CO2 Adsorption on PtCu Sub-Nanoclusters Deposited on Pyridinic N-Doped Graphene: A DFT Investigation
Abstract
:1. Introduction
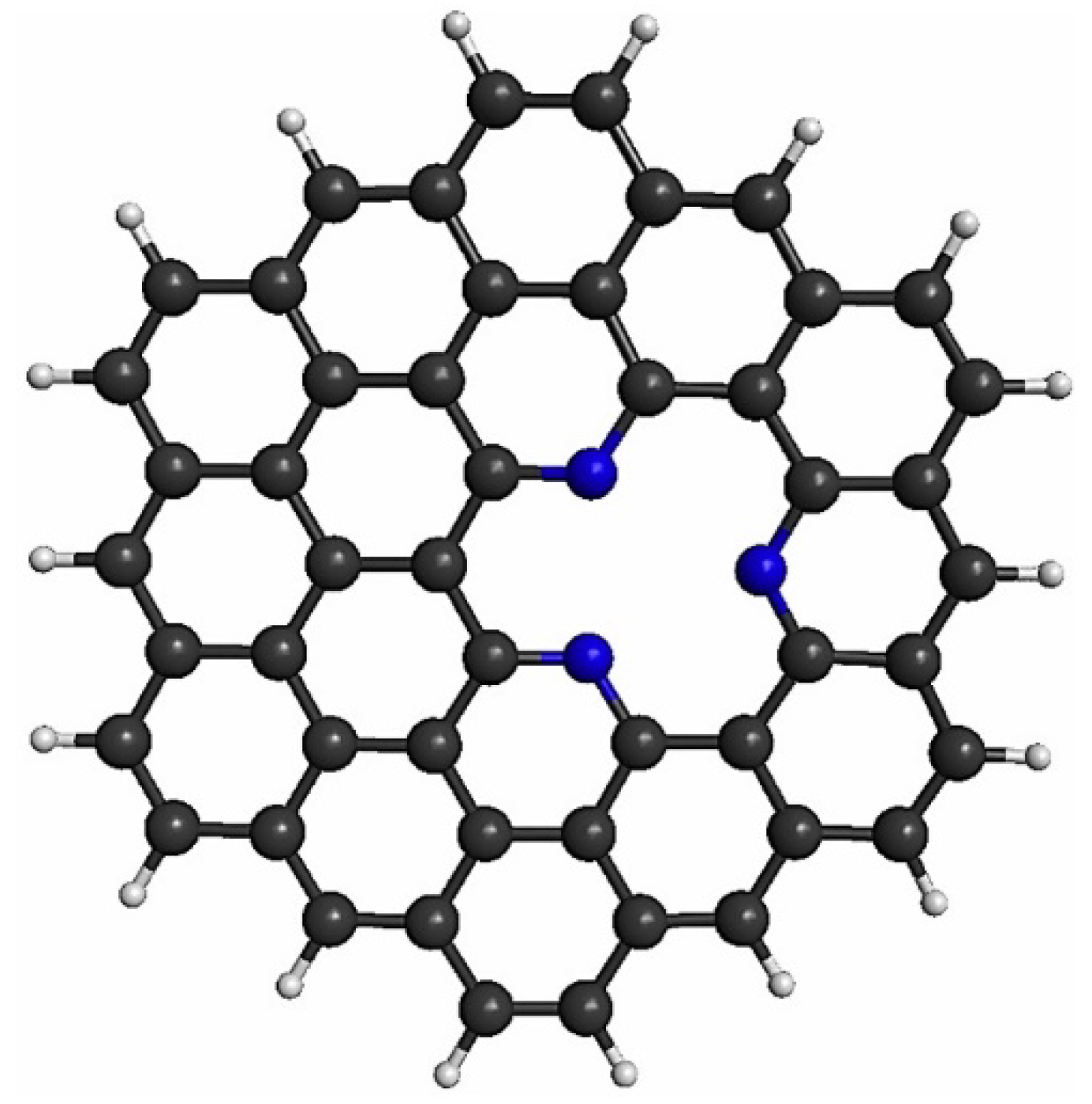
2. Computational Methodology
3. Results
3.1. Stability of Pt4-xCux (x = 0–4) Sub-Nanoclusters on PNG
3.2. CO2 Adsorption on Pt4-xCux (x = 0–4) Sub-Nanoclusters Deposited on PNG
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Duan, X.; Xu, J.; Wei, Z.; Ma, J.; Guo, S.; Wang, S.; Liu, H.; Dou, S. Metal-free carbon materials for CO2 electrochemical reduction. Adv. Mater. 2017, 29, 1701784. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Li, G.; Fang, C. Urbanization, economic growth, energy consumption, and CO2 emissions: Empirical evidence from countries with different income levels. Renew. Sustain. Energy Rev. 2018, 81, 2144–2159. [Google Scholar] [CrossRef]
- Bekun, F.V.; Emir, F.; Sarkodie, S.A. Another look at the relationship between energy consumption, carbon dioxide emissions, and economic growth in South Africa. Sci. Total Environ. 2019, 655, 759–765. [Google Scholar] [CrossRef] [PubMed]
- Kemache, N.; Pasquier, L.C.; Cecchi, E.; Mouedhen, I.; Blais, J.F.; Mercier, G. Aqueous mineral carbonation for CO2 sequestration: From laboratory to pilot scale. Fuel Process. Technol. 2017, 166, 209–216. [Google Scholar] [CrossRef]
- Kaliyavaradhan, S.K.; Ling, T.C. Potential of CO2 sequestration through construction and demolition (C&D) waste—An overview. J. CO2 Util. 2017, 20, 234–242. [Google Scholar] [CrossRef]
- Nielsen, D.U.; Hu, X.M.; Daasbjerg, K.; Skrydstrup, T. Chemically and electrochemically catalysed conversion of CO2 to CO with follow-up utilization to value-added chemicals. Nat. Catal. 2018, 1, 244–254. [Google Scholar] [CrossRef]
- Gao, Y.; Qian, K.; Xu, B.; Li, Z.; Zheng, J.; Zhao, S.; Ding, F.; Sun, Y.; Xu, Z. Recent advances in visible-light-driven conversion of CO2 by photocatalysts into fuels or value-added chemicals. Carbon Resour. Convers. 2020, 3, 46–59. [Google Scholar] [CrossRef]
- Whang, H.S.; Lim, J.; Choi, M.S.; Lee, J.; Lee, H. Heterogeneous catalysts for catalytic CO2 conversion into value-added chemicals. BMC Chem. Eng. 2019, 1, 9. [Google Scholar] [CrossRef]
- Ma, J.; Sun, N.; Zhang, X.; Zhao, N.; Xiao, F.; Wei, W.; Sun, Y. A short review of catalysis for CO2 conversion. Catal. Today 2009, 148, 221–231. [Google Scholar] [CrossRef]
- Wang, W.; Wang, S.; Ma, X.; Gong, J. Recent advances in catalytic hydrogenation of carbon dioxide. Chem. Soc. Rev. 2011, 40, 3703–3727. [Google Scholar] [CrossRef] [Green Version]
- Rahaman, M.; Dutta, A.; Broekmann, P. Size-dependent activity of palladium nanoparticles: Efficient conversion of CO2 into formate at low overpotentials. ChemSusChem 2017, 10, 1733–1741. [Google Scholar] [CrossRef]
- Dongare, S.; Singh, N.; Bhunia, H. Electrocatalytic reduction of CO2 to useful chemicals on copper nanoparticles. Appl. Surf. Sci. 2021, 537, 148020. [Google Scholar] [CrossRef]
- Bernal, M.; Bagger, A.; Scholten, F.; Sinev, I.; Bergmann, A.; Ahmadi, M.; Rossmeisl, J.; Cuenya, B.R. CO2 electroreduction on copper-cobalt nanoparticles: Size and composition effect. Nano Energy 2018, 53, 27–36. [Google Scholar] [CrossRef]
- Ismail, A.M.; Samu, G.F.; Balog, A.; Csapó, E.; Janáky, C. Composition dependent electrocatalytic behavior of Au–Sn bimetallic nanoparticles in carbon dioxide reduction. ACS Energy Lett. 2018, 4, 48–53. [Google Scholar] [CrossRef] [Green Version]
- Zhao, X.; Luo, B.; Long, R.; Wang, C.; Xiong, Y. Composition-dependent activity of Cu–Pt alloy nanocubes for electrocatalytic CO2 reduction. J. Mater. Chem. A 2015, 3, 4134–4138. [Google Scholar] [CrossRef]
- Guo, X.; Zhang, Y.; Deng, C.; Li, X.; Xue, Y.; Yan, Y.M.; Sun, K. Composition dependent activity of Cu–Pt nanocrystals for electrochemical reduction of CO2. Chem. Commun. 2015, 51, 1345–1348. [Google Scholar] [CrossRef] [PubMed]
- Varela, A.S.; Schlaup, C.; Jovanov, Z.P.; Malacrida, P.; Horch, S.; Stephens, I.E.; Chorkendorff, I. CO2 electroreduction on well-defined bimetallic surfaces: Cu overlayers on Pt (111) and Pt (211). J. Phys. Chem. C 2013, 117, 20500–20508. [Google Scholar] [CrossRef]
- Gálvez-González, L.E.; Juárez-Sánchez, J.O.; Pacheco-Contreras, R.; Garzón, I.L.; Paz-Borbón, L.O.; Posada-Amarillas, A. CO2 adsorption on gas-phase Cu4−xPtx (x= 0–4) clusters: A DFT study. Phys. Chem. Chem. Phys. 2018, 20, 17071–17080. [Google Scholar] [CrossRef]
- Martínez-Espinosa, J.A.; Cruz-Martínez, H.; Calaminici, P.; Medina, D.I. Structures and properties of Co13−xCux (x= 0–13) nanoclusters and their interaction with pyridinic N3-doped graphene nanoflake. Phys. E 2021, 134, 114858. [Google Scholar] [CrossRef]
- Rojas-Chávez, H.; Cruz-Martínez, H.; Flores-Rojas, E.; Juárez-García, J.M.; González-Domínguez, J.L.; Daneu, N.; Santoyo-Salazar, J. The mechanochemical synthesis of PbTe nanostructures: Following the Ostwald ripening effect during milling. Phys. Chem. Chem. Phys. 2018, 20, 27082–27092. [Google Scholar] [CrossRef]
- Neto, A.C.; Guinea, F.; Peres, N.M.; Novoselov, K.S.; Geim, A.K. The electronic properties of graphene. Rev. Mod. Phys. 2009, 81, 109. [Google Scholar] [CrossRef] [Green Version]
- Singh, V.; Joung, D.; Zhai, L.; Das, S.; Khondaker, S.I.; Seal, S. Graphene based materials: Past, present and future. Prog. Mater. Sci. 2011, 56, 1178–1271. [Google Scholar] [CrossRef]
- Kong, X.K.; Chen, C.L.; Chen, Q.W. Doped graphene for metal-free catalysis. Chem. Soc. Rev. 2014, 43, 2841–2857. [Google Scholar] [CrossRef]
- Montejo-Alvaro, F.; Rojas-Chávez, H.; Román-Doval, R.; Mtz-Enriquez, A.I.; Cruz-Martínez, H.; Medina, D.I. Stability of Pd clusters supported on pristine, B-doped, and defective graphene quantum dots, and their reactivity toward oxygen adsorption: A DFT analysis. Solid State Sci. 2019, 93, 55–61. [Google Scholar] [CrossRef]
- Kaushal, S.; Kaur, M.; Kaur, N.; Kumari, V.; Singh, P.P. Heteroatom-doped graphene as sensing materials: A mini review. RSC Adv. 2020, 10, 28608–28629. [Google Scholar] [CrossRef]
- Cruz-Martínez, H.; Rojas-Chávez, H.; Montejo-Alvaro, F.; Peña-Castañeda, Y.A.; Matadamas-Ortiz, P.T.; Medina, D.I. Recent Developments in Graphene-Based Toxic Gas Sensors: A Theoretical Overview. Sensors 2021, 21, 1992. [Google Scholar] [CrossRef] [PubMed]
- Rangel, E.; Sansores, E. Theoretical study of hydrogen adsorption on nitrogen doped graphene decorated with palladium clusters. Int. J. Hydrogen Energy 2014, 39, 6558–6566. [Google Scholar] [CrossRef]
- Rangel, E.; Sansores, E.; Vallejo, E.; Hernández-Hernández, A.; López-Pérez, P.A. Study of the interplay between N-graphene defects and small Pd clusters for enhanced hydrogen storage via a spill-over mechanism. Phys. Chem. Chem. Phys. 2016, 18, 33158–33170. [Google Scholar] [CrossRef]
- Sánchez-Rodríguez, E.P.; Vargas-Hernández, C.N.; Cruz-Martínez, H.; Medina, D.I. Stability, magnetic, energetic, and reactivity properties of icosahedral M@ Pd12 (M= Fe, Co, Ni, and Cu) core-shell nanoparticles supported on pyridinic N3-doped graphene. Solid State Sci. 2021, 112, 106483. [Google Scholar] [CrossRef]
- Wang, Q.; Tian, Y.; Chen, G.; Zhao, J. Theoretical insights into the energetics and electronic properties of MPt12 (M= Fe, Co, Ni, Cu, and Pd) nanoparticles supported by N-doped defective graphene. Appl. Surf. Sci. 2017, 397, 199–205. [Google Scholar] [CrossRef]
- Zhao, C.; Wu, H. Density functional investigation of mercury and arsenic adsorption on nitrogen doped graphene decorated with palladium clusters: A promising heavy metal sensing material in farmland. Appl. Surf. Sci. 2017, 399, 55–66. [Google Scholar] [CrossRef]
- Gulati, A.; Kakkar, R. DFT study of adsorption of glyphosate pesticide on Pt-Cu decorated pyridine-like nitrogen-doped graphene. J. Nanopart Res. 2020, 22, 17. [Google Scholar] [CrossRef]
- Neese, F. Software update: The ORCA program system, version 4.0. Wiley Interdiscip Rev. Comput Mol. Sci. 2018, 8, e1327. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, W. Comment on “Generalized Gradient Approximation Made Simple”. Phys. Rev. Lett. 1998, 80, 890. [Google Scholar] [CrossRef]
- Weigend, F.; Ahlrichs, R. Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: Design and assessment of accuracy. Phys. Chem. Chem. Phys. 2005, 7, 3297. [Google Scholar] [CrossRef] [PubMed]
- Hay, P.J.; Wadt, W.R. Ab initio effective core potentials for molecular calculations. Potentials for K to Au including the outermost core orbitale. J. Chem. Phys. 1985, 82, 299–310. [Google Scholar] [CrossRef]
- Zhou, X.; Chu, W.; Sun, W.; Zhou, Y.; Xue, Y. Enhanced interaction of nickel clusters with pyridinic-N (B) doped graphene using DFT simulation. Comput. Theor. Chem. 2017, 1120, 8–16. [Google Scholar] [CrossRef]
- Lu, T.; Chen, F. Multiwfn: A multifunctional wavefunction analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef]
- Liu, S.; Huang, S. Theoretical insights into the activation of O2 by Pt single atom and Pt4 nanocluster on functionalized graphene support: Critical role of Pt positive polarized charges. Carbon 2017, 115, 11–17. [Google Scholar] [CrossRef]
- Rêgo, C.R.; Tereshchuk, P.; Oliveira, L.N.; Da Silva, J.L. Graphene-supported small transition-metal clusters: A density functional theory investigation within van der Waals corrections. Phys. Rev. B 2017, 95, 235422. [Google Scholar] [CrossRef]
- Fampiou, I.; Ramasubramaniam, A. Binding of Pt nanoclusters to point defects in graphene: Adsorption, morphology, and electronic structure. J. Phys. Chem. C 2012, 116, 6543–6555. [Google Scholar] [CrossRef]
- Batista, K.E.; Ocampo-Restrepo, V.K.; Soares, M.D.; Quiles, M.G.; Piotrowski, M.J.; Da Silva, J.L. Ab Initio Investigation of CO2 Adsorption on 13-Atom 4d Clusters. J. Chem. Inf. Model. 2020, 60, 537–545. [Google Scholar] [CrossRef] [PubMed]
- Mendes, P.C.; Verga, L.G.; Da Silva, J.L. Ab initio screening of Pt-based transition-metal nanoalloys using descriptors derived from the adsorption and activation of CO2. Phys. Chem. Chem. Phys. 2021, 23, 6029–6041. [Google Scholar] [CrossRef] [PubMed]

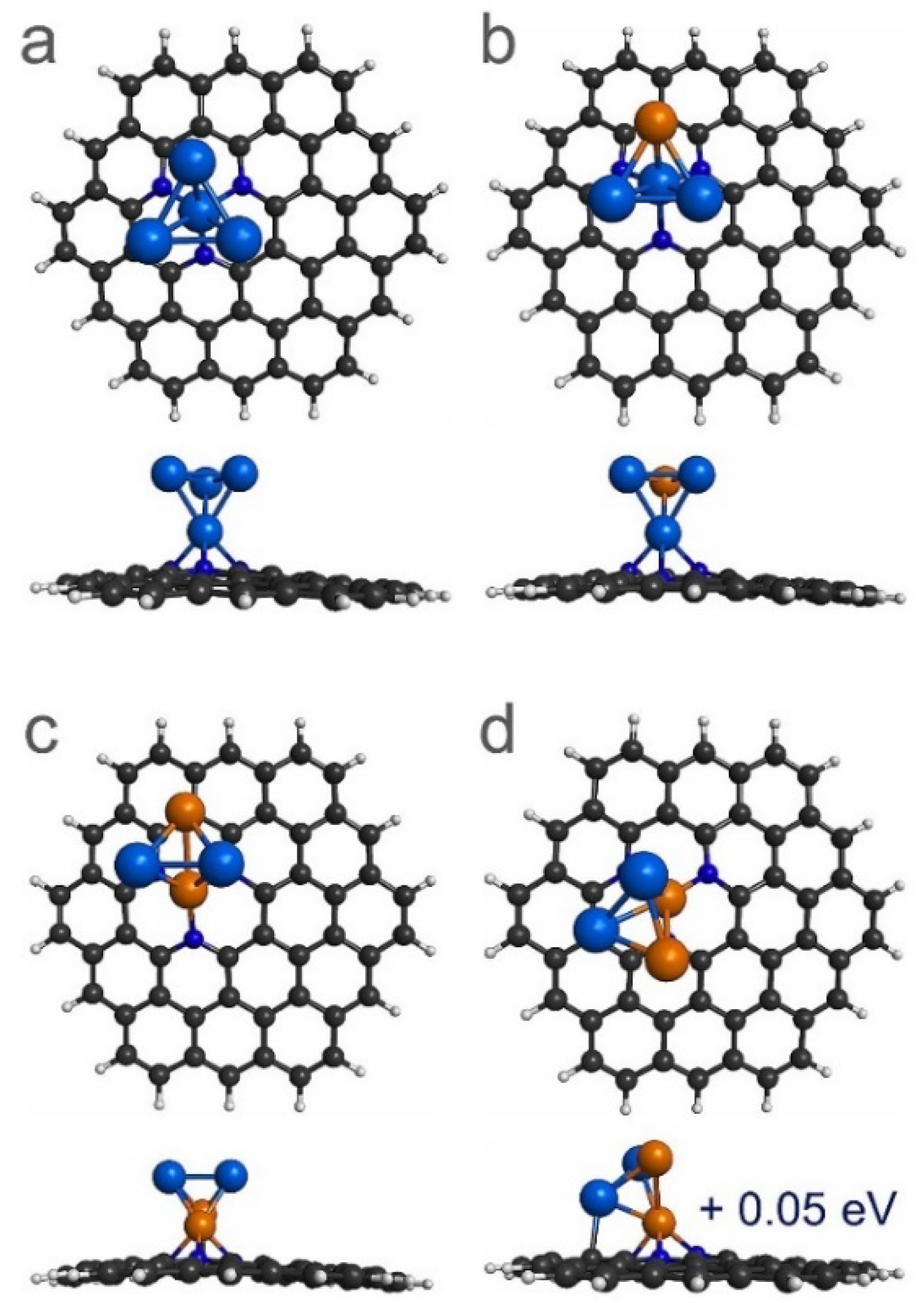

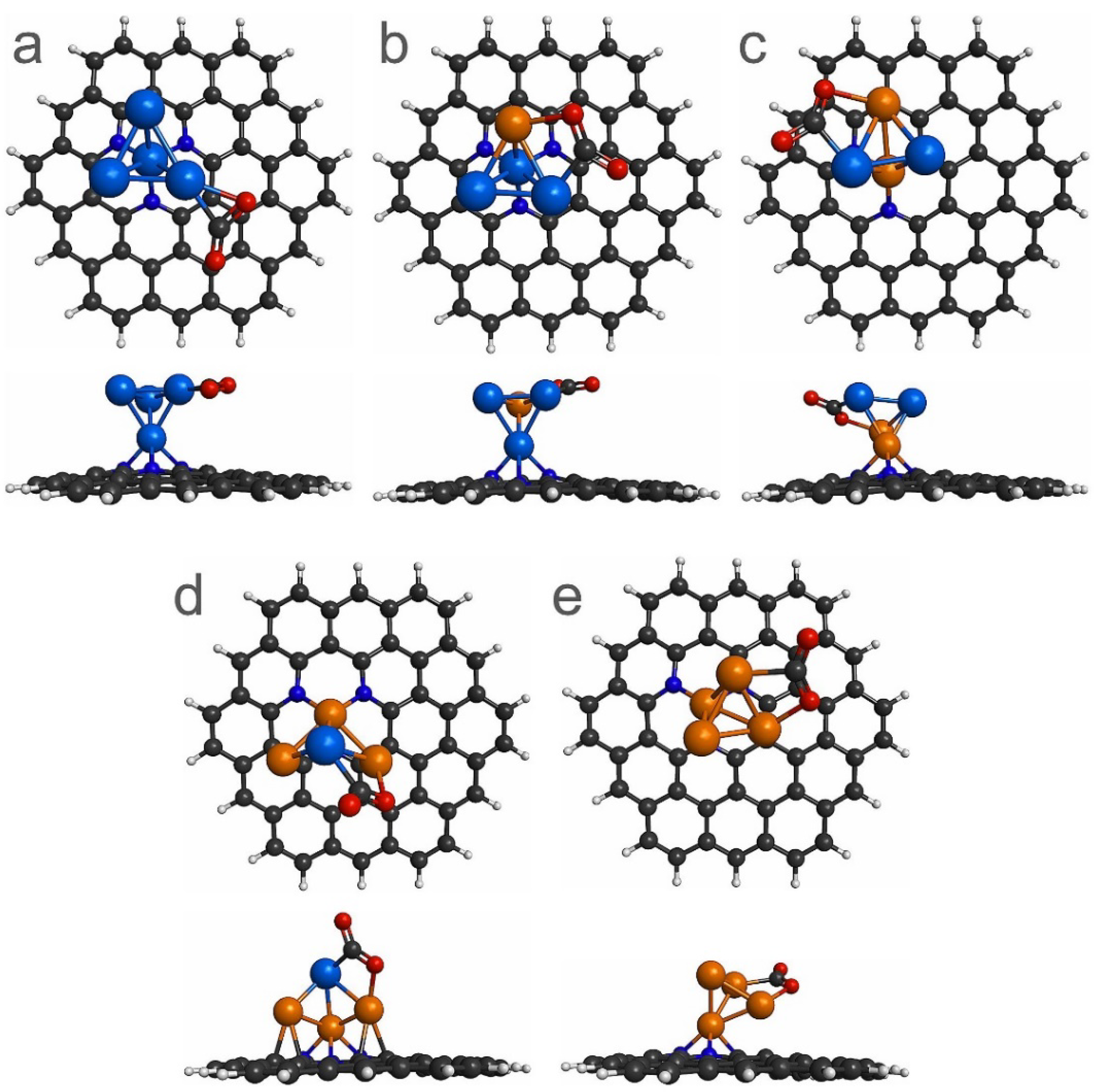
| System | Eb (eV) | QTAIM Charge (e) |
|---|---|---|
| Pt4/PNG | −3.61 | 0.23 |
| Pt3Cu/PNG | −3.01 | 0.26 |
| Pt2Cu2/PNG | −2.65 | 0.52 |
| PtCu3/PNG | −3.26 | 0.69 |
| Cu4/PNG | −2.44 | 0.57 |
| System | Eads (eV) | Charge Transfer Toward CO2 (e) | Average CO2 Bond Length (Å) | Bending Angle of CO2 (°) |
|---|---|---|---|---|
| CO2/Pt4/PNG | −1.06 | −0.37 | 1.24 | 141.25 |
| CO2/Pt3Cu/PNG | −2.21 | −0.42 | 1.24 | 140.03 |
| CO2/Pt2Cu2/PNG | −2.34 | −0.44 | 1.25 | 139.32 |
| CO2/PtCu3/PNG | −2.48 | −0.46 | 1.25 | 135.86 |
| CO2/Cu4/PNG | −1.81 | −0.58 | 1.24 | 138.27 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Montejo-Alvaro, F.; González-Quijano, D.; Valmont-Pineda, J.A.; Rojas-Chávez, H.; Juárez-García, J.M.; Medina, D.I.; Cruz-Martínez, H. CO2 Adsorption on PtCu Sub-Nanoclusters Deposited on Pyridinic N-Doped Graphene: A DFT Investigation. Materials 2021, 14, 7619. https://doi.org/10.3390/ma14247619
Montejo-Alvaro F, González-Quijano D, Valmont-Pineda JA, Rojas-Chávez H, Juárez-García JM, Medina DI, Cruz-Martínez H. CO2 Adsorption on PtCu Sub-Nanoclusters Deposited on Pyridinic N-Doped Graphene: A DFT Investigation. Materials. 2021; 14(24):7619. https://doi.org/10.3390/ma14247619
Chicago/Turabian StyleMontejo-Alvaro, Fernando, Diego González-Quijano, Jorge A. Valmont-Pineda, Hugo Rojas-Chávez, José M. Juárez-García, Dora I. Medina, and Heriberto Cruz-Martínez. 2021. "CO2 Adsorption on PtCu Sub-Nanoclusters Deposited on Pyridinic N-Doped Graphene: A DFT Investigation" Materials 14, no. 24: 7619. https://doi.org/10.3390/ma14247619
APA StyleMontejo-Alvaro, F., González-Quijano, D., Valmont-Pineda, J. A., Rojas-Chávez, H., Juárez-García, J. M., Medina, D. I., & Cruz-Martínez, H. (2021). CO2 Adsorption on PtCu Sub-Nanoclusters Deposited on Pyridinic N-Doped Graphene: A DFT Investigation. Materials, 14(24), 7619. https://doi.org/10.3390/ma14247619







