Alternative Materials for the Enrichment of Biogas with Methane
Abstract
:1. Introduction
2. Materials
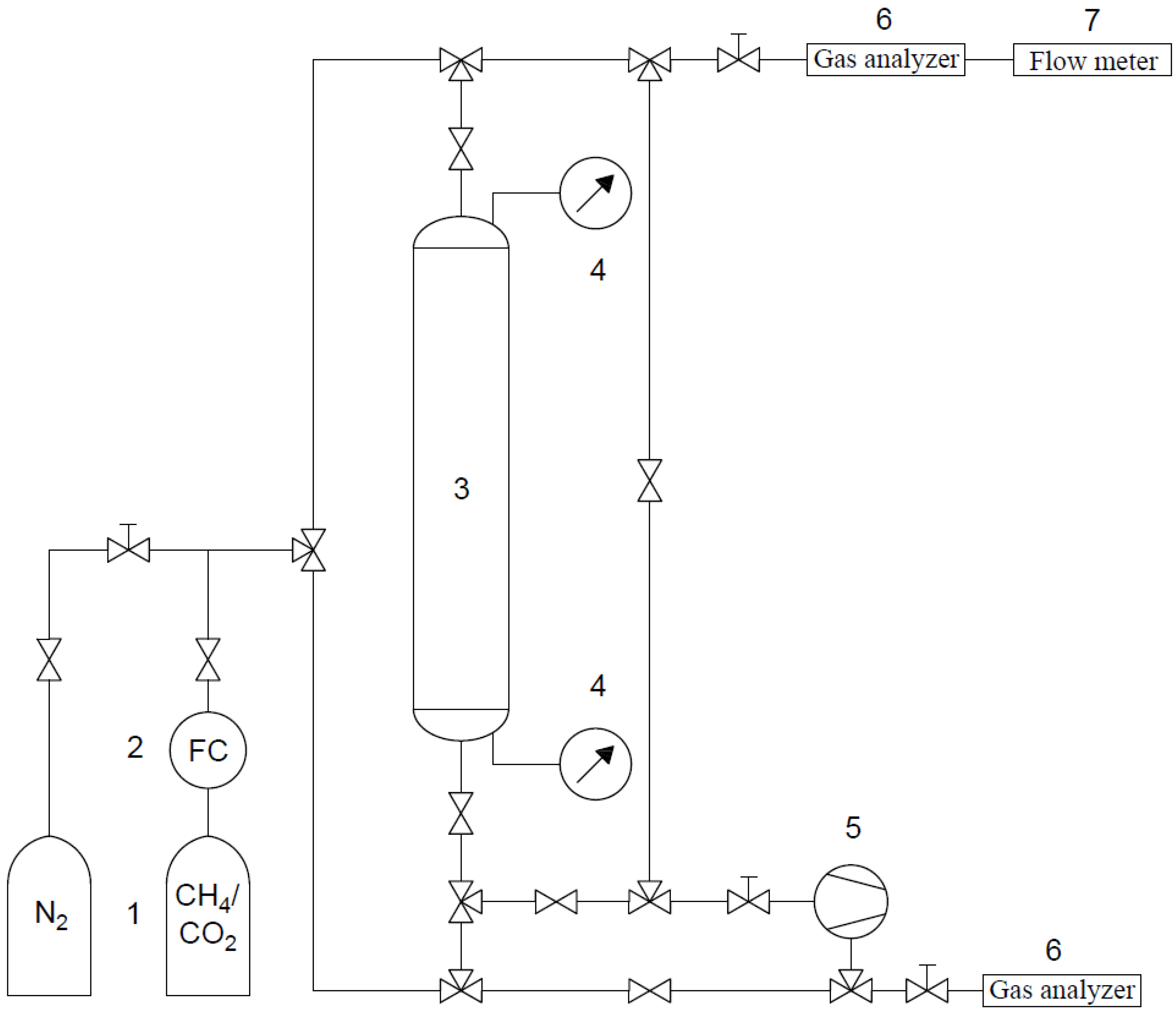
3. Experiment
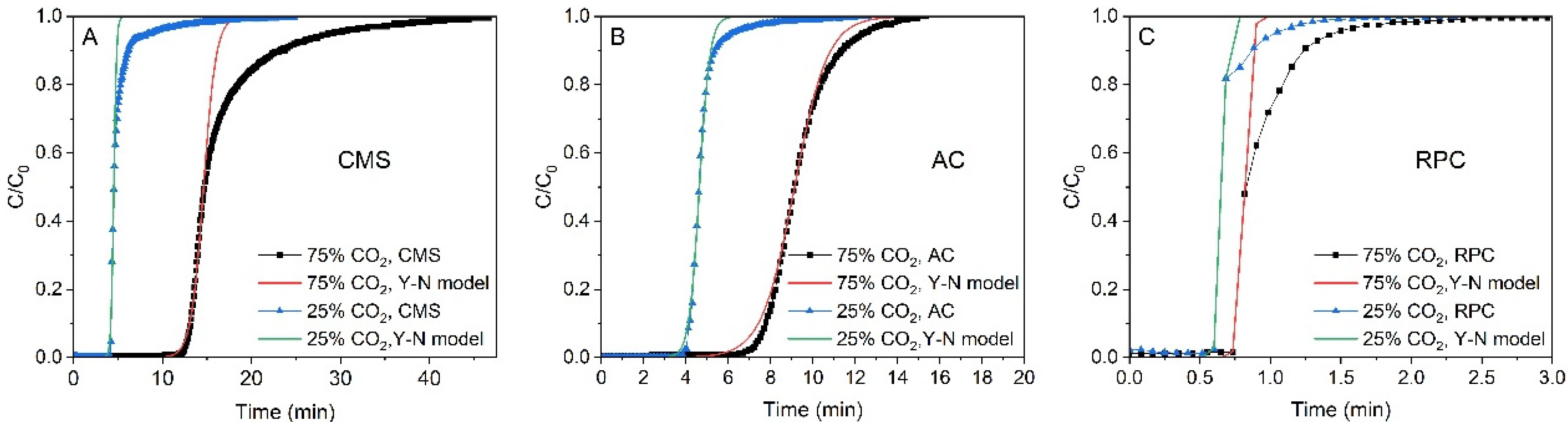
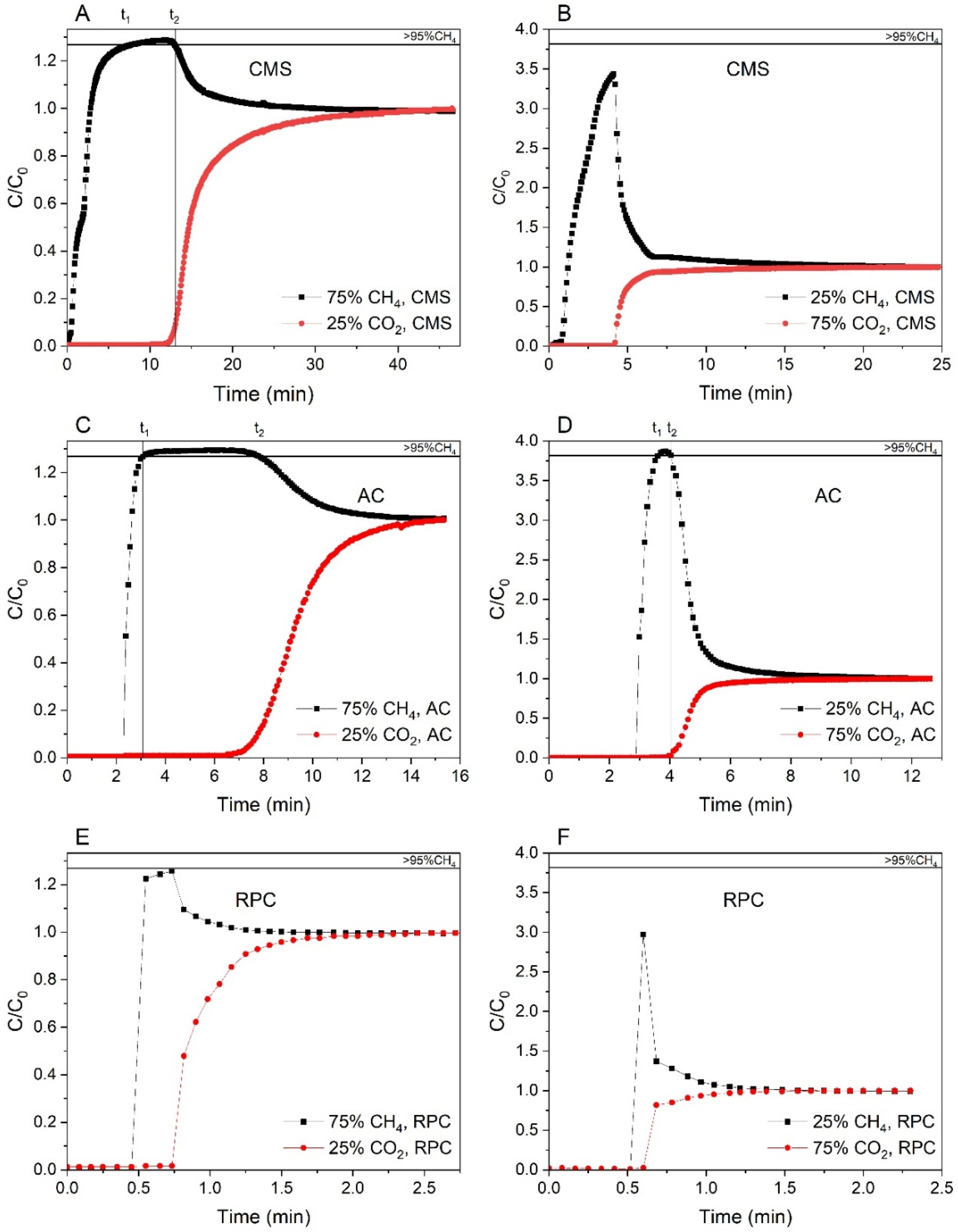
4. Column Dynamics Study
5. Results and Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Spagnolo, S.; Chinellato, G.; Cristiano, S.; Zucaro, A.; Gonella, F. Sustainability assessment of bioenergy at different scales: An emergy analysis of biogas power production. J. Clean. Prod. 2020, 277, 124038. [Google Scholar] [CrossRef]
- Toro, J.C.S.; Pérez, Y.C.; Cardona-Alzate, C.A. Evaluation of biogas and syngas as energy vectors for heat and power generation using lignocellulosic biomass as raw material. Electron. J. Biotechnol. 2018, 33, 52–62. [Google Scholar] [CrossRef]
- Gawlik, L.; Szurlej, A.; Wyrwa, A. The impact of the long-term EU target for renewables on the structure of electricity production in Poland. Energy 2015, 92, 172–178. [Google Scholar] [CrossRef]
- Damrongsak, D.; Tippayawong, N. Experimental investigation of an automotive air-conditioning system driven by a small biogas engine. Appl. Therm. Eng. 2010, 30, 400–405. [Google Scholar] [CrossRef]
- Subramanian, K.; Mathad, V.C.; Vijay, V.; Subbarao, P. Comparative evaluation of emission and fuel economy of an automotive spark ignition vehicle fuelled with methane enriched biogas and CNG using chassis dynamometer. Appl. Energy 2013, 105, 17–29. [Google Scholar] [CrossRef]
- Saadabadi, S.A.; Thattai, A.T.; Fan, L.; Lindeboom, R.E.; Spanjers, H.; Aravind, P. Solid Oxide Fuel Cells fuelled with biogas: Potential and constraints. Renew. Energy 2019, 134, 194–214. [Google Scholar] [CrossRef]
- Saadabadi, S.A.; Illathukandy, B.; Aravind, P.V. Direct internal methane reforming in biogas fuelled solid oxide fuel cell; the influence of operating parameters. Energy Sci. Eng. 2021, 9, 1232–1248. [Google Scholar] [CrossRef]
- Dudek, M.; Adamczyk, B.; Sitarz, M.; Śliwa, M.; Lach, R.; Skrzypkiewicz, M.; Raźniak, A.; Ziąbka, M.; Zuwała, J.; Grzywacz, P. The usefulness of walnut shells as waste biomass fuels in direct carbon solid oxide fuel cells. Biomass Bioenergy 2018, 119, 144–154. [Google Scholar] [CrossRef]
- Zhou, K.; Chaemchuen, S.; Verpoort, F. Alternative materials in technologies for Biogas upgrading via CO2 capture. Renew. Sustain. Energy Rev. 2017, 79, 1414–1441. [Google Scholar] [CrossRef]
- Baena-Moreno, F.M.; Rodríguez-Galán, M.; Vega, F.; Vilches, L.F.; Navarrete, B. Review: Recent advances in biogas purifying technologies. Int. J. Green Energy 2019, 16, 401–412. [Google Scholar] [CrossRef]
- Khan, I.U.; Othman, M.H.D.; Hashim, H.; Matsuura, T.; Ismail, A.F.; Rezaei-DashtArzhandi, M.; Azelee, I.W. Biogas as a renewable energy fuel—A review of biogas upgrading, utilisation and storage. Energy Convers. Manag. 2017, 150, 277–294. [Google Scholar] [CrossRef]
- Shah, G.; Ahmad, E.; Pant, K.; Vijay, V. Comprehending the contemporary state of art in biogas enrichment and CO2 capture technologies via swing adsorption. Int. J. Hydrogen Energy 2021, 46, 6588–6612. [Google Scholar] [CrossRef]
- Chouikhi, N.; Brandani, F.; Pullumbi, P.; Perre, P.; Puel, F. Biomethane production by adsorption technology: New cycle development, adsorbent selection and process optimization. Adsorption 2020, 26, 1275–1289. [Google Scholar] [CrossRef]
- Paolini, V.; Torre, M.; Giacopini, W.; Pastori, M.; Segreto, M.; Tomassetti, L.; Carnevale, M.; Gallucci, F.; Petracchini, F.; Guerriero, E. CO2/CH4 separation by hot potassium carbonate absorption for biogas upgrading. Int. J. Greenh. Gas Control 2019, 83, 186–194. [Google Scholar] [CrossRef]
- Nasir, R.; Abdulrahman, A. Polymeric amine membrane materials for carbon dioxide (CO2)/methane (CH4) separation. Materwiss. Werkst. 2020, 51, 66–72. [Google Scholar] [CrossRef]
- Baena-Moreno, F.M.; Rodríguez-Galán, M.; Vega, F.; Vilches, L.F.; Navarrete, B.; Zhang, Z. Biogas upgrading by cryogenic techniques. Environ. Chem. Lett. 2019, 17, 1251–1261. [Google Scholar] [CrossRef]
- Dębek, R.; Azzolina-Jury, F.; Travert, A.; Maugé, F. A review on plasma-catalytic methanation of carbon dioxide–Looking for an efficient catalyst. Renew. Sustain. Energy Rev. 2019, 116, 109427. [Google Scholar] [CrossRef]
- Montesano, C.; Faedda, M.; Martini, L.M.; Dilecce, G.; Tosi, P. CH4 reforming with CO2 in a nanosecond pulsed discharge. The importance of the pulse sequence. J. CO2 Util. 2021, 49, 101556. [Google Scholar] [CrossRef]
- Witte, J.; Settino, J.; Biollaz, S.M.; Schildhauer, T.J. Direct catalytic methanation of biogas—Part I: New insights into biomethane production using rate-based modelling and detailed process analysis. Energy Convers. Manag. 2018, 171, 750–768. [Google Scholar] [CrossRef]
- Kokkoli, A.; Zhang, Y.; Angelidaki, I. Microbial electrochemical separation of CO2 for biogas upgrading. Bioresour. Technol. 2018, 247, 380–386. [Google Scholar] [CrossRef] [Green Version]
- Zhong, D.-L.; Lu, Y.-Y.; Sun, D.-J.; Zhao, W.-L.; Li, Z. Performance evaluation of methane separation from coal mine gas by gas hydrate formation in a stirred reactor and in a fixed bed of silica sand. Fuel 2015, 143, 586–594. [Google Scholar] [CrossRef]
- Abd, A.A.; Othman, M.R.; Naji, S.Z.; Hashim, A.S. Methane enrichment in biogas mixture using pressure swing adsorption: Process fundamental and design parameters. Mater. Today Sustain. 2021, 11–12, 100063. [Google Scholar] [CrossRef]
- Rainone, F.; D’Agostino, O.; Erto, A.; Balsamo, M.; Lancia, A. Biogas upgrading by adsorption onto activated carbon and carbon molecular sieves: Experimental and modelling study in binary CO2/CH4 mixture. J. Environ. Chem. Eng. 2021, 9, 106256. [Google Scholar] [CrossRef]
- Chen, F.; Zhang, Z.; Yang, Q.; Yang, Y.; Bao, Z.; Ren, Q. Microporous Carbon Adsorbents Prepared by Activating Reagent-Free Pyrolysis for Upgrading Low-Quality Natural Gas. ACS Sustain. Chem. Eng. 2020, 8, 977–985. [Google Scholar] [CrossRef]
- Peredo-Mancilla, D.; Ghimbeu, C.M.; Ho, B.-N.; Jeguirim, M.; Hort, C.; Bessieres, D. Comparative study of the CH4/CO2 adsorption selectivity of activated carbons for biogas upgrading. J. Environ. Chem. Eng. 2019, 7, 103368. [Google Scholar] [CrossRef]
- Masruroh, K.; Cahyono, R.B.; Prasetyo, I.; Ariyanto, T. The Effect of Amine Types on Breakthrough Separation of Methane on Biogas. Int. J. Renew. Energy Dev. 2021, 10, 149–155. [Google Scholar] [CrossRef]
- Chidambaram, A.; Le, D.H.; Navarro, J.A.; Stylianou, K.C. Robust metal-organic frameworks for dry and wet biogas upgrading. Appl. Mater. Today 2021, 22, 100933. [Google Scholar] [CrossRef]
- Chaemchuen, S.; Alam Kabir, N.; Zhou, K.; Verpoort, F. Metal–organic frameworks for upgrading biogas via CO2 adsorption to biogas green energy. Chem. Soc. Rev. 2013, 42, 9304–9332. [Google Scholar] [CrossRef] [PubMed]
- Qiao, Z.; Zhou, J.; Lu, X. Designing new amine functionalized metal-organic frameworks for carbon dioxide/methane separation. Fluid Phase Equilib. 2014, 362, 342–348. [Google Scholar] [CrossRef]
- Xu, J.; Yu, J.; Xu, J.; Sun, C.; He, W.; Huang, J.; Li, G. High-value utilization of waste tires: A review with focus on modified carbon black from pyrolysis. Sci. Total Environ. 2020, 742, 140235. [Google Scholar] [CrossRef] [PubMed]
- Bernardo, M.; Lapa, N.; Fonseca, I.; Esteves, I.A.A.C. Biomass Valorization to Produce Porous Carbons: Applications in CO2 Capture and Biogas Upgrading to Biomethane—A Mini-Review. Front. Energy Res. 2021, 9, 625188. [Google Scholar] [CrossRef]
- Ghanbari, S.; Niu, C.H. Characteristics of oat hull based biosorbent for natural gas dehydration in a PSA process. J. Nat. Gas Sci. Eng. 2019, 61, 320–332. [Google Scholar] [CrossRef]
- Vilella, P.C.; Lira, J.A.; Azevedo, D.; Bastos-Neto, M.; Stefanutti, R. Preparation of biomass-based activated carbons and their evaluation for biogas upgrading purposes. Ind. Crop. Prod. 2017, 109, 134–140. [Google Scholar] [CrossRef]
- Guerrero-Esparza, M.M.; Medina-Valtierra, J.; Carrasco-Marín, F. Chars from waste tire rubber by catalytic pyrolysis and the statistical analysis of the adsorption of Fe in potable water. Environ. Prog. Sustain. Energy 2017, 36, 1794–1801. [Google Scholar] [CrossRef]
- Phasuphan, W.; Praphairaksit, N.; Imyim, A. Removal of ibuprofen, diclofenac, and naproxen from water using chitosan-modified waste tire crumb rubber. J. Mol. Liq. 2019, 294, 111554. [Google Scholar] [CrossRef]
- Nogueira, M.; Matos, I.; Bernardo, M.; Pinto, F.; Lapa, N.; Surra, E.; Fonseca, I. Lapa Char from Spent Tire Rubber: A Potential Adsorbent of Remazol Yellow Dye. C 2019, 5, 76. [Google Scholar] [CrossRef] [Green Version]
- Kirchherr, J.; Reike, D.; Hekkert, M. Conceptualizing the circular economy: An analysis of 114 definitions. Resour. Conserv. Recycl. 2017, 127, 221–232. [Google Scholar] [CrossRef]
- Sari, R.M.; Gea, S.; Wirjosentono, B.; Hendrana, S.; Torres, F.G. The effectiveness of coconut coir as tar adsorbent in liquid smoke integrated into the pyrolysis reactor. Case Stud. Therm. Eng. 2021, 25, 100907. [Google Scholar] [CrossRef]
- Sahu, J.; Karri, R.R.; Jayakumar, N. Improvement in phenol adsorption capacity on eco-friendly biosorbent derived from waste Palm-oil shells using optimized parametric modelling of isotherms and kinetics by differential evolution. Ind. Crop. Prod. 2021, 164, 113333. [Google Scholar] [CrossRef]
- Chan, W.H.; Mazlee, M.N.; Ahmad, Z.A.; Ishak, M.A.M.; Shamsul, J.B. The development of low cost adsorbents from clay and waste materials: A review. J. Mater. Cycles Waste Manag. 2017, 19, 1–14. [Google Scholar] [CrossRef]
- Pyrzynska, K. Removal of cadmium from wastewaters with low-cost adsorbents. J. Environ. Chem. Eng. 2019, 7, 102795. [Google Scholar] [CrossRef]
- Chakraborty, R.; Asthana, A.; Singh, A.K.; Jain, B.; Susan, A.B.H. Adsorption of heavy metal ions by various low-cost adsorbents: A review. Int. J. Environ. Anal. Chem. 2020, 1–38. [Google Scholar] [CrossRef]
- Quesada, H.B.; Baptista, A.T.A.; Cusioli, L.F.; Seibert, D.; de Oliveira Bezerra, C.; Bergamasco, R. Surface water pollution by pharmaceuticals and an alternative of removal by low-cost adsorbents: A review. Chemosphere 2019, 222, 766–780. [Google Scholar] [CrossRef]
- Hasan, R.; Ahliyasah, N.A.F.; Chong, C.C.; Jusoh, R.; Setiabudi, H.D. Egg-shell Treated Oil Palm Fronds (EG-OPF) as Low-Cost Adsorbent for Methylene Blue Removal. Bull. Chem. React. Eng. Catal. 2019, 14, 158–164. [Google Scholar] [CrossRef] [Green Version]
- Duan, X.-L.; Yuan, C.-G.; Jing, T.-T.; Yuan, X.-D. Removal of elemental mercury using large surface area micro-porous corn cob activated carbon by zinc chloride activation. Fuel 2019, 239, 830–840. [Google Scholar] [CrossRef]
- Karimi, M.; de Tuesta, J.L.D.; Gonçalves, C.N.D.P.; Gomes, H.T.; Rodrigues, A.E.; Silva, J.A.C.; Mohsen, K.; José, A.S. Compost from Municipal Solid Wastes as a Source of Biochar for CO2 Capture. Chem. Eng. Technol. 2020, 43, 1336–1349. [Google Scholar] [CrossRef]
- Mulu, E.; M’Arimi, M.M.; Ramkat, R.C. A review of recent developments in application of low cost natural materials in purification and upgrade of biogas. Renew. Sustain. Energy Rev. 2021, 145, 111081. [Google Scholar] [CrossRef]
- García, S.P.; Rodríguez, L.; Ángel, G.; Martínez, D.B.; Córdova, F.D.J.C.; Regalado, E.S.; Giraudet, S.; Guzmán, N.E.D. Siloxane removal for biogas purification by low cost mineral adsorbent. J. Clean. Prod. 2021, 286, 124940. [Google Scholar] [CrossRef]
- Rouzitalab, Z.; Maklavany, D.M.; Rashidi, A.; Jafarinejad, S. Synthesis of N-doped nanoporous carbon from walnut shell for enhancing CO2 adsorption capacity and separation. J. Environ. Chem. Eng. 2018, 6, 6653–6663. [Google Scholar] [CrossRef]
- Chomiak, K.; Gryglewicz, S.; Kierzek, K.; Machnikowski, J. Optimizing the properties of granular walnut-shell based KOH activated carbons for carbon dioxide adsorption. J. CO2 Util. 2017, 21, 436–443. [Google Scholar] [CrossRef]
- Mansurov, Z.; Lodewyckx, P.; Velasco, L.; Azat, S.; Kerimkulova, A. Modified sorbents based on walnut shell for sorption of toxic gases. Mater. Today Proc. 2021, 1–38. [Google Scholar] [CrossRef]
- Augelletti, R.; Conti, M.; Annesini, M.C. Pressure swing adsorption for biogas upgrading. A new process configuration for the separation of biomethane and carbon dioxide. J. Clean. Prod. 2017, 140, 1390–1398. [Google Scholar] [CrossRef]
- Nouri, H.; Ouederni, A. Modeling of the Dynamics Adsorption of Phenol from an Aqueous Solution on Activated Carbon Produced from Olive Stones. J. Biosens. Bioelectron. 2013, 4, 153. [Google Scholar] [CrossRef]
- Patel, H. Fixed-bed column adsorption study: A comprehensive review. Appl. Water Sci. 2019, 9, 45. [Google Scholar] [CrossRef] [Green Version]
- Chowdhury, Z.; Zain, S.M.; Rashid, A.; Rafique, R.F.; Khalid, K. Breakthrough Curve Analysis for Column Dynamics Sorption of Mn(II) Ions from Wastewater by UsingMangostana garciniaPeel-Based Granular-Activated Carbon. J. Chem. 2012, 2013, 959761. [Google Scholar] [CrossRef] [Green Version]
- Al Mesfer, M.K.; Danish, M.; Khan, M.I.; Ali, I.H.; Hasan, M.; El Jery, A. Continuous Fixed Bed CO2 Adsorption: Breakthrough, Column Efficiency, Mass Transfer Zone. Processes 2020, 8, 1233. [Google Scholar] [CrossRef]
- Pirngruber, G.D.; Hamon, L.; Bourrelly, S.; Llewellyn, P.L.; Lenoir, E.; Guillerm, V.; Serre, C.; Devic, T. A Method for Screening the Potential of MOFs as CO2 Adsorbents in Pressure Swing Adsorption Processes. ChemSusChem 2012, 5, 762–776. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Gutiérrez, N.; García, S.; Gil, M.V.; Rubiera, F.; Pevida, C. Dynamic Performance of Biomass-Based Carbons for CO2/CH4 Separation. Approximation to a Pressure Swing Adsorption Process for Biogas Upgrading. Energy Fuels 2016, 30, 5005–5015. [Google Scholar] [CrossRef] [Green Version]
- Parinyakit, S.; Worathanakul, P. Static and Dynamic Simulation of Single and Binary Component Adsorption of CO2 and CH4 on Fixed Bed Using Molecular Sieve of Zeolite 4A. Processes 2021, 9, 1250. [Google Scholar] [CrossRef]
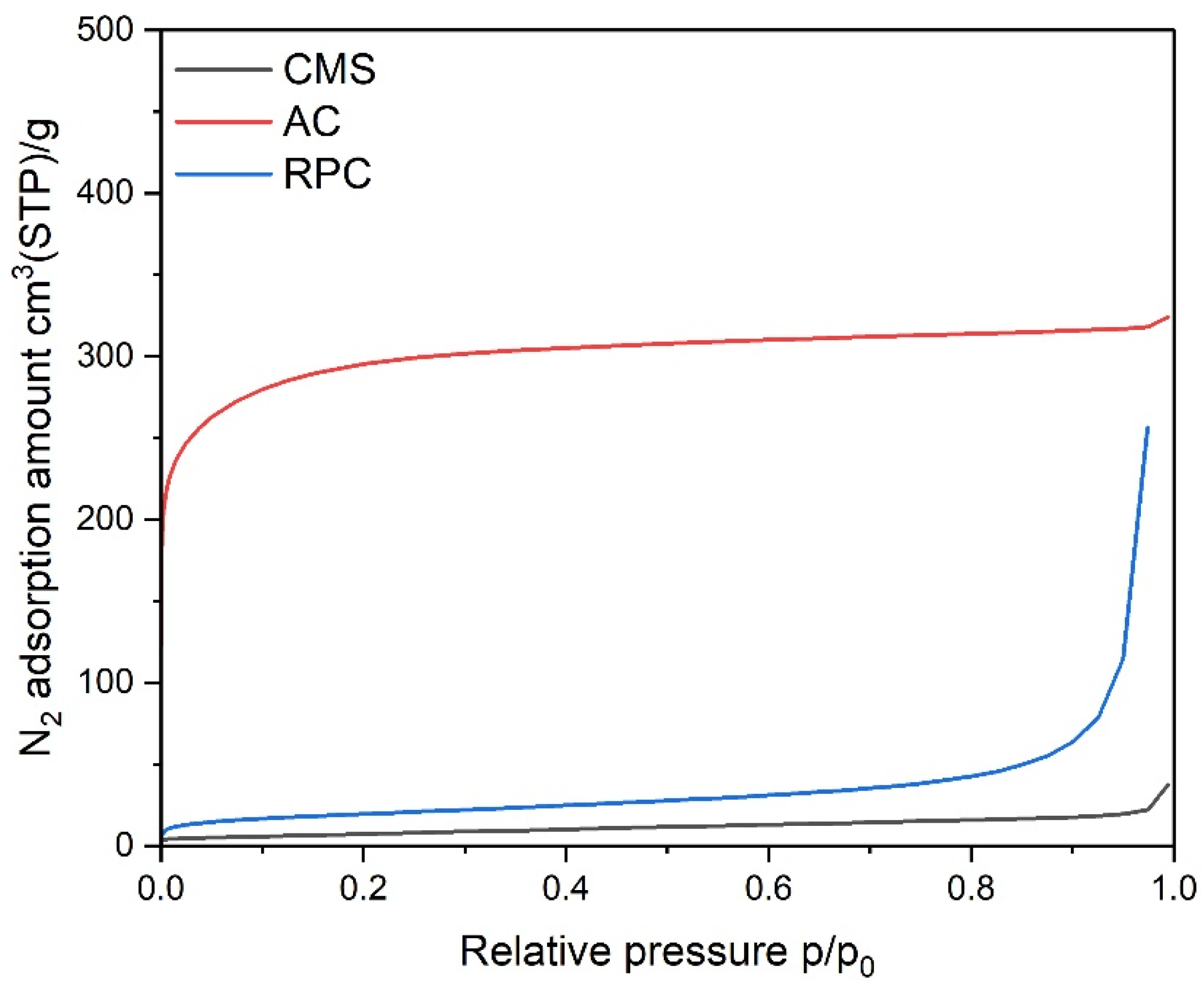
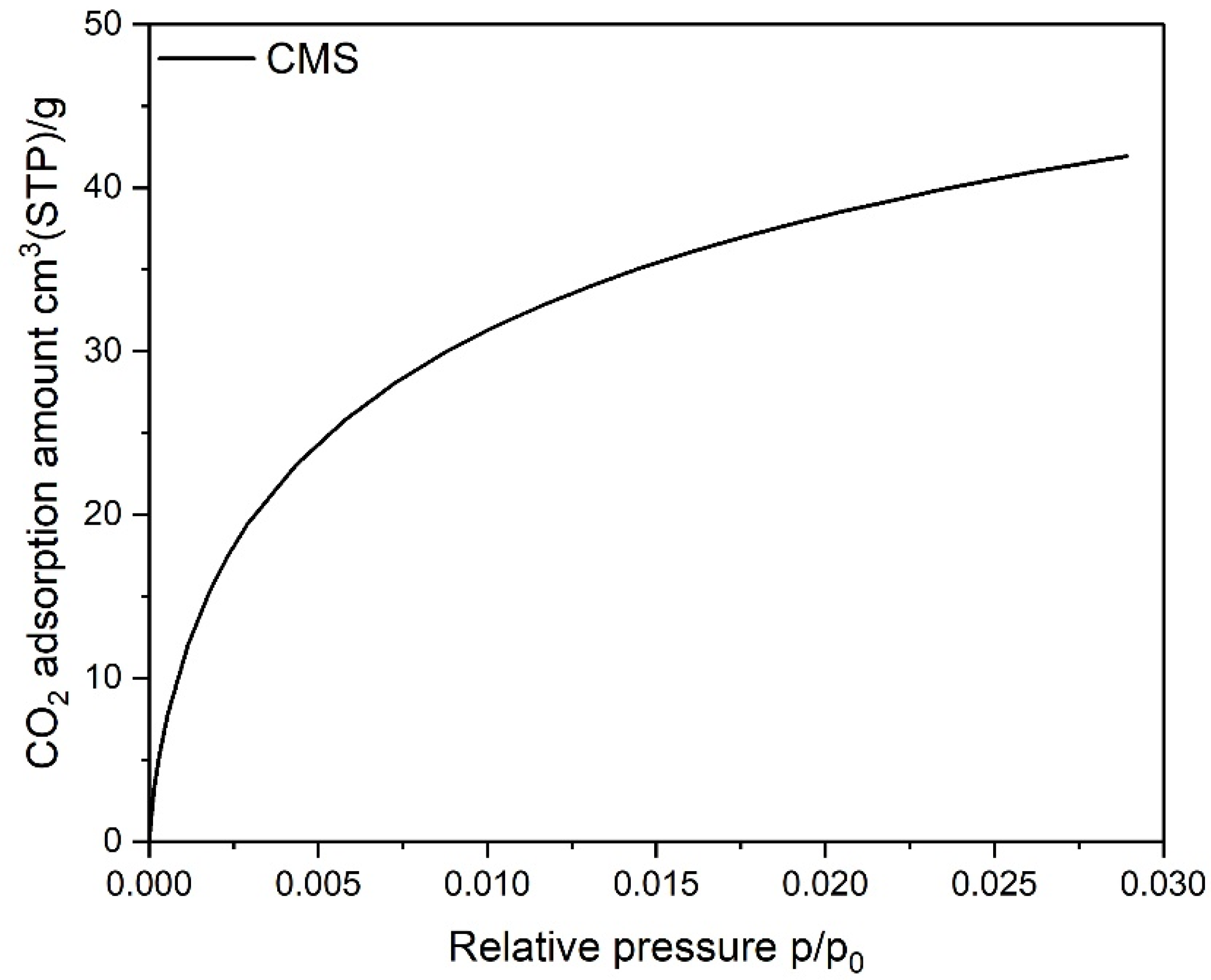

| Parameter | Unit | CMS a | AC b | RPC b |
|---|---|---|---|---|
| SBET | m2/g | - | 1118 | 70.3 |
| Vmic | cm3(STP)/g | 0.177 | 0.415 | 0.025 |
| Vmes | cm3(STP)/g | - | 0.061 | 0.390 |
| Parameter | Unit | CMS | AC | RPC | |||
|---|---|---|---|---|---|---|---|
| 75:25 a | 25:75 b | 75:25 a | 25:75 b | 75:25 a | 25:75 b | ||
| 1/min | 1.41 | 6.50 | 1.28 | 4.09 | 47.35 | 59.78 | |
| min | 14.60 | 4.53 | 9.13 | 4.64 | 0.82 | 0.66 | |
| min | 16.69 | 5.14 | 9.29 | 4.75 | 0.89 | 0.65 | |
| R2 | - | 0.984 | 0.989 | 0.997 | 0.998 | 0.951 | 0.992 |
| RMSE | - | 0.053 | 0.038 | 0.023 | 0.023 | 0.090 | 0.039 |
| Parameter | Unit | CMS | AC | RPC | |||
|---|---|---|---|---|---|---|---|
| 75:25 a | 25:75 b | 75:25 a | 25:75 b | 75:25 a | 25:75 b | ||
| mmol/g | 0.252 | 0.273 | 0.195 | 0.335 | 0.024 | 0.059 | |
| mmol/g | 0.286 | 0.309 | 0.198 | 0.344 | 0.026 | 0.059 | |
| mmol/g | 0.433 | - c | 0.467 | 0.033 | - c | - c | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bałys, M.; Brodawka, E.; Jodłowski, G.S.; Szczurowski, J.; Wójcik, M. Alternative Materials for the Enrichment of Biogas with Methane. Materials 2021, 14, 7759. https://doi.org/10.3390/ma14247759
Bałys M, Brodawka E, Jodłowski GS, Szczurowski J, Wójcik M. Alternative Materials for the Enrichment of Biogas with Methane. Materials. 2021; 14(24):7759. https://doi.org/10.3390/ma14247759
Chicago/Turabian StyleBałys, Mieczysław, Ewelina Brodawka, Grzegorz Stefan Jodłowski, Jakub Szczurowski, and Marta Wójcik. 2021. "Alternative Materials for the Enrichment of Biogas with Methane" Materials 14, no. 24: 7759. https://doi.org/10.3390/ma14247759
APA StyleBałys, M., Brodawka, E., Jodłowski, G. S., Szczurowski, J., & Wójcik, M. (2021). Alternative Materials for the Enrichment of Biogas with Methane. Materials, 14(24), 7759. https://doi.org/10.3390/ma14247759






