Enhanced Combustion of Bituminous Coal and Semicoke Mixture by Ferric Oxide with Thermographic and Kinetic Analyses
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Materials
2.2. Thermogravimetric Analysis
2.3. Kinetic Analysis
3. Results
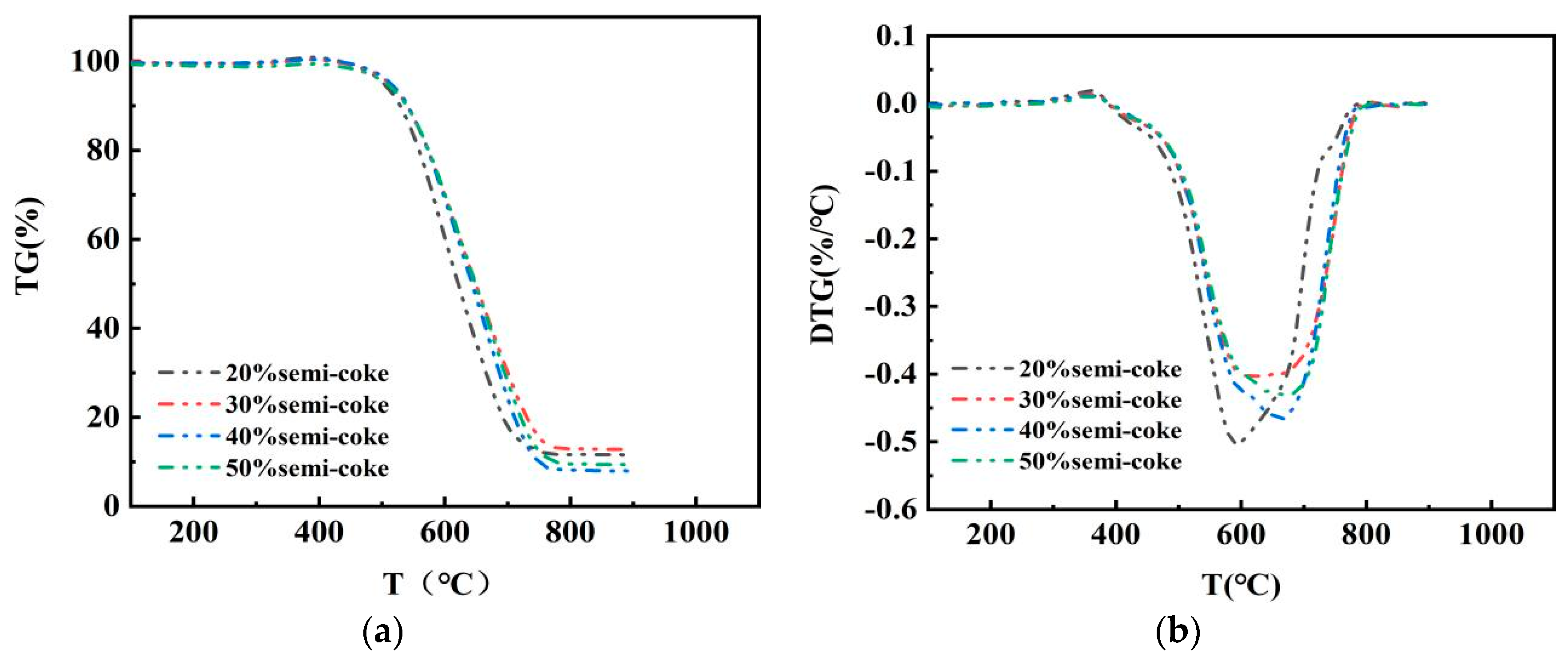
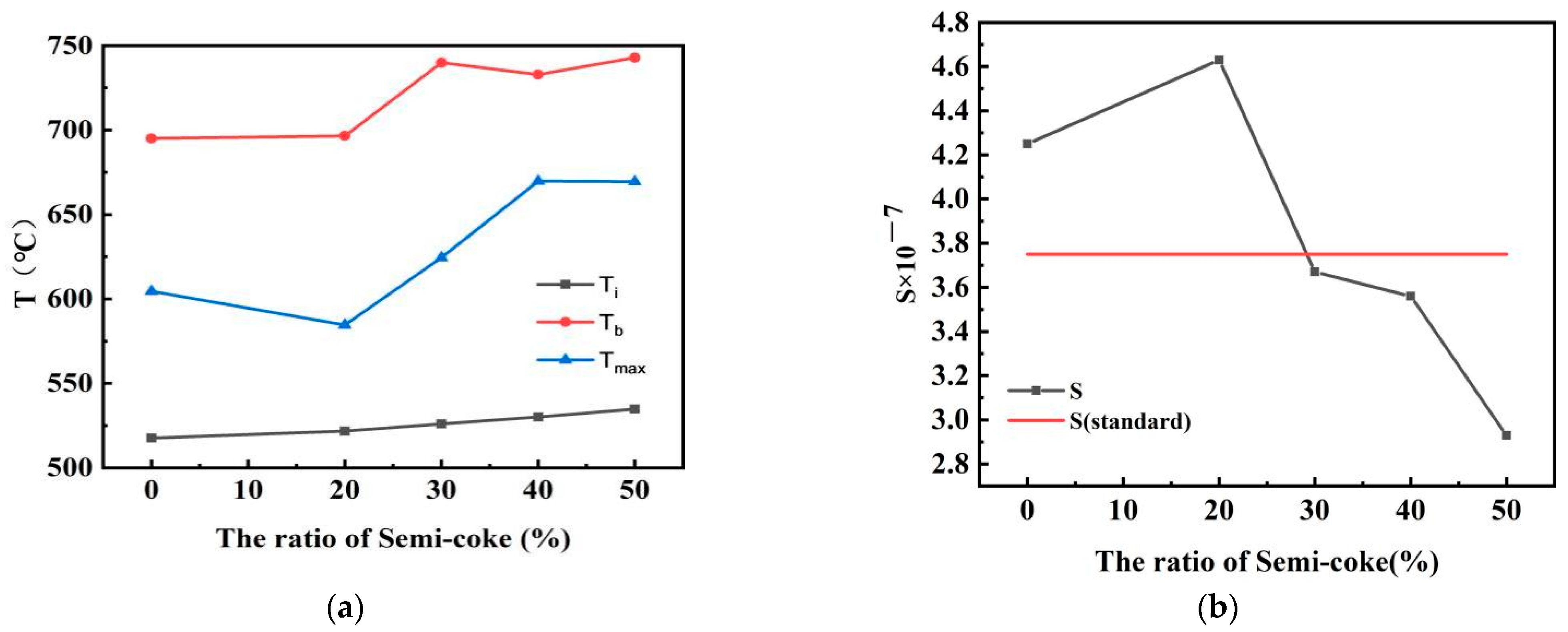
3.1. Thermal Analysis of Mixed Combustion of Semicoke and Bituminous Coal
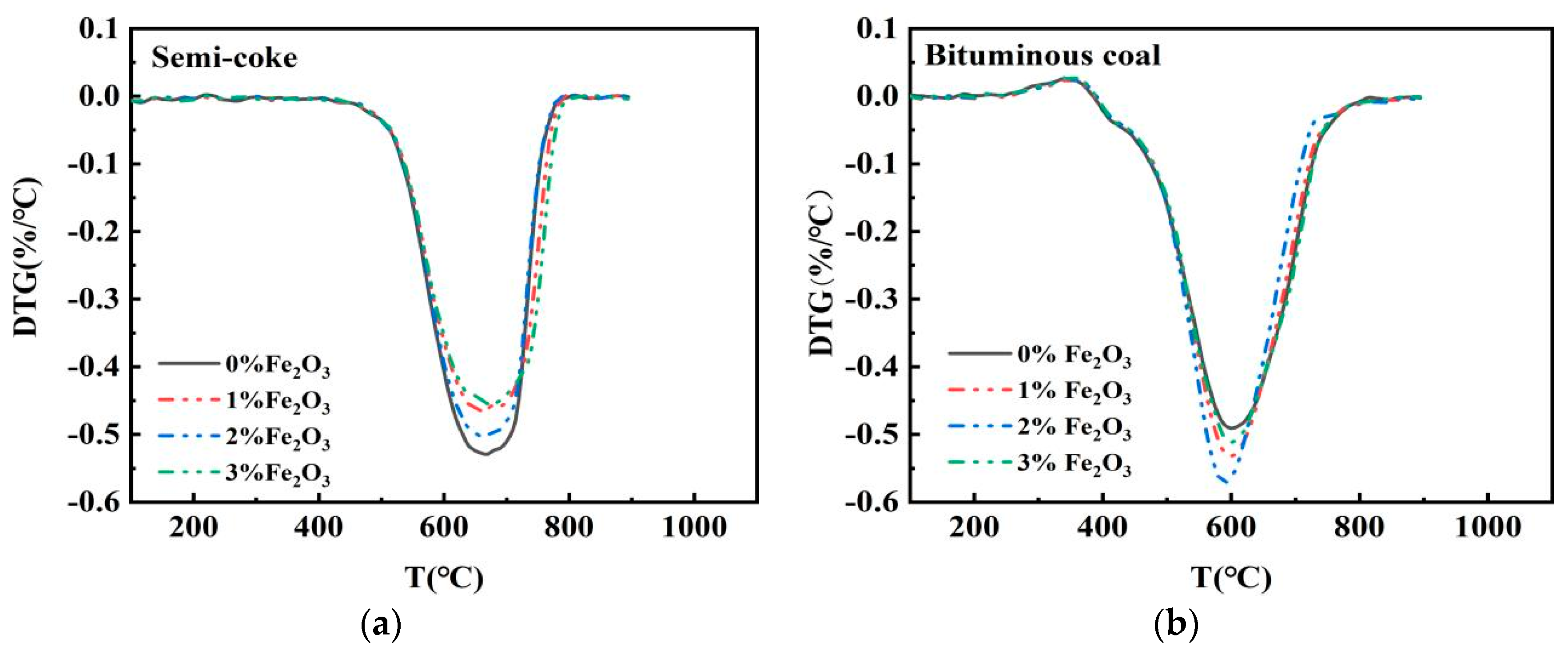
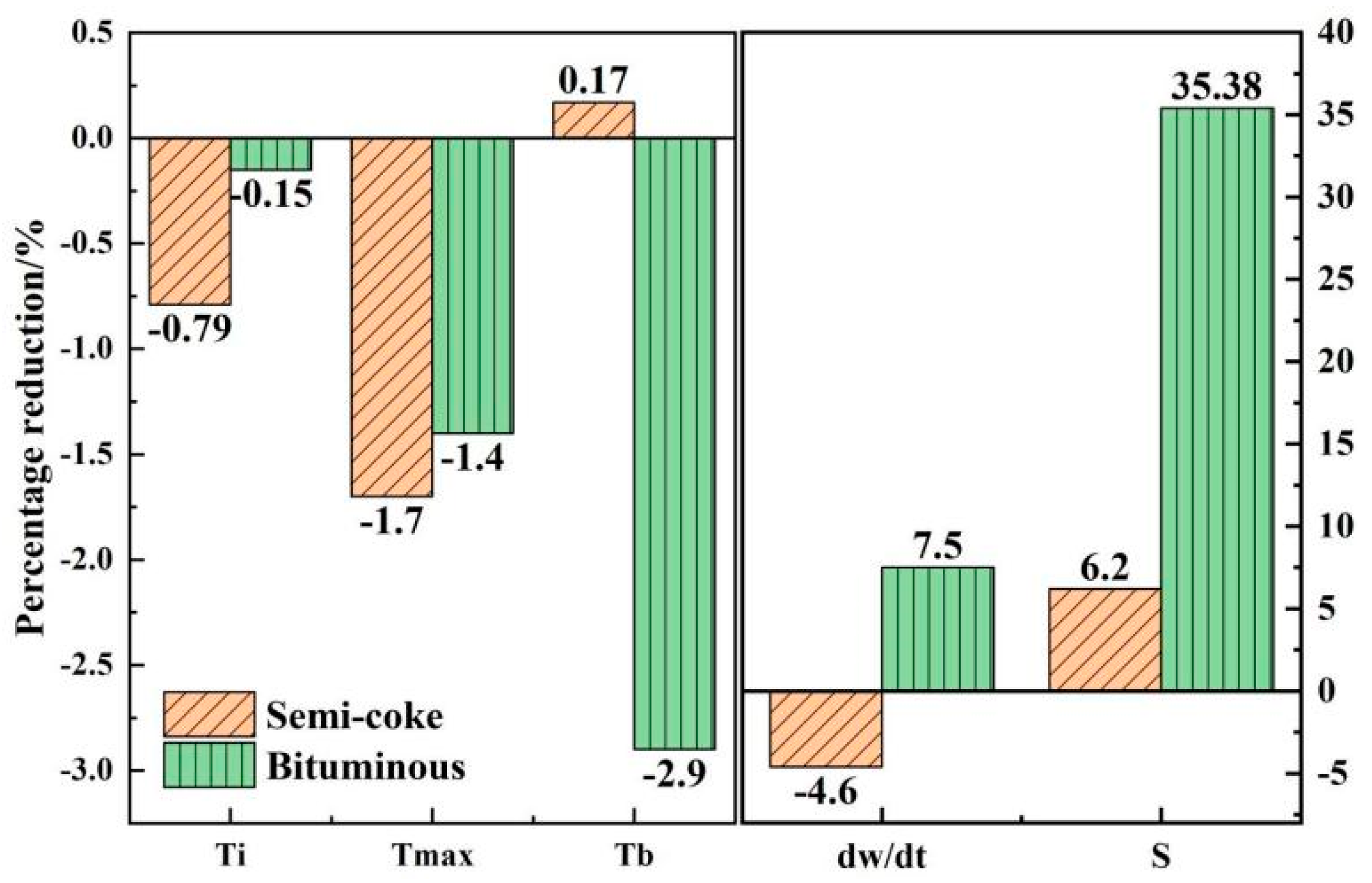
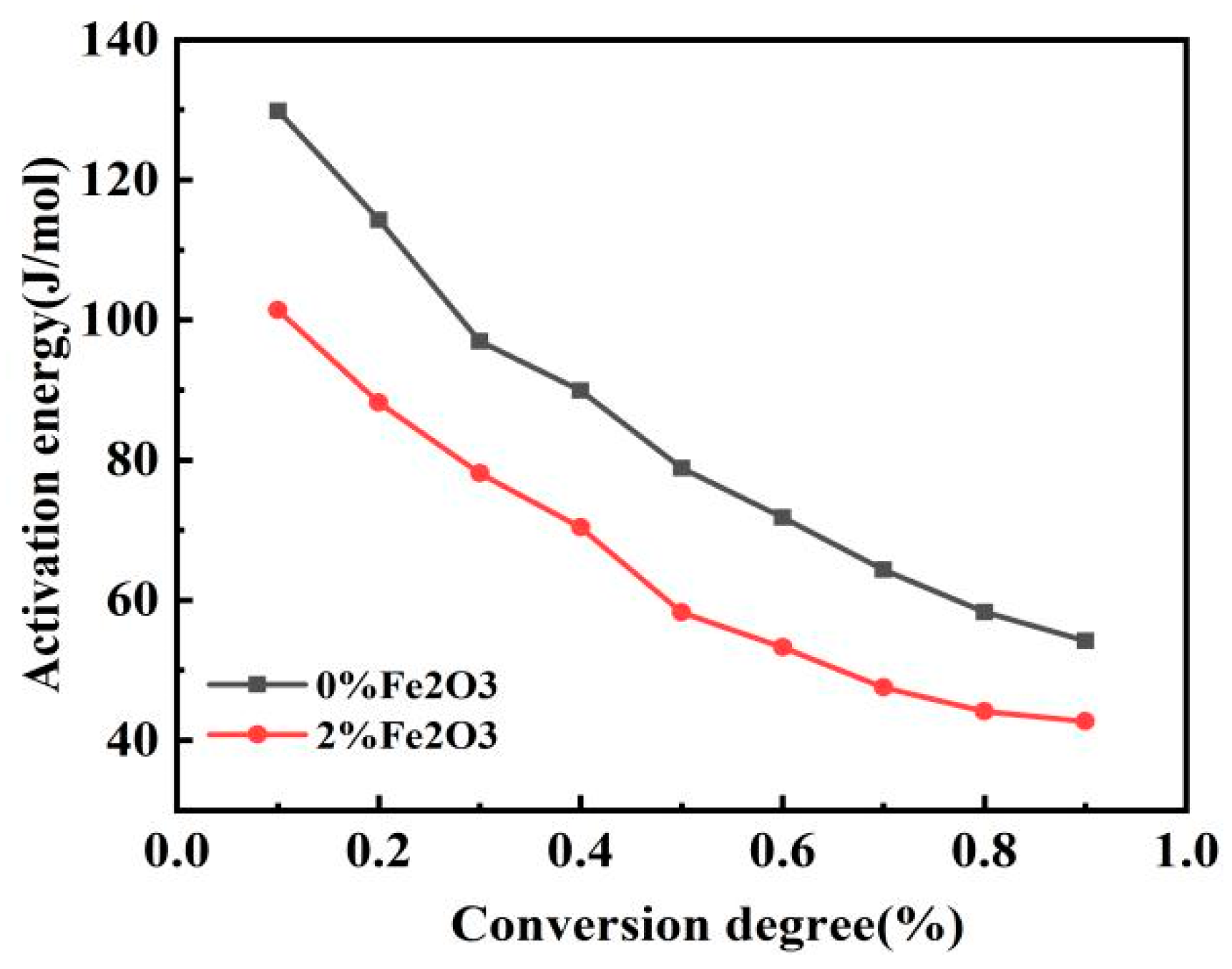
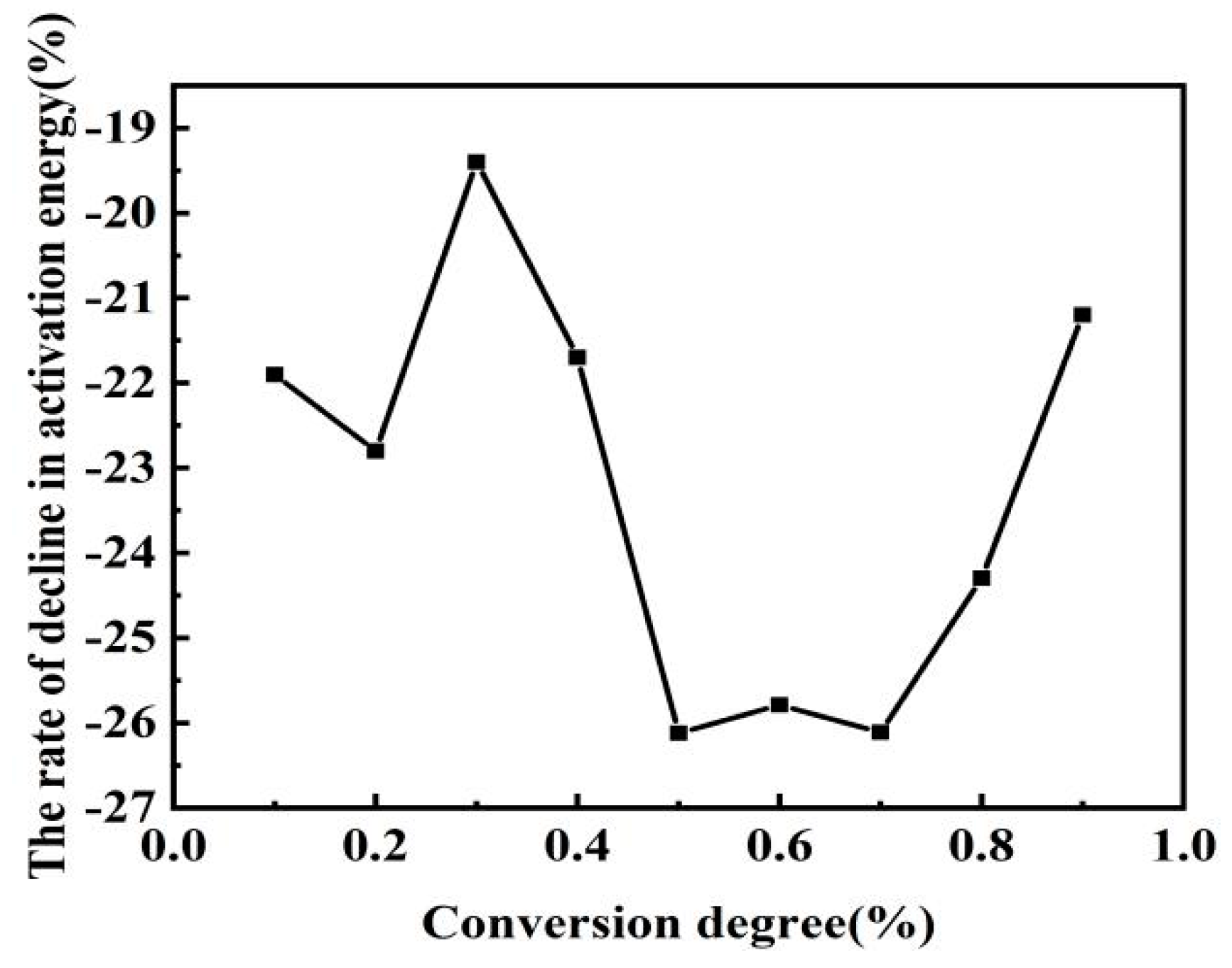
3.2. Effect of Fe2O3 on Combustion of Semicoke and Bituminous Coal
3.3. Study of Catalysts for Mixed Combustion of Bituminous Coal and Semicoke
3.4. Effect of Temperature Rise Rate on the Combustion of Pulverized Coal Mixture
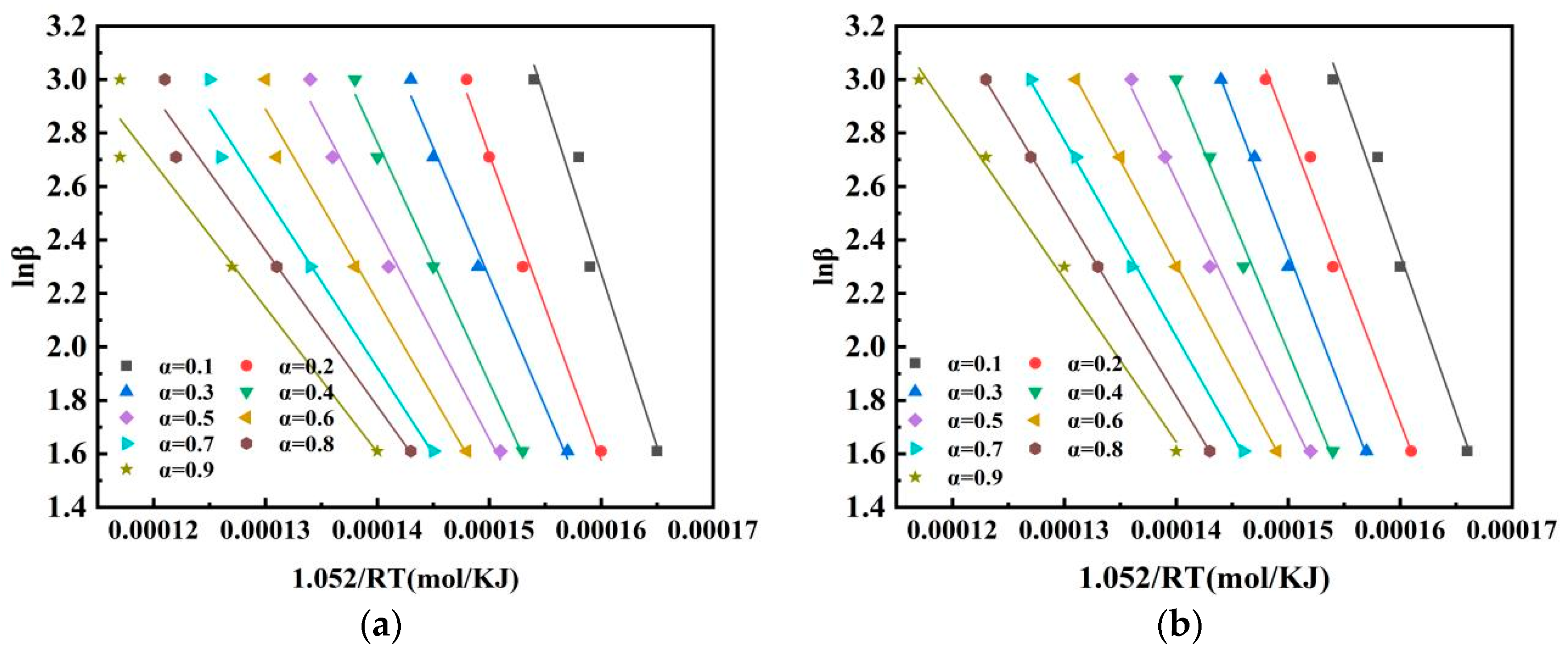
3.5. Kinetic Analysis of Pulverised Coal Combustion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| FCad | Fixed carbon |
| Mad | Moisture |
| Aad | Ash |
| Vad | Volatiles |
| Stad | Sulphur content |
| Ti | Ignition temperature |
| Tmax | Maximal burning rate temperature |
| Tb | Burnout temperature |
| ω | Mass percentage |
| S | Comprehensive combustion characteristic index |
| β | Heating rate |
| αi | Mass fraction of the sample when it catches fire |
| αb | Mass fraction of the sample when it burns out |
| t | Time |
| T | Temperature |
| A | Pre-exponential factor |
| E | Activation energy |
| R | Gas constant = 8.31447 J/molK |
| mi | Initial weights of the samples |
| mt | Weight at moment t |
| m∞ | Final weights of the samples |
| R2 | Correlation coefficient |
References
- Pan, S.; Chen, J.; Fan, F.; Li, P. Present situation analysis and prospect of low rank coal quality-based utilization conversion route. Clean Coal Technol. 2017, 23, 7–12. [Google Scholar]
- Yao, H.; He, B.; Ding, G.; Tong, W.; Kuang, Y. Thermogravimetric analyses of oxy-fuel co-combustion of semi-coke and bituminous coal. Appl. Therm. Eng. 2019, 156, 708–721. [Google Scholar] [CrossRef]
- Wu, Q.; Sun, C.; Zhu, Z.; Wang, Y.; Zhang, C. Effects of Boron Carbide on Coking Behavior and Chemical Structure of High Volatile Coking Coal during Carbonization. Materials 2021, 14, 302. [Google Scholar] [CrossRef]
- Liang, W.; Ning, X.; Wang, G.; Zhang, J.; Li, R.; Chang, W.; Wang, C. Influence mechanism and kinetic analysis of co-gasification of biomass char and semi-coke. Renew. Energy 2021, 163, 331–341. [Google Scholar] [CrossRef]
- Ouyang, Z.; Zhu, J.; Lu, Q. Experimental study on preheating and combustion characteristics of pulverized anthracite coal. Fuel 2013, 113, 122–127. [Google Scholar] [CrossRef]
- Yao, Y.; Zhu, J.; Lu, Q. Experimental Study on Nitrogen Transformation in Combustion of Pulverized Semi-coke Preheated in a Circulating Fluidized Bed. Energy Fuels 2015, 29, 3985–3991. [Google Scholar] [CrossRef]
- Wang, Q.; Zhao, W.; Liu, H.; Jia, C.; Li, C. Interactions and kinetic analysis of oil shale semi-coke with cornstalk during co-combustion. Appl. Energy 2011, 88, 2080–2087. [Google Scholar] [CrossRef]
- Yan, L.; Yue, G.; He, B. Thermodynamic analyses of a biomass-coal co-gasification power generation system. Bioresour. Technol. 2016, 205, 133–141. [Google Scholar] [CrossRef]
- Wang, G.; Zhang, J.; Shao, J.; Ren, S. Characterisation and model fitting kinetic analysis of coal/biomass co-combustion. Thermochim. Acta 2014, 591, 68–74. [Google Scholar] [CrossRef]
- Blesa, M.; Miranda, J.; Moliner, R.; Izquierdo, M.; Palacios, J. Low-temperature co-pyrolysis of a low-rank coal and biomass to preparesmokeless fuel briquettes. J. Anal. Appl. Pyrol. 2003, 70, 665–677. [Google Scholar] [CrossRef]
- Liu, Q.; Hu, H.; Zhou, Q.; Zhu, S.; Chen, G. Effect of inorganic matter on reactivity and kinetics of coal pyrolysis. Fuel 2004, 83, 713–718. [Google Scholar] [CrossRef]
- Fahmi, R.; Bridgwater, A.; Darvell, L.; Jones, J.; Yates, N.; Thain, S.; Donnison, I. The effect of alkali metals on combustion and pyrolysis of Lolium and Festuca grasses, switchgrass and willow. Fuel 2007, 86, 1560–1569. [Google Scholar] [CrossRef]
- Zhang, L.; Xu, S.; Zhao, W.; Shu, Q. Co-pyrolysis of biomass and coal in a free fall reactor. Fuel 2007, 86, 353–359. [Google Scholar] [CrossRef]
- Sonobe, T.; Worasuwannarak, N.; Pipatmanomai, S. Synergies in co-pyrolysis of Thai lignite and corncob. Fuel Process. Technol. 2008, 89, 1371–1378. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, G.; Xing, X.; Pang, Q.; Shao, J.; Ren, S. Combustion characteristics and kinetics of pulverized coal in Oxygen-Enriched environments. J. Iron Steel Res. 2013, 25, 9–14. [Google Scholar]
- Murphy, J.; Shaddix, C. Combustion kinetics of coal chars in oxygen-enriche denvironments. Combust. Flame 2006, 144, 710–729. [Google Scholar] [CrossRef]
- Sun, B.; Shi, X.; Huang, Z.; Wang, Q. Experimental investigation on combustion characteristics of oil shale semi-coke and bituminous coal blends. J. Northeast. Dianli Univ. 2012, 35, 476–480. [Google Scholar]
- Gopalakrishnan, R.; Bartholomew, C. Effect of CaO, high-temperature treatment, carbon structure, and coal rank on intrinsic char oxidation rates. Energy Fuels 1996, 10, 689–695. [Google Scholar] [CrossRef]
- Hu, Y.; Wang, Z.; Cheng, X.; Liu, M.; Ma, C. Effects of Catalysts on Combustion Characteristics and Kinetics of Coal-Char Blends. Asia Conf. Energy Environ. Eng. 2018, 133, 021023. [Google Scholar] [CrossRef]
- Ma, B.; Li, X.; Xu, L.; Wang, K.; Wang, X. Investigation on catalyzed combustion of high ash coal by thermogravimetric analysis. Thermochim. Acta 2006, 445, 19–22. [Google Scholar] [CrossRef]
- Gong, X.; Guo, Z.; Wang, Z. Reactivity of pulverized coals during combustion catalyzed by CeO2 and Fe2O3. Combust. Fuel 2010, 157, 351–356. [Google Scholar] [CrossRef]
- Jiang, P.; Meng, Y.; Parvez, A.; Dong, X.; Wu, T. Influence of co-processing of coal and oil shale on combustion characteristics, kinetics and ash fusion behaviour. Energy 2020, 216, 119–229. [Google Scholar] [CrossRef]
- Zhang, J.; Jia, X.; Wang, C.; Zhao, N.; Wang, P.; Che, D. Experimental investigation on combustion and NO formation characteristics of semi-coke and bituminous coal blends. Fuel 2019, 247, 87–96. [Google Scholar] [CrossRef]
- Zhou, J.; Zhang, H.; Lv, J. Study on combustion characteristics of a petroleum coke at different heating by using thermogravimetry. Coal Convers. 2006, 29, 39–43. [Google Scholar]
- Nie, Q.; Sun, S.; Li, Z.; Zhang, X.; Wu, S.; Qin, Y. Thermogravimetric analysis on the combustion characteristics of brown coal blends. J. Combust. Sci. Technol. 2001, 7, 72–76. [Google Scholar]
- Li, X.; Ma, B.; Xu, L.; Hu, Z.; Wang, X. Thermogravimetric analysis of the co-combustion of the blends with high ash coal and waste tyres. Thermochim. Acta 2006, 441, 79–83. [Google Scholar] [CrossRef]
- Wu, J.; Wang, B.; Cheng, F. Thermal and kinetic characteristics of combustion of coal sludge. J. Therm. Anal. Calorim. 2017, 129, 1899–1909. [Google Scholar] [CrossRef]
- Zhou, C. Mechanism of Intensified Combustion of PCI Coal by Catalysts and Its Fundamental Research for Application in Blast Furnace. Ph.D. Thesis, Chongqing University, Chongqing, China, 2014. [Google Scholar]
- Zheng, S.; Hu, Y.; Wang, Z.; Cheng, X. Experimental investigation on ignition and burnout characteristics of semi-coke and bituminous coal blends. J. Energy Inst. 2020, 93, 1373–1381. [Google Scholar] [CrossRef]
- Ozawa, T. A New Method of Analyzing Thermo-gravimetric Data. Bull. Chem. Soc. Jpn. 1965, 38, 1881–1886. [Google Scholar] [CrossRef] [Green Version]
- Flynn, J.; Wall, L. A quick, direct method for the determination of activation energy from thermogravi-metric data. J. Polym. Sci. 1966, 4, 323–328. [Google Scholar]
- Peng, Z.; Zhang, J.; Bi, C.; Ning, X.; Tan, P.; Wang, G. Effect of coke powder and semi-coke on combustion characteristics of blast furnace blended coal. Iron Steel 2019, 54, 10–18. [Google Scholar]
- Łączny, M.J.; Iwaszenko, S.; Smoliński, A. Process Kinetics of the Carbonation of Fly Ashes: A Research Study. Materials 2021, 14, 253. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhang, J.; Xu, R.; Wang, G.; Xu, T.; Tang, Q. Combustion characteristics and mechanism analysis of blended coal of semi coke and bituminous coal. China Metall. 2016, 26, 7–12, 30. [Google Scholar]
- Gong, X.; Guo, Z.; Wang, Z. Variation on anthracite coal combustion efficiency with CeO2 and Fe2O3 addition by Differential Thermal Analysis (DTA). Energy 2010, 35, 506–511. [Google Scholar] [CrossRef]
- Gong, Z.; Wen, W.; Zhao, Z.; Li, B. Combination of catalytic combustion and catalytic denitration on semi-coke with Fe2O3 and CeO2. Catal. Today 2018, 318, 59–65. [Google Scholar] [CrossRef]
- Zhu, T.; Zhang, S.; Huang, J.; Wang, Y. Effect of calcium oxide on pyrolysis of coal in a fluidized bed—ScienceDirect. Fuel Process. Technol. 2000, 64, 271–284. [Google Scholar]
- Wang, H.; Cang, D.; Wang, Y. Study on the effects of a new additive on coal combustion by TG-DTA. Metall. Energy 2008, 27, 32–34. [Google Scholar]

| Coal Type | Proximate Analysis (wt %) | ||||
|---|---|---|---|---|---|
| FCad | Mad | Aad | Vad | Stad | |
| Anthracite coal | 79.76 | 0.27 | 11.48 | 8.76 | 0.60 |
| Bituminous coal | 75.76 | 0.37 | 10.78 | 13.09 | 0.52 |
| Semicoke | 85.85 | 0.64 | 8.78 | 4.73 | 0.22 |
| Samples | Ti/°C | Tb/°C | Tmax/°C | (dw/dt)max/%·min−1 | S/×10−7 |
|---|---|---|---|---|---|
| 50% anthracite coal | 523.99 | 707.32 | 604.15 | 9.66 | 3.86 |
| Samples | Ti/°C | Tb/°C | Tmax/°C | (dw/dt)max/%·min−1 | S/×10−7 |
|---|---|---|---|---|---|
| 15% semicoke | 524.77 | 704.56 | 599.71 | 10.00 | 4.24 |
| 25% semicoke | 524.74 | 706.82 | 599.53 | 9.86 | 4.23 |
| Semicoke Ratio | Ti/°C | Tb/°C | Tmax/°C | (dm/dt)max/%·Min−1 | S/×10−7 |
|---|---|---|---|---|---|
| 30% | 524.82 | 712.46 | 594.28 | 9.6 | 4.09 |
| 35% | 524.65 | 709.19 | 624.5 | 9.52 | 3.95 |
| 40% | 521.73 | 711.83 | 589.62 | 9.02 | 3.52 |
| Conversion Rate | 0 wt% Fe2O3 | 2 wt% Fe2O3 | ||
|---|---|---|---|---|
| E | R2 | E | R2 | |
| 0.1 | 129.8 | 0.95822 | 101.4 | 0.96964 |
| 0.2 | 114.3 | 0.98655 | 88.1 | 0.97238 |
| 0.3 | 96.9 | 0.98757 | 78.1 | 0.99442 |
| 0.4 | 89.9 | 0.99106 | 70.4 | 0.98818 |
| 0.5 | 78.8 | 0.97982 | 58.2 | 0.99229 |
| 0.6 | 71.8 | 0.96669 | 53.2 | 0.99959 |
| 0.7 | 64.3 | 0.96475 | 47.5 | 0.99815 |
| 0.8 | 58.3 | 0.96298 | 44.1 | 0.99988 |
| 0.9 | 54.1 | 0.94195 | 42.6 | 0.99133 |
| Average | 84.24 | 0.971 | 64.84 | 0.98954 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lv, T.; Kou, L.; Hu, T.; Zhang, L.; Yang, L. Enhanced Combustion of Bituminous Coal and Semicoke Mixture by Ferric Oxide with Thermographic and Kinetic Analyses. Materials 2021, 14, 7696. https://doi.org/10.3390/ma14247696
Lv T, Kou L, Hu T, Zhang L, Yang L. Enhanced Combustion of Bituminous Coal and Semicoke Mixture by Ferric Oxide with Thermographic and Kinetic Analyses. Materials. 2021; 14(24):7696. https://doi.org/10.3390/ma14247696
Chicago/Turabian StyleLv, Tingting, Luyao Kou, Tu Hu, Libo Zhang, and Li Yang. 2021. "Enhanced Combustion of Bituminous Coal and Semicoke Mixture by Ferric Oxide with Thermographic and Kinetic Analyses" Materials 14, no. 24: 7696. https://doi.org/10.3390/ma14247696
APA StyleLv, T., Kou, L., Hu, T., Zhang, L., & Yang, L. (2021). Enhanced Combustion of Bituminous Coal and Semicoke Mixture by Ferric Oxide with Thermographic and Kinetic Analyses. Materials, 14(24), 7696. https://doi.org/10.3390/ma14247696







