Influence of Powder Milling and Annealing Parameters on the Formation of Cubic Li7La3Zr2O12 Compound
Abstract
:1. Introduction
2. Materials and Methods
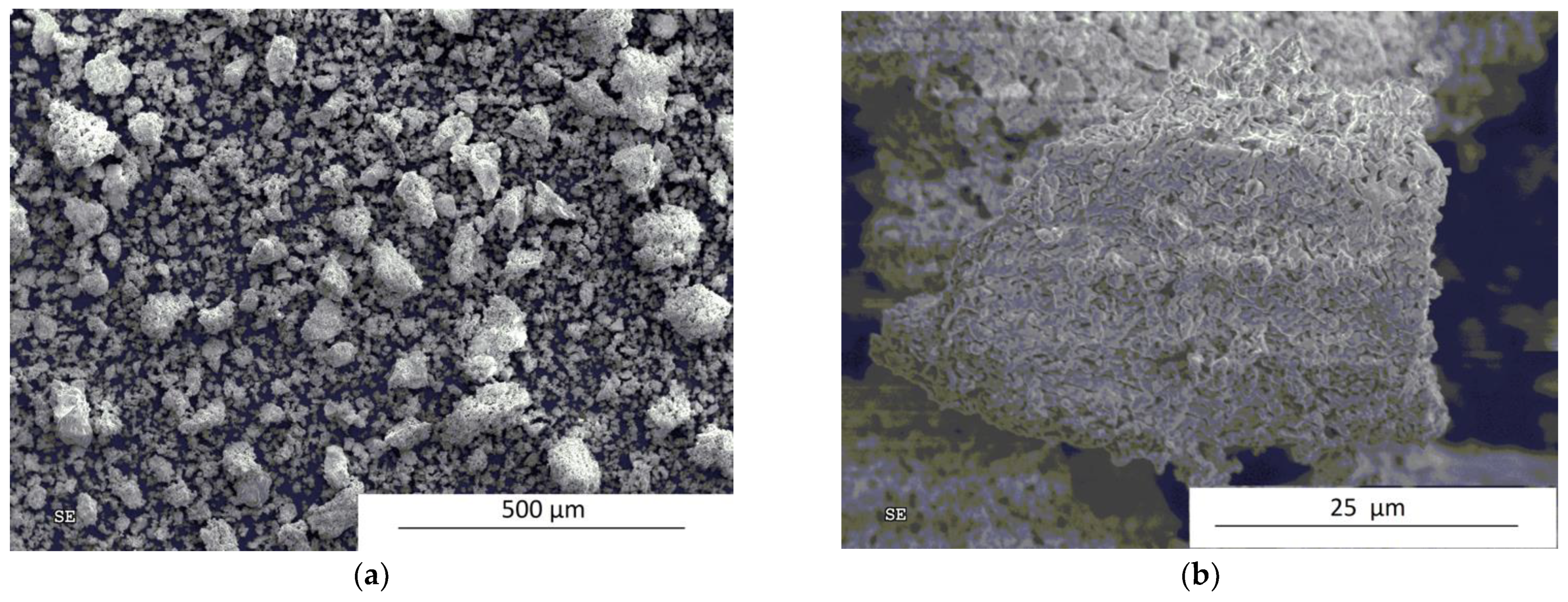
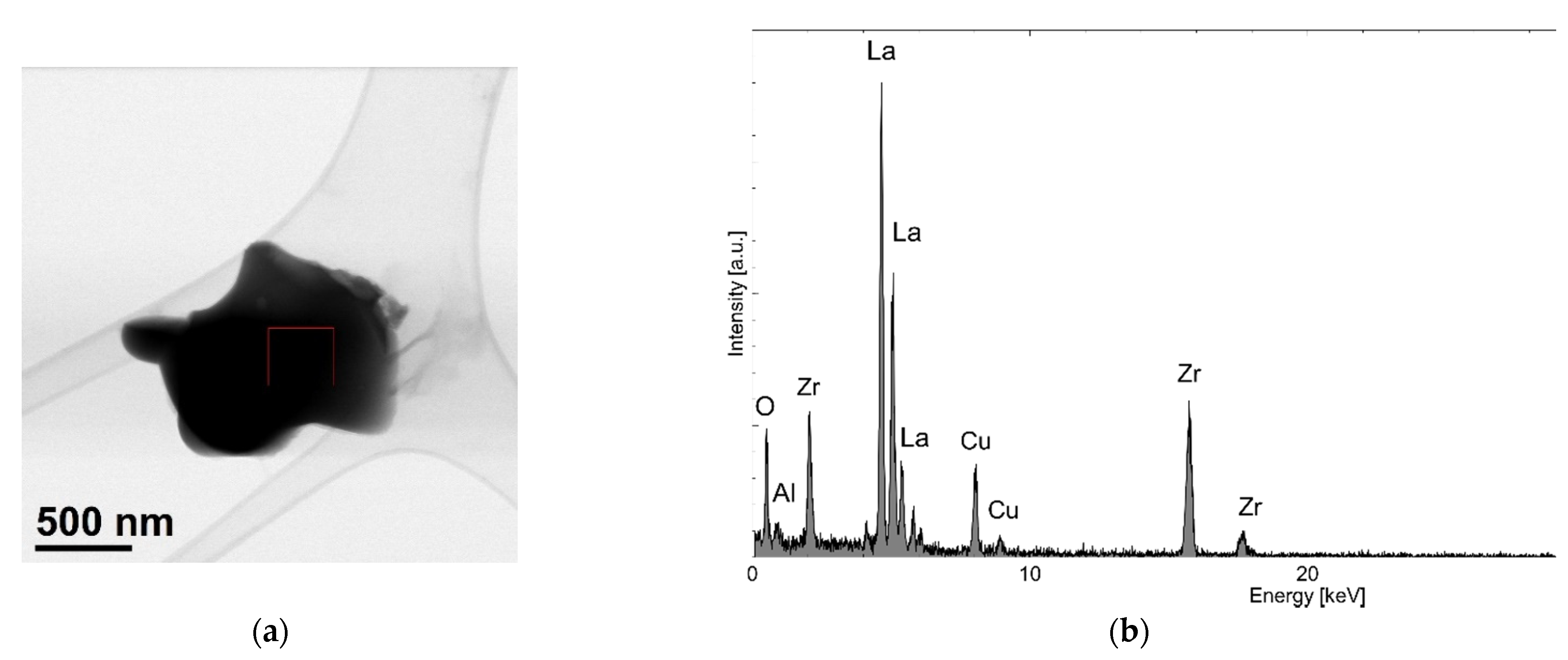
3. Results and Discussion
4. Conclusions
- -
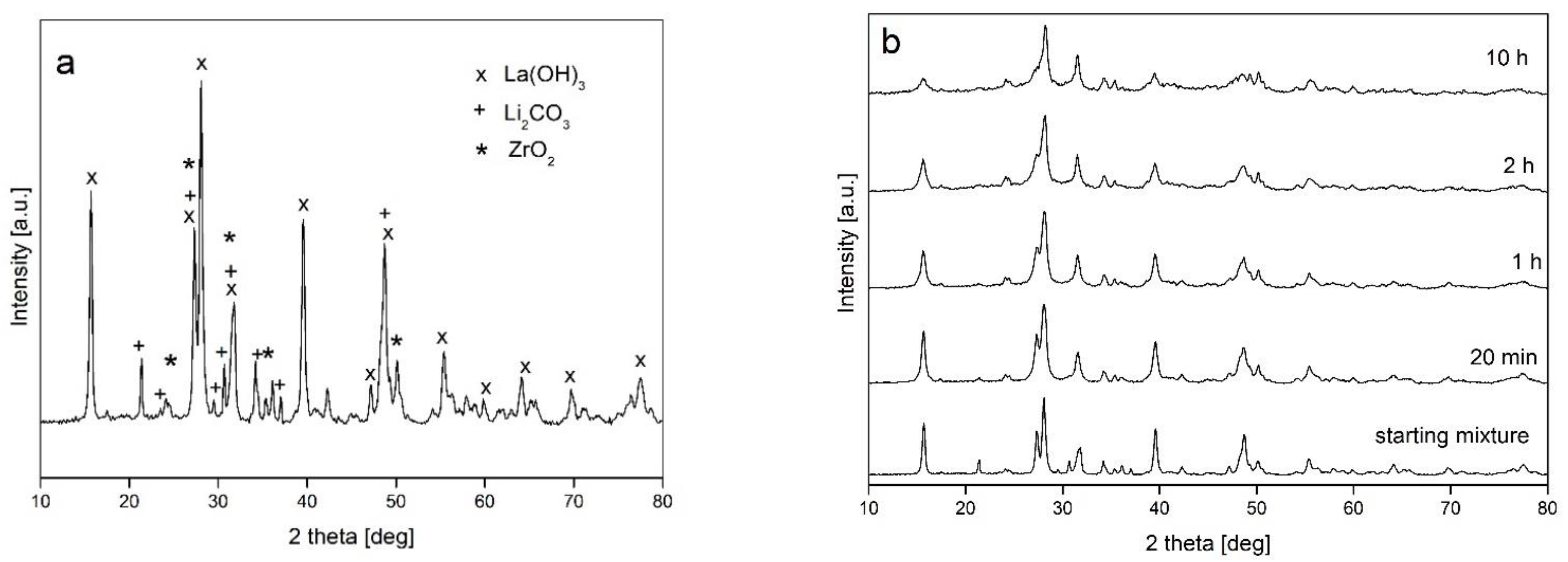
- The mechanical milling processes had no induced phase transformations of the starting powder mixture, and a broadening of the diffraction lines was observed only due to structural refinement and the generation of crystal imperfections;
- -
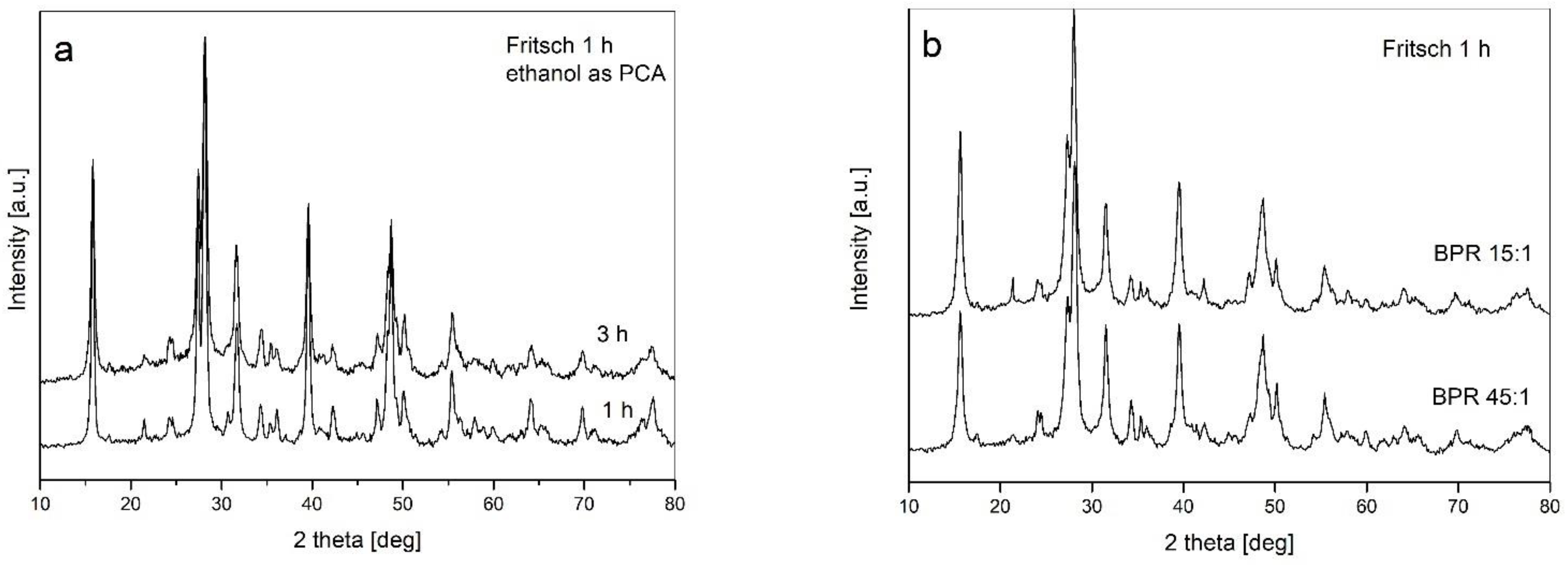
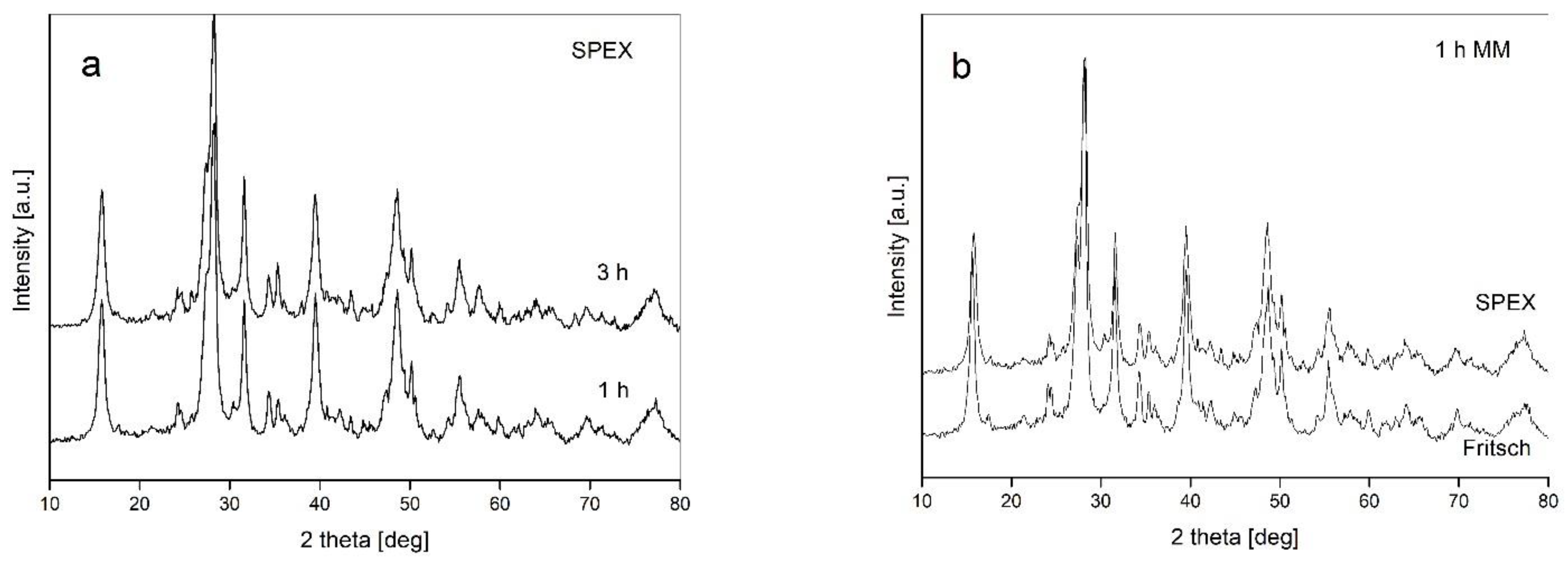
- The type of ball mill used (with different dominant modes of milling—impact and friction) has no influence on the processing of powders
- -
- Changes to other milling parameters (ball-to-powder weight ratio BPR, addition of ethanol, prolongation of milling up to 10 h) do not introduce qualitative changes in the powder structure;
- -
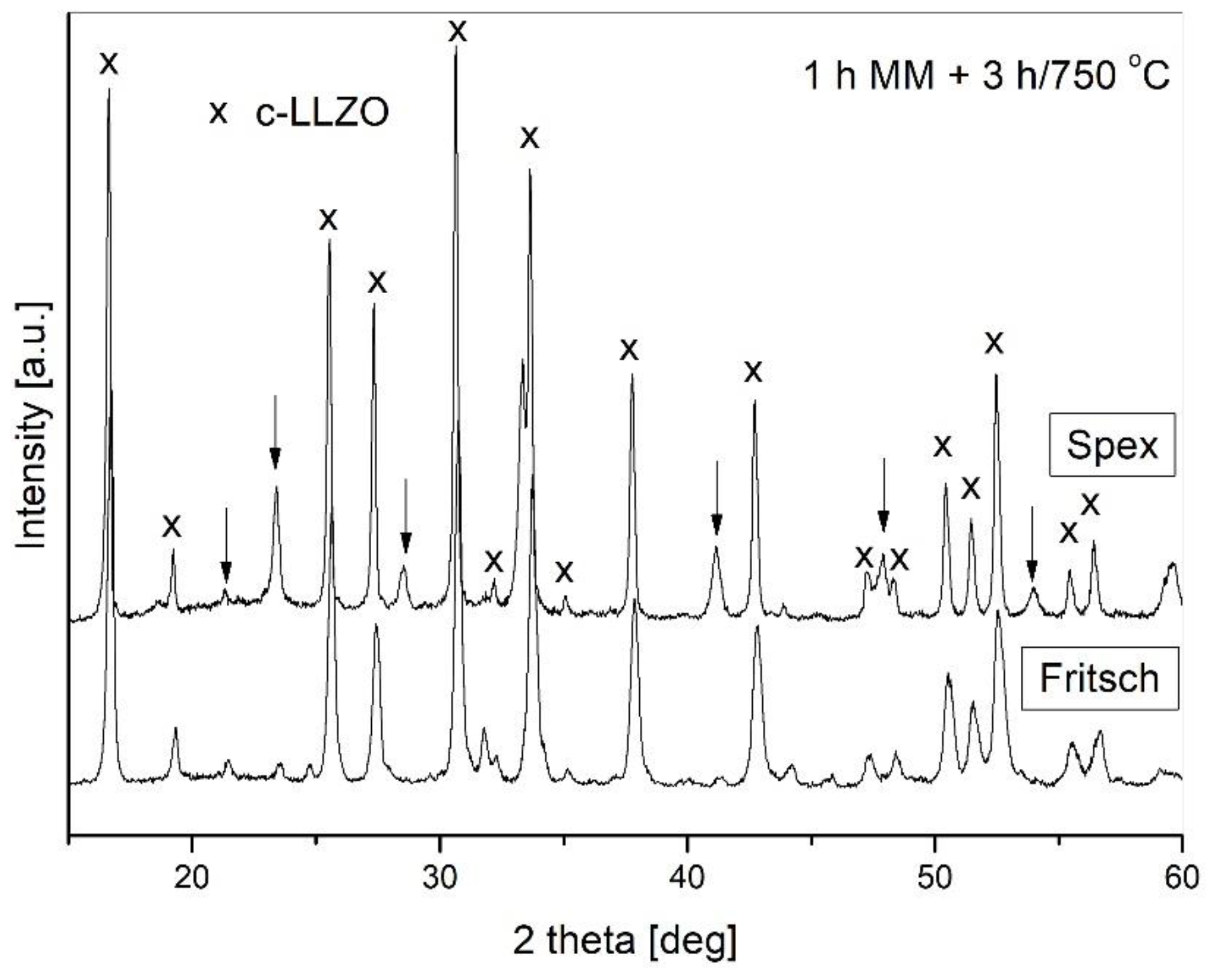
- The formation of c-LLZO was possible only after powder annealing, and the amount of the desired cubic modification of LLZO significantly depended on the annealing parameters. It was found that annealing at 750 °C resulted in a higher amount of c-LLZO than processing at 950 °C, and that the prolongation of annealing time for upward of 3 h was beneficial.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Mukhopadhyay, A.; Sheldon, B.W. Deformation and stress in electrode materials for Li-ion batteries. Prog. Mater. Sci. 2014, 63, 58–116. [Google Scholar] [CrossRef]
- Thangadurai, V.; Weppner, W. Recent progress in solid oxide and lithium ion conducting electrolytes research. Ionics 2006, 12, 81–92. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.G.; Son, B.; Mukherjee, S.; Schuppert, N.; Bates, A.; Kwon, O.; Choi, M.J.; Chung, H.Y.; Park, S. A review of lithium and non-lithium based solid state batteries. J. Power Sources 2015, 282, 299–322. [Google Scholar] [CrossRef]
- Anurova, N.A.; Blatov, V.A.; Ilyushin, G.D.; Blatova, O.A.; Ivanov-Schitz, A.K.; Demyanets, L.N. Migration maps of Li+ cations in oxygen-containing compounds. Solid State Ion. 2008, 179, 2248–2254. [Google Scholar] [CrossRef]
- Teng, S.; Tan, J.; Tiwari, A. Recent developments in garnet based solid state electrolytes for thin film batteries. Curr. Opin. Solid State Mater. Sci. 2014, 18, 29–38. [Google Scholar] [CrossRef]
- Awaka, J.; Takashima, A.; Kataoka, K.; Kojima, N.; Idemoto, Y.; Akimoto, J. Crystal structure of fast lithium-ion conducting cubic Li7La3Zr2O12. Chem. Lett. 2011, 40, 60–62. [Google Scholar] [CrossRef]
- Murugan, R.; Thangadurai, V.; Weppner, W. Fast lithium ion conduction in garnet type Li7La3Zr2O12. Angew. Chem. Int. Ed. 2007, 46, 7778–7781. [Google Scholar] [CrossRef] [PubMed]
- Narayan, J.; Fletcher, C.W.; White, W.H.; Christie, J. Melting phenomena and pulsed-laser annealing in semiconductors. J. Appl. Phys. 1981, 52, 7121–7128. [Google Scholar] [CrossRef]
- Chang, C.M.; Hong, S.H.; Park, H.M. Spark plasma sintering of Al substituted LiHf2(PO4)3 solid electrolytes. Solid State Ion. 2005, 176, 2583–2587. [Google Scholar] [CrossRef]
- Feng, J.K.; Lu, L.; Lai, M.O. Lithium storage capability of lithium ion conductor Li1.5Al0.5Ge1.5(PO4)3. J. Alloys Compd. 2010, 501, 255–258. [Google Scholar] [CrossRef]
- Kotobuki, M.; Munakata, H.; Kanamura, K.; Sato, Y.; Yoshida, T. Compatibility of Li7La3Zr2O12 Solid Electrolyte to All-Solid-State Battery Using Li Metal Anode. J. Electrochem. Soc. 2010, 157, A1076–A1079. [Google Scholar] [CrossRef]
- Awaka, J.; Kijima, N.; Hayakawa, H.; Akimoto, J. Synthesis and structure analysis of tetragonal Li7La3Zr2O12 with the garnet related type structure. J. Solid State Chem. 2009, 182, 2046–2052. [Google Scholar] [CrossRef]
- Buschmann, H.; Dolle, J.; Berendts, S.; Kuhn, A.; Bottke, P.; Wilkening, M.; Heitjans, P.; Senyshyn, A.; Ehrenberg, H.; Lotnyk, A.; et al. Structure and dynamics of the fast lithium ion conductor Li7La3Zr2O12. Phys. Chem. Chem. Phys. 2011, 13, 19378–19392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rangasamy, E.; Wolfenstine, J.; Sakamoto, J. The role of Al and Li concentration on the formation of cubic garnet solid state electrolyte of nominal composition Li7La3Zr2O12. Solid State Ion. 2012, 206, 28–32. [Google Scholar] [CrossRef]
- Jin, Y.; McGinn, P.J. Al-Doped Li7La3Zr2O12 synthesized by a polymerized complex method. J. Power Sources 2011, 196, 8683–8687. [Google Scholar] [CrossRef]
- Düvel, A.; Kuhn, A.; Robben, L.; Wilkening, M.; Heitjans, P. Mechanosynthesis of solid electrolytes: Preparation, characterization, and Li ion transport properties of garnet type Al doped Li7La3Zr2O12 crystallizing with cubic symmetry. J. Phys. Chem. C 2012, 116, 15192–15202. [Google Scholar] [CrossRef]
- Kumazaki, S.; Iriyama, Y.; Kim, K.H.; Murugan, R.; Tanabe, K.; Yamamoto, K.; Hirayama, T.; Ogumi, Z. High lithium ion conductive Li7La3Zr2O12 by inclusion of both Al and Si. Electrochem. Commun. 2011, 13, 509–512. [Google Scholar] [CrossRef]
- Kumar, P.J.; Nishimura, K.; Senna, M.; Duvel, A.; Heitjans, P.; Kawaguchi, T.; Sakamoto, N.; Wakiya, N.; Suzuki, H. A novel low-temperature solid-state route for nanostructured cubic garnet Li7La3Zr2O12 and its application to Li-ion battery. RSC Adv. 2016, 6, 62656. [Google Scholar] [CrossRef] [Green Version]
- Ishiguro, K.; Nakata, Y.; Matsui, M.; Uechi, I.; Takeda, Y.; Yamamoto, O.; Imanishi, I. Stability of Nb-doped cubic Li7La3Zr2O12 with lithium metal. J. Electrochem. Soc. 2013, 10, A1690–A1693. [Google Scholar] [CrossRef]
- Gu, W.; Ezbiri, M.; Prasada Rao, R.; Avdeev, M.; Adams, S. Effects of penta- and trivalent dopants on structure and conductivity of Li7La3Zr2O12. Solid State Ion. 2015, 274, 100–105. [Google Scholar] [CrossRef]
- Allen, J.L.; Wolfenstine, J.; Rangasamy, E.; Sakamoto, J. Effect of substitution (Ta, Al, Ga) on the conductivity of Li7La3Zr2O12. J. Power Sources 2012, 206, 315–319. [Google Scholar] [CrossRef]
- Cheng, L.; Park, S.J.; Hou, H.; Zorba, V.; Chen, G.; Richardson, T.; Cabana, J.; Russo, R.; Doeff, M. Effect of microstructure and surface impurity segregation on the electrical and electrochemical properties of dense Al-substituted Li7La3Zr2O12. J. Mater. Chem. A 2014, 2, 172–181. [Google Scholar] [CrossRef] [Green Version]
- Kotobuki, M.; Kanamura, K.; Sato, Y.; Yoshida, T. Fabrication of all-solid-state lithium battery with lithium metal anode using Al2O3-added Li7La3Zr2O12 solid electrolyte. J. Power Sources 2011, 196, 7750–7754. [Google Scholar] [CrossRef]
- Lee, J.M.; Kim, T.; Baek, S.W.; Aihara, Y.; Park, Y.; Kim, Y.I.; Doo, S.G. High lithium ion conductivity of Li7La3Zr2O12 synthesized by solid state reaction. Solid State Ion. 2014, 258, 13–17. [Google Scholar] [CrossRef]
- Chen, R.J.; Huang, M.; Huang, W.Z.; Shen, Y.; Lin, Y.H.; Nan, C.W. Effect of calcining and Al doping on structure and conductivity of Li7La3Zr2O12. Solid State Ion. 2014, 265, 7–12. [Google Scholar] [CrossRef]
- Suryanarayana, C. Mechanical alloying and milling. Prog. Mater. Sci. 2001, 46, 1–184. [Google Scholar] [CrossRef]
- Rietveld, H.M. A profile refinement method for nuclear and magnetic structures. J. Appl. Cryst. 1969, 2, 65–71. [Google Scholar] [CrossRef]
- Xie, H.; Li, Y.T.; Goodenough, J.B. Low-temperature synthesis of Li7La3Zr2O12 with cubic garnet-type structure. Mater. Res. Bull. 2012, 47, 1229–1232. [Google Scholar] [CrossRef]
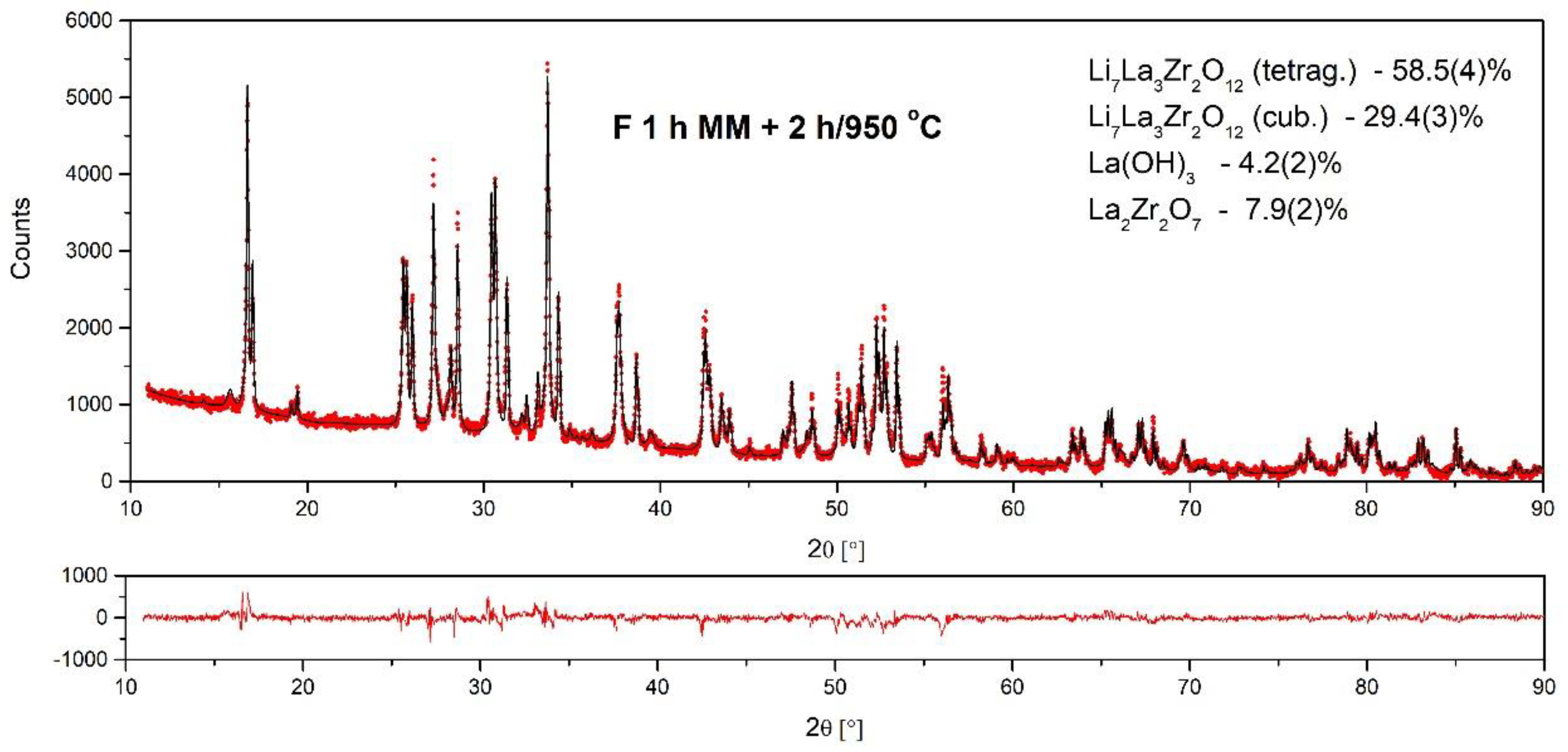
| Compound | Weight Content (%) | Cryst. Structure | Space Group | Lattice Parameters (Å) |
|---|---|---|---|---|
| Li7La3Zr2O12 | 29.4 (3) | cubic | IA–3d | a = 13.025 (1) |
| Li7La3Zr2O12 | 58.5 (4) | tetragonal | I41/acd | a = 13.1189 (8), c = 12.6698 (5) |
| La2Zr2O7 | 7.9 (2) | cubic | Fd-3m | a = 10.8226 (8) |
| La(OH)3 | 4.2 (2) | hexagonal | P63/m | a = 6.5346 (5), c = 3.8579 (5) |
| Milling and Annealing Parameters | c-LLZO Content (wt.%) ±2 (%) | Other Phases Detected |
|---|---|---|
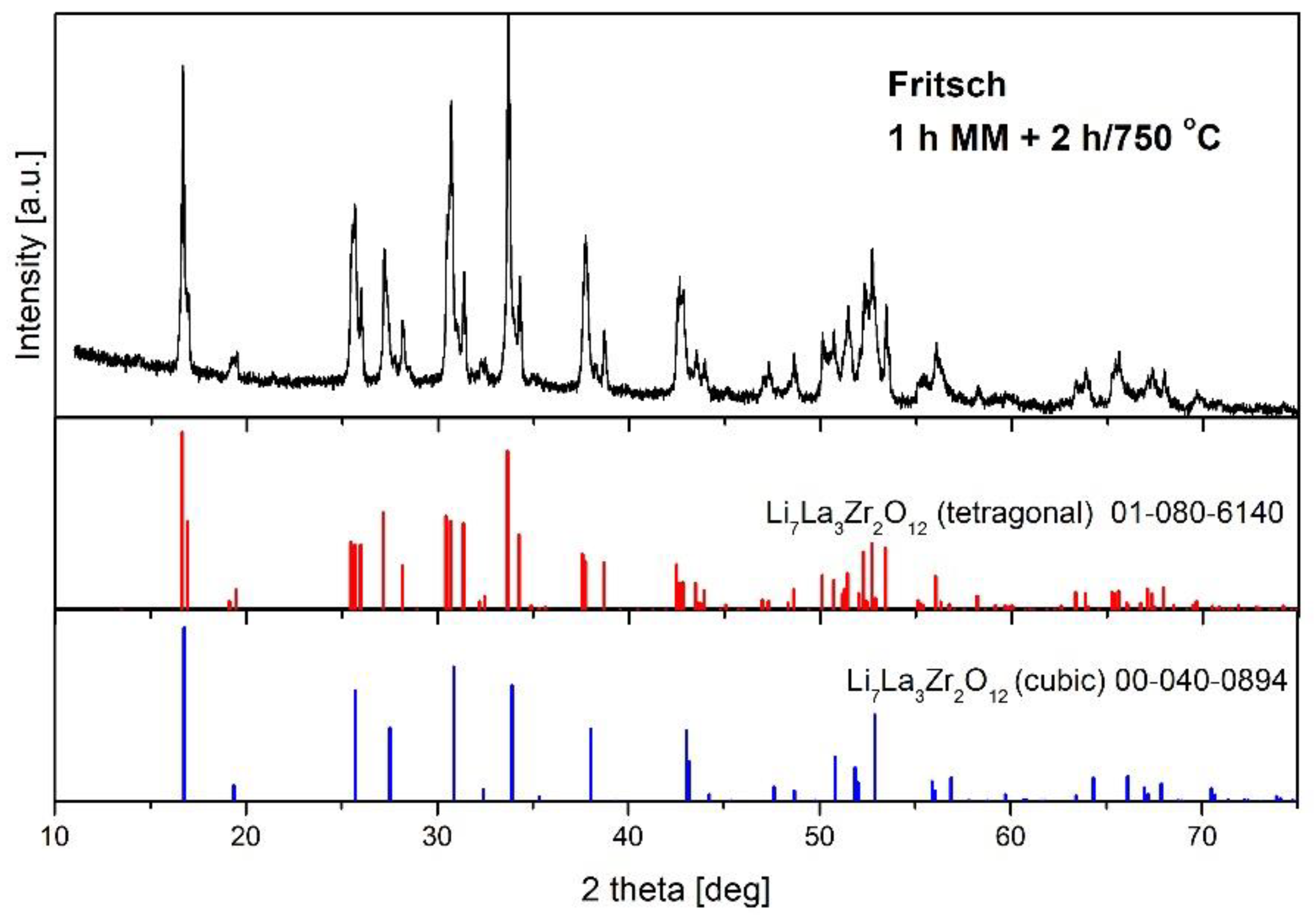
| Fritsch 1h MM + 750 °C/2h | 48 | t-LLZO Li2CO3 ZrO2 La2Zr2O7 LaAlO3 La(OH)3 La2O2CO3 La2O3 LaOOH |
| Fritsch 1 h MM + 750 °C/3 h | 90 | |
| Fritsch 1 h MM + 950 °C/2 h | 30 | |
| Fritsch 1 h MM + 950 °C/3h | 60 | |
| SPEX 1 h MM + 750 °C/3 h | 80 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oleszak, D.; Pawlyta, M.; Pikula, T. Influence of Powder Milling and Annealing Parameters on the Formation of Cubic Li7La3Zr2O12 Compound. Materials 2021, 14, 7633. https://doi.org/10.3390/ma14247633
Oleszak D, Pawlyta M, Pikula T. Influence of Powder Milling and Annealing Parameters on the Formation of Cubic Li7La3Zr2O12 Compound. Materials. 2021; 14(24):7633. https://doi.org/10.3390/ma14247633
Chicago/Turabian StyleOleszak, Dariusz, Mirosława Pawlyta, and Tomasz Pikula. 2021. "Influence of Powder Milling and Annealing Parameters on the Formation of Cubic Li7La3Zr2O12 Compound" Materials 14, no. 24: 7633. https://doi.org/10.3390/ma14247633
APA StyleOleszak, D., Pawlyta, M., & Pikula, T. (2021). Influence of Powder Milling and Annealing Parameters on the Formation of Cubic Li7La3Zr2O12 Compound. Materials, 14(24), 7633. https://doi.org/10.3390/ma14247633








