Sulfonitrocarburizing of High-Speed Steel Cutting Tools: Kinetics and Performances
Abstract
:1. Introduction
2. Materials and Methods
- Carbamide (produced by AZOMURES, Targu Mures, Romania, with particle sizes of 2 ÷ 5 mm, with 46% nitrogen content and maximum humidity of 0.3%);
- Ammonium chloride (analytically pure, purchased through Silver Chemical, Bucharest, Romania);
- Sulfur powder with particle sizes of 60 ÷ 70 μm and 99.5% purity (Jainson Lab, Mrerut, India);
- Charcoal powder (SC FANCOM IMPEX SRL, Lupeni, Romania).
- The maximum permissible cutting speed (determined by the moment when catastrophic wear occurs);
- The maximum cutting length with the maximum permissible cutting speed (until the tool wears out completely, meaning the maximum cut length between two resharpenings). The test conditions were chosen in accordance with ISO 3685/1993 International Standard—Tool-life testing with single-point turning tools.
- To determine the maximum permissible cutting speed, the feed rate was set at 0.7 mm/rot with a cutting depth of 1.5 mm, without cooling.
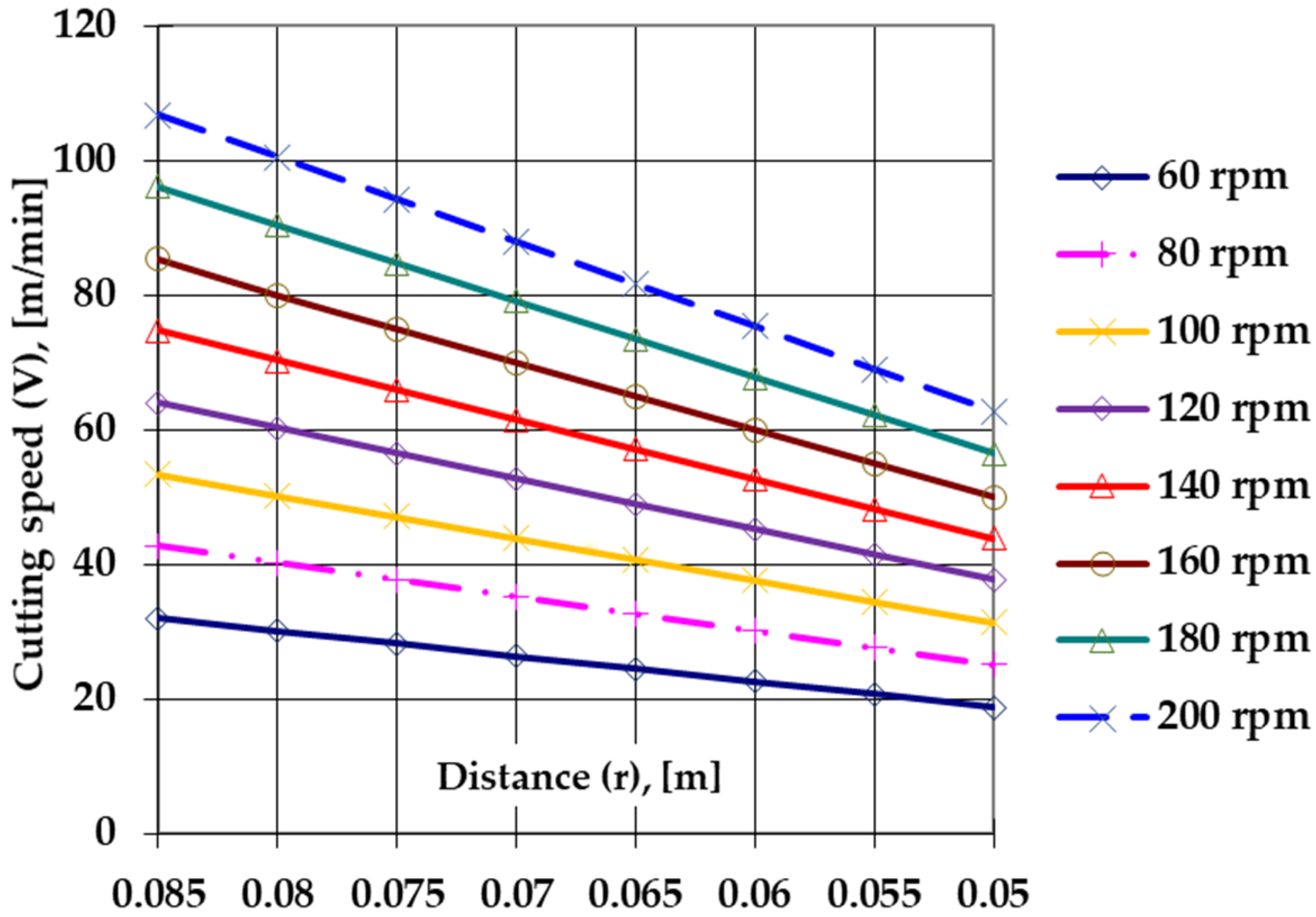
- To determine the maximum chip length, a speed range of 60 ÷ 200 m/min was tested in the conditions of a feed rate of 0.07 mm/rot and a cutting depth of 1.5 mm, without cooling. One may observe that the conditions chosen for testing the tools fall within the value ranges related to surface finishing operations.
- Quenched (austenitizing in neutral melted salt bath BaCl2 at 1280 °C for 2 min with oil) and triple-tempered (melted salt bath with NaNO3 and NaNO2 at 560 °C for 1 h and air cooling for each tempering heat treatment);
- Quenched, double tempered, and sulfonitrocarburized in powdered solid medium (30% CON2H4, 10% S, 3.5% NH4Cl, and 40% finely divided coal, Al2O3 balance) under the conditions of 550 °C for 1 h; processing took place in a furnace provided with automatic temperature regulation and control system, produced by UTTIS SA, Vidra, Romania.
- Quenched, double-tempered, and nitrocarburized in powdered solid medium (30%CON2H4 and 3.5%NH4Cl+40% finely divided coal, Al2O3 balance under the conditions of 550 °C for 1 h; processing took place in a furnace provided with the automatic regulation system and temperature control, produced by UTTIS SA, Vidra, Romania.
- Quenched, double-tempered, and nitrided in a gaseous atmosphere (35% NH3, dissociation degree), in a furnace with Φ 600 × 900 mm retort dimensions, 35 KW of power, at a temperature of 560 °C for 30 min.
- Quenched, double-tempered, and ion nitriding at 450 °C for 1.5 h in a gas mixture (NH3 and Ar) (p (NH3+Ar) = 2 mbar) in laboratory equipment with 3 KW of power; the batch cooling was performed during the installation in an argon atmosphere of up to 200 °C.
- Hardening and tempering for all the analyzed processing variants were performed in identical conditions from the point of view of thermal and temporal parameters of the chosen media.
- Thermochemical processing in powdered solid mixtures was performed by placing samples in metallic containers, sealed with metal lids and clay.
3. Results and Discussion
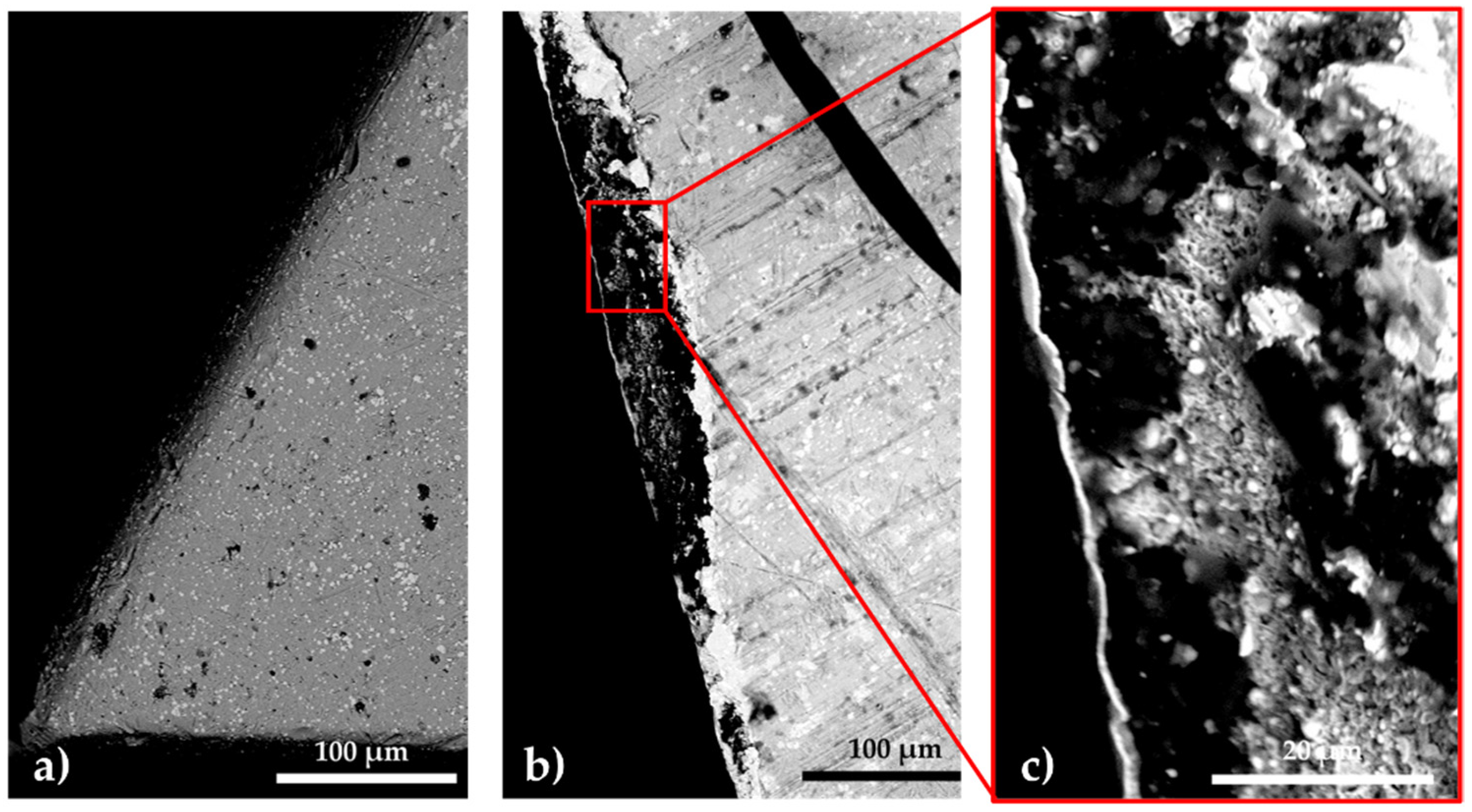
3.1. Results Regarding the Growth Kinetics of Sulfonitrocarburized Layers on T1 HSS Matrices
ΔG750 °C = −537 kJ/mol; ΔH750 °C = −1937.8 kJ/mol
ΔG750 °C = −427.6 kJ/mol; ΔH750 °C = −364.0 kJ/mol
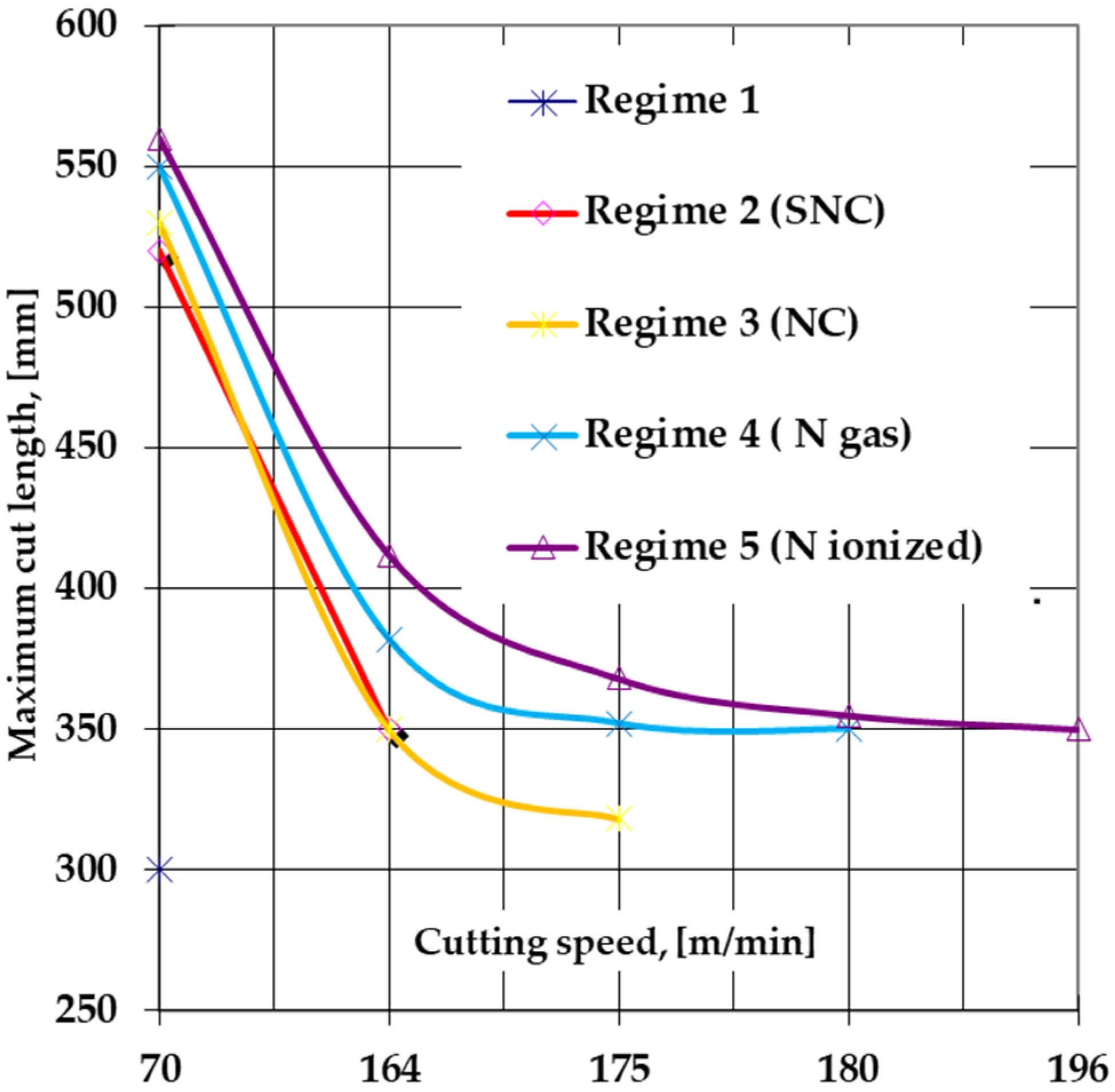
3.2. The Performances of Cutting Tools—Removable Plates—Made of T1 Sulfonitrocarburized HSS in Powdered Solid Medium Containing Carbamide, as Compared to the Same Plates Subjected to Other Types of Thermochemical Treatments—Nitrocarburized, Gas Nitrided, Ion Nitrided
4. Conclusions
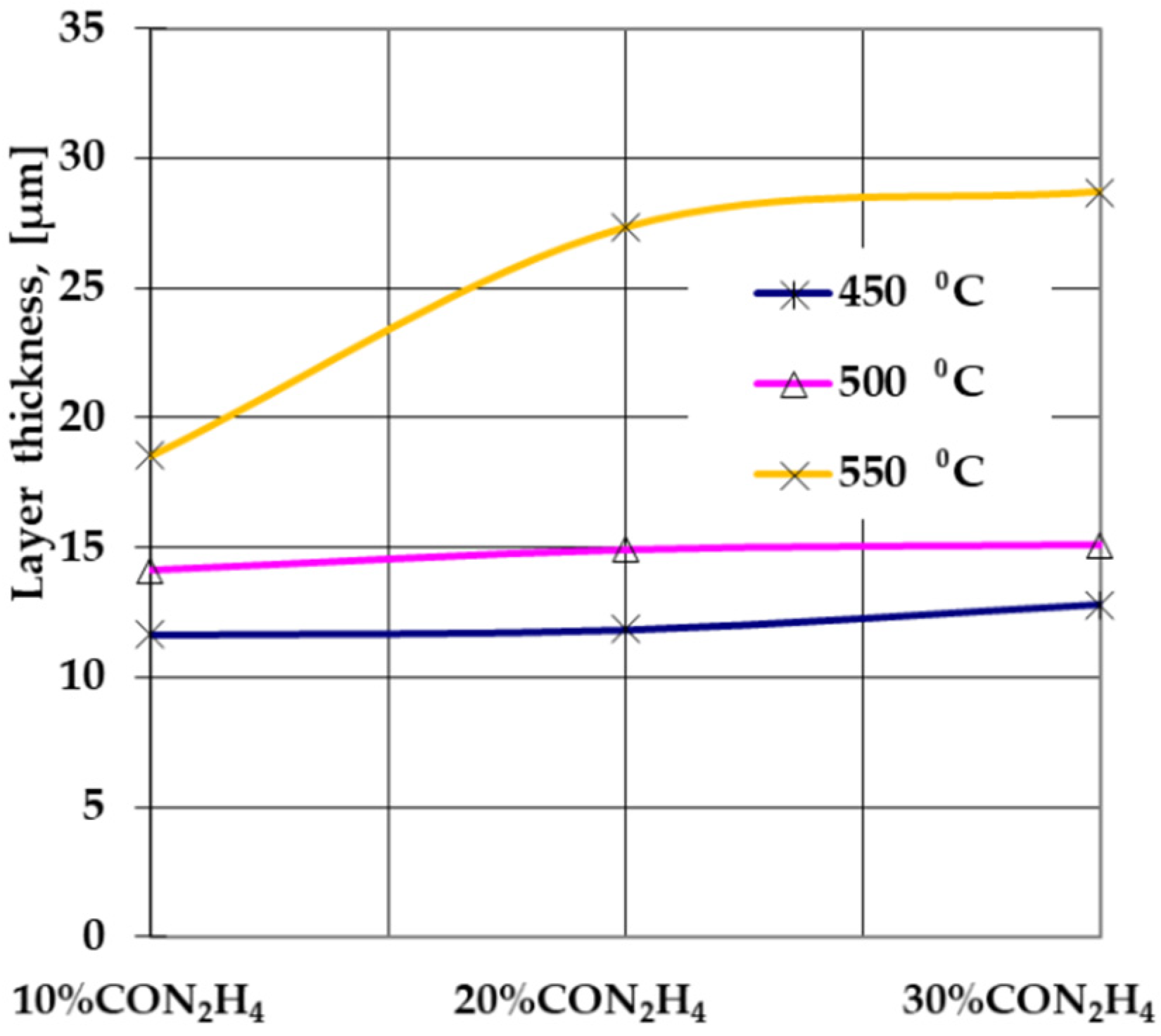
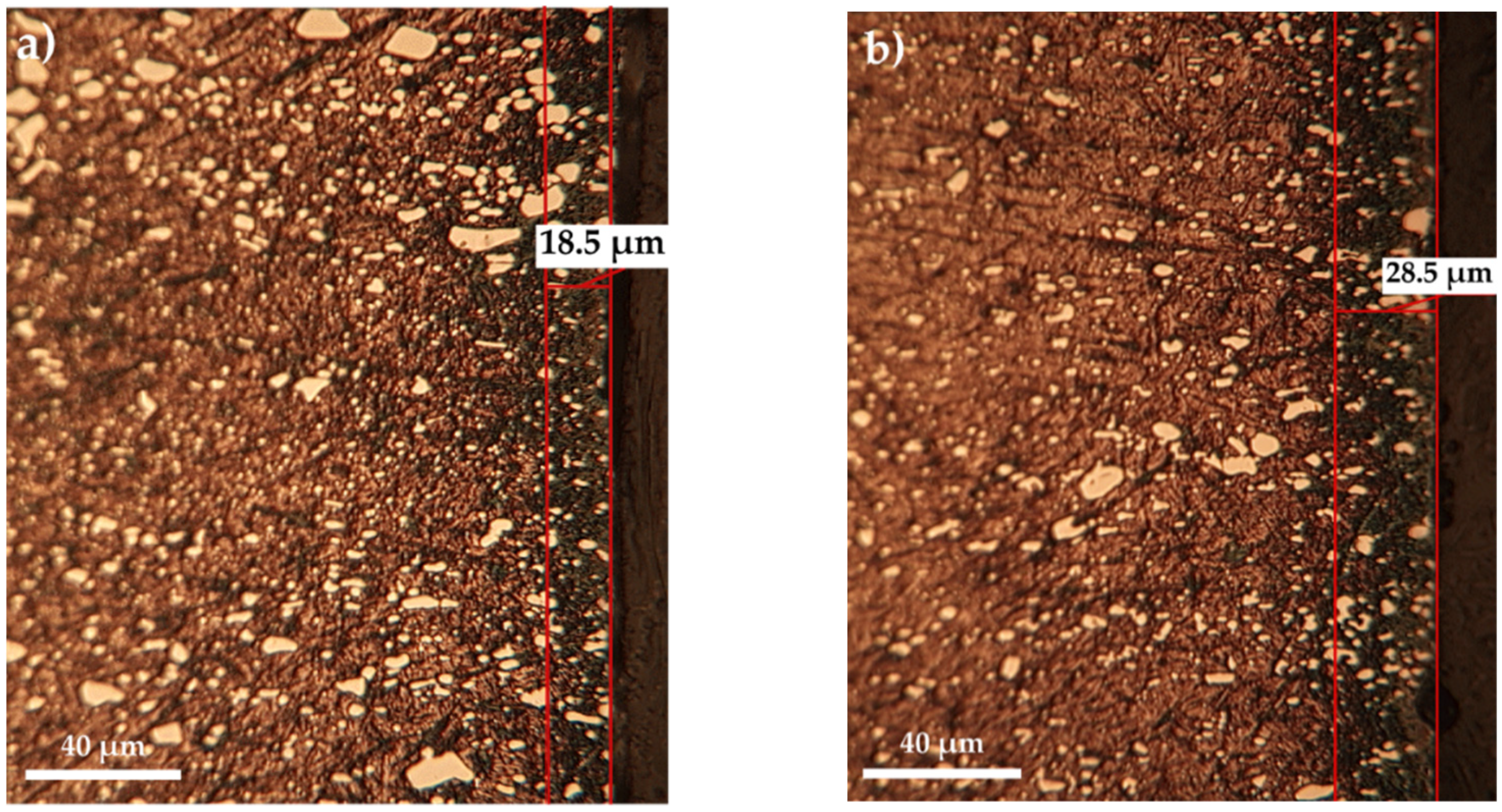
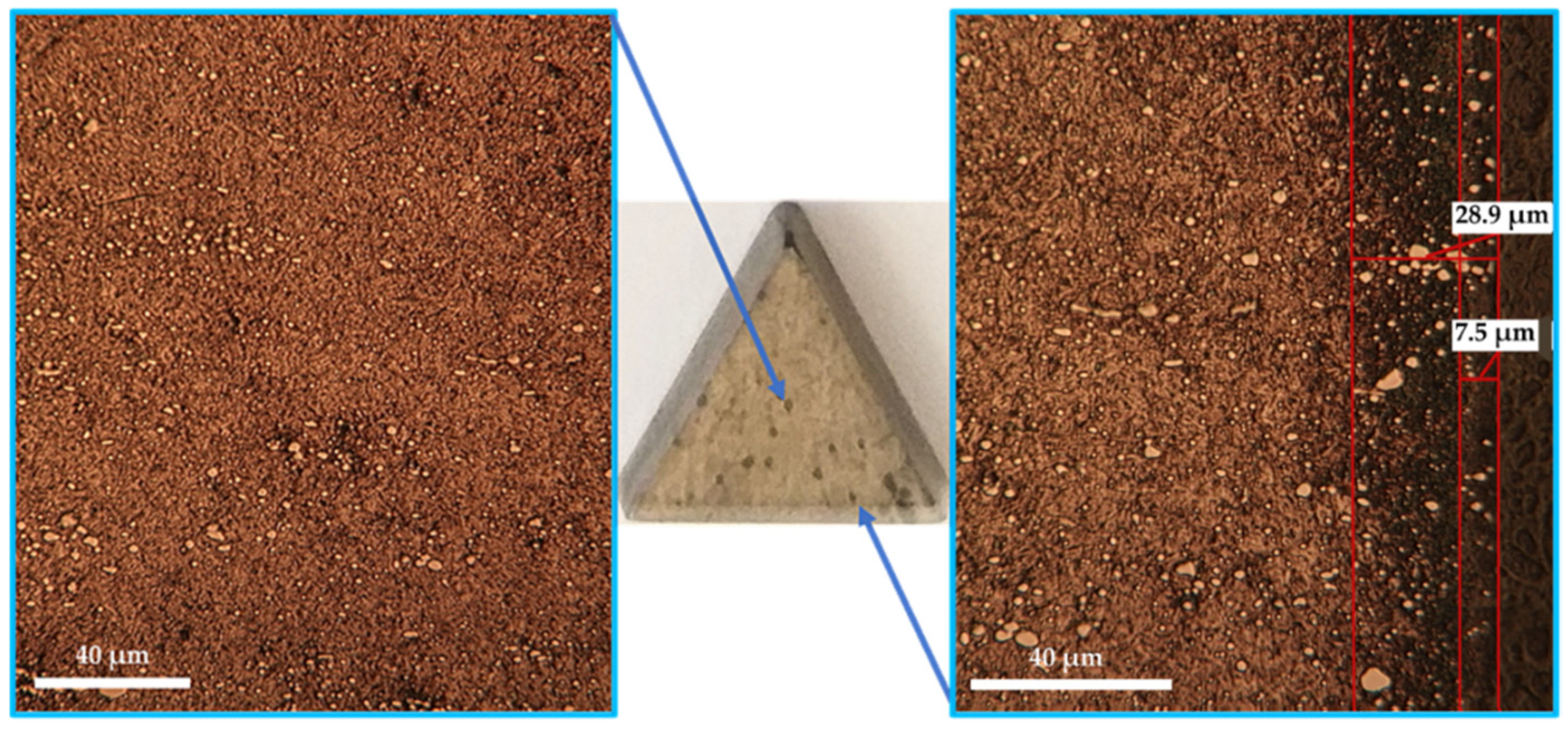
- Achieving sulfonitrocarburized layers, perfectly adherent and dimensionally uniform and within dimensional limits and phase compositions, as is recommended for T1 HSS grade, using powdered solid medium containing carbamide, is conditioned by limiting the proportion of this organic compound to about 20% in the mixture and the temperature at a maximum of 550 °C.
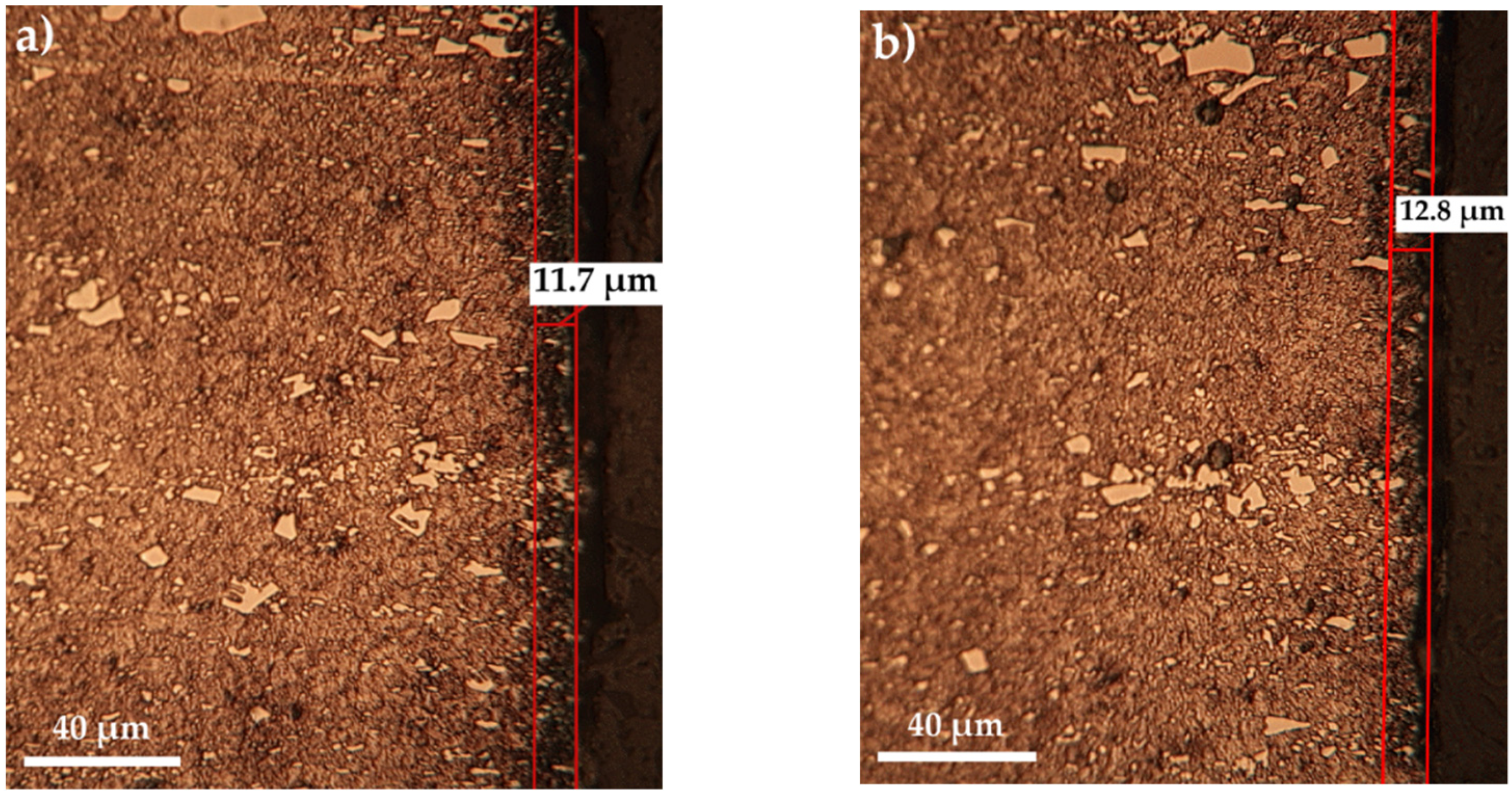
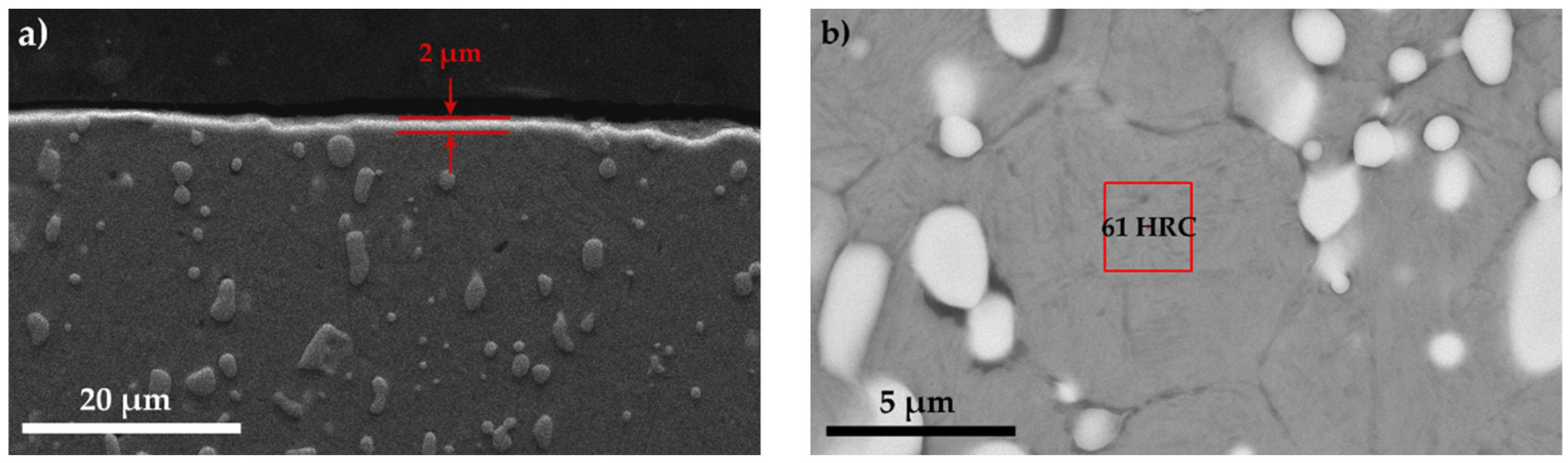
- The maximum microhardness recorded in the sulfonitrocarburized layer by T1 steel was 1027 HV0.02, at a depth of about 16 ÷ 30 microns; in the surface adjacent area, the presence of iron sulfides leads to microhardness values by about 10.7% lower as compared to those in the deeper zone, where carbonitrides presence was recorded.
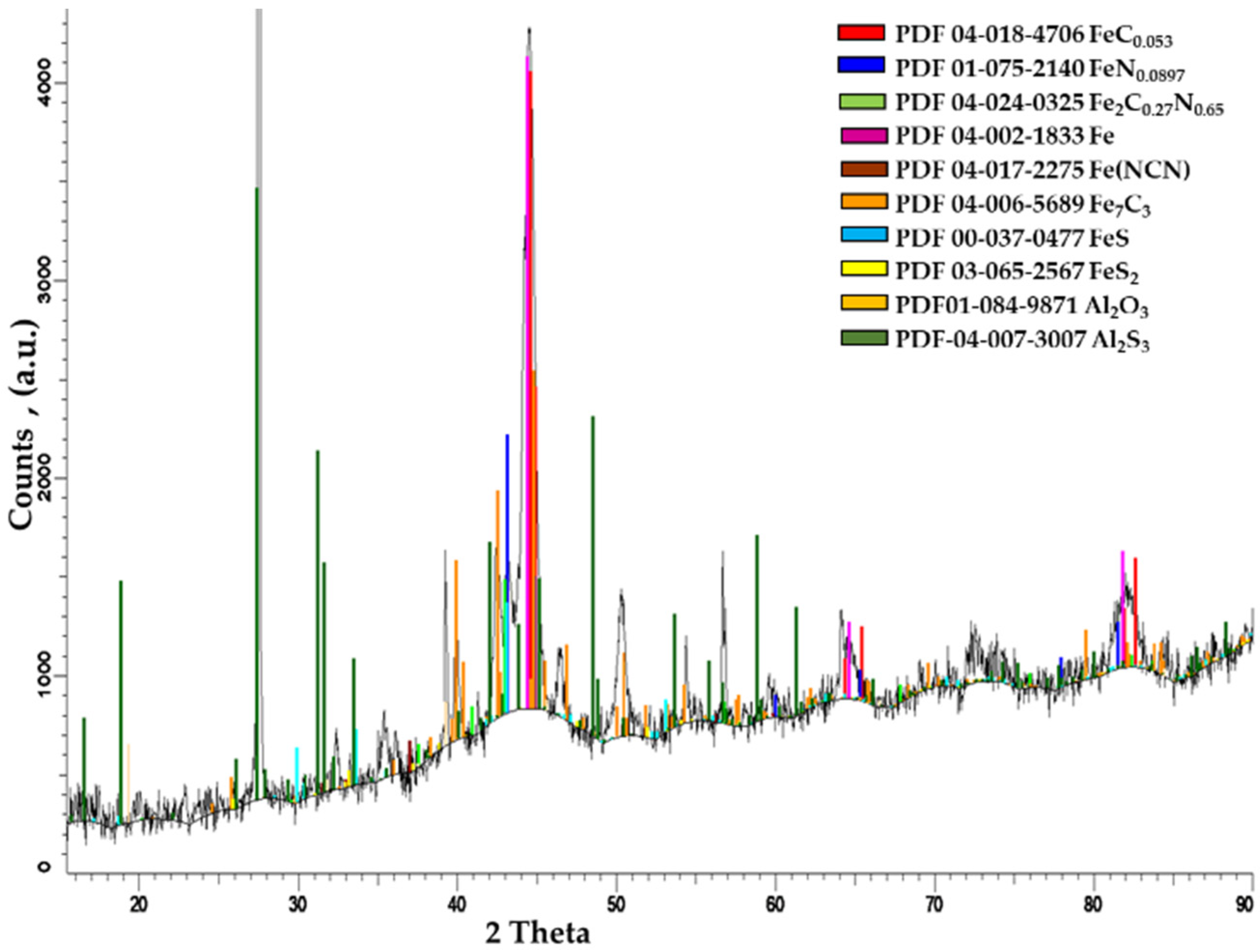
- X-ray diffraction analysis of sulfonitrocarburized layers in a powdered solid medium containing 20% carbamide showed a considerable increase in the carbide proportion when compared to that recorded in T1 steel in as–cast or annealed states (about 25%), quenched (about 16%), or quenched and tempered (about 18%).
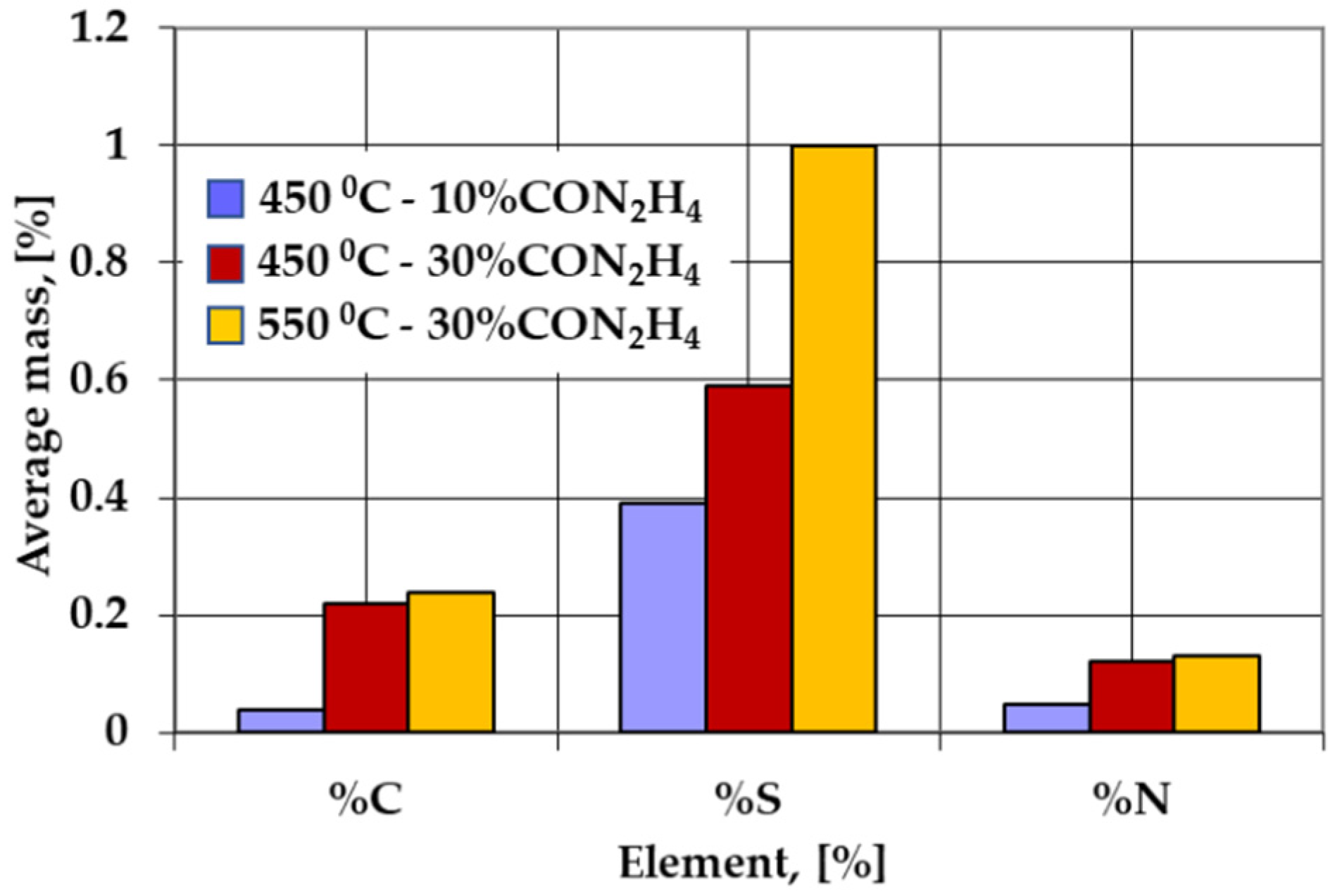
- The activity of the powdered solid medium used for sulfonitrocarburizing changed considerably when the carbamide proportion was increased to 10 ÷ 30% and the temperature was in the range of 450 ÷ 550 °C, the most significant increase being registered for carbon, sulfur, and nitrogen, in descending order.
- The use of sulfonitrocarburizing mixtures for the controlled zonal modification of the sulfur, carbon, and nitrogen concentrations in the surface layers of HSS represents a special solution from a technical and economic point of view.
- Sulfonitrocarburizing in a powdery solid medium that contains carbamide of T1 HSS cutting tools ensures a considerable increase in their performance. Thus, there was an increase of over 140% of the maximum permissible cutting speed, while the maximum cut length increased by over 80%, compared to those of thermochemically unprocessed tools working at the maximum permissible cutting speed (about 70 m/min).
- Sulfonitrocarburizing in a powdered solid medium containing carbamide performs very closely with regards to the cutting tools to thermochemical processing variants such as gas nitriding or plasma nitriding.
- The proposed process is non-polluting, both reactants and reaction products being environmentally friendly, this being an additional argument, along with the obtained performance, to apply it on an industrial scale.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gheller, L.A. Instrumentaliniie Stali (Tool Steels); Metallurghia: Moskva, Russia, 1975; pp. 506–507. [Google Scholar]
- Sergheicev, I.M.; Pecikovskii, A.M. Termiceskaia Obrabotka Rejuscevo I Izmeritelinovo Instrumenta (Heat Treatment of Cutting an Measurement Tools); Masinostroenie: Moscow, Russia, 1967; pp. 5–14. [Google Scholar]
- Dulamita, T.; Gherghescu, I.A. Oteluri de Scule. Proprietati, Tratamente Termice, Utilizari (Tool Steels. Properties, Heat Treatments, Uses); Editura Tehnica (Technical Publishing House): Bucharest, Romania, 1990; pp. 21–26. [Google Scholar]
- Shaojun, S.; Xianping, Z.; Chengtong, S. Heat-treatment and properties of high-speed steel cutting tools. In Proceedings of the IOP Conference Series: Materials Science and Engineering, Nanchang, China, 25–27 May 2018; Volume 423, pp. 1–6. [Google Scholar] [CrossRef] [Green Version]
- Budau, V.; Duma, S. Hardness and Structural Aspects of the Heat—Treated HS 18-0-1 Steel. Ann. Fac. Eng. Hunedoara Int. J. Eng. 2011, 9, 101–104. [Google Scholar]
- Bobzin, K. Review—High-performace coatings for cutting tools. CIRP J. Manuf. Sci. Technol. 2017, 18, 1–9. [Google Scholar] [CrossRef]
- Rousseau, A.F. Metallurgical Characterization and Performance of High-Speed Steel Tool Materials Used in Metal Cutting Applications. Master’s Thesis, RMIT University, Melbourne, Australia, February 2016. [Google Scholar]
- Stoicescu, M.; Ene, E.; Zara, A.; Giacomelli, I.; Berbecaru, A.C. The Influence of Thermal and Thermochemical Treatments Applied to Rapid Steels Concerning Wear Resistance. U.P.B. Sci. Bull. Ser. B 2018, 80, 157–169. [Google Scholar]
- Psyllaki, P.; Stamatiou, K.; Iliadis, I.; Mourlas, A.; Asteris, P.; Vaxevanidis, N. Surface treatment of tool steels against galling failure. MATEC Web Conf. 2018, 188, 04024. [Google Scholar] [CrossRef]
- Strahin, B. The Effect of Engineered Surfaces on the Mechanical Properties of Tool Steels Used for Industrial Cutting Tools. Master’s Thesis, The University of Akron, Akron, OH, USA, 2017. [Google Scholar]
- Kanaev, V.A.; Bebenin, A.S. Increasing the Hardness, Resistance and Wear Resistance of the Tools by carbonitration. Mod. High Technol. 2013, 8, 100–101. [Google Scholar]
- Petrovna, K.T. Improving the Operational Properties of Small-Sized Tools and Technological Equipment by Carbonitriding in Powder Activated Charcoal Mixtures. Master’s Thesis, Publishing House of FGOU VPO “KSTU”, Kaliningrad, Russia, 2011; pp. 6–14. [Google Scholar]
- Volkova, A.S.; Aculinin, A.A. Increasing the Resistance of the Cutting Tool by Carbonitrating. Adv. Mod. Nat. Sci. 2012, 6, 173–174. [Google Scholar]
- Harlamov, L.A.; Kroli, O.S. Increasing the Durability of Cutting Tools by Carbonitration; Vladimir Dal National University of Eastern Ukraine: Donetk, Ukraine, 2015; pp. 223–226. [Google Scholar]
- Araujo, A.G.F.; Naeem, M.; Araujo, L.N.M.; Costa, T.H.C.; Khan, K.H.; Diaz-Guillen, J.C.; Iqbal, I.; Liborio, M.S.; Sousa, R.R.M. Design, manufacturing, and plasma nitriding of AISI-M2 steel forming tool and its performance analysis. J. Mater. Res. Technol. 2020, 9, 14517–14527. [Google Scholar] [CrossRef]
- Uglov, V.V.; Kuleschov, A.K.; Alifanov, A.P.; Rusalsky, D.P.; Grishkevich, A.A.; Chaevskij, V.V.; Syshchenko, A.F. Improvement of Wear-Resistance of Wood-Cutting Hard Alloy Tools by Sulfocyanidation and ZrN Coating. In Proceedings of the 10th International Conference “Interaction of Radiation with Solids”, Minsk, Belarus, 24–27 September 2013; pp. 320–322. [Google Scholar]
- Araujo Junior, E.; Bandeira, R.M.; Manfrinato, M.D.; Moreto, J.A.; Borges, R.; Santos Vales, S.; Suzuki, P.A.; Rosino, L.S. Effect of ionic plasma nitriding process on the corrosion and micro-abrasive wear behavior of AISI 316L austenitic and AISI 470 super-ferritic stainless steels. J. Mater. Res. Technol. 2019, 8, 2180–2191. [Google Scholar] [CrossRef]
- Oliveira, L.F.; Rocha, A.S.; Rocha dos Santos, G. Comparison of surface properties modification by direct and active screen plasma nitriding of an ASTM M2 high-speed steel in a nitrogen rich gas mixture. Tecnol. Metal. Mater. Min. 2021, 18, 1–8. [Google Scholar] [CrossRef]
- Nikolova, M.; Nikolov, N.; Yankov, E.; Derin, B.; Topalski, S. Vacuum Oxy-nitro carburizing of tool steels: Structure and mechanical reliability. Indian J. Eng. Mater. Sci. 2020, 27, 5–18. [Google Scholar]
- Toth, L.; Nyikes, Z.; Umesh, M. Incresing tool steel surface wear resistance by surface treatment. Műszaki Tudományos Közlemények 2020, 13, 170–173. [Google Scholar] [CrossRef]
- Torodoc, N.; Giacomelli, I. Contribution of Heat and Thermochemical Treatments to the Improvement of the Performances of High-Speed Steel Tools. Ann. Dunarea Jos Univ. Galati. Fascicle IX Metall. Mater. Sci. 2007, 1, 14–17. [Google Scholar]
- Smatov, A.A. Kombinirovannoe Obemno-Poverhnostnovo Uprocinenie Stalinovo Rejuscevo-Instrumenta (Combined Volumic and Surface Hardened of Cutting Tool Steels); Vestnic Brestskovo Gosudarstvennovo Tehniceskovo Universiteta (Bulletin of Brest State Technical University): Brest, Belarus, 2008; Volume 4. [Google Scholar]
- Cojocaru, M.O.; Druga, L.N.; Pencea, I.; Branzei, M.; Ciuca, S. Procedeu de Nitrocarburare cu Sau Fara Sulf, in Mediu Solid Pulverulent, a Unor Produse Metalice (Nitrocarburization Process with or without Sulfur, in Powdery Solid Medium, of Some Metallic Products). Patent No. 132662, 27 November 2020. [Google Scholar]
- Tarasov, A.N. Cutting and Shaping-Tools from Nitrocarburized. High Speed Steels: A Possible Alternative to Hard Alloy Tools. Metal Sci. Heat Treat. 2001, 43, 169–172. [Google Scholar] [CrossRef]
- Medvedeva, A. Performance of Advanced Tool Steels for Cutting Tool Bodies. Doctoral Dissertation, Karlstad University Studies, Karlstad, Sweden, 2010. [Google Scholar]
- Doyle, E.D.; Pagson, A.M.; Hubbard, P.; Dowey, S.J.; Pilkington, A.; McCulloch, D.G.; Latham, K.; DuPlessis, J. Nitriding of high speed steels. In Proceedings of the 18th IFHTSE Congress-Proceedings, Anais Proceeding, Rio de Janeiro, Brazil, 26–30 July 2010; pp. 5531–5539. [Google Scholar]
- Lahtin, L.M.; Kogan, L.D. Azotirovanie Stali (Steel Nitriding); Maşinostroenie (Mechanical Engineering): Moscow, Russia, 1976; p. 72. [Google Scholar]
- Neustroev, G.N.; Bogdanov, V.V.; Ivanov, L.P. Nizkotemperaturnoe Tianirovanie Instrumentalinih Stalei (Low Temperature Nitrocarburizing of Tool Steels); Progressivniie metodi termiceskoi i himico-termiceskoi obrabotki (Progressive methods of thermal and chemical-thermal treatment); Masinostroenie (Mechanical Engineering): Moscow, Russia, 1972; pp. 89–91.
- King, P.C.; Reynoldson, R.W. Ferritic Nitrocarburising of Tool Steels. Surf. Eng. 2005, 21, 86–98. [Google Scholar] [CrossRef] [Green Version]
- Jadeilson, S.A.; Santos, L.P.; Silva, S.C.; Santos, S.C. Analysis of Performance of Cutting Tools in the Processes of Thinning and Finishing by Coating Milling of Alloy Mn-Si in Steel Sae 1020. J. Mech. Eng. Autom. 2018, 8, 69–77. [Google Scholar]
- Minkevici, A.N. Tratamentele Termochimice ale Metalelor si Aliajelor (Thermochemical Treatments of Metals and Alloys); Editura Tehnica (Technical Publishing House): Bucharest, Romania, 1968; p. 293. [Google Scholar]
- Zajac, J.; Duplak, J.; Duplakova, D.; Cizmar, P.; Olexa, I.; Bittner, A. Prediction of Cutting Material Durability by T = f(vc) Dependence for Turning Processes. Processes 2020, 8, 789. [Google Scholar] [CrossRef]
- Panda, A.; Duplak, J.; Hatala, M.; Krenicky, T.; Vrabel, P. Research on the Durability of Selected Cutting Materials in the Process of Turning Carbon Steel. MM Sci. J. 2016, 10, 1086–1089. [Google Scholar] [CrossRef] [Green Version]
- Darmawan, M.; Kusman, M.; Hamdani, R.A. The Material Performance of HSS Tools and Its Relation with Chemical Composition and Carbide Distribution. In Proceedings of the IOP Conference Series: Materials Science and Engineering, Bandung, Indonesia, 14 November 2015; Volume 128, pp. 197–202. [Google Scholar] [CrossRef] [Green Version]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cojocaru, M.O.; Branzei, M.; Ciuca, S.; Gherghescu, I.A.; Ion, M.; Druga, L.N.; Cotrut, C.M. Sulfonitrocarburizing of High-Speed Steel Cutting Tools: Kinetics and Performances. Materials 2021, 14, 7779. https://doi.org/10.3390/ma14247779
Cojocaru MO, Branzei M, Ciuca S, Gherghescu IA, Ion M, Druga LN, Cotrut CM. Sulfonitrocarburizing of High-Speed Steel Cutting Tools: Kinetics and Performances. Materials. 2021; 14(24):7779. https://doi.org/10.3390/ma14247779
Chicago/Turabian StyleCojocaru, Mihai Ovidiu, Mihai Branzei, Sorin Ciuca, Ioana Arina Gherghescu, Mariana Ion, Leontin Nicolae Druga, and Cosmin Mihai Cotrut. 2021. "Sulfonitrocarburizing of High-Speed Steel Cutting Tools: Kinetics and Performances" Materials 14, no. 24: 7779. https://doi.org/10.3390/ma14247779
APA StyleCojocaru, M. O., Branzei, M., Ciuca, S., Gherghescu, I. A., Ion, M., Druga, L. N., & Cotrut, C. M. (2021). Sulfonitrocarburizing of High-Speed Steel Cutting Tools: Kinetics and Performances. Materials, 14(24), 7779. https://doi.org/10.3390/ma14247779







