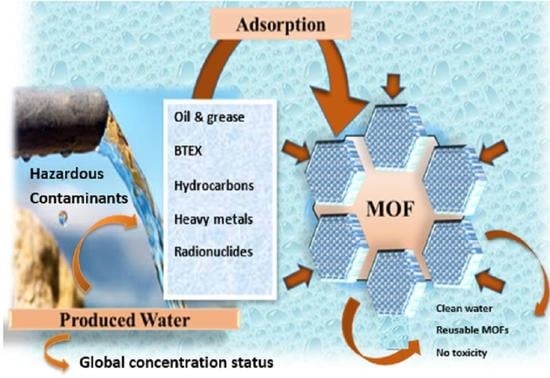
Produced Water Treatment with Conventional Adsorbents and MOF as an Alternative: A Review
Abstract
1. Introduction
2. Composition of Produced Water
2.1. Concentrations of Radioactive Compounds in Produced Water
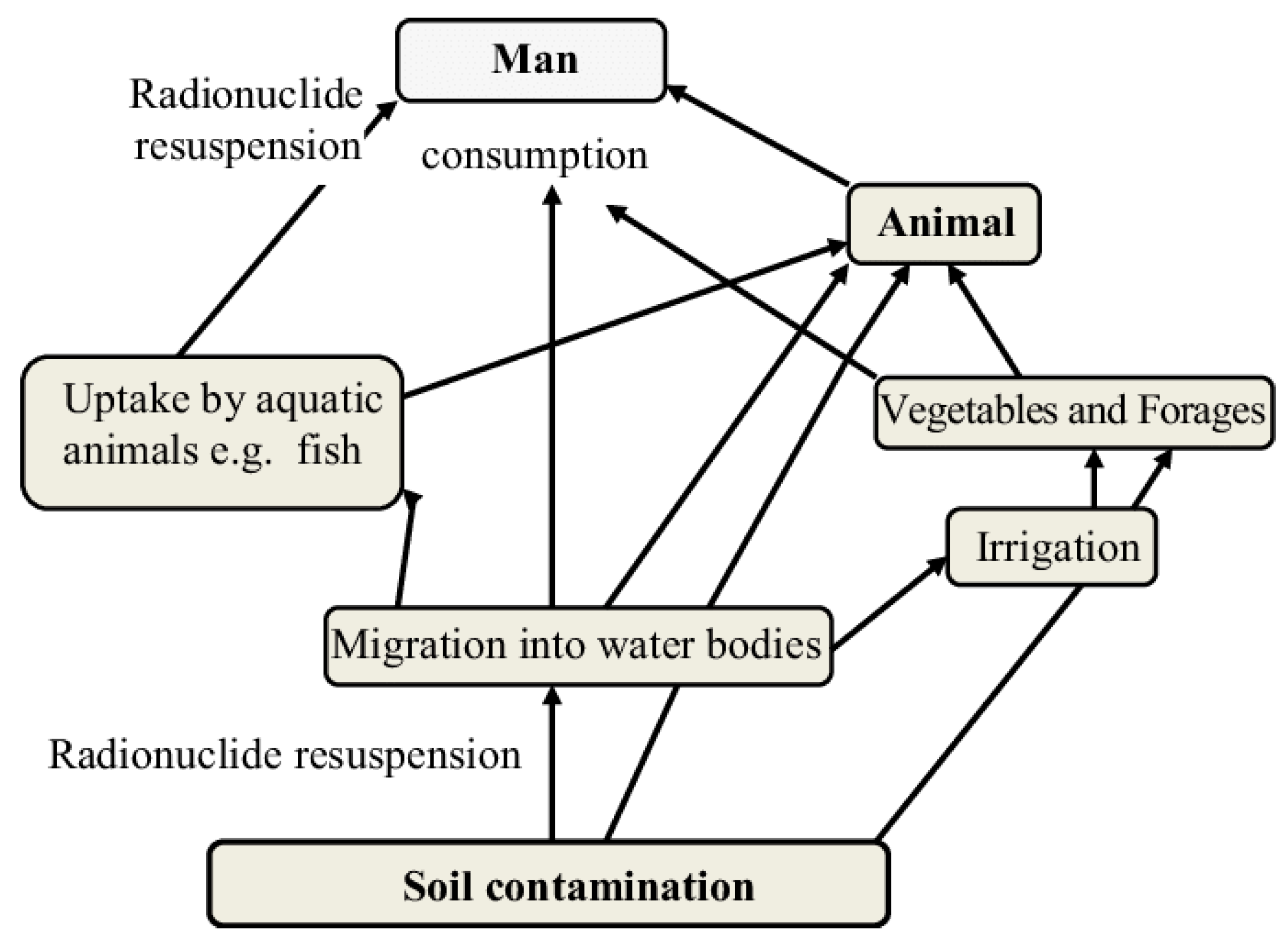
2.2. The Impact of Produced Water on the Environment
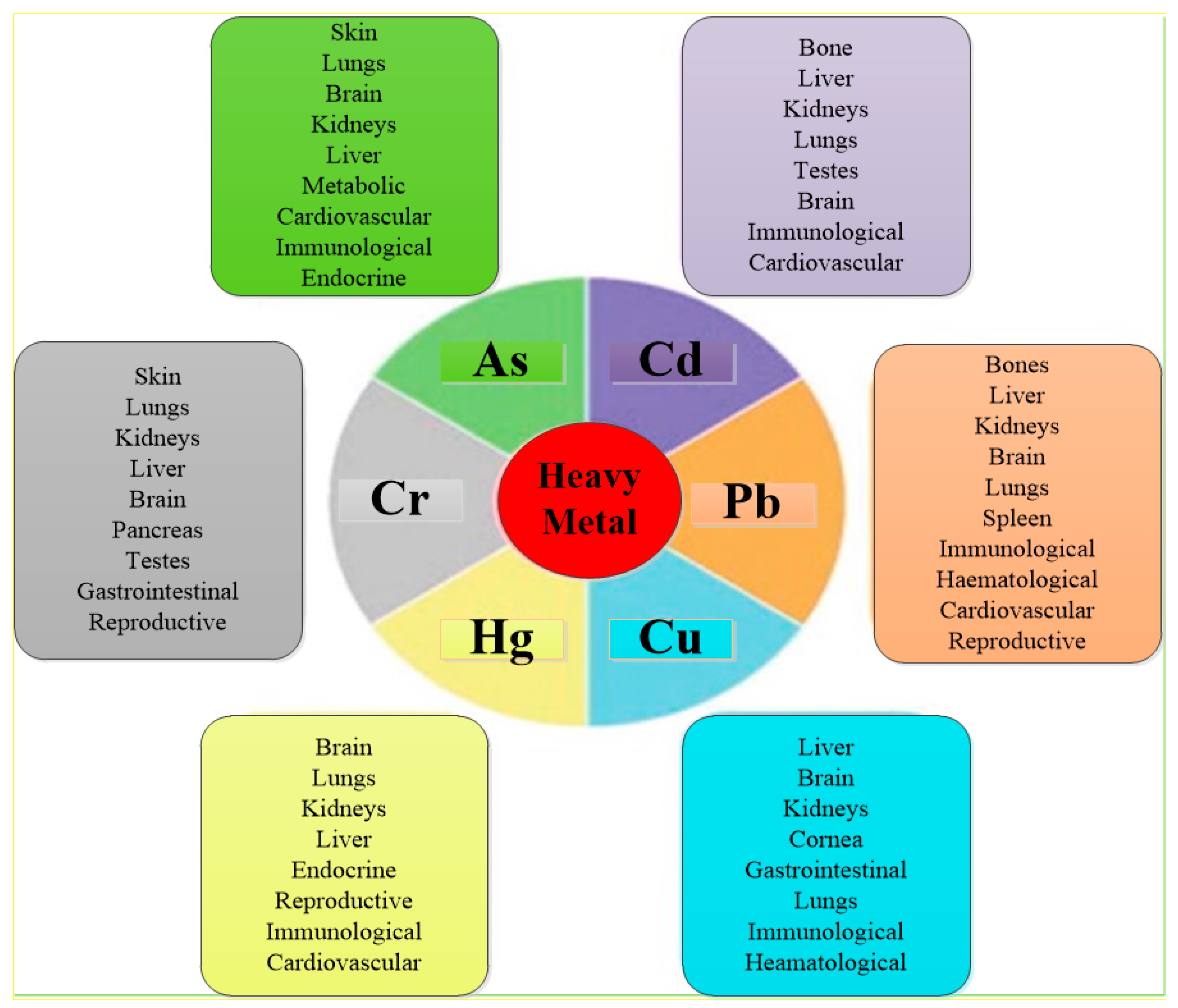
2.3. The Impact of Produced Water on Human Health
2.4. Produced Water Management—Discharge
2.5. The Reuse of Produced Water
3. Produced Water Treatment
3.1. Adsorption Classification
3.2. Factors Affecting the Adsorption Performance
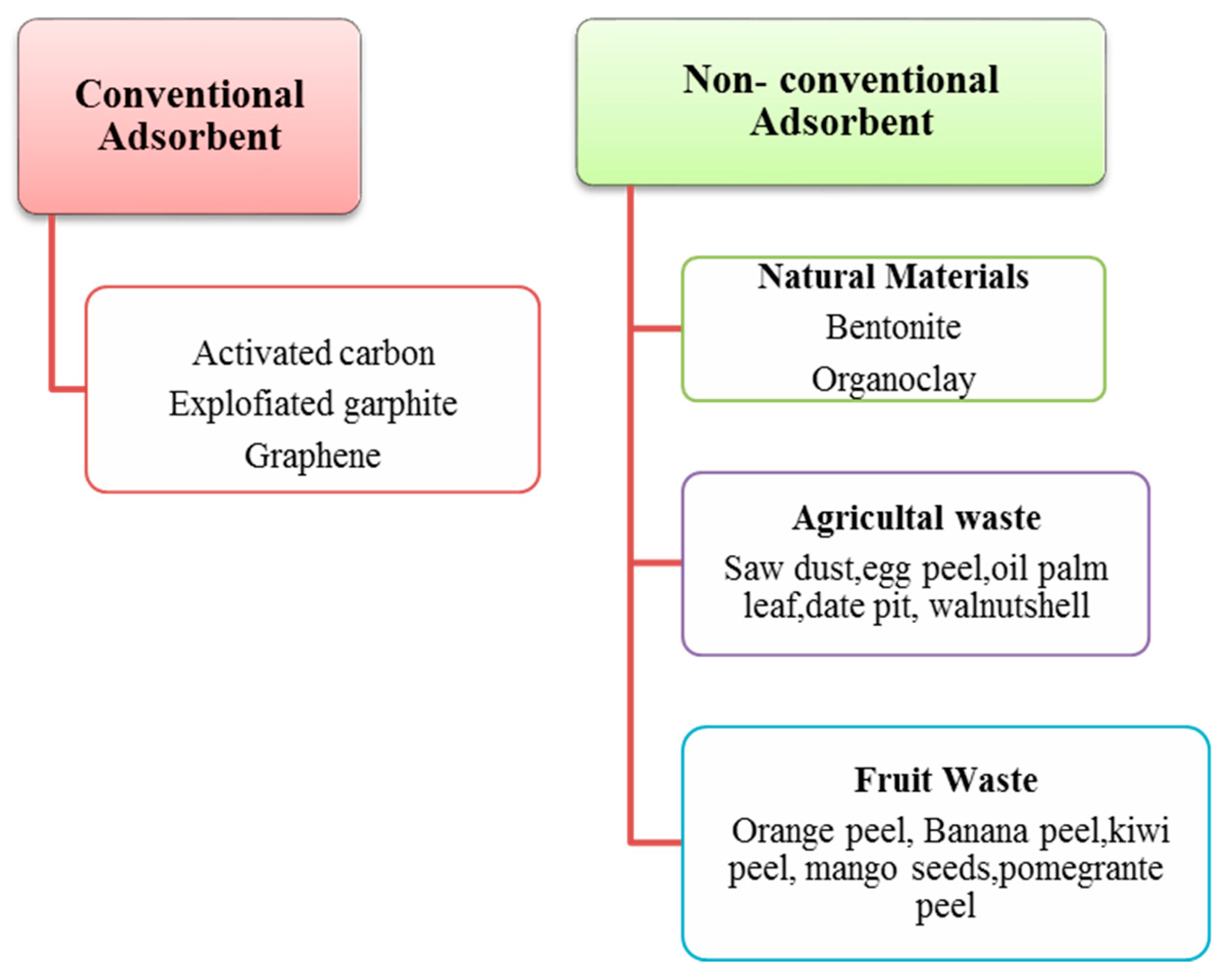
3.3. Conventional and Non-Conventional Adsorbents for Produced Water Treatment
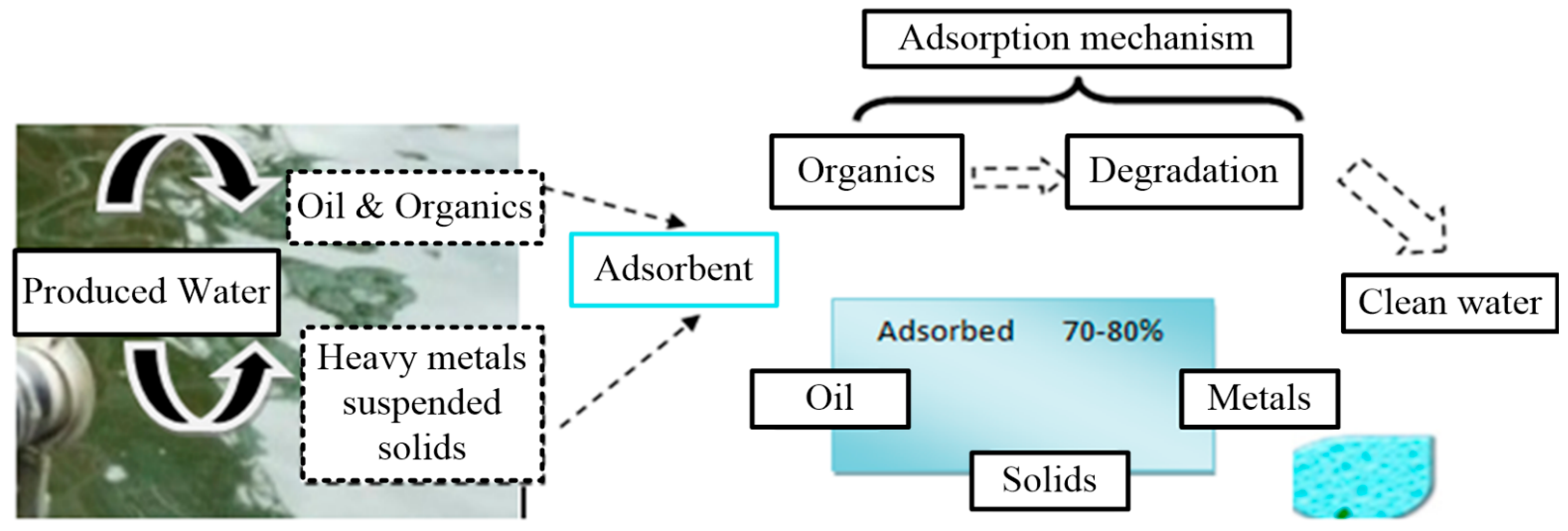
3.4. Produced Water Treatment
3.4.1. Oil Removal
3.4.2. Total Organic Carbon (TOC) Removal
3.4.3. BTEX Removal
3.4.4. Metals Removal
| Adsorbent | Targeted Pollutant | % Removal | Limitations | References |
|---|---|---|---|---|
| Sawdust | COD | 33% | Pre-treatment required to enhance efficiency | [134] |
| Walnut shell | COD | 49% | Carbon is lost during reactivation | [134] |
| Palm shell | COD | 56% | Loss of carbon during activation | [134] |
| Lime | Heavy metals | 95% | pH dependent; produces a large amount of sludge; overdose can cause poor effluent quality | [145] |
| Mxene nano adsorbent | Barium | 90% | Structure is not stable | [146] |
| Exfoliated graphite | TOC | - | Poor hydrophobicity; difficult to handle on-site because of their granular or powder forms | [122] |
| Peat and sawdust | BTEX | 67.8% and 57.8% | Mechanical strength of peat is low, and pretreatments are required to enhance the efficiency of sawdust | [137] |
| Modified organoclay | BTEX | 95.6% | Not suitable for pollutants that have a strongly acidic character; poor reusability and oil recovery | [138] |
3.5. Adsorption Limitations
3.6. Adsorption Isotherms and Kinetics
| Pollutant | Adsorbent | Isotherm Models | Kinetic Models | References |
|---|---|---|---|---|
| Oil and organic pollutant | Date pit | Langmuir | - | [120] |
| Walnut shell | Freundlich | - | ||
| Heavy metals | Fruit peel waste | Langmuir | Pseudo-second order | [113] |
| Oil | Pomegranate peel | Langmuir | Pseudo-second order | [118] |
| Oil | Amorphous carbon thin film (palm oil) | - | Thomas model | [149] |
| Oil | Banana peels | Langmuir | Pseudo-second order | [126] |
| Oil | Bentonite, PAC, and DC | Freundlich | - | [124] |
| Oil | Eggshells | - | Pseudo-second order | [125] |
| Oil | Eggplant peel | Langmuir | Pseudo-second order | [151] |
4. Recent Progress in the Development of Porous Adsorbent
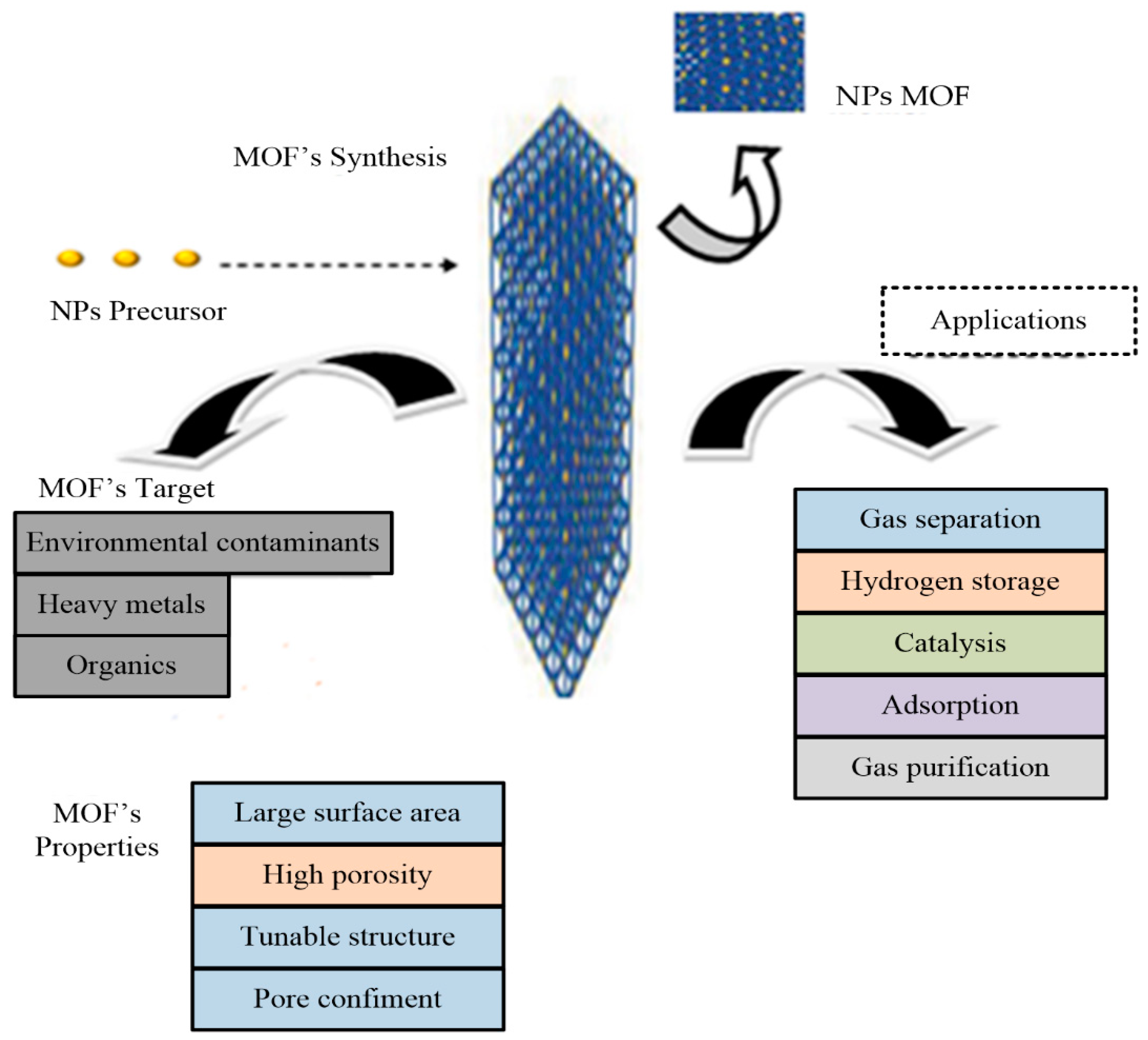
4.1. Metal–Organic Frameworks (MOFs)
4.2. The Significance of Metal–Organic Frameworks
4.3. Metal–Organic Frameworks as Adsorbents
- Water condensation in the solid pores shows steep uptake behavior.
- Facile desorption and adsorption for energy efficiency and a high uptake capacity for water.
- High water stability and cycling performance.
4.3.1. Adsorption of Organics
4.3.2. Adsorption of Heavy Metals
- Precious metal collection and recovery of these ions can contribute to the progress of their applications in industries.
- As hazardous pollutants, they can have serious negative health effects on human beings and ultimately could be a major global threat to the environment.
| MOFs | Pollutants | Removal Efficiency | References |
|---|---|---|---|
| MIL-53(Al)-GO | As (III) | 94.8% | [182] |
| 3D Cobalt MOF | Pb2+ Hg2+, Al2+, Fe3 Cd2+ | - | [184] |
| MOF-808 | As | 80.07% | [185] |
| MIL-100(Fe) | As | 98.2% | [181] |
| Cu-BTC | Hg2+ | 90.74% | [180] |
| MIL-96 | Arsenic | 80% | [190] |
| FMOF-1 | FMOF-1 | 87.7% | [175] |
| ZIF-8 | Hydroxymethylfurfura | 96.8% | [191] |
| UiO-66-NH2@MON | Toluene | 87.3% | [192] |
| UiO-66 | Methylchlorophenoxypropionic acid | 98.7% | [179] |
5. MOF Recycling
6. Future Research Perspectives
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Ac | Actinium |
| BOD | Biological oxygen demand |
| Cd | Cadmium |
| COD | Chemical oxygen demand |
| Cr | Chromium |
| Cu | Copper |
| DC | Deposited carbon |
| EG | Exfoliated graphite |
| FMOFs | Fluorous metal–organic frameworks |
| GM | Graphene magnetite |
| GN | Graphene nanoplatelets |
| HMF | Hydroxymethylfurfural |
| KW | Kiwi |
| MCPP | Methylchlorophenoxypropionic acid |
| MOFs | Metal–organic frameworks |
| NPD | Naphthalene, phenanthrene, dibenzothiophene |
| OIW | Non-polar oil in water |
| PAC | Powdered activated carbon |
| PPP | Pomegranate peel powder |
| PZC | Point of zero charge |
| Ra | Radium |
| TDS | Total dissolved solids |
| TENORM | Technologically enhanced naturally occurring radioactive materials |
| TOC | Total organic carbon |
| TSS | Total suspended solids |
| TOC | Total organic carbon |
| WS | Walnut shell |
| Zn | Zinc |
References
- Abd El-Ghaffar, M.; Abdel-Wahab, Z.; Elwakeel, K. Extraction and separation studies of silver (I) and copper (II) from their aqueous solution using chemically modified melamine resins. Hydrometallurgy 2009, 96, 27–34. [Google Scholar] [CrossRef]
- Bayati, F.; Shayegan, J.; Noorjahan, A. Treatment of oilfield produced water by dissolved air precipitation/solvent sublation. J. Pet. Sci. Eng. 2011, 80, 26–31. [Google Scholar] [CrossRef]
- Kabyl, A.; Yang, M.; Abbassi, R.; Li, S. A risk-based approach to produced water management in offshore oil and gas operations. Process. Saf. Environ. Prot. 2020, 139, 341–361. [Google Scholar] [CrossRef]
- Energy Outlook. International Energy Outlook. In Outlook; Washington, DC, USA. 2010. Available online: https://www.eia.gov/outlooks/ieo/index.php (accessed on 10 October 2021).
- Hu, L.; Yu, J.; Luo, H.; Wang, H.; Xu, P.; Zhang, Y. Simultaneous recovery of ammonium, potassium and magnesium from produced water by struvite precipitation. Chem. Eng. J. 2020, 382, 123001. [Google Scholar] [CrossRef]
- Al-Ghouti, M.A.; Al-Kaabi, M.A.; Ashfaq, M.Y.; Da’Na, D.A. Produced water characteristics, treatment and reuse: A review. J. Water Process. Eng. 2019, 28, 222–239. [Google Scholar] [CrossRef]
- Haneef, T.; Ul Mustafa, M.R.; Rasool, K.; Ho, Y.C.; Mohamed Kutty, S.R. Removal of polycyclic aromatic hydrocarbons in a heterogeneous Fenton like oxidation system using nanoscale zero-valent iron as a catalyst. Water 2020, 12, 2430. [Google Scholar] [CrossRef]
- Azetsu-Scott, K.; Yeats, P.; Wohlgeschaffen, G.; Dalziel, J.; Niven, S.; Lee, K. Precipitation of heavy metals in produced water: Influence on contaminant transport and toxicity. Mar. Environ. Res. 2007, 63, 146–167. [Google Scholar] [CrossRef] [PubMed]
- Hayes, T.; Arthur, D. Overview of Emerging Produced Water Treatment Technologies. In Proceedings of the 11th Annual International Petroleum Environmental Conference, Albuquerque, NM, USA, 12–15 October 2004. [Google Scholar]
- Estrada, J.M.; Bhamidimarri, R. A review of the issues and treatment options for wastewater from shale gas extraction by hydraulic fracturing. Fuel 2016, 182, 292–303. [Google Scholar] [CrossRef]
- Rahman, A.; Agrawal, S.; Nawaz, T.; Pan, S.; Selvaratnam, T. A review of algae-based produced water treatment for biomass and biofuel production. Water 2020, 12, 2351. [Google Scholar] [CrossRef]
- Chen, L.; Xu, Q.; Gossage, J.L.; Lou, H.H. Simulation and economic evaluation of a coupled thermal vapor compression desalination process for produced water management. J. Nat. Gas Sci. Eng. 2016, 36, 442–453. [Google Scholar] [CrossRef]
- Fakhru’L-Razi, A.; Pendashteh, A.; Abdullah, L.C.; Biak, D.R.A.; Madaeni, S.S.; Abidin, Z.Z. Review of technologies for oil and gas produced water treatment. J. Hazard. Mater. 2009, 170, 530–551. [Google Scholar] [CrossRef] [PubMed]
- Igunnu, E.T.; Chen, G.Z. Produced water treatment technologies. Int. J. Low-Carbon Technol. 2014, 9, 157–177. [Google Scholar] [CrossRef]
- Rezakazemi, M.; Amooghin, A.E.; Montazer-Rahmati, M.M.; Ismail, A.F.; Matsuura, T. State-of-the-art membrane based CO2 separation using mixed matrix membranes (MMMs): An overview on current status and future directions. Prog. Polym. Sci. 2014, 39, 817–861. [Google Scholar] [CrossRef]
- Younas, M.; Rezakazemi, M.; Daud, M.; Wazir, M.B.; Ahmad, S.; Ullah, N.; Inamuddin; Ramakrishna, S. Recent progress and remaining challenges in post-combustion CO2 capture using metal-organic frameworks (MOFs). Prog. Energy Combust. Sci. 2020, 80, 100849. [Google Scholar] [CrossRef]
- Roushani, M.; Saedi, Z.; Baghelani, Y.M. Removal of cadmium ions from aqueous solutions using TMU-16-NH2 metal organic framework. Environ. Nanotechnol. Monit. Manag. 2017, 7, 89–96. [Google Scholar]
- Gulzamana, H.; Baloo, L. Design Expert Application in the Optimization of Cadmium (II) by Chitosan from Produced water. Ann. Rom. Soc. Cell Biol. 2021, 25, 4687–4695. [Google Scholar]
- Ghosh, D.; Saha, R.; Ghosh, A.; Nandi, R.; Saha, B. A review on toxic cadmium biosorption from contaminated wastewater. Desalin. Water Treat. 2015, 53, 413–420. [Google Scholar] [CrossRef]
- NPC. Management of Produced Water from Oil and Gas Wells. National Petroleum Council. Available online: https://www.npc.org/Prudent_Development-Topic_Papers/2-17_Management_of_Produced_Water_Paper.pdf (accessed on 6 November 2021).
- Hoelzer, K.; Sumner, A.; Karatum, O.; Nelson, R.; Drollette, B.D.; O’Connor, M.P.; D’Ambro, E.L.; Getzinger, G.; Ferguson, P.L.; Reddy, C.M.; et al. Indications of transformation products from hydraulic fracturing additives in shale-gas wastewater. Environ. Sci. Technol. 2016, 50, 8036–8048. [Google Scholar] [CrossRef]
- Lee, K.; Cobanli, S.E.; Robinson, B.J.; Wohlgeschaffen, G. Application of Microbiological Methods to Assess the Potential Impact of Produced Water Discharges. In Produced Water: Environmental Risks and Mitigation Technologies; Springer Publishing: New York, NY, USA, 2011. [Google Scholar]
- Benko, K.L.; Drewes, J.E. Produced water in the Western United States: Geographical distribution, occurrence, and composition. Environ. Eng. Sci. 2008, 25, 239–246. [Google Scholar] [CrossRef]
- Faber, A.-H.; Annevelink, M.; Gilissen, H.K.; Schot, P.; van Rijswick, M.; de Voogt, P.; van Wezel, A. How to Adapt Chemical Risk Assessment for Unconventional Hydrocarbon Extraction Related to the Water System. In Reviews of Environmental Contamination and Toxicology; Springer: Singapore, 2017; Volume 246, pp. 1–32. [Google Scholar]
- Jiménez, S.; Andreozzi, M.; Micó, M.M.; Alvarez, M.G.; Contreras, S. Produced water treatment by advanced oxidation processes. Sci. Total Environ. 2019, 666, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Eriksen, D.Ø.; Sidhu, R.; Strålberg, E.; Iden, K.I.; Hylland, K.; Ruus, A.; Rye, H. Radionuclides in produced water from Norwegian oil and gas installations—Concentrations and bioavailability. Czechoslov. J. Phys. 2006, 56, D43–D48. [Google Scholar] [CrossRef]
- Hamlat, M.; Djeffal, S.; Kadi, H. Assessment of radiation exposures from naturally occurring radioactive materials in the oil and gas industry. Appl. Radiat. Isot. 2001, 55, 141–146. [Google Scholar] [CrossRef]
- Othman, I.M.E.-S.A.; Saleh, I.H.; Ghatass, Z.F.; Metwally, M.A.-A. Radiological Risk Assessment in a Type of Complex Petroleum Refinery in Egypt. Arab. J. Nucl. Sci. Appl. 2018, 51, 31–43. [Google Scholar] [CrossRef]
- Kh, K.H. Research into the radionuclide pollution of ecosystem on the territory of oil fields of Absheron peninsula. Kim. Probl. 2016, 3, 233–237. [Google Scholar]
- Shawky, S.; Amer, H.; Nada, A.; El-Maksoud, T.A.; Ibrahiem, N. Characteristics of NORM in the oil industry from Eastern and Western deserts of Egypt. Appl. Radiat. Isot. 2001, 55, 135–139. [Google Scholar] [CrossRef]
- Ali, M.M.; Zhao, H.; Li, Z.; Ayoub, A.A. A review about radioactivity in TENORMs of produced water waste from petroleum industry and its environmental and health effects. EES 2020, 467, 012120. [Google Scholar] [CrossRef]
- McDevitt, B.; McLaughlin, M.; Cravotta, C.A.; Ajemigbitse, M.A.; Van Sice, K.J.; Blotevogel, J.; Borch, T.; Warner, N.R. Emerging investigator series: Radium accumulation in carbonate river sediments at oil and gas produced water discharges: Implications for beneficial use as disposal management. Environ. Sci. Process. Impacts 2019, 21, 324–338. [Google Scholar] [CrossRef]
- Kraemer, T.F.; Reid, D.F. The occurrence and behavior of radium in saline formation water of the U.S. Gulf Coast region. Chem. Geol. 1984, 46, 153–174. [Google Scholar] [CrossRef]
- Jodłowski, P.; Macuda, J.; Nowak, J.; Dinh, C.N. Radioactivity in wastes generated from shale gas exploration and production—North-Eastern Poland. J. Environ. Radioact. 2017, 175, 34–38. [Google Scholar] [CrossRef] [PubMed]
- Lagera, L.; Hart, A.; Graham, B. Radionuclides in Oil and gas Operational Discharges and Environmental Samples Associated with Offshore Oil and Gas Production Facilities. In Radionuclides, Metals, and Hydrocarbons in Oil and gas Operational Discharges and Environmental Samples Associated with Offshore Production Facilities on the Texas/Louisiana Continental Shelf with an Environmental Assessment of Metals and Hydrocarbons; Prepared for US Department of Energy; Continental Shelf Associates, Inc.: Jupiter, FL, USA, 1999. Available online: https://www.osti.gov/servlets/purl/9730#page=96 (accessed on 10 October 2021).
- Gaefvert, T.; Færevik, I. Natural Radioactivity in Produced Water from the Norwegian Oil and Gas Industry in 2003; Statens Straalevern: Oesteraas, Norway, 2005. [Google Scholar]
- Kpeglo, D.O. Radiation Exposure to Natural Radioactivity in Crude Oil and Petroleum Waste from Oil Fields in Ghana; Modelling, Risk Assessment and Regulatory Control. Ph.D. Thesis, University of Ghana, Accra, Ghana, 2015. [Google Scholar]
- Agbalagba, E.; Avwiri, G.; Ononugbo, C. Activity concentration and radiological impact assessment of 226Ra, 228Ra and 40K in drinking waters from (OML) 30, 58 and 61 oil fields and host communities in Niger Delta region of Nigeria. J. Environ. Radioact. 2013, 116, 197–200. [Google Scholar] [CrossRef]
- Avwiri Gregory, O.; Emmanuel, E.; Ezekiel, O.A. Gamma spectroscopy analysis of produced water from selected flow stations in delta state, Nigeria. Int. J. Environ. Monit. Anal. 2013, 1, 167–174. [Google Scholar]
- Parmaksız, A.; Ağuş, Y.; Bulgurlu, F.; Bulur, E.; Öncü, T.; Özkök, Y. Measurement of enhanced radium isotopes in oil production wastes in Turkey. J. Environ. Radioact. 2015, 141, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Faanu, A.; Ephraim, J.H.; Darko, E.O. Assessment of public exposure to naturally occurring radioactive materials from mining and mineral processing activities of Tarkwa Goldmine in Ghana. Environ. Monit. Assess. 2011, 180, 15–29. [Google Scholar] [CrossRef] [PubMed]
- Canoba, A.; Gnoni, G.; Truppa, W.; Nuclear, A.R. Norm measurements in the oil and gas industry in Argentina. In PARTE I. 2007. Available online: https://www.argentina.gob.ar/sites/default/files/mt_2007a.pdf#page=34 (accessed on 10 October 2021).
- Pillay, A.E.; Salih, F.M.; Maleek, M.I. Radioactivity in oily sludge and produced waste water from oil: Environmental concerns and potential remedial measures. Sustainability 2010, 2, 890–901. [Google Scholar] [CrossRef]
- Khadhim, N.F.; Adnan, O.H. Measurement of natural radioactivity in Al-Dora Refinery by using (HPGe) detector. Adv. Appl. Sci. Res. 2016, 7, 197–208. [Google Scholar]
- Ali, K.K.; Shafik, S.S.; Husain, H.A. Radiological Assessment of NORM Resulting From Oil and Gas Production Processing in South Rumaila Oil Field, Southern Iraq. Iraqi J. Sci. 2017, 58, 1037–1050. [Google Scholar]
- Moatar, F.; Shadizadeh, S.R.; Karbassi, A.R.; Ardalani, E.; Derakhshi, R.A.; Asadi, M. Determination of naturally occurring radioactive materials (NORM) in formation water during oil exploration. J. Radioanal. Nucl. Chem. 2010, 283, 3–7. [Google Scholar] [CrossRef]
- Botezatu, E.; Grecea, C. Radiological impact assessment on behalf of Oil/Gas Industry. J. Prev. Med. 2004, 12, 16–21. [Google Scholar]
- Zakaria, K.M. Radiological Impacts of Norm and Poly Aromatic Hydrocarbon in Petroleum Industry Process on Marine Ecosystem at the Red Sea, Egypt. Environ. Anal. Ecol. Stud. 2018, 1. [Google Scholar] [CrossRef]
- Testa, C.; Desideri, D.; Meli, M.A.; Roselli, C.; Bassignani, A.; Colombo, G.; Fantoni, R.F. Radiation protection and radioactive scales in oil and gas production. Health Phys. 1994, 67, 34–38. [Google Scholar] [CrossRef] [PubMed]
- Otton, J.K. Environmental Aspects of Produced-Water Salt Releases in Onshore and Coastal Petroleum-Producing Areas of the Conterminous US—A Bibliography; USGS Publication Warehouse; US Geological Survey: Reston, VA, USA, 2006. Available online: https://pubs.er.usgs.gov/publication/ofr20061154 (accessed on 6 November 2021).
- Hedar, Y. Pollution Impact and Alternative Treatment for Produced Water. In Proceedings of the 2nd International Conference on Energy, Environmental and Information System (ICENIS 2017), Semarang, Indonesia, 15–16 August 2018; Volume 31, p. 03004. [Google Scholar] [CrossRef]
- Jiménez, S.; Micó, M.M.; Arnaldos, M.; Medina, F.; Contreras, S. State of the art of produced water treatment. Chemosphere 2018, 192, 186–208. [Google Scholar] [CrossRef] [PubMed]
- Bostick, D.T. Characterization of Soluble Organics in Produced Water; ORNL Oak Ridge National Laboratory (US): Oak Ridge, TN, USA, 2002. Available online: https://www.osti.gov/biblio/814231 (accessed on 10 October 2021).
- Krause, P. Spatial and temporal variability in receiving water toxicity near an oil effluent discharge site. Arch. Environ. Contam. Toxicol. 1995, 29, 523–529. [Google Scholar] [CrossRef]
- Guerra, K.; Dahm, K.; Dundorf, S. Oil and Gas Produced Water Management and Beneficial Use in the Western United States; US Department of the Interior, Bureau of Reclamation: Denver, CO, USA, 2011. Available online: https://www.usbr.gov/research/dwpr/reportpdfs/report157.pdf (accessed on 10 October 2021).
- Gazali, A.K. Environmental Impact opf Produced Water and Drilling Waste Discharges from the Niger Delta Petroleum Industry. IOSR J. Eng. 2017, 7, 22–29. [Google Scholar] [CrossRef]
- Neff, J.; Lee, K.; DeBlois, E.M. Produced Water: Overview of Composition, Fates, and Effects. In Produced Water; Springer: Springer, 2011; pp. 3–54. [Google Scholar]
- Neff, J.M. Bioaccumulation in Marine Organisms: Effect of Contaminants from Oil Well Produced Water; Elsevier: Amsterdam, The Netherlands, 2002. [Google Scholar]
- Elias-Samlalsingh, N.; Agard, J.B. Application of toxicity identification evaluation procedures for characterizing produced water using the tropical mysid, Metamysidopsis insularis. Environ. Toxicol. Chem. Int. J. 2004, 23, 1194–1203. [Google Scholar] [CrossRef]
- Schifter, I.; González-Macías, C.; Salazar-Coria, L.; Sánchez-Reyna, G.; González-Lozano, C. Long-term effects of discharges of produced water the marine environment from petroleum-related activities at Sonda de Campeche, Gulf of México. Environ. Monit. Assess. 2015, 187, 723. [Google Scholar] [CrossRef]
- Taqi, A.; Al-Ani, L.A.A.; Ali, A.M. Assessment of the natural radioactivity levels in Kirkuk oil field. J. Radiat. Res. Appl. Sci. 2016, 9, 337–344. [Google Scholar] [CrossRef]
- Mountford, P.; Temperton, D. Recommendations of the International Commission on Radiological Protection (ICRP) 1990; Springer: Berlin/Heidelberg, Germany, 1992. [Google Scholar]
- Taheri, A.; Taheri, A.; Fathivand, A.A.; Mansouri, N. Risk assessment of naturally occurring radioactive materials (NORM) in the hydrocarbon sludge extracted from the south pars gas field in Iran. Process. Saf. Environ. Prot. 2019, 125, 102–120. [Google Scholar] [CrossRef]
- Ali, M.M.; Zhao, H.; Li, Z.; Maglas, N.N. Concentrations of TENORMs in the petroleum industry and their environmental and health effects. RSC Adv. 2019, 9, 39201–39229. [Google Scholar] [CrossRef]
- Abo-Elmagd, M.; Soliman, H.; Salman, K.; El-Masry, N. Radiological hazards of TENORM in the wasted petroleum pipes. J. Environ. Radioact. 2009, 101, 51–54. [Google Scholar] [CrossRef] [PubMed]
- Al-Saleh, F.; Al-Harshan, G. Measurements of radiation level in petroleum products and wastes in Riyadh City Refinery. J. Environ. Radioact. 2008, 99, 1026–1031. [Google Scholar] [CrossRef] [PubMed]
- Olobatoke, R.Y.; Mathuthu, M. Radionuclide exposure in animals and the public health implications. Turk. J. Vet. Anim. Sci. 2015, 39, 381–388. [Google Scholar] [CrossRef]
- Masindi, V.; Muedi, K.L. Environmental Contamination by Heavy Metals. Heavy Met. 2018, 10, 115–132. [Google Scholar]
- Grant, A.; Briggs, A.D. Toxicity of sediments from around a North Sea oil platform: Are metals or hydrocarbons responsible for ecological impacts? Mar. Environ. Res. 2002, 53, 95–116. [Google Scholar] [CrossRef]
- Somma, S.; Reverchon, E.; Baldino, L. Water Purification of Classical and Emerging Organic Pollutants: An Extensive Review. ChemEngineering 2021, 5, 47. [Google Scholar] [CrossRef]
- Holdway, D.A. The acute and chronic effects of wastes associated with offshore oil and gas production on temperate and tropical marine ecological processes. Mar. Pollut. Bull. 2002, 44, 185–203. [Google Scholar] [CrossRef]
- Ahmad, F.; Morris, K.; Law, G.T.W.; Taylor, K.G.; Shaw, S. Fate of radium on the discharge of oil and gas produced water to the marine environment. Chemosphere 2021, 273, 129550. [Google Scholar] [CrossRef]
- McCormack, P. Analysis of oilfield produced waters and production chemicals by electrospray ionisation multi-stage mass spectrometry (ESI-MSn). Water Res. 2001, 35, 3567–3578. [Google Scholar] [CrossRef]
- Veil, J. US Produced Water Volumes and Management Practices in 2012; Groundwater Protection Council: Oklahoma City, OK, USA, 2015. [Google Scholar]
- Sweet, D. Energy in the United States: An Industry Analysis; Appalachian State University: Boone, NC, USA, 2020. [Google Scholar]
- Lin, L.; Jiang, W.; Chen, L.; Xu, P.; Wang, H. Treatment of Produced Water with Photocatalysis: Recent Advances, Affecting Factors and Future Research Prospects. Catalysts 2020, 10, 924. [Google Scholar] [CrossRef]
- Mondal, S.; Wickramasinghe, S.R. Produced water treatment by nanofiltration and reverse osmosis membranes. J. Membr. Sci. 2008, 322, 162–170. [Google Scholar] [CrossRef]
- Khatib, Z.; Verbeek, P. Water to Value—Produced Water Management for Sustainable Field Development of Mature and Green Fields. J. Pet. Technol. 2003, 55, 26–28. [Google Scholar] [CrossRef]
- Burnett, D.B.; Siddiqui, M. Recovery of Fresh Water Resources from Desalination of Brine Produced during Oil and Gas Production Operations; Texas Engineering Experiment Station: College Station, TX, USA, 2006. [Google Scholar] [CrossRef][Green Version]
- Wang, Z.; Gong, Z.; Wang, Z.; Li, X.; Chu, Z. Application and development of pyrolysis technology in petroleum oily sludge treatment. Environ. Eng. Res. 2020, 26, 190460. [Google Scholar] [CrossRef]
- Al-abri, O.H.; Lakkimsetty, N.R.; Shaik, F. pretreatment of oil produced water using low cost adsorbents. Int. J. of Mech. Prod. Eng. Res. Dev. IJMPERD 2020, 10, 2435–2444. [Google Scholar]
- United States Environmental Protection Agency. Oil and Gas Extraction Effluent Guidelines; EPA: Washington, DC, USA, 2019. Available online: https://www.epa.gov/eg/oiland-gas-extraction-effluent-guidelines (accessed on 20 November 2021).
- Clark, C.E.; Veil, J.A. Produced Water Volumes and Management Practices in the United States; Argonne National Lab.(ANL): Argonne, IL, USA, 2009.
- Muherei, M.; Junin, R. Potential of surfactant washing to solve drilling waste environmental problems off shore. J. Eng. Res. 2007, 12, 1–10. [Google Scholar]
- CAPP. Technical Report: Offshore Produced Water Waste Management. CAPP (Canadian Association of Petroleum). Available online: http://www.capp.ca (accessed on 19 November 2021).
- Hildenbrand, Z.L.; Santos, I.; Liden, T.; Carlton, D.D.; Varona-Torres, E.; Martin, M.S.; Reyes, M.L.; Mulla, S.R.; Schug, K.A. Characterizing variable biogeochemical changes during the treatment of produced oilfield waste. Sci. Total Environ. 2018, 634, 1519–1529. [Google Scholar] [CrossRef] [PubMed]
- Thompson, A.W.; Prokopy, L.S. Tracking urban sprawl: Using spatial data to inform farmland preservation policy. Land Use Policy 2009, 26, 194–202. [Google Scholar] [CrossRef]
- Sheikholeslami, Z.; Kebria, D.Y.; Qaderi, F. Nanoparticle for degradation of BTEX in produced water; an experimental procedure. J. Mol. Liq. 2018, 264, 476–482. [Google Scholar] [CrossRef]
- Drewes, J.E.; Cath, T.Y.; Xu, P.; Graydon, J.; Veil, J.; Snyder, S. An Integrated Framework for Treatment and Management of Produced Water; RPSEA Project; 2009; p. 07122-12. Available online: http://aqwatec.mines.edu/research/projects/Tech_Assessment_PW_Treatment_Tech.pdf (accessed on 10 October 2021).
- Li, Y.S.; Yan, L.; Xiang, C.B.; Hong, L.J. Treatment of oily wastewater by organic–inorganic composite tubular ultrafiltration (UF) membranes. Desalination 2006, 196, 76–83. [Google Scholar] [CrossRef]
- Lusinier, N.; Seyssiecq, I.; Sambusiti, C.; Jacob, M.; Lesage, N.; Roche, N. Biological Treatments of Oilfield Produced Water: A Comprehensive Review. SPE J. 2019, 24, 2135–2147. [Google Scholar] [CrossRef]
- Qu, F.; Zhu, L.; Yang, K. Adsorption behaviors of volatile organic compounds (VOCs) on porous clay heterostructures (PCH). J. Hazard. Mater. 2009, 170, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Daigle, T.P.; Cox, L.D. Ultra deep water discharge of produced water and/or solids at the seabed. In Research Partnership to Secure Energy for America (RPSEA); USA. 2016. Available online: www.rpsea.org (accessed on 10 October 2021).
- Abesekara, M.S.; Kosvinna, K.N.R.; Amarasinghe, B. Adsorption and desorption studies of Ni2+ ions on to coconut shell char. In Proceedings of the 2nd International Symposium on Water Pollution and Treatment, Bangkok, Thailand, 17–18 October 2019; Volume 427, p. 012005. [Google Scholar] [CrossRef]
- Ayob, S.; Othman, N.; Altowayti, W.A.H.; Khalid, F.S.; Abu Bakar, N.; Tahir, M.; Soedjono, E.S. A Review on Adsorption of Heavy Metals from Wood-Industrial Wastewater by Oil Palm Waste. J. Ecol. Eng. 2021, 22, 249–265. [Google Scholar] [CrossRef]
- Mathew, B.B.; Jaishankar, M.; Biju, V.G.; Beeregowda, K.N. Role of Bioadsorbents in Reducing Toxic Metals. J. Toxicol. 2016, 2016, 4369604. [Google Scholar] [CrossRef]
- Sims, R.A.; Harmer, S.L.; Quinton, J.S. The role of physisorption and chemisorption in the oscillatory adsorption of organosilanes on aluminium oxide. Polymers 2019, 11, 410. [Google Scholar] [CrossRef] [PubMed]
- Olanipekun, O.; Oyefusi, A.; Neelgund, G.M.; Oki, A. Adsorption of lead over graphite oxide. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 118, 857–860. [Google Scholar] [CrossRef]
- Lasheen, M.; El-Sherif, I.Y.; El-Wakeel, S.T.; Sabry, D.Y.; El-Shahat, M.F. Heavy metals removal from aqueous solution using magnetite Dowex 50WX4 resin nanocomposite. JMES 2017, 8, 503–511. [Google Scholar]
- Doyle, D.; Brown, A. Produced water treatment and hydrocarbon removal with organoclay. In Proceedings of the SPE Annual Technical Conference and Exhibition, Society of Petroleum Engineers, Dallas, TX, USA, 1–4 October 2000. [Google Scholar] [CrossRef]
- Carvalho, M.; Clarisse, M.; Lucas, E.; Barbosa, C.; Barbosa, L. Evaluation of the polymeric materials (DVB copolymers) for produced water treatment. In Proceedings of the International Petroleum Exhibition and Conference, Abu Dhabi, United Arab Emirates, 13–16 October 2002. [Google Scholar]
- Janks, J.S.; Cadena, F. Investigations into the Use of Modified Zeolites for Removing Benzene, Toluene, and Xylene from Saline Produced Water. In Produced Water; Springer: Singapore, 1992; pp. 473–487. [Google Scholar]
- Arthur, J.D.; Langhus, B.G.; Patel, C. Technical Summary of Oil & Gas Produced Water Treatment Technologies; All Consulting, LLC: Tulsa, Oklahoma, 2005. [Google Scholar]
- Elsayed, A.E.; Osman, D.I.; Attia, S.K.; Ahmed, H.M.; Shoukry, E.M.; Mostafa, Y.M.; Taman, A.R. A study on the removal characteristics of organic and inorganic pollutants from wastewater by low cost biosorbent. Egypt. J. Chem. 2020, 63, 1429–1442. [Google Scholar] [CrossRef]
- Imamoglu, M.; Tekir, O. Removal of copper (II) and lead (II) ions from aqueous solutions by adsorption on activated carbon from a new precursor hazelnut husks. Desalination 2008, 228, 108–113. [Google Scholar] [CrossRef]
- Krishnan, K.A.; Anirudhan, T. Removal of cadmium(II) from aqueous solutions by steam-activated sulphurised carbon prepared from sugar-cane bagasse pith: Kinetics and equilibrium studies. Water SA 2003, 29, 147–156. [Google Scholar] [CrossRef]
- Gusain, R.; Kumar, N.; Ray, S.S. Recent advances in carbon nanomaterial-based adsorbents for water purification. Coord. Chem. Rev. 2020, 405, 213111. [Google Scholar] [CrossRef]
- Renugadevi, N.; Sangeetha, R.; Lalitha, P. Kinetics of the adsorption of methylene blue from an industrial dyeing effluent onto activated carbon prepared from the fruits of Mimusops elengi. Arch. Appl. Sci. Res. 2011, 3, 492–498. [Google Scholar]
- Abechi, E.; Gimba, C.E.; Uzairu, A.; Kagbu, J.A. Kinetics of adsorption of methylene blue onto activated carbon prepared from palm kernel shell. Arch. Appl. Sci. Res. 2011, 3, 154–164. [Google Scholar]
- Ghane Ghanad, I. Atmospheric Leaching of Enargite in Iron Sulfate Solutions Catalyzed by Activated Carbon. Ph.D. Thesis, University of British Columbia, Vancouver, BC, Cananda, 2011. [Google Scholar]
- Li, X.; Chen, S.; Fan, X.; Quan, X.; Tan, F.; Zhang, Y.; Gao, J. Adsorption of ciprofloxacin, bisphenol and 2-chlorophenol on electrospun carbon nanofibers: In comparison with powder activated carbon. J. Colloid Interface Sci. 2015, 447, 120–127. [Google Scholar] [CrossRef]
- Matsui, M.; Kiyozumi, Y.; Yamamoto, T.; Mizushina, Y.; Mizukami, F.; Sakaguchi, K. Selective adsorption of biopolymers on zeolites. Chem. A Eur. J. 2001, 7, 1555–1560. [Google Scholar] [CrossRef]
- Pathak, P.; Mandavgane, S.; Kulkarni, B.D. Fruit peel waste as a novel low-cost bio adsorbent. Rev. Chem. Eng. 2015, 31, 361–381. [Google Scholar] [CrossRef]
- Mahmudi, M.; Arsad, S.; Amalia, M.C.; Rohmaningsih, H.A.; Prasetiya, F.S. An Alternative Activated Carbon from Agricultural Waste on Chromium Removal. J. Ecol. Eng. 2020, 21, 1–9. [Google Scholar] [CrossRef]
- Bhatnagar, A.; Minocha, A. Adsorptive removal of 2,4-dichlorophenol from water utilizing Punica granatum peel waste and stabilization with cement. J. Hazard. Mater. 2009, 168, 1111–1117. [Google Scholar] [CrossRef]
- Fillo, J.P.; Koraido, S.M.; Evans, J.M. Sources, Characteristics, and Management of Produced Waters from Natural Gas Production and Storage Operations. In Produced Water; Springer: Singapore, 1992; pp. 151–161. [Google Scholar]
- Johnson, B.M.; Kanagy, L.E.; Rodgers Jr, J.H.; Castle, J.W. Feasibility of a pilot-scale hybrid constructed wetland treatment system for simulated natural gas storage produced waters. Environ. Geosci. 2008, 15, 91–104. [Google Scholar] [CrossRef]
- Ibrahim, T.H.; Gulistan, A.S.; Khamis, M.I.; Ahmed, H.; Aidan, A. Produced water treatment using naturally abundant pomegranate peel. Desalin. Water Treat. 2016, 57, 6693–6701. [Google Scholar] [CrossRef]
- Jafer, A.S.; Hassan, A.A. Removal of oil content in oilfield produced water using chemically modified kiwi peels as efficient low-cost adsorbent. J. Phys. Conf. Ser. 2019, 1294, 072013. [Google Scholar] [CrossRef]
- Alsulaili, A.D.; Fahim, A.M. Oil removal from produced water by agriculture waste adsorbents. Int. J. Environ. Waste Manag. 2020, 25, 12–31. [Google Scholar] [CrossRef]
- El-Sayed, M.; Ramzi, M.; Hosny, R.; Fathy, M.; Moghny, T.A. Breakthrough curves of oil adsorption on novel amorphous carbon thin film. Water Sci. Technol. 2016, 73, 2361–2369. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, K.; Kitazawa, H.; Fujishige, M.; Akuzawa, N.; Ortiz-Medina, J.; Morelos-Gomez, A.; Cruz-Silva, R.; Araki, T.; Hayashi, T.; Endo, M. Oil removing properties of exfoliated graphite in actual produced water treatment. J. Water Process. Eng. 2017, 20, 226–231. [Google Scholar] [CrossRef]
- Chacra, L.A.; Sabri, M.A.; Ibrahim, T.H.; Khamis, M.; Hamdan, N.; Al-Asheh, S.; AlRefai, M.; Fernandez, C. Application of graphene nanoplatelets and graphene magnetite for the removal of emulsified oil from produced water. J. Environ. Chem. Eng. 2018, 6, 3018–3033. [Google Scholar] [CrossRef]
- Okiel, K.; El-Sayed, M.; El-Kady, M.Y. Treatment of oil–water emulsions by adsorption onto activated carbon, bentonite and deposited carbon. Egypt. J. Pet. 2011, 20, 9–15. [Google Scholar] [CrossRef]
- Muhammad, I.; El-Nafaty, U.A.; Abdulsalam, S.; Makarfi, Y.I. Removal of oil from oil produced water using eggshell. Civ. Environ. Res. 2012, 2, 52–63. [Google Scholar]
- El-Nafaty, U.; Muhammad, I.; Abdulsalam, S. Biosorption and kinetic studies on oil removal from produced water using banana peel. Civ. Environ. Res. 2013, 3, 125–136. [Google Scholar]
- El-Nafaty, U.; Abdulsalam Sirajuddeen, I.M. Isotherm studies on oil removal from produced water using mango seed kernel powder as sorbent material. Chem. Process. Eng. Res. 2014, 23, 55–65. [Google Scholar]
- Alther, G.R. Organically modified clay removes oil from water. Waste Manag. 1995, 15, 623–628. [Google Scholar] [CrossRef]
- Alther, G.R. How to Remove Emulsified Oil from Wastewater with Organoclays. Water Eng. Manag. 2001, 148, 27–29. [Google Scholar]
- Gabardo, I.T.; Platte, E.B.; Araujo, A.S.; Pulgatti, F.H. Evaluation of Produced Water from Brazilian Offshore Platforms. In Produced Water; Springer: Singapore, 2011; pp. 89–113. [Google Scholar]
- Neff, J.; Sauer, T.; Hart, A. Bioaccumulation of Hydrocarbons from Produced Water Discharged to Offshore Waters of the US Gulf of Mexico. In Produced Water; Springer: Singapore, 2011; pp. 441–477. [Google Scholar]
- Ayers, R.; Parker, M. Produced Water Waste Management: Technical Report, Canada. 2001. Available online: www.capp.ca (accessed on 6 November 2021).
- Veil, J.A.; Kimmell, T.A.; Rechner, A.C. Characteristics of Produced Water Discharged to the Gulf of Mexico Hypoxiczone; Argonne National Lab. (ANL): Argonne, IL, USA, 2005. [Google Scholar]
- Gallo-Cordova, A.; Silva-Gordillo, M.D.M.; Muñoz, G.A.; Arboleda-Faini, X.; Streitwieser, D.A. Comparison of the adsorption capacity of organic compounds present in produced water with commercially obtained walnut shell and residual biomass. J. Environ. Chem. Eng. 2017, 5, 4041–4050. [Google Scholar] [CrossRef]
- Ascrizzi, R.; Cioni, P.L.; Amadei, L.; Maccioni, S.; Flamini, G. Geographical patterns of in vivo spontaneously emitted volatile organic compounds in Salvia species. Microchem. J. 2017, 133, 13–21. [Google Scholar] [CrossRef]
- Nasrollahpour, S.; Kebria, D.Y.; Ghavami, M.; Ghasemi-Fare, O. Application of Organically Modified Clay in Removing BTEX from Produced Water. In Geo-Congress 2020: Geo-Systems, Sustainability, Geoenvironmental Engineering, and Unsaturated Soil Mechanics, Minneapolis, Minnesota; American Society of Civil Engineers: Reston, VA, USA, 2020; pp. 275–283. [Google Scholar]
- Costa, A.; Romão, L.; Araújo, B.R.; Lucas, S.; Maciel, S.; Wisniewski, A.; Alexandre, M.D.R. Environmental strategies to remove volatile aromatic fractions (BTEX) from petroleum industry wastewater using biomass. Bioresour. Technol. 2012, 105, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, M.; Da Motta, M.; Benachour, M.; Sales DC, S.; Abreu, C.A.M. Evaluation of BTEX and phenol removal from aqueous solution by multi-solute adsorption onto smectite organoclay. J. Hazard. Mater. 2012, 239, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Egbuchunam, T.; Obi, C.; Okieimen, F.E.; Tihminlioglu, F. Removal of BTEX from aqueous solution using organokaolinite. Int. J. Appl. Environ. Sci. 2016, 11, 505–513. [Google Scholar]
- SharmaSarkar, S.; Jaynes, W.F.; Vance, G.F. BTEX Sorption by Montmorillonite Organo-Clays: TMPA, Adam, HDTMA. Water Air Soil Pollut. 2000, 119, 257–273. [Google Scholar] [CrossRef]
- Stephenson, M. A Survey of Produced Water Studies. In Produced Water; Springer: Singapore, 1992; pp. 1–11. [Google Scholar]
- Bánfalvi, G. Heavy Metals, Trace Elements and Their Cellular Effects. In Cellular Effects of Heavy Metals; Springer: Singapore, 2011; pp. 3–28. [Google Scholar]
- Spellman, F.R. Handbook of Water and Wastewater Treatment Plant Operations; CRC Press: Boca Raton, FL, USA, 2013. [Google Scholar]
- Kose-Mutlu, B.; Ersahin, M.E.; Ozgun, H.; Kaya, R.; Kinaci, C.; Koyuncu, I. Influence of powdered and granular activated carbon system as a pre-treatment alternative for membrane filtration of produced water. J. Chem. Technol. Biotechnol. 2017, 92, 283–291. [Google Scholar] [CrossRef]
- Houcine, M. Solution for heavy metals decontamination in produced water/case study in southern Tunisia. In SPE International Conference on Health, Safety and Environment in Oil and Gas Exploration and Production; Society of Petroleum Engineers: Kuala Lumpur, Malaysia, 2002. [Google Scholar]
- Fard, A.K.; Mckay, G.; Chamoun, R.; Rhadfi, T.; Preud’Homme, H.; Atieh, M.A. Barium removal from synthetic natural and produced water using MXene as two dimensional (2-D) nanosheet adsorbent. Chem. Eng. J. 2017, 317, 331–342. [Google Scholar] [CrossRef]
- Mon, M.; Bruno, R.; Ferrando-Soria, J.; Armentano, D.; Pardo, E. Metal–organic framework technologies for water remediation: Towards a sustainable ecosystem. J. Mater. Chem. A 2018, 6, 4912–4947. [Google Scholar] [CrossRef]
- de Azevedo, A.R.; Marvila, M.T.; Ali, M.; Khan, M.I.; Masood, F.; Vieira, C.M.F. Effect of the addition and processing of glass polishing waste on the durability of geopolymeric mortars. Case Stud. Constr. Mater. 2021, 15, e00662. [Google Scholar] [CrossRef]
- Fathy, M.; El-Sayed, M.; Ramzi, M.; Abdelraheem, O.H. Adsorption separation of condensate oil from produced water using ACTF prepared of oil palm leaves by batch and fixed bed techniques. Egypt. J. Pet. 2018, 27, 319–326. [Google Scholar] [CrossRef]
- Mahmoud, A.M.; Ibrahim, F.A.; Shaban, S.; Youssef, N.A. Adsorption of heavy metal ion from aqueous solution by nickel oxide nano catalyst prepared by different methods. Egypt. J. Pet. 2015, 24, 27–35. [Google Scholar] [CrossRef]
- Gulistan, A.S.; Ibrahim, T.H.; Khamis, M.I.; Elsayed, Y. Application of eggplant peels powder for the removal of oil from produced water. Desalin. Water Treat. 2015, 57, 15724–15732. [Google Scholar] [CrossRef]
- Blanchard, G.; Maunaye, M.; Martin, G. Removal of heavy metals from waters by means of natural zeolites. Water Res. 1984, 18, 1501–1507. [Google Scholar] [CrossRef]
- Giusti, L. A review of waste management practices and their impact on human health. Waste Manag. 2009, 29, 2227–2239. [Google Scholar] [CrossRef]
- Kanoo, P.; Matsuda, R.; Higuchi, M.; Kitagawa, S.; Maji, T.K. New Interpenetrated Copper Coordination Polymer Frameworks having Porous Properties. Chem. Mater. 2009, 21, 5860–5866. [Google Scholar] [CrossRef]
- Fujita, M.; Kwon, Y.J.; Washizu, S.; Ogura, K. Preparation, clathration ability, and catalysis of a two-dimensional square network material composed of cadmium (II) and 4, 4′-bipyridine. J. Am. Chem. Soc. 1994, 116, 1151–1152. [Google Scholar] [CrossRef]
- Yaghi, O.; Li, G.; Li, H. Selective binding and removal of guests in a microporous metal–organic framework. Nature 1995, 378, 703–706. [Google Scholar] [CrossRef]
- Li, J.-R.; Kuppler, R.J.; Zhou, H.-C. Selective gas adsorption and separation in metal–organic frameworks. Chem. Soc. Rev. 2009, 38, 1477–1504. [Google Scholar] [CrossRef]
- Ramaswamy, P.; Wong, N.E.; Shimizu, G.K.H. MOFs as proton conductors–challenges and opportunities. Chem. Soc. Rev. 2014, 43, 5913–5932. [Google Scholar] [CrossRef]
- Shimizu, G.K.; Taylor, J.M.; Kim, S. Proton conduction with metal-organic frameworks. Science 2013, 341, 354–355. [Google Scholar] [CrossRef]
- Campbell, M.G.; Sheberla, D.; Liu, S.F.; Swager, T.M.; Dincă, M. Cu3(hexaiminotriphenylene)2: An Electrically Conductive 2D Metal-Organic Framework for Chemiresistive Sensing. Angew. Chem. Int. Ed. 2015, 54, 4349–4352. [Google Scholar] [CrossRef]
- Pardo, E.; Cangussu, D.; Dul, M.-C.; Lescouëzec, R.; Herson, P.; Journaux, Y.; Pedroso, E.F.; Pereira, C.; Muñoz, M.C.; Ruiz-García, R.; et al. A Metallacryptand-Based Manganese(II)–Cobalt(II) Ferrimagnet with a Three-Dimensional Honeycomb Open-Framework Architecture. Angew. Chem. Int. Ed. 2008, 47, 4211–4216. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.; Buswell, R.; Le, T.; Austin, S.; Gibb, A.; Thorpe, T. Developments in construction-scale additive manufacturing processes. Autom. Constr. 2012, 21, 262–268. [Google Scholar] [CrossRef]
- Rubio-Martinez, M.; Avci-Camur, C.; Thornton, A.W.; Imaz, I.; Maspoch, D.; Hill, M.R. New synthetic routes towards MOF production at scale. Chem. Soc. Rev. 2017, 46, 3453–3480. [Google Scholar] [CrossRef] [PubMed]
- Sharifi, S.; Behzadi, S.; Laurent, S.; Forrest, M.L.; Stroeve, P.; Mahmoudi, M. Toxicity of nanomaterials. Chem. Soc. Rev. 2012, 41, 2323–2343. [Google Scholar] [CrossRef]
- Zhang, Z.; Lin, Y.H.; Tang, Z.L.; Zhang, J.Y. Nanometer materials & nanotechnology and their application prospect. J. Mater. Eng. 2000, 3, 42–48. [Google Scholar]
- Chaikittisilp, W.; Ariga, K.; Yamauchi, Y. A new family of carbon materials: Synthesis of MOF-derived nanoporous carbons and their promising applications. J. Mater. Chem. A 2013, 1, 14–19. [Google Scholar] [CrossRef]
- Lu, G.; Li, S.; Guo, Z.; Farha, O.K.; Hauser, B.G.; Qi, X.; Wang, Y.; Wang, X.; Han, S.; Liu, X.; et al. Imparting functionality to a metal–organic framework material by controlled nanoparticle encapsulation. Nat. Chem. 2012, 4, 310–316. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.C.; Cheetham, A.K. Mechanical properties of hybrid inorganic–organic framework materials: Establishing fundamental structure–property relationships. Chem. Soc. Rev. 2011, 40, 1059–1080. [Google Scholar] [CrossRef]
- Fediuk, R.S.; Mochalov, A.V.; Pezin, D.N.; Liseitsev, Y.L. Composite Binders for Concretes with Improved Impact Endurance. Inorg. Mater. Appl. Res. 2019, 10, 1177–1184. [Google Scholar] [CrossRef]
- Barreto, E.D.S.; Stafanato, K.V.; Marvila, M.T.; de Azevedo, A.R.G.; Ali, M.; Pereira, R.M.L.; Monteiro, S.N. Clay Ceramic Waste as Pozzolan Constituent in Cement for Structural Concrete. Materials 2021, 14, 2917. [Google Scholar] [CrossRef]
- Gu, X.; Lu, Z.-H.; Jiang, H.-L.; Akita, T.; Xu, Q. Synergistic Catalysis of Metal–Organic Framework-Immobilized Au–Pd Nanoparticles in Dehydrogenation of Formic Acid for Chemical Hydrogen Storage. J. Am. Chem. Soc. 2011, 133, 11822–11825. [Google Scholar] [CrossRef]
- Wu, J.; Xu, J.W.; Liu, W.C.; Yang, S.Z.; Luo, M.M.; Han, Y.Y.; Batten, S.R. Designed metal-organic framework based on metal-organic polyhedron: Drug delivery. Inorg. Chem. Commun. 2016, 71, 32–34. [Google Scholar] [CrossRef]
- Song, Z.; Cheng, N.; Lushington, A.; Sun, X. Recent Progress on MOF-Derived Nanomaterials as Advanced Electrocatalysts in Fuel Cells. Catalysts 2016, 6, 116. [Google Scholar] [CrossRef]
- Furukawa, H.; Cordova, K.E.; O’Keeffe, M.; Yaghi, O.M. The Chemistry and Applications of Metal-Organic Frameworks. Science 2013, 341, 1230444. [Google Scholar] [CrossRef]
- Yang, C.; Kaipa, U.; Mather, Q.Z.; Wang, X.; Nesterov, V.; Venero, A.F.; Omary, M.A. Fluorous Metal–Organic Frameworks with Superior Adsorption and Hydrophobic Properties toward Oil Spill Cleanup and Hydrocarbon Storage. J. Am. Chem. Soc. 2011, 133, 18094–18097. [Google Scholar] [CrossRef]
- Chun, J.; Kang, S.; Park, N.; Park, E.J.; Jin, X.; Kim, K.-D.; Seo, H.O.; Lee, S.M.; Kim, H.J.; Kwon, W.H.; et al. Metal–Organic Framework@Microporous Organic Network: Hydrophobic Adsorbents with a Crystalline Inner Porosity. J. Am. Chem. Soc. 2014, 136, 6786–6789. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; Liu, D.; Huang, H.; Yang, Q.; Zhong, C. Efficient capture of nitrobenzene from waste water using metal–organic frameworks. Chem. Eng. J. 2014, 246, 142–149. [Google Scholar] [CrossRef]
- Jin, H.; Li, Y.; Liu, X.; Ban, Y.; Peng, Y.; Jiao, W.; Yang, W. Recovery of HMF from aqueous solution by zeolitic imidazolate frameworks. Chem. Eng. Sci. 2015, 124, 170–178. [Google Scholar] [CrossRef]
- Seo, Y.S.; Khan, N.A.; Jhung, S.H. Adsorptive removal of methylchlorophenoxypropionic acid from water with a metal-organic framework. Chem. Eng. J. 2015, 270, 22–27. [Google Scholar] [CrossRef]
- Ke, F.; Qiu, L.-G.; Yuan, Y.-P.; Peng, F.-M.; Jiang, X.; Xie, A.-J.; Shen, Y.-H.; Zhu, J. Thiol-functionalization of metal-organic framework by a facile coordination-based postsynthetic strategy and enhanced removal of Hg2+ from water. J. Hazard. Mater. 2011, 196, 36–43. [Google Scholar] [CrossRef]
- Zhu, B.-J.; Yu, X.-Y.; Jia, Y.; Peng, F.-M.; Sun, B.; Zhang, M.-Y.; Luo, T.; Liu, J.-H.; Huang, X.-J. Iron and 1,3,5-Benzenetricarboxylic Metal–Organic Coordination Polymers Prepared by Solvothermal Method and Their Application in Efficient As(V) Removal from Aqueous Solutions. J. Phys. Chem. C 2012, 116, 8601–8607. [Google Scholar] [CrossRef]
- Chowdhury, T.; Zhang, L.; Zhang, J.; Aggarwal, S. Removal of Arsenic(III) from Aqueous Solution Using Metal Organic Framework-Graphene Oxide Nanocomposite. Nanomaterials 2018, 8, 1062. [Google Scholar] [CrossRef]
- Qian, X.; Yadian, B.; Wu, R.; Long, Y.; Zhou, K.; Zhu, B.; Huang, Y. Structure stability of metal-organic framework MIL-53 (Al) in aqueous solutions. Int. J. Hydrog. Energy 2013, 38, 16710–16715. [Google Scholar] [CrossRef]
- Abbasi, A.; Moradpour, T.; van Hecke, K. A new 3D cobalt (II) metal–organic framework nanostructure for heavy metal adsorption. Inorg. Chim. Acta 2015, 430, 261–267. [Google Scholar] [CrossRef]
- Li, Z.-Q.; Yang, J.-C.; Sui, K.-W.; Yin, N. Facile synthesis of metal-organic framework MOF-808 for arsenic removal. Mater. Lett. 2015, 160, 412–414. [Google Scholar] [CrossRef]
- Mi, L.; Hou, H.; Song, Z.; Han, H.; Fan, Y. Polymeric Zinc Ferrocenyl Sulfonate as a Molecular Aspirator for the Removal of Toxic Metal Ions. Chem. A Eur. J. 2008, 14, 1814–1821. [Google Scholar] [CrossRef]
- Mi, L.; Hou, H.; Song, Z.; Han, H.; Xu, H.; Fan, Y.; Ng, S.-W. Rational Construction of Porous Polymeric Cadmium Ferrocene-1,1′-disulfonates for Transition Metal Ion Exchange and Sorption. Cryst. Growth Des. 2007, 7, 2553–2561. [Google Scholar] [CrossRef]
- Das, S.; Kim, H.; Kim, K. Metathesis in single crystal: Complete and reversible exchange of metal ions constituting the frameworks of metal-organic frameworks. J. Am. Chem. Soc. 2009, 131, 3814–3815. [Google Scholar] [CrossRef]
- Prasad, T.K.; Hong, D.H.; Suh, M.P. High Gas Sorption and Metal-Ion Exchange of Microporous Metal-Organic Frameworks with Incorporated Imide Groups. Chem. A Eur. J. 2010, 16, 14043–14050. [Google Scholar] [CrossRef]
- Loiseau, T.; Lecroq, L.; Volkringer, C.; Marrot, J.; Férey, G.; Haouas, M.; Taulelle, F.; Bourrelly, S.; Llewellyn, A.P.L.; Latroche, M. MIL-96, a Porous Aluminum Trimesate 3D Structure Constructed from a Hexagonal Network of 18-Membered Rings and μ3-Oxo-Centered Trinuclear Units. J. Am. Chem. Soc. 2006, 128, 10223–10230. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Yan, G.; Wang, T.; Jia, W.; Zeng, X.; Sperry, J.; Lin, L. Cu 1–Cu 0 bicomponent CuNPs@ ZIF-8 for highly selective hydrogenation of biomass derived 5-hydroxymethylfurfural. Green Chem. 2019, 21, 4319–4323. [Google Scholar] [CrossRef]
- Li, C.-Y.; Liu, J.M.; Wang, Z.H.; Lv, S.W.; Zhao, N.; Wang, S. Integration of Fe3O4@ UiO-66-NH2@ MON core-shell structured adsorbents for specific preconcentration and sensitive determination of aflatoxins against complex sample matrix. J. Hazard. Mater. 2020, 384, 121348. [Google Scholar] [CrossRef]
- Srinivas, G.; Krungleviciute, V.; Guo, Z.-X.; Yildirim, T. Exceptional CO2capture in a hierarchically porous carbon with simultaneous high surface area and pore volume. Energy Environ. Sci. 2014, 7, 335–342. [Google Scholar] [CrossRef]
- Li, P. Groundwater Quality in Western China: Challenges and Paths Forward for Groundwater Quality Research in Western China; Springer: Berlin/Heidelberg, Germany, 2016. [Google Scholar]
- Bobbitt, N.S.; Mendonca, M.L.; Howarth, A.J.; Islamoglu, T.; Hupp, J.T.; Farha, O.K.; Snurr, R.Q. Metal–organic frameworks for the removal of toxic industrial chemicals and chemical warfare agents. Chem. Soc. Rev. 2017, 46, 3357–3385. [Google Scholar] [CrossRef]


| Parameter | Units | Ranges |
|---|---|---|
| pH | - | 4.3–10 |
| Total Dissolved Solids | mg/L | 100–400,000 |
| Total Suspended Solids | mg/L | 1.2–1000 |
| Chemical Oxygen Demand | mg/L | 1220–2600 |
| Total Organic Carbon | - | 1500 |
| Salinity | mg/L | 5000–300,000 |
| Conductivity | μS/cm | 4200–58,600 |
| Surface Tension | dyn/cm | 43–78 |
| Density | kg/m3 | 1014–1140 |
| TENORMS | Concentration Bq. L-1 | Countries | References |
|---|---|---|---|
| 226 Ra | 5.1–14.8 | Algeria | [26,27,28] |
| 0.5–16 | Norway | [22] | |
| 13.8–111.2 | Syria | [29] | |
| 1.07–34.15, 5–40 | Egypt | [30,31] | |
| (<0.002–58) | USA | [32,33] | |
| 210 Pb | <5 | Poland | [34,35] |
| 2.6–16.7 | USA | [32,33] | |
| 228 Ra | <0.05–12.0 | Brazil | [28,36] |
| <0.02–13.26 | Egypt | [32,33,37,38,39] | |
| <2 | Poland | [34] | |
| <1–4 | Turkey | [40] | |
| 6.40–35.50 | Ghana | [37,41] | |
| 8.1 | Nigeria | [31,38] | |
| <1.1 × 10−3–9.6 | Argentina | [42] | |
| 35–763, 0.02–59 | USA | [32,33] | |
| 40 K | 39.8 | Nigeria | [31,38] |
| 1.65–11.99 | Ghana | [37,41] | |
| 1522–1535 | Oman | [43] | |
| 221–899 | Romania | [43] | |
| 4.4–43.7 632.5–1448.7 | Egypt | [28,30] | |
| 14.6 | Iraq | [44,45] | |
| 3.6–15.37 | Azerbaijan | [29] | |
| 7.3 | Iran | [46] | |
| 238 U | <4.5 × 10−3 | Congo | [41,42,46,47,48] |
| 7.3 × 10−3–1.5 × 10−2 | Italy | [49] | |
| 9.47–25.2 | Egypt | [28,30] | |
| 4.12 | Iraq | [44,45] | |
| 0.043–1.1 | Ghana | [37,41] |
| Country | Effluent Limits | Reporting Routine | |
|---|---|---|---|
| Monthly | Daily | ||
| Canada | 40 ppm monthly avg. | 80 ppm 2-day avg | Annual |
| USA | 29 mg/L monthly avg. | 42 mg/L daily max | Monthly |
| UK | 40 ppm monthly avg. | - | Annual |
| Western Australia | 30 ppm monthly avg. | 50 mg/L daily max | - |
| Mediterranean Sea | 40 ppm monthly avg. | - | - |
| Advantages | Disadvantages |
|---|---|
| It is feasible for all the contaminants present in PW | It cannot remove TDS and salt concentrations |
| It can considerably reduce TOC, BTEX, and oil concentrations | For media regeneration, expensive chemicals are required |
| It is used as a polishing step in PW to achieve the best results | It cannot be used as a major treatment process due to the rapid consumption of adsorbent material |
| It uses compact, packed bed modules, and is cheaper, efficient, and requires minimal energy | A disposal system is required for waste generated by used adsorbent media, or some form of regeneration |
| It can remove 80% of heavy metals | It has a high retention time |
| Nearly 100% of water recovery can be achieved | Less efficient at a higher feed concentration |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gul Zaman, H.; Baloo, L.; Pendyala, R.; Singa, P.K.; Ilyas, S.U.; Kutty, S.R.M. Produced Water Treatment with Conventional Adsorbents and MOF as an Alternative: A Review. Materials 2021, 14, 7607. https://doi.org/10.3390/ma14247607
Gul Zaman H, Baloo L, Pendyala R, Singa PK, Ilyas SU, Kutty SRM. Produced Water Treatment with Conventional Adsorbents and MOF as an Alternative: A Review. Materials. 2021; 14(24):7607. https://doi.org/10.3390/ma14247607
Chicago/Turabian StyleGul Zaman, Humaira, Lavania Baloo, Rajashekhar Pendyala, Pradeep Kumar Singa, Suhaib Umer Ilyas, and Shamsul Rahman Mohamed Kutty. 2021. "Produced Water Treatment with Conventional Adsorbents and MOF as an Alternative: A Review" Materials 14, no. 24: 7607. https://doi.org/10.3390/ma14247607
APA StyleGul Zaman, H., Baloo, L., Pendyala, R., Singa, P. K., Ilyas, S. U., & Kutty, S. R. M. (2021). Produced Water Treatment with Conventional Adsorbents and MOF as an Alternative: A Review. Materials, 14(24), 7607. https://doi.org/10.3390/ma14247607