Ultra-Short Laser Surface Properties Optimization of Biocompatibility Characteristics of 3D Poly-ε-Caprolactone and Hydroxyapatite Composite Scaffolds
Abstract
:1. Introduction
2. Materials and Methods
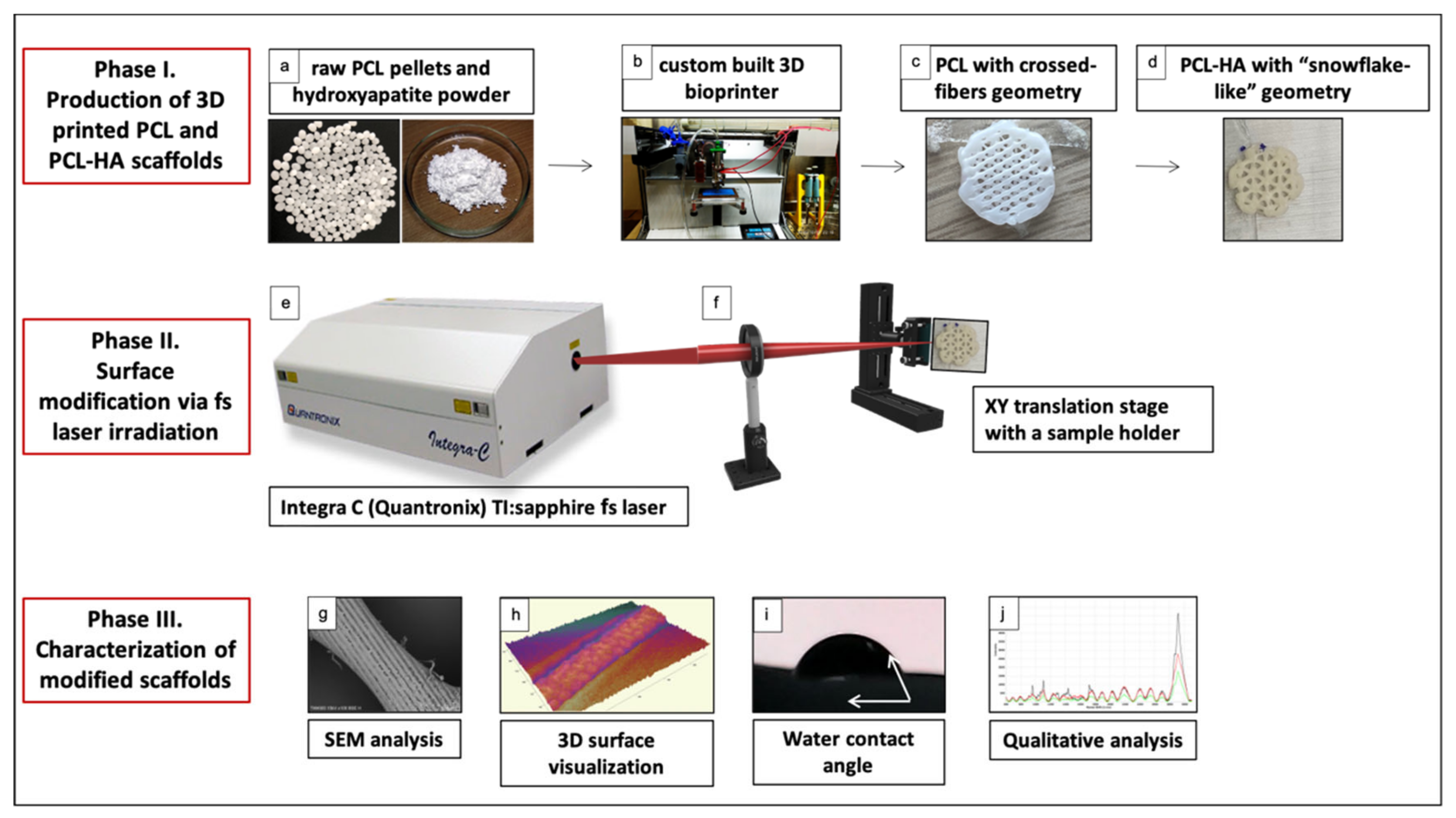
2.1. Fabrication of 3D-Printed PCL and PCL/HA Scaffolds
2.2. Irradiation of PCL and PCL-HA Scaffolds with Femtosecond Laser Pulses
2.3. Characterization of Laser-Irradiated PCL and PCL/HA Scaffolds
2.3.1. Scanning Electron Microscopy and Analysis of Elemental Composition via Energy-Dispersive X-ray Spectroscopy (EDX)
2.3.2. Analysis of Surface Modifications via Optical Profilometer
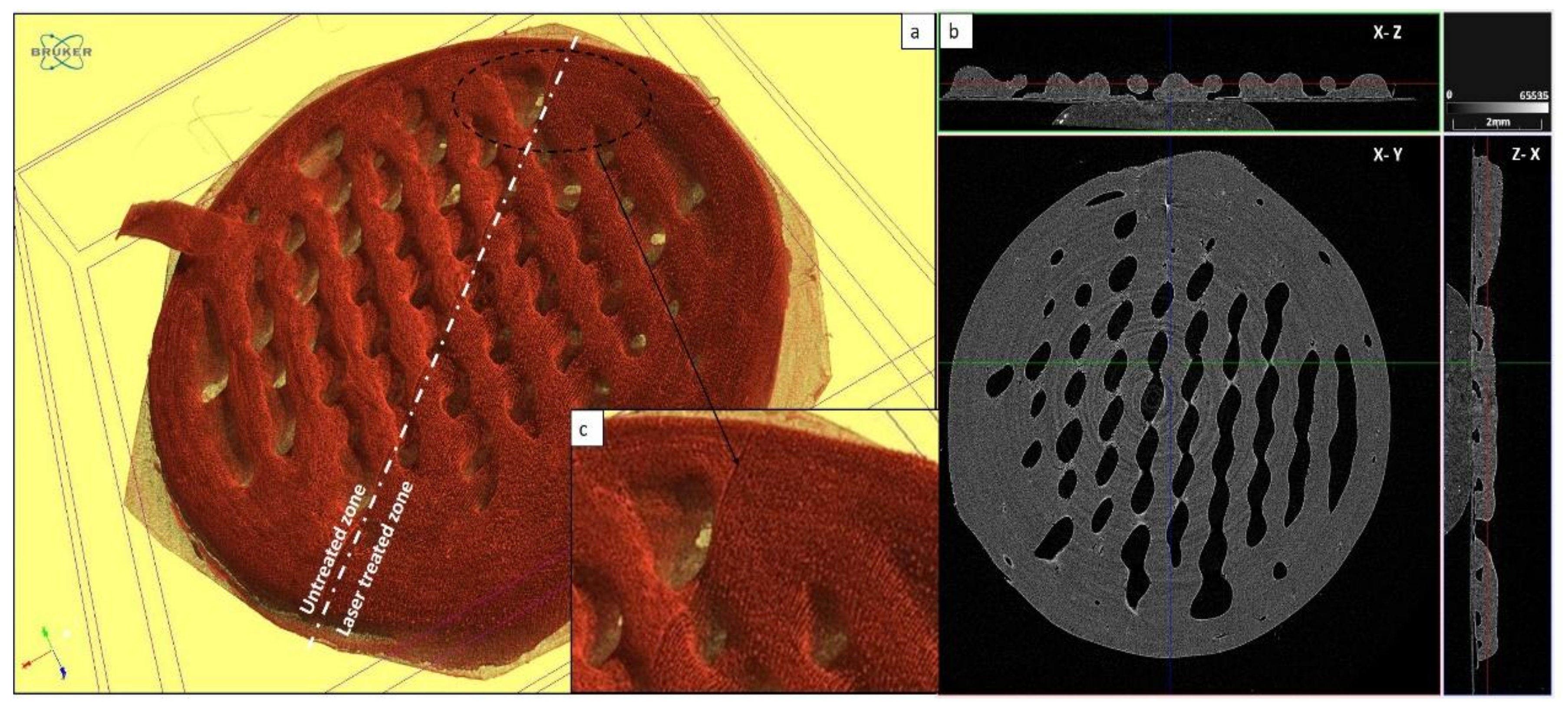
2.4. Computed X-ray Tomography for Evaluation of Surface Roughness after Femtosecond Laser Ablation
2.5. Raman Spectroscopy and X-ray Diffraction Analysis (XRD) for Assessment of Changes in Structural Conformation of PCL and PCL-HA
2.6. Wettability Assessment of PCL and PCL/HA Scaffolds via Water Contact Angle (WCA) Measurement
2.7. Microhardness Test of PCL and PCL/HA 3D Scaffold
3. Results
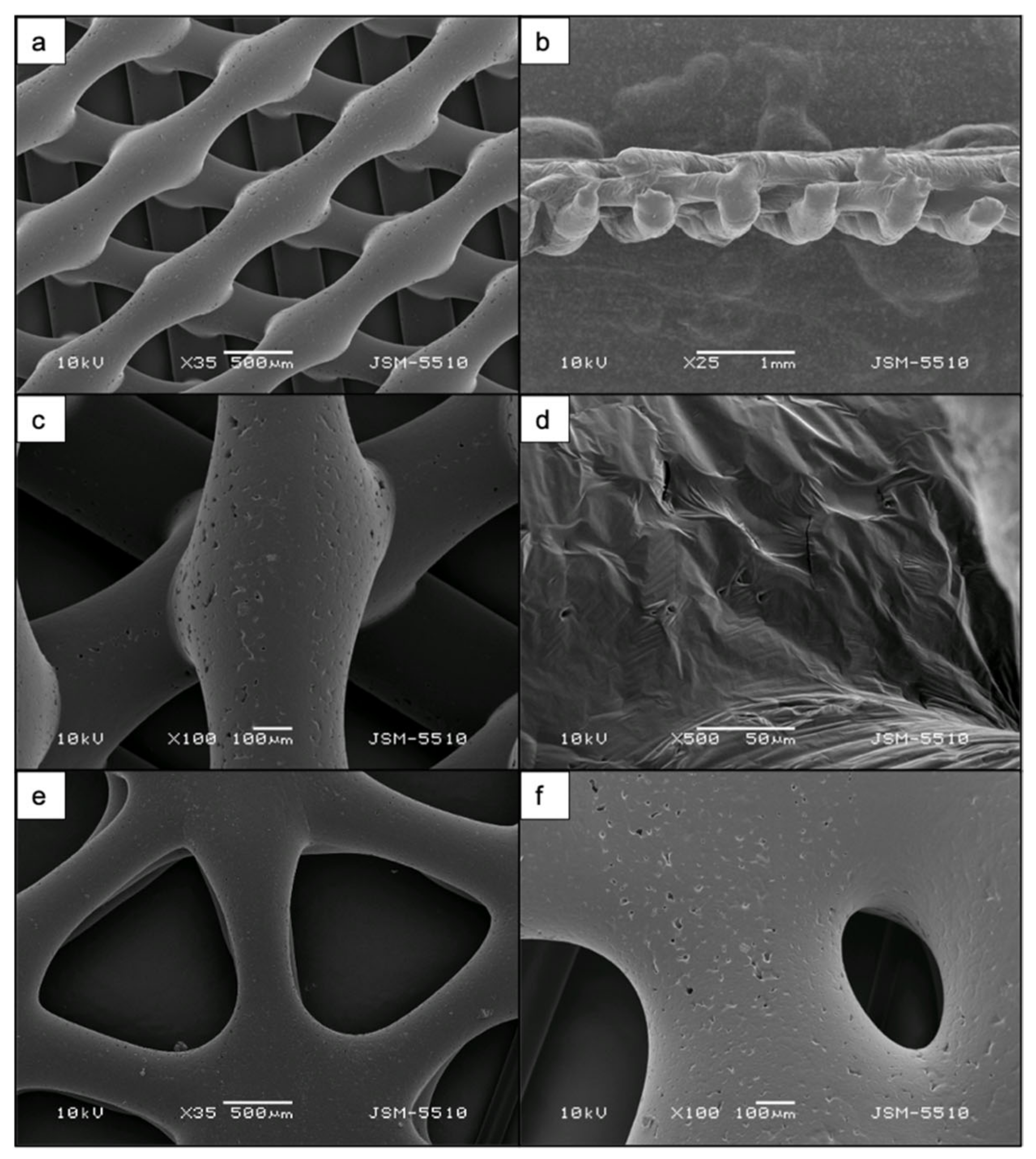
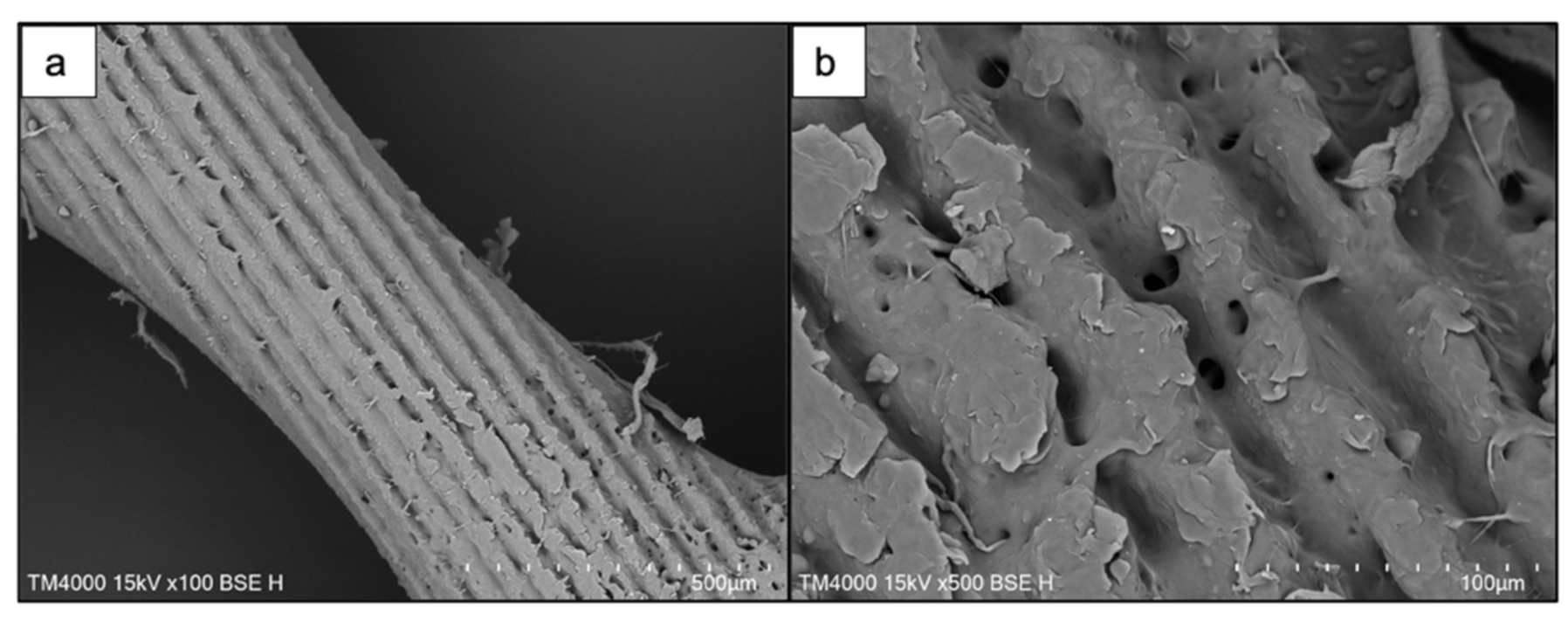
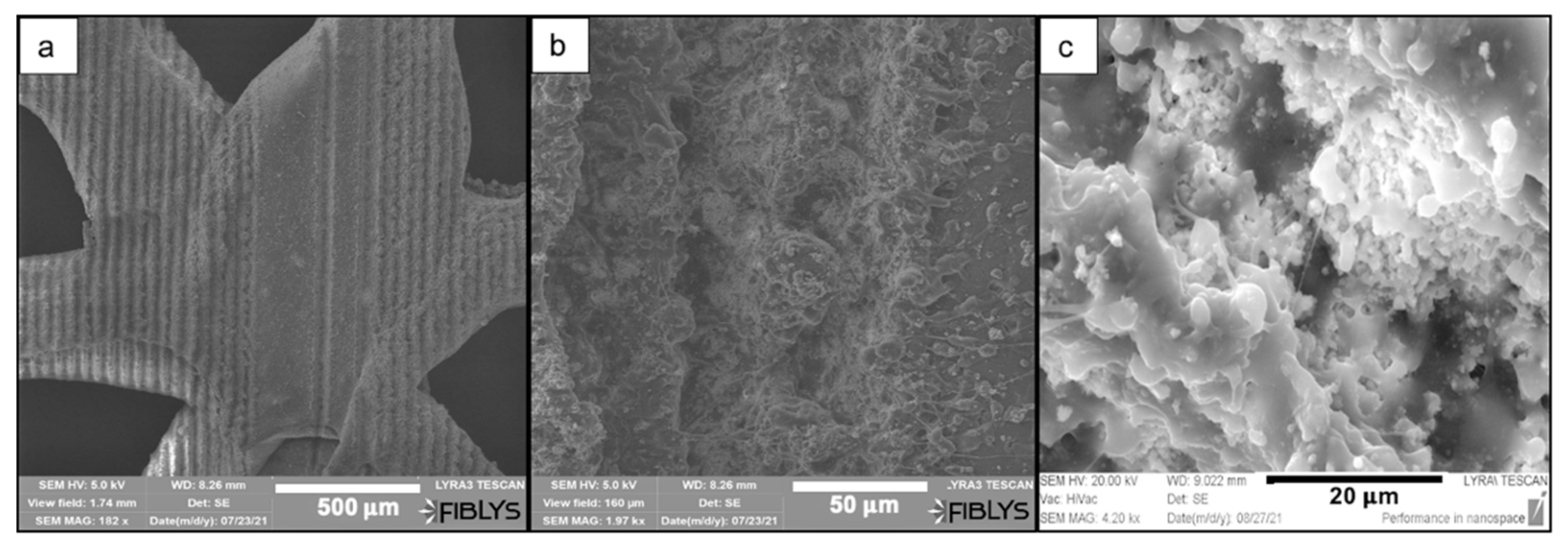
3.1. Morphological Studies of PCL and PCL/HA Matrices
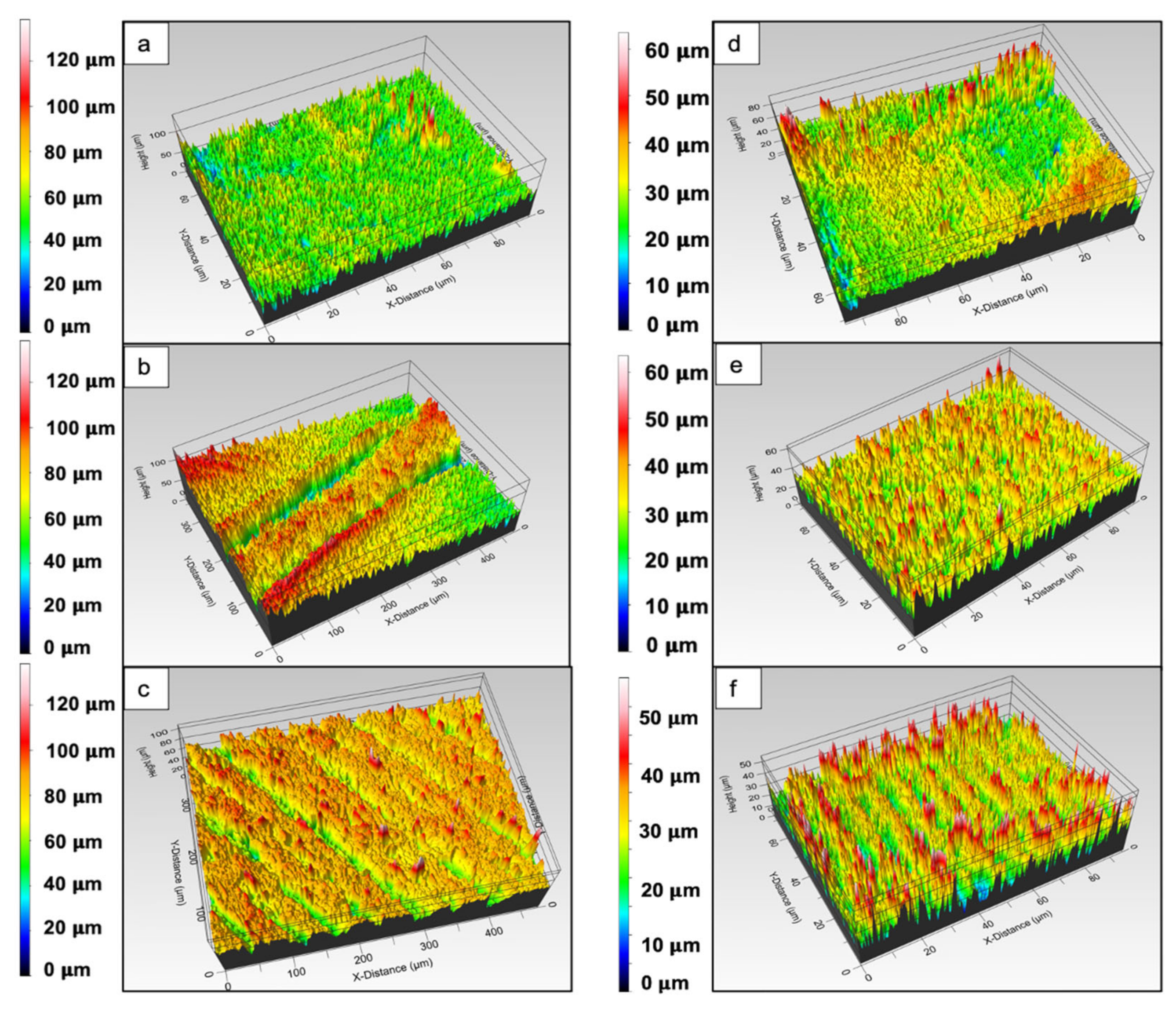
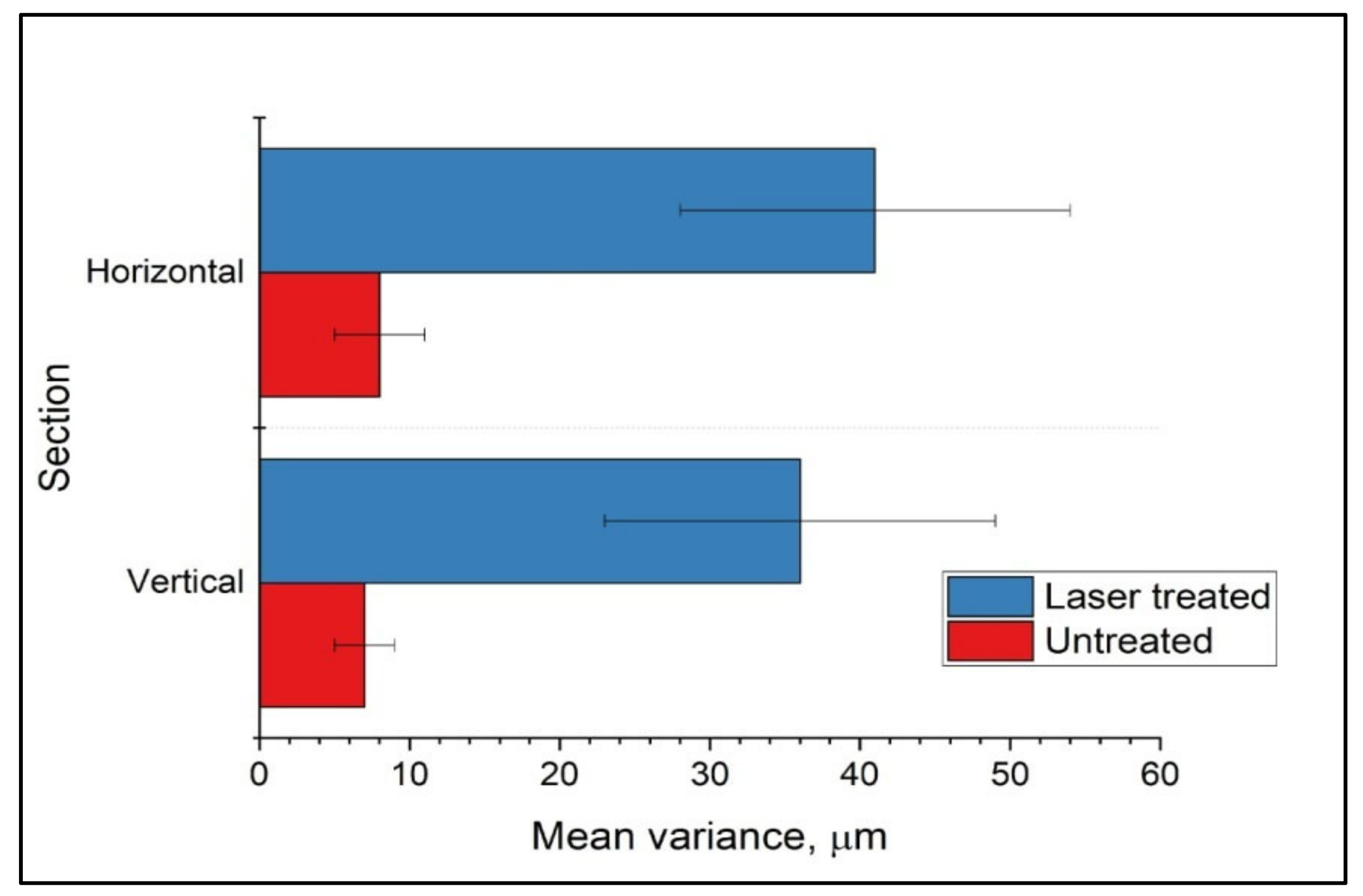
3.2. Surface Roughness Assessment of Femtosecond Laser-Processed Scaffolds
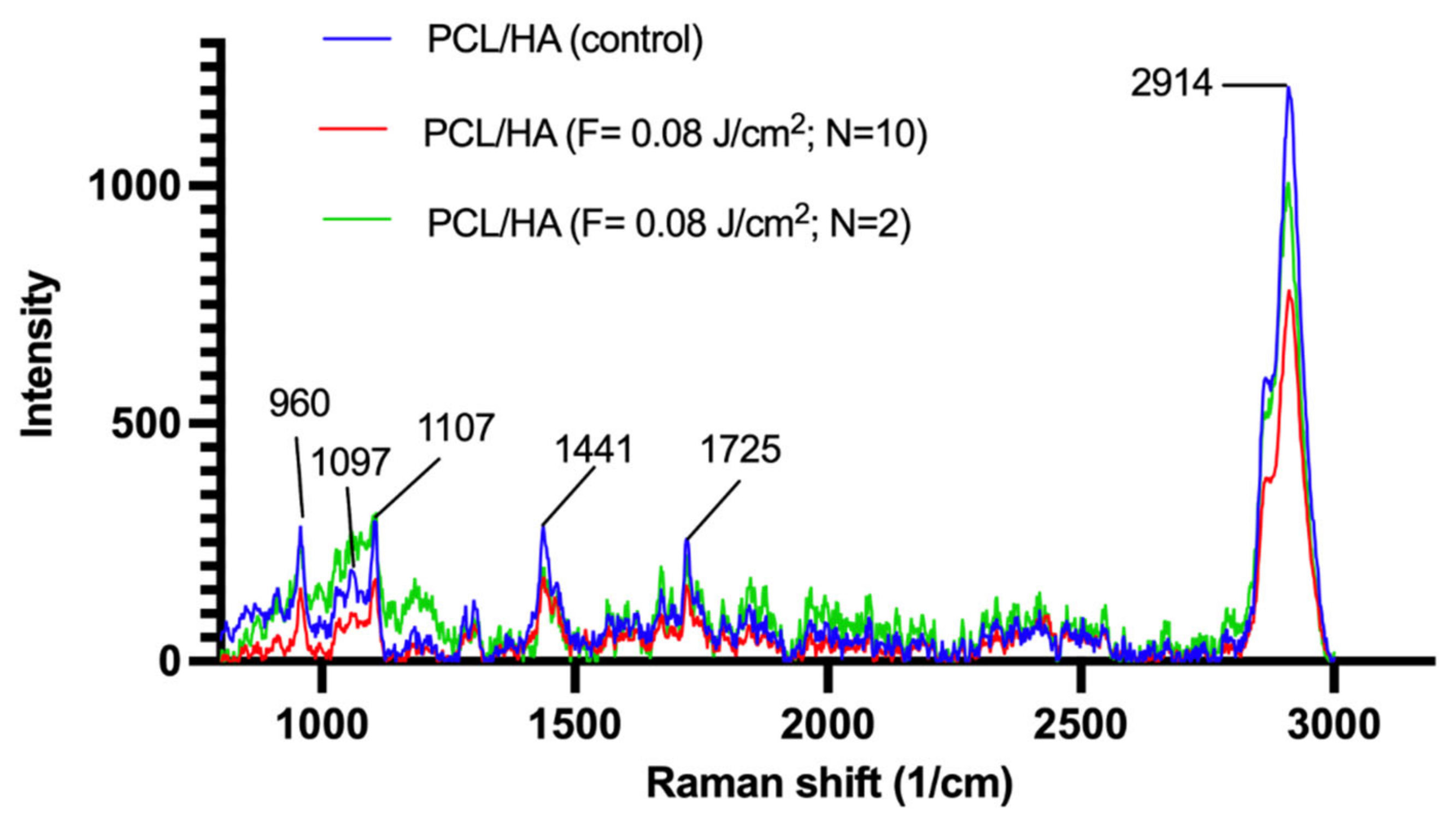
3.3. Changes in Crystallinity of PCL in Relation to Femtosecond Laser Treatment
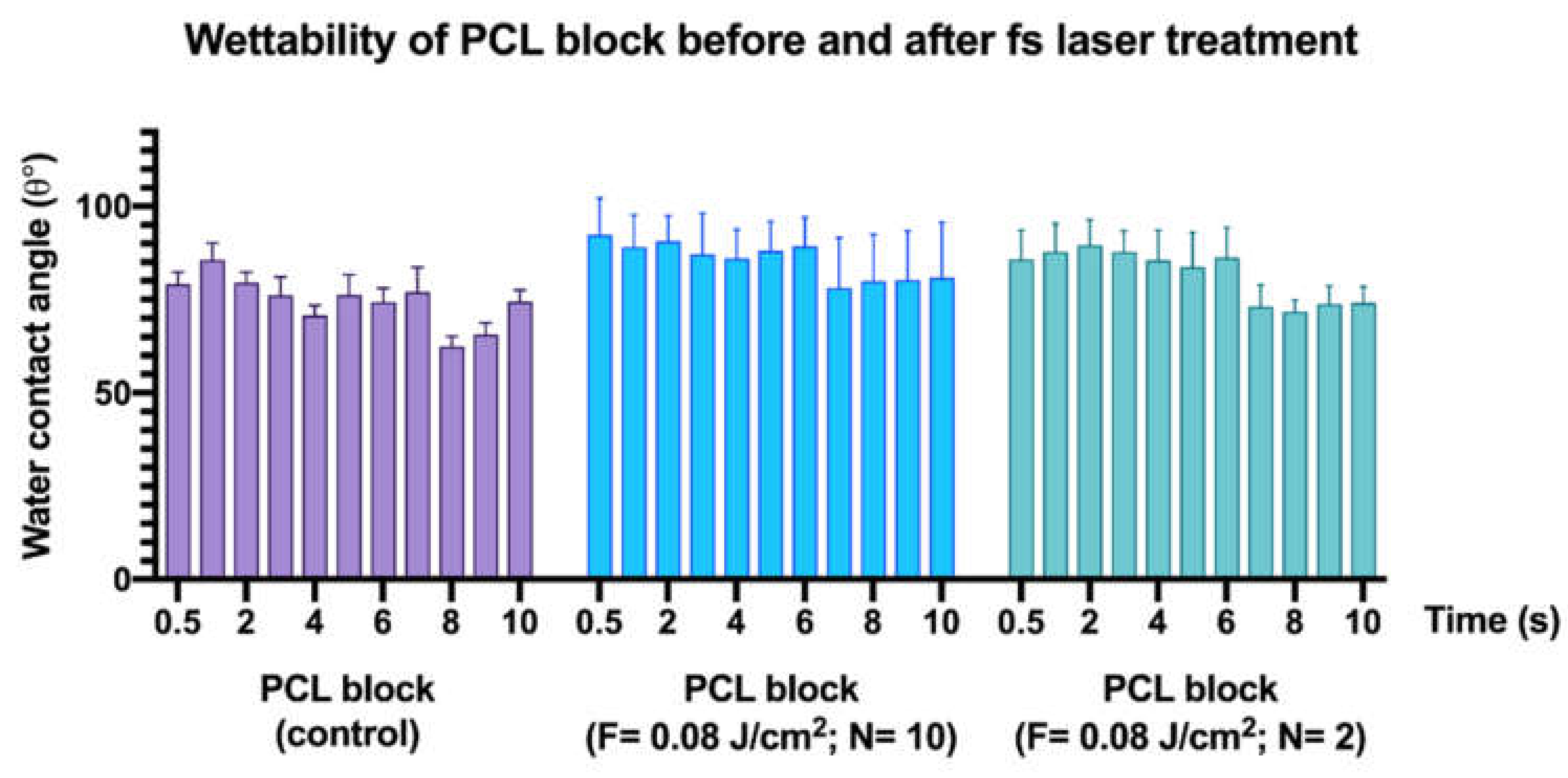
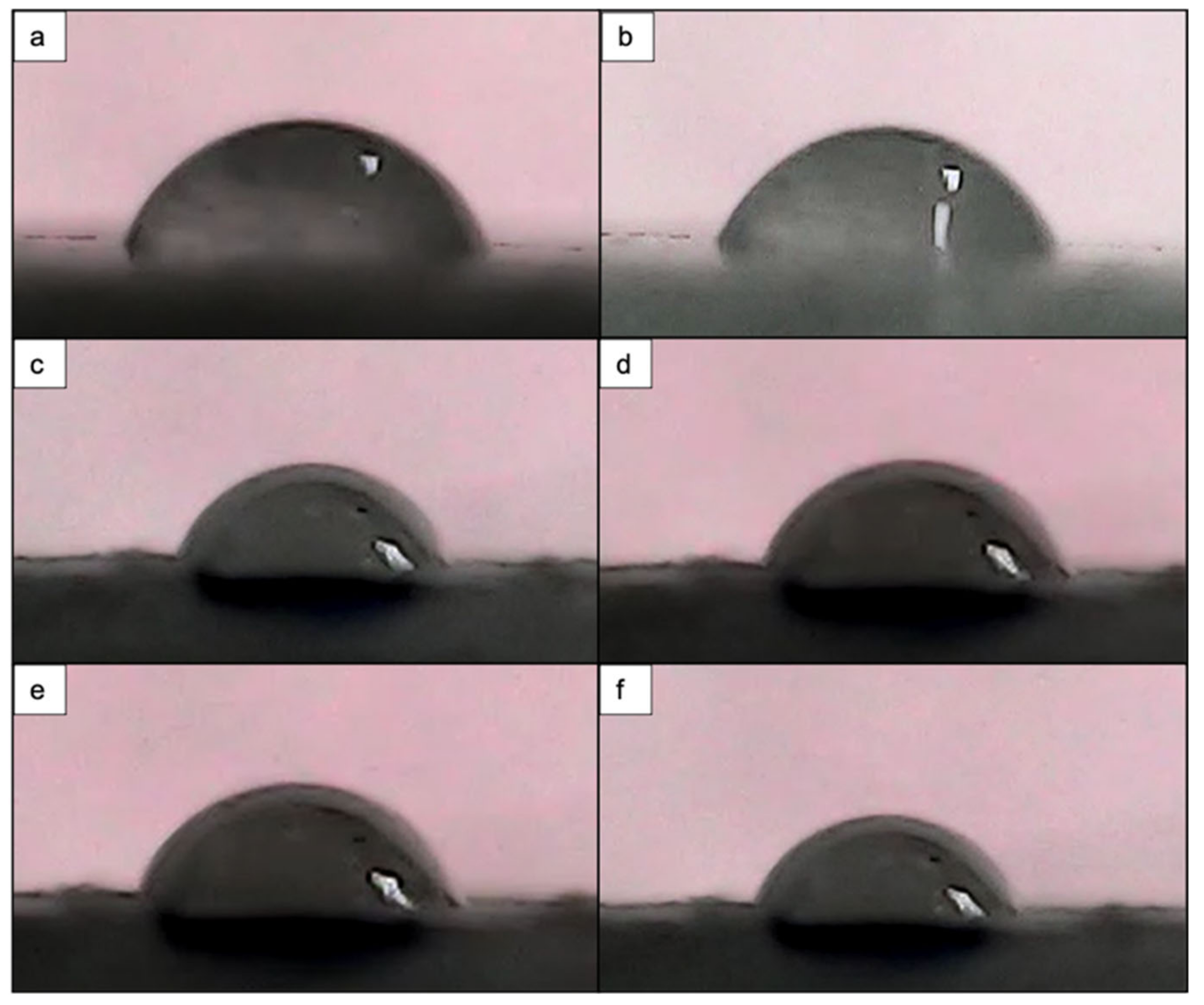
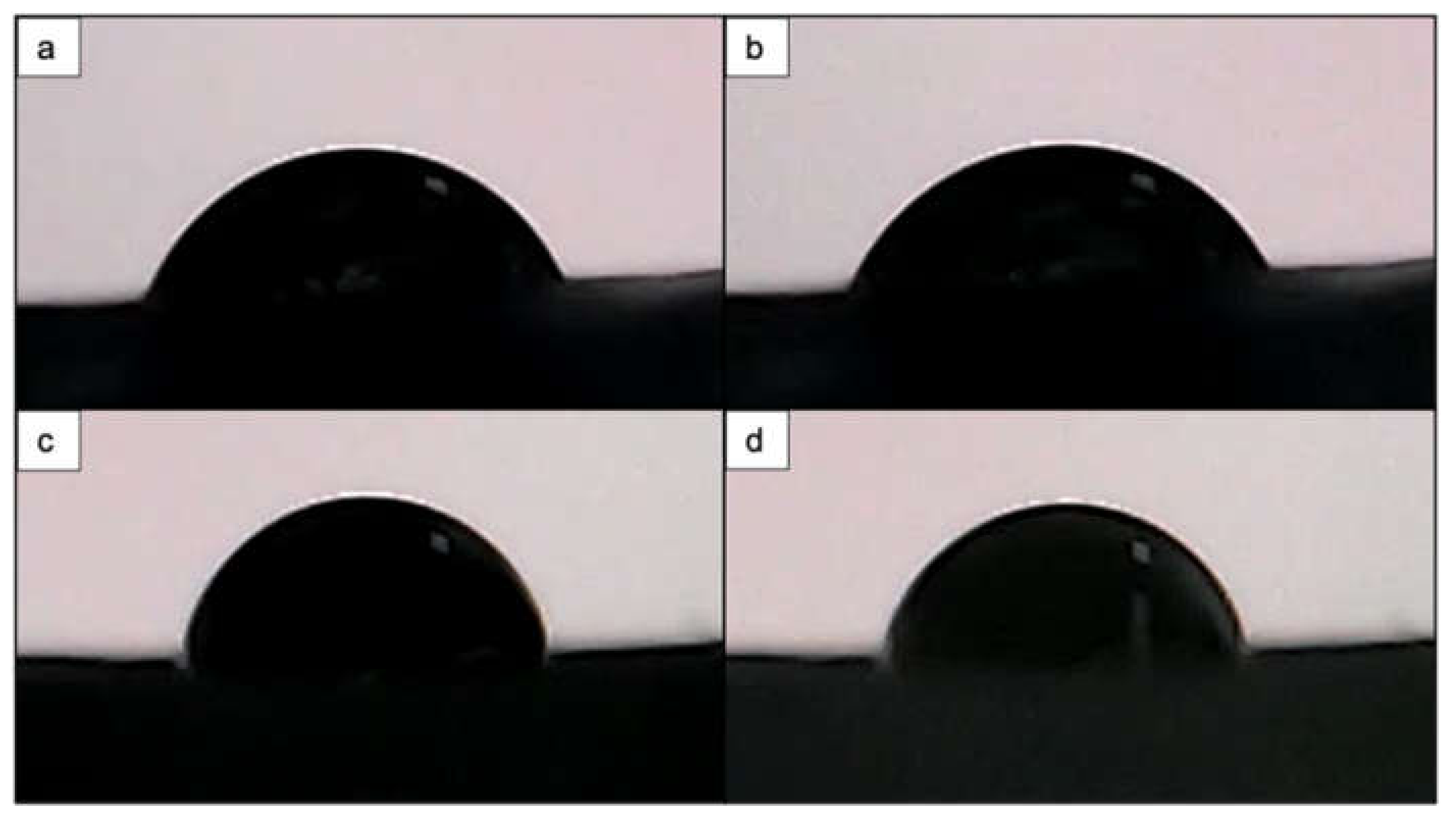
3.4. Effects of Femtosecond Laser Treatment on PCL and PCL/HA Scaffold Wettability
3.5. Evaluation of Microhardness before and after Femtosecond Laser Processing of PCL and PCL/HA Scaffolds
4. Discussion
4.1. Laser Texturing and Structures Morphology
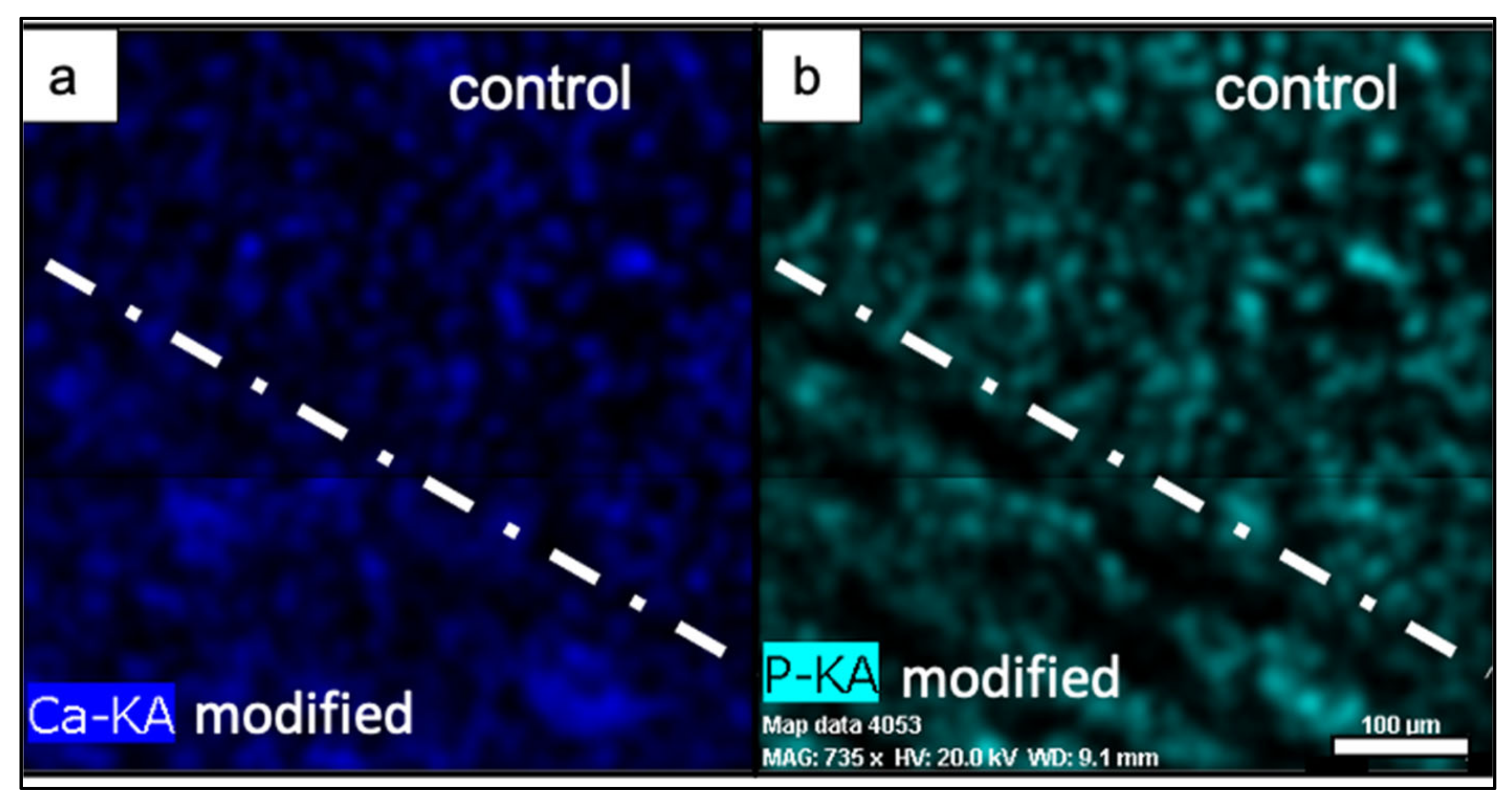
4.2. Chemical Composition
4.3. Wettability Evolution and Mechanical Stability
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Arciola, C.R.; Campoccia, D.; Montanaro, L. Implant Infections: Adhesion, Biofilm Formation and Immune Evasion. Nat. Rev. Microbiol. 2018, 16, 397–409. [Google Scholar] [CrossRef]
- Veerachamy, S.; Yarlagadda, T.; Manivasagam, G.; Yarlagadda, P.K. Bacterial Adherence and Biofilm Formation on Medical Implants: A Review. Proc. Inst. Mech. Eng. 2014, 228, 1083–1099. [Google Scholar] [CrossRef]
- Arciola, C.R.; Campoccia, D.; Ehrlich, G.D.; Montanaro, L. Biofilm-Based Implant Infections in Orthopaedics. In Biofilm-Based Healthcare-Associated Infections; Advances in Experimental Medicine and Biology; Donelli, G., Ed.; Springer International Publishing: Cham, Switzerland, 2015; Volume 830, pp. 29–46. [Google Scholar] [CrossRef]
- Carniello, V.; Peterson, B.W.; van der Mei, H.C.; Busscher, H.J. Physico-Chemistry from Initial Bacterial Adhesion to Surface-Programmed Biofilm Growth. Adv. Colloid Interface Sci. 2018, 261, 1–14. [Google Scholar] [CrossRef]
- Zheng, S.; Bawazir, M.; Dhall, A.; Kim, H.-E.; He, L.; Heo, J.; Hwang, G. Implication of Surface Properties, Bacterial Motility, and Hydrodynamic Conditions on Bacterial Surface Sensing and Their Initial Adhesion. Front. Bioeng. Biotechnol. 2021, 9, 643722. [Google Scholar] [CrossRef]
- Ribeiro, M.; Monteiro, F.J.; Ferraz, M.P. Infection of Orthopedic Implants with Emphasis on Bacterial Adhesion Process and Techniques Used in Studying Bacterial-Material Interactions. Biomatter 2012, 2, 176–194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, J.; Gao, P.; Han, S.; Kao, R.Y.T.; Wu, S.; Liu, X.; Qian, S.; Chu, P.K.; Cheung, K.M.C.; Yeung, K.W.K. A Tailored Positively-Charged Hydrophobic Surface Reduces the Risk of Implant Associated Infections. Acta Biomater. 2020, 114, 421–430. [Google Scholar] [CrossRef]
- Renner, L.D.; Weibel, D.B. Physicochemical Regulation of Biofilm Formation. MRS Bull. 2011, 36, 347–355. [Google Scholar] [CrossRef] [Green Version]
- Tuson, H.H.; Weibel, D.B. Bacteria–Surface Interactions. Soft Matter 2013, 9, 4368. [Google Scholar] [CrossRef] [Green Version]
- Preedy, E.; Perni, S.; Nipiĉ, D.; Bohinc, K.; Prokopovich, P. Surface Roughness Mediated Adhesion Forces between Borosilicate Glass and Gram-Positive Bacteria. Langmuir 2014, 30, 9466–9476. [Google Scholar] [CrossRef] [Green Version]
- Perera-Costa, D.; Bruque, J.M.; González-Martín, M.L.; Gómez-García, A.C.; Vadillo-Rodríguez, V. Studying the Influence of Surface Topography on Bacterial Adhesion Using Spatially Organized Microtopographic Surface Patterns. Langmuir 2014, 30, 4633–4641. [Google Scholar] [CrossRef]
- Xing, R.; Lyngstadaas, S.P.; Ellingsen, J.E.; Taxt-Lamolle, S.; Haugen, H.J. The Influence of Surface Nanoroughness, Texture and Chemistry of TiZr Implant Abutment on Oral Biofilm Accumulation. Clin. Oral Implant. Res. 2015, 26, 649–656. [Google Scholar] [CrossRef]
- Yao, W.-L.; Lin, J.C.Y.; Salamanca, E.; Pan, Y.-H.; Tsai, P.-Y.; Leu, S.-J.; Yang, K.-C.; Huang, H.-M.; Huang, H.-Y.; Chang, W.-J. Er, Cr: YSGG Laser Performance Improves Biological Response on Titanium Surfaces. Materials 2020, 13, 756. [Google Scholar] [CrossRef] [Green Version]
- Singh, A.V.; Vyas, V.; Patil, R.; Sharma, V.; Scopelliti, P.E.; Bongiorno, G.; Podestà, A.; Lenardi, C.; Gade, W.N.; Milani, P. Quantitative Characterization of the Influence of the Nanoscale Morphology of Nanostructured Surfaces on Bacterial Adhesion and Biofilm Formation. PLoS ONE 2011, 6, e25029. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, Y.; Feng, G.; Moraru, C.I. Micro- and Nanotopography Sensitive Bacterial Attachment Mechanisms: A Review. Front. Microbiol. 2019, 10, 191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crawford, R.J.; Webb, H.K.; Truong, V.K.; Hasan, J.; Ivanova, E.P. Surface Topographical Factors Influencing Bacterial Attachment. Adv. Colloid Interface Sci. 2012, 179–182, 142–149. [Google Scholar] [CrossRef]
- Cheng, Y.-T.; Rodak, D.E. Is the Lotus Leaf Superhydrophobic? Appl. Phys. Lett. 2005, 86, 144101. [Google Scholar] [CrossRef]
- Ge, X.; Ren, C.; Ding, Y.; Chen, G.; Lu, X.; Wang, K.; Ren, F.; Yang, M.; Wang, Z.; Li, J.; et al. Micro/Nano-Structured TiO2 Surface with Dual-Functional Antibacterial Effects for Biomedical Applications. Bioact. Mater. 2019, 4, 346–357. [Google Scholar] [CrossRef] [PubMed]
- Robotti, F.; Bottan, S.; Fraschetti, F.; Mallone, A.; Pellegrini, G.; Lindenblatt, N.; Starck, C.; Falk, V.; Poulikakos, D.; Ferrari, A. A Micron-Scale Surface Topography Design Reducing Cell Adhesion to Implanted Materials. Sci. Rep. 2018, 8, 10887. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Zha, G.; Luo, Q.; Zhang, J.; Zhang, F.; Li, X.; Zhao, S.; Zhu, W.; Li, X. The Construction of Hierarchical Structure on Ti Substrate with Superior Osteogenic Activity and Intrinsic Antibacterial Capability. Sci. Rep. 2015, 4, 6172. [Google Scholar] [CrossRef] [Green Version]
- Francone, A.; Merino, S.; Retolaza, A.; Ramiro, J.; Alves, S.A.; de Castro, J.V.; Neves, N.M.; Arana, A.; Marimon, J.M.; Torres, C.M.S.; et al. Impact of Surface Topography on the Bacterial Attachment to Micro- and Nano-Patterned Polymer Films. Surf. Interfaces 2021, 27, 101494. [Google Scholar] [CrossRef]
- Wang, J.; Guo, C. Ultrafast Dynamics of Femtosecond Laser-Induced Periodic Surface Pattern Formation on Metals. Appl. Phys. Lett. 2005, 87, 251914. [Google Scholar] [CrossRef]
- Hergenröder, R.; Samek, O.; Hommes, V. Femtosecond Laser Ablation Elemental Mass Spectrometry. Mass Spectrom. Rev. 2006, 25, 551–572. [Google Scholar] [CrossRef]
- Toyserkani, E.; Rasti, N. Ultrashort Pulsed Laser Surface Texturing. In Laser Surface Engineering; Elsevier: Rotterdam, The Netherlands, 2015; pp. 441–453. [Google Scholar] [CrossRef]
- Hamad, A.H. Effects of Different Laser Pulse Regimes (Nanosecond, Picosecond and Femtosecond) on the Ablation of Materials for Production of Nanoparticles in Liquid Solution. In High Energy and Short Pulse Lasers; Viskup, R., Ed.; InTech: London, UK, 2016. [Google Scholar] [CrossRef] [Green Version]
- Samanta, A.; Wang, Q.; Shaw, S.K.; Ding, H. Roles of Chemistry Modification for Laser Textured Metal Alloys to Achieve Extreme Surface Wetting Behaviors. Mater. Des. 2020, 192, 108744. [Google Scholar] [CrossRef]
- Amin Yavari, S.; Castenmiller, S.M.; Strijp, J.A.G.; Croes, M. Combating Implant Infections: Shifting Focus from Bacteria to Host. Adv. Mater. 2020, 32, 2002962. [Google Scholar] [CrossRef]
- Dwivedi, R.; Kumar, S.; Pandey, R.; Mahajan, A.; Nandana, D.; Katti, D.S.; Mehrotra, D. Polycaprolactone as Biomaterial for Bone Scaffolds: Review of Literature. J. Oral Biol. Craniofacial Res. 2020, 10, 381–388. [Google Scholar] [CrossRef]
- Mondal, D.; Griffith, M.; Venkatraman, S.S. Polycaprolactone-Based Biomaterials for Tissue Engineering and Drug Delivery: Current Scenario and Challenges. Int. J. Polym. Mater. Polym. Biomater. 2016, 65, 255–265. [Google Scholar] [CrossRef]
- Xu, L.-C.; Siedlecki, C.A. Staphylococcus epidermidis Adhesion on Hydrophobic and Hydrophilic Textured Biomaterial Surfaces. Biomed. Mater. 2014, 9, 035003. [Google Scholar] [CrossRef]
- Maikranz, E.; Spengler, C.; Thewes, N.; Thewes, A.; Nolle, F.; Jung, P.; Bischoff, M.; Santen, L.; Jacobs, K. Different Binding Mechanisms of Staphylococcus aureus to Hydrophobic and Hydrophilic Surfaces. Nanoscale 2020, 12, 19267–19275. [Google Scholar] [CrossRef]
- Lotz, E.M.; Olivares-Navarrete, R.; Berner, S.; Boyan, B.D.; Schwartz, Z. Osteogenic Response of Human MSCs and Osteoblasts to Hydrophilic and Hydrophobic Nanostructured Titanium Implant Surfaces: HYDROPHILIC VERSUS HYDROPHOBIC NANOSTRUCTURED SURFACES. J. Biomed. Mater. Res. A 2016, 104, 3137–3148. [Google Scholar] [CrossRef]
- Zareidoost, A.; Yousefpour, M.; Ghaseme, B.; Amanzadeh, A. The Relationship of Surface Roughness and Cell Response of Chemical Surface Modification of Titanium. J. Mater. Sci. Mater. Med. 2012, 23, 1479–1488. [Google Scholar] [CrossRef]
- Rho, J.Y.; Kuhn-Spearing, L.; Zioupos, P. Mechanical properties and the hierarchical structure of bone. Med. Eng. Phys. 1998, 20, 92–102. [Google Scholar] [CrossRef]
- Meskinfam, M.; Bertoldi, S.; Albanese, N.; Cerri, A.; Tanzi, M.C.; Imani, R.; Baheiraei, N.; Farokhi, M.; Fare, S. Polyurethane foam/nano hydroxyapatite composite as a suitable scaffold for bone tissue regeneration. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 82, 130–140. [Google Scholar] [CrossRef]
- Zheng, P.; Hu, X.; Lou, Y.; Tang, K. A Rabbit Model of Osteochondral Regeneration Using Three-Dimensional Printed Polycaprolactone-Hydroxyapatite Scaffolds Coated with Umbilical Cord Blood Mesenchymal Stem Cells and Chondrocytes. Med. Sci. Monit. 2019, 25, 7361–7369. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Chen, M.; Zheng, T.; Yang, X. Effect of Surface Roughness on the Initial Response of MC3T3-E1 Cells Cultured on Polished Titanium Alloy. Biomed. Mater. Eng. 2015, 26, S155–S164. [Google Scholar] [CrossRef] [Green Version]
- Murphy, C.M.; Haugh, M.G.; O’Brien, F.J. The Effect of Mean Pore Size on Cell Attachment, Proliferation and Migration in Collagen–Glycosaminoglycan Scaffolds for Bone Tissue Engineering. Biomaterials 2010, 31, 461–466. [Google Scholar] [CrossRef]
- Holthaus, M.G.; Stolle, J.; Treccani, L.; Rezwan, K. Orientation of Human Osteoblasts on Hydroxyapatite-Based Microchannels. Acta Biomater. 2012, 8, 394–403. [Google Scholar] [CrossRef]
- Harris, L.; Foster, S.; Richards, R. An Introduction to Staphylococcus Aureus, and Techniques for Identifying and Quantifying S. aureus Adhesins in Relation to Adhesion to Biomaterials: Review. Eur. Cell. Mater. 2002, 4, 39–60. [Google Scholar] [CrossRef]
- Kotula, A.P.; Snyder, C.R.; Migler, K.B. Determining Conformational Order and Crystallinity in Polycaprolactone via Raman Spectroscopy. Polymer 2017, 117, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Baranowska-Korczyc, A.; Warowicka, A.; Jasiurkowska-Delaporte, M.; Grześkowiak, B.; Jarek, M.; Maciejewska, B.M.; Jurga-Stopa, J.; Jurga, S. Antimicrobial Electrospun Poly(ε-Caprolactone) Scaffolds for Gingival Fibroblast Growth. RSC Adv. 2016, 6, 19647–19656. [Google Scholar] [CrossRef]
- Kister, G.; Cassanas, G.; Bergounhon, M.; Hoarau, D.; Vert, M. Structural Characterization and Hydrolytic Degradation of Solid Copolymers of d,l-Lactide-Co-ε-Caprolactone by Raman Spectroscopy. Polymer 2000, 41, 925–932. [Google Scholar] [CrossRef]
- Ciobanu, C.S.; Massuyeau, F.; Constantin, L.V.; Predoi, D. Structural and Physical Properties of Antibacterial Ag-Doped Nano-Hydroxyapatite Synthesized at 100 °C. Nanoscale Res. Lett. 2011, 6, 613. [Google Scholar] [CrossRef] [Green Version]
- Esmaeilkhanian, A.; Sharifianjazi, F.; Abouchenari, A.; Rouhani, A.; Parvin, N.; Irani, M. Synthesis and Characterization of Natural Nano-Hydroxyapatite Derived from Turkey Femur-Bone Waste. Appl. Biochem. Biotechnol. 2019, 189, 919–932. [Google Scholar] [CrossRef] [PubMed]
- Medeiros, G.S.; Muñoz, P.A.R.; de Oliveira, C.F.P.; da Silva, L.C.E.; Malhotra, R.; Gonçalves, M.C.; Rosa, V.; Fechine, G.J.M. Polymer Nanocomposites Based on Poly(ε-Caprolactone), Hydroxyapatite and Graphene Oxide. J. Polym. Environ. 2020, 28, 331–342. [Google Scholar] [CrossRef]
- Yong, J.; Chen, F.; Yang, Q.; Hou, X. Femtosecond Laser Controlled Wettability of Solid Surfaces. Soft Matter 2015, 11, 8897–8906. [Google Scholar] [CrossRef] [Green Version]
- Murakami, D.; Jinnai, H.; Takahara, A. Wetting Transition from the Cassie–Baxter State to the Wenzel State on Textured Polymer Surfaces. Langmuir 2014, 30, 2061–2067. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Shi, J.; Li, W.; Sun, K. Morphology, Wettability, and Mechanical Properties of Polycaprolactone/Hydroxyapatite Composite Scaffolds with Interconnected Pore Structures Fabricated by a Mini-Deposition System. Polym. Eng. Sci. 2012, 52, 2396–2402. [Google Scholar] [CrossRef]
- Rajzer, I. Fabrication of Bioactive Polycaprolactone/Hydroxyapatite Scaffolds with Final Bilayer Nano-/Micro-Fibrous Structures for Tissue Engineering Application. J. Mater. Sci. 2014, 49, 5799–5807. [Google Scholar] [CrossRef] [Green Version]
- Tang, M.; Chen, C.; Zhu, J.; Allcock, H.R.; Siedlecki, C.A.; Xu, L.-C. Inhibition of Bacterial Adhesion and Biofilm Formation by a Textured Fluorinated Alkoxyphosphazene Surface. Bioact. Mater. 2021, 6, 447–459. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.-R.; Weeks, E.R.; Ducker, W.A. Surface Topography Hinders Bacterial Surface Motility. ACS Appl. Mater. Interfaces 2018, 10, 9225–9234. [Google Scholar] [CrossRef] [PubMed]
- Sjöström, T.; Nobbs, A.H.; Su, B. Bactericidal Nanospike Surfaces via Thermal Oxidation of Ti Alloy Substrates. Mater. Lett. 2016, 167, 22–26. [Google Scholar] [CrossRef] [Green Version]
- Van Bael, S.; Chai, Y.C.; Truscello, S.; Moesen, M.; Kerckhofs, G.; Van Oosterwyck, H.; Kruth, J.-P.; Schrooten, J. The Effect of Pore Geometry on the In Vitro Biological Behavior of Human Periosteum-Derived Cells Seeded on Selective Laser-Melted Ti6Al4V Bone Scaffolds. Acta Biomater. 2012, 8, 2824–2834. [Google Scholar] [CrossRef]
- Paul, C.D.; Shea, D.J.; Mahoney, M.R.; Chai, A.; Laney, V.; Hung, W.; Konstantopoulos, K. Interplay of the Physical Microenvironment, Contact Guidance, and Intracellular Signaling in Cell Decision Making. FASEB J. 2016, 30, 2161–2170. [Google Scholar] [CrossRef] [Green Version]
- Murphy, C.M.; O’Brien, F.J. Understanding the Effect of Mean Pore Size on Cell Activity in Collagen-Glycosaminoglycan Scaffolds. Cell Adhes. Migr. 2010, 4, 377–381. [Google Scholar] [CrossRef] [Green Version]
- Kosik-Kozioł, A.; Graham, E.; Jaroszewicz, J.; Chlanda, A.; Kumar, P.T.S.; Ivanovski, S.; Święszkowski, W.; Vaquette, C. Surface Modification of 3D Printed Polycaprolactone Constructs via a Solvent Treatment: Impact on Physical and Osteogenic Properties. ACS Biomater. Sci. Eng. 2019, 5, 318–328. [Google Scholar] [CrossRef]
- Wibowo, A.; Vyas, C.; Cooper, G.; Qulub, F.; Suratman, R.; Mahyuddin, A.I.; Dirgantara, T.; Bartolo, P. 3D Printing of Polycaprolactone–Polyaniline Electroactive Scaffolds for Bone Tissue Engineering. Materials 2020, 13, 512. [Google Scholar] [CrossRef] [Green Version]
- Qin, X.-H.; Senturk, B.; Valentin, J.; Malheiro, V.; Fortunato, G.; Ren, Q.; Rottmar, M.; Maniura-Weber, K. Cell-Membrane-Inspired Silicone Interfaces That Mitigate Proinflammatory Macrophage Activation and Bacterial Adhesion. Langmuir 2019, 35, 1882–1894. [Google Scholar] [CrossRef]
- Ishizaki, T.; Saito, N.; Takai, O. Correlation of Cell Adhesive Behaviors on Superhydrophobic, Superhydrophilic, and Micropatterned Superhydrophobic/Superhydrophilic Surfaces to Their Surface Chemistry. Langmuir 2010, 26, 8147–8154. [Google Scholar] [CrossRef]
- Briscoe, B.J.; Sinha, S.K. Hardness and Normal Indentation of Polymers. In Mechanical Properties and Testing of Polymers; Swallowe, G.M., Brewis, D., Briggs, D., Eds.; Polymer Science and Technology Series; Springer: Dordrecht, The Netherlands, 1999. [Google Scholar] [CrossRef]
- Wang, J.; Yin, B.; Liu, G.; Li, S.; Zhang, X.; Hu, Z.; Wu, W.; Zhang, Y. Microhardness Distribution of the Tibial Diaphysis and Test Site Selection for Reference Point Indentation Technique. Medicine 2019, 98, e16523. [Google Scholar] [CrossRef] [PubMed]
- Liang, W.; Ding, P.; Li, G.; Lu, E.; Zhao, Z. Hydroxyapatite Nanoparticles Facilitate Osteoblast Differentiation and Bone Formation within Sagittal Suture during Expansion in Rats. Drug Des. Devel. Ther. 2021, 15, 905–917. [Google Scholar] [CrossRef]




| Element | PCL/HA Scaffold Weight (%) | PCL Scaffold Weight (%) | ||
|---|---|---|---|---|
| Control | Ablated | Control | Ablated | |
| C | 49.71 | 57.88 | 50.92 | 72.42 |
| O | 47.28 | 22.59 | 49.98 | 27.58 |
| Ca | 2.21 | 13.13 | N/A | N/A |
| P | 0.80 | 6.40 | N/A | N/A |
| № | cPCL- Block | 3D cPCL | 3D fsPCL | 3D cPCL + HA | 3D fsPCL + HA |
|---|---|---|---|---|---|
| 1 | 23.0 | 12.4 | 11.1 | 12.1 | 8.1 |
| 2 | 16.9 | 11.9 | 9.8 | 12.6 | 11.4 |
| 3 | 18.3 | 12.5 | 12.3 | 11.4 | 12.5 |
| 4 | 21.1 | 13.0 | 13.1 | 13.3 | 10.7 |
| 5 | 14.8 | 11.1 | 10.9 | 9.9 | 9.8 |
| Averaged value | 18.82 | 12.18 | 11.44 | 11.86 | 10.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Daskalova, A.; Filipov, E.; Angelova, L.; Stefanov, R.; Tatchev, D.; Avdeev, G.; Sotelo, L.; Christiansen, S.; Sarau, G.; Leuchs, G.; et al. Ultra-Short Laser Surface Properties Optimization of Biocompatibility Characteristics of 3D Poly-ε-Caprolactone and Hydroxyapatite Composite Scaffolds. Materials 2021, 14, 7513. https://doi.org/10.3390/ma14247513
Daskalova A, Filipov E, Angelova L, Stefanov R, Tatchev D, Avdeev G, Sotelo L, Christiansen S, Sarau G, Leuchs G, et al. Ultra-Short Laser Surface Properties Optimization of Biocompatibility Characteristics of 3D Poly-ε-Caprolactone and Hydroxyapatite Composite Scaffolds. Materials. 2021; 14(24):7513. https://doi.org/10.3390/ma14247513
Chicago/Turabian StyleDaskalova, Albena, Emil Filipov, Liliya Angelova, Radostin Stefanov, Dragomir Tatchev, Georgi Avdeev, Lamborghini Sotelo, Silke Christiansen, George Sarau, Gerd Leuchs, and et al. 2021. "Ultra-Short Laser Surface Properties Optimization of Biocompatibility Characteristics of 3D Poly-ε-Caprolactone and Hydroxyapatite Composite Scaffolds" Materials 14, no. 24: 7513. https://doi.org/10.3390/ma14247513
APA StyleDaskalova, A., Filipov, E., Angelova, L., Stefanov, R., Tatchev, D., Avdeev, G., Sotelo, L., Christiansen, S., Sarau, G., Leuchs, G., Iordanova, E., & Buchvarov, I. (2021). Ultra-Short Laser Surface Properties Optimization of Biocompatibility Characteristics of 3D Poly-ε-Caprolactone and Hydroxyapatite Composite Scaffolds. Materials, 14(24), 7513. https://doi.org/10.3390/ma14247513









