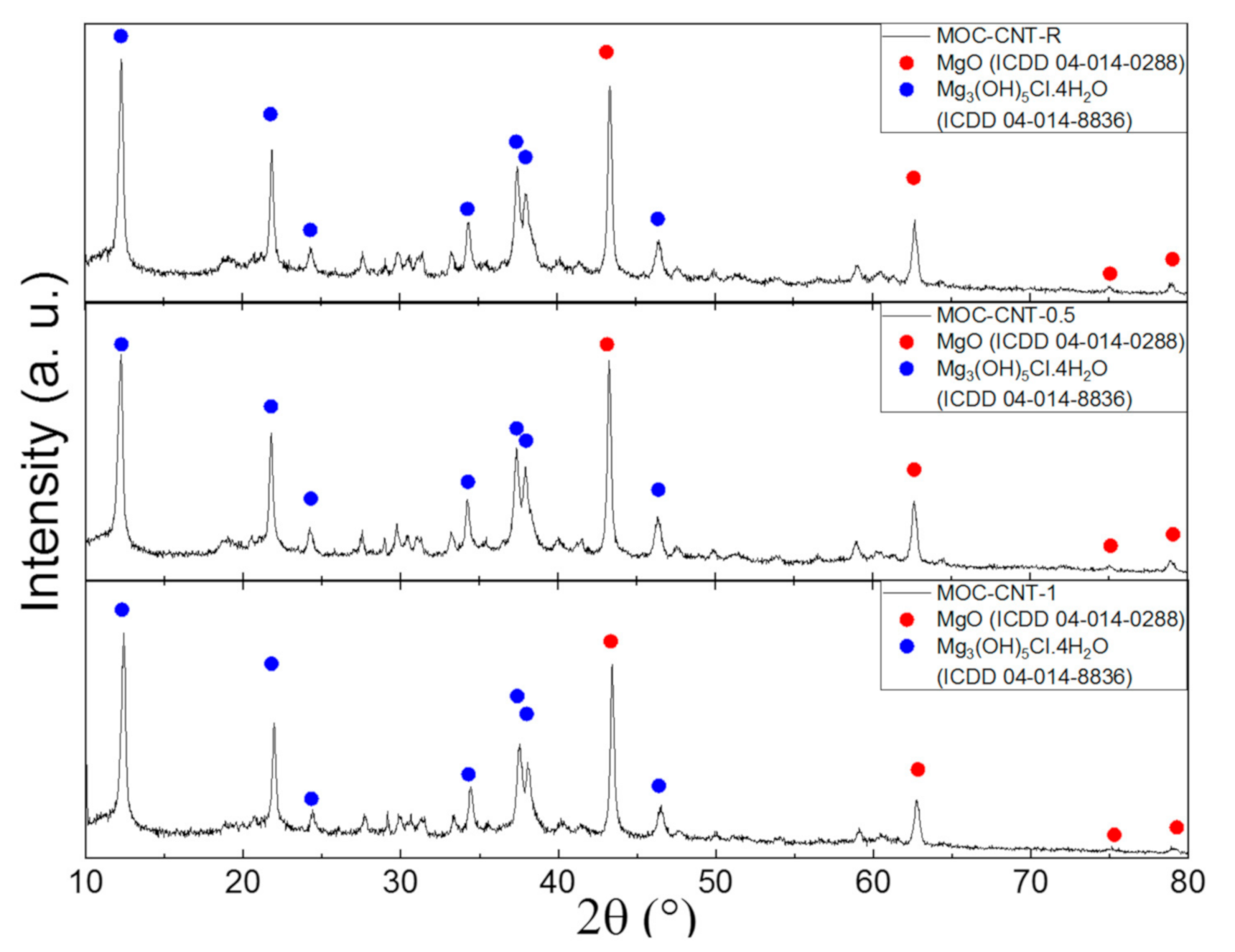
- Stable and durable MOC phase 5, formed properly and with no crystalline impurities, were present in the sample (except for the parent MgO acting as a filler);
- (ii).
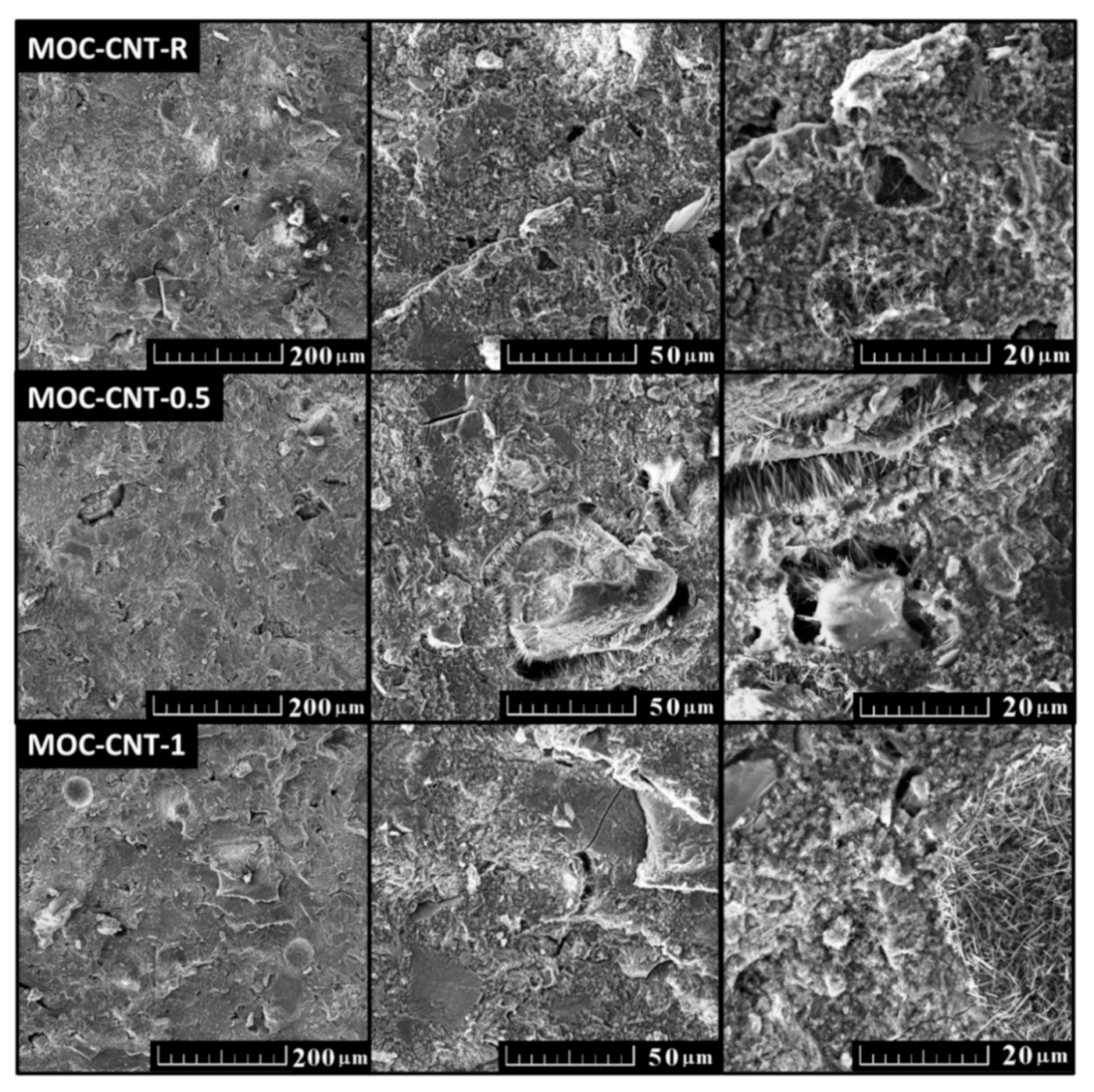
- The structure of the developed composites was highly compacted without any visible defects;
- (iii).
- The thermal behavior of the hardened materials was presumably comparable to the behavior of MOC phase 5 alone, with the exception of MWCNT oxidation, which was observed in the temperature region between 450–600 °C;
- (iv).
- The MWCNT-doped composites exhibited increased mechanical resistance and stiffness, which was due to the lower porosity, average particles size, and excellent mechanical parameters of MWCNT;
- (v).
- The incorporation of MWCNTs resulted in greatly reduced water ingress. which is positive for material durability in the presence of moisture;
- (vi).
- The heat transport and storage were moderately increased by the incorporation of MWCNTs into the composites.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Huntzinger, D.N.; Eatmon, T.D. A life-cycle assessment of portland cement manufacturing: Comparing the traditional process with alternative technologies. J. Clean. Prod. 2009, 17, 668–675. [Google Scholar] [CrossRef]
- Ruan, S.; Unluer, C. Comparative life cycle assessment of reactive MgO and portland cement production. J. Clean. Prod. 2016, 137, 258–273. [Google Scholar] [CrossRef]
- Liska, M.; Al-Tabbaa, A. Ultra-green construction: Reactive magnesia masonry products. Proc. Inst. Civ. Eng. Waste Resour. Manag. 2009, 162, 185–196. [Google Scholar] [CrossRef]
- Al-Tabbaa, A. Reactive magnesia cement. In Eco-Efficient Concrete; Pacheco-Torgal, F., Jalali, S., Labrincha, J., John, V.M., Eds.; Woodhead Publishing: Cambridge, UK, 2013; pp. 523–543. [Google Scholar]
- Bilinski, H.; Matkovic, B.; Mazuranic, C.; Zunic, T.B. The formation of magnesium oxychloride phases in the systems MgO-MgCl2-H2O and NaOH-MgCl2-H2O. J. Am. Ceram. Soc. 1984, 67, 266–269. [Google Scholar] [CrossRef]
- Urwongse, L.; Sorrell, C.A. The system MgO-MgCl2-H2O at 23 °C. J. Am. Ceram. Soc. 1980, 63, 501–504. [Google Scholar] [CrossRef]
- Christensen, A.N.; Norby, P.; Hanson, J.C. Chemical reactions in the system MgO-MgCl2-H2O followed by time-resolved synchrotron X-ray powder diffraction. J. Solid State Chem. 1995, 114, 556–559. [Google Scholar] [CrossRef]
- Matkovic, B.; Popovic, S.; Rogic, V.; Zunic, T.; Young, J.F. Reaction products in magnesium oxychloride cement pastes. System MgO-MgCl2-H2O. J. Am. Ceram. Soc. 1977, 60, 504–507. [Google Scholar] [CrossRef]
- Dinnebier, R.E.; Freyer, D.; Bette, S.; Oestreich, M. 9Mg(OH)2·MgCl2·4H2O, a high temperature phase of the magnesia binder system. Inorg. Chem. 2010, 49, 9770–9776. [Google Scholar] [CrossRef]
- Dinnebier, R.E.; Oestreich, M.; Bette, S.; Freyer, D. 2Mg(OH)2·MgCl2·2H2O and 2Mg(OH)2·MgCl2·4H2O, two high temperature phases of the magnesia cement system. Z. Anorg. Allg. Chem. 2012, 638, 628–633. [Google Scholar] [CrossRef]
- Pannach, M.; Bette, S.; Freyer, D. Solubility equilibria in the system MgO-MgCl2-H2O from 298 to 393 K. J. Chem. Eng. Data 2017, 62, 1384–1396. [Google Scholar] [CrossRef]
- Montle, J.F.; Mayhan, K.G. The role of magnesium oxychloride as a fire-resistive material. Fire Technol. 1974, 10, 201–210. [Google Scholar] [CrossRef]
- Xu, B.; Ma, H.; Hu, C.; Yang, S.; Li, Z.J.C.; Materials, B. Influence of curing regimes on mechanical properties of magnesium oxychloride cement-based composites. Constr. Build. Mater. 2016, 102, 613–619. [Google Scholar] [CrossRef]
- Beaudoin, J.J.; Ramachandran, V.S. Strength development in magnesium oxychloride and other cements. Cem. Concr. Res. 1975, 5, 617–630. [Google Scholar] [CrossRef]
- Qiao, H.; Cheng, Q.; Jinlei, W.; Yingying, S. The application review of magnesium oxychloride cement. J. Chem. Pharm. Res. 2014, 6, 180–185. [Google Scholar]
- Wang, Y.; Wei, L.; Yu, J.; Yu, K. Mechanical properties of high ductile magnesium oxychloride cement-based composites after water soaking. Cem. Concr. Compos. 2019, 97, 248–258. [Google Scholar] [CrossRef]
- Deng, D. The mechanism for soluble phosphates to improve the water resistance of magnesium oxychloride cement. Cem. Concr. Res. 2003, 33, 1311–1317. [Google Scholar] [CrossRef]
- Luo, X.; Fan, W.; Li, C.; Wang, Y.; Yang, H.; Liu, X.; Yang, S. Effect of hydroxyacetic acid on the water resistance of magnesium oxychloride cement. Constr. Build. Mater. 2020, 246, 118428. [Google Scholar] [CrossRef]
- He, P.; Poon, C.S.; Tsang, D.C.W. Using incinerated sewage sludge ash to improve the water resistance of magnesium oxychloride cement (MOC). Constr. Build. Mater. 2017, 147, 519–524. [Google Scholar] [CrossRef]
- Guo, Y.; Zhang, Y.; Soe, K.; Hutchison, W.D.; Timmers, H.; Poblete, M.R. Effect of fly ash on mechanical properties of magnesium oxychloride cement under water attack. Struct. Concr. 2020, 21, 1181–1199. [Google Scholar] [CrossRef]
- Feng, C.; Guimarães, A.S.; Ramos, N.; Sun, L.; Gawin, D.; Konca, P.; Hall, C.; Zhao, J.; Hirsch, H.; Grunewald, J.; et al. Hygric properties of porous building materials (VI): A round robin campaign. Build. Environ. 2020, 185, 107242. [Google Scholar] [CrossRef]
- Jirickova, A.; Lojka, M.; Lauermannova, A.M.; Antonacik, F.; Sedmidubsky, D.; Pavlikova, M.; Zaleska, M.; Pavlik, Z.; Jankovsky, O. Synthesis, structure, and thermal stability of magnesium oxychloride 5Mg(OH)2·MgCl2·8H2O. Appl. Sci. 2020, 10, 15. [Google Scholar] [CrossRef]
- Lojka, M.; Jankovský, O.; Jiříčková, A.; Lauermannová, A.-M.; Antončík, F.; Sedmidubský, D.; Pavlík, Z. Thermal stability and kinetics of formation of magnesium oxychloride phase 3Mg(OH)2∙MgCl2∙8H2O. Materials 2020, 13, 767. [Google Scholar] [CrossRef] [PubMed]
- Eubank, W.R. Calcination studies of magnesium oxides. J. Am. Ceram. Soc. 1951, 34, 225–229. [Google Scholar] [CrossRef]
- Fischer, H.C. Calcination of calcite: I, Effect of heating rate and temperature on bulk density of calcium oxide. J. Am. Ceram. Soc. 1955, 38, 245–251. [Google Scholar] [CrossRef]
- Fischer, H.C. Calcination of calcite: II, Size and growth rate of calcium oxide crystallites. J. Am. Ceram. Soc. 1955, 38, 284–288. [Google Scholar] [CrossRef]
- Jankovský, O.; Lojka, M.; Lauermannová, A.-M.; Antončík, F.; Pavlíková, M.; Pavlík, Z.; Sedmidubský, D. Carbon dioxide uptake by MOC-based materials. Appl. Sci. 2020, 10, 2254. [Google Scholar] [CrossRef]
- Pivák, A.; Pavlíková, M.; Záleská, M.; Lojka, M.; Jankovský, O.; Pavlík, Z.J.M. Magnesium oxychloride cement composites with silica filler and coal fly ash admixture. Materials 2020, 13, 2537. [Google Scholar] [CrossRef]
- Pivák, A.; Pavlíková, M.; Záleská, M.; Lojka, M.; Lauermannová, A.-M.; Jankovský, O.; Pavlík, Z.J.P. Low-carbon composite based on MOC, silica sand and ground porcelain insulator waste. Processes 2020, 8, 829. [Google Scholar] [CrossRef]
- Jankovský, O.; Lojka, M.; Lauermannová, A.-M.; Antončík, F.; Pavlíková, M.; Záleská, M.; Pavlík, Z.; Pivák, A.; Sedmidubský, D. Towards novel building materials: High-strength nanocomposites based on graphene, graphite oxide and magnesium oxychloride. Appl. Mater. Today 2020, 20, 100766. [Google Scholar] [CrossRef]
- Chuah, S.; Pan, Z.; Sanjayan, J.G.; Wang, C.M.; Duan, W.H. Nano reinforced cement and concrete composites and new perspective from graphene oxide. Constr. Build. Mater. 2014, 73, 113–124. [Google Scholar] [CrossRef]
- Du, H.; Gao, H.J.; Pang, S.D. Improvement in concrete resistance against water and chloride ingress by adding graphene nanoplatelet. Cem. Concr. Res. 2016, 83, 114–123. [Google Scholar] [CrossRef]
- Mohammed, A.; Sanjayan, J.G.; Nazari, A.; Al-Saadi, N.T.K. Effects of graphene oxide in enhancing the performance of concrete exposed to high-temperature. Aust. J. Civ. Eng. 2017, 15, 61–71. [Google Scholar] [CrossRef]
- Devasena, M.; Karthikeyan, J. Investigation on strength properties of graphene oxide concrete. Int. J. Eng. Sci. Invent. Res. Dev. 2015, 1, 307–310. [Google Scholar]
- Khare, R.; Bose, S. Carbon nanotube based composites—A review. J. Miner. Mater. Charact. Eng. 2005, 4, 16. [Google Scholar] [CrossRef]
- Baddour, C.E.; Briens, C. Carbon nanotube synthesis: A review. Int. J. Chem. React. Eng. 2005, 3. [Google Scholar] [CrossRef]
- Shokrieh, M.M.; Saeedi, A.; Chitsazzadeh, M. Mechanical properties of multi-walled carbon nanotube/polyester nanocomposites. J. Nanostruct. Chem. 2013, 3, 20. [Google Scholar] [CrossRef]
- Jankovský, O.; Storti, E.; Moritz, K.; Luchini, B.; Jiříčková, A.; Aneziris, C.G. Nano-functionalization of carbon-bonded alumina using graphene oxide and mwcnts. J. Eur. Ceram. Soc. 2018, 38, 4732–4738. [Google Scholar] [CrossRef]
- Jankovský, O.; Storti, E.; Schmidt, G.; Dudczig, S.; Sofer, Z.; Aneziris, C.G. Unique wettability phenomenon of carbon-bonded alumina with advanced nanocoating. Appl. Mater. Today 2018, 13, 24–31. [Google Scholar] [CrossRef]
- Chen, S.J.; Collins, F.G.; Macleod, A.J.N.; Pan, Z.; Duan, W.H.; Wang, C.M. Carbon nanotube–cement composites: A retrospect. IES J. Part A Civ. Struct. Eng. 2011, 4, 254–265. [Google Scholar] [CrossRef]
- Choi, H.; Kang, D.; Seo, G.S.; Chung, W. Effect of some parameters on the compressive strength of MWCNT-cement composites. Adv. Mater. Sci. Eng. 2015, 2015, 340808. [Google Scholar] [CrossRef]
- Kim, G.M.; Nam, I.W.; Yang, B.; Yoon, H.N.; Lee, H.K.; Park, S. Carbon nanotube (CNT) incorporated cementitious composites for functional construction materials: The state of the art. Compos. Struct. 2019, 227, 111244. [Google Scholar] [CrossRef]
- Yakovlev, G.; Pervushin, G.; Maeva, I.; Keriene, J.; Pudov, I.; Shaybadullina, A.; Buryanov, A.; Korzhenko, A.; Senkov, S. Modification of construction materials with multi-walled carbon nanotubes. Procedia Eng. 2013, 57, 407–413. [Google Scholar] [CrossRef]
- Makar, J.; Beaudoin, J. Carbon nanotubes and their application in the construction industry. Spec. Publ. R. Soc. Chem. 2004, 292, 331–342. [Google Scholar]
- EN 1015-10. Methods of Test for Mortar for Masonry—Part 10: Determination of Dry Bulk Density of Hardened 676 Mortar; European Committee for Standardization: Brussels, Belgium, 1999. [Google Scholar]
- Pavlíková, M.; Zemanová, L.; Pokorný, J.; Záleská, M.; Jankovský, O.; Lojka, M.; Sedmidubský, D.; Pavlík, Z. Valorization of wood chips ash as an eco-friendly mineral admixture in mortar mix design. Waste Manag. 2018, 80, 89–100. [Google Scholar] [CrossRef]
- Záleská, M.; Pavlíková, M.; Pokorný, J.; Jankovský, O.; Pavlík, Z.; Černý, R. Structural, mechanical and hygrothermal properties of lightweight concrete based on the application of waste plastics. Constr. Build. Mater. 2018, 180, 1–11. [Google Scholar] [CrossRef]
- EN 1015-11. Methods of Test for Mortar for Masonry—Part 10: Determination of Flexural and Compressive Strength 678 of Hardened Mortar; European Committee for Standardization: Brussels, Belgium, 1999. [Google Scholar]
- Wei, L.; Wang, Y.; Yu, J.; Xiao, J.; Xu, S. Feasibility study of strain hardening magnesium oxychloride cement-based composites. Constr. Build. Mater. 2018, 165, 750–760. [Google Scholar] [CrossRef]
- Wang, L. Study on the water resistance and mechanism of improving for magnesium oxychloride cement with phosphate and polymer. J. Funct. Mater. 2015, 46, 13066–13069. [Google Scholar]
- EN 13755. Natural Stone Test Methods: Determination of Water Absorption at Atmospheric Pressure; British Standards Institution: London, UK, 2008. [Google Scholar]
- EN 1015–18. Methods of Test for Mortar for Masonry. Part 18: Determination of Water Absorption Coefficient Due to Capillary Action of Hardened Mortar; European Committee for Standardization: Brussels, Belgium, 2002. [Google Scholar]
- Adhikary, S.K.; Rudžionis, Ž.; Rajapriya, R. The effect of carbon nanotubes on the flowability, mechanical, microstructural and durability properties of cementitious composite: An overview. Sustainability 2020, 12, 8362. [Google Scholar] [CrossRef]
- Jianli, M.; Youcai, Z.; Jinmei, W.; Li, W. Effect of magnesium oxychloride cement on stabilization/solidification of sewage sludge. Constr. Build. Mater. 2010, 24, 79–83. [Google Scholar] [CrossRef]
- Hall, D.A.; Stevens, R.; El-Jazairi, B.J.C. The effect of retarders on the microstructure and mechanical properties of magnesia–phosphate cement mortar. Cem. Concr. Res. 2001, 31, 455–465. [Google Scholar] [CrossRef]
- Manzur, T.; Yazdani, N.; Emon, M.A.B. Effect of carbon nanotube size on compressive strengths of nanotube reinforced cementitious composites. J. Mater. 2014, 2014, 1–8. [Google Scholar] [CrossRef]
- Zu, M.; Lu, W.; Li, Q.-W.; Zhu, Y.; Wang, G.; Chou, T.-W. Characterization of carbon nanotube fiber compressive properties using tensile recoil measurement. ACS Nano 2012, 6, 4288–4297. [Google Scholar] [CrossRef] [PubMed]
- Esmaeilzadeh, H.; Su, J.; Charmchi, M.; Sun, H. Effect of hydrophobicity on the water flow in carbon nanotube—A molecular dynamics study. Theor. Appl. Mech. Lett. 2018, 8, 284–290. [Google Scholar] [CrossRef]
- Han, Z.; Fina, A. Thermal conductivity of carbon nanotubes and their polymer nanocomposites: A review. Prog. Polym. Sci. 2011, 36, 914–944. [Google Scholar] [CrossRef]


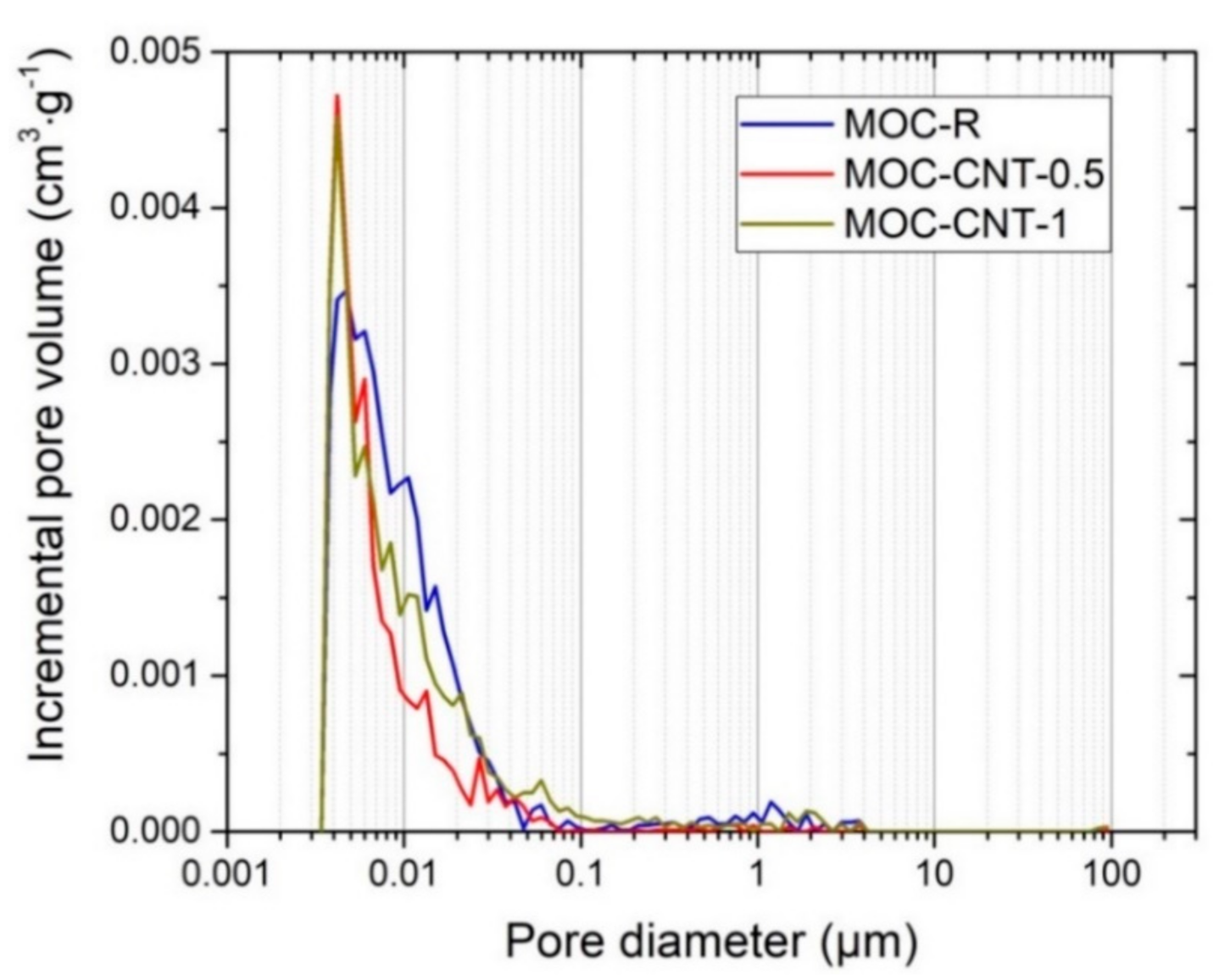
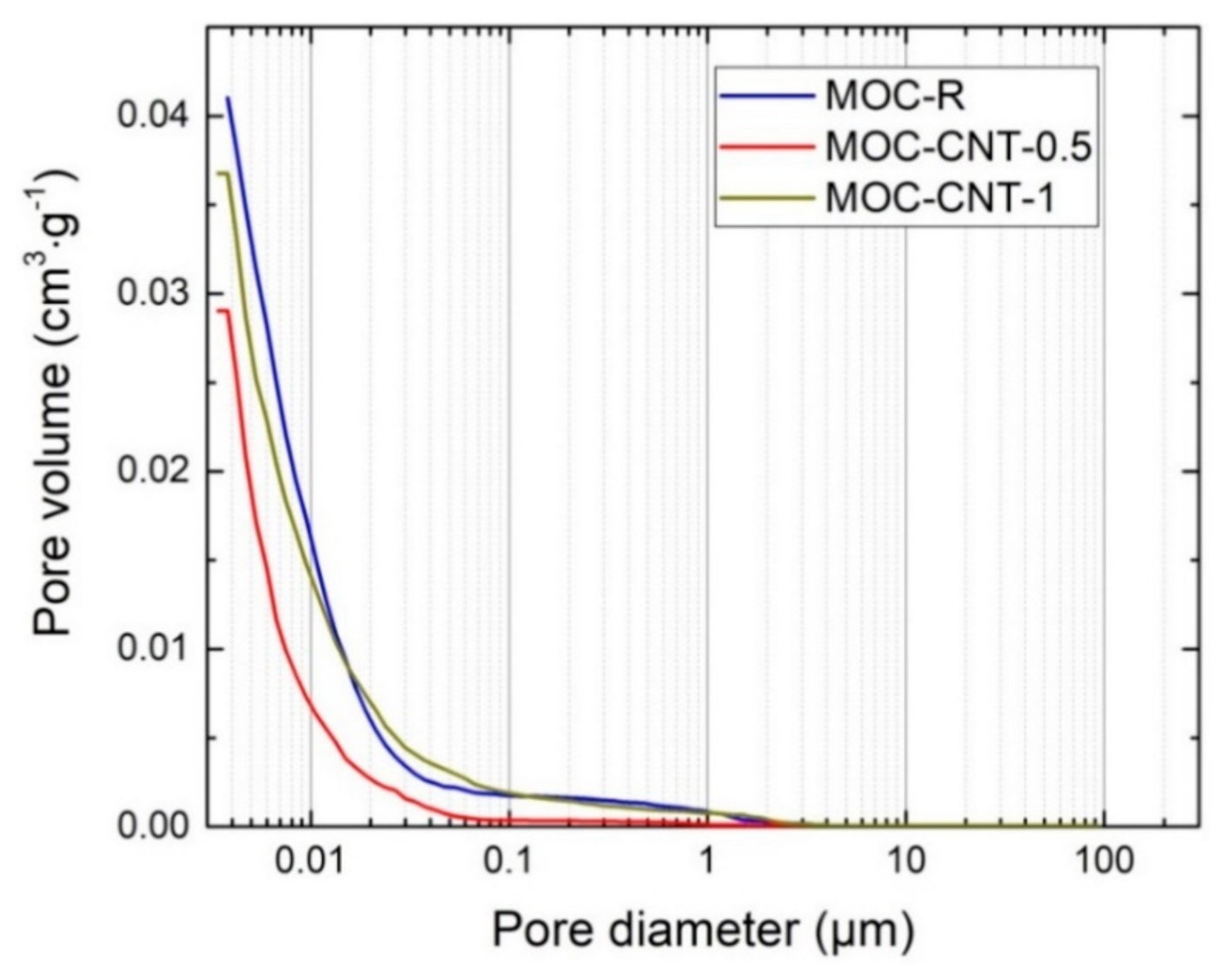
| Mixture | Mass (g) | |||
|---|---|---|---|---|
| MgO | MgCl2∙6H2O | Water | MWCNT | |
| MOC-CNT-R | 1318.6 | 584.1 | 388.3 | 0 |
| MOC-CNT-0.5 | 1318.6 | 584.1 | 388.3 | 9.5 |
| MOC-CNT-1 | 1318.6 | 584.1 | 388.3 | 19.0 |
| Material | MOC-R | MOC-CNT-0.5 | MOC-CNT-1 |
|---|---|---|---|
| Bulk density ρb (kg.m−3) | 1913 ± 27 | 1907 ± 27 | 1885 ± 26 |
| Specific density ρs (kg∙m−3) | 1975 ± 24 | 1957 ± 24 | 1943 ± 23 |
| Total open porosity Ψ (%) | 3.14 ± 0.06 | 2.55 ± 0.05 | 2.99 ± 0.06 |
| Flexural strength ff (MPa) | 14.1 ± 0.2 | 15.7 ± 0.2 | 16.5 ± 0.2 |
| Compressive strength fc (MPa) | 71.4 ± 1.0 | 77.5 ± 1.1 | 80.2 ± 1.1 |
| Young’s modulus Ed (GPa) | 24.7 ± 0.6 | 25.8 ± 0.6 | 26.1 ± 0.6 |
| Parameter | MOC-R | MOC-CNT-0.5 | MOC-CNT-1 |
|---|---|---|---|
| 24-h water absorption (%) | 1.19 ± 0.01 | 1.06 ± 0.01 | 1.05 ± 0.01 |
| Water absorption coefficient (kg∙m−2∙s−1/2) | 0.0016 | 0.0010 | 0.0008 |
| Thermal conductivity (W∙m−1∙K−1) | 1.519 | 1.531 | 1.618 |
| Thermal diffusivity × 10−5 (m2∙s−1) | 0.772 | 0.811 | 0.802 |
| Volumetric heat capacity × 105 (J∙m−3∙K−1) | 1.967 | 1.888 | 2.010 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lojka, M.; Lauermannová, A.-M.; Sedmidubský, D.; Pavlíková, M.; Záleská, M.; Pavlík, Z.; Pivák, A.; Jankovský, O. Magnesium Oxychloride Cement Composites with MWCNT for the Construction Applications. Materials 2021, 14, 484. https://doi.org/10.3390/ma14030484
Lojka M, Lauermannová A-M, Sedmidubský D, Pavlíková M, Záleská M, Pavlík Z, Pivák A, Jankovský O. Magnesium Oxychloride Cement Composites with MWCNT for the Construction Applications. Materials. 2021; 14(3):484. https://doi.org/10.3390/ma14030484
Chicago/Turabian StyleLojka, Michal, Anna-Marie Lauermannová, David Sedmidubský, Milena Pavlíková, Martina Záleská, Zbyšek Pavlík, Adam Pivák, and Ondřej Jankovský. 2021. "Magnesium Oxychloride Cement Composites with MWCNT for the Construction Applications" Materials 14, no. 3: 484. https://doi.org/10.3390/ma14030484
APA StyleLojka, M., Lauermannová, A.-M., Sedmidubský, D., Pavlíková, M., Záleská, M., Pavlík, Z., Pivák, A., & Jankovský, O. (2021). Magnesium Oxychloride Cement Composites with MWCNT for the Construction Applications. Materials, 14(3), 484. https://doi.org/10.3390/ma14030484