Effect of the Addition of Agribusiness and Industrial Wastes as a Partial Substitution of Portland Cement for the Carbonation of Mortars
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sampling Materials and Selection
2.2. Elaboration of the Specimens
2.3. Carbonation Analysis
2.4. Compressive Strength
2.5. Electrical Resistivity Test
3. Results
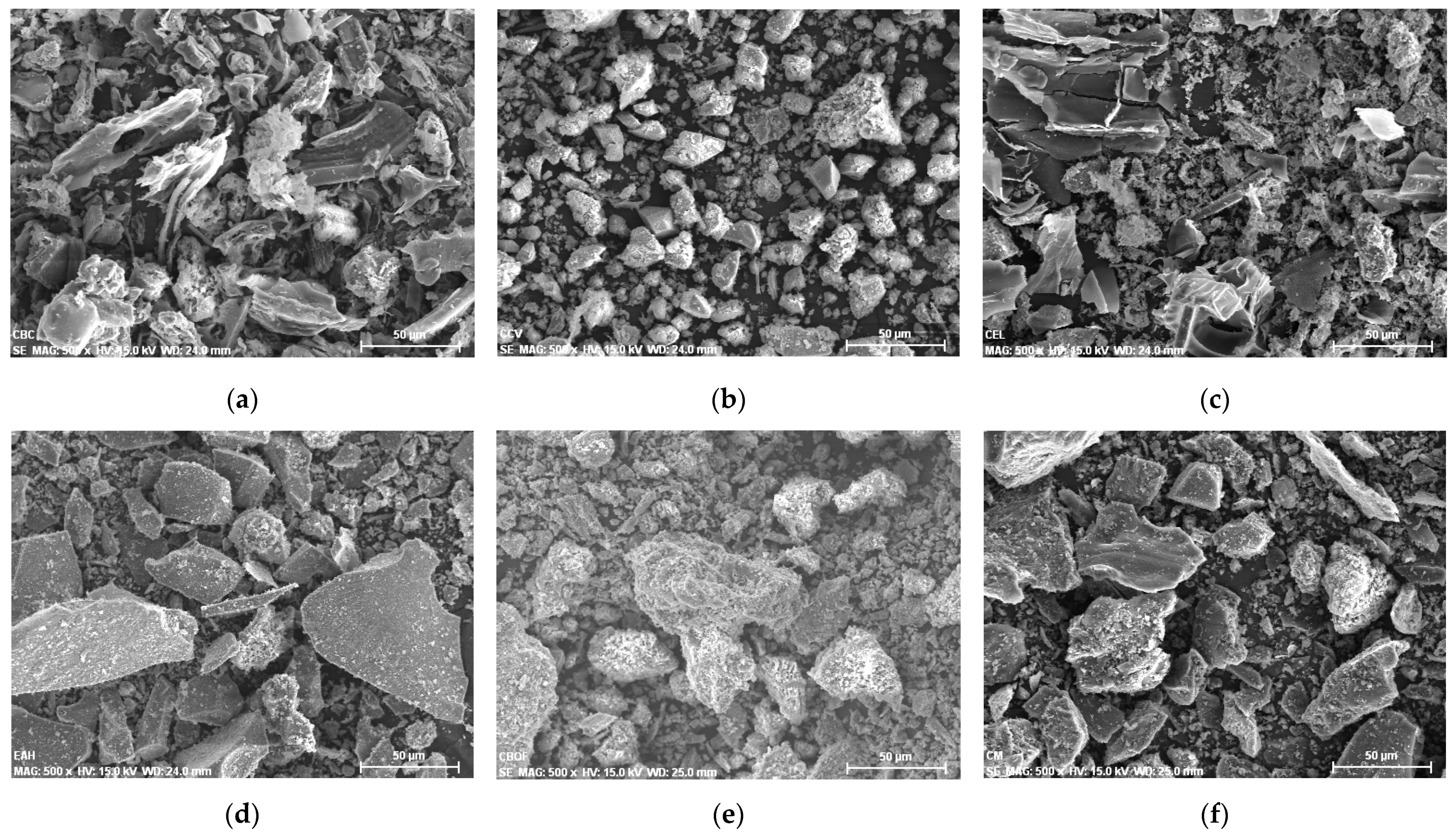
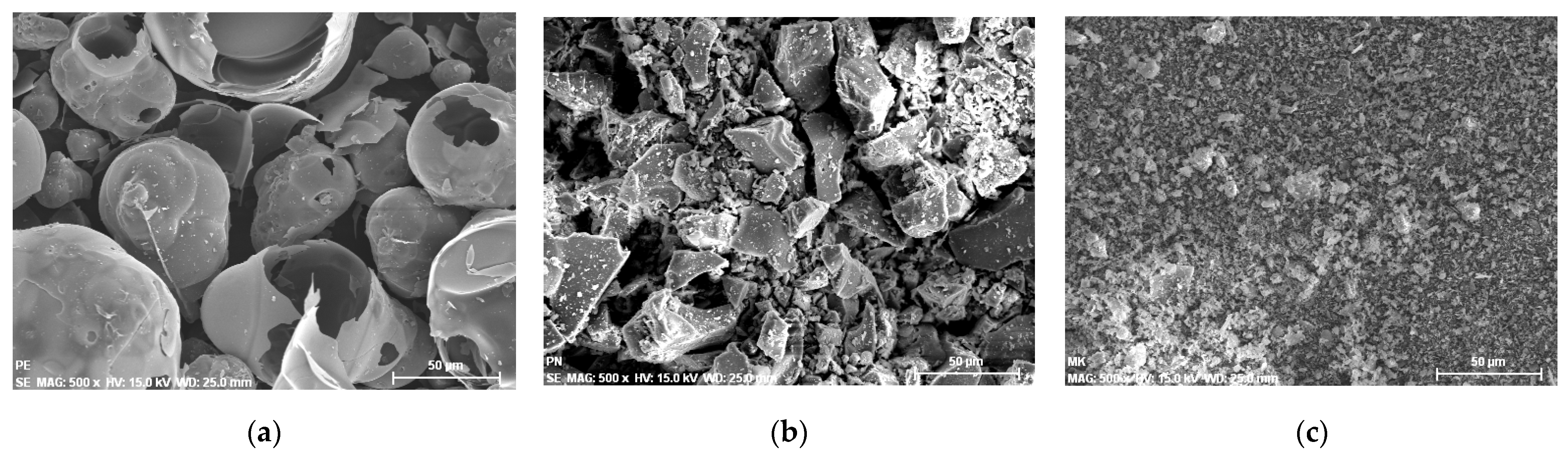
3.1. Characterization of the Aggregates
3.2. Characterization of the Additions
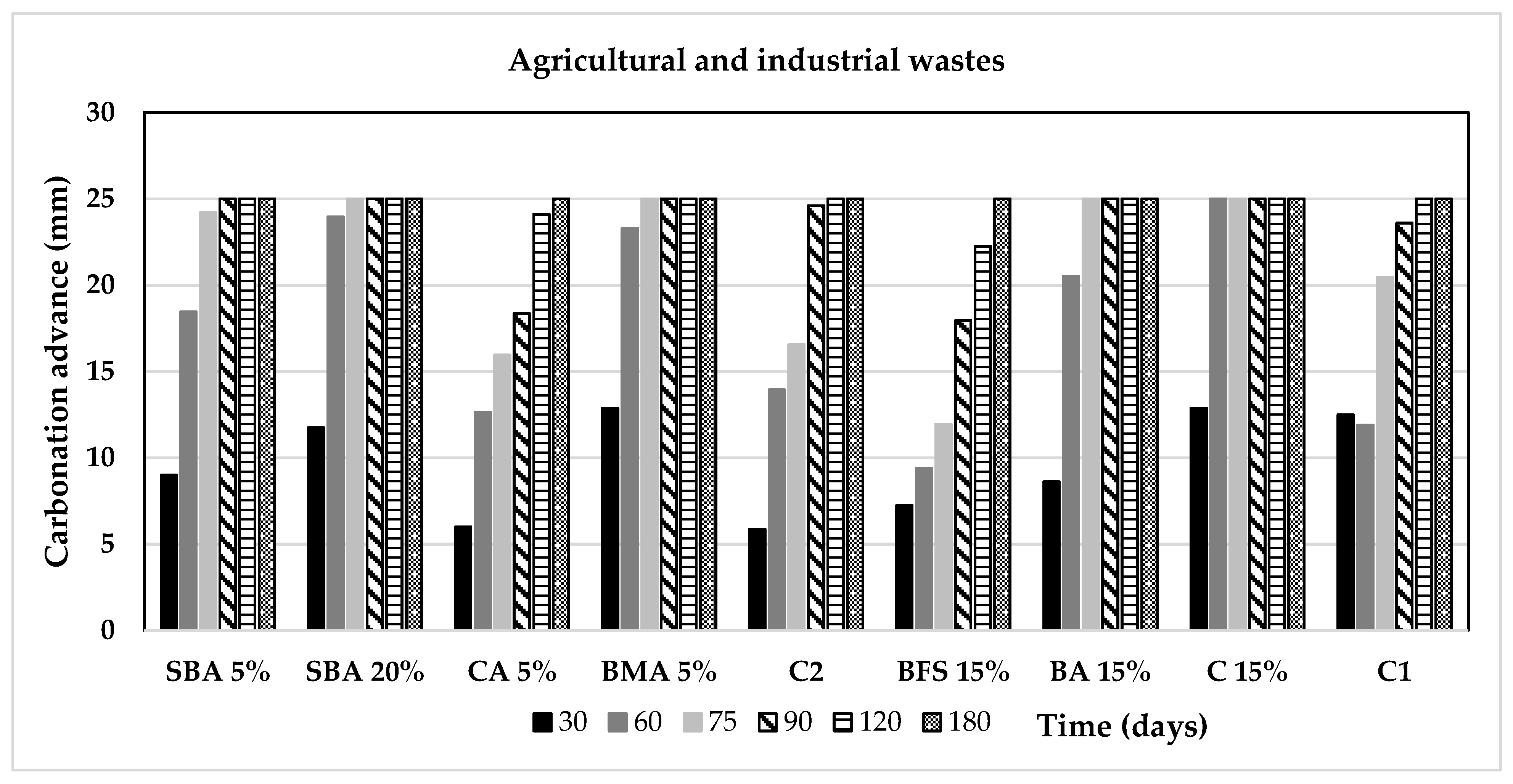
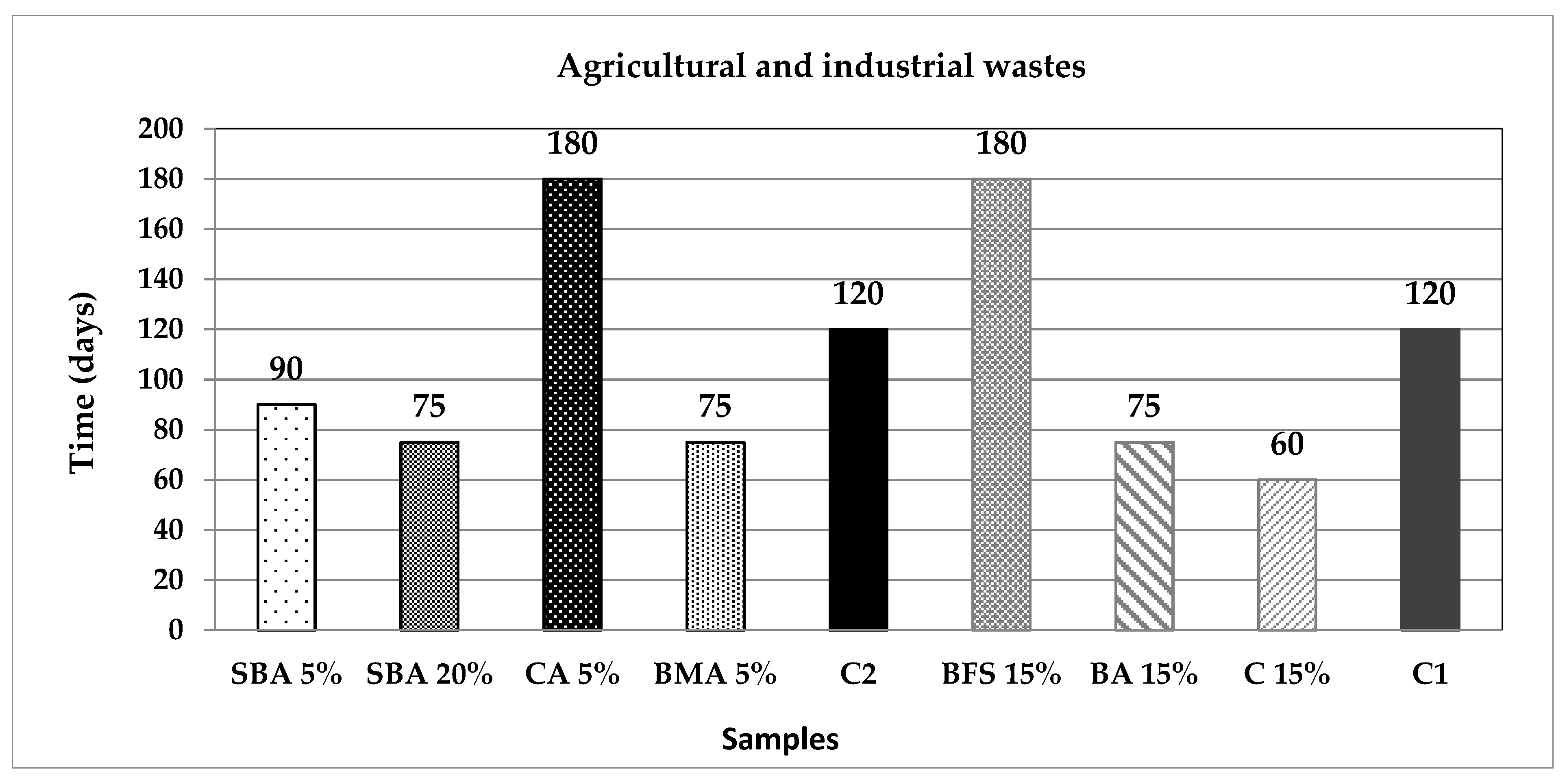
3.3. Carbonation Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rosewitz, J.A.; Wang, S.; Scarlata, S.F.; Rahbar, N. An enzymatic self-healing cementitious material. Appl. Mater. Today 2021, 23, 101035. [Google Scholar] [CrossRef]
- International Energy Agency (IEA). World Energy Outlook. 2019. Available online: https://iea.blob.core.windows.net/assets/98909c1b-aabc-4797-9926-35307b418cdb/WEO2019-free.pdf (accessed on 22 November 2021).
- USGS Mineral Commodity Summaries (Commodity statistics and information); United States Geological Survey (USGS). Mineral Yearbooks; USGS: Washington, DC, USA, 2019. [Google Scholar]
- NMX-C-530-ONNCCE. Durabilidad-Norma General de Durabilidad de Estructuras de Concreto Reforzado-Criterios y Especificaciones; Industria de la Normalización y Certificación de la Construccion y Edificación: Mexico City, Mexico, 2018. [Google Scholar]
- Noushini, A.; Castel, A. Performance-based criteria to assess the suitability of geopolymer concrete in marine environments using modified ASTM C1202 and ASTM C1556 methods. Mater. Struc. 2018, 51, 146. [Google Scholar] [CrossRef]
- Ziane, S.; Khelifa, M.-R.; Mezhoud, S.; Medaoud, S. Durability of concrete reinforced with alfa fibres exposed to external sulphate attack and thermal stresses. Asian J. Civ. Eng. 2020, 21, 555–567. [Google Scholar] [CrossRef]
- Collepardi, M. The New Concrete, 1st ed.; Titoretto: Villorba, Italy, 2006. [Google Scholar]
- European Committee for Standardization. Concrete—Specification, Performance, Production and Conformity; European Committee for Standardization: Brussels, Belgium, 2013. [Google Scholar]
- European Committee for Standardization. Eurocode 2: Design of Concrete Structures—Part 1-1: General Rules and Rules for Buildings; European Committee for Standardization: Brussels, Belgium, 2004. [Google Scholar]
- Bertolini, L.; Elsenes, B.; Pedeferri, P.; Redaelli, E.; Polder, R. Corrosion of Steel in Concrete: Prevention, Diagnosis, Repair, 2nd ed.; Wiley-VCH: Weinheim, Germany, 2013. [Google Scholar]
- Chen, T.; Gao, X. Effect of carbonation curing regime on strength and microstructure of Portland cement paste. J. CO2 Util. 2019, 34, 74–86. [Google Scholar] [CrossRef]
- Neves Junior, A.; Toledo Filho, R.D.; de Moraes Rego Fairbairn, E.; Dweck, J. The effects of the early carbonation curing on the mechanical and porosity properties of high initial strength Portland cement pastes. Constr. Build. Mater. 2015, 77, 448–454. [Google Scholar] [CrossRef]
- Zhutovsky, S.; Shishkin, A. Recycling of hydrated Portland cement paste into new clinker. Constr. Build. Mater. 2021, 280, 122510. [Google Scholar] [CrossRef]
- Talukdar, S.; Banthia, N. Carbonation in concrete infrastructure in the context of global climate change: Development of a service lifespan model. Constr. Build. Mater. 2013, 40, 775–782. [Google Scholar] [CrossRef]
- Chávez-Ulloa, E.; López, T.P.; Trujeque, J.R.; Pérez, F.C. Deterioration of concrete structures due to carbonation in tropical marine environment and accelerated carbonation chamber. Rev. Tec. Fac. Ing. Univ. Zulia 2013, 36, 104–113. [Google Scholar]
- Malhotra, V.M.; Metha, P. Pozzolanic and Cementitious Materials, 1st ed.; CRC Press: Ottawa, ON, Canada, 1996. [Google Scholar]
- Escalante, J.I.; Navarro, A.; Gomez, L.Y. Caracterización de morteros de cemento portland substituido por metacaolín de baja pureza. Rev. ALCONPAT 2012, 1, 186–199. [Google Scholar] [CrossRef] [Green Version]
- Memon, M.J.; Jhatial, A.A.; Murtaza, A.; Raza, M.S.; Phulpoto, K.B. Production of eco-friendly concrete incorporating rice husk ash and polypropylene fibres. Environ. Sci. Pollut. Res. 2021, 28, 39168–39184. [Google Scholar] [CrossRef]
- Mustakim, S.M.; Das, S.K.; Mishra, J.; Aftab, A.; Alomayri, T.S.; Assaedi, H.S.; Kaze, C.R. Improvement in Fresh, Mechanical and Microstructural Properties of Fly Ash- Blast Furnace Slag Based Geopolymer Concrete by Addition of Nano and Micro Silica. Silicon 2021, 13, 2415–2428. [Google Scholar] [CrossRef]
- Kannur, B.; Chore, H.S. Utilization of sugarcane bagasse ash as cement-replacing materials for concrete pavement: An overview. Innov. Infrastruct. Solut. 2021, 6, 184. [Google Scholar] [CrossRef]
- Chandrasekhar Reddy, K. Investigation of mechanical and durable studies on concrete using waste materials as hybrid reinforcements: Novel approach to minimize material cost. Innov. Infrastruct. Solut. 2021, 6, 197. [Google Scholar] [CrossRef]
- Martinez-Molina, W.; Torres-Acosta, A.A.; Martínez-Peña, G.E.; Alonso-Guzmán, E.; Mendoza-Pérez, I.N. Cement-based, materials-enhanced durability from opuntia ficus indica mucilage additions. ACI Mater. J. 2015, 112, 165–172. [Google Scholar] [CrossRef]
- Islam, G.M.S.; Rahman, M.H.; Kazi, N. Waste glass powder as partial replacement of cement for sustainable concrete practice. Int. J. Sustain. Built Environ. 2017, 6, 37–44. [Google Scholar] [CrossRef] [Green Version]
- Janfeshan Araghi, H.; Nikbin, I.M.; Rahimi Reskati, S.; Rahmani, E.; Allahyari, H. An experimental investigation on the erosion resistance of concrete containing various PET particles percentages against sulfuric acid attack. Constr. Build. Mater. 2015, 77, 461–471. [Google Scholar] [CrossRef] [Green Version]
- Anderson, D.J.; Smith, S.T.; Au, F.T.K. Mechanical properties of concrete utilizing waste ceramic as coarse aggregate. Constr. Build. Mater. 2016, 117, 20–28. [Google Scholar] [CrossRef]
- Tavakoli, D.; Heidari, A.; Pilehrood, S.H. Properties of concrete made with waste clay brick as sand incorporating nano SiO2. Indian J. Sci. Technol. 2014, 7, 1899–1905. [Google Scholar] [CrossRef]
- Gupta, T.; Chaudhary, S.; Sharma, R.K. Assessment of mechanical and durability properties of concrete containing waste rubber tire as fine aggregate. Constr. Build. Mater. 2014, 73, 562–574. [Google Scholar] [CrossRef]
- Cartuxo, F.; de Brito, J.; Evangelista, L.; Jiménez, J.R.; Ledesma, E.F. Rheological behaviour of concrete made with fine recycled concrete aggregates—Influence of the superplasticizer. Constr. Build. Mater. 2015, 89, 36–47. [Google Scholar] [CrossRef]
- Mo, K.H.; Alengaram, U.J.; Jumaat, M.Z.; Yap, S.P.; Lee, S.C. Green concrete partially comprised of farming waste residues: A review. J. Clean. Prod. 2016, 117, 122–138. [Google Scholar] [CrossRef]
- Devadiga, D.G.; Bhat, K.S.; Mahesha, G.T. Sugarcane bagasse fiber reinforced composites: Recent advances and applications. Cogent Eng. 2020, 7, 1823159. [Google Scholar] [CrossRef]
- Han-Seung, L.; Wang, X.-Y. Evaluation of compressive strength development and carbonation depth of high-volume slag-blended concrete. Constr. Build. Mater. 2016, 124, 45–54. [Google Scholar] [CrossRef]
- Miah, M.J.; Hossain Patoary, M.M.; Paul, S.C.; Babafemi, A.J.; Panda, B. Enhancement of mechanical properties and porosity of concrete using steel slag coarse aggregate. Materials 2020, 13, 2865. [Google Scholar] [CrossRef] [PubMed]
- Landa-Sánchez, A.; Bosch, J.; Baltazar-Zamora, M.A.; Croche, R.; Landa-Ruiz, L.; Santiago-Hurtado, G.; Moreno-Landeros, V.M.; Olguín-Coca, J.; López-Léon, L.; Bastidas, J.M.; et al. Corrosion behavior of steel-reinforced green concrete containing recycled coarse aggregate additions in sulfate media. Materials 2020, 13, 4345. [Google Scholar] [CrossRef]
- Irshidat, M.R.; Al-Nuaimi, N. Industrial waste utilization of carbon dust in sustainable cementitious composites production. Materials 2020, 13, 3295. [Google Scholar] [CrossRef]
- Srimahachota, T.; Yokota, H.; Akira, Y. Recycled nylon fiber from waste fishing nets as reinforcement in polymer cement mortar for the repair of corroded RC beams. Materials 2020, 13, 4276. [Google Scholar] [CrossRef]
- Ulewicz, M.; Pietrzak, A. Properties and structure of concretes doped with production waste of thermoplastic elastomers from the production of car floor mats. Materials 2021, 14, 872. [Google Scholar] [CrossRef]
- Kanojia, A.; Jain, S.K. Performance of coconut shell as coarse aggregate in concrete. Constr. Build. Mater. 2017, 140, 150–156. [Google Scholar] [CrossRef]
- Morandeau, A.; Thiéry, M.; Dangla, P. Impact of accelerated carbonation on OPC cement paste blended with fly ash. Cem. Concr. Res. 2015, 67, 226–236. [Google Scholar] [CrossRef]
- Tavakoli, D.; Hashempour, M.; Heidari, A. Use of waste materials in concrete: A review. Pertanika J. Sci. Technol. 2018, 26, 499–522. [Google Scholar]
- Criado, M.C.; Vera, C.; Downey, P.; Soto, M.C. Influences of the fiber glass in physical-mechanical properties of the concrete. Rev. Ing. Construcción 2005, 20, 201–212. [Google Scholar]
- Page, J.; Khadraoui, F.; Gomina, M.; Boutouil, M. Influence of different surface treatments on the water absorption capacity of flax fibres: Rheology of fresh reinforced-mortars and mechanical properties in the hardened state. Constr. Build. Mater. 2019, 199, 424–434. [Google Scholar] [CrossRef]
- El-Nadoury, W.W. Applicability of Using Natural Fibers for Reinforcing Concrete. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2020; Volume 809. [Google Scholar] [CrossRef]
- He, J.; Kawasaki, S.; Achal, V. The utilization of agricultural waste as agro-cement in concrete: A review. Sustainability 2020, 12, 6971. [Google Scholar] [CrossRef]
- Trocónis de Rincón, O. DURAR Network Members, Manual for Inspecting, Evaluating and Diagnosing Corrosion in Reinforced Concrete Structures, 1st ed.; CYTED: Maracaibo, Venezuela, 2000. [Google Scholar]
- Gómez-Zamorano, L.Y.; Escalante-García, J.I.; Mendoza-Suárez, G. Geothermal waste: An alternative replacement material of portland cement. J. Mater. Sci. 2004, 39, 4021–4025. [Google Scholar] [CrossRef]
- Morandeau, A.; Thiéry, M.; Dangla, P. Investigation of the carbonation mechanism of CH and C-S-H in terms of kinetics, microstructure changes and moisture properties. Cem. Concr. Res. 2014, 56, 153–170. [Google Scholar] [CrossRef] [Green Version]
- de Oliveira, A.M.; Cascudo, O. Effect of mineral additions incorporated in concrete on thermodynamic and kinetic parameters of chloride-induced reinforcement corrosion. Constr. Build. Mater. 2018, 192, 467–477. [Google Scholar] [CrossRef]
- Galan, I.; Andrade, C.; Mora, P.; Sanjuan, M.A. Sequestration of CO2 by Concrete Carbonation. Environ. Sci. Technol. 2010, 44, 3181–3186. [Google Scholar] [CrossRef] [PubMed]
- Possan, E.; Cristiano Fogaça, J.; Catiussa, E.; Pazuch, M. Sequestro de CO2 Devido à Carbonatação do Concreto: Potencialidades da Barragem de Itaipu. Rev. Estud. Ambient. 2012, 14, 28–38. [Google Scholar]
- Jacobsen, S.; Jahren, P. Binding of CO2 by carbonation of Norwegian Concrete. In Proceedings of the CANMET/ACI International Conference on Sustainability and Concrete Technology, Lyon, France, 1 September 2001; pp. 329–338. [Google Scholar]
- Kim, S.-K.; Jang, I.-Y.; Yang, H.-J. An Empirical Study on the Absorption of Carbon Dioxide in OLED-Mixed Concrete through Carbonation Reaction. KSCE J. Civ. Eng. 2020, 24, 2495–2504. [Google Scholar] [CrossRef]
- Guedes de Paiva, F.F.; Tamashiro, J.R.; Pereira Silva, L.H.; Kinoshita, A. Utilization of inorganic solid wastes in cementitious materials—A systematic literature review. Const. Build. Mater. 2021, 285, 122833. [Google Scholar] [CrossRef]
- Berenguer, R.; Lima, N.; Cruz, R.; Pinto, L.; Lima, N. Thermodynamic, microstructural and chemometric analyses of the reuse of sugarcane ashes in cement manufacturing. J. Environ. Chem. Eng. 2021, 9, 105350. [Google Scholar] [CrossRef]
- Liu, G.; Florea, M.; Brouwers, H. The role of recycled waste glass incorporation on the carbonation behaviour of sodium carbonate activated slag mortar. J. Clean. Prod. 2021, 292, 126050. [Google Scholar] [CrossRef]
- Mi, R.; Pan, G.; Li, Y.; Kuang, T.; Lu, X. Distinguishing between new and old mortars in recycled aggregate concrete under carbonation using iron oxide red. Const. Build. Mater. 2019, 222, 601–609. [Google Scholar] [CrossRef]
- Zhang, S.; Ghouleh, Z.; Shao, Y. Green concrete made from MSWI residues derived eco-cement and bottom ash aggregates. Const. Build. Mater. 2021, 297, 123818. [Google Scholar] [CrossRef]
- Duygu, E.; Fort, R. Accelerating carbonation in lime-based mortar in high CO2 environments. Const. Build. Mater. 2018, 188, 314–325. [Google Scholar] [CrossRef]
- Sharma, D.; Goyal, S. Accelerated carbonation curing of cement mortars containing cement kiln dust: An effective way of CO2 sequestration and carbon footprint reduction. J. Clean. Prod. 2018, 192, 844–854. [Google Scholar] [CrossRef]
- Rigopoulos, I.; Kyriakou, L.; Vasiliades, M.A.; Kyratsi, T.; Efstathiou, A.M.; Ioannou, I. Improving the carbonation of air lime mortars at ambient conditions via the incorporation of ball-milled quarry waste. Const. Build. Mater. 2021, 301, 124703. [Google Scholar] [CrossRef]
- Liu, S.; Hao, Y.; Ma, G. Approaches to enhance the carbonation resistance of fly ash and slag-based alkali-activated mortar- experimental evaluations. J. Clean. Prod. 2021, 280, 124321. [Google Scholar] [CrossRef]
- NMX-C-414-ONNCCE-2017. Cementantes Hidraulicos. Especificaciones y Métodos de Ensayo; ONNCCE: Mexico City, Mexico, 2017. [Google Scholar]
- ASTM C150/C150M-21. Standard Specification for Portland Cement; ASTM International: West Conshohocken, PA, USA, 2021. [Google Scholar]
- ASTM C31/C31M-21a. Standard Practice for Making and Curing Concrete Test Specimens in the Field; ASTM International: West Conshohocken, PA, USA, 2021. [Google Scholar]
- ASTM C109/C109M-21. Standard Test Method for Compressive Strength of Hydraulic Cement Mortars (Using 2-in. or [50 mm] Cube Specimens); ASTM International: West Conshohocken, PA, USA, 2021. [Google Scholar]
- ASTM C1876-19. Standard Test Method for Bulk Electrical Resistivity or Bulk Conductivity of Concrete; ASTM International: West Conshohocken, PA, USA, 2019. [Google Scholar]
- ASTM D75-03. Standard Practice for Sampling Aggregates; ASTM International: West Conshohocken, PA, USA, 2003. [Google Scholar]
- ASTM C702 / C702M-18. Standard Practice for Reducing Samples of Aggregate to Testing Size; ASTM International: West Conshohocken, PA, USA, 2018. [Google Scholar]
- ASTM C29 / C29M-17a. Standard Test Method for Bulk Density (“Unit Weight”) and Voids in Aggregate; ASTM International: West Conshohocken, PA, USA, 2017. [Google Scholar]
- ASTM C128-15. Standard Test Method for Relative Density (Specific Gravity) and Absorption of Fine Aggregate; ASTM International: West Conshohocken, PA, USA, 2015. [Google Scholar]
- ASTM C70-20. Standard Test Method for Surface Moisture in Fine Aggregate; ASTM International: West Conshohocken, PA, USA, 2020. [Google Scholar]
- ASTM C566-19. Standard Test Method for Total Evaporable Moisture Content of Aggregate by Drying; ASTM International: West Conshohocken, PA, USA, 2019. [Google Scholar]
- ASTM D2419-14. Standard Test Method for Sand Equivalent Value of Soils and Fine Aggregate; ASTM International: West Conshohocken, PA, USA, 2014. [Google Scholar]
- ASTM C142 / C142M-17. Standard Test Method for Clay Lumps and Friable Particles in Aggregates; ASTM International: West Conshohocken, PA, USA, 2017. [Google Scholar]
- NMX-C-515-ONNCCE-2016. Industria de la Construcción, Concreto Hidráulico, Durabilidad, Determinación de la Profundidad de Carbonatación en Concreto Hidráulico, Especificaciones y Método de Ensayo; Industria de la Normalización y Certificación de la Construccion y Edificación: Mexico City, Mexico, 2016. [Google Scholar]
- Ali, S.; Kumar, A.; Rizvi, S.H.; Ali, M.; Ahmed, I. Effect of Sugarcane Bagasse Ash as Partial Cement Replacement on the Compressive Strength of Concrete. Quaid-E-Awam Univ. Res. J. Eng. Sci. Technol. 2018, 18, 44–49. [Google Scholar] [CrossRef]
- El-Diadamony, H.; Amer, A.A.; Sokkary, T.M.; El-Hoseny, S. Hydration and characteristics of metakaolin pozzolanic cement pastes. HBRC J. 2018, 14, 150–158. [Google Scholar] [CrossRef] [Green Version]
- Guzmán-Torres, J.A.; Domínguez-Mota, F.J.; Alonso-Guzmán, E.M. A multi-layer approach to classify the risk of corrosion in concrete specimens that contain different additives. Case Stud. Constr. Mater. 2021, 15, e00719. [Google Scholar] [CrossRef]
- Silva, A.; de Brito, J.; Lima Gaspar, P. Methodologies for Service Life Prediction of Buildings. With a Focus on Façade Claddings, 1st ed.; Springer: Cham, Switzerland, 2016. [Google Scholar] [CrossRef]








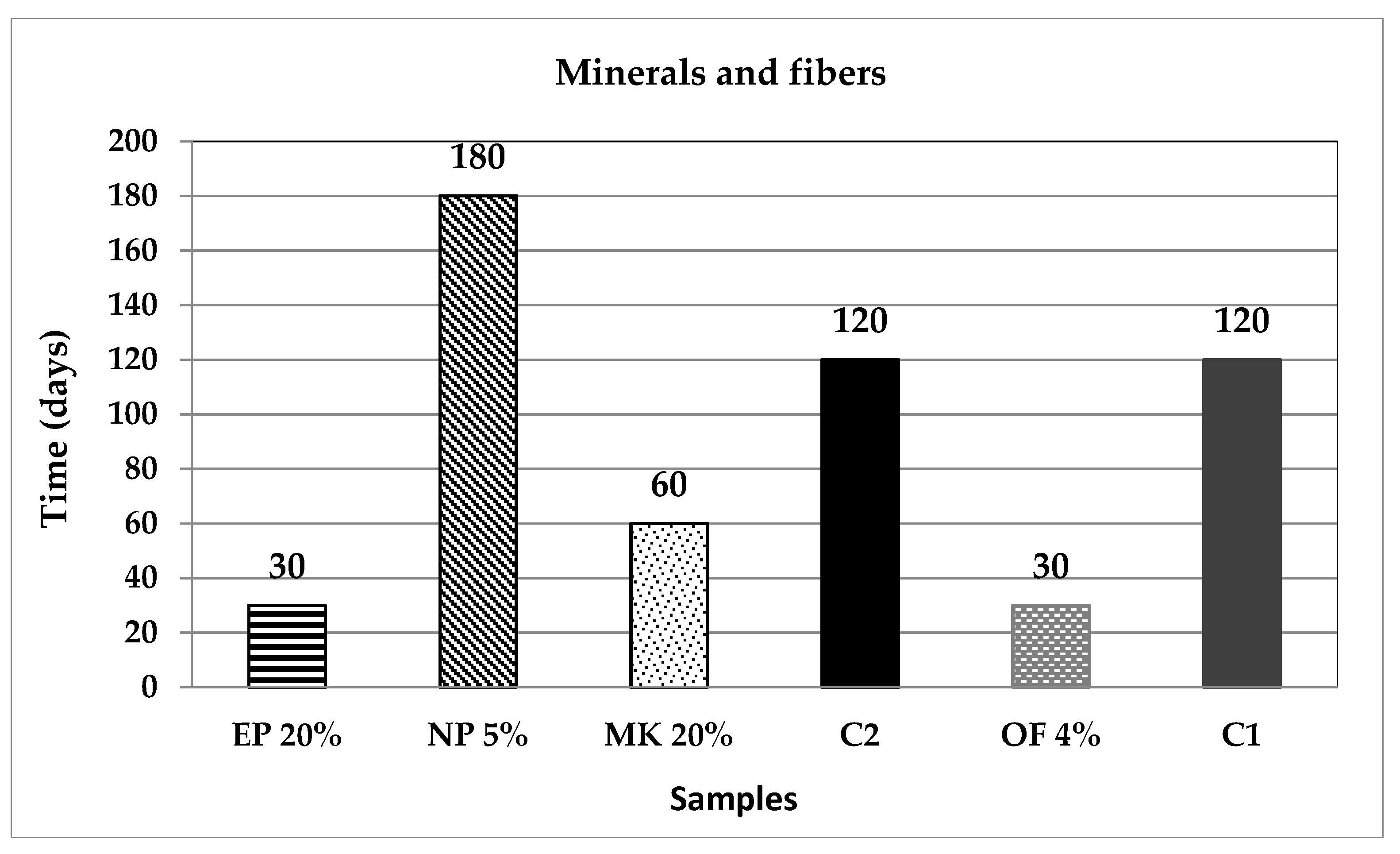
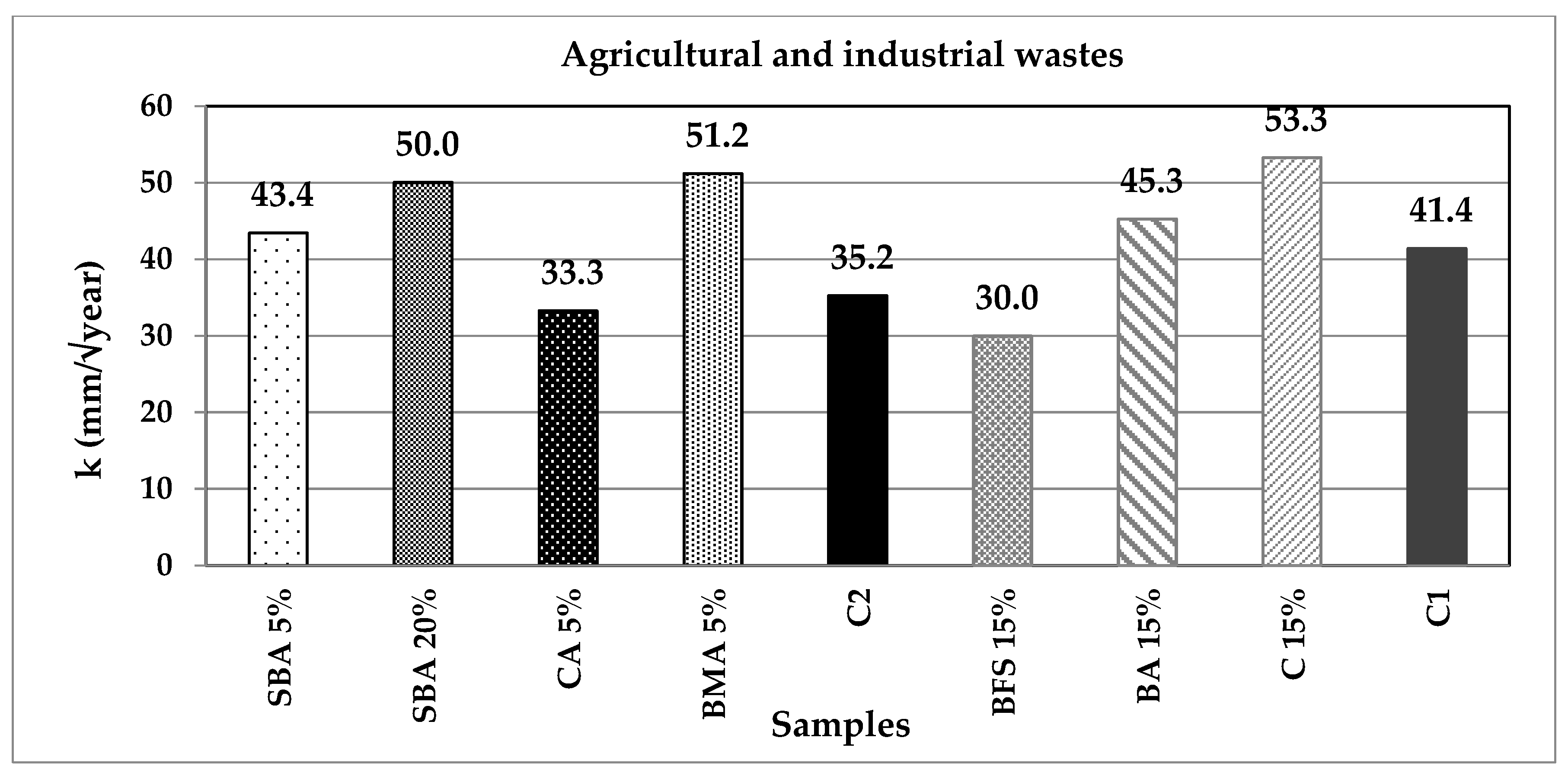
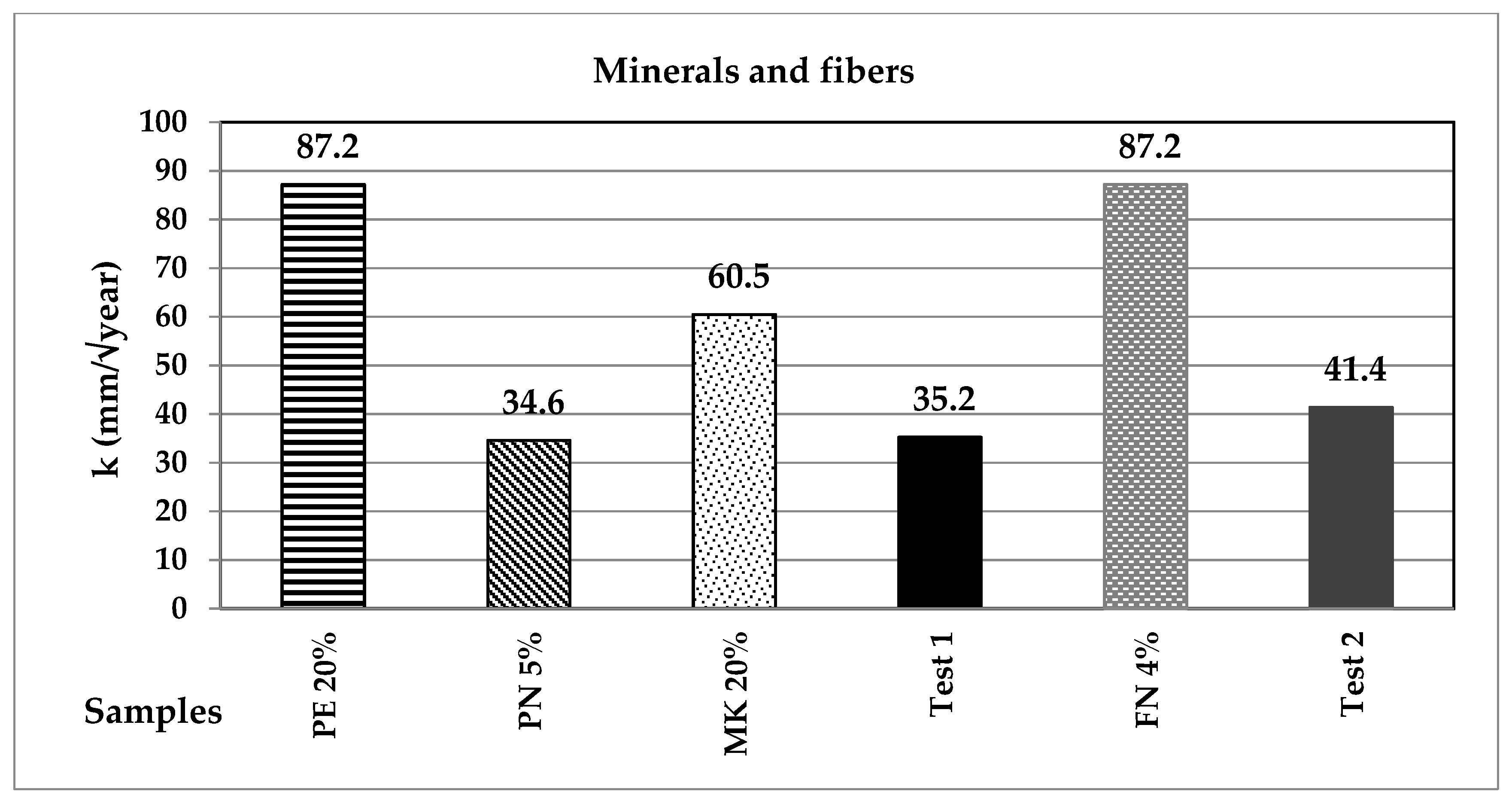
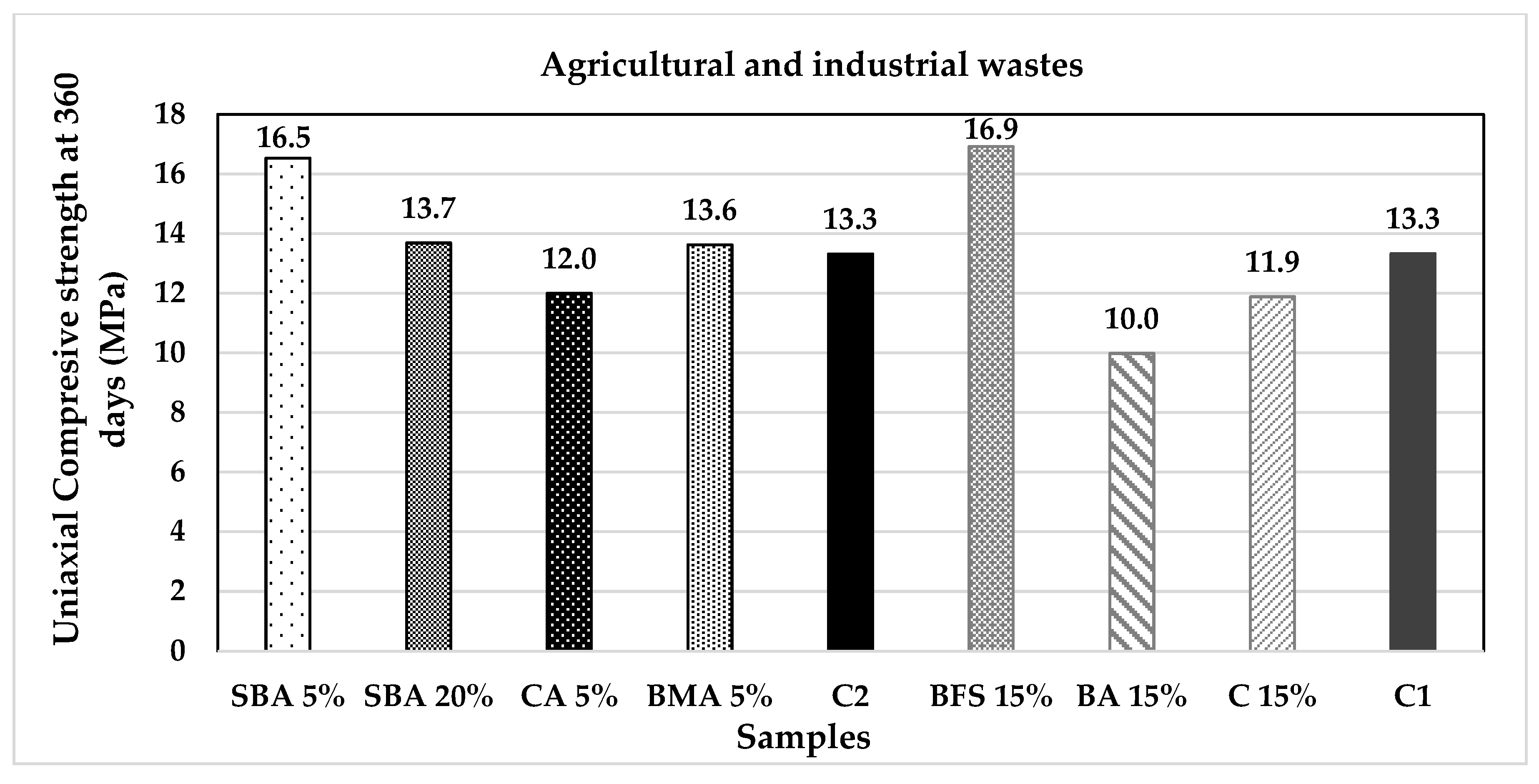
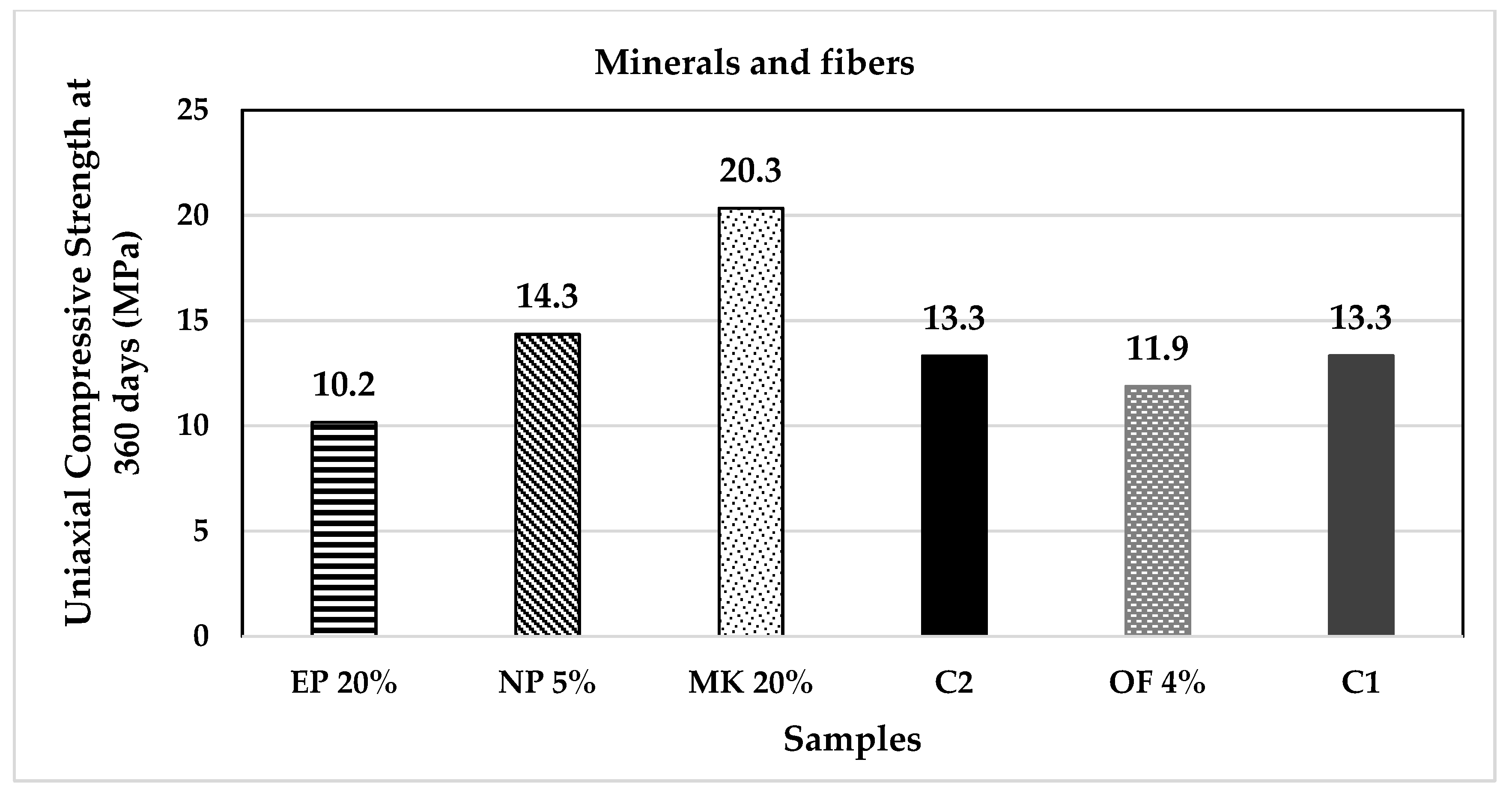
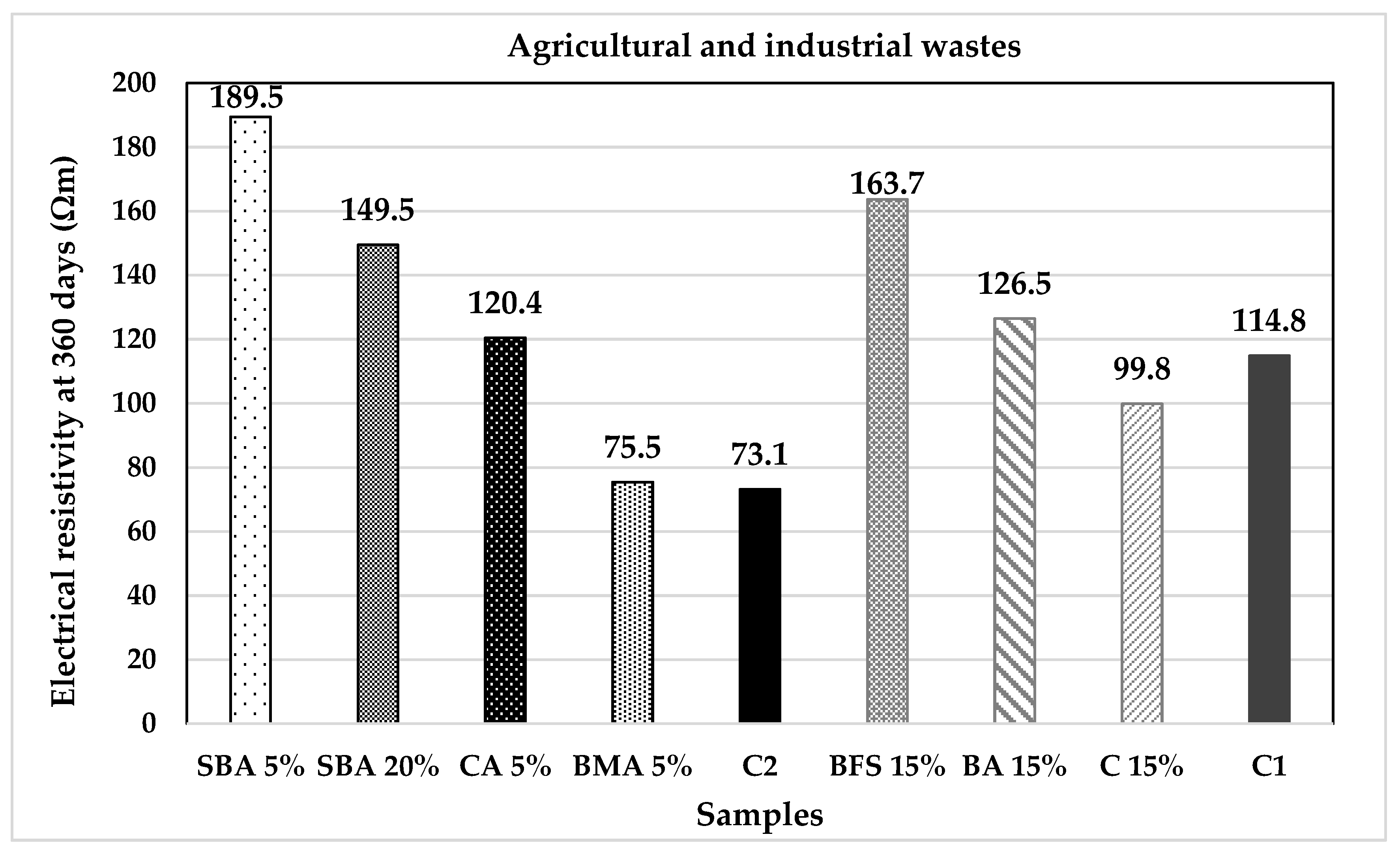
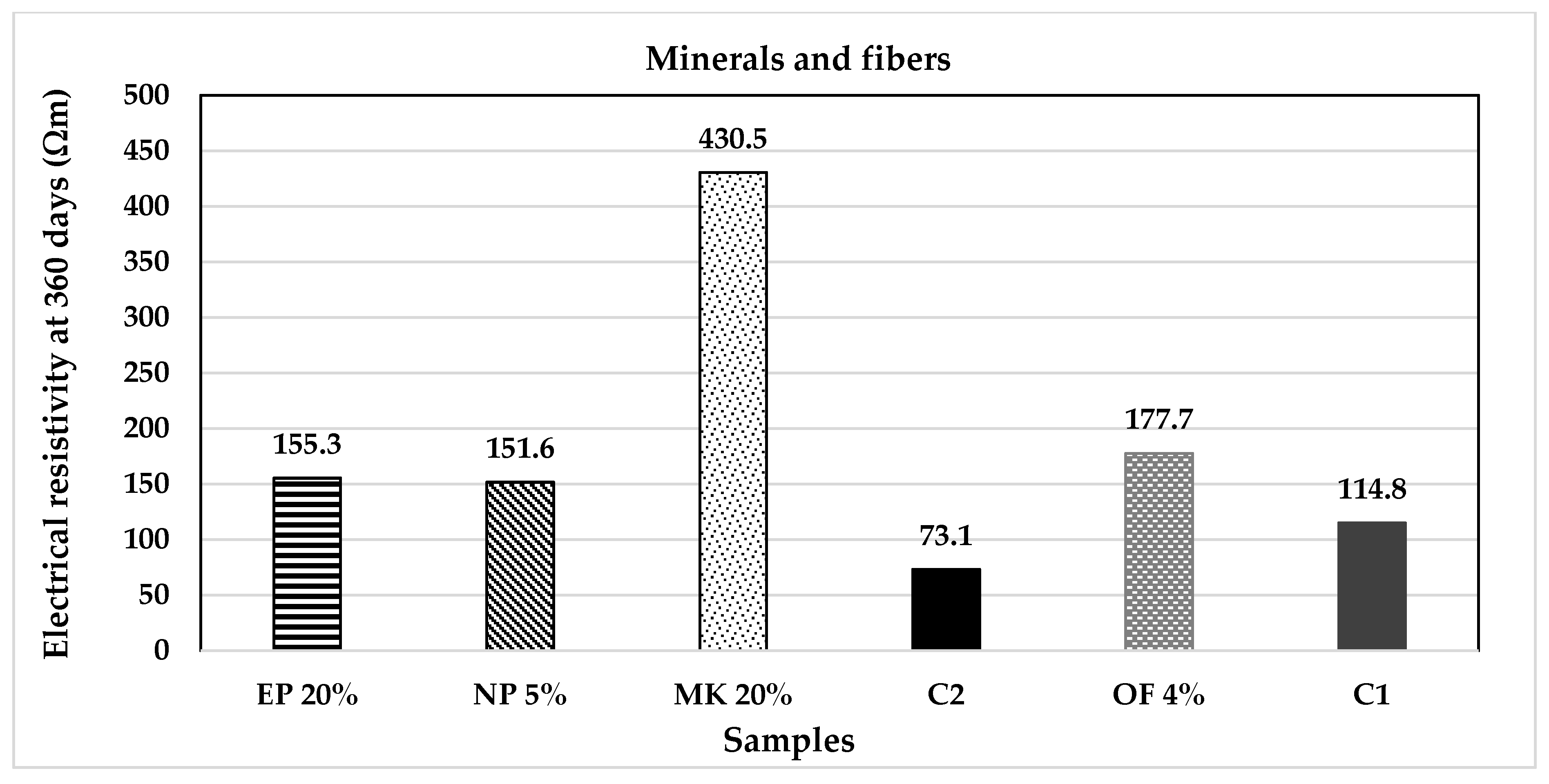
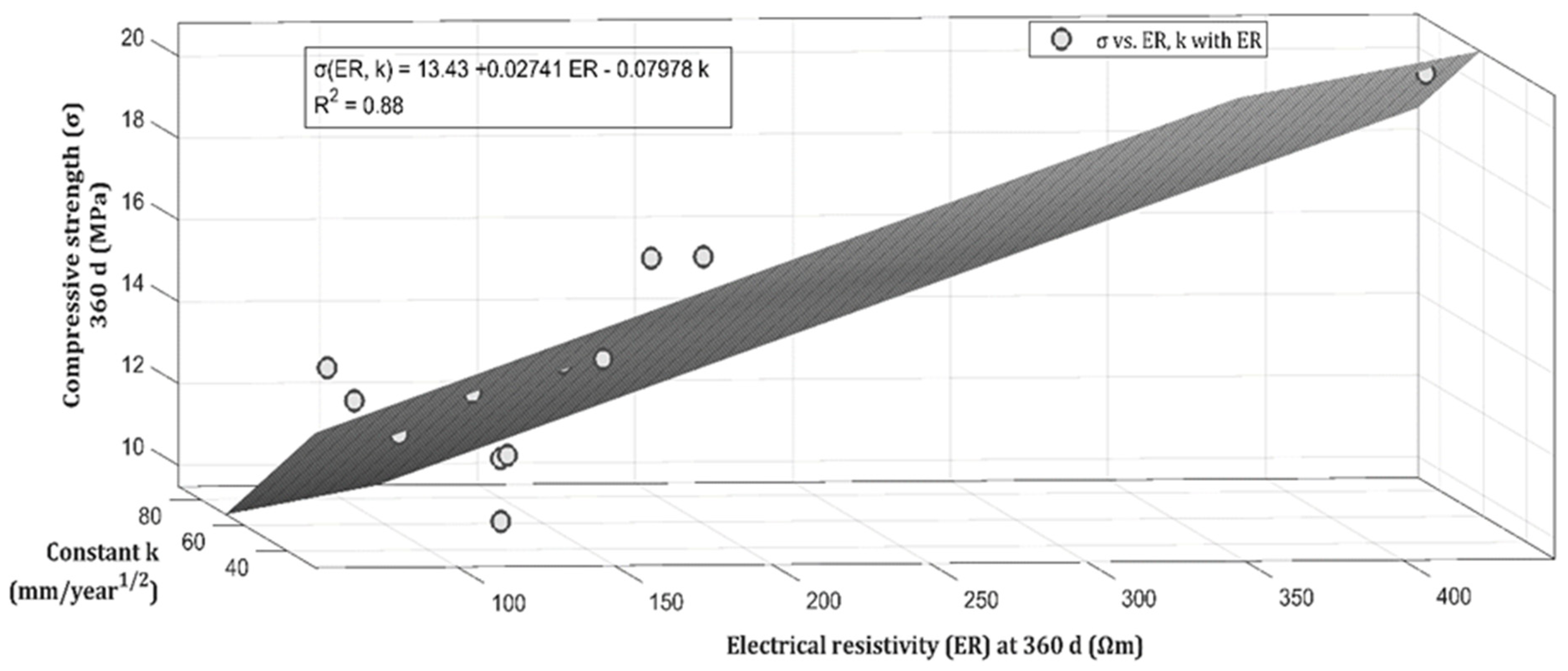
| Classification | Description | Code | (%) | Origin |
|---|---|---|---|---|
| Agricultural and industrial wastes | Sugarcane bagasse ash | SBA5 | 5 | Sugar cane mill of Taretan, Michoacan, Mexico |
| SBA20 | 20 | |||
| Charcoal ashes | CA | 5 | Cuitzeo lake margins | |
| Brick manufacturing ash | BMA | 5 | Santiago Undameo, Michoacan, Mexico | |
| Blast-furnace slag | BFS | 15 | Arcelor Mittal Industry | |
| Bottom ash | BA | 15 | Arcelor Mittal Industry | |
| Coal | C | 15 | Arcelor Mittal Industry | |
| Minerals and fibres | Expanded perlite | EP | 20 | Construction products with high-purity standards and quality |
| Natural perlite | NP | 5 | ||
| Metakaolin | MK | 20 | ||
| Opuntia ficus-indica dehydrated fibers | OF | 4 | Food grade product | |
| Fine Aggregates | Control 1, ARL and ARL2 | C1 | - | Lerma river margins |
| Control 2, HUAJ | C2 | - | Huajumbaro river margins |
| Test | Standard | Lerma Sand | Huajumbaro Sand |
|---|---|---|---|
| Sampling | ASTM D-75-03 [66] | 250 kg | 250 kg |
| Reducing sampling | ASTM C-702 [67] | 0.500 kg | 0.500 kg |
| Bulk density (unit weight and voids) | ASTM C-29/C-29M [68] | 1.353 | 1.226 |
| Bulk density (unit weight) | ASTM C-29/C-29M [68] | 1.444 | 1.331 |
| Relative density | ASTM C-128 [69] | 2–40 | 2.31 |
| Specific gravity | ASTM C-128 [69] | 2.39–2.48 | 2.24–2.36 |
| Surface moisture (%) | ASTM C-128 [69] ASTM C-70 [70] | 0.748 | 0.741 |
| Absorption percentage (%) | ASTM C-128 [69] ASTM C-566 [71] | 1.89 | 3.18 |
| Sand equivalent value (%) | ASTM D-2419 [72] | 86.97 | 98.25 |
| Clay lumps and friable particles (%) | ASTM C-142 [73] | 8.165 | 2.498 |
| SiO2 | TiO2 | Al2O3 | Fe2O3 | MnO | MgO | CaO | Na2O | K2O | P2O5 | SO3 | PXC | Total | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SBA | 60.04 | 0.43 | 6.289 | 3.145 | 0.13 | 1.825 | 1.64 | 0.446 | 1.856 | 0.786 | - | 23.6 | 100 |
| OF | 14.58 | 0.83 | 4.287 | 26.77 | 2.423 | 5.995 | 37.456 | 0.032 | 0.024 | 0.814 | 0.8 | 4.79 | 98 |
| CA | 32.52 | 0.76 | 13.55 | 5.371 | 0.112 | 2.108 | 18.759 | 0.67 | 1.027 | 0.541 | - | 22.2 | 97.6 |
| BA | 27.93 | 0.2 | 6.437 | 2.217 | 0.083 | 1.301 | 49.773 | 0.669 | 1.255 | 0.118 | 3.37 | 5.12 | 95.1 |
| BMA | 19.10 | 0.32 | 8.776 | 2.008 | 0.538 | 4.243 | 27.874 | 0.545 | 6.051 | 1.763 | 0.8 | 27.3 | 98.5 |
| BFS | 36.38 | 0.56 | 10.63 | 0.335 | 0.417 | 10.107 | 37.551 | 0.298 | 0.424 | 0.053 | 1.93 | 0.72 | 96 |
| MK | 49.75 | 1.53 | 44.71 | 0.509 | 0.013 | 0.159 | 0.044 | 0.23 | 0.141 | 0.033 | - | 0.77 | 97.9 |
| EP | 73.59 | 0.13 | 13.43 | 1.166 | 0.045 | 0.197 | 1.333 | 2.918 | 5.013 | 0.02 | - | 1.18 | 99 |
| NP | 72.20 | 0.12 | 13.58 | 1.011 | 0.073 | 0.538 | 1.052 | 3.216 | 4.285 | 0.025 | - | 3.99 | 100 |
| HUAJ | 78.19 | 0.2 | 11.56 | 1.567 | 0.03 | 0.239 | 1.015 | 2.666 | 3.577 | 0.036 | - | 1.19 | 100 |
| ARL | 77.57 | 0.28 | 10.67 | 2.384 | 0.025 | 0.479 | 1.338 | 2.232 | 3.083 | 0.075 | - | 2.2 | 100.3 |
| ARL2 | 75.25 | 0.32 | 11.92 | 2.689 | 0.026 | 0.568 | 1.482 | 2.219 | 3.124 | 0.077 | - | 2.5 | 100 |
| Sample | SiO2 | Al2O3 | Fe2O3 | Pozzolanic Activity | Acid Character |
|---|---|---|---|---|---|
| SBA | 60.04 | 6.289 | 3.145 | 69.47 | 66.32 |
| OF | 14.58 | 4.287 | 26.77 | 45.64 | 19.37 |
| CA | 32.52 | 13.55 | 5.371 | 51.44 | 46.07 |
| BA | 27.93 | 6.437 | 2.217 | 36.58 | 34.41 |
| BMA | 19.1 | 8.776 | 2.008 | 29.88 | 27.87 |
| BFS | 36.38 | 10.63 | 0.335 | 47.35 | 47.01 |
| MK | 49.75 | 44.71 | 0.509 | 94.97 | 94.46 |
| EP | 73.59 | 13.43 | 1.166 | 88.19 | 87.03 |
| NP | 72.2 | 13.58 | 1.011 | 86.79 | 85.78 |
| HUAJ | 78.19 | 11.56 | 1.567 | 91.32 | 89.75 |
| ARL | 77.57 | 10.67 | 2.384 | 90.62 | 88.24 |
| ARL2 | 75.25 | 11.92 | 2.689 | 89.86 | 87.16 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martinez-Molina, W.; Chavez-Garcia, H.L.; Perez-Lopez, T.; Alonso-Guzman, E.M.; Arreola-Sanchez, M.; Navarrete-Seras, M.A.; Borrego-Perez, J.A.; Sanchez-Calvillo, A.; Guzman-Torres, J.A.; Perez-Quiroz, J.T. Effect of the Addition of Agribusiness and Industrial Wastes as a Partial Substitution of Portland Cement for the Carbonation of Mortars. Materials 2021, 14, 7276. https://doi.org/10.3390/ma14237276
Martinez-Molina W, Chavez-Garcia HL, Perez-Lopez T, Alonso-Guzman EM, Arreola-Sanchez M, Navarrete-Seras MA, Borrego-Perez JA, Sanchez-Calvillo A, Guzman-Torres JA, Perez-Quiroz JT. Effect of the Addition of Agribusiness and Industrial Wastes as a Partial Substitution of Portland Cement for the Carbonation of Mortars. Materials. 2021; 14(23):7276. https://doi.org/10.3390/ma14237276
Chicago/Turabian StyleMartinez-Molina, Wilfrido, Hugo L. Chavez-Garcia, Tezozomoc Perez-Lopez, Elia M. Alonso-Guzman, Mauricio Arreola-Sanchez, Marco A. Navarrete-Seras, Jorge A. Borrego-Perez, Adria Sanchez-Calvillo, Jose A. Guzman-Torres, and Jose T. Perez-Quiroz. 2021. "Effect of the Addition of Agribusiness and Industrial Wastes as a Partial Substitution of Portland Cement for the Carbonation of Mortars" Materials 14, no. 23: 7276. https://doi.org/10.3390/ma14237276
APA StyleMartinez-Molina, W., Chavez-Garcia, H. L., Perez-Lopez, T., Alonso-Guzman, E. M., Arreola-Sanchez, M., Navarrete-Seras, M. A., Borrego-Perez, J. A., Sanchez-Calvillo, A., Guzman-Torres, J. A., & Perez-Quiroz, J. T. (2021). Effect of the Addition of Agribusiness and Industrial Wastes as a Partial Substitution of Portland Cement for the Carbonation of Mortars. Materials, 14(23), 7276. https://doi.org/10.3390/ma14237276






