Structural Adaptive, Self-Separating Material for Removing Ibuprofen from Waters and Sewage
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Techniques
2.2. Synthesis of β−CD−M
2.3. Determination of Grafted β−CD Able to Form Complex
2.4. Water Regain Analysis
2.5. Adsorption Procedure
2.5.1. Analytical Method of Pollutant Concentration Determination
2.5.2. Adsorption Kinetics in Batch Experiments
2.5.3. Adsorption Isotherms
2.5.4. Thermodynamic Analysis
2.5.5. Effects of the Dosage of the Adsorbent, pH, Humic Acid and Ionic Strength
2.5.6. Sewage Treatment
2.5.7. Material Regeneration
3. Results and Discussion
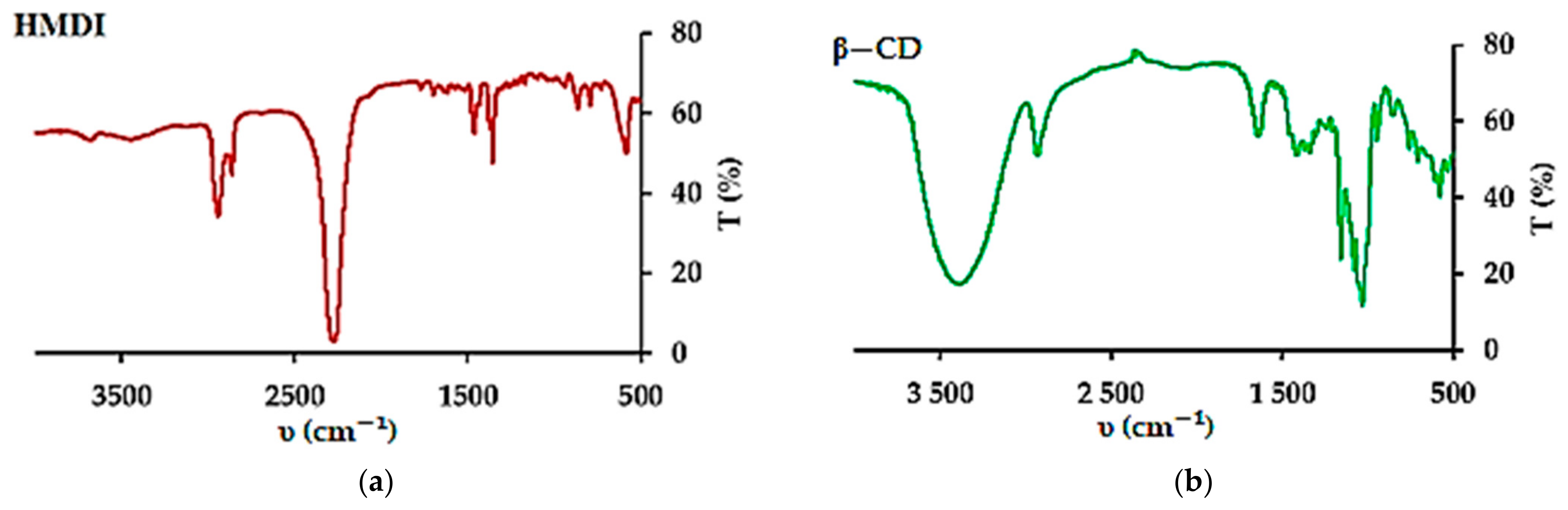
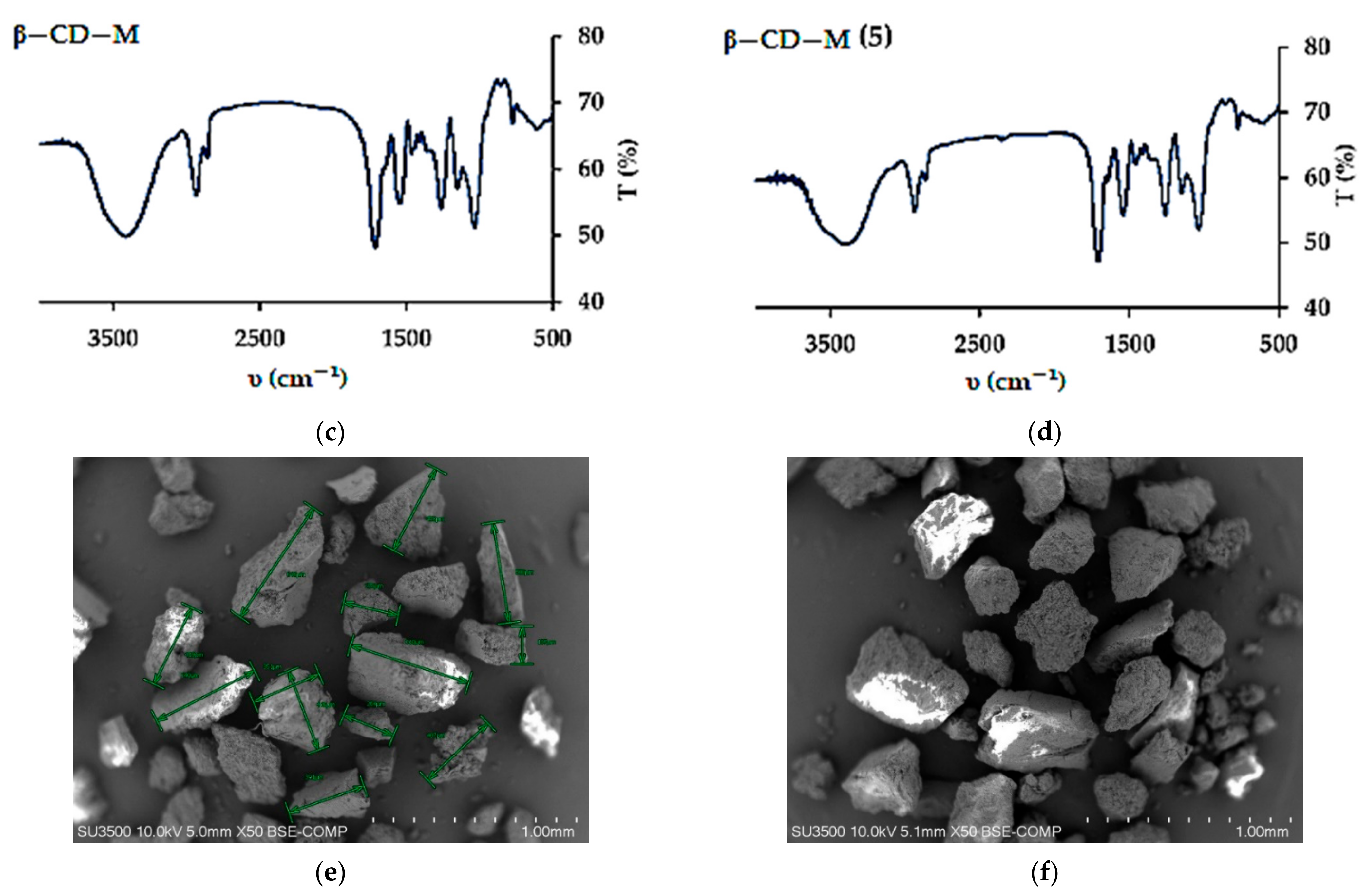
3.1. Adsorbents Properties
3.2. Adsorption Experiments
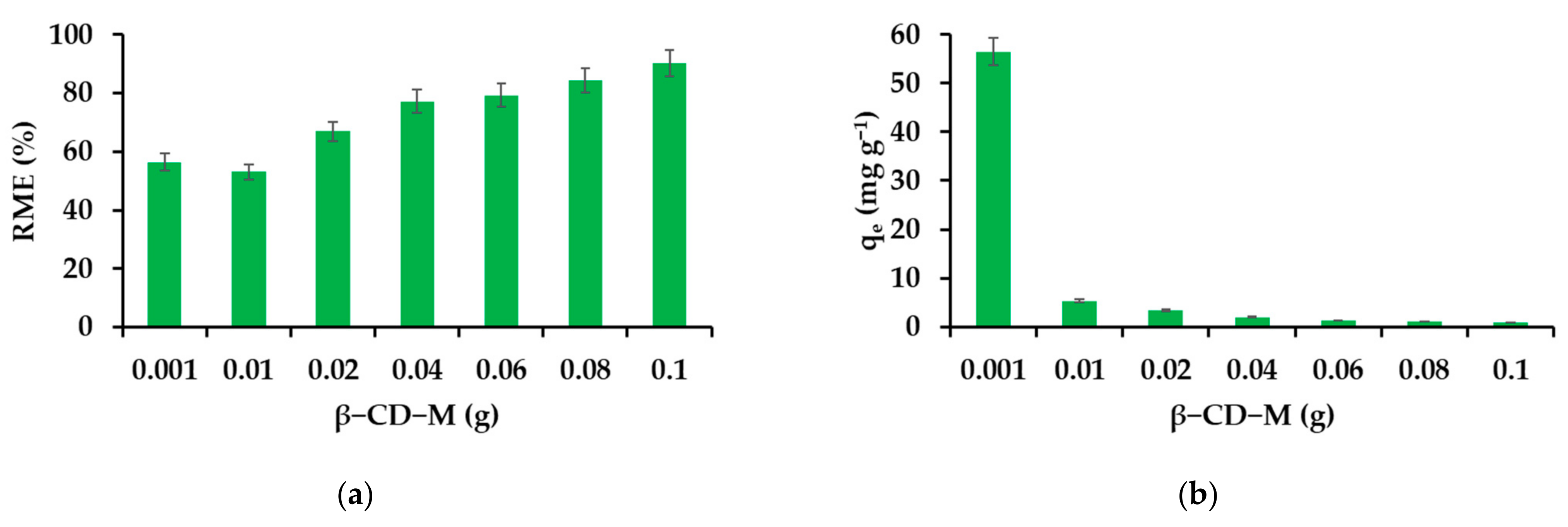
3.2.1. Effect of Adsorbent Mass on the Removal Efficiency
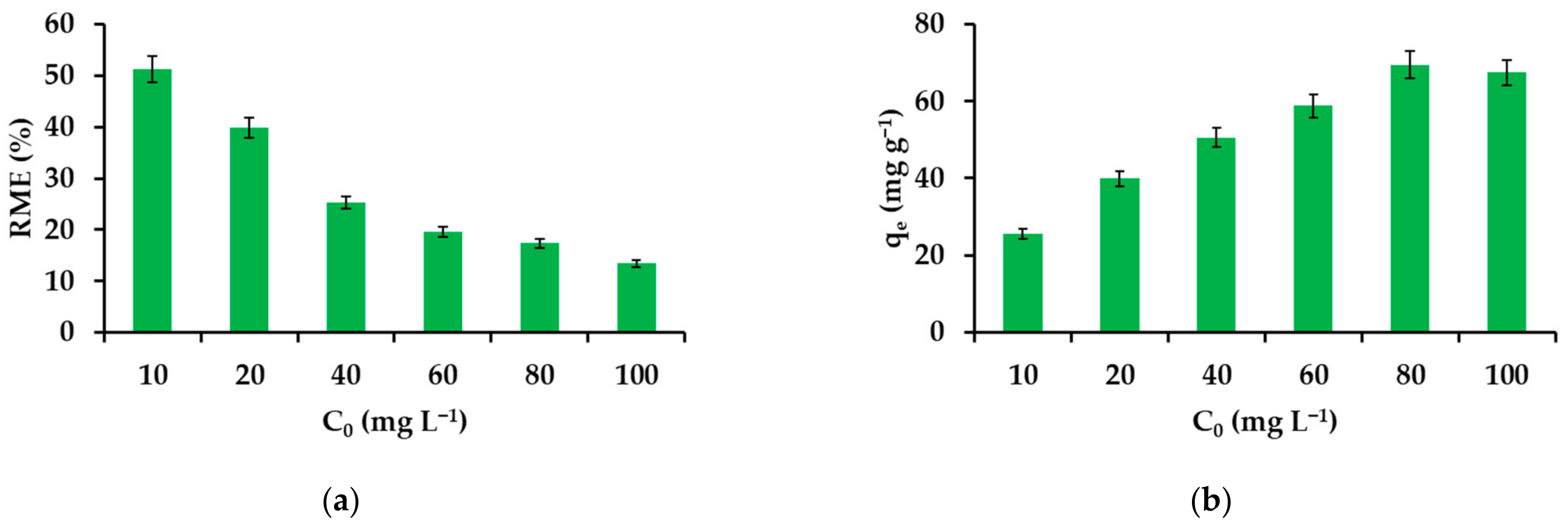
3.2.2. Effect of IBU Initial Concentration on the Removal Efficiency
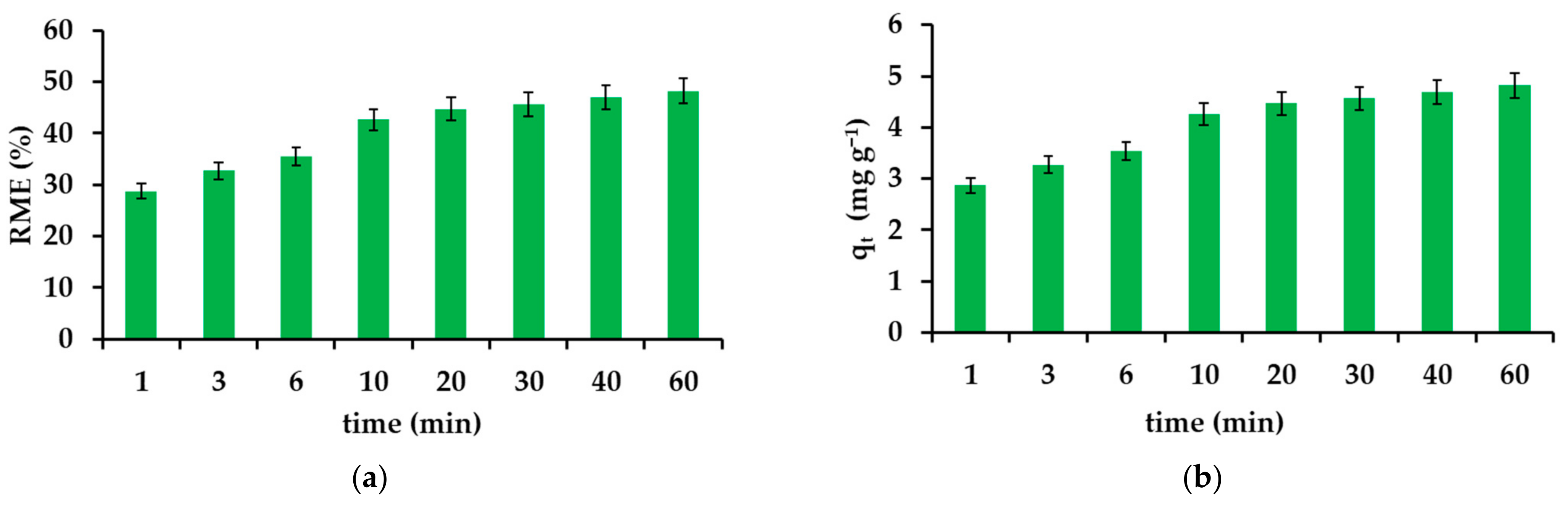
3.2.3. Effect of Contact Time in the Adsorption Experiments
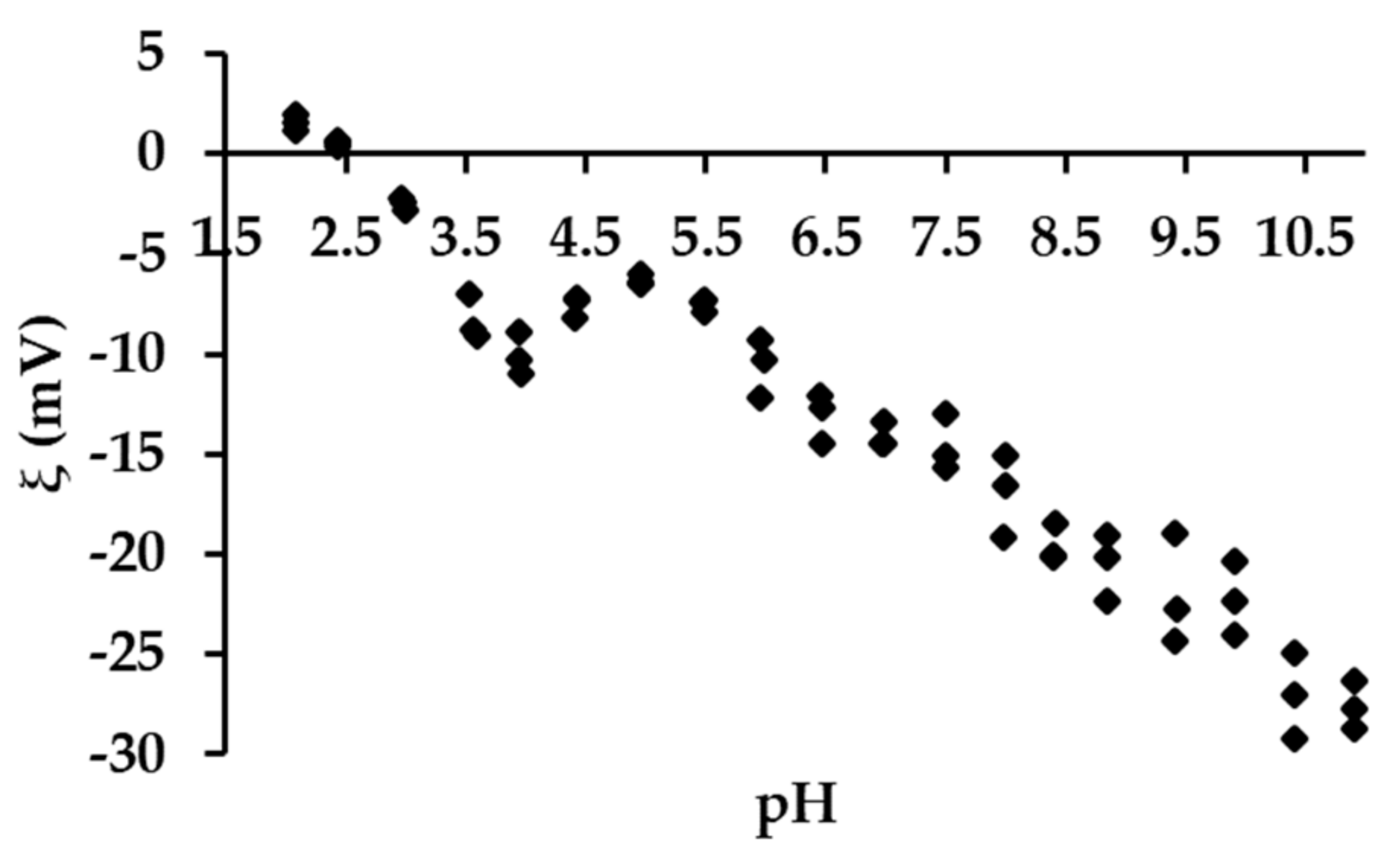
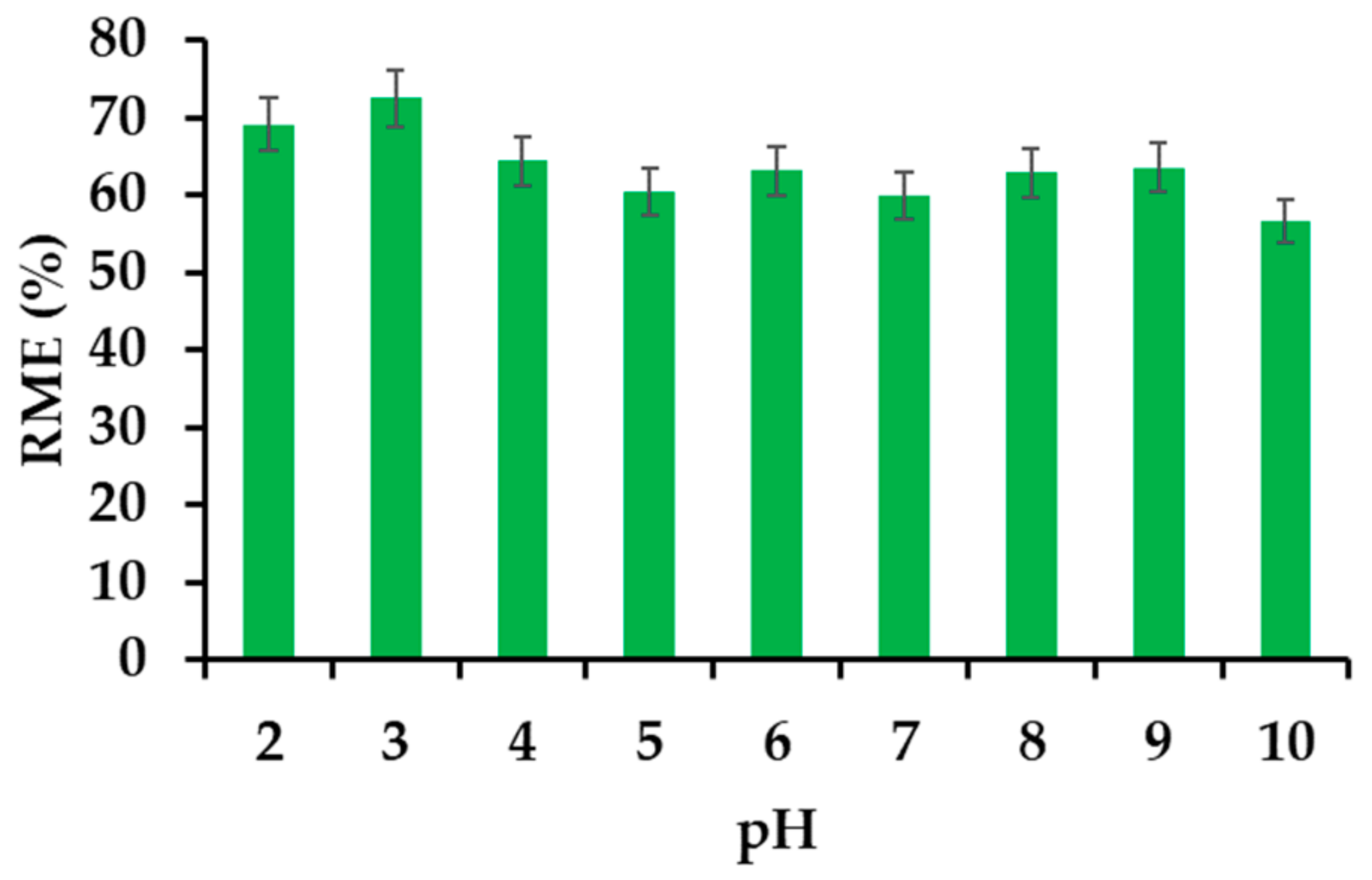
3.2.4. Effect of the pH on the Removal Efficiency
3.2.5. Effect of Salts, Humic and Fulvic Acids on the Removal Efficiency
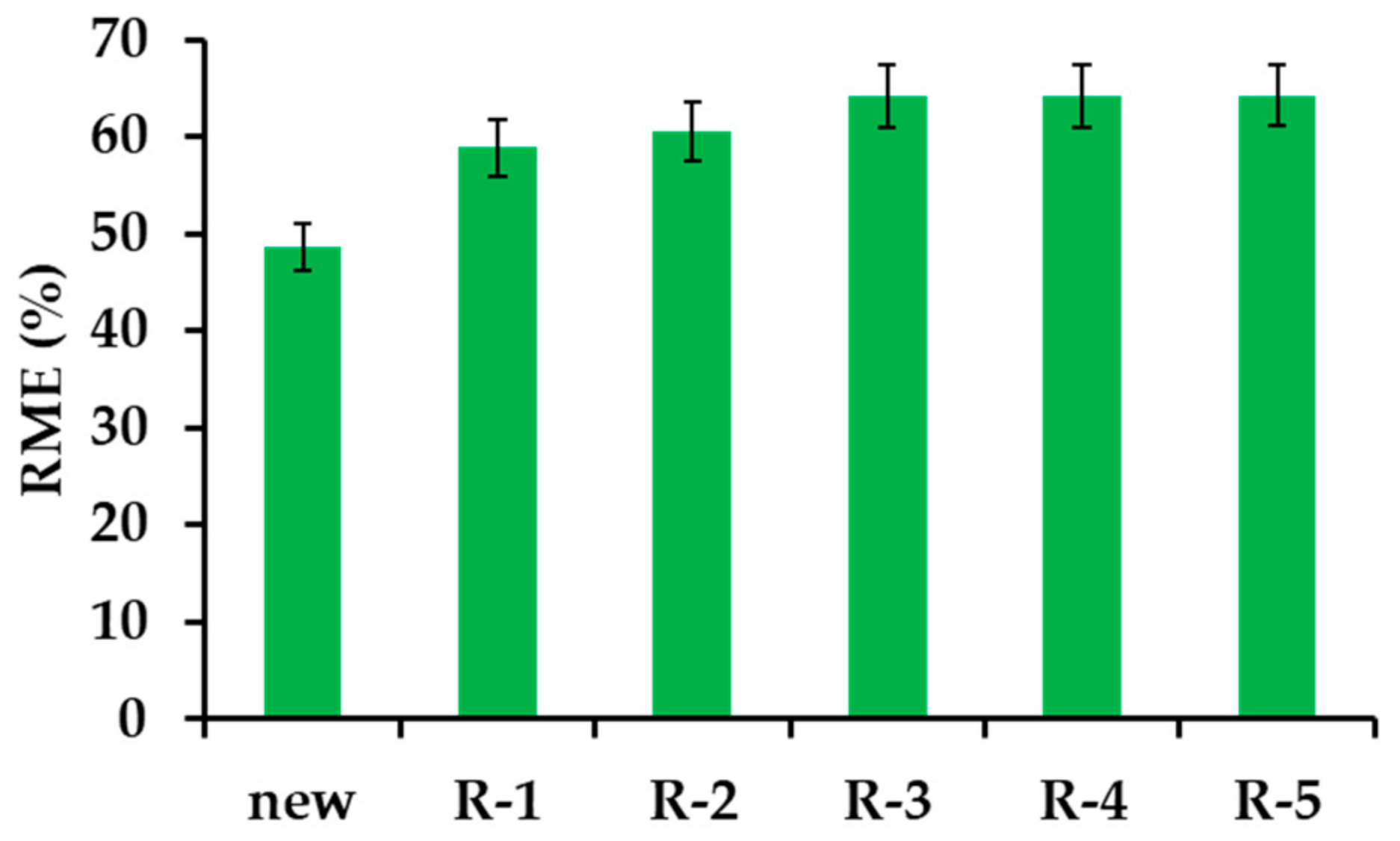
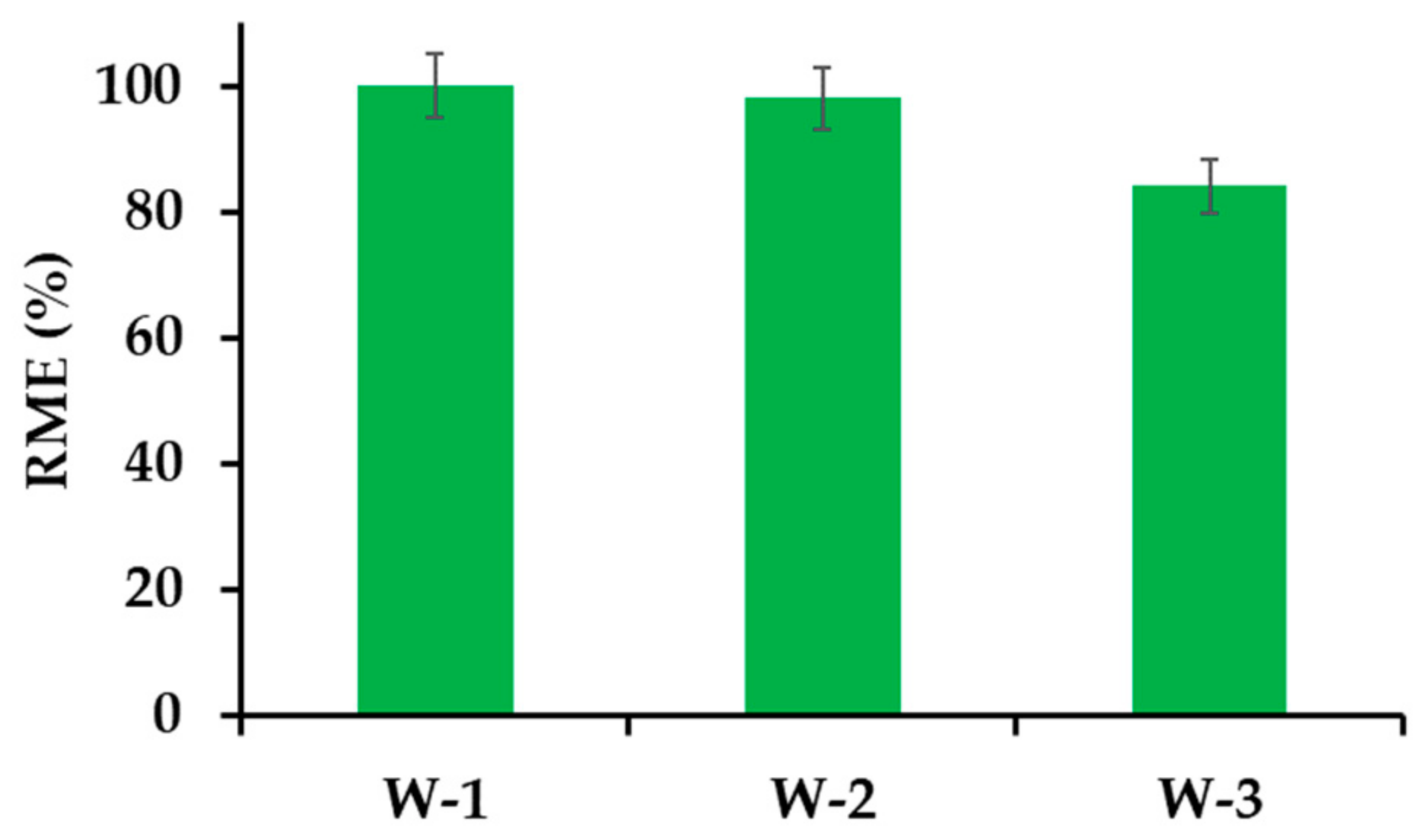
3.2.6. β−CD−M Regeneration
3.2.7. Adsorption of Pharmaceuticals Present in Biologically Treated Sewage of Municipal Type
3.2.8. Adsorption Isotherms
3.3. Kinetic Models
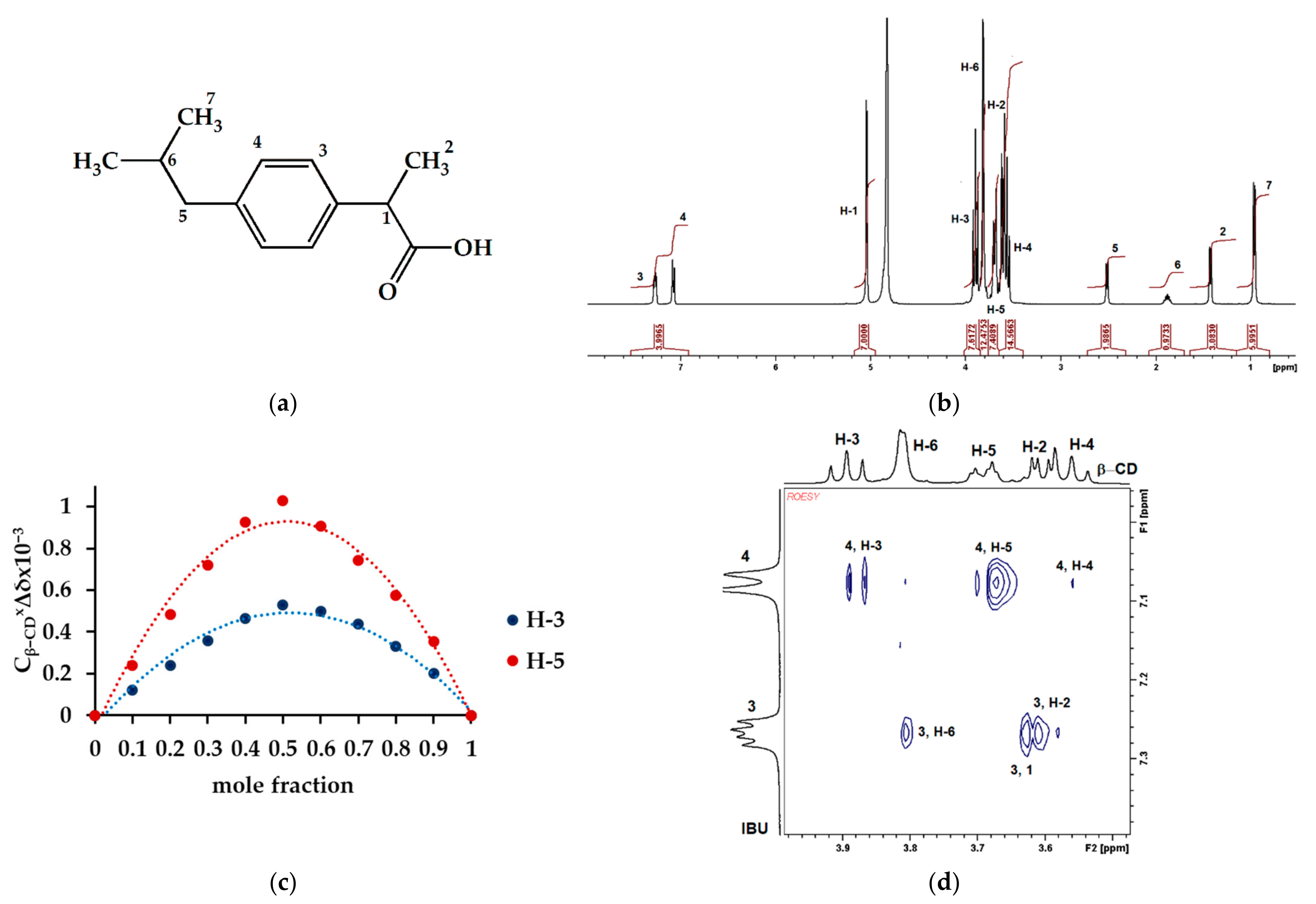
3.4. Mechanism of Adsorption
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gibson, R.; Durán-Álvarez, J.C.; Estrada, K.L.; Chávez, A.; Jiménez Cisneros, B. Accumulation and leaching potential of some pharmaceuticals and potential endocrine disruptors in soils irrigated with wastewater in the Tula Valley, Mexico. Chemosphere 2010, 81, 1437–1445. [Google Scholar] [CrossRef] [PubMed]
- Nunes, B.; Carvalho, F.; Guilhermino, L. Acute toxicity of widely used pharmaceuticals in aquatic species: Gambusia holbrooki, Artemia parthenogenetica and Tetraselmis chuii. Ecotoxicol. Environ. Saf. 2005, 61, 413–419. [Google Scholar] [CrossRef] [PubMed]
- Hossain, A.; Nakamichi, S.; Mamun, H.A.; Tani, K.; Masunaga, S.; Matsuda, H. Occurrence and ecological risk of pharmaceuticals in river surface water of Bangladesh. Environ. Res. 2018, 165, 258–266. [Google Scholar] [CrossRef] [PubMed]
- Marmon, P.; Owen, S.F.; Margiotta-Casaluci, L. Pharmacology-informed prediction of the risk posed to fish by mixtures of non-steroidal anti-inflammatory drugs (NSAIDs) in the environment. Environ. Int. 2020, 146, 106222. [Google Scholar] [CrossRef]
- Patel, N.; Khan, Z.A.; Shahane, S.; Rai, D.; Chauhan, D.; Kant, C.; Chaudhary, V.K. Emerging Pollutants in Aquatic Environment: Source, Effect, and Challenges in Biomonitoring and Bioremediation—A Review. Pollution 2020, 6, 99–113. [Google Scholar] [CrossRef]
- Herrmann, M.; Olsson, O.; Fiehn, R.; Herrel, M.; Kümmerer, K. The significance of different health institutions and their respective contributions of active pharmaceutical ingredients to wastewater. Environ. Int. 2015, 85, 61–76. [Google Scholar] [CrossRef]
- Al Aukidy, M.; Verlicchi, P.; Voulvoulis, N. A framework for the assessment of the environmental risk posed by pharmaceuticals originating from hospital effluents. Sci. Total Environ. 2014, 493, 54–64. [Google Scholar] [CrossRef]
- Verlicchi, P.; Al Aukidy, M.; Galletti, A.; Petrovic, M.; Barceló, D. Hospital effluent: Investigation of the concentrations and distribution of pharmaceuticals and environmental risk assessment. Sci. Total Environ. 2012, 430, 109–118. [Google Scholar] [CrossRef]
- Streit, A.F.; Collazzo, G.C.; Druzian, S.P.; Verdi, R.S.; Foletto, E.L.; Oliveira, L.F.; Dotto, G.L. Adsorption of ibuprofen, ketoprofen, and paracetamol onto activated carbon prepared from effluent treatment plant sludge of the beverage industry. Chemosphere 2020, 262, 128322. [Google Scholar] [CrossRef]
- Langford, K.H.; Thomas, K.V. Determination of pharmaceutical compounds in hospital effluents and their contribution to wastewater treatment works. Environ. Int. 2009, 35, 766–770. [Google Scholar] [CrossRef]
- Verlicchi, P.; Al Aukidy, M.; Zambello, E. Occurrence of pharmaceutical compounds in urban wastewater: Removal, mass load and environmental risk after a secondary treatment—A review. Sci. Total Environ. 2012, 429, 123–155. [Google Scholar] [CrossRef]
- Leung, H.W.; Jin, L.; Wei, S.; Tsui, M.M.P.; Zhou, B.; Jiao, L.; Cheung, P.C.; Chun, Y.K.; Murphy, M.B.; Lam, K.S.P. Pharmaceuticals in Tap Water: Human Health Risk Assessment and Proposed Monitoring Framework in China. Environ. Health Perspect. 2013, 121, 839–846. [Google Scholar] [CrossRef] [Green Version]
- Bottoni, P.; Caroli, S. Presence of residues and metabolites of pharmaceuticals in environmental compartments, food commodities and workplaces: A review spanning the three-year period 2014–2016. Microchem. J. 2018, 136, 2–24. [Google Scholar] [CrossRef]
- Richardson, S.D.; Kimura, S.Y. Water Analysis: Emerging Contaminants and Current Issues. Anal. Chem. 2019, 92, 473–505. [Google Scholar] [CrossRef]
- Carvalho, I.T.; Santos, L. Antibiotics in the aquatic environments: A review of the European scenario. Environ. Int. 2016, 94, 736–757. [Google Scholar] [CrossRef]
- Sathishkumar, P.; Meena, R.A.A.; Palanisami, T.; Ashokkumar, V.; Palvannan, T.; Gu, F.L. Occurrence, interactive effects and ecological risk of diclofenac in environmental compartments and biota—A review. Sci. Total Environ. 2019, 698, 134057. [Google Scholar] [CrossRef]
- Izadi, P.; Salem, R.; Papry, S.A.; Magdouli, S.; Pulicharla, R.; Brar, S.K. Non-steroidal anti-inflammatory drugs in the environment: Where were we and how far we have come? Environ. Pollut. 2020, 267, 115370. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, S.-Q.; Zhang, M.-Y.; He, B.-S. Targeted eco-pharmacovigilance for ketoprofen in the environment: Need, strategy and challenge. Chemosphere 2018, 194, 450–462. [Google Scholar] [CrossRef]
- Tyumina, E.A.; Bazhutin, G.A.; Gómez, A.D.P.C.; Ivshina, I.B. Nonsteroidal Anti-inflammatory Drugs as Emerging Contaminants. Microbiology 2020, 89, 148–163. [Google Scholar] [CrossRef]
- Wang, Y.; He, L.; Dang, G.; Li, H.; Li, X. Polypyrrole-functionalized magnetic Bi2MoO6 nanocomposites as a fast, efficient and reusable adsorbent for removal of ketoprofen and indomethacin from aqueous solution. J. Colloid Interface Sci. 2021, 592, 51–65. [Google Scholar] [CrossRef]
- Smiljanić, D.; de Gennaro, B.; Izzo, F.; Langella, A.; Daković, A.; Germinario, C.; Rottinghaus, G.E.; Spasojević, M.; Mercurio, M. Removal of emerging contaminants from water by zeolite-rich composites: A first approach aiming at diclofenac and ketoprofen. Microporous Mesoporous Mater. 2020, 298, 110057. [Google Scholar] [CrossRef]
- Garcia, J.J.M.; Nuñez, J.A.P.; Salapare, H.S.; Vasquez, M.R. Adsorption of diclofenac sodium in aqueous solution using plasma-activated natural zeolites. Results Phys. 2019, 15, 102629. [Google Scholar] [CrossRef]
- Vatankhah, H.; Szczuka, A.; Mitch, W.A.; Almaraz, N.; Brannum, J.; Bellona, C. Evaluation of Enhanced Ozone–Biologically Active Filtration Treatment for the Removal of 1,4−Dioxane and Disinfection Byproduct Precursors from Wastewater Effluent. Environ. Sci. Technol. 2019, 53, 2720–2730. [Google Scholar] [CrossRef]
- Jothinathan, L.; Hu, J. Kinetic evaluation of graphene oxide based heterogenous catalytic ozonation for the removal of ibuprofen. Water Res. 2018, 134, 63–73. [Google Scholar] [CrossRef]
- Al-Rimawi, F.; Daana, M.; Khamis, M.; Karaman, R.; Khoury, H.; Qurie, M. Removal of Selected Pharmaceuticals from Aqueous Solutions Using Natural Jordanian Zeolite. Arab. J. Sci. Eng. 2018, 44, 209–215. [Google Scholar] [CrossRef]
- Tam, N.T.M.; Liu, Y.; Bashir, H.; Yin, Z.; He, Y.; Zhou, X. Efficient Removal of Diclofenac from Aqueous Solution by Potassium Ferrate-Activated Porous Graphitic Biochar: Ambient Condition Influences and Adsorption Mechanism. Int. J. Environ. Res. Public Health 2019, 17, 291. [Google Scholar] [CrossRef] [Green Version]
- Phasuphan, W.; Praphairaksit, N.; Imyim, A. Removal of ibuprofen, diclofenac, and naproxen from water using chitosan−modified waste tire crumb rubber. J. Mol. Liq. 2019, 294, 111554. [Google Scholar] [CrossRef]
- Shahnaz, T.; Priyan, V.V.; Pandian, S.; Narayanasamy, S. Use of Nanocellulose extracted from grass for adsorption abatement of Ciprofloxacin and Diclofenac removal with phyto, and fish toxicity studies. Environ. Pollut. 2020, 268, 115494. [Google Scholar] [CrossRef]
- Yu, C.; Bahashi, J.; Bi, E. Mechanisms and quantification of adsorption of three anti-inflammatory pharmaceuticals onto goethite with/without surface-bound organic acids. Chemosphere 2019, 222, 593–602. [Google Scholar] [CrossRef]
- Hiew, B.Y.Z.; Lee, L.Y.; Lai, K.C.; Gan, S.; Thangalazhy-Gopakumar, S.; Pan, G.-T.; Yang, T.C.-K. Adsorptive decontamination of diclofenac by three-dimensional graphene-based adsorbent: Response surface methodology, adsorption equilibrium, kinetic and thermodynamic studies. Environ. Res. 2018, 168, 241–253. [Google Scholar] [CrossRef]
- Wang, J.; Li, H.; Shuang, C.; Li, A.; Wang, C.; Huang, Y. Effect of pore structure on adsorption behavior of ibuprofen by magnetic anion exchange resins. Microporous Mesoporous Mater. 2015, 210, 94–100. [Google Scholar] [CrossRef]
- Kurczewska, J.; Cegłowski, M.; Schroeder, G. PAMAM-halloysite Dunino hybrid as an effective adsorbent of ibuprofen and naproxen from aqueous solutions. Appl. Clay Sci. 2020, 190, 105603. [Google Scholar] [CrossRef]
- Iovino, P.; Canzano, S.; Capasso, S.; Erto, A.; Musmarra, D. A modeling analysis for the assessment of ibuprofen adsorption mechanism onto activated carbons. Chem. Eng. J. 2015, 277, 360–367. [Google Scholar] [CrossRef]
- Mestre, A.; Pires, J.; Nogueira, J.; Carvalho, A. Activated carbons for the adsorption of ibuprofen. Carbon 2007, 45, 1979–1988. [Google Scholar] [CrossRef]
- Jodeh, S.; Abdelwahab, F.; Jaradat, N.; Warad, I.; Jodeh, W. Adsorption of diclofenac from aqueous solution using Cyclamen persicum tubers based activated carbon (CTAC). J. Assoc. Arab. Univ. Basic Appl. Sci. 2016, 20, 32–38. [Google Scholar] [CrossRef] [Green Version]
- Mlunguza, N.Y.; Ncube, S.; Mahlambi, P.N.; Chimuka, L.; Madikizela, L.M. Adsorbents and removal strategies of non−steroidal anti−inflammatory drugs from contaminated water bodies. J. Environ. Chem. Eng. 2019, 7, 103142. [Google Scholar] [CrossRef]
- Wei, H.; Deng, S.; Huang, Q.; Nie, Y.; Wang, B.; Huang, J.; Yu, G. Regenerable granular carbon nanotubes/alumina hybrid adsorbents for diclofenac sodium and carbamazepine removal from aqueous solution. Water Res. 2013, 47, 4139–4147. [Google Scholar] [CrossRef]
- Schneider, H.-J.; Hacket, F.; Rüdiger, V.; Ikeda, H. NMR Studies of Cyclodextrins and Cyclodextrin Complexes. Chem. Rev. 1998, 98, 1755–1786. [Google Scholar] [CrossRef]
- Crini, G. Review: A History of Cyclodextrins. Chem. Rev. 2014, 114, 10940–10975. [Google Scholar] [CrossRef]
- Sandilya, A.A.; Natarajan, U.; Priya, M.H. Molecular View into the Cyclodextrin Cavity: Structure and Hydration. ACS Omega 2020, 5, 25655–25667. [Google Scholar] [CrossRef]
- Cova, T.F.; Murtinho, D.; Aguado, R.; Pais, A.A.C.C.; Valente, A.J.M. Cyclodextrin Polymers and Cyclodextrin−Containing Polysaccharides for Water Remediation. Polysaccharides 2021, 2, 2. [Google Scholar] [CrossRef]
- Hădărugă, N.G.; Hădărugă, D.I.; Isengard, H.-D. Water content of natural cyclodextrins and their essential oil complexes: A comparative study between Karl Fischer titration and thermal methods. Food Chem. 2011, 132, 1741–1748. [Google Scholar] [CrossRef]
- Saifi, A.; Joseph, J.P.; Singh, A.P.; Pal, A.; Kumar, K. Complexation of an Azo Dye by Cyclodextrins: A Potential Strategy for Water Purification. ACS Omega 2021, 6, 4776–4782. [Google Scholar] [CrossRef]
- Fang, Z.; Comino, P.; Bhandari, B. Effect of encapsulation of d-limonene on the moisture adsorption property of β-cyclodextrin. LWT 2013, 51, 164–169. [Google Scholar] [CrossRef]
- Kfoury, M.; Landy, D.; Fourmentin, S. Characterization of Cyclodextrin/Volatile Inclusion Complexes: A Review. Molecules 2018, 23, 1204. [Google Scholar] [CrossRef] [Green Version]
- Przybyla, M.A.; Yilmaz, G.; Remzi Becer, C. Natural cyclodextrins and their derivatives for polymer synthesis. Polym. Chem. 2020, 11, 7582–7602. [Google Scholar] [CrossRef]
- Hu, X.; Xu, G.; Zhang, H.; Li, M.; Tu, Y.; Xie, X.; Zhu, Y.; Jiang, L.; Zhu, X.; Ji, X.; et al. Multifunctional β-Cyclodextrin Polymer for Simultaneous Removal of Natural Organic Matter and Organic Micropollutants and Detrimental Microorganisms from Water. ACS Appl. Mater. Interfaces 2020, 12, 12165–12175. [Google Scholar] [CrossRef]
- Xia, D.; Wang, P.; Ji, X.; Khashab, N.M.; Sessler, J.L.; Huang, F. Functional Supramolecular Polymeric Networks: The Marriage of Covalent Polymers and Macrocycle-Based Host-Guest Interactions. Chem. Rev. 2020, 120, 6070–6123. [Google Scholar] [CrossRef]
- Szejtli, J. Utilization of cyclodextrins in industrial products and processes. J. Mater. Chem. 1997, 7, 575–587. [Google Scholar] [CrossRef]
- Salazar, S.; Guerra, D.; Yutronic, N.; Jara, P. Removal of Aromatic Chlorinated Pesticides from Aqueous Solution Using β-Cyclodextrin Polymers Decorated with Fe3O4 Nanoparticles. Polymers 2018, 10, 1038. [Google Scholar] [CrossRef] [Green Version]
- Taka, A.L.; Pillay, K.; Mbianda, X.Y. Nanosponge cyclodextrin polyurethanes and their modification with nanomaterials for the removal of pollutants from waste water: A review. Carbohydr. Polym. 2017, 159, 94–107. [Google Scholar] [CrossRef]
- Sherje, A.P.; Dravyakar, B.R.; Kadam, D.; Jadhav, M. Cyclodextrin-based nanosponges: A critical review. Carbohydr. Polym. 2017, 173, 37–49. [Google Scholar] [CrossRef]
- Krabicová, I.; Appleton, S.L.; Tannous, M.; Hoti, G.; Caldera, F.; Pedrazzo, A.R.; Cecone, C.; Cavalli, R.; Trotta, F. History of Cyclodextrin Nanosponges. Polymers 2020, 12, 1122. [Google Scholar] [CrossRef]
- Morin-Crini, N.; Winterton, P.; Fourmentin, S.; Wilson, L.D.; Fenyvesi, E.; Crini, G. Water-insoluble β-Cyclodextrin–epichlorohydrin polymers for removal of pollutants from aqueous solutions by sorption processes using batch studies: A review of inclusion mechanisms. Prog. Polym. Sci. 2018, 78, 1–23. [Google Scholar] [CrossRef]
- Crini, G.; Morcellet, M. Synthesis and applications of adsorbents containing cyclodextrins. J. Sep. Sci. 2002, 25, 789–813. [Google Scholar] [CrossRef]
- Wang, J.; Cheng, G.; Lu, J.; Chen, H.; Zhou, Y. PDA-cross-linked beta-cyclodextrin: A novel adsorbent for the removal of BPA and cationic dyes. Water Sci. Technol. 2020, 81, 2337–2350. [Google Scholar] [CrossRef]
- Li, X.; Nie, X.-J.; Zhu, Y.-N.; Ye, W.-C.; Jiang, Y.-L.; Su, S.-L.; Yan, B.-T. Adsorption behaviour of Eriochrome Black T from water onto a cross-linked β-Cyclodextrin polymer. Colloids Surf. A Physicochem. Eng. Asp. 2019, 578, 123582. [Google Scholar] [CrossRef]
- Kekes, T.; Tzia, C. Adsorption of indigo carmine on functional chitosan and β-Cyclodextrin/chitosan beads: Equilibrium, kinetics and mechanism studies. J. Environ. Manag. 2020, 262, 110372. [Google Scholar] [CrossRef]
- Badruddoza, A.Z.M.; Bhattarai, B.; Suri, R.P.S. Environmentally Friendly β-Cyclodextrin–Ionic Liquid Polyurethane-Modified Magnetic Sorbent for the Removal of PFOA, PFOS, and Cr(VI) from Water. ACS Sustain. Chem. Eng. 2017, 5, 9223–9232. [Google Scholar] [CrossRef]
- Karoyo, A.H.; Yang, J.; Wilson, L.D. Cyclodextrin-Based Polymer-Supported Bacterium for the Adsorption and in-situ Biodegradation of Phenolic Compounds. Front. Chem. 2018, 6, 403. [Google Scholar] [CrossRef]
- Liu, H.; Cai, X.; Wang, Y.; Chen, J. Adsorption mechanism-based screening of cyclodextrin polymers for adsorption and separation of pesticides from water. Water Res. 2011, 45, 3499–3511. [Google Scholar] [CrossRef] [PubMed]
- Chabalala, M.B.; Al-Abri, M.Z.; Mamba, B.B.; Nxumalo, E.N. Mechanistic aspects for the enhanced adsorption of bromophenol blue and atrazine over cyclodextrin modified polyacrylonitrile nanofiber membranes. Chem. Eng. Res. Des. 2021, 169, 19–32. [Google Scholar] [CrossRef]
- Karoyo, A.H.; Wilson, L.D. Nano-Sized Cyclodextrin-Based Molecularly Imprinted Polymer Adsorbents for Perfluorinated Compounds—A Mini-Review. Nanomaterials 2015, 5, 981–1003. [Google Scholar] [CrossRef] [Green Version]
- Errico, M.J. The Cyclodextrin-Perfluorinated Surfactant Host-Guest Complex: Fundamental Studies for Potential Environmental Remediation and Therapeutic Applications. Ph.D. Thesis, Florida International University, Miami, FL, USA, May 2018. [Google Scholar] [CrossRef]
- Danquah, M.K.; Aruei, R.C.; Wilson, L.D. Phenolic Pollutant Uptake Properties of Molecular Templated Polymers Containing β-Cyclodextrin. J. Phys. Chem. B 2018, 122, 4748–4757. [Google Scholar] [CrossRef]
- Ateia, M.; Alsbaiee, A.; Karanfil, T.; Dichtel, W.R. Efficient PFAS Removal by Amine-Functionalized Sorbents: Critical Review of the Current Literature. Environ. Sci. Technol. Lett. 2019, 6, 688–695. [Google Scholar] [CrossRef]
- Hoti, G.; Caldera, F.; Cecone, C.; Pedrazzo, A.; Anceschi, A.; Appleton, S.; Monfared, Y.; Trotta, F. Effect of the Cross-Linking Density on the Swelling and Rheological Behavior of Ester-Bridged β-Cyclodextrin Nanosponges. Materials 2021, 14, 478. [Google Scholar] [CrossRef]
- Mamba, B.B.; Krause, R.W.; Malefetse, T.J.; Sithole, S.P. Sithole, Degradation studies of β-Cyclodextrin polyurethane polymers using soil burial experiments. S. Afr. J. Chem. 2008, 61, 133–140. [Google Scholar]
- Bazyari-Delavar, S.; Badalkhani-Khamseh, F.; Ebrahim-Habibi, A.; Hadipour, N.L. Investigation of host-guest interactions between polyester dendrimers and ibuprofen using density functional theory (DFT). Comput. Theor. Chem. 2020, 1189, 112983. [Google Scholar] [CrossRef]
- Heydari, S.; Kakhki, R.M. Thermodynamic study of complex formation of β-Cyclodextrin with ibuprofen by conductometric method and determination of ibuprofen in pharmaceutical drugs. Arab. J. Chem. 2017, 10, S1223–S1226. [Google Scholar] [CrossRef] [Green Version]
- Sumarno; Rahim, R.; Trisanti, P.N. Ketoprofen-β-Cyclodextrin inclusion complexes formation by supercritical process technology. AIP Conf. Proc. 2017, 1840, 80010. [Google Scholar] [CrossRef]
- Gómez, M.J.; Petrovic, M.; Fernández-Alba, A.R.; Barceló, D. Determination of pharmaceuticals of various therapeutic classes by solid-phase extraction and liquid chromatography-tandem mass spectrometry analysis in hospital effluent wastewaters. J. Chromatogr. A 2006, 1114, 224–233. [Google Scholar] [CrossRef]
- Hładoń, T.; Ćwiertnia, B. The effect of humidity on the stability of diclofenac sodium in inclusion complex with β-Cyclodextrin in the solid state. Pharmazie 1999, 54, 943–944. [Google Scholar]
- Das, S.; Subuddhi, U. Studies on the complexation of diclofenac sodium with β-cyclodextrin: Influence of method of preparation. J. Mol. Struct. 2015, 1099, 482–489. [Google Scholar] [CrossRef]
- Wang, J.; Yang, F. Preparation of 2-hydroxypropyl-β-Cyclodextrin polymers crosslinked by poly(acrylic acid) for efficient removal of ibuprofen. Mater. Lett. 2020, 284, 128882. [Google Scholar] [CrossRef]
- Moulahcene, L.; Skiba, M.; Bounoure, F.; Benamor, M.; Milon, N.; Hallouard, F.; Lahiani-Skiba, M. New Polymer Inclusion Membrane Containing β-Cyclodextrin Polymer: Application for Pharmaceutical Pollutant Removal from Waste Water. Int. J. Environ. Res. Public Health 2019, 16, 414. [Google Scholar] [CrossRef] [Green Version]
- Mphahlele, K.; Onyango, M.S.; Mhlanga, S.D. Adsorption of aspirin and paracetamol from aqueous solution using Fe/N-CNT/β-Cyclodextrin nanocomopsites synthesized via a benign microwave assisted method. J. Environ. Chem. Eng. 2015, 3, 2619–2630. [Google Scholar] [CrossRef]
- Alipoori, S.; Rouhi, H.; Linn, E.; Stumpfl, H.; Mokarizadeh, H.; Esfahani, M.R.; Koh, A.; Weinman, S.T.; Wujcik, E.K. Polymer-Based Devices and Remediation Strategies for Emerging Contaminants in Water. ACS Appl. Polym. Mater. 2021, 3, 549–577. [Google Scholar] [CrossRef]
- Jurecska, L.; Dobosy, P.; Barkács, K.; Fenyvesi, É.; Záray, G. Characterization of cyclodextrin containing nanofilters for removal of pharmaceutical residues. J. Pharm. Biomed. Anal. 2014, 98, 90–93. [Google Scholar] [CrossRef]
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of Gases in Multimolecular Layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Barrett, E.P.; Joyner, L.G.; Halenda, P.P. The Determination of Pore Volume and Area Distributions in Porous Substances. I. Computations from Nitrogen Isotherms. J. Am. Chem. Soc. 1951, 73, 373–380. [Google Scholar] [CrossRef]
- Ding, S.; Liu, P.; Zhang, S.; Gao, C.; Wang, F.; Ding, Y.; Yang, M. Crosslinking of β-Cyclodextrin and combining with ammonium polyphosphate for flame-retardant polypropylene. J. Appl. Polym. Sci. 2019, 137, 48320. [Google Scholar] [CrossRef]
- Goel, A.; Nene, S.N. Modifications in the Phenolphthalein Method for Spectrophotometric Estimation of Beta Cyclodextrin. Starch-Stärke 1995, 47, 399–400. [Google Scholar] [CrossRef]
- De Souza, Í.F.T.; Petri, D.F.S. β-Cyclodextrin hydroxypropyl methylcellulose hydrogels for bisphenol A adsorption. J. Mol. Liq. 2018, 266, 640–648. [Google Scholar] [CrossRef]
- Cadena, P.G.; Oliveira, E.C.; Araujo, A.N.; Montenegro, M.C.B.S.M.; Pimentel, M.C.B.; Filho, J.L.L.; Silva, V.L. Simple Determination of Deoxycholic and Ursodeoxycholic Acids by Phenolphthalein-β-Cyclodextrin Inclusion Complex. Lipids 2009, 44, 1063–1070. [Google Scholar] [CrossRef]
- Mohamed, M.H.; Wilson, L.D.; Pratt, D.Y.; Guo, R.; Wu, C.; Headley, J.V. Evaluation of the accessible inclusion sites in copolymer materials containing β-Cyclodextrin. Carbohydr. Polymers 2012, 87, 1241–1248. [Google Scholar] [CrossRef]
- Pu, H.; Tang, P.; Zhao, L.; Sun, Q.; Zhai, Y.; Li, Z.; Gan, N.; Liu, Y.; Ren, X.; Li, H. Preparation of a carboxymethyl β-Cyclodextrin polymer and its rapid adsorption performance for basic fuchsin. RSC Adv. 2020, 10, 20905–20914. [Google Scholar] [CrossRef]
- Caldera, F.; Tannous, M.; Cavalli, R.; Zanetti, M.; Trotta, F. Evolution of Cyclodextrin Nanosponges. Int. J. Pharm. 2017, 531, 470–479. [Google Scholar] [CrossRef]
- Ascar, L.; Ahumada, I.; Lopez, A.; Quintanilla, F.; Leiva, K. Nonsteroidal Anti-Inflammatory Drug Determination in Water Samples by HPLC-DAD under Isocratic Conditions. J. Braz. Chem. Soc. 2013, 24, 1160–1166. [Google Scholar] [CrossRef]
- Hanapi, N.S.M.; Sanagi, M.M.; Ismail, A.K.; Saim, N.; Ibrahim, W.N.W.; Marsin, F.M. Rapid Determination of Non-steroidal Anti-inflammatory Drugs in Aquatic Matrices by Two-phase Micro-electrodriven Membrane Extraction Combined with Liquid Chromatography. J. Chromatogr. Sci. 2017, 56, 166–176. [Google Scholar] [CrossRef]
- Santos, J.L.; Aparicio, I.; Alonso, E.; Callejón, M. Simultaneous determination of pharmaceutically active compounds in wastewater samples by solid phase extraction and high-performance liquid chromatography with diode array and fluorescence detectors. Anal. Chim. Acta 2005, 550, 116–122. [Google Scholar] [CrossRef]
- Bello, O.S.; Alao, O.C.; Alagbada, T.C.; Agboola, O.S.; Omotoba, O.T.; Abikoye, O.R. A renewable, sustainable and low-cost adsorbent for ibuprofen removal. Water Sci. Technol. 2020, 83, 111–122. [Google Scholar] [CrossRef]
- Nworie, F.S.; Nwabue, F.; Oti, W.; Mbam, E.; Nwali, B. Removal of methylene blue from aqueous solution using activated rice husk biochar: Adsorption isotherms, kinetics and error analysis. J. Chil. Chem. Soc. 2019, 64, 4365–4376. [Google Scholar] [CrossRef] [Green Version]
- Alothman, Z.A.; Badjah, A.Y.; Alduhaish, O.M.; Rathinam, K.; Panglisch, S.; Ali, I. Synthesis, characterization, kinetics and modeling studies of new generation pollutant ketoprofen removal in water using copper nanoparticles. J. Mol. Liq. 2020, 323, 115075. [Google Scholar] [CrossRef]
- Alothman, Z.A.; Badjah, A.Y.; Alharbi, O.M.; Ali, I. Synthesis of chitosan composite iron nanoparticles for removal of diclofenac sodium drug residue in water. Int. J. Biol. Macromol. 2020, 159, 870–876. [Google Scholar] [CrossRef]
- Yaneva, Z.L.; Koumanova, B.K.; Allen, S.J. Applicability comparison of different kinetic/diffusion models for 4-nitrophenol sorption on Rhizopus oryzae dead biomass. Bulg. Chem. Commun. 2013, 45, 161–168. [Google Scholar]
- Martín, J.; Orta, M.D.M.; Medina-Carrasco, S.; Santos, J.L.; Aparicio, I.; Alonso, E. Evaluation of a modified mica and montmorillonite for the adsorption of ibuprofen from aqueous media. Appl. Clay Sci. 2019, 171, 29–37. [Google Scholar] [CrossRef]
- Varank, G.; Demir, A.; Yetilmezsoy, K.; Top, S.; Sekman, E.; Bilgili, M.S. Removal of 4-nitrophenol from aqueous solution by natural low-cost adsorbents. Indian J. Chem. Technol. 2012, 19, 7–25. [Google Scholar]
- Langmuir, I. The constitution and fundamental properties of solids and liquids. II. Liquids. J. Am Chem. Soc. 1917, 39, 1848–1906. [Google Scholar] [CrossRef] [Green Version]
- Freundlich, H.M. Uber die adsorption in losungen. Z. Phys. Chem. 1906, 57, 385–470. [Google Scholar] [CrossRef]
- Halsey, G. Physical Adsorption on Non-Uniform Surfaces. J. Chem. Phys. 1948, 16, 931–937. [Google Scholar] [CrossRef]
- Hill, T.L. Theory of Physical Adsorption. Adv. Catal. 1952, 4, 211–258. [Google Scholar] [CrossRef]
- Sips, R. On the Structure of a Catalyst Surface. J. Chem. Phys. 1948, 16, 490–495. [Google Scholar] [CrossRef]
- Lima, E.C.; Hosseini-Bandegharaei, A.; Moreno-Piraján, J.C.; Anastopoulos, I. A critical review of the estimation of the thermodynamic parameters on adsorption equilibria. Wrong use of equilibrium constant in the Van’t Hoof equation for calculation of thermodynamic parameters of adsorption. J. Mol. Liq. 2018, 273, 425–434. [Google Scholar] [CrossRef]
- Higuti, I.H.; Da Silva, P.A.; Papp, J.; Okiyama, V.M.D.E.; De Andrade, E.A.; Marcondes, A.D.A.; Nascimento, A.J.D. Colorimetric determination of alpha and beta-cyclodextrins and studies on optimization of CGTase production from B. firmus using factorial designs. Braz. Arch. Biol. Technol. 2004, 47, 837–841. [Google Scholar] [CrossRef] [Green Version]
- Anne, J.M.; Boon, Y.H.; Saad, B.; Miskam, M.; Yusoff, M.M.; Shahriman, M.S.; Zain, N.N.M.; Lim, V.; Raoov, M. β-Cyclodextrin conjugated bifunctional isocyanate linker polymer for enhanced removal of 2,4-dinitrophenol from environmental waters. R. Soc. Open Sci. 2018, 5, 180942. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rima, J.; Assakera, K. β-Cyclodextrin Polyurethanes Copolymerized with Beetroot Fibers (Bio-Polymer) to Clean-Up Water Polluted by Organics and Spilled-Oil. J. Pet. Environ. Biotechnol. 2018, 9, 1–7. [Google Scholar] [CrossRef]
- Roberts, P.H.; Thomas, K.V. The occurrence of selected pharmaceuticals in wastewater effluent and surface waters of the lower Tyne catchment. Sci. Total Environ. 2006, 356, 143–153. [Google Scholar] [CrossRef] [PubMed]
- Giles, C.H.; MacEwan, T.H.; Nakhwa, S.N.; Smith, D. Studies in adsorption. Part XI. A system of classification of solution adsorption isotherms, and its use in diagnosis of adsorption mechanisms and in measurement of specific surface areas of solids. J. Chem. Soc. (Resumed) 1960, 786, 3973–3993. [Google Scholar] [CrossRef]
- Almjadleh, M.; Alasheh, S.; Raheb, I. Use of natural and modified Jordanian zeolitic tuff for removal of cadmium(II) from aqueous solutions. Jordan J. Civ. Eng. 2014, 8, 332–343. [Google Scholar]
- Vicente-Martínez, Y.; Caravaca, M.; Soto-Meca, A.; Solana-González, R. Magnetic core-modified silver nanoparticles for ibuprofen removal: An emerging pollutant in waters. Sci. Rep. 2020, 10, 18288. [Google Scholar] [CrossRef]
- Hanbali, G.; Jodeh, S.; Hamed, O.; Bol, R.; Khalaf, B.; Qdemat, A.; Samhan, S. Enhanced Ibuprofen Adsorption and Desorption on Synthesized Functionalized Magnetic Multiwall Carbon Nanotubes from Aqueous Solution. Materials 2020, 13, 3329. [Google Scholar] [CrossRef]
- Al-Yousef, H.A.; Alotaibi, B.M.; Aouaini, F.; Sellaoui, L.; Bonilla-Petriciolet, A. Adsorption of ibuprofen on cocoa shell biomass-based adsorbents: Interpretation of the adsorption equilibrium via statistical physics theory. J. Mol. Liq. 2021, 331, 115697. [Google Scholar] [CrossRef]
- Husein, D.Z.; Hassanien, R.; Al-Hakkani, M. Green-synthesized copper nano-adsorbent for the removal of pharmaceutical pollutants from real wastewater samples. Heliyon 2019, 5, e02339. [Google Scholar] [CrossRef] [Green Version]
- Ulfa, M.; Worabay, N.S.; Pradipta, M.F.; Prasetyoko, D. Removal of ibuprofen from aqueous solutions by adsorption on tiny zinc oxide sheet-like structure. AIP Conf. Proc. 2019, 2194, 020131. [Google Scholar] [CrossRef]
- Al-Ghouti, M.A.; Da’Ana, D.A. Guidelines for the use and interpretation of adsorption isotherm models: A review. J. Hazard. Mater. 2020, 393, 122383. [Google Scholar] [CrossRef]
- Haghseresht, F.; Lu, G. Adsorption Characteristics of Phenolic Compounds onto Coal-Reject-Derived Adsorbents. Energy Fuels 1998, 12, 1100–1107. [Google Scholar] [CrossRef]
- Weber, W.J.; Morris, J.C. Kinetics of Adsorption on Carbon from Solution. J. Sanit. Eng. Div. 1964, 90, 31–60. [Google Scholar] [CrossRef]
- Weber, W.J.; Morris, C.J. Advances in Water Pollution Research. In Proceedings of the 1st International Conference on Water Pollution Research, London, UK, 3–7 September 1962; Pregamon Press: Oxford, UK, 1962; Volume 2, p. 231. [Google Scholar]




| Kinetic Model | Linear Form | Plot | Ref. |
|---|---|---|---|
| Pseudo-first-order | [92] | ||
| Pseudo-second-order | [20] | ||
| Weber and Morris | [93] | ||
| Liquid film diffusion | [94,95] | ||
| Bangham’s diffusion | [96] | ||
| Elovich | [97] | ||
| Modified Freundlich | [98] |
| Model * | Model in Linear Form * | Plot | Ref. | |
|---|---|---|---|---|
| [99] | ||||
| Freundlich | [100] | |||
| Halsey | [101] | |||
| Hill | [102] | |||
| Sips | [103] | |||
| Parameter | Second Stage |
|---|---|
| BOD5 (mg L−1 O2) | 30 |
| COD (mg L−1 O2) | 114 |
| TOC (mg L−1 C) | 2.2 |
| Total suspended solids (mg L−1) | 40 |
| Total nitrogen (mg L−1) | 10 |
| total phosphorus (mg L−1) | 0.5 |
| Conductivity (μS cm−1) | 1120 |
| pH | 6.2 |
| Isotherm Model | Parameters | IBU |
|---|---|---|
| Langmuir | KL (L mg−1) | 0.082 |
| qmax (mg g−1) | 77.52 | |
| R2 | 0.990 | |
| 0.11–0.55 | ||
| Freundlich | KF (L g−1) | 15.823 |
| n | 2.937 | |
| R2 | 0.9763 | |
| Halsey | KH (L g−1) | 1.001 |
| nH | 0.0003 | |
| R2 | 0.9763 | |
| Hill | KD (L g−1) | −0.994 |
| nH | 0.0014 | |
| R2 | 0.9818 | |
| Sips | KS (L mg−1) | 0.129 |
| n | 1.33 | |
| qmax (mg g−1) | 86.21 | |
| R2 | 0.9934 |
| Adsorbent | Qmax (mg g−1) | Adsorption Conditions | Specific Surface Area (m2 g−1) | Reference | |||
|---|---|---|---|---|---|---|---|
| pH | Temp. (°C) | Time (h) | C0 (mg L−1) | ||||
| Magnetic core-modified silver nanoparticles | 0.29 | 7 | 25 | 0.75 | 0.2 | 116.48 | [111] |
| Multiwall carbon nanotubes | 1.15 | 4 | 25 | 2 | 200 | 151 | [112] |
| Jordanian zeolite | 1.23 | 2 | 25 | 1.33 | 510 | 41.20 | [35,36] |
| Cocoa Shell biomass | 21.43 | 2 | 30 | 10 | 30 | no data | [113] |
| Acid-modified kola nut husk (KNHA) | 28.22 | not adjusted | 30 | 3 | 20 | 712 | [92] |
| Copper nanoparticles (Cu NPs) | 33.9 | 4.5 | 25 | 1 | 10–40 | no data | [114] |
| Chitosan-modified waste tire crumb rubber | 70.00 | 6 | 25 | 24 | 40 | no data | [27] |
| β−CD−M | 86.21 | 4.54 | 25 | 1 | 1–100 | 7.31 | this work |
| 2−hydroxypropyl-β-Cyclodextrin polymers | 87.50 | 5 | 25 | 2 | 50 | no data | [75] |
| Activated Carbon | 105.91 | 4 | 25 | 3 | 50 | 642 | [9] |
| Zinc oxide sheet | 220.00 | no data | 25 | 0.83 | 100 | no data | [115] |
| Temperature (K) | ΔH0 (kJ mol−1) | ΔS0 (J K−1 mol−1) | ΔG0 (kJ mol−1) |
|---|---|---|---|
| 293 | −19.32 | ||
| 313 | −11.37 | 27.13 | −19.83 |
| 333 | −20.40 | ||
| linear equation | value | SE | R2 |
| intercept | 3.2628 | 0.02007 | 0.9989 |
| slop | 1367.1 | 15.04890 |
| Isotherm Model | Intercept and Slop The Time Interval (min) | Parameters | IBU |
|---|---|---|---|
| First-order kinetic model | −0.0513 | qe (mg g−1) | 0.0513 |
| 0.446 | k1 (min−1) | 1.56 | |
| 1–60 | R2 | 0.965 | |
| Second-order kinetic model | 0.2043 | qe (mg g−1) | 4.89 |
| 0.3105 | k2 (g min−1 mg−1) | 77.162 | |
| 1–60 | R2 | 0.9994 | |
| Bangham’s pore diffusion model | 0.1647 | δ | 0.1647 |
| −3.2093 | KB (mL L−1 g−1) | 0.712 | |
| 1–10 | R2 | 0.9936 | |
| 0.2588 | δ | 0.0702 | |
| −3.466 | KB (mL L−1 g−1) | 0.905 | |
| 10–60 | R2 | 0.9891 | |
| Weber and Morris | 0.6165 | C1 | 2.269 |
| 2.2692 | Kid−1 (g mg−1 min−1/2) | 0.616 | |
| 1–10 | R2 | 0.9988 | |
| 0.109 | C2 | 3.983 | |
| 3.9827 | Kid−2 (g mg−1 min−1/2) | 0.109 | |
| 10–60 | R2 | 0.9901 | |
| Liquid film diffusion | −0.1173 −0.7814 1–10 | Klf (g mg−1 min−1) | 0.117 |
| −0.0322 −0.5524 | R2 | 0.9988 | |
| 10–60 | Klf (g mg−1 min−1) | 0.043 | |
| R2 | 0.9896 | ||
| Elovich | 0.4936 | β (g mg−1) | 2.026 |
| 0.9835 | α (g mg−1 min−1) | 176.080 | |
| 1–10 | R2 | 0.9835 | |
| modified Freundlich | 0.1364 | 1/m | 0.136 |
| 1.0731 | kF (L g−1 min−1) | 0.146 | |
| 1–10 | R2 | 0.9767 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Skwierawska, A.M.; Nowacka, D.; Nowicka, P.; Rosa, S.; Kozłowska-Tylingo, K. Structural Adaptive, Self-Separating Material for Removing Ibuprofen from Waters and Sewage. Materials 2021, 14, 7697. https://doi.org/10.3390/ma14247697
Skwierawska AM, Nowacka D, Nowicka P, Rosa S, Kozłowska-Tylingo K. Structural Adaptive, Self-Separating Material for Removing Ibuprofen from Waters and Sewage. Materials. 2021; 14(24):7697. https://doi.org/10.3390/ma14247697
Chicago/Turabian StyleSkwierawska, Anna Maria, Dominika Nowacka, Paulina Nowicka, Sandra Rosa, and Katarzyna Kozłowska-Tylingo. 2021. "Structural Adaptive, Self-Separating Material for Removing Ibuprofen from Waters and Sewage" Materials 14, no. 24: 7697. https://doi.org/10.3390/ma14247697
APA StyleSkwierawska, A. M., Nowacka, D., Nowicka, P., Rosa, S., & Kozłowska-Tylingo, K. (2021). Structural Adaptive, Self-Separating Material for Removing Ibuprofen from Waters and Sewage. Materials, 14(24), 7697. https://doi.org/10.3390/ma14247697






