Antimicrobial, Antibiofilm, and Antioxidant Activity of Functional Poly(Butylene Succinate) Films Modified with Curcumin and Carvacrol
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of PBS-Based Films
2.3. Determination of Films Antimicrobial Activity
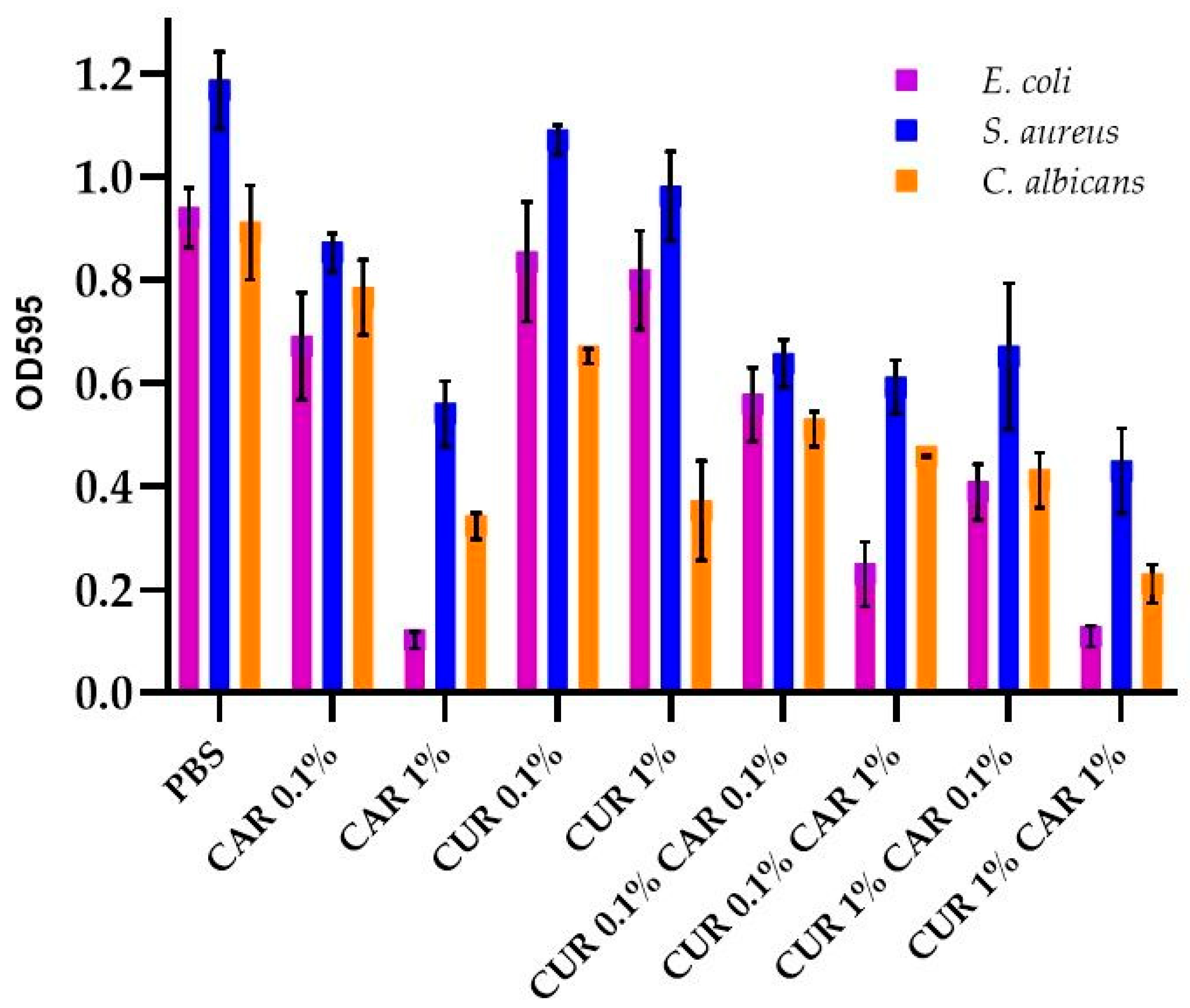
2.4. Determination of Biofilm Formation on Films
2.5. Determination of Reducing Power and Free Radicals Scavenging Activity
2.6. Statistical Analysis
3. Results
3.1. Antimicrobial and Antibiofilm Activity
3.2. Radicals Scavenging Activities and Reducing Power
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Geyer, R.; Jambeck, J.R.; Law, K.L. Production, Use, and Fate of All Plastics Ever Made. Sci. Adv. 2017, 3, 1207–1221. [Google Scholar] [CrossRef] [Green Version]
- Brouwer, M.T.; van Velzen, E.U.T.; Ragaert, K.; Klooster, R. ten Technical Limits in Circularity for Plastic Packages. Sustainability 2020, 12, 10021. [Google Scholar] [CrossRef]
- Siracusa, V.; Rocculi, P.; Romani, S.; Rosa, M.D. Biodegradable Polymers for Food Packaging: A Review. Trends Food Sci. Technol. 2008, 19, 634–643. [Google Scholar] [CrossRef]
- Aeschelmann, F.; Carus, M. Biobased Building Blocks and Polymers in the World: Capacities, Production, and Applications-Status Quo and Trends towards 2020. Ind. Biotechnol. 2015, 11, 154–159. [Google Scholar] [CrossRef]
- Jiang, M.; Ma, J.; Wu, M.; Liu, R.; Liang, L.; Xin, F.; Zhang, W.; Jia, H.; Dong, W. Progress of Succinic Acid Production from Renewable Resources: Metabolic and Fermentative Strategies. Bioresour. Technol. 2017, 245, 1710–1717. [Google Scholar] [CrossRef] [PubMed]
- Zarei, M.; el Fray, M. Synthesis of Hydrophilic Poly(Butylene Succinate-Butylene Dilinoleate) (PBS-DLS) Copolymers Containing Poly(Ethylene Glycol) (PEG) of Variable Molecular Weights. Polymers 2021, 13, 3177. [Google Scholar] [CrossRef]
- Cho, H.S.; Moon, H.S.; Kim, M.; Nam, K.; Kim, J.Y. Biodegradability and Biodegradation Rate of Poly(Caprolactone)-Starch Blend and Poly(Butylene Succinate) Biodegradable Polymer under Aerobic and Anaerobic Environment. Waste Manag. 2011, 31, 475–480. [Google Scholar] [CrossRef]
- Cheung, H.-y.; Ho, M.-p.; Lau, K.-t.; Cardona, F.; Hui, D. Natural Fibre-Reinforced Composites for Bioengineering and Environmental Engineering Applications. Compos. Part B Eng. 2009, 40, 655–663. [Google Scholar] [CrossRef]
- Ojijo, V.; Sinha Ray, S.; Sadiku, R. Role of Specific Interfacial Area in Controlling Properties of Immiscible Blends of Biodegradable Polylactide and Poly[(Butylene Succinate)-Co-Adipate]. ACS Appl. Mater. Interfaces 2012, 4, 6690–6701. [Google Scholar] [CrossRef]
- Alfei, S.; Marengo, B.; Zuccari, G. Nanotechnology Application in Food Packaging: A Plethora of Opportunities versus Pending Risks Assessment and Public Concerns. Food Res. Int. 2020, 137, 109664. [Google Scholar] [CrossRef]
- Ordon, M.; Nawrotek, P.; Stachurska, X.; Mizielí Nska, M. Polyethylene Films Coated with Antibacterial and Antiviral Layers Based on CO2 Extracts of Raspberry Seeds, of Pomegranate Seeds and of Rosemary. Coatings 2021, 11, 1179. [Google Scholar] [CrossRef]
- Ma, Q.; Ren, Y.; Wang, L. Investigation of Antioxidant Activity and Release Kinetics of Curcumin from Tara Gum/ Polyvinyl Alcohol Active Film. Food Hydrocoll. 2017, 70, 286–292. [Google Scholar] [CrossRef]
- Lukic, I.; Vulic, J.; Ivanovic, J. Antioxidant Activity of PLA/PCL Films Loaded with Thymol and/or Carvacrol Using ScCO2 for Active Food Packaging. Food Packag. Shelf Life 2020, 26, 100578. [Google Scholar] [CrossRef]
- Mizielińska, M.; Łopusiewicz, Ł.; Mȩżyńska, M.; Bartkowiak, A. The Influence of Accelerated UV-A and Q-Sun Irradiation on the Antimicrobial Properties of Coatings Containing ZnO Nanoparticles. Molecules 2017, 22, 1556. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Souza, A.G.; dos Santos, N.M.A.; da Silva Torin, R.F.; dos Santos Rosa, D. Synergic Antimicrobial Properties of Carvacrol Essential Oil and Montmorillonite in Biodegradable Starch Films. Int. J. Biol. Macromol. 2020, 164, 1737–1747. [Google Scholar] [CrossRef] [PubMed]
- Jahdkaran, E.; Hosseini, S.E.; Mohammadi Nafchi, A.; Nouri, L. The Effects of Methylcellulose Coating Containing Carvacrol or Menthol on the Physicochemical, Mechanical, and Antimicrobial Activity of Polyethylene Films. Food Sci. Nutr. 2021, 9, 2768–2778. [Google Scholar] [CrossRef] [PubMed]
- Lozano-Navarro, J.I.; Díaz-Zavala, N.P.; Velasco-Santos, C.; Melo-Banda, J.A.; Páramo-García, U.; Paraguay-Delgado, F.; García-Alamilla, R.; Martínez-Hernández, A.L.; Zapién-Castillo, S. Chitosan-Starch Films with Natural Extracts: Physical, Chemical, Morphological and Thermal Properties. Materials 2018, 11, 120. [Google Scholar] [CrossRef] [Green Version]
- Marchese, A.; Arciola, C.R.; Coppo, E.; Barbieri, R.; Barreca, D.; Chebaibi, S.; Sobarzo-Sánchez, E.; Nabavi, S.F.; Nabavi, S.M.; Daglia, M. The Natural Plant Compound Carvacrol as an Antimicrobial and Anti-Biofilm Agent: Mechanisms, Synergies and Bio-Inspired Anti-Infective Materials. Biofouling 2018, 34, 630–656. [Google Scholar] [CrossRef]
- Scaffaro, R.; Maio, A.; Nostro, A. Poly(Lactic Acid)/Carvacrol-Based Materials: Preparation, Physicochemical Properties, and Antimicrobial Activity. Appl. Microbiol. Biotechnol. 2020, 104, 1823–1835. [Google Scholar] [CrossRef] [PubMed]
- Niza, E.; Božik, M.; Bravo, I.; Clemente-Casares, P.; Lara-Sanchez, A.; Juan, A.; Klouček, P.; Alonso-Moreno, C. PEI-Coated PLA Nanoparticles to Enhance the Antimicrobial Activity of Carvacrol. Food Chem. 2020, 328, 127131. [Google Scholar] [CrossRef]
- Krepker, M.; Prinz-Setter, O.; Shemesh, R.; Vaxman, A.; Alperstein, D.; Segal, E. Antimicrobial Carvacrol-Containing Polypropylene Films: Composition, Structure and Function. Polymers 2018, 10, 79. [Google Scholar] [CrossRef] [Green Version]
- Łopusiewicz, Ł.; Kwiatkowski, P.; Drozłowska, E.; Trocer, P.; Kostek, M.; Śliwiński, M.; Polak-Śliwińska, M.; Kowalczyk, E.; Sienkiewicz, M. Preparation and Characterization of Carboxymethyl Cellulose-Based Bioactive Composite Films Modified with Fungal Melanin and Carvacrol. Polymers 2021, 13, 499. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Olsen, C.; McHugh, T.; Friedman, M.; Levin, C.E.; Jaroni, D.; Ravishankar, S. Edible Films Containing Carvacrol and Cinnamaldehyde Inactivate Escherichia coli O157:H7 on Organic Leafy Greens in Sealed Plastic Bags. J. Food Saf. 2020, 40, e12758. [Google Scholar] [CrossRef]
- Akram, M.; Mohiuddin, E. Curcuma Longa and Curcumin: A Review Article. Rom. J. Biol. Plant Biol. 2010, 55, 65–70. [Google Scholar]
- Goel, A.; Kunnumakkara, A.B.; Aggarwal, B.B. Curcumin as “Curecumin”: From Kitchen to Clinic. Biochem. Pharmacol. 2008, 75, 787–809. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aliabbasi, N.; Fathi, M.; Emam-Djomeh, Z. Curcumin: A Promising Bioactive Agent for Application in Food Packaging Systems. J. Environ. Chem. Eng. 2021, 9, 105520. [Google Scholar] [CrossRef]
- Roy, S.; Rhim, J.W. Preparation of Antimicrobial and Antioxidant Gelatin/Curcumin Composite Films for Active Food Packaging Application. Colloids Surf. B Biointerfaces 2020, 188, 110761. [Google Scholar] [CrossRef]
- Roy, S.; Rhim, J.W. Curcumin Incorporated Poly(Butylene Adipate-Co-Terephthalate) Film with Improved Water Vapor Barrier and Antioxidant Properties. Materials 2020, 13, 4369. [Google Scholar] [CrossRef]
- Roy, S.; Rhim, J.W. Preparation of Bioactive Functional Poly(Lactic Acid)/Curcumin Composite Film for Food Packaging Application. Int. J. Biol. Macromol. 2020, 162, 1780–1789. [Google Scholar] [CrossRef] [PubMed]
- Barclay, L.R.C.; Vinqvist, M.R.; Mukai, K.; Goto, H.; Hashimoto, Y.; Tokunaga, A.; Uno, H. On the Antioxidant Mechanism of Curcumin: Classical Methods Are Needed to Determine Antioxidant Mechanism and Activity. Org. Lett. 2000, 2, 2841–2843. [Google Scholar] [CrossRef]
- Yun, D.G.; Lee, D.G. Antibacterial Activity of Curcumin via Apoptosis-like Response in Escherichia coli. Appl. Microbiol. Biotechnol. 2016, 100, 5505–5514. [Google Scholar] [CrossRef]
- Zia, J.; Paul, U.C.; Heredia-Guerrero, J.A.; Athanassiou, A.; Fragouli, D. Low-Density Polyethylene/Curcumin Melt Extruded Composites with Enhanced Water Vapor Barrier and Antioxidant Properties for Active Food Packaging. Polymer 2019, 175, 137–145. [Google Scholar] [CrossRef]
- Chen, Y.; Lin, J.; Fei, Y.; Wang, H.; Gao, W. Preparation and characterization of electrospinning PLA/curcumin composite membranes. Fibers Polym. 2010, 11, 1128–1131. [Google Scholar] [CrossRef]
- Luo, N.; Varaprasad, K.; Reddy, G.V.S.; Rajulu, A.V.; Zhang, J. Preparation and Characterization of Cellulose/Curcumin Composite Films. RSC Adv. 2012, 2, 8483–8488. [Google Scholar] [CrossRef]
- Liu, J.; Wang, H.; Wang, P.; Guo, M.; Jiang, S.; Li, X.; Jiang, S. Films Based on κ-Carrageenan Incorporated with Curcumin for Freshness Monitoring. Food Hydrocoll. 2018, 83, 134–142. [Google Scholar] [CrossRef]
- Suwantong, O.; Opanasopit, P.; Ruktanonchai, U.; Supaphol, P. Electrospun Cellulose Acetate Fiber Mats Containing Curcumin and Release Characteristic of the Herbal Substance. Polymer 2007, 48, 7546–7557. [Google Scholar] [CrossRef]
- Gopinath, D.; Ahmed, M.R.; Gomathi, K.; Chitra, K.; Sehgal, P.K.; Jayakumar, R. Dermal Wound Healing Processes with Curcumin Incorporated Collagen Films. Biomaterials 2004, 25, 1911–1917. [Google Scholar] [CrossRef]
- de Campos, S.S.; de Oliveira, A.; Moreira, T.F.M.; da Silva, T.B.V.; da Silva, M.V.; Pinto, J.A.; Bilck, A.P.; Gonçalves, O.H.; Fernandes, I.P.; Barreiro, M.F.; et al. TPCS/PBAT Blown Extruded Films Added with Curcumin as a Technological Approach for Active Packaging Materials. Food Packag. Shelf Life 2019, 22, 100424. [Google Scholar] [CrossRef] [Green Version]
- Varshosaz, J.; Jajanian-Najafabadi, A.; Soleymani, A.; Khajavinia, A. Poly (Butylene Adipate-Co-Terephthalate) Electrospun Nanofibers Loaded with 5-Fluorouracil and Curcumin in Treatment of Colorectal Cancer Cells. Polym. Test. 2018, 65, 217–230. [Google Scholar] [CrossRef]
- Ezati, P.; Rhim, J.W. PH-Responsive Pectin-Based Multifunctional Films Incorporated with Curcumin and Sulfur Nanoparticles. Carbohydr. Polym. 2020, 230, 115638. [Google Scholar] [CrossRef]
- Kavoosi, G.; Dadfar, S.M.M.; Mohammadi Purfard, A.; Mehrabi, R. Antioxidant and Antibacterial Properties of Gelatin Films Incorporated with Carvacrol. J. Food Saf. 2013, 33, 423–432. [Google Scholar] [CrossRef]
- Musso, Y.S.; Salgado, P.R.; Mauri, A.N. Smart Edible Films Based on Gelatin and Curcumin. Food Hydrocoll. 2017, 66, 8–15. [Google Scholar] [CrossRef] [Green Version]
- Łopusiewicz, Ł.; Zdanowicz, M.; Macieja, S.; Kowalczyk, K.; Bartkowiak, A. Development and Characterization of Bioactive Poly(Butylene-Succinate) Films Modified with Quercetin for Food Packaging Applications. Polymers 2021, 13, 1798. [Google Scholar] [CrossRef] [PubMed]
- Barros, C.H.N.; Devlin, H.; Hiebner, D.W.; Vitale, S.; Quinn, L.; Casey, E. Enhancing Curcumin’s Solubility and Antibiofilm Activity: Via Silica Surface Modification. Nanoscale Adv. 2020, 2, 1694–1708. [Google Scholar] [CrossRef] [Green Version]
- Łopusiewicz, Ł.; Drozłowska, E.; Trocer, P.; Kostek, M.; Śliwiński, M.; Henriques, M.H.F.; Bartkowiak, A.; Sobolewski, P. Whey Protein Concentrate/Isolate Biofunctional Films Modified with Melanin from Watermelon (Citrullus lanatus) Seeds. Materials 2020, 13, 3876. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Moser, D.; Han, F.; Leonhard, M.; Schneider-Stickler, B.; Tan, Y. Preparation and Antibiofilm Studies of Curcumin Loaded Chitosan Nanoparticles against Polymicrobial Biofilms of Candida albicans and Staphylococcus aureus. Carbohydr. Polym. 2020, 241, 116254. [Google Scholar] [CrossRef] [PubMed]
- Qiao, Y.; Duan, L. Curcumin-Loaded Polyvinyl Butyral Film with Antibacterial Activity. E-Polymers 2020, 20, 673–681. [Google Scholar] [CrossRef]
- Abdel-Lateef, E.; Mahmoud, F.; Hammam, O.; El-Ahwany, E.; El-Wakil, E.; Kandil, S.; Abu Taleb, H.; El-Sayed, M.; Hassenein, H. Bioactive Chemical Constituents of Curcuma longa L. Rhizomes Extract Inhibit the Growth of Human Hepatoma Cell Line (HepG2). Acta Pharm. 2016, 66, 387–398. [Google Scholar] [CrossRef] [Green Version]
- Vamanu, E.; Gatea, F.; Sârbu, I.; Pelinescu, D. An in Vitro Study of the Influence of Curcuma longa Extracts on the Microbiota Modulation Process, in Patients with Hypertension. Pharmaceutics 2019, 11, 191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verderosa, A.D.; Totsika, M.; Fairfull-Smith, K.E. Bacterial Biofilm Eradication Agents: A Current Review. Front. Chem. 2019, 7, 824. [Google Scholar] [CrossRef] [Green Version]
- Nahar, S.; Mizan, F.R.; Ha, A.J.-w.; Ha, S.-D. Advances and Future Prospects of Enzyme-Based Biofilm Prevention Approaches in the Food Industry. Compr. Rev. Food Sci. Food Saf. 2018, 17, 1484–1502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chan, S.; Pullerits, K.; Keucken, A.; Persson, K.M.; Paul, C.J.; Rådström, P. Bacterial Release from Pipe Biofilm in a Full-Scale Drinking Water Distribution System. NPJ Biofilms Microbiomes 2019, 5, 1–8. [Google Scholar] [CrossRef] [PubMed]
- di Salle, A.; Viscusi, G.; di Cristo, F.; Valentino, A.; Gorrasi, G.; Lamberti, E.; Vittoria, V.; Calarco, A.; Peluso, G. Antimicrobial and Antibiofilm Activity of Curcumin-Loaded Electrospun Nanofibers for the Prevention of the Biofilm-Associated Infections. Molecules 2021, 26, 4866. [Google Scholar] [CrossRef]
- Luzi, F.; Fortunati, E.; Giovanale, G.; Mazzaglia, A.; Torre, L.; Balestra, G.M. Cellulose Nanocrystals from Actinidia Deliciosa Pruning Residues Combined with Carvacrol in PVA_CH Films with Antioxidant/Antimicrobial Properties for Packaging Applications. Int. J. Biol. Macromol. 2017, 104, 43–55. [Google Scholar] [CrossRef] [PubMed]



| Sample Name | CUR Concentration (w/w) | CAR Concentration (w/w) |
|---|---|---|
| PBS | 0% | 0% |
| CAR1% | 0% | 1% |
| CAR0.1% | 0% | 0.1% |
| CUR0.1% | 0.1% | 0% |
| CUR1% | 1% | 0% |
| CUR0.1%CAR0.1% | 0.1% | 0.1% |
| CUR0.1%CAR 1% | 0.1% | 1% |
| CUR1%CAR0.1% | 1% | 0.1% |
| CUR1%CAR 1% | 1% | 1% |
| Sample | RP (700 nm) | DPPH (%) | ABTS (%) |
|---|---|---|---|
| PBS | 0.000 ± 0.000 f | 0.00 ± 0.00 e | 0.00 ± 0.00 f |
| CAR1% | 0.169 ± 0.004 de | 47.90 ± 3.68 b | 88.84 ± 1.89 b |
| CAR0.1% | 0.169 ± 0.001 de | 20.38 ± 2.78 c | 28.72 ± 1.89 c |
| CUR0.1% | 0.173 ± 0.001 cd | 25.14 ± 3.76 c | 51.93 ± 4.00 d |
| CUR1% | 0.190 ± 0.001 b | 88.43 ± 2.74 a | 98.21 ± 0.42 a |
| CUR0.1%CAR0.1% | 0.175 ± 0.002 c | 52.99 ± 4.78 b | 76.93 ± 0.63 e |
| CUR0.1%CAR 1% | 0.176 ± 0.002 c | 68.22 ± 1.25 d | 91.07 ± 0.00 b |
| CUR1%CAR0.1% | 0.193 ± 0.004 ab | 89.31 ± 0.23 a | 97.77 ± 0.21 a |
| CUR1%CAR 1% | 0.196 ± 0.007 a | 91.47 ± 0.00 a | 99.21 ± 0.00 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Łopusiewicz, Ł.; Macieja, S.; Bartkowiak, A.; El Fray, M. Antimicrobial, Antibiofilm, and Antioxidant Activity of Functional Poly(Butylene Succinate) Films Modified with Curcumin and Carvacrol. Materials 2021, 14, 7882. https://doi.org/10.3390/ma14247882
Łopusiewicz Ł, Macieja S, Bartkowiak A, El Fray M. Antimicrobial, Antibiofilm, and Antioxidant Activity of Functional Poly(Butylene Succinate) Films Modified with Curcumin and Carvacrol. Materials. 2021; 14(24):7882. https://doi.org/10.3390/ma14247882
Chicago/Turabian StyleŁopusiewicz, Łukasz, Szymon Macieja, Artur Bartkowiak, and Mirosława El Fray. 2021. "Antimicrobial, Antibiofilm, and Antioxidant Activity of Functional Poly(Butylene Succinate) Films Modified with Curcumin and Carvacrol" Materials 14, no. 24: 7882. https://doi.org/10.3390/ma14247882
APA StyleŁopusiewicz, Ł., Macieja, S., Bartkowiak, A., & El Fray, M. (2021). Antimicrobial, Antibiofilm, and Antioxidant Activity of Functional Poly(Butylene Succinate) Films Modified with Curcumin and Carvacrol. Materials, 14(24), 7882. https://doi.org/10.3390/ma14247882








