A Study of the Impact of Graphene Oxide on Viral Infection Related to A549 and TC28a2 Human Cell Lines
Abstract
:1. Introduction
2. Materials and Methods
2.1. Graphene Oxide (GO) Synthesis
2.2. Physicochemical Characterization
2.3. Tissue Cultures
2.4. Cytological Investigations Tested on A549 and TC28a2 Cell Lines
2.5. Morphometrical Analysis
2.6. Statistical Analysis
3. Results
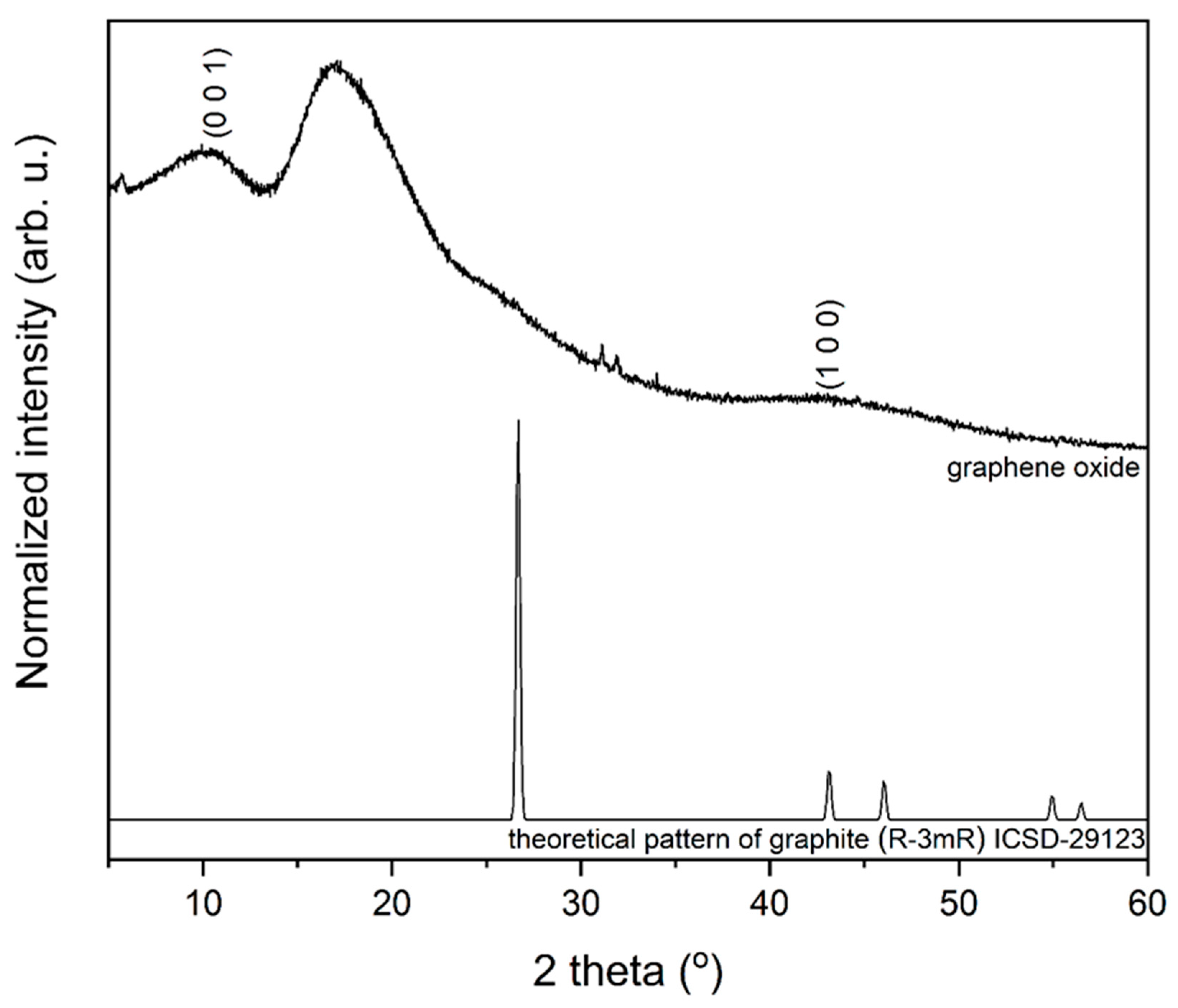
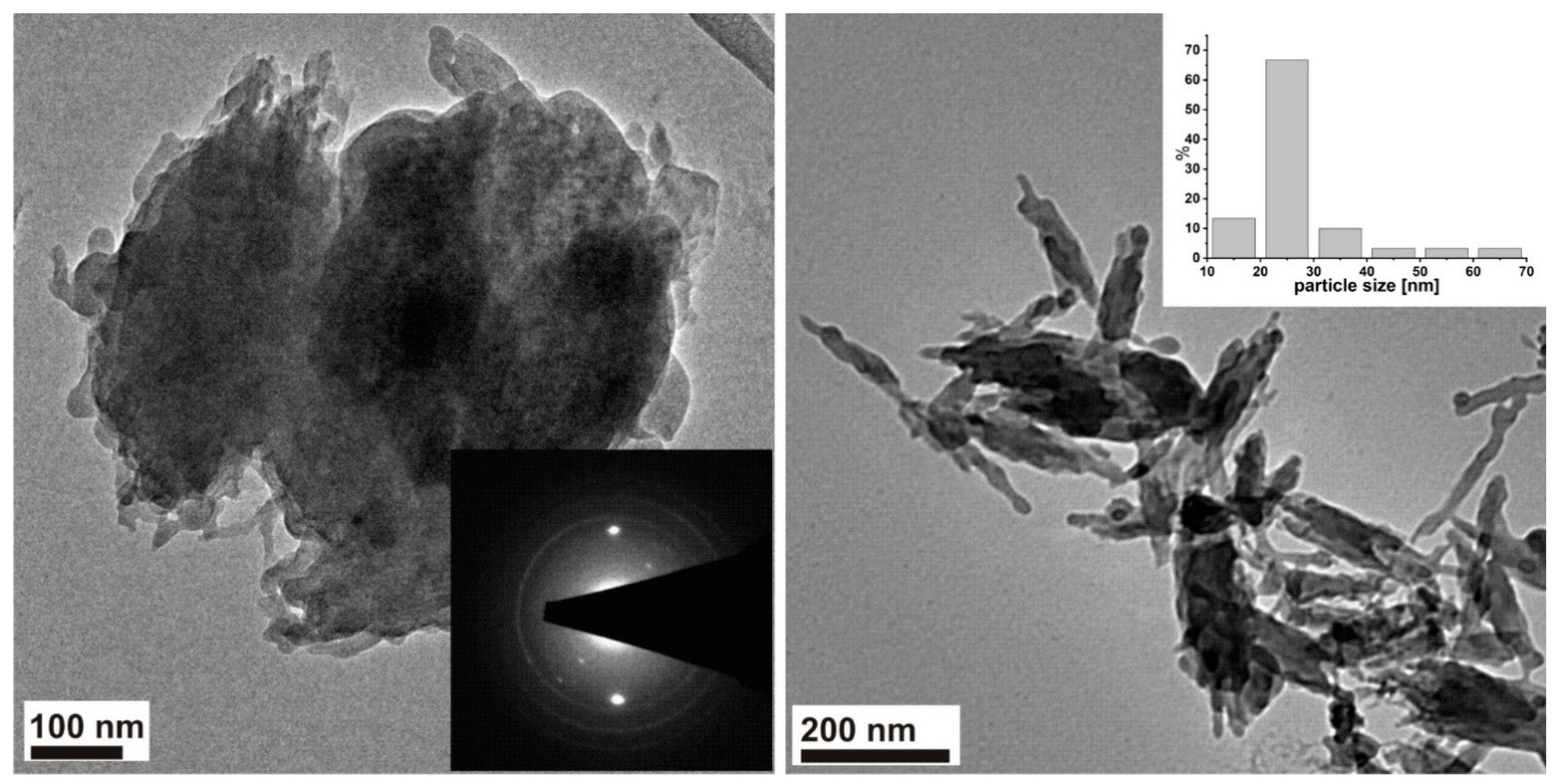
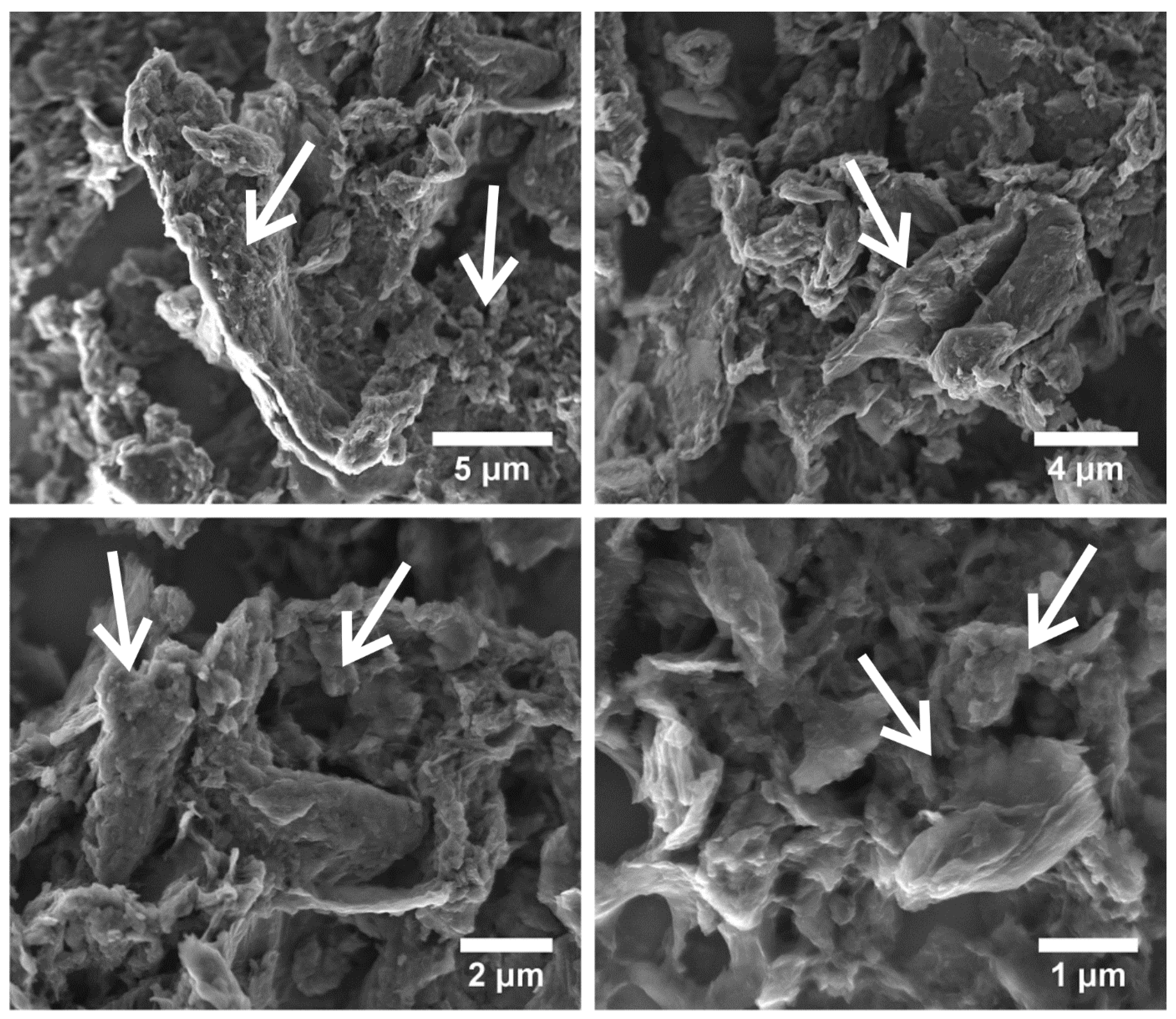
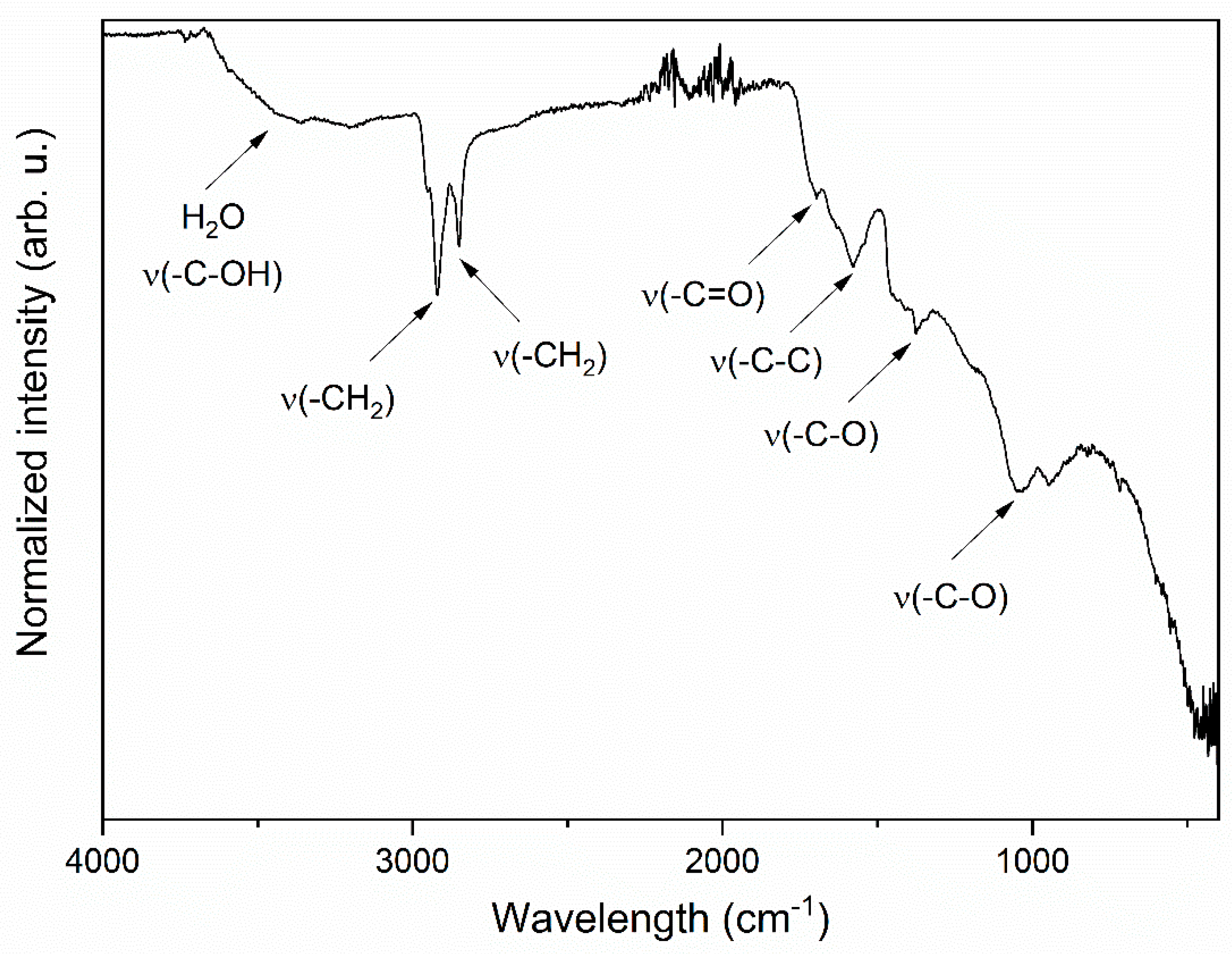
3.1. Structure and Morphology Analysis
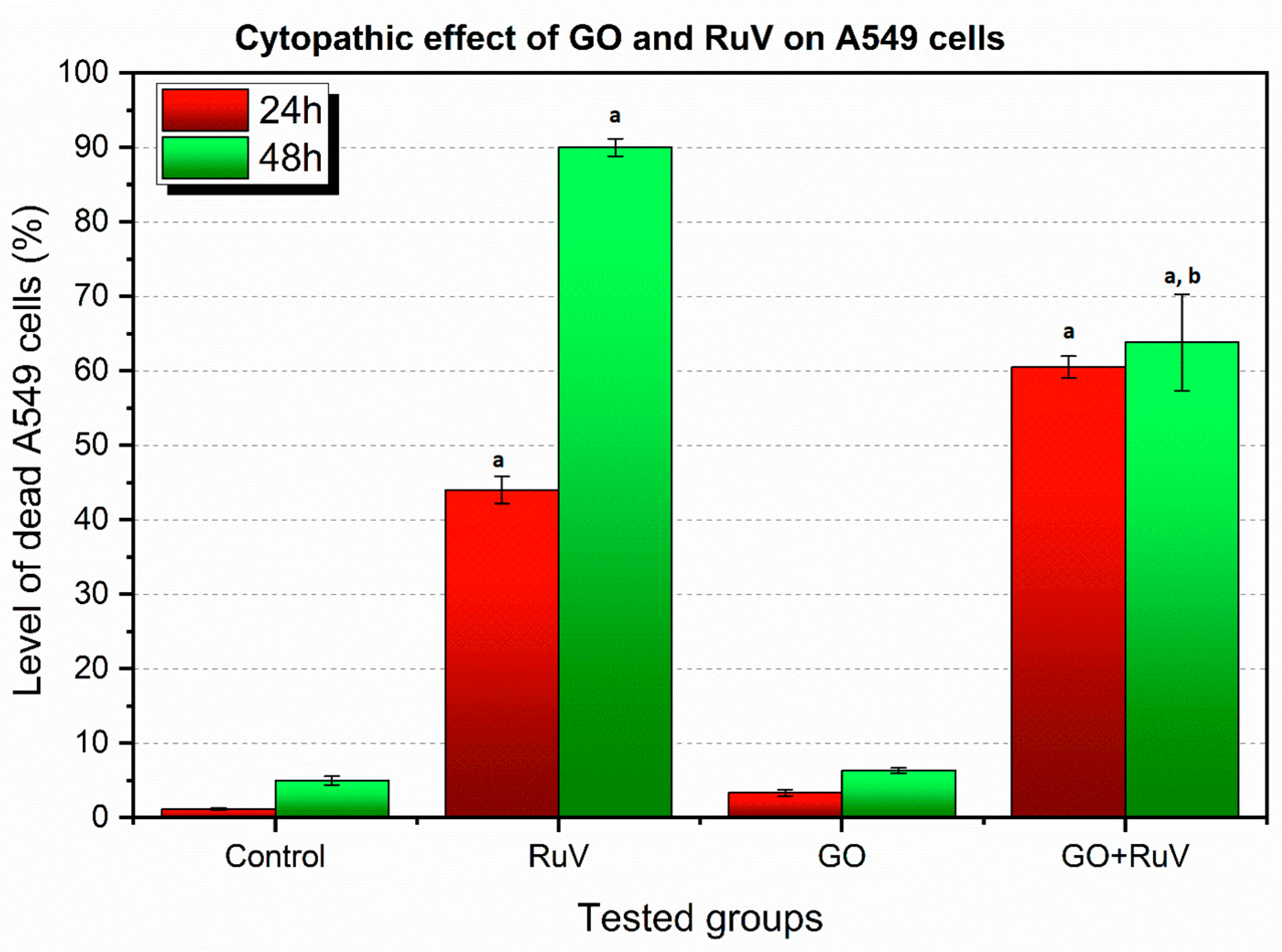
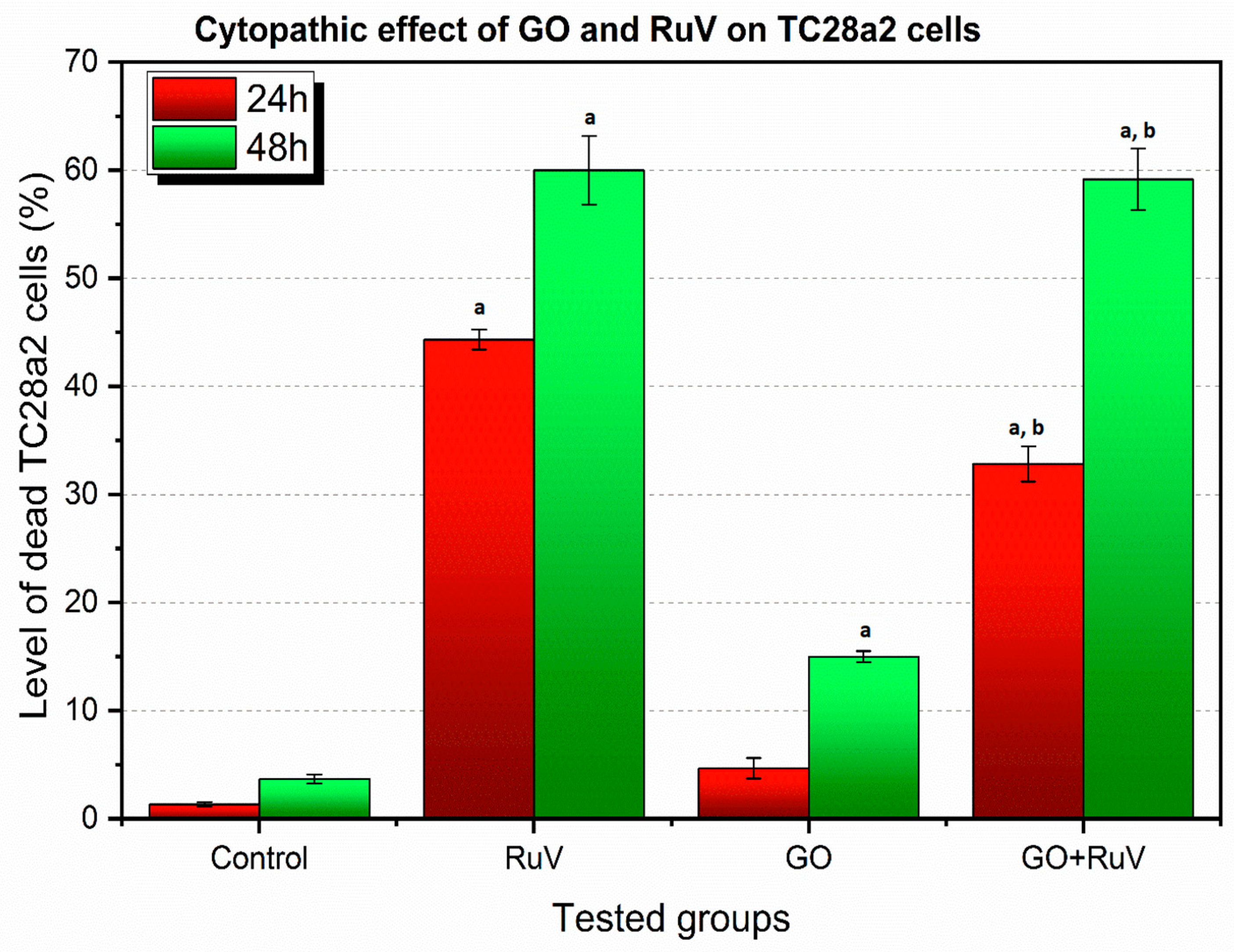
3.2. Cytopathic Effect Assay Results
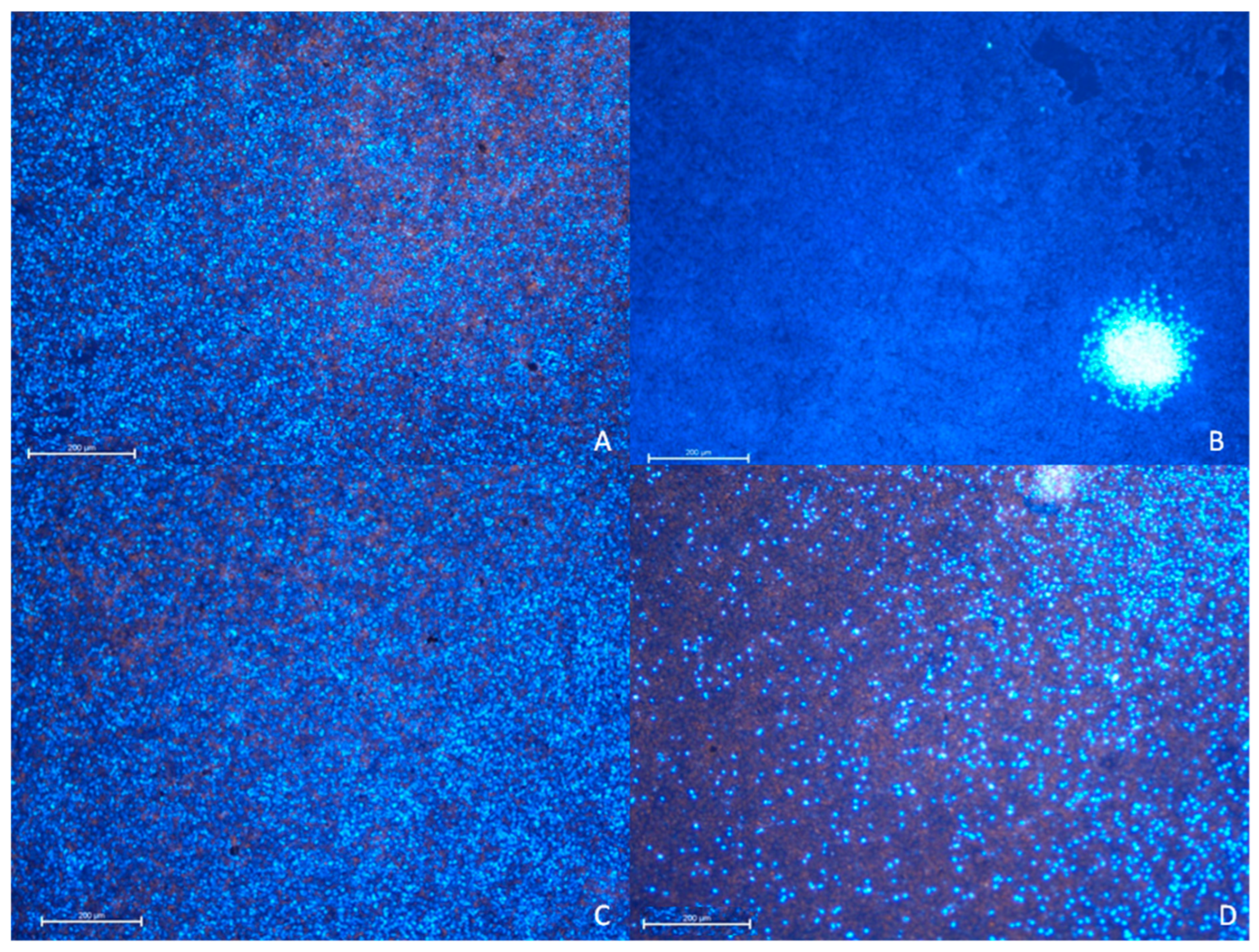
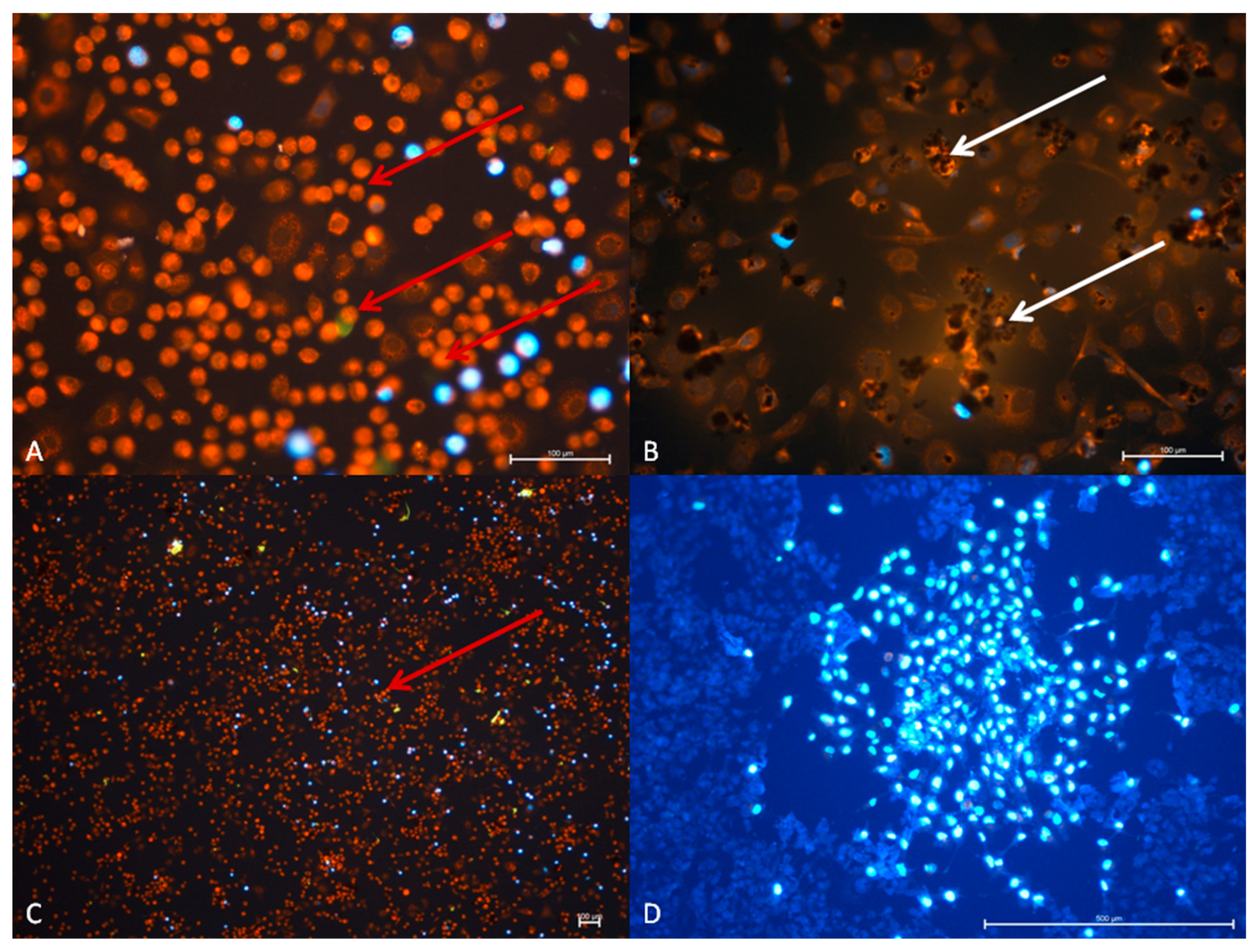
3.2.1. The A549 Cell Line—Cytopathic Changes in Cells after 24 h of Incubation
3.2.2. TC28a2 Cell Line—Cytopathic Changes in Cells after 24 h of Incubation
3.2.3. A549 Cell Line—Cytopathic Changes in Cells after 48 h of Incubation
3.2.4. TC28a2 Cell Line—Cytopathic Changes in Cells after 48 h of Incubation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Geim, A.K.; Novoselov, K.S. The Rise of Graphene. In Nanoscience and Technology; Co-Published with Macmillan Publishers Ltd.: London, UK, 2009; pp. 11–19. [Google Scholar]
- Liu, J.; Cui, L.; Losic, D. Graphene and Graphene Oxide as New Nanocarriers for Drug Delivery Applications. Acta Biomater. 2013, 9, 9243–9257. [Google Scholar] [CrossRef]
- Kalman, J.; Merino, C.; Fernández-Cruz, M.L.; Navas, J.M. Usefulness of Fish Cell Lines for the Initial Characterization of Toxicity and Cellular Fate of Graphene-Related Materials (Carbon Nanofibers and Graphene Oxide). Chemosphere 2019, 218, 347–358. [Google Scholar] [CrossRef] [PubMed]
- Arvidsson, R.; Boholm, M.; Johansson, M.; de Montoya, M.L. “Just Carbon”: Ideas About Graphene Risks by Graphene Researchers and Innovation Advisors. NanoEthics 2018, 12, 199–210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bengtson, S.; Kling, K.; Madsen, A.M.; Noergaard, A.W.; Jacobsen, N.R.; Clausen, P.A.; Alonso, B.; Pesquera, A.; Zurutuza, A.; Ramos, R.; et al. No Cytotoxicity or Genotoxicity of Graphene and Graphene Oxide in Murine Lung Epithelial FE1 Cells in Vitro. Environ. Mol. Mutagenesis 2016, 57, 469–482. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karimzadeh, Z.; Javanbakht, S.; Namazi, H. Carboxymethylcellulose/MOF-5/Graphene Oxide Bio-Nanocomposite as Antibacterial Drug Nanocarrier Agent. BioImpacts 2018, 9, 5–13. [Google Scholar] [CrossRef]
- Wang, S.-B.; Ma, Y.-Y.; Chen, X.-Y.; Zhao, Y.-Y.; Mou, X.-Z. Ceramide-Graphene Oxide Nanoparticles Enhance Cytotoxicity and Decrease HCC Xenograft Development: A Novel Approach for Targeted Cancer Therapy. Front. Pharmacol. 2019, 10, 69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahlinder, L.; Henych, J.; Lindström, S.W.; Ekstrand-Hammarström, B.; Stengl, V.; Österlund, L. Graphene Oxide Nanoparticle Attachment and Its Toxicity on Living Lung Epithelial Cells. RSC Adv. 2015, 5, 59447–59457. [Google Scholar] [CrossRef]
- Di Carlo, R.; Di Crescenzo, A.; Pilato, S.; Ventrella, A.; Piattelli, A.; Recinella, L.; Chiavaroli, A.; Giordani, S.; Baldrighi, M.; Camisasca, A.; et al. Osteoblastic Differentiation on Graphene Oxide-Functionalized Titanium Surfaces: An In Vitro Study. Nanomaterials 2020, 10, 654. [Google Scholar] [CrossRef] [Green Version]
- Paul, A.; Hasan, A.; Al Kindi, H.; Gaharwar, A.K.; Rao, V.T.S.; Nikkhah, M.; Shin, S.R.; Krafft, D.; Dokmeci, M.R.; Shum-Tim, D.; et al. Injectable Graphene Oxide/Hydrogel-Based Angiogenic Gene Delivery System for Vasculogenesis and Cardiac Repair. ACS Nano 2014, 8, 8050–8062. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, B.; Wei, P.; Zhou, Z.; Wei, T. Interactions of Graphene with Mammalian Cells: Molecular Mechanisms and Biomedical Insights. Adv. Drug Deliv. Rev. 2016, 105, 145–162. [Google Scholar] [CrossRef] [Green Version]
- Lee, W.C.; Lim, C.H.Y.X.; Shi, H.; Tang, L.A.L.; Wang, Y.; Lim, C.T.; Loh, K.P. Origin of Enhanced Stem Cell Growth and Differentiation on Graphene and Graphene Oxide. ACS Nano 2011, 5, 7334–7341. [Google Scholar] [CrossRef] [PubMed]
- Miao, W.; Shim, G.; Lee, S.; Lee, S.; Choe, Y.S.; Oh, Y.-K. Safety and Tumor Tissue Accumulation of Pegylated Graphene Oxide Nanosheets for Co-Delivery of Anticancer Drug and Photosensitizer. Biomaterials 2013, 34, 3402–3410. [Google Scholar] [CrossRef] [PubMed]
- Liao, C.; Li, Y.; Tjong, S. Graphene Nanomaterials: Synthesis, Biocompatibility, and Cytotoxicity. Int. J. Mol. Sci. 2018, 19, 3564. [Google Scholar] [CrossRef] [Green Version]
- Akhavan, O.; Ghaderi, E.; Akhavan, A. Size-Dependent Genotoxicity of Graphene Nanoplatelets in Human Stem Cells. Biomaterials 2012, 33, 8017–8025. [Google Scholar] [CrossRef] [PubMed]
- Batiuskaite, D.; Grinceviciute, N.; Snitka, V. Impact of Graphene Oxide on Viability of Chinese Hamster Ovary and Mouse Hepatoma MH-22A Cells. Toxicol. In Vitro 2015, 29, 1195–1200. [Google Scholar] [CrossRef] [PubMed]
- Seifi, T.; Reza Kamali, A. Antiviral Performance of Graphene-Based Materials with Emphasis on COVID-19: A Review. Med. Drug Discov. 2021, 11, 100099. [Google Scholar] [CrossRef]
- Schinwald, A.; Murphy, F.A.; Jones, A.; MacNee, W.; Donaldson, K. Graphene-Based Nanoplatelets: A New Risk to the Respiratory System as a Consequence of Their Unusual Aerodynamic Properties. ACS Nano 2012, 6, 736–746. [Google Scholar] [CrossRef] [PubMed]
- Demir, E.; Marcos, R. Toxic and Genotoxic Effects of Graphene and Multi-Walled Carbon Nanotubes. J. Toxicol. Environ. Health Part A 2018, 81, 645–660. [Google Scholar] [CrossRef]
- Dhurandhar, N.V. Contribution of Pathogens in Human Obesity. Drug News Perspect. 2004, 17, 307–313. [Google Scholar] [CrossRef]
- Lambert, N.; Strebel, P.; Orenstein, W.; Icenogle, J.; Poland, G.A. Rubella. Lancet 2015, 385, 2297–2307. [Google Scholar] [CrossRef] [Green Version]
- Claus, C.; Manssen, L.; Hübner, D.; Roßmark, S.; Bothe, V.; Petzold, A.; Große, C.; Reins, M.; Mankertz, A.; Frey, T.; et al. Activation of the Mitochondrial Apoptotic Signaling Platform during Rubella Virus Infection. Viruses 2015, 7, 6108–6126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trinh, Q.D.; Pham, N.T.K.; Takada, K.; Komine-Aizawa, S.; Hayakawa, S.; Trinh, Q.D.; Pham, N.T.K.; Takada, K.; Komine-Aizawa, S.; Hayakawa, S. Myelin Oligodendrocyte Glycoprotein-Independent Rubella Infection of Keratinocytes and Resistance of First-Trimester Trophoblast Cells to Rubella Virus In Vitro. Viruses 2018, 10, 23. [Google Scholar] [CrossRef] [Green Version]
- Märker-Hermann, E.; Schütz, N.; Bauer, H. Virale Arthritiden. Z. Rheumatol. 2010, 69, 871–878. [Google Scholar] [CrossRef]
- Lazar, M.; Perelygina, L.; Martines, R.; Greer, P.; Paddock, C.D.; Peltecu, G.; Lupulescu, E.; Icenogle, J.; Zaki, S.R. Immunolocalization and Distribution of Rubella Antigen in Fatal Congenital Rubella Syndrome. EBioMedicine 2016, 3, 86–92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perelygina, L.; Zheng, Q.; Metcalfe, M.; Icenogle, J. Persistent Infection of Human Fetal Endothelial Cells with Rubella Virus. PLoS ONE 2013, 8, e73014. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, G.; Li, J.; Ren, X.; Chen, C.; Wang, X. Few-Layered Graphene Oxide Nanosheets as Superior Sorbents for Heavy Metal Ion Pollution Management. Environ. Sci. Technol. 2011, 45, 10454–10462. [Google Scholar] [CrossRef]
- Krishnamoorthy, K.; Veerapandian, M.; Yun, K.; Kim, S.-J.J. The Chemical and Structural Analysis of Graphene Oxide with Different Degrees of Oxidation. Carbon 2013, 53, 38–49. [Google Scholar] [CrossRef]
- Saladino, M.L.; Markowska, M.; Carmone, C.; Cancemi, P.; Alduina, R.; Presentato, A.; Scaffaro, R.; Biały, D.; Hasiak, M.; Hreniak, D.; et al. Graphene Oxide Carboxymethylcellulose Nanocomposite for Dressing Materials. Materials 2020, 13, 1980. [Google Scholar] [CrossRef]
- Zhang, C.; Zhu, X.; Wang, Z.; Sun, P.; Ren, Y.; Zhu, J.; Zhu, J.; Xiao, D. Facile Synthesis and Strongly Microstructure-Dependent Electrochemical Properties of Graphene/Manganese Dioxide Composites for Supercapacitors. Nanoscale Res. Lett. 2014, 9, 490. [Google Scholar] [CrossRef] [Green Version]
- Song, M.; Yu, L.; Wu, Y. Simple Synthesis and Enhanced Performance of Graphene Oxide-Gold Composites. J. Nanomater. 2012, 2012, 37. [Google Scholar] [CrossRef]
- Paulchamy, B.; Arthi, G.; Lignesh, B. A Simple Approach to Stepwise Synthesis of Graphene Oxide Nanomaterial. J. Nanomed. Nanotechnol. 2015, 6, 1–4. [Google Scholar] [CrossRef]
- Shahriary, L.; Athawale, A.A. Graphene Oxide Synthesized by Using Modified Hummers Approach. Int. J. Renew. Energy Environ. Eng. 2014, 2, 58–63. [Google Scholar]
- Bykkam, S.; Rao, K.; Chakra, C.; Thunugunta, T. Synthesis and Characterization of Graphene Oxide and Its Antimicrobial Activity against Klebsiella and Staphylococus. Int. J. Adv. Biotechnol. Res. 2013, 4, 1005–1009. [Google Scholar]
- Tsai, S.-M.; Bangalore, P.; Chen, E.Y.; Lu, D.; Chiu, M.-H.; Suh, A.; Gehring, M.; Cangco, J.P.; Garcia, S.G.; Chin, W.-C. Graphene-Induced Apoptosis in Lung Epithelial Cells through EGFR. J. Nanopart. Res. 2017, 19, 262. [Google Scholar] [CrossRef]
- Park, E.-J.; Lee, G.-H.; Han, B.S.; Lee, B.-S.; Lee, S.; Cho, M.-H.; Kim, J.-H.; Kim, D.-W. Toxic Response of Graphene Nanoplatelets in Vivo and in Vitro. Arch. Toxicol. 2015, 89, 1557–1568. [Google Scholar] [CrossRef]
- Mittal, S.; Kumar, V.; Dhiman, N.; Chauhan, L.K.S.; Pasricha, R.; Pandey, A.K. Physico-Chemical Properties Based Differential Toxicity of Graphene Oxide/Reduced Graphene Oxide in Human Lung Cells Mediated through Oxidative Stress. Sci. Rep. 2016, 6, 39548. [Google Scholar] [CrossRef] [PubMed]
- Jarosz, A.; Skoda, M.; Dudek, I.; Szukiewicz, D. Oxidative Stress and Mitochondrial Activation as the Main Mechanisms Underlying Graphene Toxicity against Human Cancer Cells. Oxidative Med. Cell. Longev. 2016, 2016, 5851035. [Google Scholar] [CrossRef] [Green Version]
- Gc, J.B.; Gerstman, B.S.; Stahelin, R.V.; Chapagain, P.P. The Ebola Virus Protein VP40 Hexamer Enhances the Clustering of PI(4,5)P2 Lipids in the Plasma Membrane. Phys. Chem. Chem. Phys. PCCP 2016, 18, 28409–28417. [Google Scholar] [CrossRef] [Green Version]
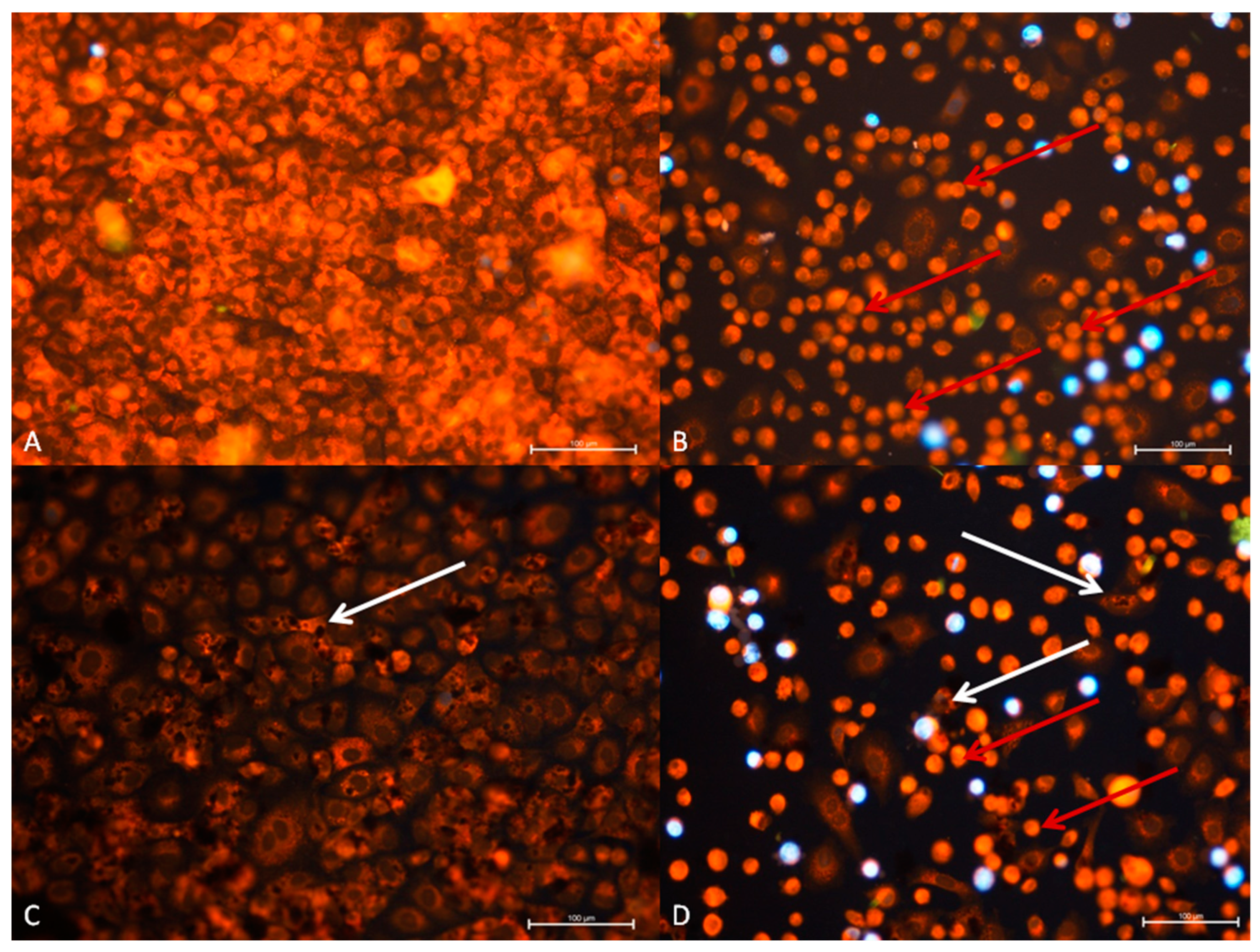
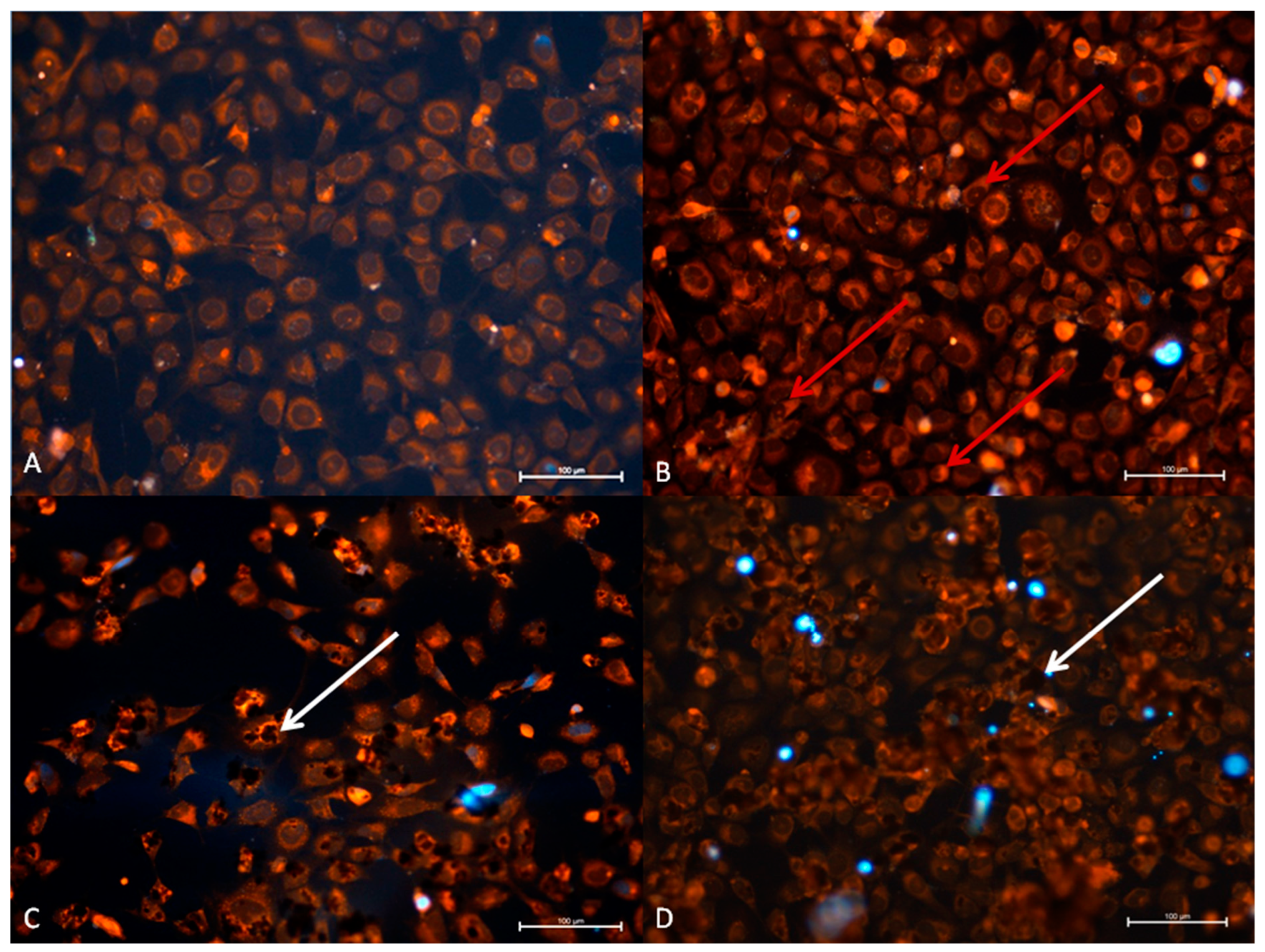
| Cell Line | Control after 24 h Control after 48 h | RuV after 24 h RuV after 48 h | GO after 24 h GO after 48 h | GO and RuV after 24 h GO and RuV after 48 h |
|---|---|---|---|---|
| A549 | 1/24 1/48 | 2/24 2/48 | 3/24 3/48 | 4/24 4/48 |
| TC28a2 | 5/24 5/48 | 6/24 6/48 | 7/24 7/48 | 8/24 8/48 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuropka, P.; Dobrzynski, M.; Bazanow, B.; Stygar, D.; Gebarowski, T.; Leskow, A.; Tarnowska, M.; Szyszka, K.; Malecka, M.; Nowak, N.; et al. A Study of the Impact of Graphene Oxide on Viral Infection Related to A549 and TC28a2 Human Cell Lines. Materials 2021, 14, 7788. https://doi.org/10.3390/ma14247788
Kuropka P, Dobrzynski M, Bazanow B, Stygar D, Gebarowski T, Leskow A, Tarnowska M, Szyszka K, Malecka M, Nowak N, et al. A Study of the Impact of Graphene Oxide on Viral Infection Related to A549 and TC28a2 Human Cell Lines. Materials. 2021; 14(24):7788. https://doi.org/10.3390/ma14247788
Chicago/Turabian StyleKuropka, Piotr, Maciej Dobrzynski, Barbara Bazanow, Dominika Stygar, Tomasz Gebarowski, Anna Leskow, Malgorzata Tarnowska, Katarzyna Szyszka, Malgorzata Malecka, Nicole Nowak, and et al. 2021. "A Study of the Impact of Graphene Oxide on Viral Infection Related to A549 and TC28a2 Human Cell Lines" Materials 14, no. 24: 7788. https://doi.org/10.3390/ma14247788
APA StyleKuropka, P., Dobrzynski, M., Bazanow, B., Stygar, D., Gebarowski, T., Leskow, A., Tarnowska, M., Szyszka, K., Malecka, M., Nowak, N., Strek, W., & Wiglusz, R. J. (2021). A Study of the Impact of Graphene Oxide on Viral Infection Related to A549 and TC28a2 Human Cell Lines. Materials, 14(24), 7788. https://doi.org/10.3390/ma14247788











