Chemical Modification of Chitosan for Removal of Pb(II) Ions from Aqueous Solutions
Abstract
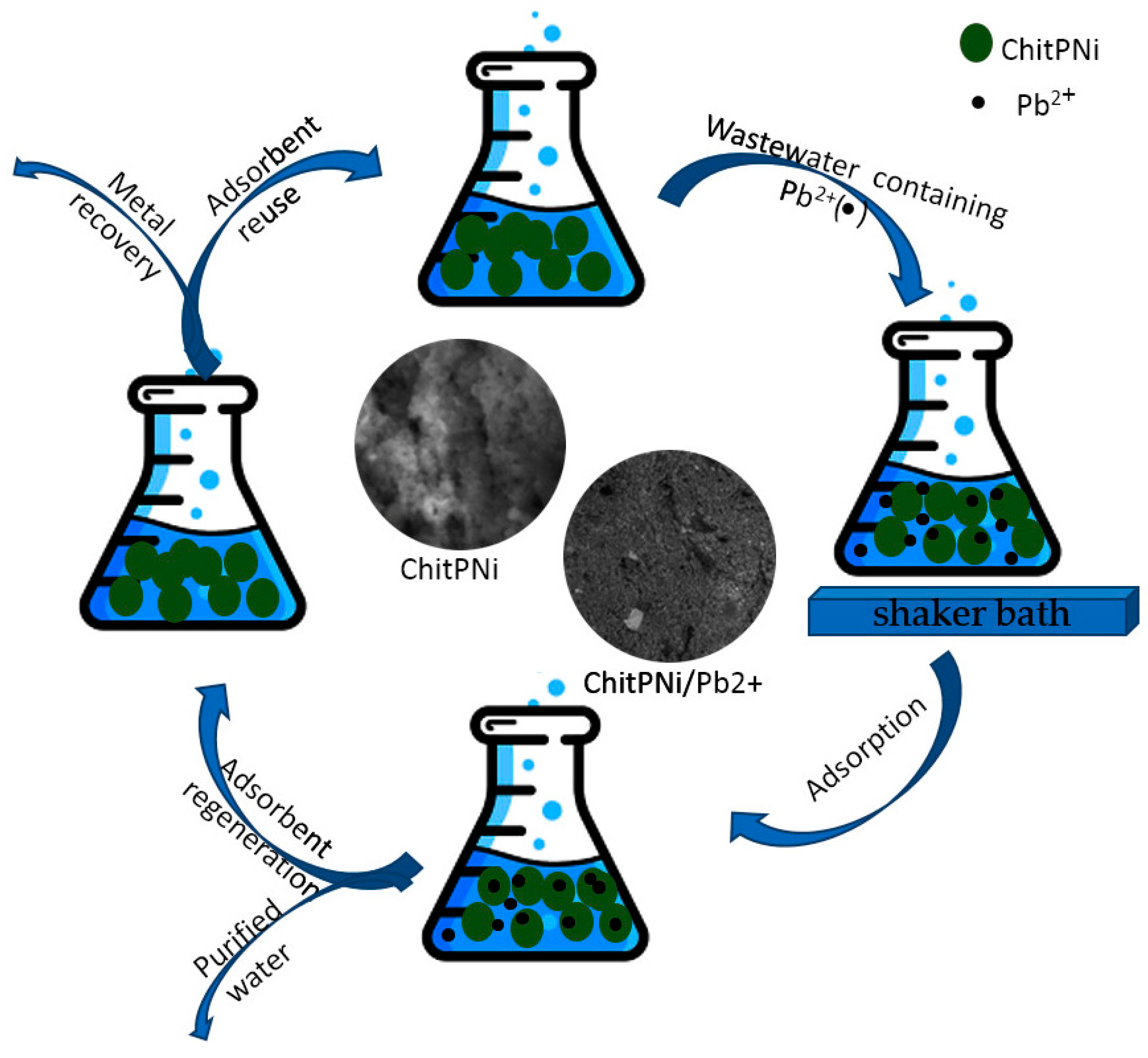
:1. Introduction
2. Materials and Methods
2.1. Materials
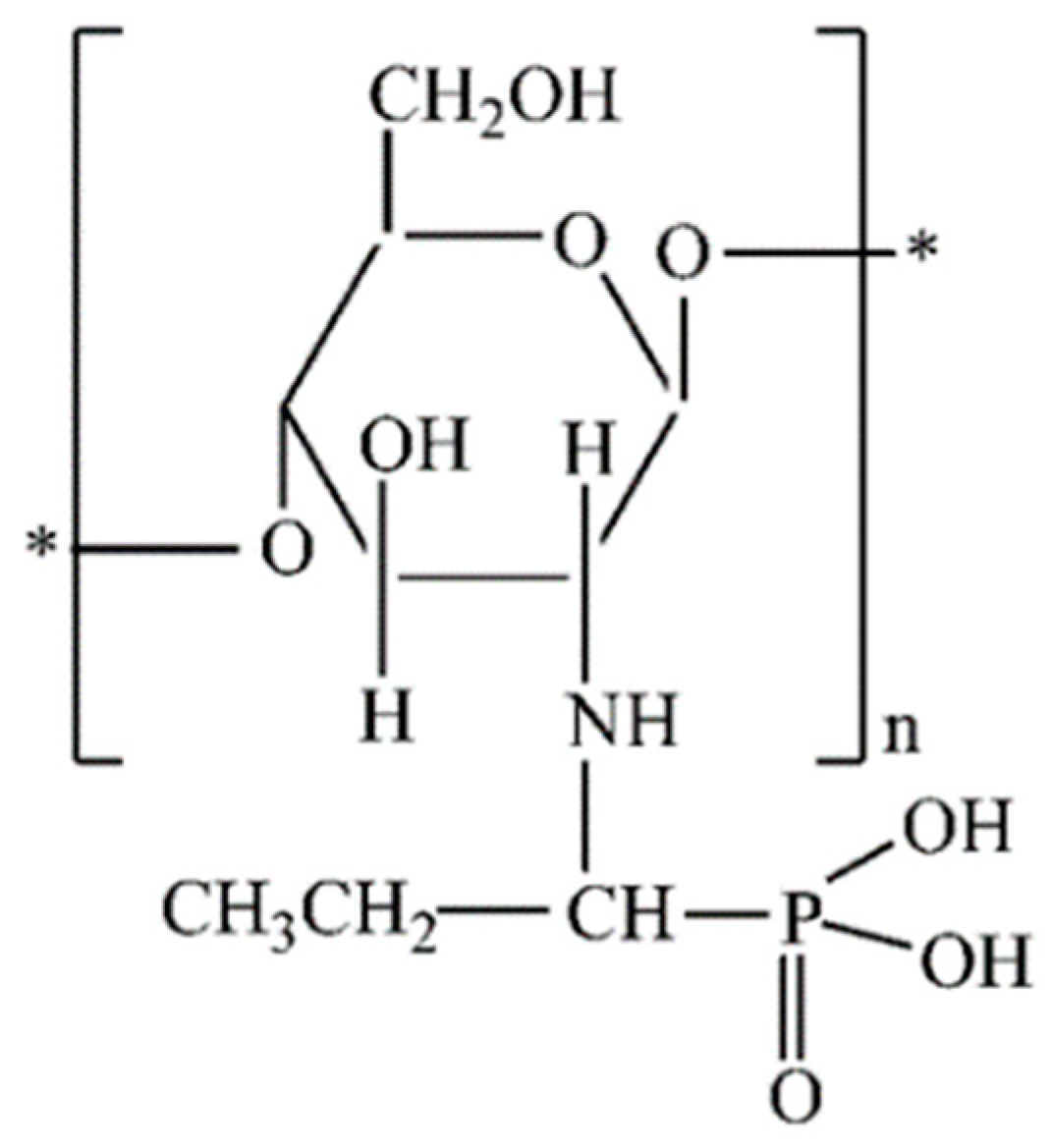
2.2. The Chemical Modification of Chitosan with Aminophosphonic Groups Using the “One-Pot” Kabachnik-Fields Reaction
2.3. The Impregnation with Ni(II) Ions of Chemically Modified Chitosan
2.4. Characterization
2.5. Adsorption Studies of the Modified Chitosan with Aminophosphonic Acid Groups and Ni(II) Ions (ChitPNi)
2.6. Adsorption Theory Background
2.6.1. Kinetic Studies
2.6.2. Equilibrium Studies
3. Results and Discussion
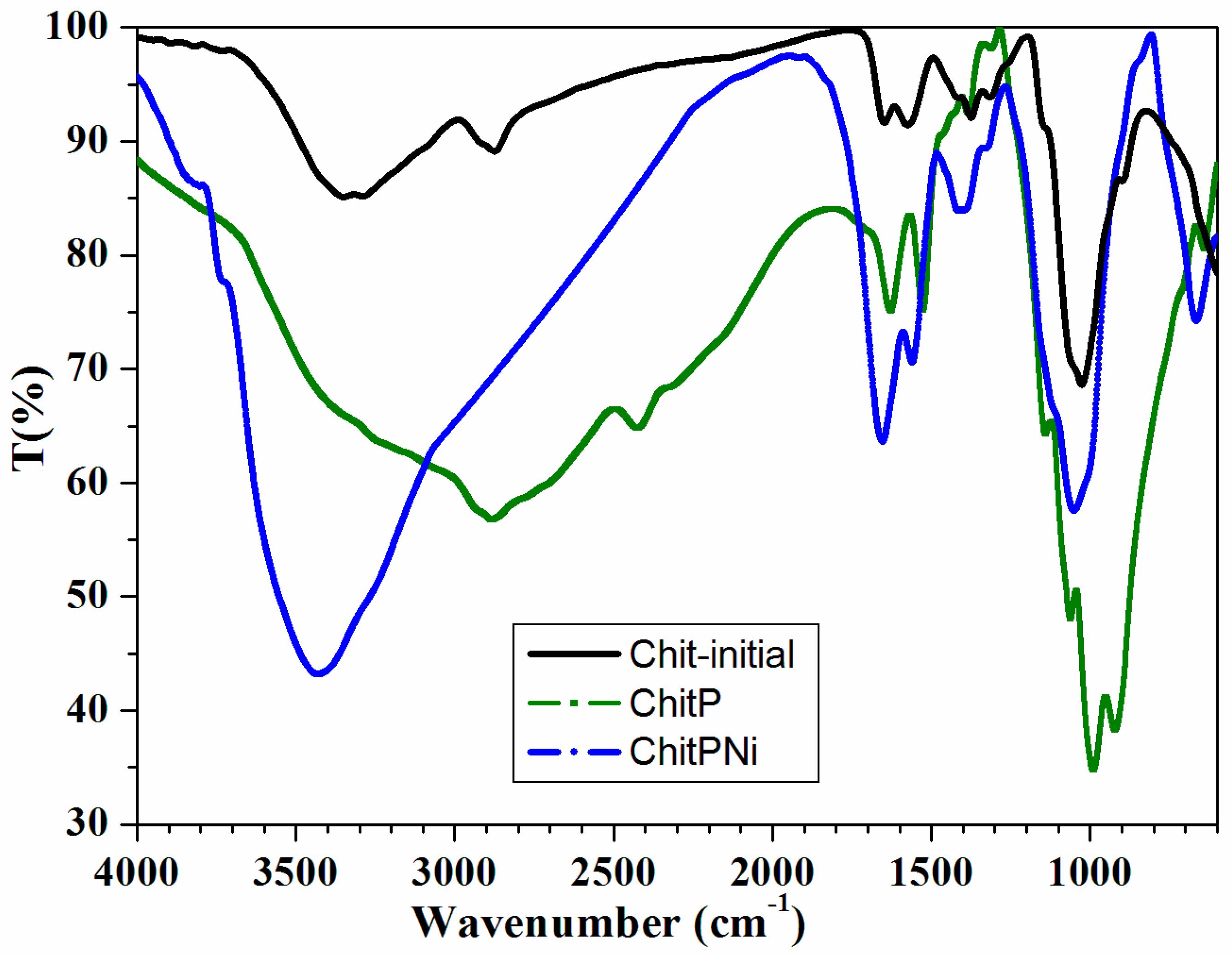
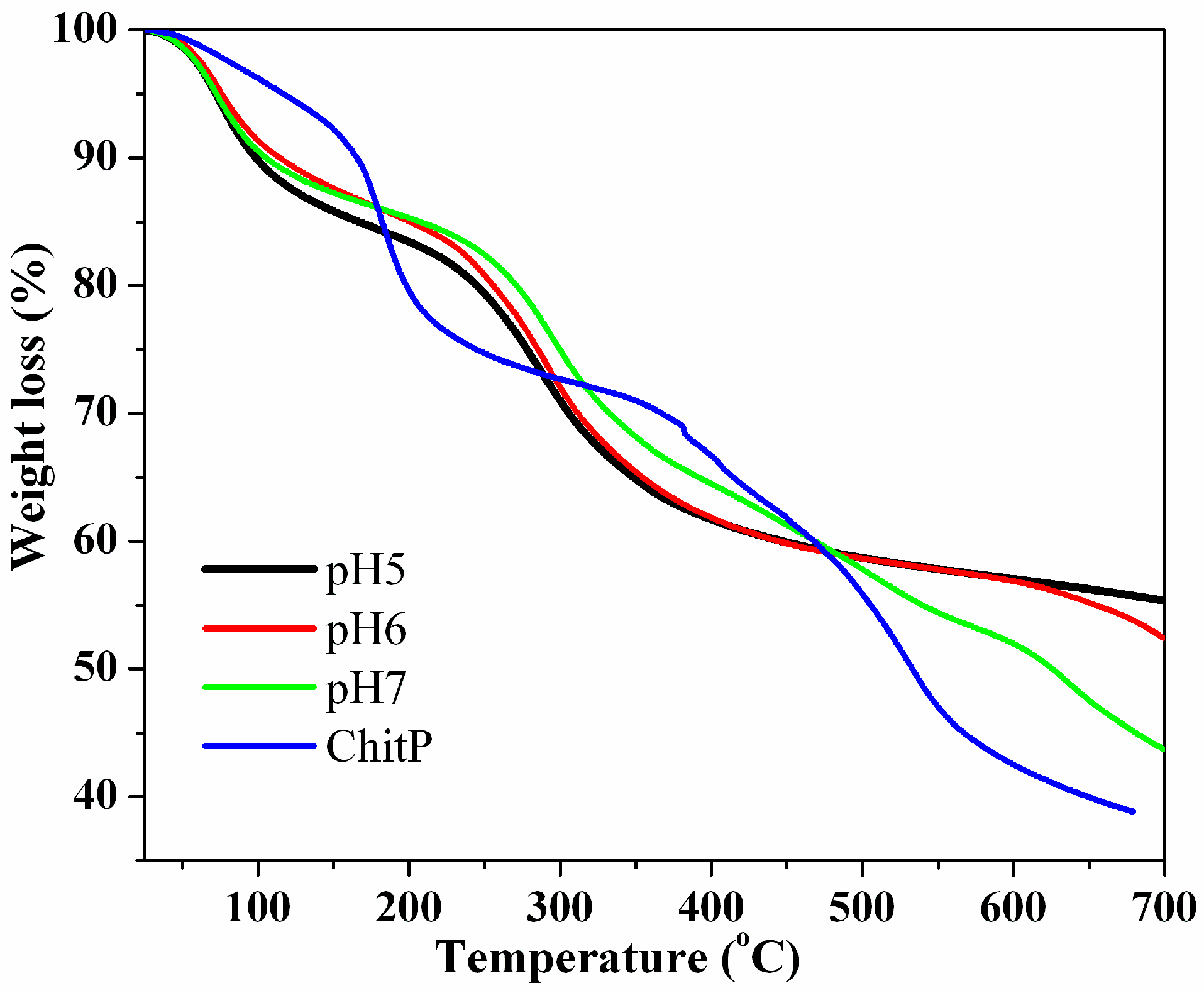
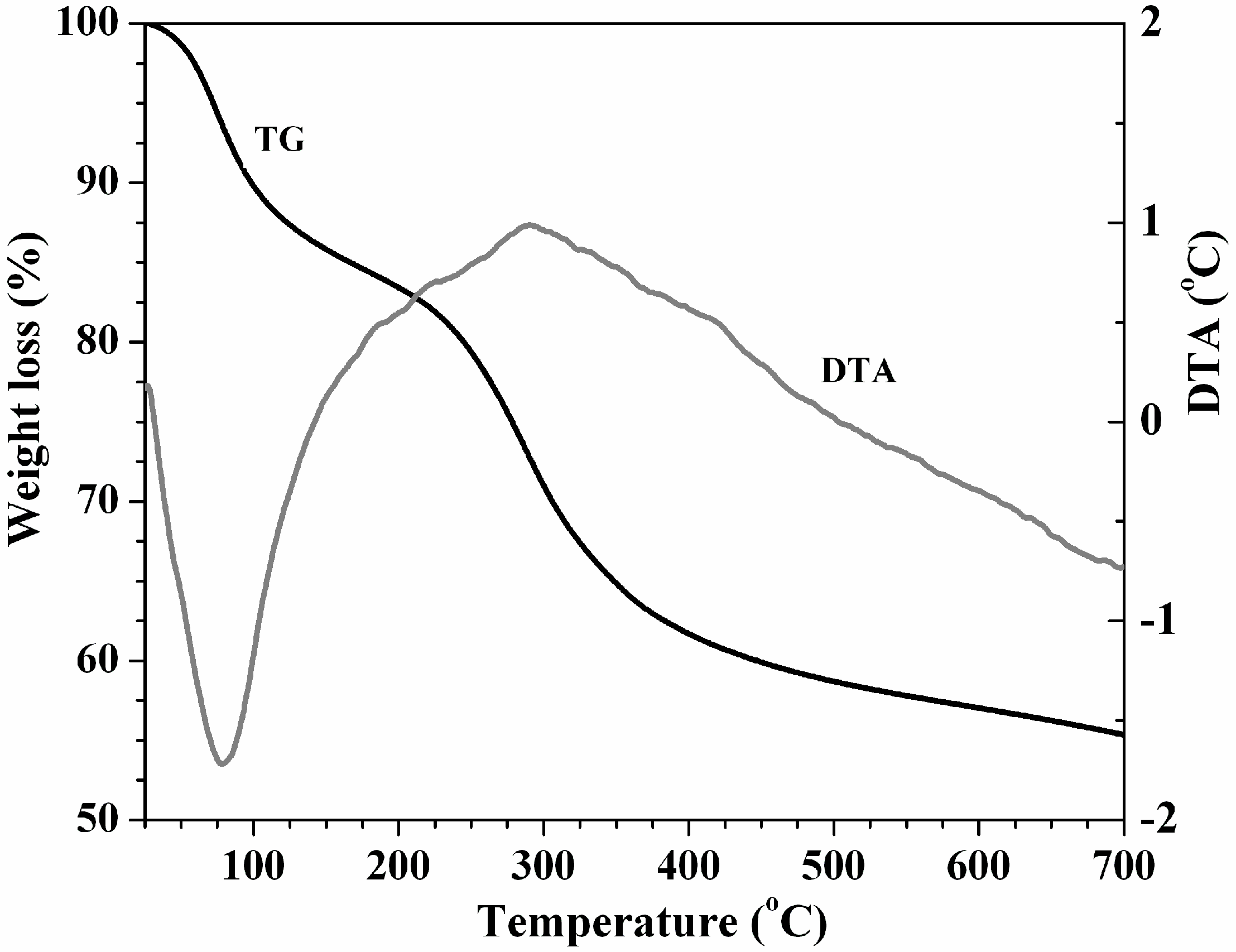
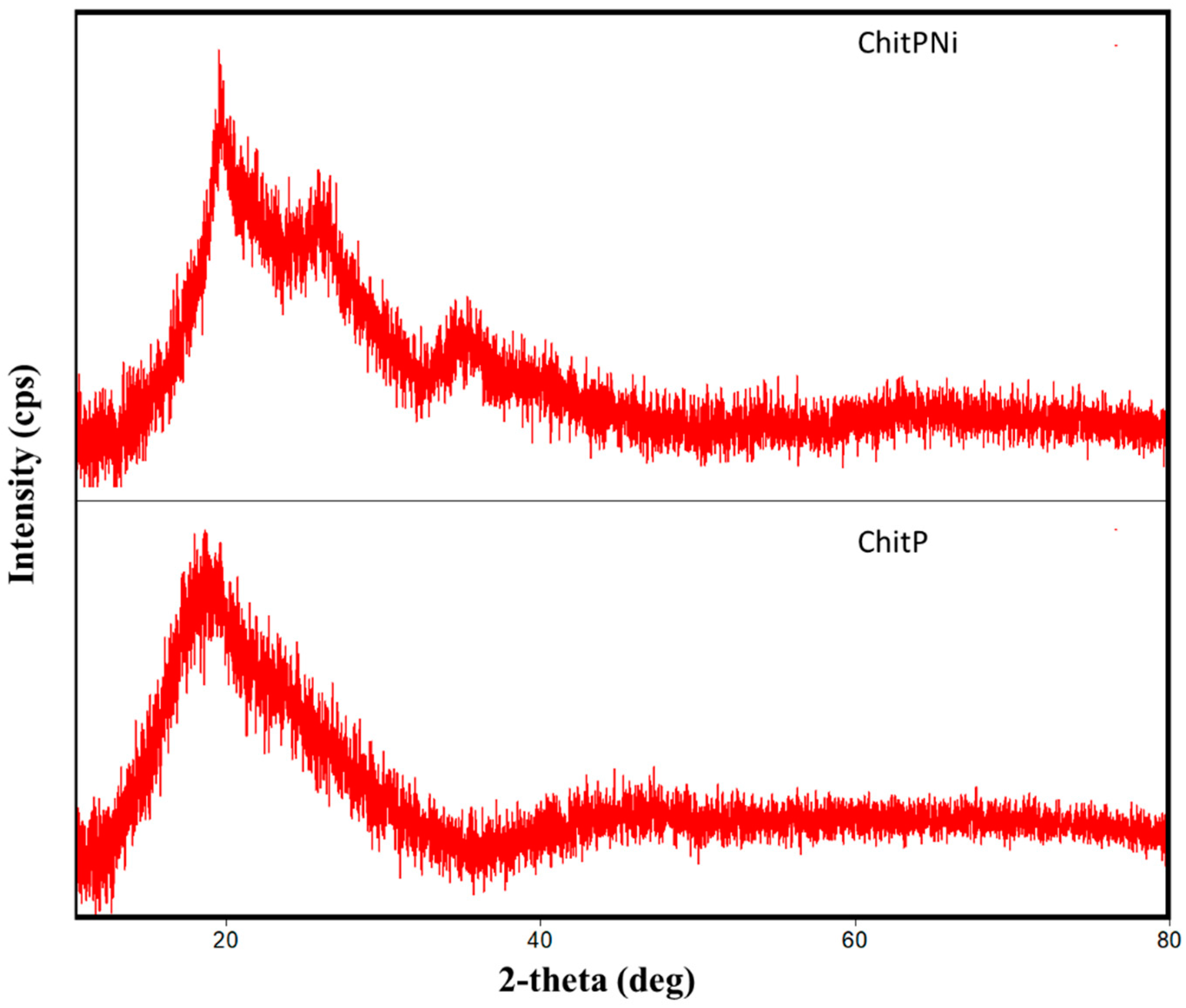
3.1. Adsorbent Characterization
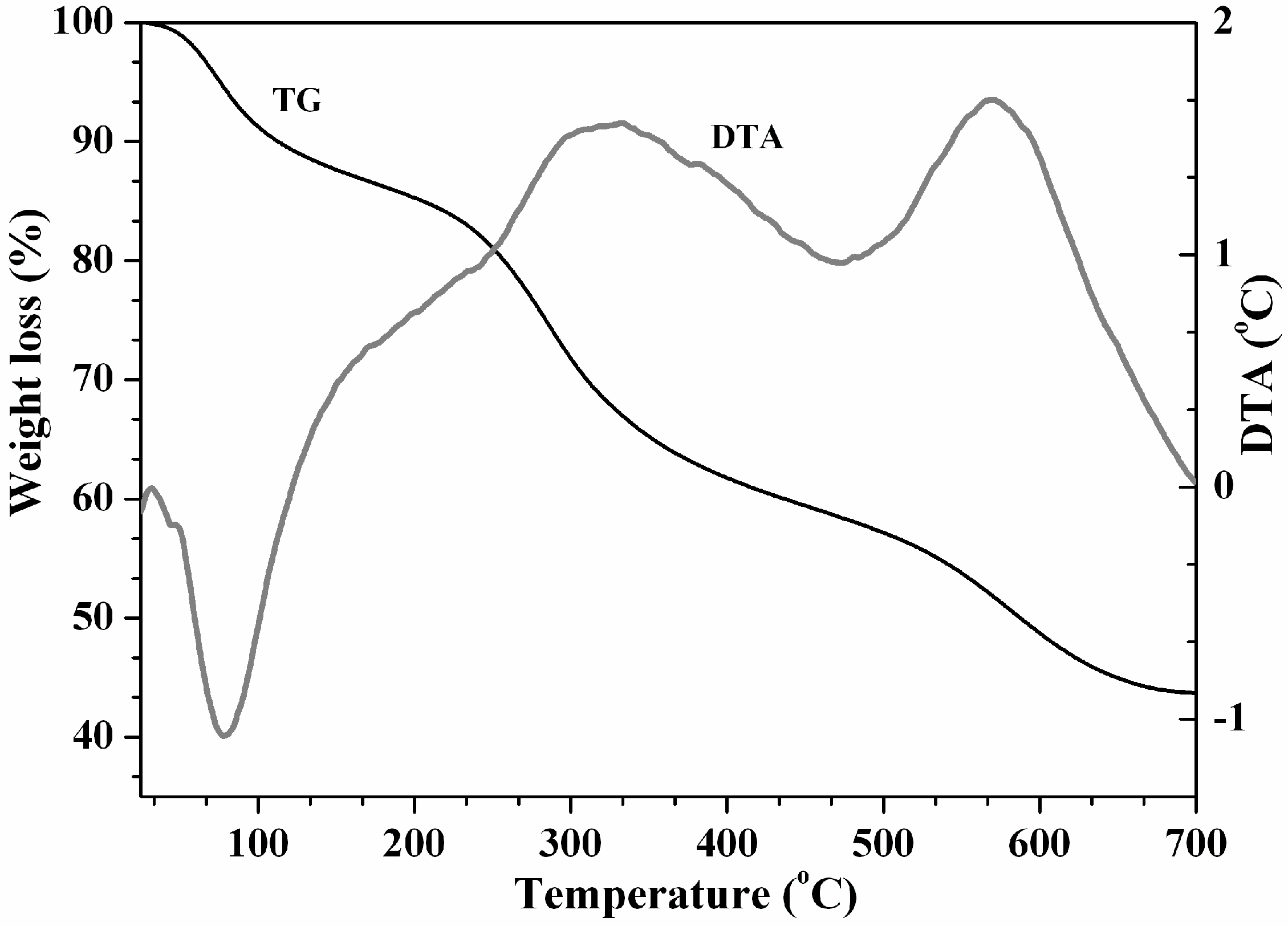
3.2. Adsorbent (ChitPNipH5) Characterization after Pb(II) Ions Adsorption
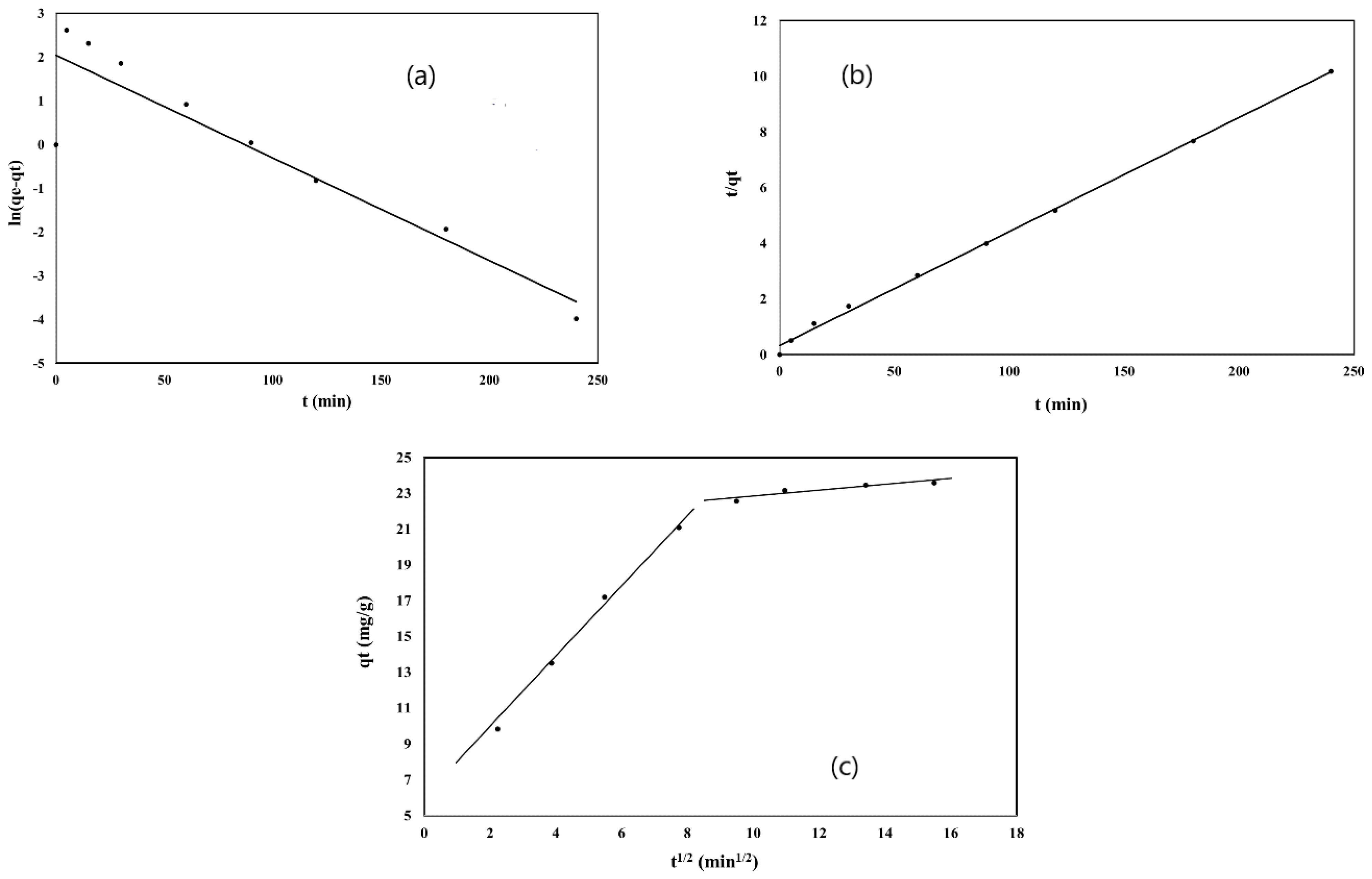
3.3. Kinetic Studies
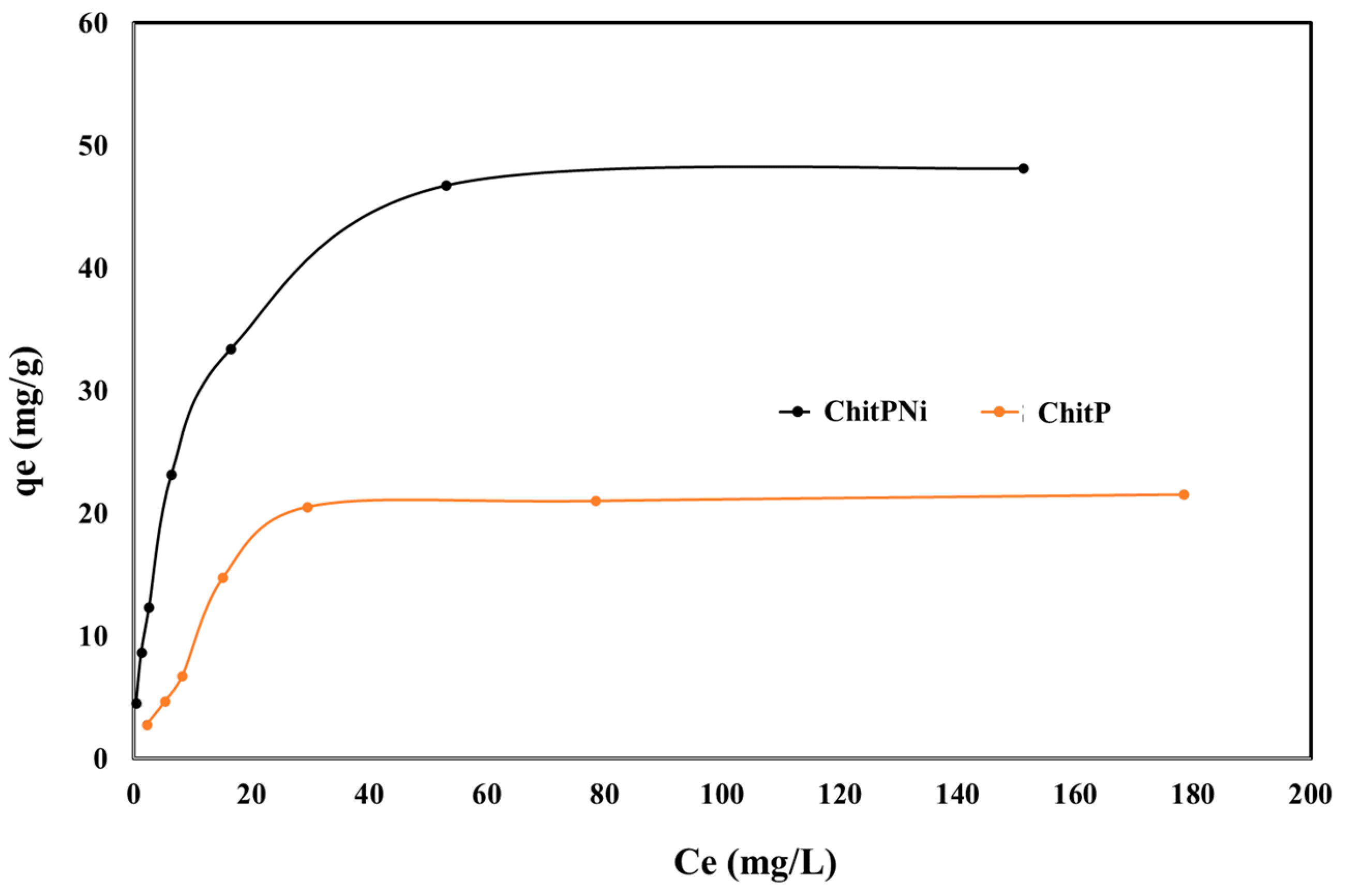
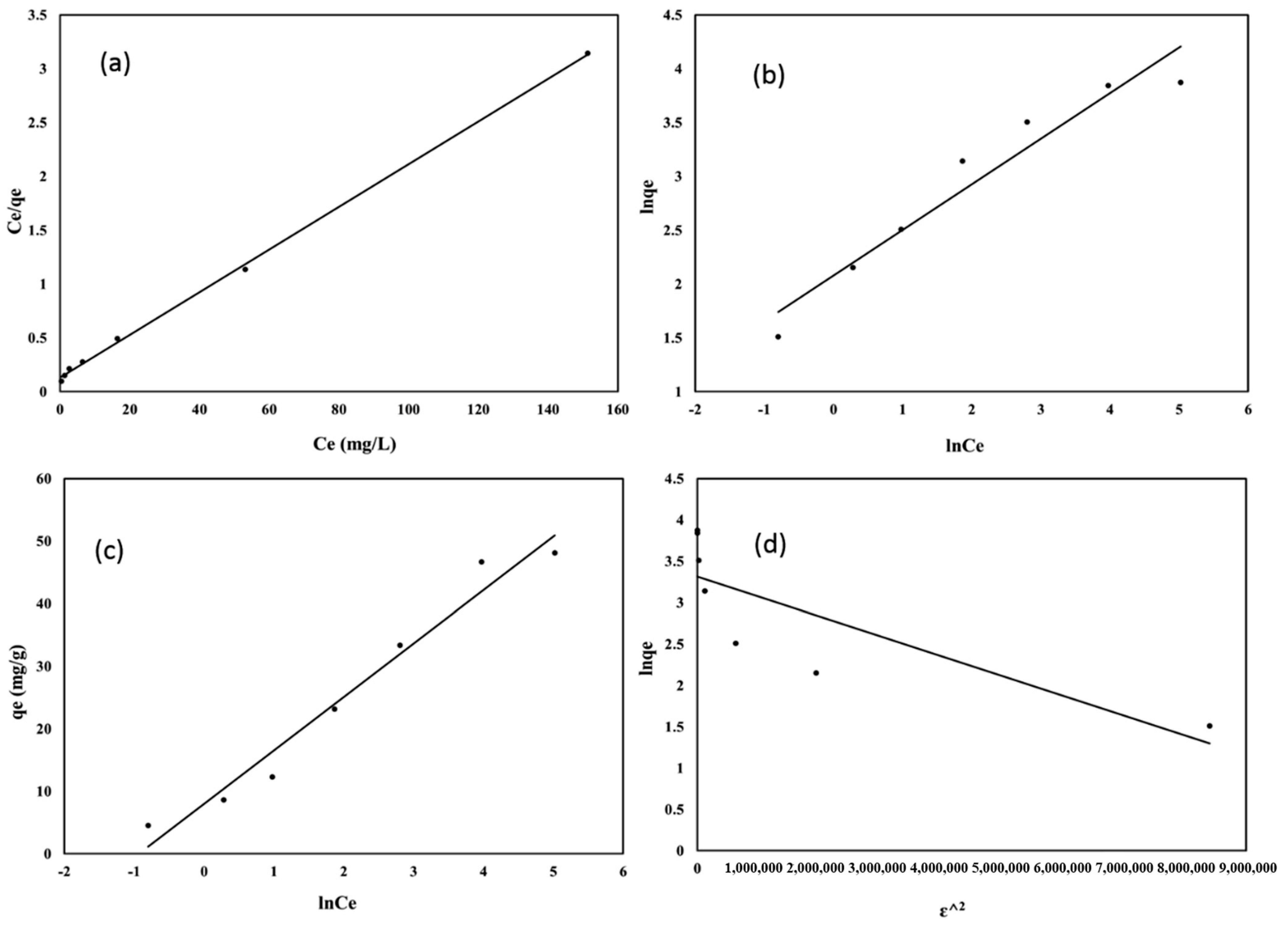
3.4. Equilibrium Studies
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Krishnapriya, K.R.; Kandaswamy, M. Synthesis and Characterization of a Crosslinked Chitosan Derivative with a Complexing Agent and Its Adsorption Studies Toward Metal (II) Ions. Carbohyd. Res. 2009, 344, 1632–1638. [Google Scholar] [CrossRef]
- Hamza, M.F.; Hamad, N.A.; Hamad, D.M.; Khalafalla, M.S.; Abdel-Rahman, A.A.-H.; Zeid, I.F.; Wei, Y.; Hessien, M.M.; Fouda, A.; Salem, W.M. Synthesis of Eco-Friendly Biopolymer, Alginate-Chitosan Composite to Adsorb the Heavy Metals, Cd(II) and Pb(II) from Contaminated Effluents. Materials 2021, 14, 2189. [Google Scholar] [CrossRef]
- Budnyak, T.; Tertykh, V.; Yanovska, E. Chitosan Immobilized on Silica Surface for Wastewater Treatment. J. Mater. Sci. 2014, 20, 177–182. [Google Scholar] [CrossRef] [Green Version]
- Hirano, S.; Seino, H.; Akiyama, Y.; Nonaka, I. Chitosan: A Biocompatible Material for Oral and Intravenous Administrations. In Progress in Biomedical Polymers; Gebelein, C.G., Dunn, R.L., Eds.; Springer: Boston, MA, USA, 1990; pp. 283–290. [Google Scholar] [CrossRef]
- Muzzarelli, R.; Muzzarelli, C. Chitosan Chemistry: Relevance to the Biomedical Sciences. Adv. Polym. Sci. 2005, 186, 151–209. [Google Scholar] [CrossRef]
- Hoppe-Seiler, F. Chitin and Chitosan. Ber. Dtsch. Chem. Ges. 1994, 27, 3329–3331. [Google Scholar]
- Agrawal, P.; Strijkers, G.J.; Nicolay, K. Chitosan-Based Systems for Molecular Imaging. Adv. Drug. Deliv. Rev. 2009, 62, 42–58. [Google Scholar] [CrossRef] [PubMed]
- Muxika, A.; Etxabide, A.; Uranga, J.; Guerrero, K.; de la Caba, P. Chitosan as a Bioactive Polymer: Processing, Properties and Applications. Int. J. Biol. Macromol. 2017, 105, 1358–1368. [Google Scholar] [CrossRef]
- Dash, M.; Chiellini, F.; Ottenbrite, R.M.; Chiellini, E. Chitosan-A Versatile Semi-Synthetic Polymer in Biomedical Applications. Prog. Polym. Sci. 2011, 36, 981–1014. [Google Scholar] [CrossRef]
- Bhatnagar, A.; Sillanpää, M. Applications of Chitin-and Chitosan-Derivatives for the Detoxification of Water and Wastewater-A Short Review. Adv. Colloid. Interfac. 2009, 152, 26–38. [Google Scholar] [CrossRef]
- Maranescu, B.; Popa, A.; Lupa, L.; Maranescu, V.; Visa, A. Use of Chitosan Complex with Aminophosphonic Groups and Cobalt for the Removal of Sr2+ Ions. Sep. Sci. Technol. 2018, 53, 1058–1064. [Google Scholar] [CrossRef]
- Prabhua, S.M.; Viswanathanb, N.; Meenakshi, S. Defluoridation of Water Using Chitosan Assisted Ethylenediamine Functionalized Synthetic Polymeric Blends. Int. J. Biol. Macromol. 2014, 70, 621–627. [Google Scholar] [CrossRef] [PubMed]
- Cho, D.W.; Jeon, B.H.; Jeong, Y.; Nam, I.H.; Choi, U.K.; Kumar, R.; Song, H. Synthesis of Hydrous Zirconium Oxide-Impregnated Chitosan Beads and Their Application for Removal of Fluoride and Lead. Appl. Surf. Sci. 2016, 372, 13–19. [Google Scholar] [CrossRef]
- Salehi, E.; Daraei, P.; Shamsabadi, A.A. A Review on Chitosan-Based Adsorptive Membranes. Carbohydr. Polym. 2016, 152, 419–432. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Xiang, Z.; Liu, Q.; Chen, Y.; Lu, F. Polyethyleneimine-Bacterial Cellulose Bioadsorbent for Effective Removal of Copper and Lead Ions from Aqueous Solution. Bioresour. Technol. 2017, 244, 844–849. [Google Scholar] [CrossRef]
- Lu, J.; Jin, R.N.; Liu, C.; Wang, Y.F.; Ouyang, X.K. Magnetic Carboxylated Cellulose Nanocrystals as Adsorbent for the Removal of Pb(II) from Aqueous Solution. Int. J. Biol. Macromol. 2016, 93, 547–556. [Google Scholar] [CrossRef]
- Kumar, R.; Sharma, R.K. Synthesis and Characterization of Cellulose Based Adsorbents for Removal of Ni(II), Cu(II) and Pb(II) Ions from Aqueous Solutions, React. Funct. Polym. 2019, 140, 82–92. [Google Scholar] [CrossRef]
- Popa, A.; Ilia, G.; Iliescu, S.; Dehelean, G.; Pascariu, A.; Bora, A.; Davidescu, C.M. Mixed Quaternary Ammonium and Phosphonium Salts Bound to Macromolecular Supports for Removal Bacteria from Water. Mol. Cryst. Liq. Cryst. 2004, 418, 923–931. [Google Scholar] [CrossRef]
- Popa, A.; Parvulescu, V.; Tablet, C.; Ilia, G.; Iliescu, S.; Pascariu, A. Heterogeneous Catalysts Obtained by Incorporation of Polymer-Supported Phosphonates into Silica Used in Oxidation Reactions. Polym. Bull. 2008, 60, 149–158. [Google Scholar] [CrossRef]
- Lazar, M.M.; Dinu, I.A.; Silion, M.; Dragan, E.S.; Dinu, M.V. Could the Porous Chitosan-Based Composite Materials Have a Chance to a “NEW LIFE” after Cu(II) ion Binding? Int. J. Biol. Macromol. 2019, 131, 134–146. [Google Scholar] [CrossRef]
- Maranescu, B.; Plesu, N.; Visa, A. Phosphonic Acid vs. Phosphonate Metal Organic Framework Influence on Mild Steel Corrosion Protection. Appl. Surf. Sci. 2019, 497, 143734. [Google Scholar] [CrossRef]
- Sahee, I.O.; Oh, W.D.; Suah, F.B.M. Chitosan Modifications for Adsorption of Pollutants—A Review. J. Hazard. Mater. 2021, 408, 12488. [Google Scholar] [CrossRef]
- Abd El-Hack, M.E.; El-Saadony, M.T.; Shafi, M.E.; Zabermawi, N.M.; Arif, M.; Batiha, G.E.; Khafaga, A.F.; Abd El-Hakim, Y.M.; Al-Sagheer, A.A. Antimicrobial and Antioxidant Properties of Chitosan and Its Derivatives and Their Applications: A Review. Int. J. Biol. Macromol. 2020, 164, 2726–2744. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Meng, Q.; Li, Q.; Liu, J.; Zhou, M.; Jin, Z.; Zhao, K. Chitosan Derivatives and Their Application in Biomedicine. Int. J. Mol. Sci. 2020, 21, 487. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wan Ngah, W.S.; Fatinathan, S. Pb(II) Biosorption Using Chitosan and Chitosan Derivatives Beds: Equilibrium, Ion Exchange and Mechanism Studies. J. Environ. Sci. 2010, 22, 338–346. [Google Scholar] [CrossRef]
- Ridha, A.M. Removal of Lead(II) from Aqueous Solution Using Chitosan Impregnated Granular Activated Carbon. J. Eng. 2017, 3, 46–60. [Google Scholar] [CrossRef]
- Gunay, A.; Arslankaya, E.; Tosun, I. Lead Removal from Queous Solution by Natural and Pretreated Clinoptilolite: Adsorption Equilibrium and Kinetics. J. Hazard. Mater. 2007, 146, 362–371. [Google Scholar] [CrossRef] [PubMed]
- Maranescu, B.; Lupa, L.; Visa, A. Synthesis Characterisations and Pb(II) Sorption Properties of Cobalt Phosphonate Materials. Pure Appl. Chem. 2016, 88, 979–992. [Google Scholar] [CrossRef] [Green Version]
- Anitha, T.; Senthil Kumar, P.; Kumar Sathish, K.; Sriram, K.; Feroze Ahmed, J. Biosorption of Lead(II) Ions onto Nano-Sized Chitosan Particle Blended Polyvinyl Alcohol (PVA): Adsorption Isotherms, Kinetics and Equilibrium Studies. Desalin. Water Treat. 2016, 57, 13711–13721. [Google Scholar] [CrossRef]
- Senthil Kumar, P.; Gayathri, R. Adsorption of Pb(II) Ions from Aqueous Solutions onto Bael Tree Leaf Powder: Isotherms, Kinetics and Thermodynamics Study. J. Eng. Sci. Technol. 2009, 4, 381–399. [Google Scholar]
- Bayuo, J.; Pelig-Ba, K.B.; Abukari, M.A. Isotherm Modelling of Lead(II) Adsorption from Aqueous Solution Using Groundnut Shell as a Low-Cost Adsorbent IOSR. J. Appl. Chem. 2018, 11, 18–23. [Google Scholar] [CrossRef]
- Asandei, D.; Bulgariu, L.; Bobu, E. Lead (II) Removal from Aqueous Solutions by Adsorption onto Chitosan. Cellul. Chem. Technol. 2009, 43, 211–216. [Google Scholar]
- Grade, C.; Vallejo, W.; Zuluaga, F. Equilibrium and Kinetic Study of Lead and Copper Ions Adsorption on Chitosan-Grafted-Poly Acrylic Acid Synthesized by Surface Initiated Atomic Transfer Polymerisation. Molecules 2018, 23, 2218. [Google Scholar] [CrossRef] [Green Version]
- Ghaffari, H.R.; Pasalari, H.; Tajvar, A.; Dindarloo, K.; Gudarzi, B.; Alipour, V.; Ghanbarneajd, A. Linear and Nonlinear Two-Parameter Adsorption Isotherm Modelng: A Case Study. Int. J. Eng. Sci. 2017, 6, 1–11. [Google Scholar] [CrossRef]
- Visa, A.; Maranescu, B.; Bucur, A.; Iliescu, S.; Demadis, K.D. Synthesis and Characterization of a Novel Phosphonate Metal Organic Framework Starting from Copper Salts. Phosphorus Sulfur Silicon Relat. Elem. 2014, 189, 630–639. [Google Scholar] [CrossRef]
- Hu, S.; Song, L.; Pan, H.; Hu, Y. Thermal Properties and Combustion Behaviors of Chitosan Based Flame Retardant Combining Phosphorus and Nickel. Ind. Eng. Chem. Res. 2012, 51, 3663–3669. [Google Scholar] [CrossRef]
- Hamza, M.F.; Wei, Y.; Mira, H.I.; Abdel-Rahman, A.-H.; Guibal, E. Synthesis and Adsorption Characteristics of Grafted Hydrazinyl Amine Magnetite-Chitosan for Ni(II) and Pb(II) Recovery. Chem. Eng. J. 2019, 362, 310–324. [Google Scholar] [CrossRef] [Green Version]
- Jayakumar, R.; Nagahama, H.; Furuike, T.; Tamura, H. Synthesis of Phosphorylated Chitosan by Novel Method and Its Characterization. Int. J. Biol. Macromol. 2008, 42, 335–339. [Google Scholar] [CrossRef]
- Lupa, L.; Cocheci, L.; Trica, B.; Coroaba, A.; Popa, A. Photodegradation of Phenolic Compounds from Water in the Presence of a Pd-Containing Exhausted Adsorbent. Appl. Sci. 2020, 10, 8440. [Google Scholar] [CrossRef]
- De Jesus, J.C.; Gonzalez, I.; Quevedo, A.; Puerta, T. Termal Decomposition of Nickel Acetate Tetrahydrate: An Integrated Study by TGA, QMS and XPS Techniques. J. Mol. Catal. 2005, 228, 283–291. [Google Scholar] [CrossRef]
- Morshedy, A.S.; Galhoum, A.A.; Aleem, A.A.H.A.; El-din, M.T.S.; Okaba, D.M.; Mostafa, M.S.; Mira, H.I.; Yang, Z.; Ibrahim, E.T. Functionalized Aminophosphonate Chitosan-Magnetic Nanocomposites for Cd(II) Removal from Aqueous solutions: Performance and Mechanisms of Sorption. Appl. Surf. Sci. 2021, 561, 150069. [Google Scholar] [CrossRef]
- Rathore, B.S.; Chauhan, N.P.S.; Jadoun, S.; Ameta, S.C.; Ameta, R. Synthesis and Characterization of Chitosan-Polyaniline-Nickel(II) Oxide Nanocomposite. J. Mol. Struct. 2021, 1242, 130750. [Google Scholar] [CrossRef]
- Popa, A.; Ilia, G.; Iliescu, S.; Plesu, N.; Ene, R.; Parvulescu, V. Styrene-Co-Divinylbenzene/Silica Hybrid Supports for Immobilization Transitional Metals and Their Application in Catalysis. Polym. Bull. 2019, 76, 139–152. [Google Scholar] [CrossRef]
- Rajesh, N.; Rajesh, V. An Indigenous Halomonas BVR1 Strain Immobilized in Crosslinked Chitosan for Adsorption of Lead and Cadmium. Int. J. Biol. Macromol. 2015, 79, 300–308. [Google Scholar] [CrossRef]
- Karthik, R.; Meenakshi, S. Removal of Pb(II) and Cd(II) Ions from Aqueous Solutions Using Polyaniline Grafted Chitosan. Chem. Eng. J. 2015, 263, 168–177. [Google Scholar] [CrossRef]
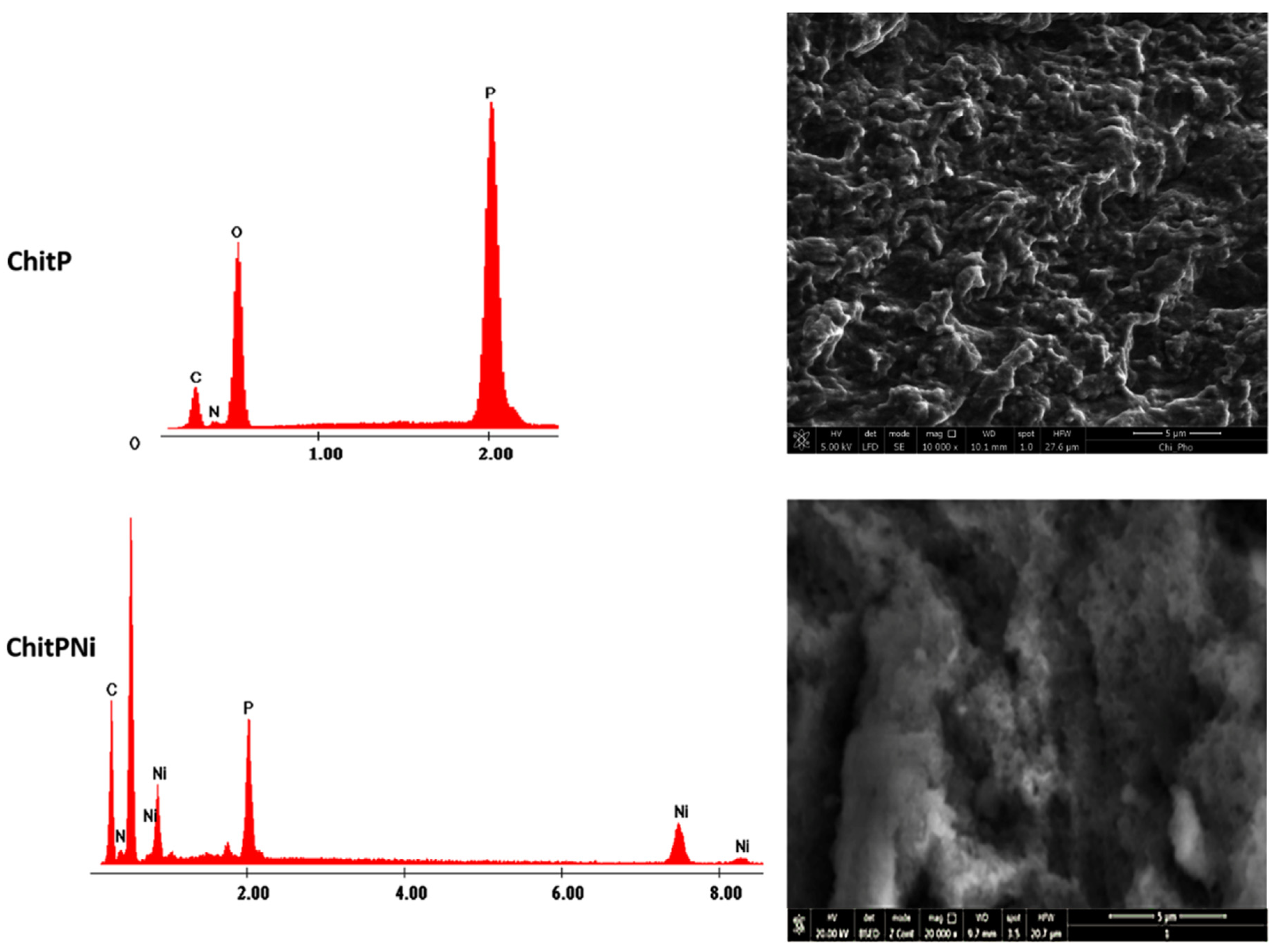
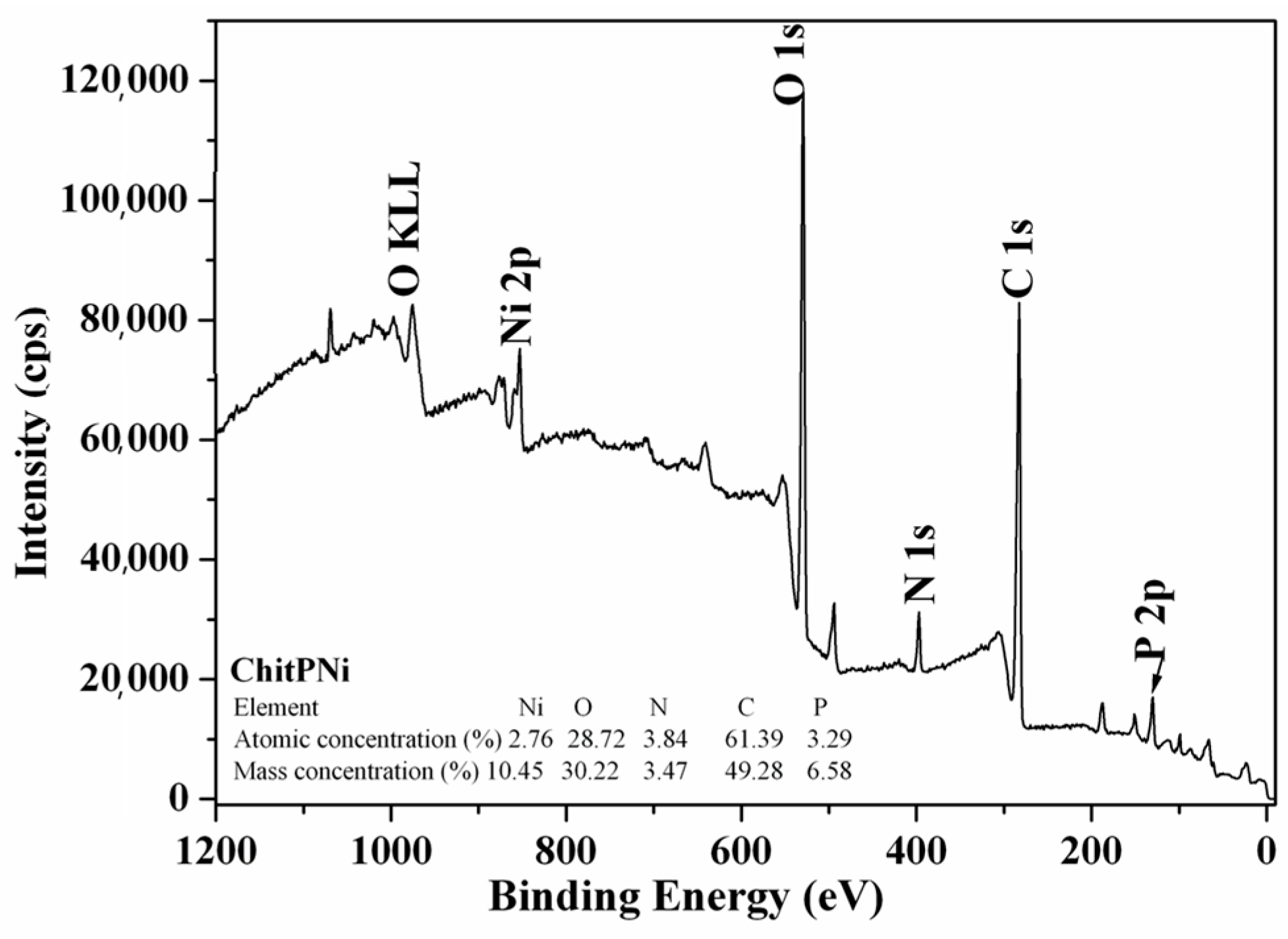
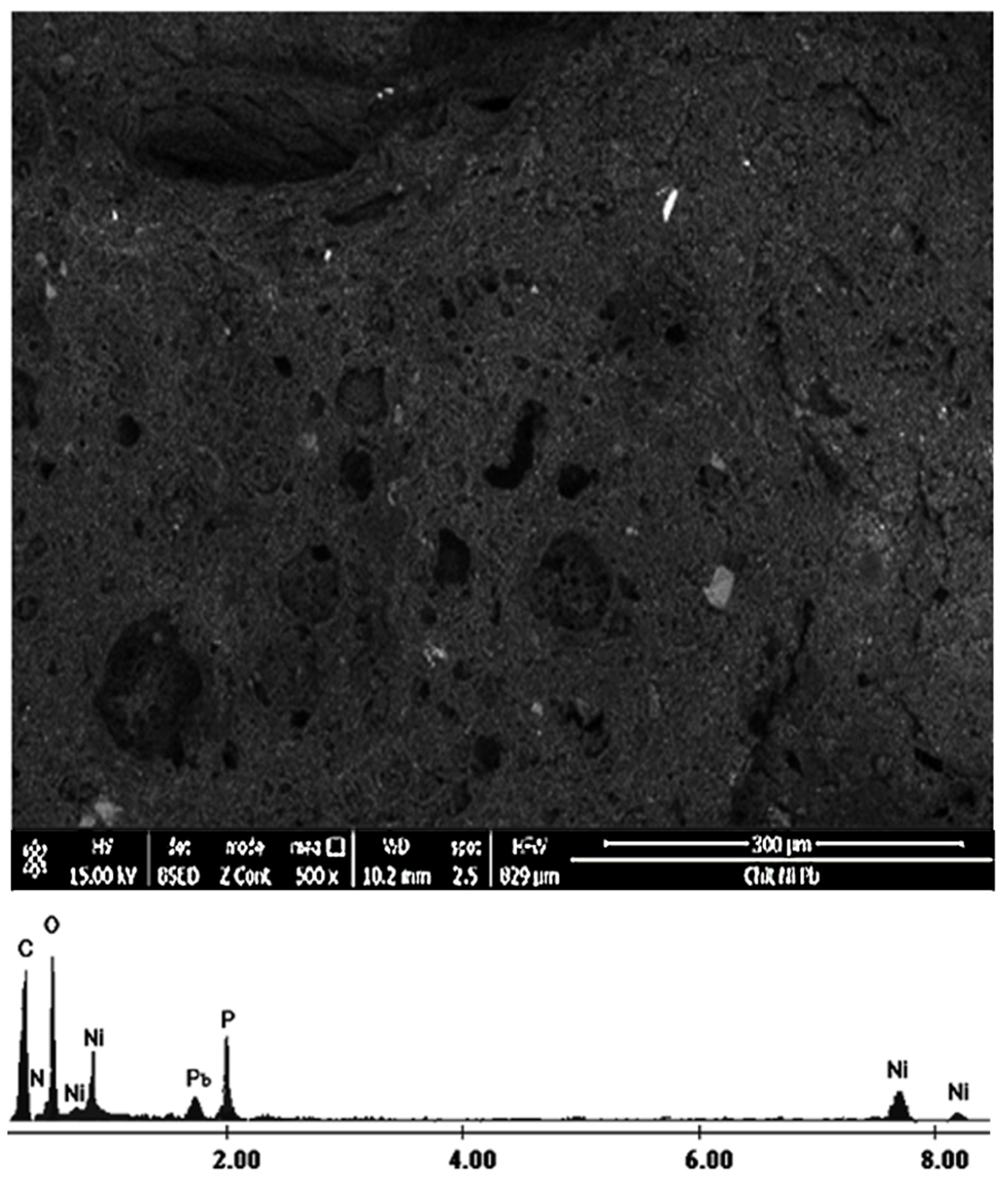

| Sample/Elem. | ChitP | ChitPNi pH 5 | ChitPNi pH 6 | ChitPNi pH 7 |
|---|---|---|---|---|
| Wt% | Wt% | Wt% | Wt% | |
| C | 26.21 | 30.88 | 24.38 | 33.47 |
| N | 5.23 | 5.90 | 6.04 | 5.29 |
| O | 54.13 | 57.32 | 60.19 | 55.38 |
| P | 14.43 | 3.34 | 4.17 | 1.47 |
| Ni | - | 2.13 | 3.25 | 2.54 |
| Sample/Elem. | Modified Chitosan (ChitPNi) after Pb(II) Adsorption | |
|---|---|---|
| Wt% | At% | |
| C | 59.91 | 67.63 |
| N | 4.52 | 4.37 |
| O | 31.62 | 26.80 |
| P | 0.15 | 0.07 |
| Ni | 2.14 | 0.33 |
| Pb | 1.66 | 0.47 |
| Model | Parameter | Value |
|---|---|---|
| Pseudo-first order | qeexp (mg g−1) | 23.6 |
| K1 (min−1) | 0.0235 | |
| qecalc (mg g−1) | 7.72 | |
| R2 | 0.8456 | |
| Pseudo-second order | K2 (min (mg g−1)−1) | 0.128 |
| qecalc (mg g−1) | 24.4 | |
| R2 | 0.9981 | |
| Intra particle diffusion | Kint | 2.053 |
| C | 5.47 | |
| R2 | 0.9947 |
| Type of Isotherm | Parameter | Value | Errors Values | ||
|---|---|---|---|---|---|
| χ2 | RMSE | ERRSQ | |||
| Langmuir | KL, L (mg−1) | 0.150 | 1.23 | 1.63 | 18.7 |
| qmcalc (mg g−1) | 50.3 | ||||
| R2 | 0.9991 | ||||
| Freundlich | KF (mg g−1) | 7.995 | 14.4 | 25.8 | 461 |
| 1/n | 0.4243 | ||||
| R2 | 0.9380 | ||||
| Temkin | KT (L g−1) | 2.556 | 11.4 | 3.013 | 63.6 |
| bT (J mol−1) | 289.85 | ||||
| R2 | 0.9670 | ||||
| Dubinin-Radushkevich | Kad (mol2 kJ−2) | 2.4 ∗ 10−7 | 41.9 | 12.4 | 1084 |
| qm (mg g−1) | 27.6 | ||||
| R2 | 0.6721 | ||||
| E (kJ mol−1) | 1.44 | ||||
| Adsorbent | qm (mg g−1) | References |
|---|---|---|
| Chitosan beads | 34.98 | [25] |
| Chitosan-GLA beads | 14.24 | |
| Chitosan-alginate beads | 60.27 | |
| Groundnut shell | 42.6 | [31] |
| Chitosan | 47.39 | [32] |
| Chitosan-grafted-poly acrylic acid | 54 | [33] |
| Crosslinked chitosan | 24.2 | [44] |
| Polyaniline grafted chitosan | 16.1 | [45] |
| Modified chitosan (ChitPNi) | 50.25 | Present paper |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Popa, A.; Visa, A.; Maranescu, B.; Hulka, I.; Lupa, L. Chemical Modification of Chitosan for Removal of Pb(II) Ions from Aqueous Solutions. Materials 2021, 14, 7894. https://doi.org/10.3390/ma14247894
Popa A, Visa A, Maranescu B, Hulka I, Lupa L. Chemical Modification of Chitosan for Removal of Pb(II) Ions from Aqueous Solutions. Materials. 2021; 14(24):7894. https://doi.org/10.3390/ma14247894
Chicago/Turabian StylePopa, Adriana, Aurelia Visa, Bianca Maranescu, Iosif Hulka, and Lavinia Lupa. 2021. "Chemical Modification of Chitosan for Removal of Pb(II) Ions from Aqueous Solutions" Materials 14, no. 24: 7894. https://doi.org/10.3390/ma14247894
APA StylePopa, A., Visa, A., Maranescu, B., Hulka, I., & Lupa, L. (2021). Chemical Modification of Chitosan for Removal of Pb(II) Ions from Aqueous Solutions. Materials, 14(24), 7894. https://doi.org/10.3390/ma14247894










