Corrosion Resistance of Electrochemically Synthesized Modified Zaccagnaite LDH-Type Films on Steel Substrates
Abstract
:1. Introduction
2. Materials and Methods
2.1. Film Synthesis
2.2. Characterization
2.3. Immersion Test and Corrosion Measurements
3. Results and Discussion
3.1. Film Deposition Mechanism
3.2. Structural Characterization
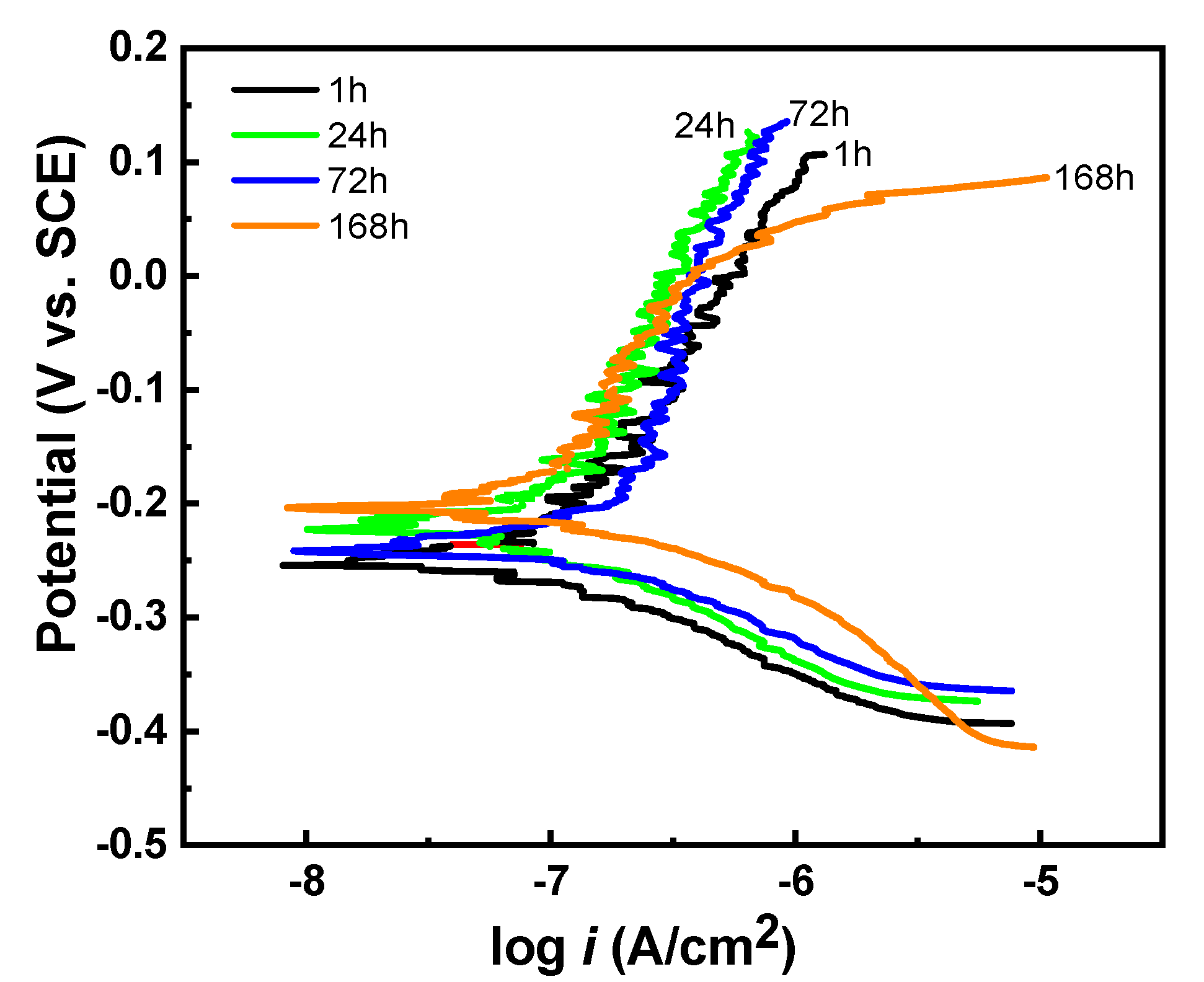
3.3. Corrosion Resistance
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Baddoo, N.R. Stainless steel in construction: A review of research, applications, challenges and opportunities. J. Constr. Steel Res. 2008, 64, 1199–1206. [Google Scholar] [CrossRef]
- Bekmurzayeva, A.; Duncanson, W.J.; Azevedo, H.S.; Kanayeva, D. Surface modification of stainless steel for biomedical applications: Revisiting a century-old material. Mater. Sci. Eng. C 2018, 93, 1073–1089. [Google Scholar] [CrossRef]
- Huynh, V.; Ngo, N.; Golden, T.D. Review article: Surface activation and pretreatments for biocompatible metals and alloys used in biomedical applications. Int. J. Biomater. 2019, 2019, 3806504. [Google Scholar] [CrossRef] [PubMed]
- Mesquita, T.J.; Chauveau, E.; Mantel, M.; Bouvier, N.; Koschel, D. Corrosion and metallurgical investigation of two supermartensitic stainless steels for oil and gas environment. Corros. Sci. 2014, 81, 152–161. [Google Scholar] [CrossRef]
- Herting, G.; Wallinder, I.O.; Leygraf, C. Corrosion-induced release of chromium and iron from ferritic stainless steel grade AISI 430 in simulated food contact. J. Food Eng. 2008, 87, 291–300. [Google Scholar]
- Bitondo, C.; Bossio, A.; Monetta, T.; Curioni, M.; Bellucci, F. The effect of annealing on the corrosion behavior of 444 stainless steel for drinking water applications. Corros. Sci. 2014, 87, 6–10. [Google Scholar] [CrossRef]
- Sugimoto, K.; Hojo, T.; Srivastava, A.K. Review: Low and medium carbon advanced high-strength forging steels for automotive applications. Metals 2019, 9, 1263. [Google Scholar] [CrossRef] [Green Version]
- Streicher, M.A.; Grubb, J.F. Austenitic and Ferritic Stainless Steels, in Uhlig’s Corrosion Handbook, 3rd ed.; Revie, R.W., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2011; pp. 657–693. [Google Scholar]
- Hu, Q.; Zhang, G.; Qiu, Y.; Guo, X. The crevice corrosion behaviour of stainless steel in sodium chloride solution. Corros. Sci. 2011, 53, 4065–4072. [Google Scholar] [CrossRef]
- Zaffora, A.; Di Franco, F.; Santamaria, M. Corrosion of stainless steel in food and pharmaceutical industry. Curr. Opin. Electrochem. 2021, 29, 100760. [Google Scholar]
- Ngo, N.; Shao, S.; Conrad, H.; Sanders, S.F.; D’Souza, F.; Golden, T.D. Synthesis, characterization, and the effects of organo-grafted nanoparticles in nickel coatings for enhanced corrosion protection. Mater. Today Commun. 2020, 25, 101628. [Google Scholar] [CrossRef]
- González, M.B.; Saidman, S.B. Electrodeposition of bilayered polypyrrole on 316 L stainless steel for corrosion prevention. Prog. Org. Coat. 2015, 78, 21–27. [Google Scholar] [CrossRef]
- Yasakau, K.A.; Kuznetsova, A.; Kallip, S.; Starykevich, M.; Tedim, J.; Ferreira, M.G.S.; Zheludkevich, M.L. A novel bilayer system comprising LDH conversion layer and sol-gel coating for active corrosion protection of AA2024. Corros. Sci. 2018, 143, 299–313. [Google Scholar] [CrossRef]
- Daugherty, R.E.; Zumbach, M.M.; Golden, T.D. Design challenges in electrodepositing metal-anionic clay nanocomposites: Synthesis, characterization, and corrosion resistance of nickel-LDH nanocomposite coatings. Surf. Coat. Technol. 2018, 349, 773–782. [Google Scholar] [CrossRef]
- Iqbal, M.A.; Sun, L.; Barrett, A.T.; Fedel, M. Layered Double Hydroxide protective films developed on aluminum and aluminum alloys: Synthetic methods and anti-corrosion mechanisms. Coatings 2020, 10, 428. [Google Scholar] [CrossRef]
- Kaseem, M.; Ramachandraiah, K.; Hossain, S.; Dikici, B. A review on LDH-smart functionalization of anodic films of Mg alloys. Nanomaterials 2021, 11, 536. [Google Scholar] [CrossRef] [PubMed]
- Evans, D.G.; Slade, R.C.T. Structural aspects of layered double hydroxides. Struct. Bond. 2006, 119, 1–87. [Google Scholar]
- Salak, A.N.; Lisenkov, A.D.; Zheludkevich, M.L.; Ferreira, M.G.S. Carbonate-free Zn-Al (1:1) layered double hydroxide film directly grown on zinc-aluminum alloy coating. ECS Electrochem. Lett. 2014, 3, C9–C11. [Google Scholar] [CrossRef]
- Mills, S.J.; Christy, A.G.; Génin, J.M.R.; Kameda, T.; Colombo, F. Nomenclature of the hydrotalcite supergroup: Natural layered double hydroxides. Mineral. Mag. 2012, 76, 1289–1336. [Google Scholar] [CrossRef] [Green Version]
- Tedim, J.; Kuznetsova, A.; Salak, A.N.; Montemor, F.; Snihirova, D.; Pilz, M.; Zheludkevich, M.L.; Ferreira, M.G.S. Zn–Al layered double hydroxides as chloride nanotraps in active protective coatings. Corros. Sci. 2012, 55, 1–4. [Google Scholar] [CrossRef]
- Syu, J.; Uan, J.; Lin, M.; Lin, Z. Optically transparent Li-Al-CO3 layered double hydroxide thin films on an AZ31 Mg alloy formed by electrochemical deposition and their corrosion resistance in a dilute chloride environment. Corros. Sci. 2013, 68, 238–248. [Google Scholar] [CrossRef]
- Zhang, F.Z.; Sun, M.; Xu, S.L.; Zhao, L.L.; Zhang, B.W. Fabrication of oriented layered double hydroxide films by spin coating and their use in corrosion protection. Chem. Eng. J. 2008, 141, 362–367. [Google Scholar] [CrossRef]
- Zhang, W.; Buchheit, R.G. Hydrotalcite coating formation on Al-Cu-Mg alloys from oxidizing bath chemistries. Corrosion 2002, 58, 591–600. [Google Scholar] [CrossRef]
- Chen, J.; Song, Y.W.; Shan, D.Y.; Han, E.H. Study of the in situ growth mechanism of Mg–Al hydrotalcite conversion film on AZ31 magnesium alloy. Corros. Sci. 2012, 63, 148–158. [Google Scholar] [CrossRef]
- Chen, J.; Song, Y.W.; Shan, D.Y.; Han, E.H. Study of the corrosion mechanism of the in situ grown Mg–Al–CO32− hydrotalcite film on AZ31 alloy. Corros. Sci. 2012, 65, 268–277. [Google Scholar] [CrossRef]
- Huang, M.; Lu, G.; Pu, J.; Qiang, Y. Superhydrophobic and smart MgAl-LDH anti-corrosion coating on AZ31 Mg surface. J. Ind. Eng. Chem. 2021, 103, 154–164. [Google Scholar] [CrossRef]
- Zhang, F.; Liua, Z.; Zeng, R.; Li, S.; Cui, H.; Song, L.; Han, E. Corrosion resistance of Mg–Al-LDH coating on magnesium alloy AZ31. Surf. Coat. Technol. 2014, 258, 1152–1158. [Google Scholar] [CrossRef]
- Ishizaki, T.; Chiba, S.; Watanabe, K.; Suzuki, H. Corrosion resistance of Mg–Al layered double hydroxide container-containing magnesium hydroxide films formed directly on magnesium alloy by chemical-free steam coating. J. Mater. Chem. A 2013, 1, 8968–8977. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, X.; Yin, M.; Pu, J.; Yuan, N.; Ding, J. Superhydrophobic and self-healing Mg-Al Layered Double Hydroxide/Silane composite coatings on the Mg alloy surface with a long-term anti-corrosion lifetime. Langmuir 2021, 37, 8129–8138. [Google Scholar] [CrossRef] [PubMed]
- Kahl, M.; Golden, T.D. Electrochemical determination of phenolic acids at a Zn/Al layered double hydroxide film modified glassy carbon electrode. Electroanalysis 2014, 26, 1664–1670. [Google Scholar] [CrossRef]
- Wang, A.Q.; Golden, T.D. Electrochemical formation of Cerium oxide/Layered Silicate nanocomposite films. J. Nanotechnol. 2016, 2016, 8459374. [Google Scholar] [CrossRef] [Green Version]
- DeLeon, V.; Golden, T.D. Effect of electrochemical parameters on the morphology and Ca/P ratios of deposited apatite coatings on metal and alloy substrates. ECS Trans. 2011, 33, 43–50. [Google Scholar] [CrossRef]
- Singh, A.P.; Roccapriore, K.; Algarni, Z.; Salloom, R.; Golden, T.D.; Philipose, U. Structure and electronic properties of InSb nanowires grown in flexible polycarbonate membranes. Nanomaterials 2019, 9, 1260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yarger, M.S.; Steinmiller, E.M.P.; Choi, K.S. Electrochemical synthesis of Zn-Al layered double hydroxide (LDHs) films. Inorg. Chem. 2008, 47, 5859–5865. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Liang, J.; Peng, Z.; Liu, B. Electrochemical deposition and characterization of Zn-Al layered double hydroxides (LDHs) films on magnesium alloy. Appl. Surf. Sci. 2014, 313, 834–840. [Google Scholar] [CrossRef]
- He, Q.-Q.; Zhou, M.-J.; Hu, J.-M. Electrodeposited Zn-Al layered double hydroxide films for corrosion protection of aluminum alloys. Electrochim. Acta 2020, 355, 136796. [Google Scholar] [CrossRef]
- Gualandi, I.; Monti, M.; Scavetta, E.; Tonelli, D.; Prevot, V.; Mousty, C. Electrodeposition of layered double hydroxides on platinum: Insights into the reactions sequence. Electrochim. Acta 2015, 152, 75–83. [Google Scholar] [CrossRef]
- Jayaraj, J.; Raj, S.A.; Srinivasan, A.; Ananthakumar, S.; Pillai, U.T.S.; Dhaipule, N.G.K.; Mudali, U.K. Composite magnesium phosphate coatings for improved corrosionresistance of magnesium AZ31 alloy. Corros. Sci. 2016, 113, 104–115. [Google Scholar] [CrossRef]
- Kaseem, M.; Hussain, T.; Baek, S.H.; Ko, Y.G. Formation of stable coral reef-like structures via self-assembly of functionalized polyvinyl alcohol for superior corrosion performance of AZ31 Mg alloy. Mater. Des. 2020, 193, 108823. [Google Scholar] [CrossRef]
- Fischer, D.A.; Vargas, I.T.; Pizarro, G.E.; Armijo, F.; Walczak, M. The effect of scan rate on the precision of determining corrosion current by Tafel extrapolation: A numerical study on the example of pure Cu in chloride containing medium. Electrochim. Acta 2019, 313, 457–467. [Google Scholar] [CrossRef]
- Therese, G.H.A.; Kamath, P.V. Electrochemical synthesis of metal oxides and hydroxides. Chem. Mater. 2000, 12, 1195–1204. [Google Scholar] [CrossRef]
- De Roy, A.; Forano, C.; Besse, J.P. Layered Double Hydroxides: Synthesis and Post-Synthesis Modification; Rives, V., Ed.; Layered Double Hydroxides: Present and Future; Nova Science Publishers, Inc.: New York, NY, USA, 2001; pp. 1–39. [Google Scholar]
- Lei, X.; Wang, L.; Zhao, X.; Chang, Z.; Jiang, M.; Yan, D.; Sun, X. Oriented CuZnAl ternary layered double hydroxide films: In situ hydrothermal growth and anticorrosion properties. Ind. Eng. Chem. Res. 2013, 52, 17934–17940. [Google Scholar] [CrossRef]
- Gupta, V.; Gupta, S.; Miura, N. Potentiostatically deposited nanostructured CoxNi1−x layered double hydroxides as electrode materials for redox-supercapacitors. J. Power Source 2008, 175, 680–685. [Google Scholar] [CrossRef]
- Kloprogge, J.T.; Frost, R.L.; Hickey, L. FT-Raman and FT-IR spectroscopic study of synthetic Mg/Zn/Al-hydrotalcites. J. Raman Spectrosc. 2004, 35, 967–974. [Google Scholar] [CrossRef] [Green Version]
- Lin, Y.; Wang, J.; Evans, D.G.; Li, D. Layered and intercalated hydrotalcite-like materials as thermal stabilizers in PVC resin. J. Phys. Chem. Solids 2006, 67, 998–1001. [Google Scholar] [CrossRef]
- Li, P.; Xu, Z.P.; Hampton, M.A.; Vu, D.T.; Huang, L.; Rudolph, V.; Nguyen, A.V. Control preparation of zinc hydroxide nitrate nanocrystals and examination of the chemical and structural stability. J. Phys. Chem. C 2012, 116, 10325–10332. [Google Scholar] [CrossRef]
- Hu, G.; Wang, N.; O’Hare, D.; Davis, J. Synthesis of magnesium aluminium layered double hydroxides in reverse microemulsions. J. Mater. Chem. 2007, 17, 2257–2266. [Google Scholar] [CrossRef]
- Rhee, S.W.; Kang, M.J. Kinetics on dehydration reaction during thermal treatment of MgAl-CO3-LDHs. Korean J. Chem. Eng. 2002, 19, 653–657. [Google Scholar] [CrossRef]
- Martin, J.; Jack, M.; Hakimian, A.; Vaillancourt, N.; Villemure, G. Electrodeposition of Ni-Al layered double hydroxide thin films having an inversed opal structure: Application as electrochromic coatings. J. Electroanal. Chem. 2016, 780, 217–224. [Google Scholar] [CrossRef]
- Uan, J.-Y.; Lin, J.-K.; Tung, Y.-S. Direct growth of oriented Mg–Al layered double hydroxide film on Mg alloy in aqueous HCO3−/CO32− solution. J. Mater. Chem. 2010, 20, 761–766. [Google Scholar] [CrossRef]
- Lu, Z.; Qian, L.; Xu, W.; Tian, Y.; Jiang, M.; Li, Y.; Sun, X.; Duan, X. Dehydrated layered double hydroxides: Alcohothermal synthesis and oxygen evolution activity. Nano Res. 2016, 9, 3152–3161. [Google Scholar] [CrossRef]
- Nakamura, K.; Shimada, Y.; Miyashita, T.; Serizawa, A.; Ishizaki, T. Effect of vapor pressure during the steam coating treatment on structure and corrosion resistance of the Mg(OH)2/Mg-Al LDH composite film formed on Mg alloy AZ61. MDPI Mater. 2018, 11, 1659. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xue, L.; Cheng, Y.; Sun, X.; Zhou, Z.; Xiao, X.; Hu, Z.; Liu, X. The formation mechanism and photocatalytic activity of hierarchical NiAl–LDH films on an Al substrate prepared under acidic conditions. Chem. Commun. 2014, 50, 2301–2303. [Google Scholar] [CrossRef] [PubMed]
- Monti, M.; Benito, P.; Basile, F.; Fornasari, G.; Gazzano, M.; Scavetta, E.; Tonelli, D.; Vaccari, A. Electrosynthesis of Ni/Al and Mg/Al layered double hydroxides on Pt and FeCrAlloy supports: Study and control of the pH near the electrode surface. Electrochim. Acta 2013, 108, 596–604. [Google Scholar] [CrossRef]
- Yan, T.; Xu, S.; Peng, Q.; Zhao, L.; Zhao, X.; Lei, X.; Zhang, F. Self-healing of layered double hydroxide film by dissolution/recrystallization for corrosion protection of aluminum. J. Electrochem. Soc. 2013, 160, C480–C486. [Google Scholar] [CrossRef]
- Jobbágy, M.; Regazzoni, A.E. Dissolution of nano-size Mg-Al-Cl hydrotalcite in aqueous media. Appl. Clay Sci. 2011, 51, 366–369. [Google Scholar] [CrossRef]
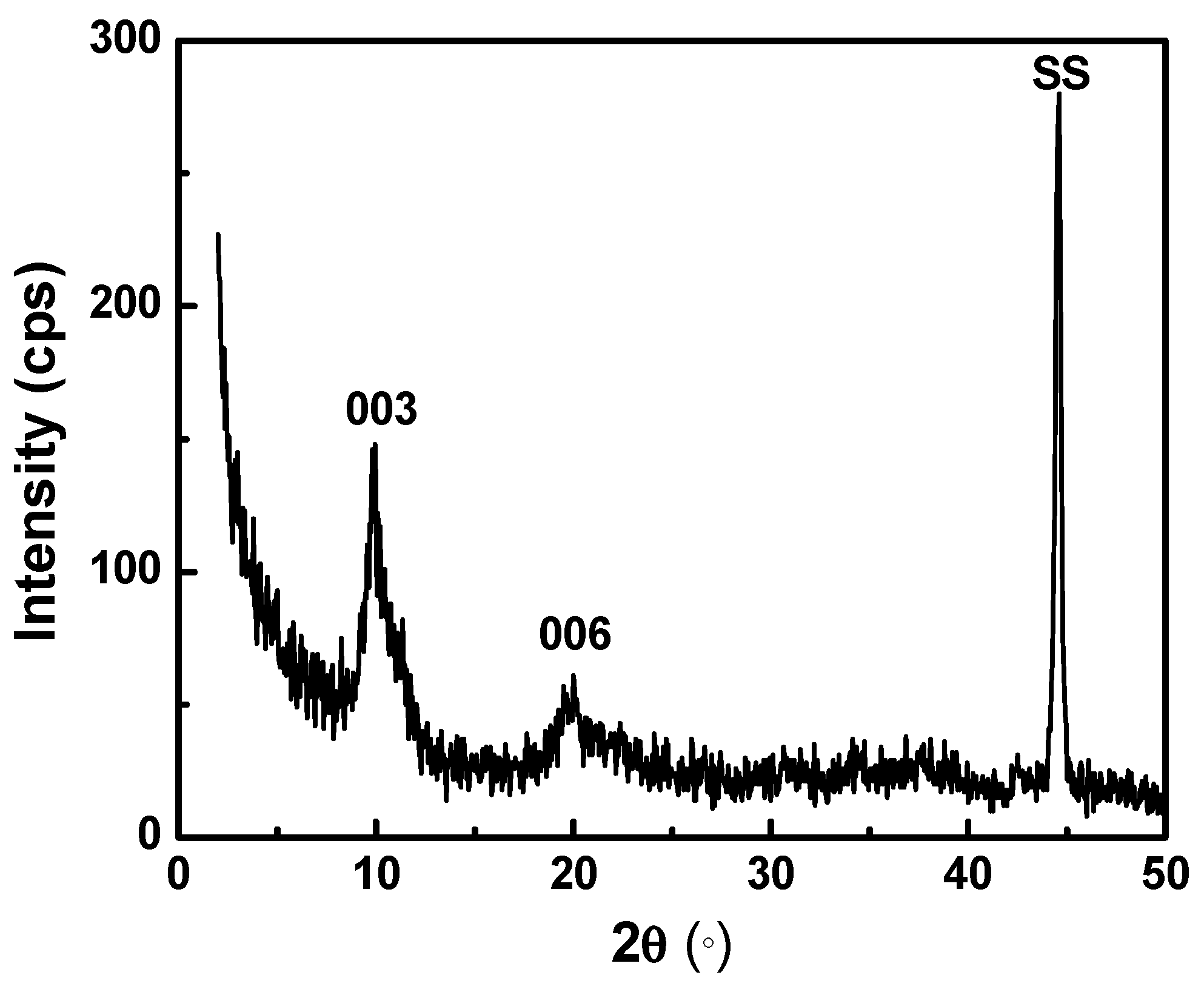







| Number of Layers | Zinc Atomic % | Aluminum Atomic % | Thickness (nm) |
|---|---|---|---|
| 1 | 41 ± 4 | 59 ± 4 | 431 ± 50 |
| 2 | 24 ± 2 | 76 ± 2 | 612 ± 46 |
| 5 | 14 ± 3 | 86 ± 3 | 2814 ± 45 |
| Sample | Ecorr (V vs. SCE) | icorr (μA/cm2) | Rp (kΩ∙cm2) |
|---|---|---|---|
| substrate | −0.363 ± 0.027 | 0.66 ± 0.01 | 19 ± 6 |
| 1L | −0.269 ± 0.020 | 0.61 ± 0.19 | 57 ± 27 |
| 2L | −0.240 ± 0.008 | 0.38 ± 0.11 | 137 ± 40 |
| 5L | −0.237 ± 0.013 | 0.12 ± 0.01 | 230 ± 54 |
| Immersion Time (h) | Ecorr (V vs. SCE) | icorr (μA/cm2) | Rp (kΩ∙cm2) |
|---|---|---|---|
| 1 | −0.239 ± 0.017 | 0.12 ± 0.02 | 218 ± 59 |
| 24 | −0.245 ± 0.031 | 0.08 ± 0.02 | 232 ± 37 |
| 72 | −0.235 ± 0.020 | 0.18 ± 0.03 | 309 ± 138 |
| 168 | −0.178 ± 0.013 | 0.18 ± 0.02 | 233 ± 72 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kahl, M.; Golden, T.D. Corrosion Resistance of Electrochemically Synthesized Modified Zaccagnaite LDH-Type Films on Steel Substrates. Materials 2021, 14, 7389. https://doi.org/10.3390/ma14237389
Kahl M, Golden TD. Corrosion Resistance of Electrochemically Synthesized Modified Zaccagnaite LDH-Type Films on Steel Substrates. Materials. 2021; 14(23):7389. https://doi.org/10.3390/ma14237389
Chicago/Turabian StyleKahl, Michael, and Teresa D. Golden. 2021. "Corrosion Resistance of Electrochemically Synthesized Modified Zaccagnaite LDH-Type Films on Steel Substrates" Materials 14, no. 23: 7389. https://doi.org/10.3390/ma14237389
APA StyleKahl, M., & Golden, T. D. (2021). Corrosion Resistance of Electrochemically Synthesized Modified Zaccagnaite LDH-Type Films on Steel Substrates. Materials, 14(23), 7389. https://doi.org/10.3390/ma14237389





