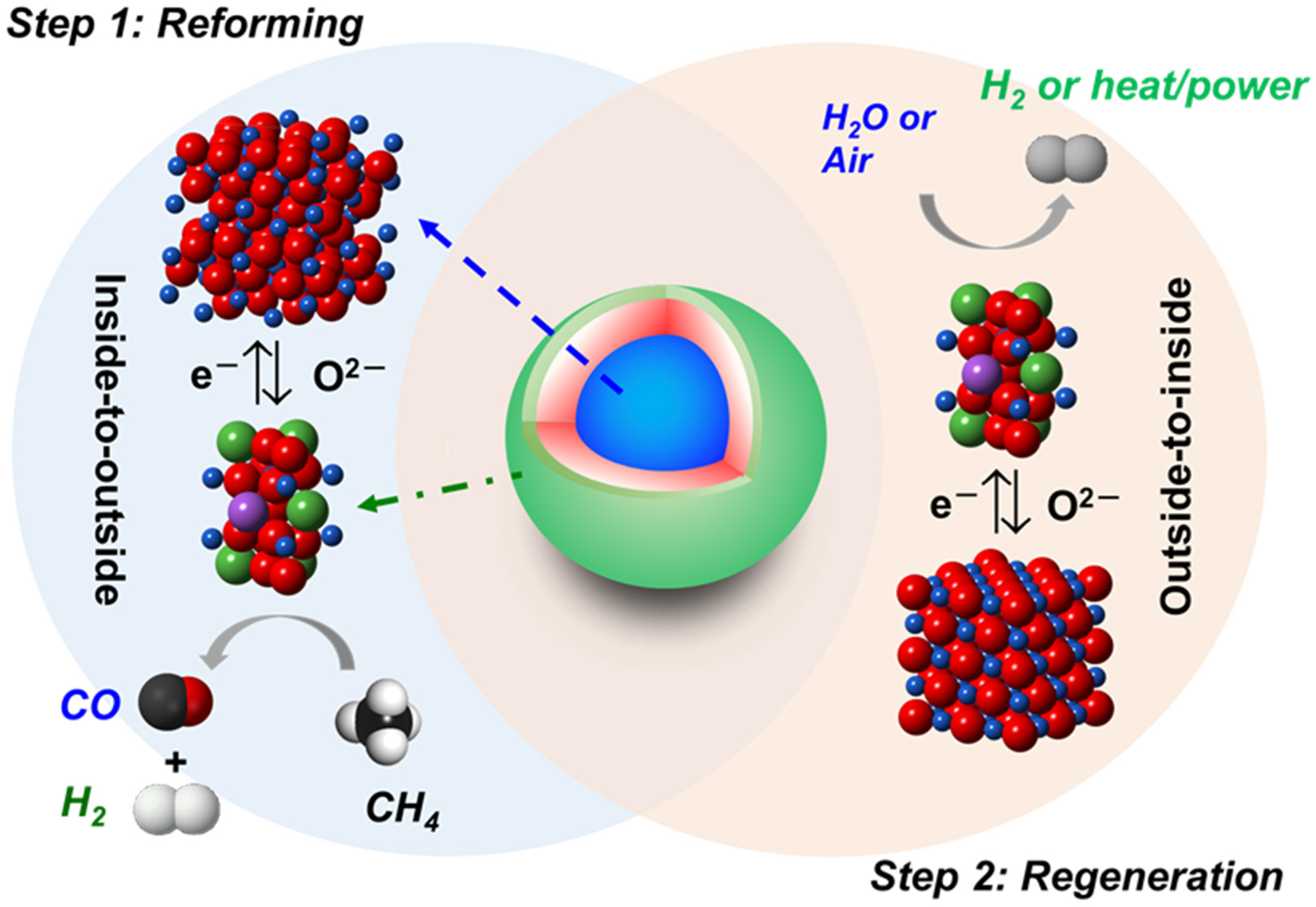
Core-Shell Fe2O3@La1−xSrxFeO3−δ Material for Catalytic Oxidations: Coverage of Iron Oxide Core, Oxygen Storage Capacity and Reactivity of Surface Oxygens
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Materials
2.1.1. Preparation of Pure LSF Perovskite
2.1.2. Fe2O3 Hematite Source
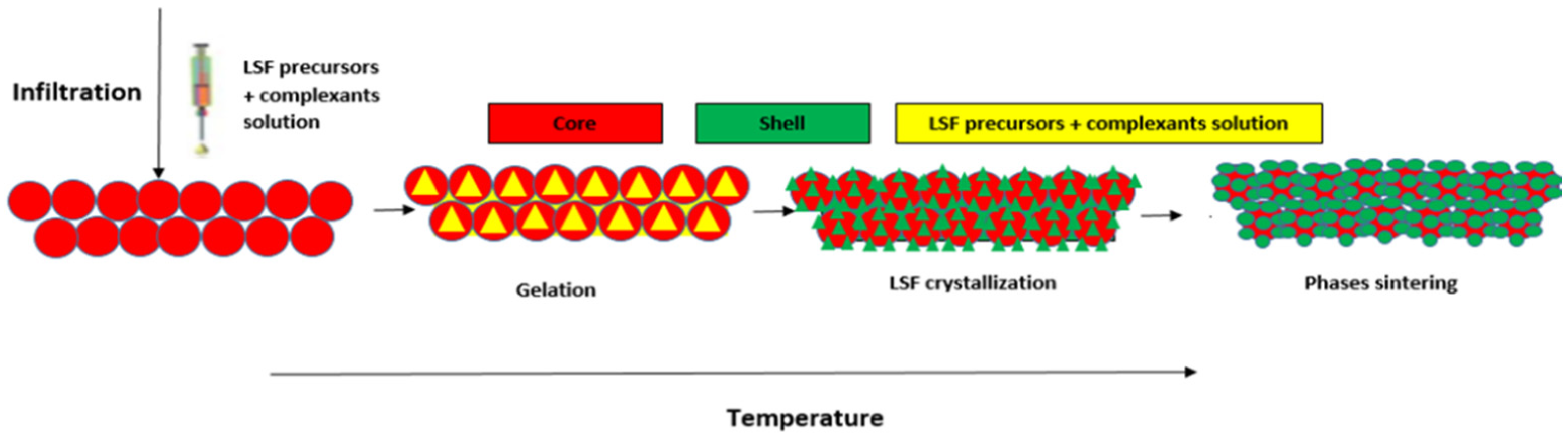
2.1.3. Preparation of Fe2O3@LSF Core-Shell Materials
2.2. Materials Characterization
3. Results and Discussion
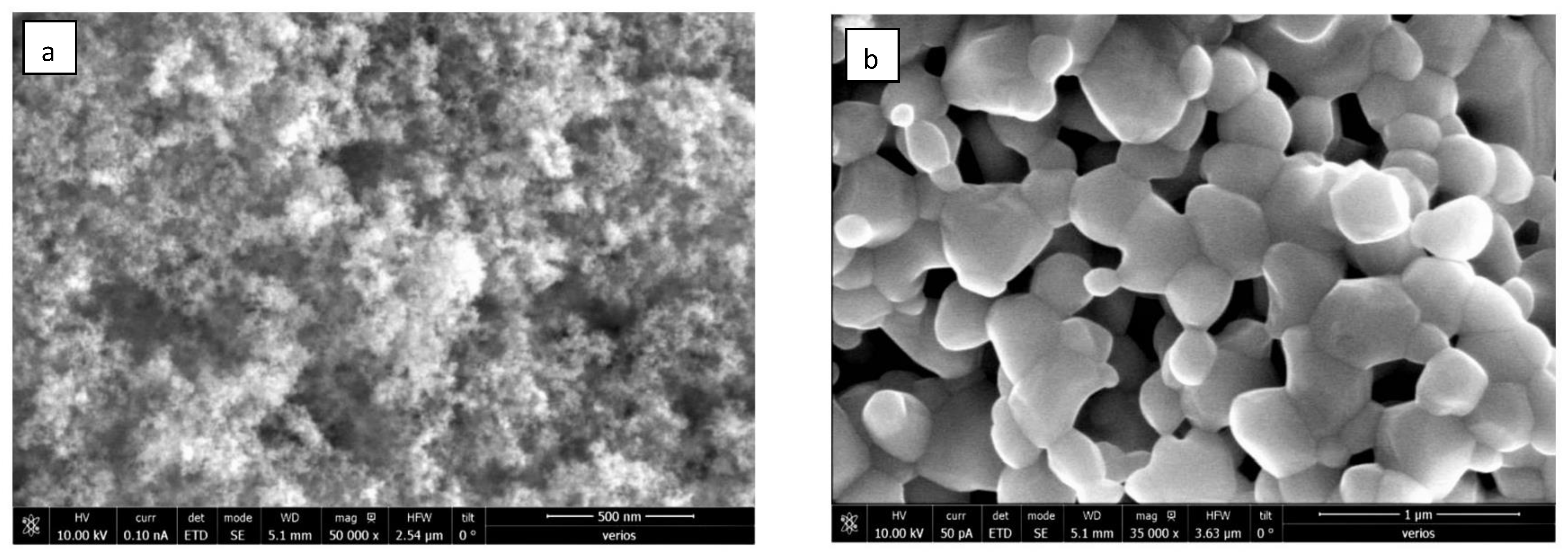
3.1. Structure and Morphology of Synthesized Core-Shell Materials
3.2. Content-State-Distribution-Reactivity of Surface Oxygen Atoms in Hematite, LSF and Core-Shell Hematite@LSF
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sun, Z.; Zhou, X.; Luo, W.; Yue, Q.; Zhang, Y.; Cheng, X.; Li, W.; Kong, B.; Deng, Y.; Zhao, D. Interfacial engineering of magnetic particles with porous shells: Towards magnetic core—Porous shell microparticles. Nano Today 2016, 11, 464–482. [Google Scholar] [CrossRef]
- Su, H.; Tian, Q.; Hurd Price, C.A.; Xu, L.; Qian, K.; Liu, J. Nanoporous core@shell particles: Design, preparation, applications in bioadsorption and biocatalysis. Nano Today 2020, 31, 100834. [Google Scholar] [CrossRef]
- Das, S.; Perez-Ramirez, J.; Gong, J.; Dewangan, N.; Hidajat, K.; Gates, B.C.; Kawi, S. Core-shell structured catalysts for thermocatalytic, photocatalytic, and electrocatalytic conversion of CO2. Chem. Soc. Rev. 2020, 49, 2937–3004. [Google Scholar] [CrossRef]
- Malayil Gopalan, S.; Deepak, V.; Jaehoon, K. Magnetic core-shell nanocatalysts: Promising versatile catalysts for organic and photocatalytic reactions. Catal. Rev. Sci. Eng. 2020, 62, 163–311. [Google Scholar] [CrossRef]
- Walker, J.S.; Rees, N.V.; Mendes, P.M. Progress towards the ideal core@shell nanoparticle for fuel cell electrocatalysis. J. Exp. Nanosci. 2018, 13, 258–271. [Google Scholar] [CrossRef]
- Chuanping, L.; Yongdong, J. Shell-Isolated Plasmonic Nanostructures for Biosensing, Catalysis, and Advanced Nanoelectronics. Adv. Funct. Mater. 2021, 31, 2008031. [Google Scholar] [CrossRef]
- Wang, Z.; Qi, J.; Yang, N.; Yu, R.; Wang, D. Core-shell nano/microstructures for heterogeneous tandem catalysis. Mater. Chem. Front. 2021, 5, 1126–1139. [Google Scholar] [CrossRef]
- Abdalwadood, H.E.; Ge, L.; Li, Z.; Liu, S.; Wang, S.; Zonghua, Z. Catalytic partial oxidation of methane to syngas: Review of perovskite catalysts and membrane reactors. Catal. Rev. Sci. Eng. 2021, 63, 1–67. [Google Scholar] [CrossRef]
- Yang, C.; Grimaud, A. Factors Controlling the Redox Activity of Oxygen in Perovskites: From Theory to Application for Catalytic Reactions. Catalyst 2017, 7, 149. [Google Scholar] [CrossRef]
- He, F.; Chen, J.; Liu, S.; Huang, Z.; Wei, G.; Wang, G.; Cao, Y.; Zhao, K. La1-xSrxFeO3 perovskite-type oxides for chemical looping steam methane reforming: Identification of the surface elements and redox cyclic performance. Int. J. Hydrogen Energy 2019, 44, 10265–10276. [Google Scholar] [CrossRef]
- Protasova, L.; Snijkers, F. Recent developments in oxygen carrier materials for hydrogen production via chemical looping processes. Fuel 2016, 181, 75–93. [Google Scholar] [CrossRef]
- Imtiaz, Q.; Hosseini, D.; Mueller, C.R. Review of oxygen carriers for chemical looping with oxygen uncoupling (CLOU): Thermodynamics, material development and synthesis. Energy Technol. 2013, 1, 633–647. [Google Scholar] [CrossRef]
- Galinsky, L.N.; Huang, Y.; Shafiefarhood, A.; Li, F. Iron Oxide with Facilitated O2–Transport for Facile Fuel Oxidation and CO2 Capture in a Chemical Looping Scheme. ACS Sustain. Chem. Eng. 2013, 1, 364–373. [Google Scholar] [CrossRef]
- Neal, L.M.; Shafiefarhood, A.; Li, F. Dynamic Methane Partial Oxidation Using a Fe2O3@La0.8Sr0.2FeO3−δ Core−Shell Redox Catalyst in the Absence of Gaseous Oxygen. ACS Catal. 2014, 4, 3560–3569. [Google Scholar] [CrossRef]
- Lobera, M.P.; Escolástico, S.; Garcia-Fayos, S.; Serra, J.M. Ethylene Production by ODHE in Catalytically Modified Ba0.5Sr0.5Co0.8Fe0.2O3−δ Membrane Reactors. ChemSusChem 2012, 5, 1587–1596. [Google Scholar] [CrossRef]
- Ten Elshof, J.E.; Bouwmeester, H.J.M.; Verweij, H. Oxygen transport through La1−xSrxFeO3−δ membranes. II. Permeation in air/CO, CO2 gradients. Solid State Ionics. 1996, 89, 81–92. [Google Scholar] [CrossRef]
- Ten Elshof, J.E.; Lankhorst, M.H.R.; Bouwmeester, H.J.M. Oxygen Exchange and Diffusion Coefficients of Strontium-Doped Lanthanum Ferrites by Electrical Conductivily Relaxation. J. Electrochem. Soc. 1997, 144, 1060–1067. [Google Scholar] [CrossRef]
- Patrakeev, M.V.; Bahteeva, J.A.; Mitberg, E.B.; Leonidov, I.A.; Kozhevnikov, V.L.; Poeppelmeierb, K.R. Electron/hole and ion transport in La1-xSrxFeO3-δ. Solid State Chem. 2003, 172, 219–231. [Google Scholar] [CrossRef]
- He, F.; Trainham, J.; Parsons, G.; Newman, J.S.; Li, F. A hybrid solar-redox scheme for liquid fuel and hydrogen coproduction. Energy Environ. Sci. 2014, 7, 2033–2042. [Google Scholar] [CrossRef]
- Ghosh Chaudhuri, R.; Paria, S. Core/shell nanoparticles: Classes, properties, synthesis mechanisms, characterization, and applications. Chem. Rev. 2012, 112, 2373–2433. [Google Scholar] [CrossRef]
- Liu, R.; Zhao, Y.; Huang, R.; Zhao, Y.; Zhou, H. Multiferroic ferrite/perovskite oxide core/shell nanostructure. J. Mater. Chem. 2010, 20, 10655–10670. [Google Scholar] [CrossRef]
- Chang, H.Y.; Wang, S.H.; Wang, Y.M.; Lai, C.W.; Lin, C.H.; Cheng, S.Y. Novel core-shell structure of perovskite anode and characterization. Int. J. Hydrogen Energy 2012, 37, 1771–1778. [Google Scholar] [CrossRef]
- Oliveira, P.N.; Alanis, D.; Bini, R.D.; Silva, D.M.; Dias, G.S.; Santos, I.A.; Cótica, L.F.; Guo, R.; Bhalla, A.S. Synthesis and characterization of structural, microstructural and ferroic properties of CoFe2O4 nanoparticles and CoFe2O4:BaTiO3 core-shell nanocomposites. Integr. Ferroelectr. 2016, 174, 88–97. [Google Scholar] [CrossRef]
- Lee, J.H.; Jang, J.T.; Choi, J.S.; Moon, S.H.; Noh, S.H.; Kim, J.W.; Kim, J.G.; Kim, I.S.; Park, K.I.; Cheon, J. Exchange-coupled magnetic nanoparticles for efficient heat induction. Nat. Nanotechnol. 2011, 6, 418–422. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.Y.; Cheng, S.Y.; Sheu, C.I.; Wang, Y.H. Core–shell structure of strontium titanate self-grown by a hydrothermal process for use in grain boundary barrier layers. Nanotechnology 2003, 14, 603–608. [Google Scholar] [CrossRef]
- Zeng, G.; Shao, J.; Gu, R.; Li, Y. Facile fabrication of a highly active shell–core LaNi(Mg, Al)O3@Mg–Al catalyst for ethanol steam reforming. Catal. Today 2014, 233, 31–37. [Google Scholar] [CrossRef]
- Huang, C.; Wu, J.; Chen, Y.T.; Tian, M.; Rykov, A.I.; Hou, B.; Lin, J.; Chang, C.R.; Pan, X.; Wang, J.; et al. In situ encapsulation of iron (0) for solar thermochemical syngas production over iron-based perovskite material. Commun. Chem. (Nat. Commun.) 2018, 1, 55. [Google Scholar] [CrossRef]
- Koch, G.; Hävecker, M.; Teschner, D.; Carey, S.J.; Wang, Y.; Kube, P.; Hetaba, W.; Lunkenbein, T.; Auffermann, G.; Timpe, O.; et al. Surface Conditions That Constrain Alkane Oxidation on Perovskites. ACS Catal. 2020, 10, 7007–07020. [Google Scholar] [CrossRef]
- Suib, S.L.; Prech, J.; Cejka, J.; Kuwahara, Y.; Mori, K.; Yamashita, H. Some novel porous materials for selective catalytic oxidations. Mater. Today 2020, 32, 244–259. [Google Scholar] [CrossRef]
- Anpo, M.; Costentin, G.; Giamello, E.; Lauron-Pernot, H.; Sojka, Z. Characterization and reactivity of oxygen species at the surface of metal oxides. J. Catal. 2021, 393, 259–280. [Google Scholar] [CrossRef]
- Zhong, Z.; Chen, K.; Ji, Y.; Yan, Q. Methane combustion over B-site partially substituted perovskite-type LaFeO3 prepared by sol-gel method. Appl. Catal. A Gen. 1997, 156, 29–41. [Google Scholar] [CrossRef]
- Chen, D.; Yang, G.; Ciucci, F.; Tadé, M.O.; Shao, Z. 3D core-shell architecture from infiltration and beneficial reactive sintering as highly efficient and thermally stable oxygen reduction electrode. J. Mater. Chem. A 2014, 2, 1284–1293. [Google Scholar] [CrossRef]
- Wang, W.; Qu, J.; Zhao, B.; Yang, G.; Shao, Z. Core-shell structured Li0.33La0.56TiO3 perovskite as a highly efficient and sulfur-tolerant anode for solid-oxide fuel cells. J. Mater. Chem. A 2015, 3, 8545–8551. [Google Scholar] [CrossRef][Green Version]
- Roduner, E. Size matters: Why nanomaterials are different. Chem. Soc. Rev. 2006, 35, 583–592. [Google Scholar] [CrossRef] [PubMed]
- Schwaminger, S.P.; García, P.F.; Merck, G.K.; Bodensteiner, F.A.; Heissler, S.; Günther, S.; Berensmeier, S. Nature of Interactions of Amino Acids with Bare Magnetite Nanoparticles. J. Phys. Chem. C 2015, 119, 23032–23041. [Google Scholar] [CrossRef]
- Bürger, A.; Magdans, U.; Gies, H. Adsorption of amino acids on the magnetite-(111)-surface: A force field study. J. Mol. Modeling 2013, 19, 851–857. [Google Scholar] [CrossRef]
- Biesingera, M.C.; Laua, L.W.M.; Gerson, A.R.; Smart, R.S.C. Resolving surface chemical states in XPS analysis of first row transition metals oxides and hydroxides: Sc, Ti, V, Cu and Zn. Appl. Surf. Sci. 2010, 257, 887–898. [Google Scholar] [CrossRef]
- Mierwaldt, D.; Mildner, S.; Arrigo, R.; Knop-Gericke, A.; Franke, E.; Blumenstein, A.; Hoffmann, J.; Jooss, C. In Situ XANES/XPS Investigation of Doped Manganese Perovskite Catalysts. Catalysts 2014, 4, 129–145. [Google Scholar] [CrossRef]
- Stoerzinger, K.A.; Hong, W.T.; Crumlin, E.J.; Bluhm, H.; Biegalski, M.D. Water Reactivity on the LaCoO3 (001) Surface: An Ambient Pressure X-ray Photoelectron Spectroscopy Study. J. Phys. Chem. C 2014, 118, 19733–19741. [Google Scholar] [CrossRef]
- Stoerzinger, K.A.; Hong, W.T.; Azimi, G.; Giordano, L.; Lee, Y.-L.; Crumlin, E.J.; Biegalski, M.D.; Bluhm, H.; Varanasi, K.K.; Shao-Horn, Y. Reactivity of Perovskites with Water: Role of Hydroxylation in Wetting and Implications for Oxygen Electrocatalysis. J. Phys. Chem. C 2015, 119, 18504–18512. [Google Scholar] [CrossRef]
- Ma, H.Q.; Tan, X.; Zhu, H.M.; Zhang, J.Y.; Zhang, L. XPS Characterization of La1–xCexFeO3 perovskite as high temperature water-gas shift catalysts. J. Chin. Soc. Rare Earths 2003, 21, 412–445. [Google Scholar] [CrossRef]
- Symianakis, E.; Malko, D.; Ahmad, E.; Mamede, A.S.; Paul, J.F.; Harrison, N.; Kucernak, A. Electrochemical characterization and quantified surface termination obtained by low energy ion scattering and X-ray photoelectron spectroscopy of orthorhombic and rhombohedral LaMnO3 powders. J. Phys. Chem. C 2015, 119, 12209–12217. [Google Scholar] [CrossRef]
- Graat, P.C.J.; Somers, M.A.J. Simultaneous determination of composition and thickness of thin iron-oxide films from XPS Fe 2p spectra. Appl. Surf. Sci. 1996, 100/101, 36–40. [Google Scholar] [CrossRef]
- Roosendaal, S.J.; van Asselen, B.J.; Elsenaar, W.M.; Vredenberg, A.; Habraken, F.H.P.M. The oxidation state of Fe(100) after initial oxidation in O2. Surf. Sci. 1999, 442, 329–337. [Google Scholar] [CrossRef]
- Widatallah, H.M.; Al-Rawas, A.D.; Johnson, C.; Al-Harthi, S.H.; Gismelseed, A.M.; Moore, E.A.; Stewart, S.J. The formation of nanocrystalline SrFeO3−δ using mechano-synthesis and subsequent sintering: Structure and mössbauer studies. J. Nanosci. Nanotechnol. 2009, 9, 2510–2517. [Google Scholar] [CrossRef]
- Abdel-Khalek, E.K.; Motawea, M.A.; Aboelnasr, M.A.; El-Bahnasawy, H.H. Study the oxygen vacancies and Fe oxidation states in CaFeO3-δ perovskite nanomaterial. Physica B 2022, 624, 413415. [Google Scholar] [CrossRef]
- Zhao, K.; Chen, J.; Li, H.; Zheng, A.; He, F. Effects of Co-substitution on the reactivity of double perovskite oxides LaSrFe2-xCoxO6 for the chemical-looping steam methane reforming. J. Energy Inst. 2019, 92, 594–603. [Google Scholar] [CrossRef]
- Berry, F.J.; Marco, J.F.; Ren, X. Reduction properties of phases in the system La0.5Sr0.5MO3 (M = Fe, Co). J. Solid State Chem. 2005, 178, 961–969. [Google Scholar] [CrossRef]
- Zhong, H.; Zeng, R. Structure of LaSrMO4 (M = Mn, Fe, Co, Ni, Cu) and their catalytic properties in the total oxidation of hexane. J. Serb. Chem. Soc. 2006, 71, 1049–1059. [Google Scholar] [CrossRef]
- Haibel, E.; Füglein, E.; Schulze, A.S.; Walter, D. Thermal decomposition of carbonated lanthanum hydroxide. J. Therm. Anal. Calorim. 2019, 138, 3571–3575. [Google Scholar] [CrossRef]
- Haibel, E.; Berendts, S.; Walter, D. Thermogravimetric and X-ray diffraction investigation on carbonated lanthanum oxide and lanthanum hydroxide formed in humid CO2 atmosphere. J. Therm. Anal. Calorim. 2018, 134, 261–267. [Google Scholar] [CrossRef]
- Füglein, E.; Walter, D. Thermal analysis of lanthanum hydroxide. J. Therm. Anal. Calorim. 2012, 110, 199–202. [Google Scholar] [CrossRef]
- Parkinson, G.S. Iron oxide surfaces. Surf. Sci. Rep. 2016, 71, 272–365. [Google Scholar] [CrossRef]
- Tang, J.J.; Liu, B. Reactivity of the Fe2O3 (0001) Surface for Methane Oxidation: A GGA+ U Study. J. Phys. Chem. C 2016, 120, 6642–6650. [Google Scholar] [CrossRef]
- Eltouny, N.; Ariya, P.A. Competing reactions of selected atmospheric gases on Fe3O4 nanoparticles surfaces. Phys. Chem. Chem. Phys. 2014, 16, 23056–23066. [Google Scholar] [CrossRef]
- Tortes Sanchez, R.M.; Curt, E.M.; Volzone, C.; Mercader, R.C.; Cavalieri, A.L. Study of Fe (II) oxidation in ground magnetite. Mat. Res. Bull. 1990, 25, 553–561. [Google Scholar] [CrossRef]
- De Alwin, C.; Leftwich, T.R.; Mukherjee, P.; Denofre, A.K.; Perrine, A. Spontaneous selective deposition of iron oxide nanoparticles on graphite as model catalysts. Nanoscale Adv. 2019, 1, 4729–4744. [Google Scholar] [CrossRef]
- Jozwiak, W.K.; Kaczmarek, E.; Maniecki, T.P.; Ignaczak, W.; Maniukiewicz, W. Reduction behavior of iron oxides in hydrogen and carbon monoxide atmospheres. Appl. Catal. A. 2007, 326, 17–27. [Google Scholar] [CrossRef]
- Feng, Y.; Wang, N.; Guo, X.; Zhang, S. Characteristics of dopant distribution and surface oxygen vacancy formation for modified Fe2O3 in chemical looping combustion. Fuel 2020, 276, 117942. [Google Scholar] [CrossRef]
- Albrecht, M.; Rodemerck, U.; Schneider, M.; Bröring, M.; Baabe, D.; Kondratenko, E.V. Unexpectedly efficient CO2 hydrogenation to higher hydrocarbons over non-doped Fe2O3. Appl. Catal. B 2017, 204, 119–126. [Google Scholar] [CrossRef]
- Chen, T.; Lyu, J.; Wang, Q.; Bai, P.; Wu, Y.; Xiangh, G. Mechanistic study on boron adsorption and isotopic separation with magnetic magnetite nanoparticles. J. Mater Sci 2021, 56, 4624–4640. [Google Scholar] [CrossRef]
- Ossowski, T.; Kiejna, A. Oxygen adsorption on Fe(110) surface revisited. Surf. Sci. 2015, 637–638, 35–41. [Google Scholar] [CrossRef]
- Papagno, L.; Caputi, L.S.; Chiarello, G. Structural study of clean and oxygen-covered Fe(110) by surface extended energy-loss fine-structure technique. Surf. Sci. 1986, 175, L767–L772. [Google Scholar] [CrossRef]
- Mani, P.; Kim, Y.; Lakhera, S.K.; Neppolian, B.H.; Choi, H. Complete arsenite removal from groundwater by UV activated potassium persulfate and iron oxide impregnated granular activated carbon. Chemosphere 2021, 277, 130225. [Google Scholar] [CrossRef]
- Zhang, Z.; Lu, J.; Yun, T.; Zheng, M.; Pan, J.; Sow, C.H.; Tok, E.S. Desorption of Ambient Gas Molecules and Phase Transformation of α-Fe2O3 Nanostructures during Ultrahigh Vacuum Annealing. J. Phys. Chem. C 2013, 117, 1509–1517. [Google Scholar] [CrossRef]
- Ivanov, D.V.; Pinaeva, L.G.; Isupova, L.A.; Sadovskaya, E.M.; Prosvirin, I.P.; Gerasimov, E.Y.; Yakovleva, I.S. Effect of surface decoration with LaSrFeO4 on oxygen mobiity and catalytic activity of La0.4Sr0.6FeO3−ı in high-temperature N2O decomposition, methane combustion and ammonia oxidation. Appl. Catal. A Gen. 2013, 457, 42–51. [Google Scholar] [CrossRef]

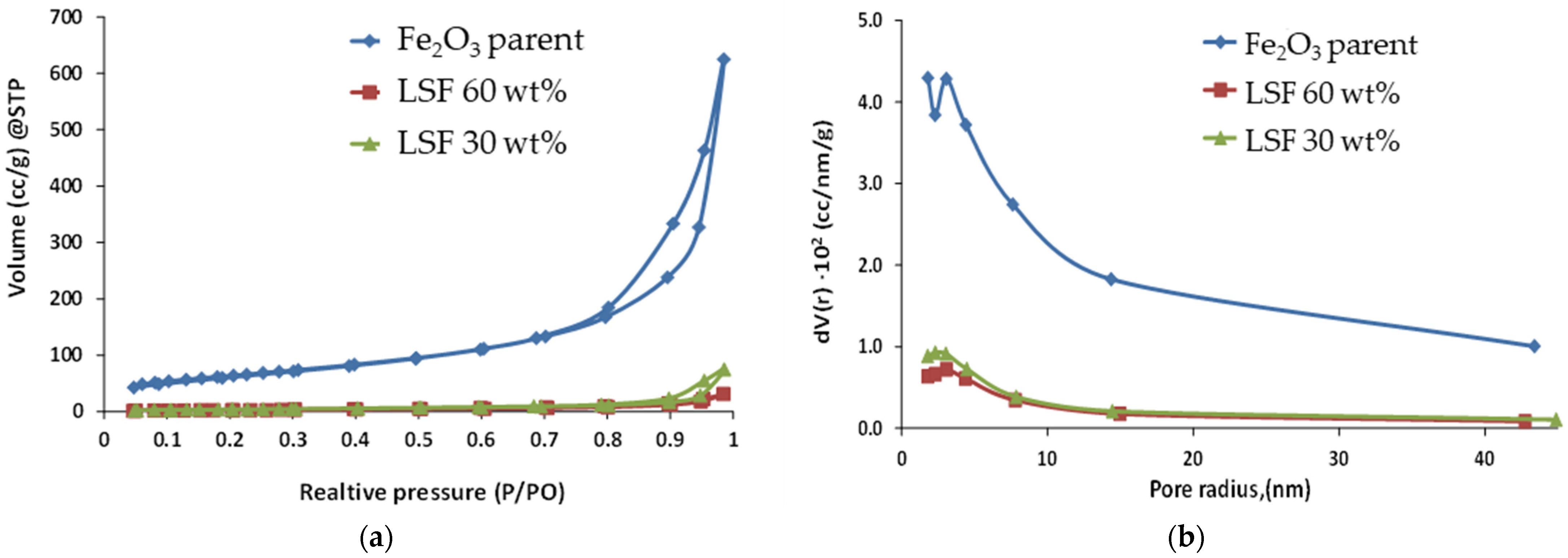
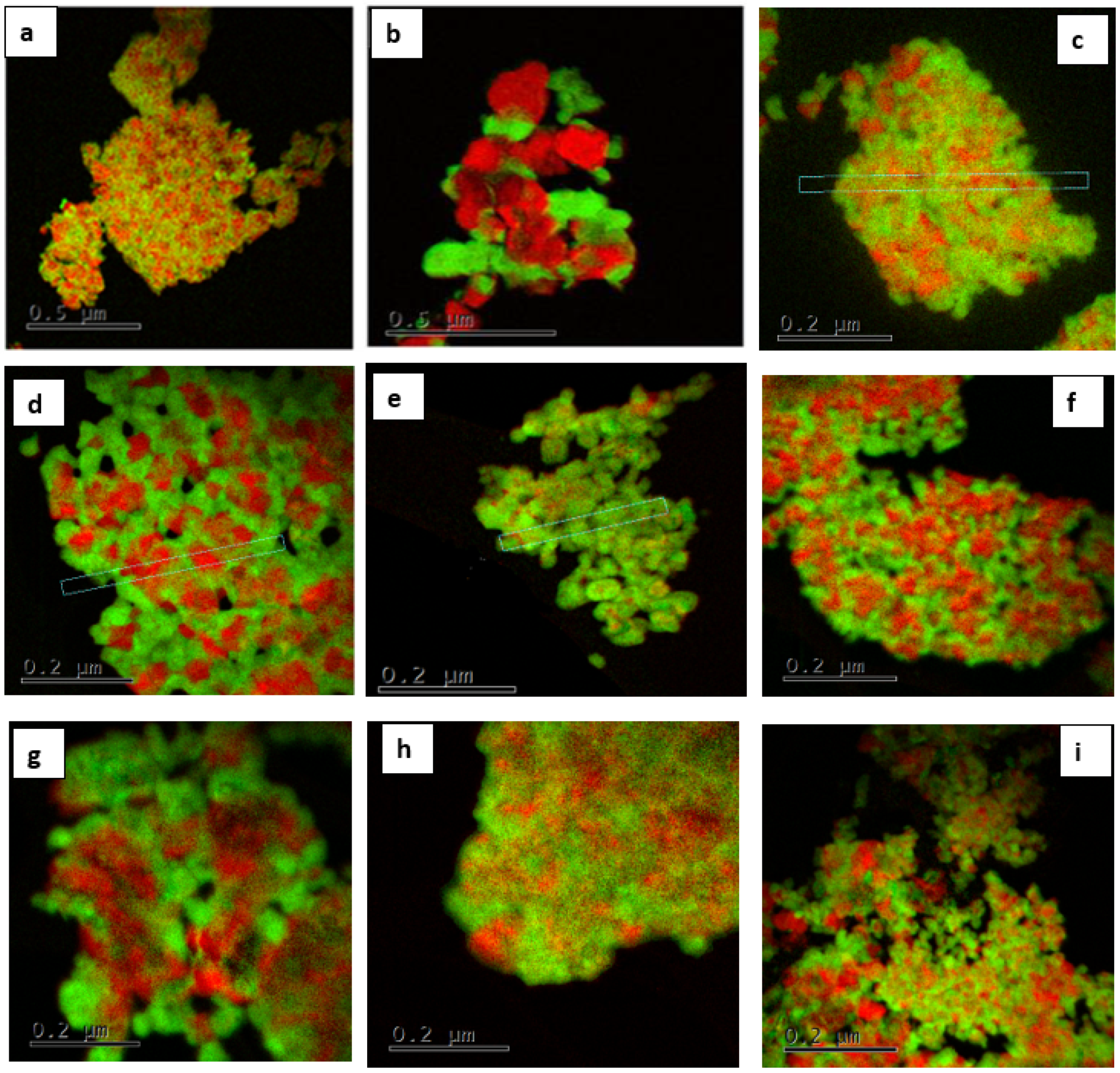
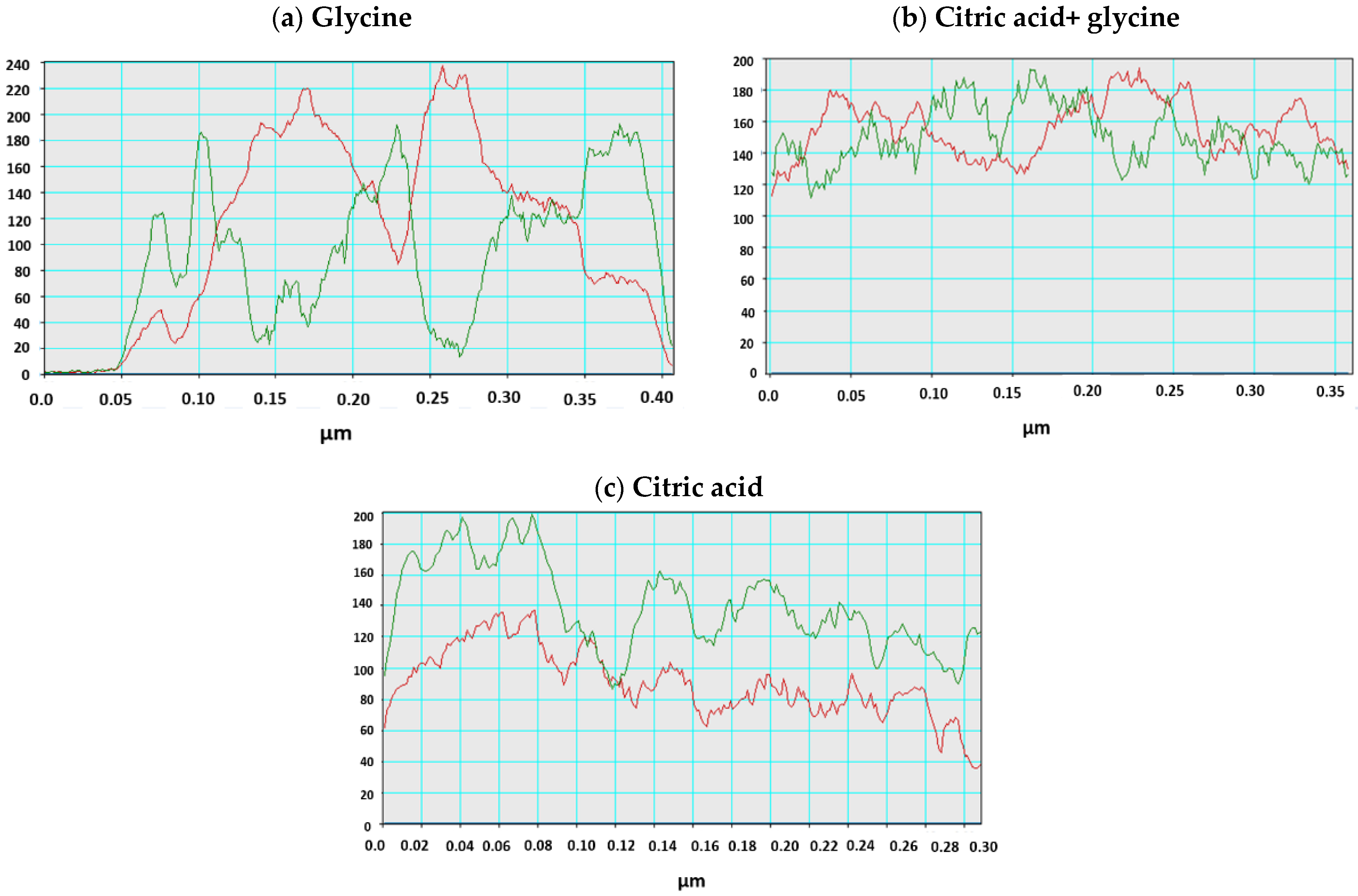
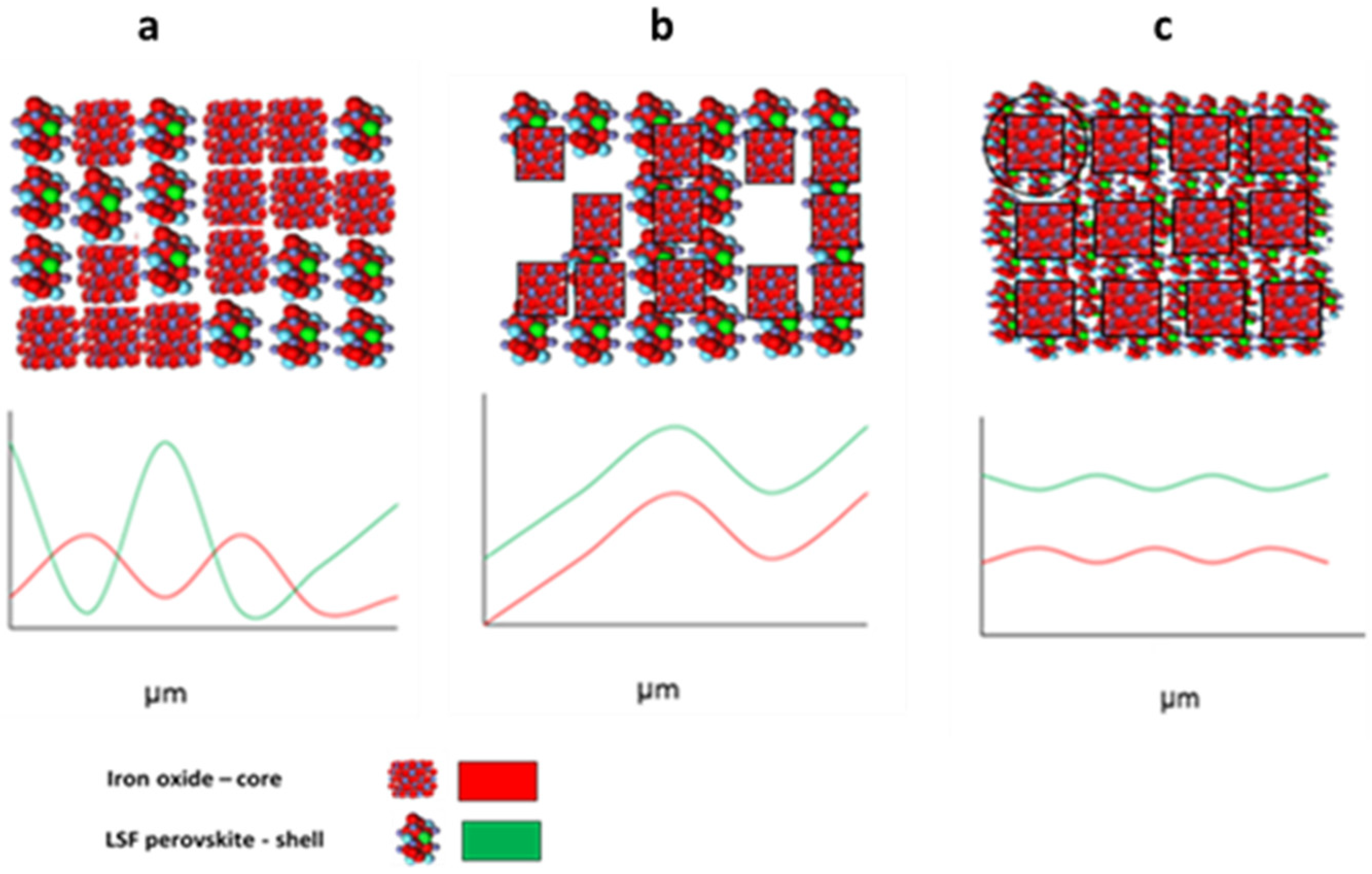
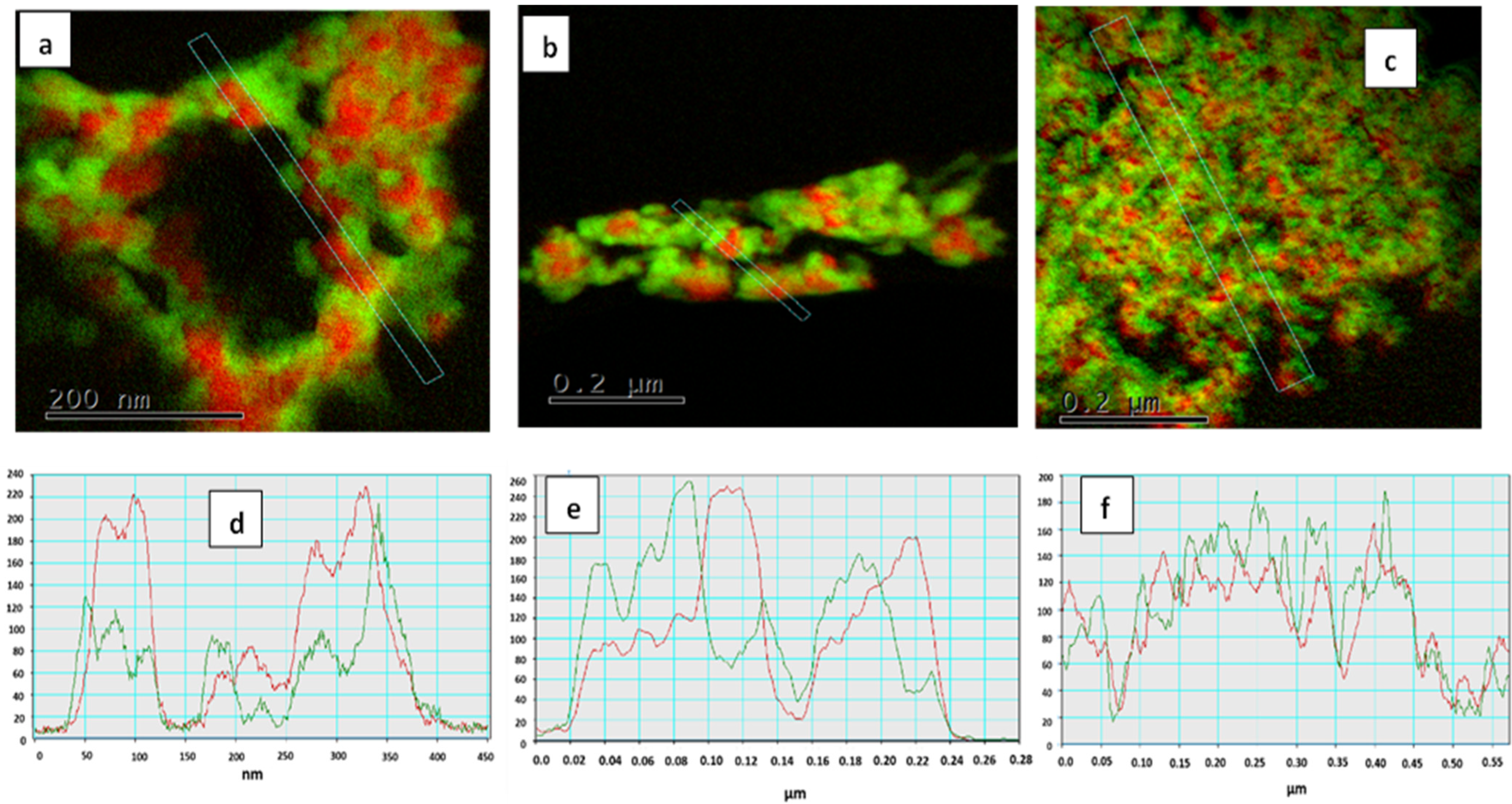
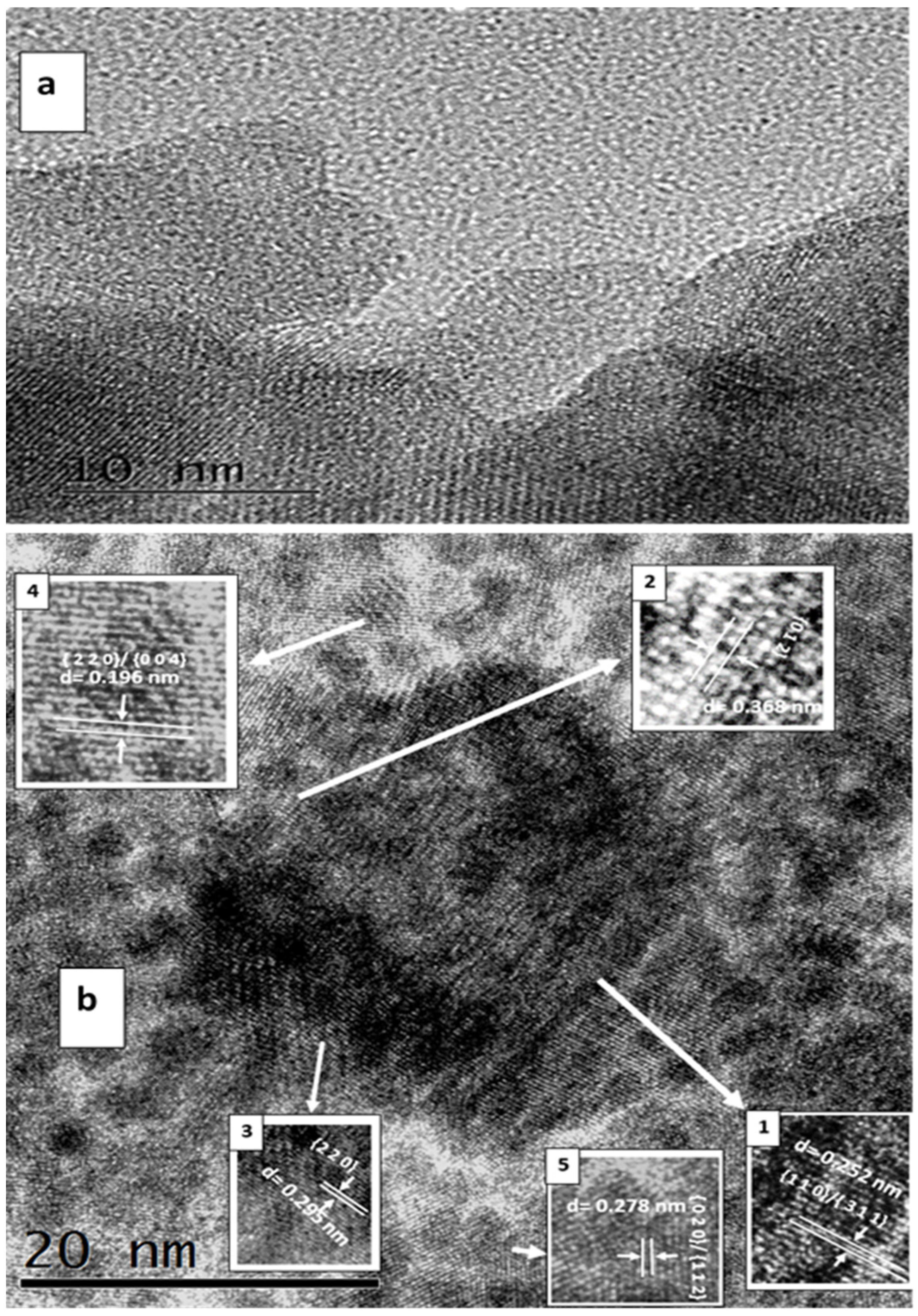

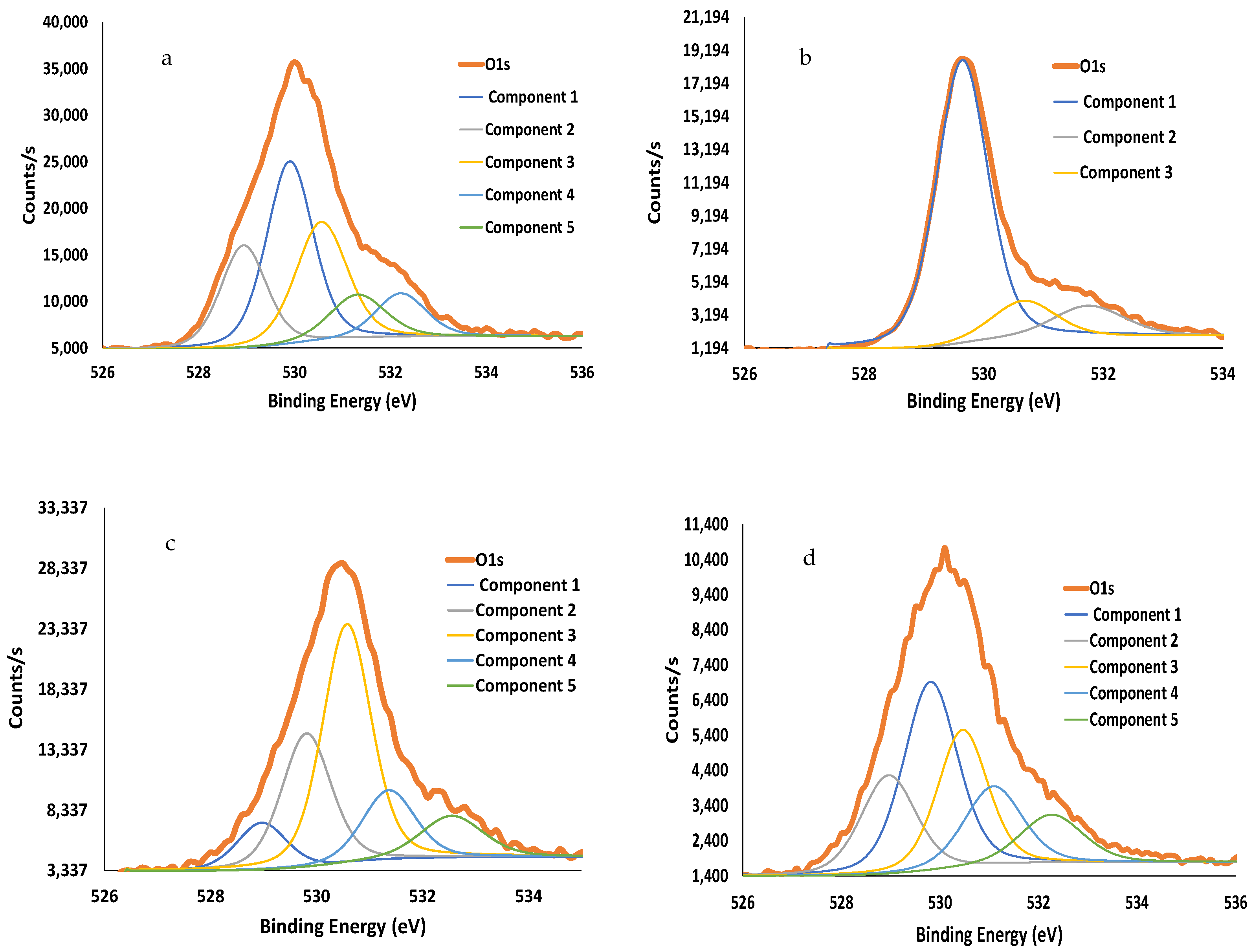
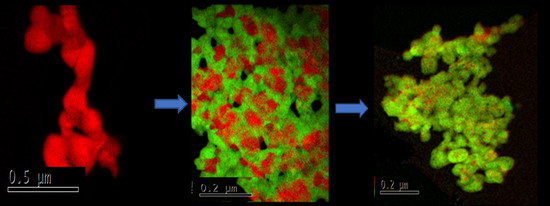
| Calcination Temperature, °C | 25 | 275 | 300 | 350 | 600 | 700 | 800 | 1000 |
|---|---|---|---|---|---|---|---|---|
| Content of hematite phases, wt% | ||||||||
| - well crystallized | 74 | 81 | 97 | 100 | 100 | 100 | 100 | 100 |
| - small nanocrystals < 4 nm | 26 | 19 | 3 | -- | -- | -- | -- | -- |
| Crystal size of well crystallized hematite phase, nm | 10 | 20 | 20 | 25 | 45 | 50 | >50 | >>50 |
| Surface area, m2/g | 229 | 127 | 99 | 50 | 45 | 8 | 2.7 | 1.0 |
| Pore volume, cm3/g | 0.97 | 0.64 | 0.42 | 0.23 | 0.1 | 0.06 | 0.01 | 0.004 |
| Average pore diameter, nm | 8.46 | 10.1 | 8.5 | 9.1 | 7.3 | 9.2 | 7.4 | 8.0 |
| LSF Shell Thickness (nm) | LSF Content, wt% | Total OSC, wt% O |
|---|---|---|
| 3 | 32 | 23.6 |
| 4 | 39 | 22.2 |
| 5 | 45 | 21.0 |
| 6 | 57 | 18.6 |
| Materials | Normalized Surface Concentrations of Metal Atoms, XPS, (at. %) | Normalized Bulk Concentrations of Metal Atoms (at. %) | Core Surface Coverage with LSF, % | ||||
|---|---|---|---|---|---|---|---|
| La | Sr | Fe | La | Sr | Fe | ||
| Fe2O3 | -- | -- | 100 | -- | -- | 100 | 0 |
| LSF | 35 | 21 | 44 | 33 | 12 | 55 | 100 |
| CS-4 | 20 | 19 | 61 | 22 | 6 | 72 | 70 |
| CS-3 | 20 | 23 | 57 | 20 | 5 | 75 | 77 |
| CS-5 | 40 | 15 | 46 | 21 | 5 | 74 | 98 |
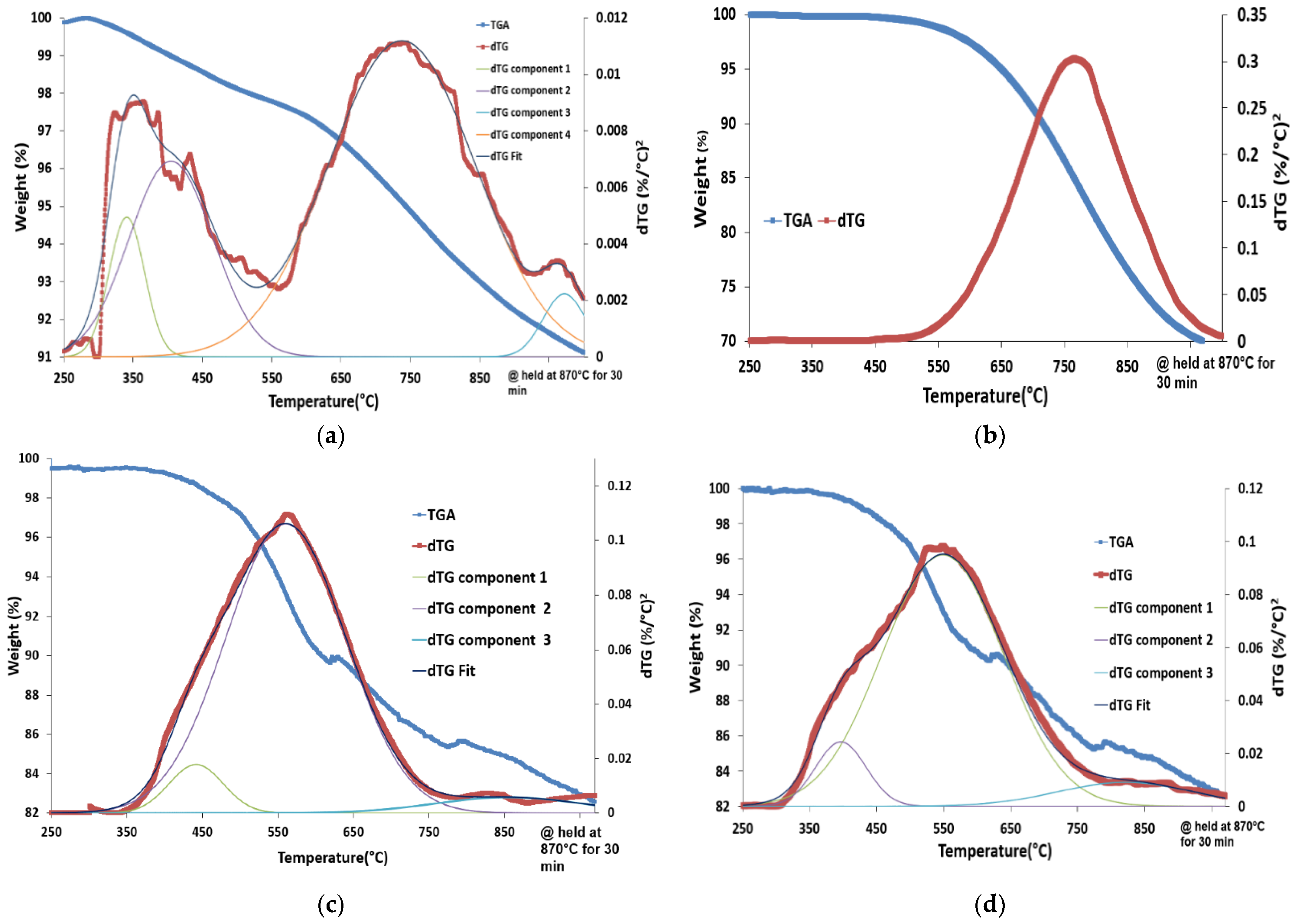
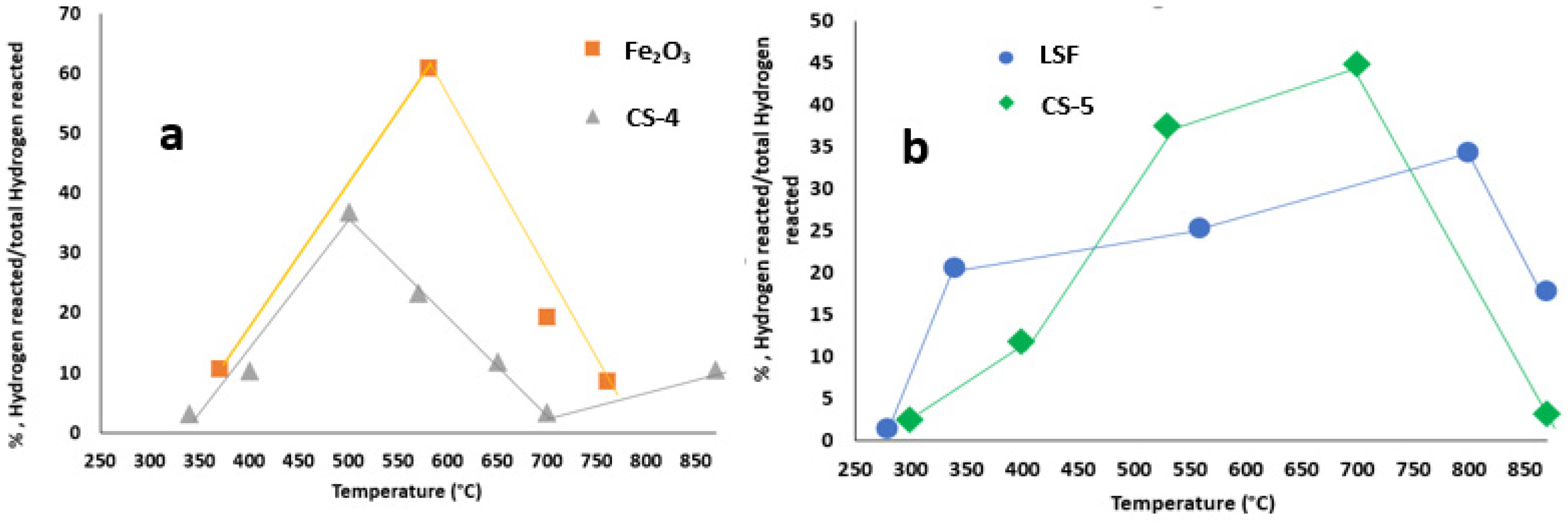
| Materials | Surface Area, m2/g | Total Amount of Reacted Hydrogen, (mmol/g) | Equivalent Amount of Removed Oxygen, (mmol/g) | TPR Peaks Positions, °C |
|---|---|---|---|---|
| LSF | 17 | 2.4 | 1.2 | 280,340,560,800,870 |
| Fe2O3 | 8 | 16.3 | 8.1 | 370,580,700,760 |
| CS-4 | 11 | 6.1 | 3.0 | 340,400,500,570,650,700,870 |
| CS-5 | 20 | 6.0 | 3.0 | 300,400,530,700,870 |
| Material | Phase Composition: Phase/wt%. | ||
|---|---|---|---|
| After H2-TPR Up to 450 °C | After H2-TPR Up to 650 °C | After H2-TPR Up to 870 °C | |
| Fe2O3 | Fe3O4/87 | Fe0/100 | Fe0/100 |
| FeO/19 | |||
| Fe0/4 | |||
| LSF | LSF/93 Fe0/7 | LSF/15 | |
| LSF/97 | Fe0/25 | ||
| Fe0/3 | La(OH)3/27 | ||
| SrLaFeO4/33 | |||
| CS-4 | LSF/72 | LSF/70 Fe0/30 | LSF/10 |
| Fe0/13 | Fe0/46 | ||
| Fe3O4/8 | La(OH)3/24 | ||
| FeO/7 | SrLaFeO4/20 | ||
| CS-5 | LSF/63 | LSF/66 Fe0/26 FeO/8 | LSF/20 |
| FeO/19 | Fe0/44 | ||
| Fe3O4/13 | La(OH)3/18 | ||
| Fe0/5 | SrLaFeO4/18 | ||
| Materials | Lattice Oxygen | Defect-Affected Oxygen | Surface Oxygen | Carbonate and Organics Oxygen | Oxygen in Hydroxyl Groups | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| B.E., eV | % at. | B.E., eV | % at. | B.E., eV | % at. | B.E., eV | % at. | B.E., eV | % at. | |
| Fe2O3 | 529.6 | 75.7 | ----- | ----- | 530.7 | 12.5 | ---- | ----- | 532.8 | 11.7 |
| LSF | 528.9 | 19.6 | 529.9 | 35.4 | 530.5 | 25.6 | 531.3 | 10.2 | 532.2 | 9.31 |
| Core-shell CS-4 | 529.0 | 8.6 | 529.8 | 24.8 | 530.6 | 43.3 | 531.4 | 13.6 | 532.6 | 9.7 |
| Core-shell CS-5 | 529.0 | 18.0 | 529.8 | 33.7 | 530.5 | 23.0 | 531.1 | 15.2 | 532.3 | 10.2 |
| Materials | Lattice Oxygen | Defect-Affected Oxygen | Surface Oxygen | Carbonate and Organics Oxygen | Oxygen in Hydroxyl Groups | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| B.E., eV | % at. | B.E., eV | % at. | B.E., eV | % at. | B.E., eV | % at. | B.E., eV | % at. | |
| LSF fresh | 528.9 | 19.6 | 529.9 | 35.4 | 530.5 | 25.6 | 531.3 | 10.2 | 532.2 | 9.31 |
| H2-TPR 450 °C | ----- | ----- | 530.2 | 35.4 | ----- | ----- | 531.9 | 51.8 | 533.7 | 12.8 |
| H2-TPR 650 °C | ----- | ----- | 529.5 | 70.5 | ----- | ----- | 531.7 | 25.6 | 532.7 | 3.93 |
| H2-TPR 870 °C | 528.7 | 29.2 | ----- | ----- | ----- | ----- | 531.1 | 57.3 | 533.4 | 12.8 |
| CS-4 fresh | 529.0 | 8.6 | 529.8 | 24.8 | 530.6 | 43.3 | 531.4 | 13.6 | 532.6 | 9.7 |
| H2-TPR 450 °C | 528.3 | 36.0 | 529.6 | 50.0 | ----- | ------ | 531.0 | 14.0 | ----- | ----- |
| H2-TPR 650 °C | 528.9 | 36.5 | 530.1 | 43.2 | ----- | ----- | 531.6 | 20.3 | ----- | ----- |
| H2-TPR 870 °C | 529.2 | 35.5 | ---- | ---- | ---- | ----- | 531.0 | 40.6 | 532.7 | 23.9 |
| CS-5 fresh | 529.0 | 18.0 | 529.8 | 33.7 | 530.5 | 23.0 | 531.1 | 15.2 | 532.3 | 10.2 |
| H2-TPR 450 °C | ----- | ----- | 529.4 | 48.9 | 530.3 | 38.3 | 531.8 | 12.7 | ----- | ----- |
| H2-TPR 650 °C | ----- | ----- | 529.4 | 87.7 | ----- | ----- | 531.5 | 12.3 | ----- | ----- |
| H2-TPR 870 °C | ----- | ----- | 529.6 | 45.2 | ----- | ----- | 531.4 | 40.1 | 533.0 | 14.6 |
| Fe2O3 fresh | 529.6 | 75.7 | ----- | ----- | 530.7 | 12.5 | ---- | --- | 532.8 | 11.7 |
| H2-TPR 450 °C | ----- | ----- | ----- | ----- | 530.4 | 73.1 | --- | ---- | 532.6 | 26.7 |
| H2-TPR 650 °C | ------ | ------ | ------ | ------ | 530.2 | 68.2 | ------ | ------ | 532.6 | 31.8 |
| H2-TPR 870 °C | ----- | ----- | ----- | ----- | 530.3 | 64.3 | --- | ---- | 532.7 | 35.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ohayon Dahan, H.; Landau, M.V.; Vidruk Nehemya, R.; Edri, E.; Herskowitz, M.; Ruan, C.; Li, F. Core-Shell Fe2O3@La1−xSrxFeO3−δ Material for Catalytic Oxidations: Coverage of Iron Oxide Core, Oxygen Storage Capacity and Reactivity of Surface Oxygens. Materials 2021, 14, 7355. https://doi.org/10.3390/ma14237355
Ohayon Dahan H, Landau MV, Vidruk Nehemya R, Edri E, Herskowitz M, Ruan C, Li F. Core-Shell Fe2O3@La1−xSrxFeO3−δ Material for Catalytic Oxidations: Coverage of Iron Oxide Core, Oxygen Storage Capacity and Reactivity of Surface Oxygens. Materials. 2021; 14(23):7355. https://doi.org/10.3390/ma14237355
Chicago/Turabian StyleOhayon Dahan, Hen, Miron V. Landau, Roxana Vidruk Nehemya, Eran Edri, Moti Herskowitz, Chongyan Ruan, and Fanxing Li. 2021. "Core-Shell Fe2O3@La1−xSrxFeO3−δ Material for Catalytic Oxidations: Coverage of Iron Oxide Core, Oxygen Storage Capacity and Reactivity of Surface Oxygens" Materials 14, no. 23: 7355. https://doi.org/10.3390/ma14237355
APA StyleOhayon Dahan, H., Landau, M. V., Vidruk Nehemya, R., Edri, E., Herskowitz, M., Ruan, C., & Li, F. (2021). Core-Shell Fe2O3@La1−xSrxFeO3−δ Material for Catalytic Oxidations: Coverage of Iron Oxide Core, Oxygen Storage Capacity and Reactivity of Surface Oxygens. Materials, 14(23), 7355. https://doi.org/10.3390/ma14237355