Post-Fatigue Fracture and Marginal Behavior of Endodontically Treated Teeth: Partial Crown vs. Full Crown vs. Endocrown vs. Fiber-Reinforced Resin Composite
Abstract
:1. Introduction
- Is there a difference between partial crowns and full crowns?
- What is the status of endocrowns in that context?
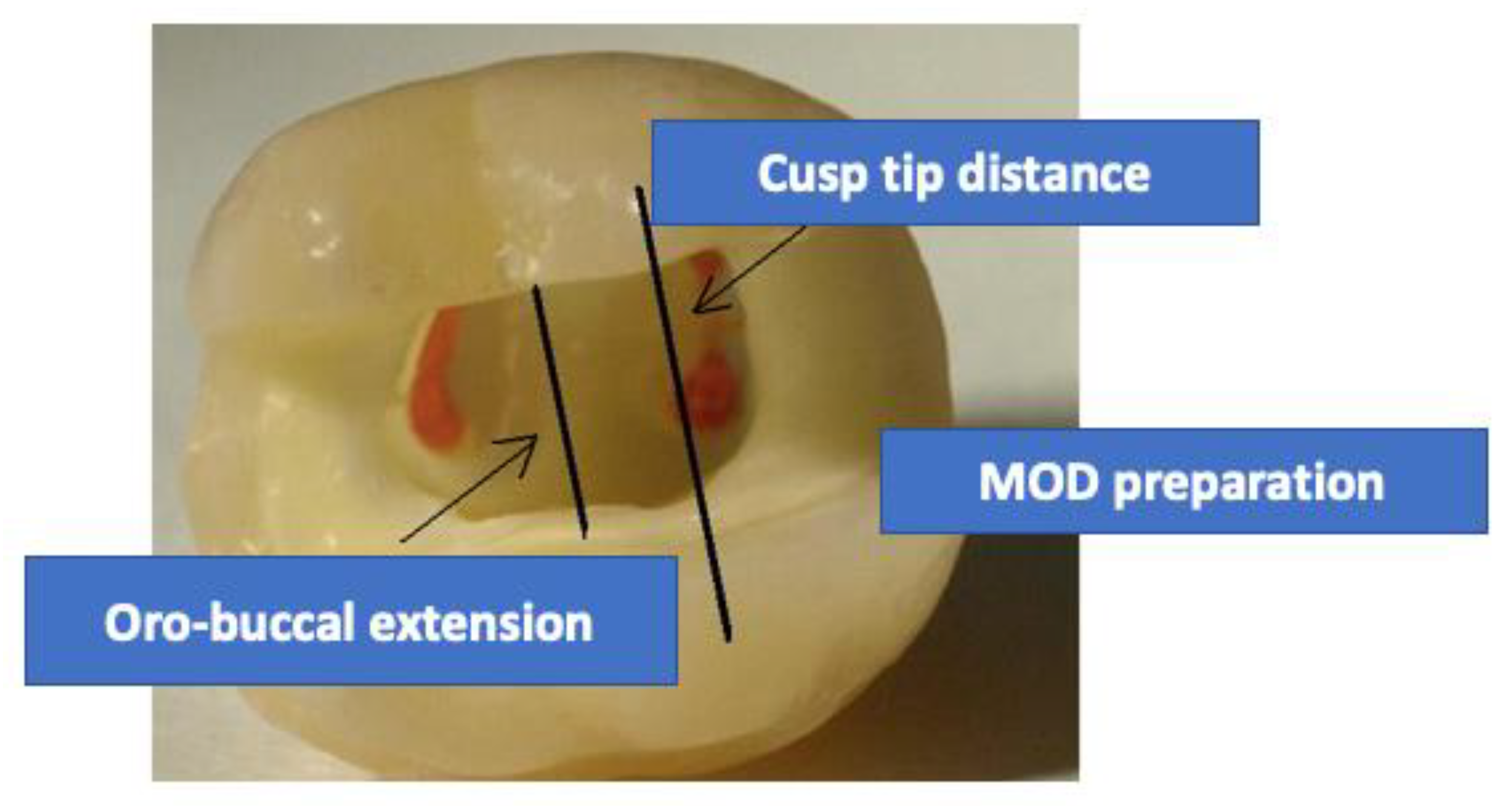
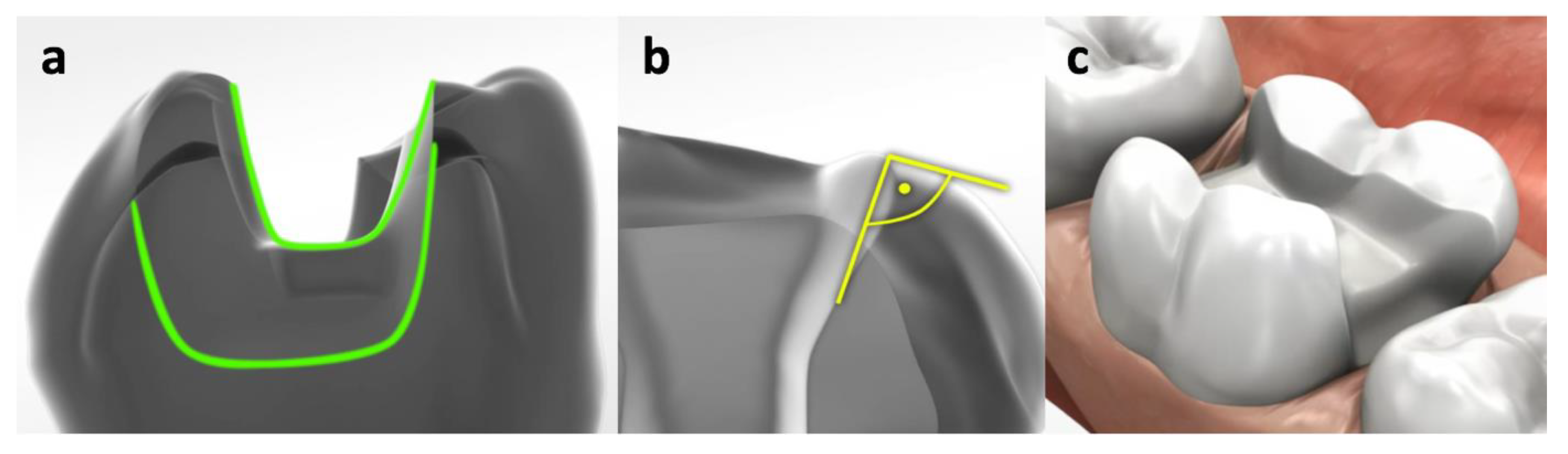
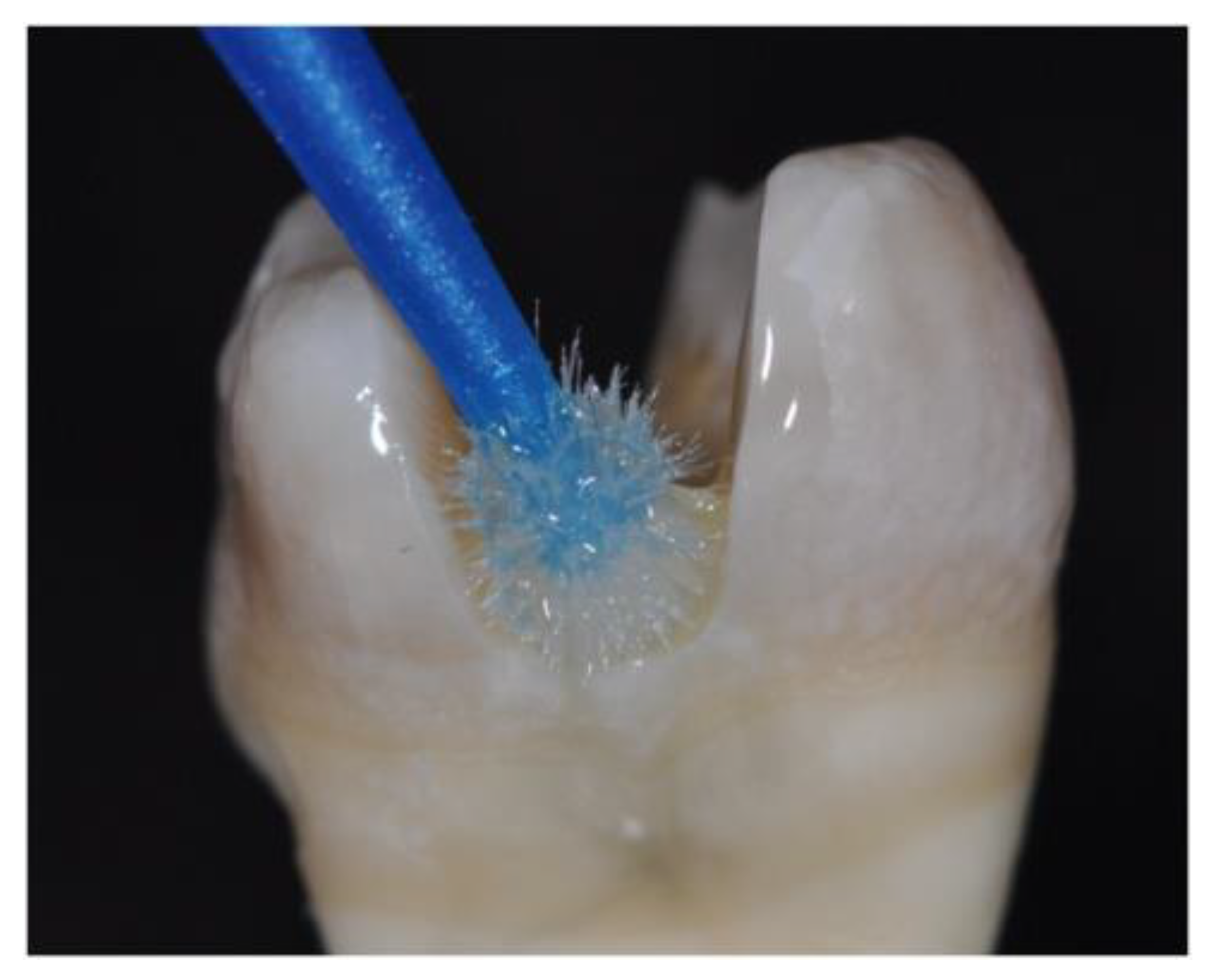
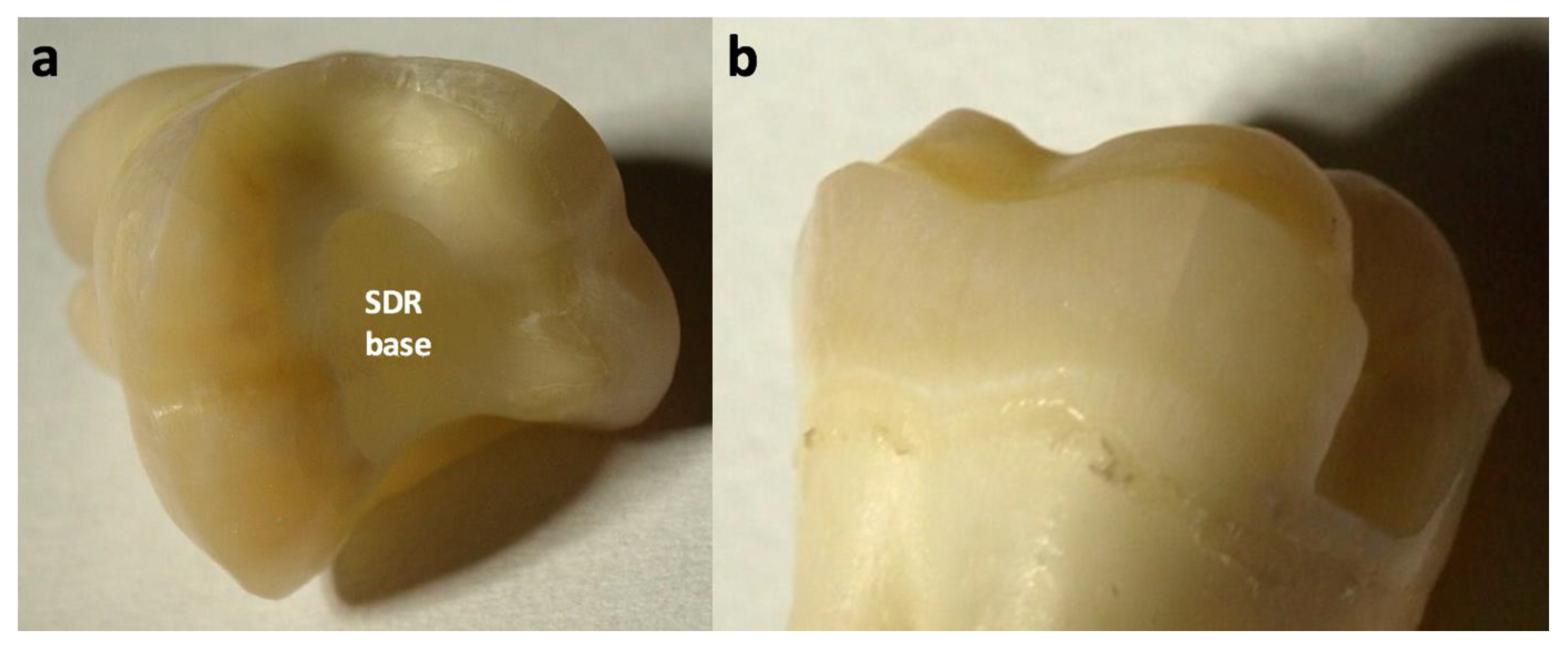
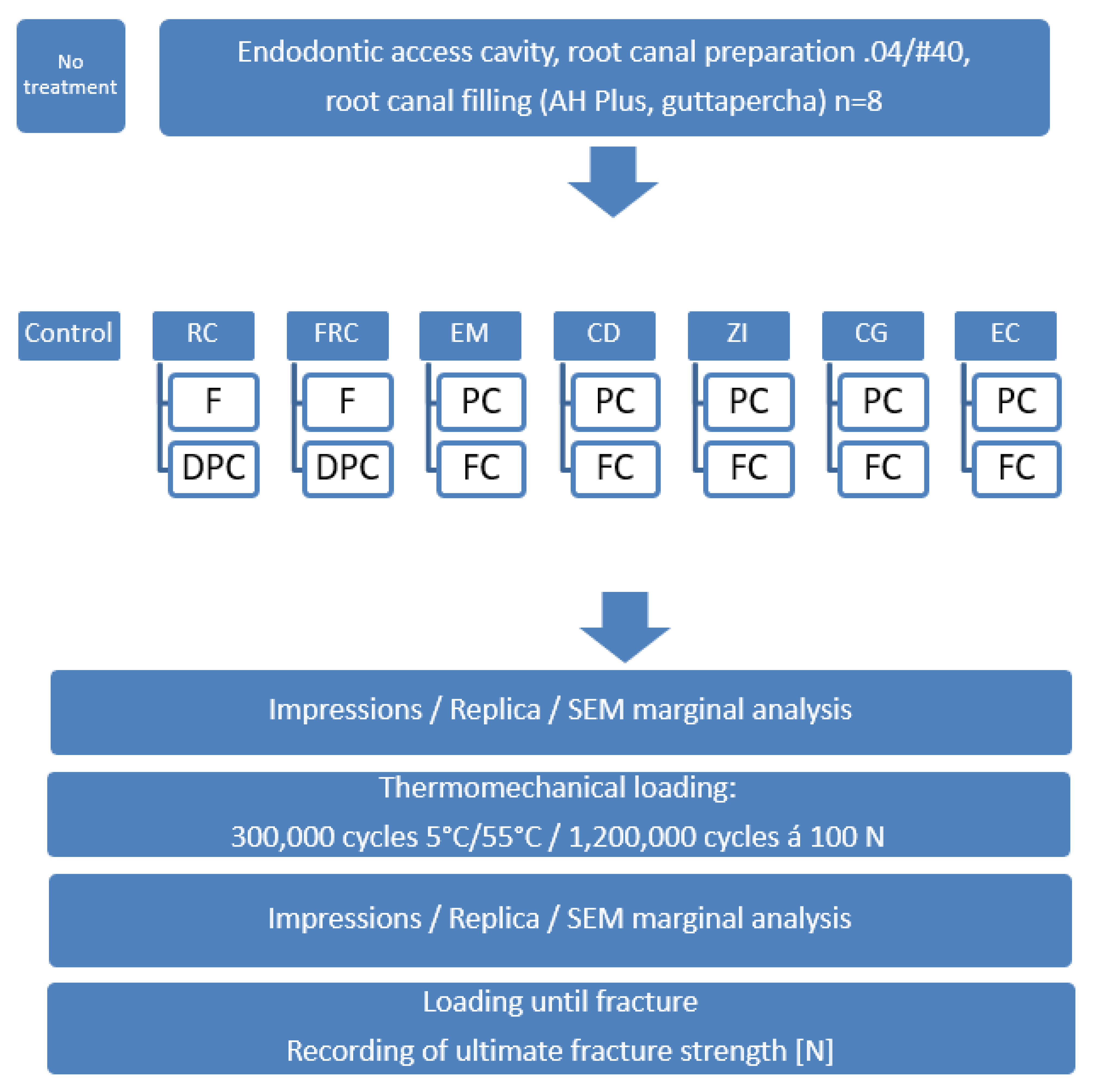
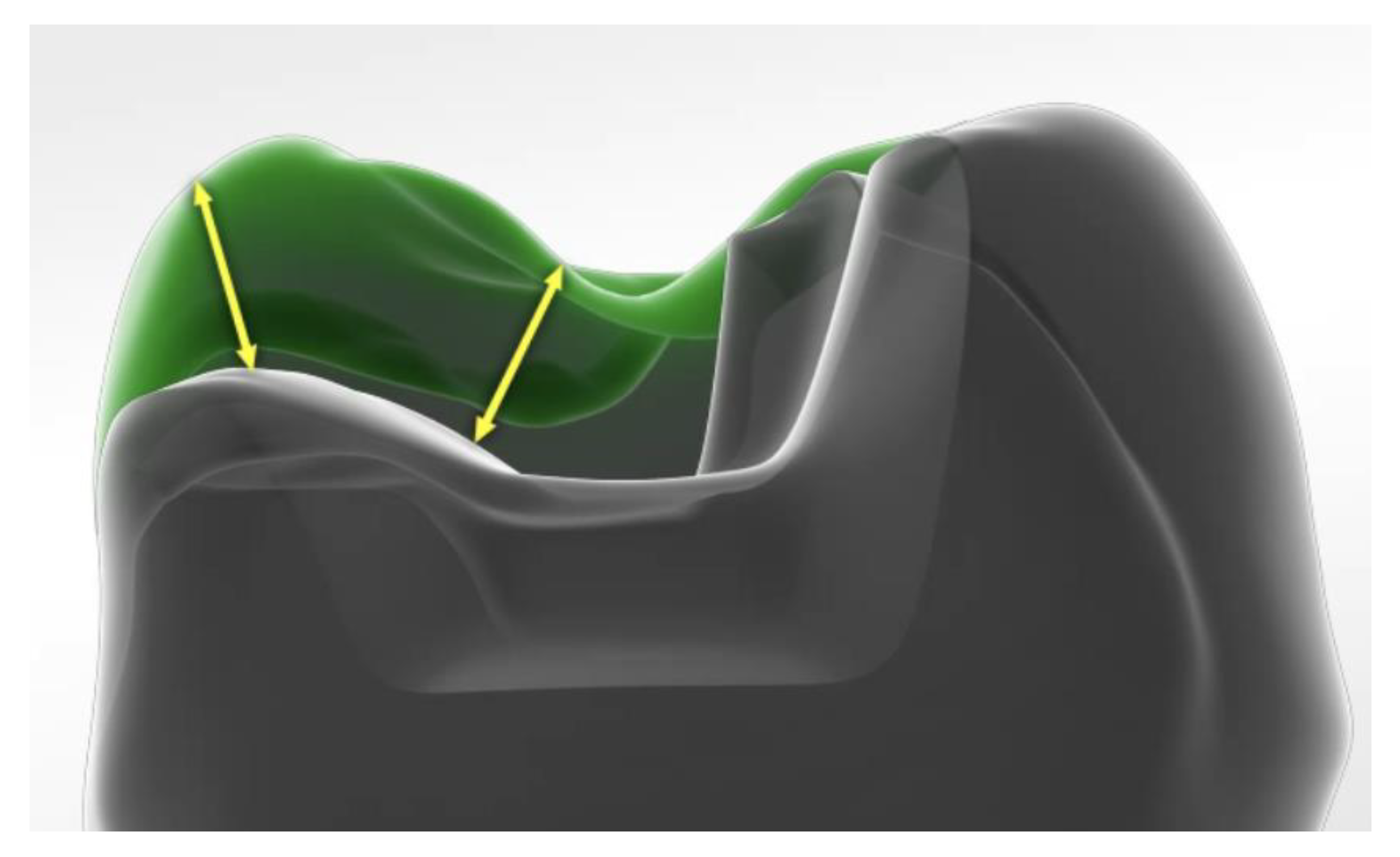
2. Methods and Materials
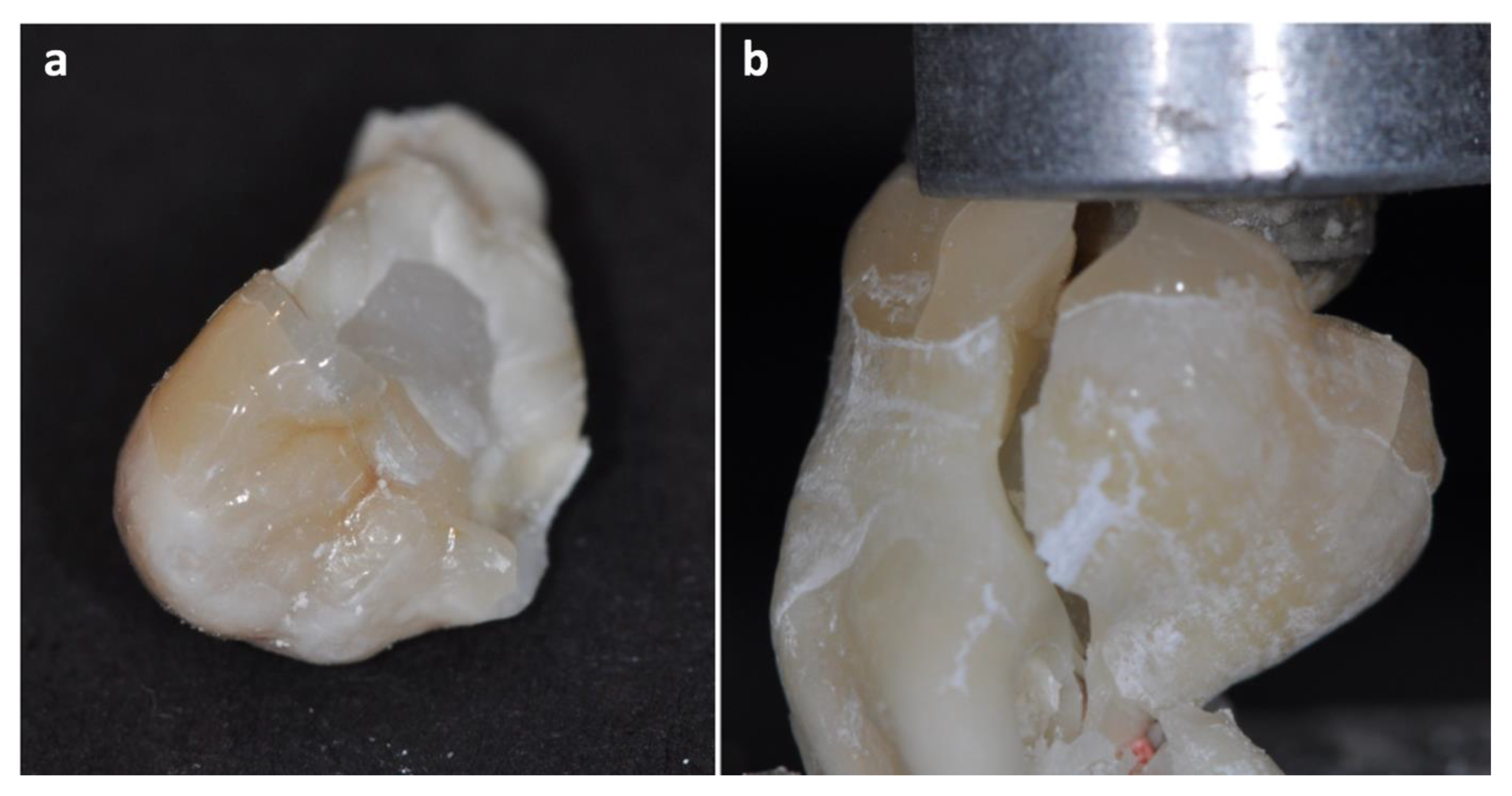
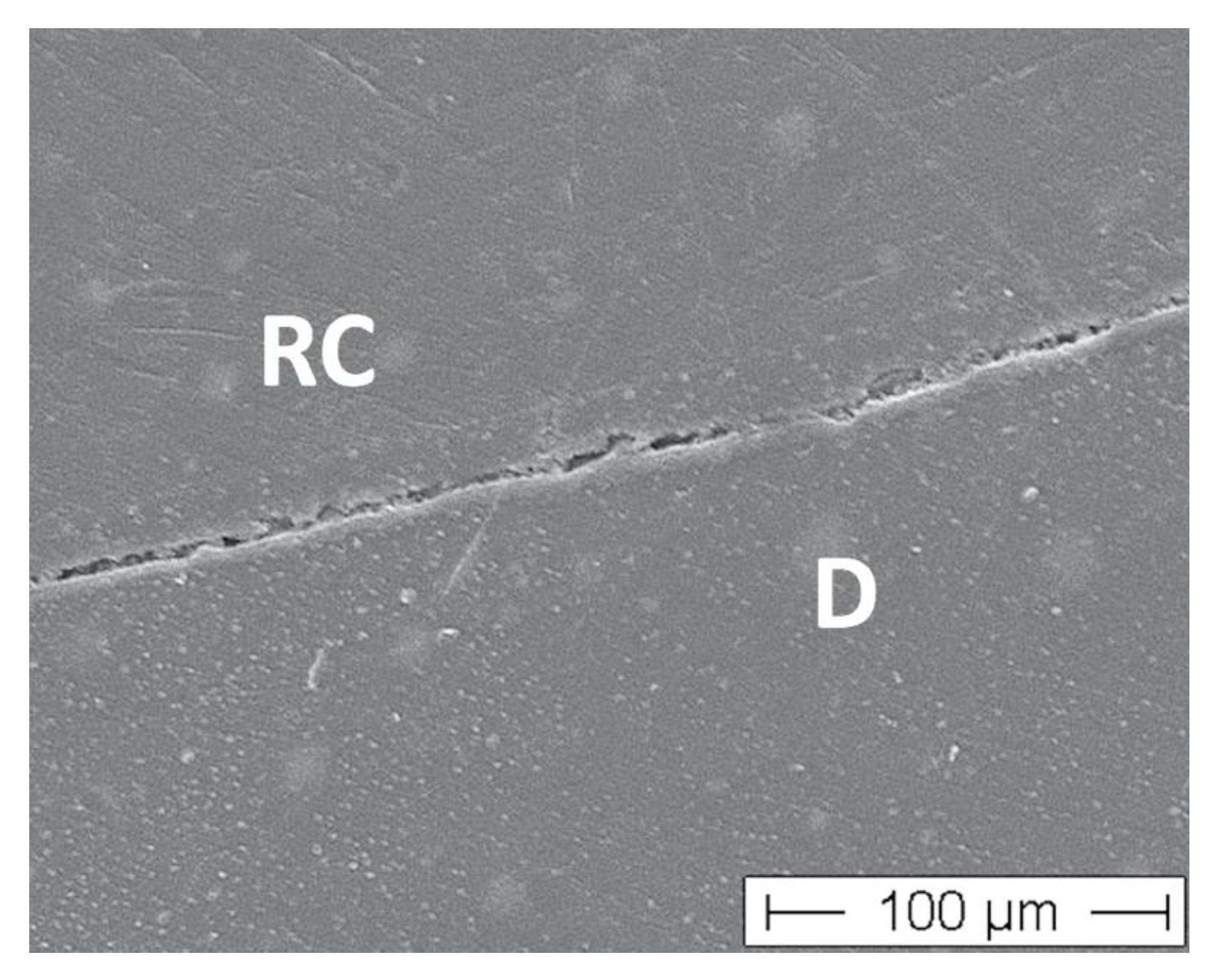
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Opdam, N.J.; Bronkhorst, E.M.; Loomans, B.A.; Huysmans, M.C. 12-year Survival of Composite vs. Amalgam Restorations. J. Dent. Res. 2010, 89, 1063–1067. [Google Scholar] [CrossRef] [PubMed]
- Opdam, N.J.; van de Sande, F.H.; Bronkhorst, E.; Cenci, M.S.; Bottenberg, P.; Pallesen, U.; Gaengler, P.; Lindberg, A.; Huysmans, M.C.; van Dijken, J.W. Longevity of posterior composite restorations: A systematic review and meta-analysis. J. Dent. Res. 2014, 93, 943–949. [Google Scholar] [CrossRef]
- Van Dijken, J.W.; Pallesen, U. A randomized 10-year prospective follow-up of Class II nanohybrid and conventional hybrid resin composite restorations. J. Adhes Dent. 2014, 16, 585–592. [Google Scholar] [PubMed]
- Da Rosa Rodolpho, P.A.; Donassollo, T.A.; Cenci, M.S.; Loguercio, A.D.; Moraes, R.R.; Bronkhorst, E.M.; Opdam, N.J.; Demarco, F.F. 22-Year clinical evaluation of the performance of two posterior composites with different filler characteristics. Dent. Mater. 2011, 27, 955–963. [Google Scholar] [CrossRef] [PubMed]
- Dammaschke, T.; Nykiel, K.; Sagheri, D.; Schäfer, E. Influence of coronal restorations on the fracture resistance of root canal-treated premolar and molar teeth: A retrospective study. Aust. Endod. J. 2013, 39, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Dietschi, D.; Duc, O.; Krejci, I.; Sadan, A. Biomechanical considerations for the restoration of endodontically treated teeth: A systematic review of the literature—Part 1. Composition and micro- and macrostructure alterations. Quintessence Int. 2007, 38, 733–743. [Google Scholar]
- Dietschi, D.; Duc, O.; Krejci, I.; Sadan, A. Biomechanical considerations for the restoration of endodontically treated teeth: A systematic review of the literature, Part II (Evaluation of fatigue behavior, interfaces, and in vivo studies). Quintessence Int. 2008, 39, 117–129. [Google Scholar]
- Eliyas, S.; Jalili, J.; Martin, N. Restoration of the root canal treated tooth. Br. Dent. J. 2015, 218, 53–62. [Google Scholar] [CrossRef]
- Fuss, Z.; Lustig, J.; Tamse, A. Prevalence of vertical root fractures in extracted endodontically treated teeth. Int. Endod. J. 1999, 32, 283–286. [Google Scholar] [CrossRef] [PubMed]
- Gutmann, J.L. The dentin-root complex: Anatomic and biologic considerations in restoring endodontically treated teeth. J. Prosthet. Dent. 1992, 67, 458–467. [Google Scholar] [CrossRef]
- Borén, D.L.; Jonasson, P.; Kvist, T. Long-term Survival of Endodontically Treated Teeth at a Public Dental Specialist Clinic. J. Endod. 2015, 41, 176–181. [Google Scholar] [CrossRef] [PubMed]
- Mannocci, F. Research that matters: Restoration of endodontically treated teeth. Int. Endod. J. 2013, 46, 389–390. [Google Scholar] [CrossRef]
- Mannocci, F.; Cowie, J. Restoration of endodontically treated teeth. Br. Dent. J. 2014, 216, 341–346. [Google Scholar] [CrossRef]
- Touré, B.; Faye, B.; Kane, A.W.; Lo, C.M.; Niang, B.; Boucher, Y. Analysis of Reasons for Extraction of Endodontically Treated Teeth: A Prospective Study. J. Endod. 2011, 37, 1512–1515. [Google Scholar] [CrossRef] [PubMed]
- Nagasiri, R.; Chitmongkolsuk, S. Long-term survival of endodontically treated molars without crown coverage: A retrospective cohort study. J. Prosthet. Dent. 2005, 93, 164–170. [Google Scholar] [CrossRef]
- Schneider, B.-J.; Freitag-Wolf, S.; Kern, M. Tactile sensitivity of vital and endodontically treated teeth. J. Dent. 2014, 42, 1422–1427. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seow, L.L.; Toh, C.G.; Wilson, N.H. Strain measurements and fracture resistance of endodontically treated premolars restored with all-ceramic restorations. J. Dent. 2015, 43, 126–132. [Google Scholar] [CrossRef]
- Yu, W.; Guo, K.; Zhang, B.; Weng, W. Fracture resistance of endodontically treated premolars restored with lithium disilicate CAD/CAM crowns or onlays and luted with two luting agents. Dent. Mater. J. 2014, 33, 349–354. [Google Scholar] [CrossRef] [Green Version]
- Zamin, C.; Silva-Sousa, Y.T.C.; Souza-Gabriel, A.E.; Messias, D.F.; Sousa-Neto, M.D. Fracture susceptibility of endodontically treated teeth. Dent. Traumatol. 2012, 28, 282–286. [Google Scholar] [CrossRef] [PubMed]
- Frankenberger, R.; Zeilinger, I.; Krech, M.; Mörig, G.; Naumann, M.; Braun, A.; Krämer, N.; Roggendorf, M.J. Stability of endodontically treated teeth with differently invasive restorations: Adhesive vs. non-adhesive cusp stabilization. Dent. Mater. 2015, 31, 1312–1320. [Google Scholar] [CrossRef]
- Einhorn, M.; DuVall, N.; Wajdowicz, M.; Brewster, J.; Roberts, H. Preparation Ferrule Design Effect on Endocrown Failure Resistance. J. Prosthodont. 2019, 28, e237–e242. [Google Scholar] [CrossRef] [Green Version]
- Kassis, C.; Khoury, P.; Mehanna, C.Z.; Baba, N.Z.; Chebel, F.B.; Daou, M.; Hardan, L. Effect of Inlays, Onlays and Endocrown Cavity Design Preparation on Fracture Resistance and Fracture Mode of Endodontically Treated Teeth: An In Vitro Study. J. Prosthodont. 2021, 30, 625–631. [Google Scholar] [CrossRef]
- Papalexopoulos, D.; Samartzi, T.-K.; Sarafianou, A. A Thorough Analysis of the Endocrown Restoration: A Literature Review. J. Contemp. Dent. Pract. 2021, 22, 422–426. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.; He, Y.; Ruan, W.; Ling, Z.; Zheng, C.; Gai, Y.; Yan, W. Biomechanical behavior of endocrown restorations with different CAD-CAM materials: A 3D finite element and in vitro analysis. J. Prosthet. Dent. 2021, 125, 890–899. [Google Scholar] [CrossRef] [PubMed]
- Sedrez-Porto, J.A.; Rosa, W.L.; da Silva, A.F.; Münchow, E.A.; Pereira-Cenci, T. Endocrown restorations: A systematic review and meta-analysis. J. Dent. 2016, 52, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Tribst, J.P.M.; Lo Giudice, R.; Dos Santos, A.F.C.; Borges, A.L.S.; Silva-Concílio, L.R.; Amaral, M.; Lo Giudice, G. Lithium Disilicate Ceramic Endocrown Biomechanical Response According to Different Pulp Chamber Extension Angles and Filling Materials. Materials 2021, 14, 1307. [Google Scholar] [CrossRef]
- Frankenberger, R.; Kramer, N.; Lohbauer, U.; Nikolaenko, S.A.; Reich, S.M. Marginal integrity: Is the clinical performance of bonded restorations predictable in vitro? J. Adhes Dent. 2007, 9 (Suppl. 1), 107–116. [Google Scholar]
- Setzer, F.C.; Boyer, K.R.; Jeppson, J.R.; Karabucak, B.; Kim, S. Long-term prognosis of endodontically treated teeth: A retrospective analysis of preoperative factors in molars. J. Endod. 2011, 37, 21–25. [Google Scholar] [CrossRef]
- Skupien, J.A.; Opdam, N.; Winnen, R.; Bronkhorst, E.; Kreulen, C.; Pereira-Cenci, T.; Huysmans, M.C. A practice-based study on the survival of restored endodontically treated teeth. J. Endod. 2013, 39, 1335–1340. [Google Scholar] [CrossRef]
- Carvalho, M.A.; Lazari, P.C.; Gresnigt, M.; Del Bel Cury, A.A.; Magne, P. Current options concerning the endodontically-treated teeth restoration with the adhesive approach. Braz. Oral Res. 2018, 32, e74. [Google Scholar] [CrossRef] [Green Version]
- Scotti, N.; Michelotto Tempesta, R.; Pasqualini, D.; Baldi, A.; Vergano, E.A.; Baldissara, P.; Alovisi, M.; Comba, A. 3D Interfacial Gap and Fracture Resistance of Endodontically Treated Premolars Restored with Fiber-reinforced Composites. J. Adhes Dent. 2020, 22, 215–224. [Google Scholar] [CrossRef] [PubMed]
- Eapen, A.M.; Amirtharaj, L.V.; Sanjeev, K.; Mahalaxmi, S. Fracture Resistance of Endodontically Treated Teeth Restored with 2 Different Fiber-reinforced Composite and 2 Conventional Composite Resin Core Buildup Materials: An in Vitro Study. J. Endod. 2017, 43, 1499–1504. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.; Shilpa-Jain, D.P.; Velmurugan, N.; Sooriaprakas, C.; Krithikadatta, J. Performance of fibre reinforced composite as a post-endodontic restoration on different endodontic cavity designs- an in-vitro study. J. Mech. Behav. Biomed. Mater. 2020, 104, 103650. [Google Scholar] [CrossRef] [PubMed]
| Restorative Material | Classification | Composition (%wt) | Manufacturer |
|---|---|---|---|
| Tetric EvoCeram Bulk Fill | Nanohybrid resin composite | Dimethacrylate, prepolymer, Barium glass, Ytterbiumtrifluoride, mixed oxides, initiators, stabilizators | Ivoclar Vivadent, Schaan, Principality of Liechtenstein |
| everX flow posterior | Fiber-reinforced bulk-fill composite | (1-Methylethyliden) bis [4,1-phenyleneoxy (2-hydroxy-3,1- propanediyl)] bismethacrylate, 2,2′-Ethylenedioxydiethyldimethacrylat, Diphenyl(2,4,6-trimethylbenzoyl) phosphinoxid, 6-T ert-butyl-2,4-xylenol 0.2%, short glass fibers, barium glass | GC Germany, Bad Homburg, Germany |
| Essentia Universal | Fine hybrid resin composite | 7,7,9(or 7,9,9)-trimethyl-4,13-dioxo-3,14-dioxa-5,12-diazahexadecane-1,16-diyl bismethacrylate, Ytterbium trifluoride, (octahydro-4,7-methano-1H-indenediyl) bis (methylene) bismethacrylate, Esterification products of 4,4′-isopropylidenediphenol, ethoxylated and 2- methylprop-2-enoic acid, 2-(2H-benzotriazol-2-yl)-p-cresol, glass fillers | |
| e.max CAD | Lithium disilicate ceramic | SiO2, Li2O, K2O, P2O5, ZrO2, ZnO, ZnO, Al2O3, MgO | Ivoclar Vivadent, Schaan, Principality of Liechtenstein |
| Celtra Duo | Zirconia-reinforced lithium silicate ceramic | Lithium silicate with 10% ZrO2 | Dentsply Sirona, Konstanz, Germany |
| Cercon ht | Zirconia | zirconium oxide, yttrium oxide, hafnium oxide, Aluminum oxide, Silicon oxide | Dentsply Sirona, Konstanz, Germany |
| Degunorm | Cast gold | 73.8% Au, 9% Pt, 9.2% Ag, 4.4% Cu, 2% Zn, 1.5% In, 0.1% Ir | Degudent, Hanau, Germany |
| Luting Material | |||
| G-Premio Bond | 2-step universal adhesive | Etchant: 36% phosphoric acid Universal adhesive: 10-MDP, 4-META, 10-MDTP, methacrylate acid ester, distilled water, acetone, initiators fine powdered silica | GC Germany, Bad Homburg, Germany |
| AdheSE Universal | 2-step universal adhesive | Etchant: 36% phosphoric acid Universal adhesive: 2-hydroxyethyl methacrylate, Bis-GMA, Ethanol, 1,10-decandiol dimethacrylate, methacrylated phosphoric acid ester, campherquinone, 2-dimethylaminoethyl mathacrylate | Ivoclar Vivadent, Schaan, Principality of Liechtenstein |
| Variolink Esthetic | Luting resin composite | Base: Bis-GMA, UDMA, TEGDMA, fillers, ytterbium trifluoride, stabilizers, pigments Catalyst: Bis-GMA, UDMA, TEGDMA, fillers, ytterbium trifluoride, stabilizers, pigments, benzoyl peroxide | |
| RelyX Unicem 2 | Self-adhesive resin cement | Base: methacrylate monomers with phosphpric acid groups, methacrylate monomers, silanated fillers, initiator components, rheological additives Catalyst: methacrylate monomers, alkaline fillers, silanated fillers, initiator compoments, stabilizers, pigments, rheological additives | 3M Oral Healthcare, Seefeld, Germany |
| Ketac Cem | Luting glass ionomer cement | Powder: calcium FASG Liquid: copolymer of acrylic and maleic acid + water | |
| Group | Fracture Strength after TML in N ± SD | Gap-Free Margins Enamel Initial in %(SD) | Gap-Free Margins Enamel after TML in %(SD) | Gap-Free Margins Dentin Initial in %(SD) | Gap-Free Margins Dentin after TML in %(SD) |
|---|---|---|---|---|---|
| Control | 806 ± 190 B | n/a | n/a | 100 | n/a |
| RC-F | 382 ± 83 D | 100 | 82 (13) B | 100 | n/a |
| RC-DPC | 688 ± 186 C | 100 | 88 (9) A | 100 | n/a |
| FRC-F | 402 ± 110 D | 100 | 89 (10) A | 100 | n/a |
| FRC-DPC | 699 ± 178 C | 100 | 93 (9) A | 100 | n/a |
| EM-PC | 723 ± 188 C | 100 | 95 (7) A | 100 | n/a |
| EM-FC | 736 ± 160 C | n/a | n/a | 100 | 95 (5) A |
| CD-PC | 702 ± 167 C | 100 | 93 (9) A | 100 | n/a |
| CD-FC | 733 ± 152 C | n/a | n/a | 100 | 96 (4) A |
| ZI-PC | 702 ± 143 C | 100 | 76 (23) C* | 100 | n/a |
| ZI-FC | 921 ± 102 A | n/a | n/a | 100 | 94 (8) A |
| CG-PC | 934 ± 172 A | 100 | 90 (5) A | 100 | n/a |
| CG-FC | 956 ± 200 A | n/a | n/a | 100 | 93 (5) A |
| EC-PC | 689 ± 175 C | 100 | 88 (12) A | 100 | n/a |
| EC-FC | 734 ± 197 C | n/a | n/a | 100 | 94 (5) A |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Frankenberger, R.; Winter, J.; Dudek, M.-C.; Naumann, M.; Amend, S.; Braun, A.; Krämer, N.; Roggendorf, M.J. Post-Fatigue Fracture and Marginal Behavior of Endodontically Treated Teeth: Partial Crown vs. Full Crown vs. Endocrown vs. Fiber-Reinforced Resin Composite. Materials 2021, 14, 7733. https://doi.org/10.3390/ma14247733
Frankenberger R, Winter J, Dudek M-C, Naumann M, Amend S, Braun A, Krämer N, Roggendorf MJ. Post-Fatigue Fracture and Marginal Behavior of Endodontically Treated Teeth: Partial Crown vs. Full Crown vs. Endocrown vs. Fiber-Reinforced Resin Composite. Materials. 2021; 14(24):7733. https://doi.org/10.3390/ma14247733
Chicago/Turabian StyleFrankenberger, Roland, Julia Winter, Marie-Christine Dudek, Michael Naumann, Stefanie Amend, Andreas Braun, Norbert Krämer, and Matthias J. Roggendorf. 2021. "Post-Fatigue Fracture and Marginal Behavior of Endodontically Treated Teeth: Partial Crown vs. Full Crown vs. Endocrown vs. Fiber-Reinforced Resin Composite" Materials 14, no. 24: 7733. https://doi.org/10.3390/ma14247733
APA StyleFrankenberger, R., Winter, J., Dudek, M.-C., Naumann, M., Amend, S., Braun, A., Krämer, N., & Roggendorf, M. J. (2021). Post-Fatigue Fracture and Marginal Behavior of Endodontically Treated Teeth: Partial Crown vs. Full Crown vs. Endocrown vs. Fiber-Reinforced Resin Composite. Materials, 14(24), 7733. https://doi.org/10.3390/ma14247733








