Enzymatically Crosslinked In Situ Synthesized Silk/Gelatin/Calcium Phosphate Hydrogels for Drug Delivery
Abstract
:1. Introduction
2. Materials and Methods
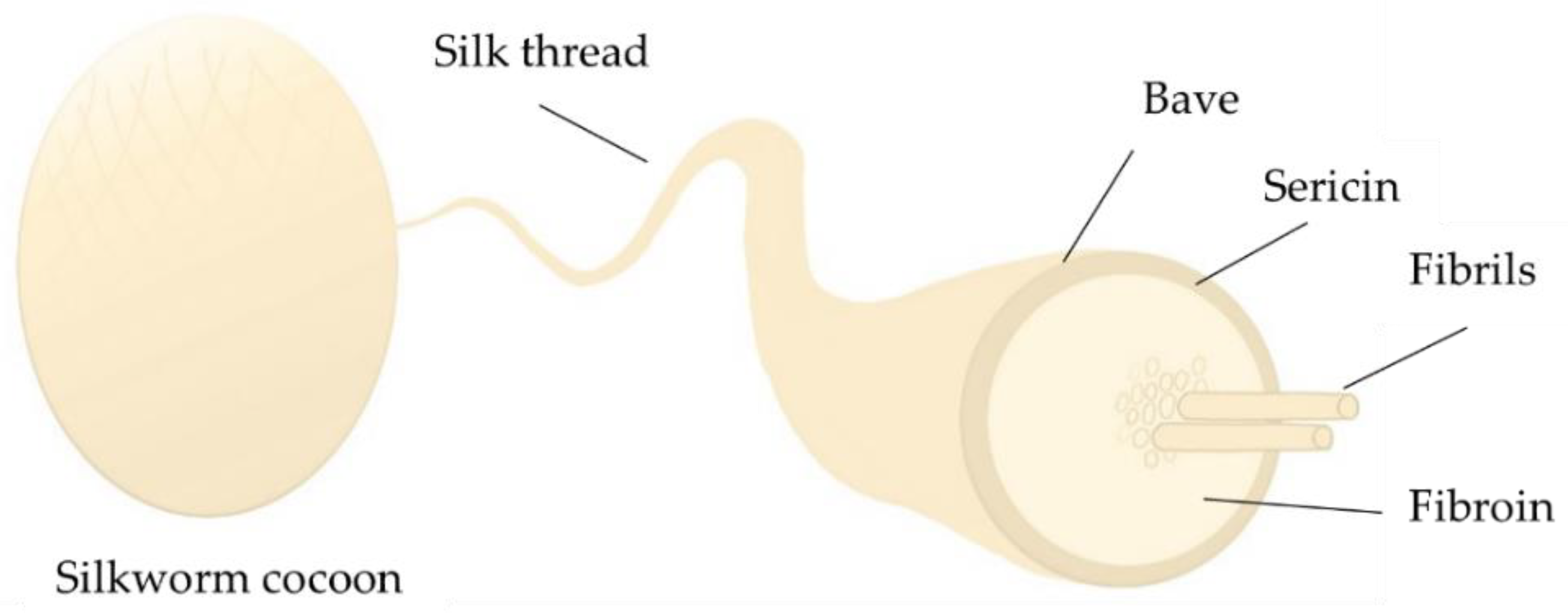
2.1. Preparation of SF
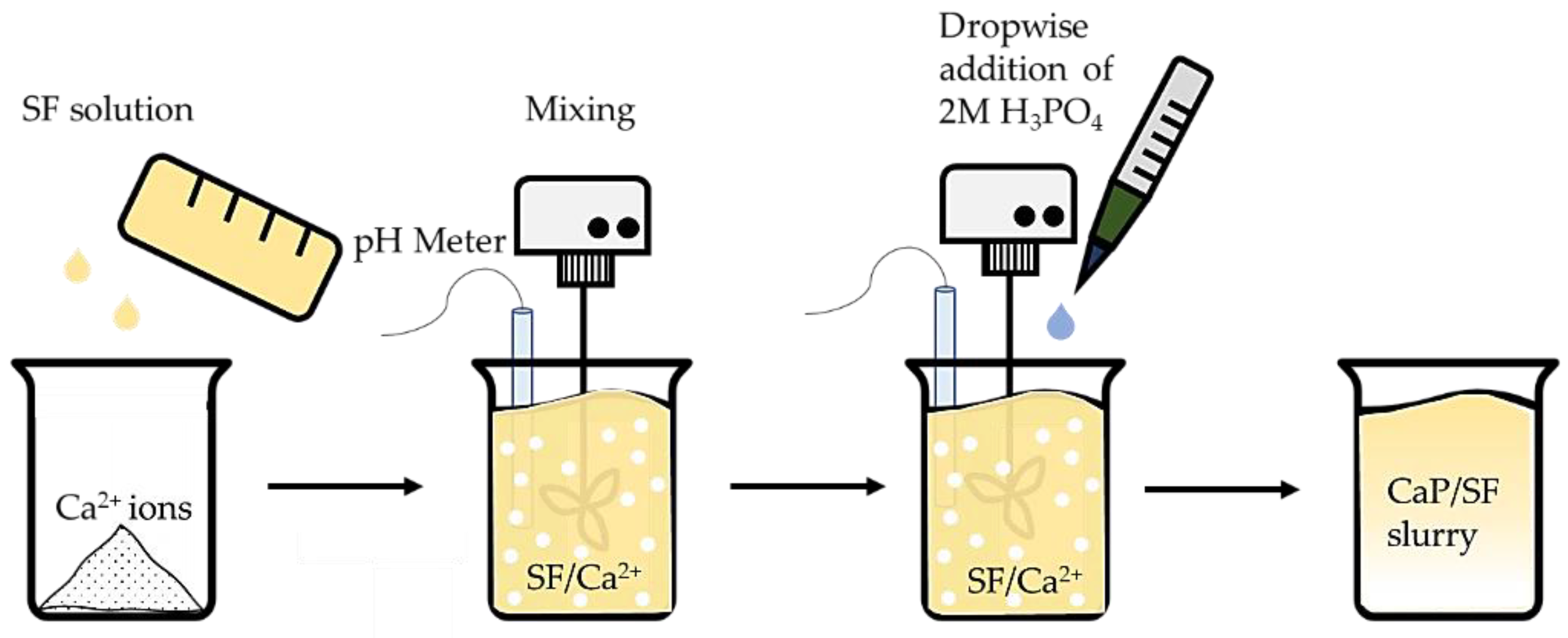
2.2. Calcium Phosphate In Situ Synthesis in the Silk Solution
2.3. Silk Fibroin/Gelatin and CaP Hydrogel Preparation
2.4. Characterization of Obtained Samples
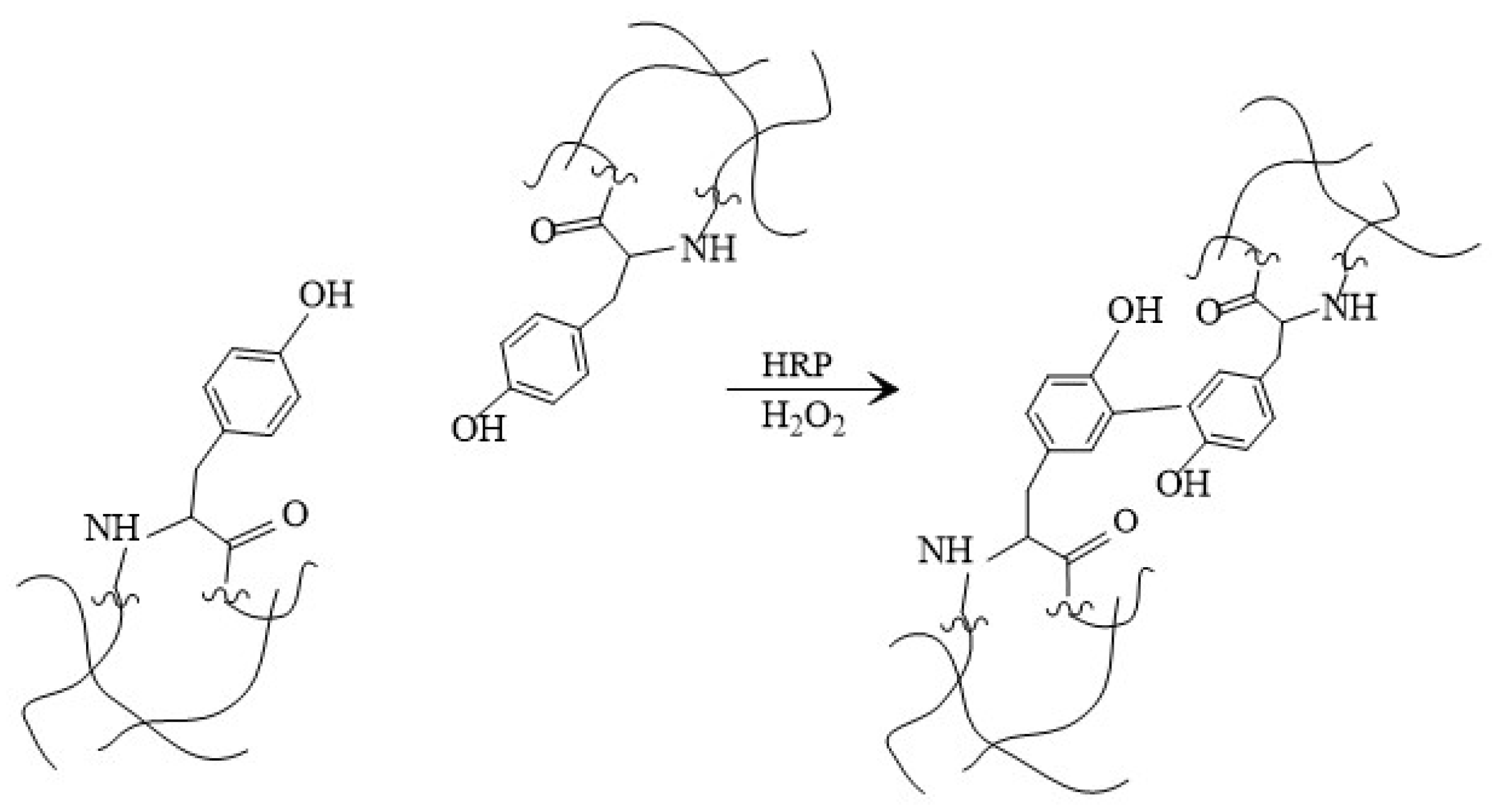
2.4.1. Attenuated Total Reflection—Fourier Transformation Infrared Spectroscopy
2.4.2. X-ray Diffraction for CaP Composite Phase Analysis
2.4.3. Brunauer–Emmett–Teller Method
2.4.4. SF/CaP Hydrogel Gel Fraction and Swelling Degree
2.4.5. Hydrogel Drug Release
2.4.6. Micro-Computed Tomography (μ-CT)
2.4.7. Scanning Electron Microscopy
2.4.8. Cell Cytotoxicity Tests
2.5. Statistic Analysis
3. Results
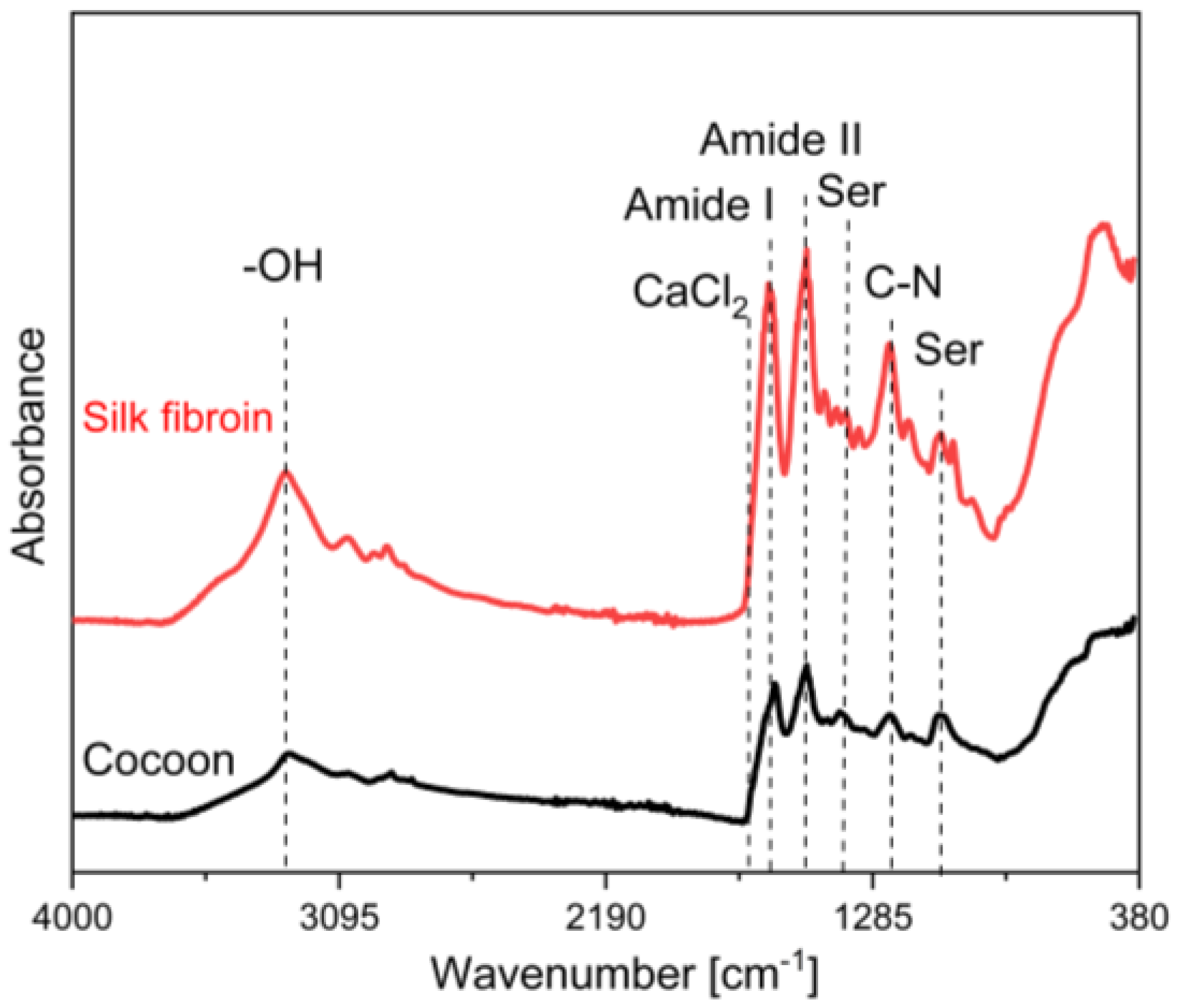
3.1. Raw Silk Fibroin Characterization
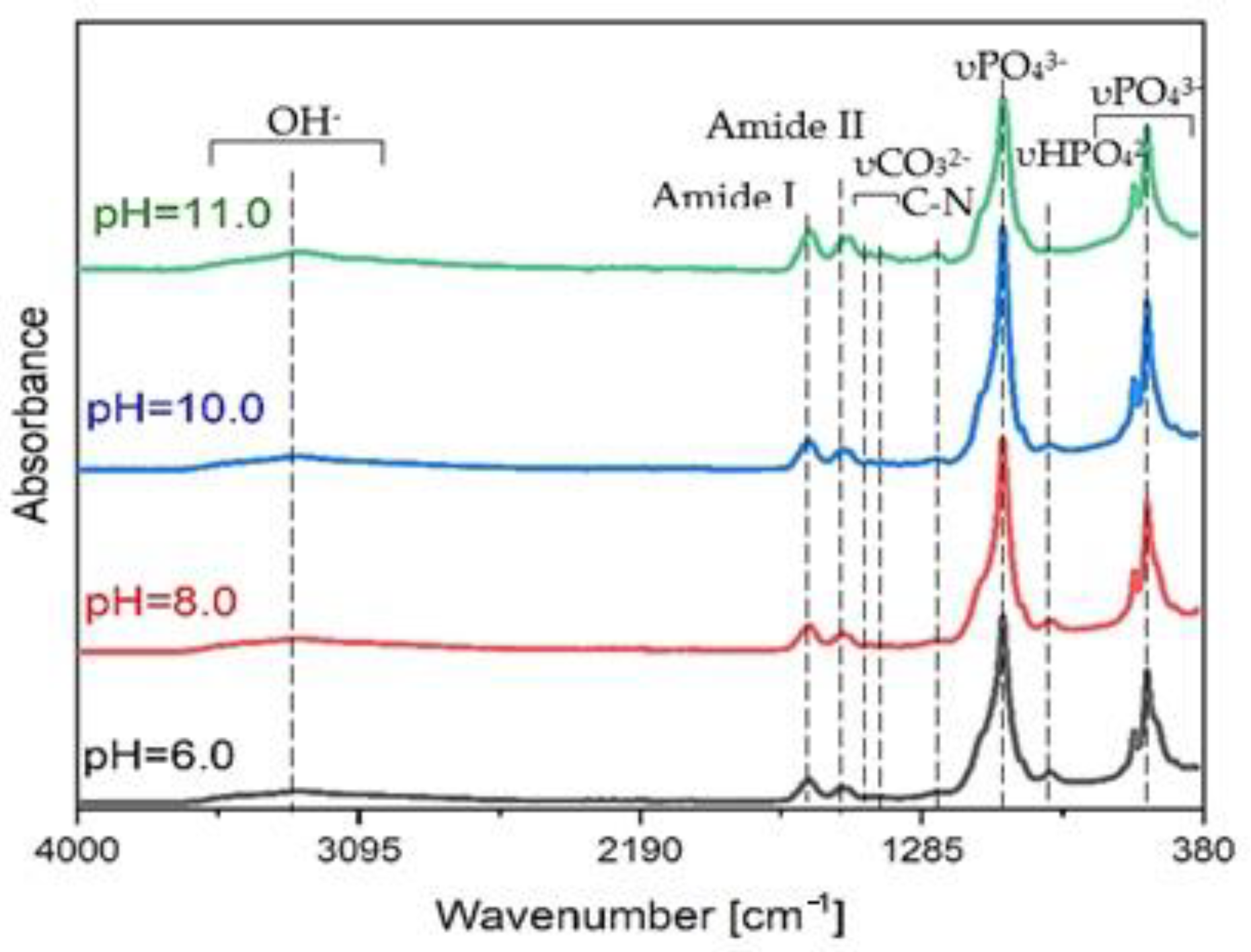
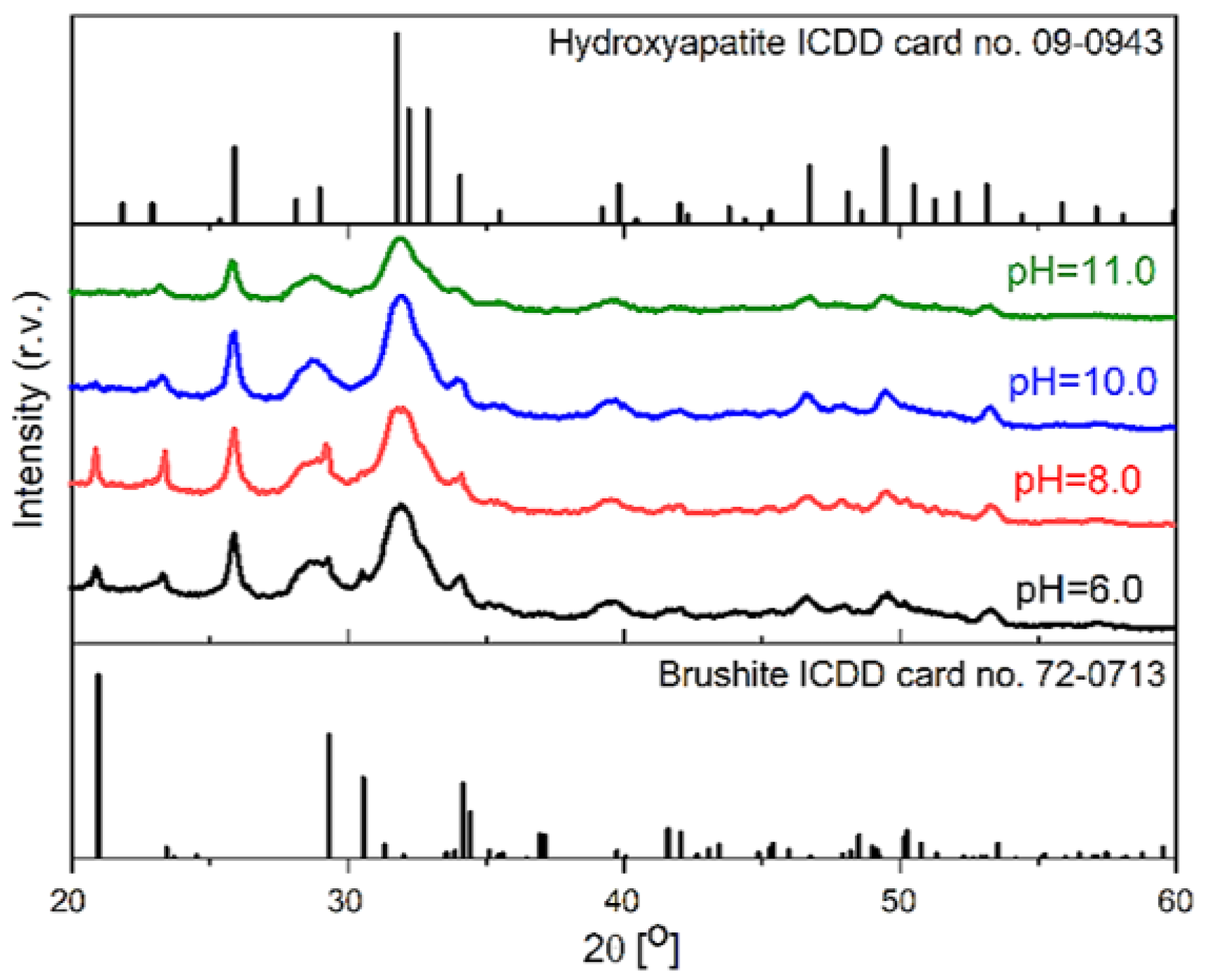
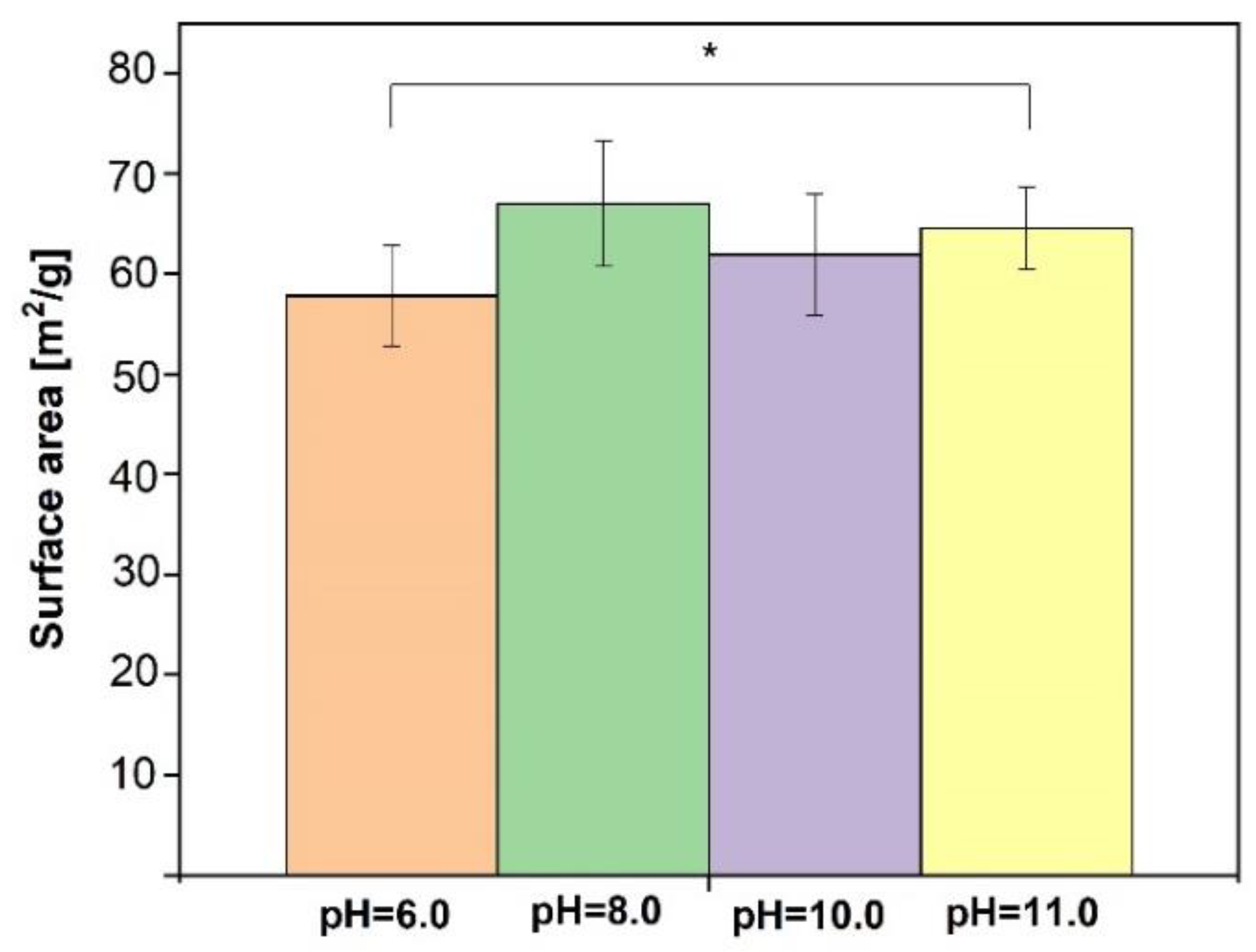
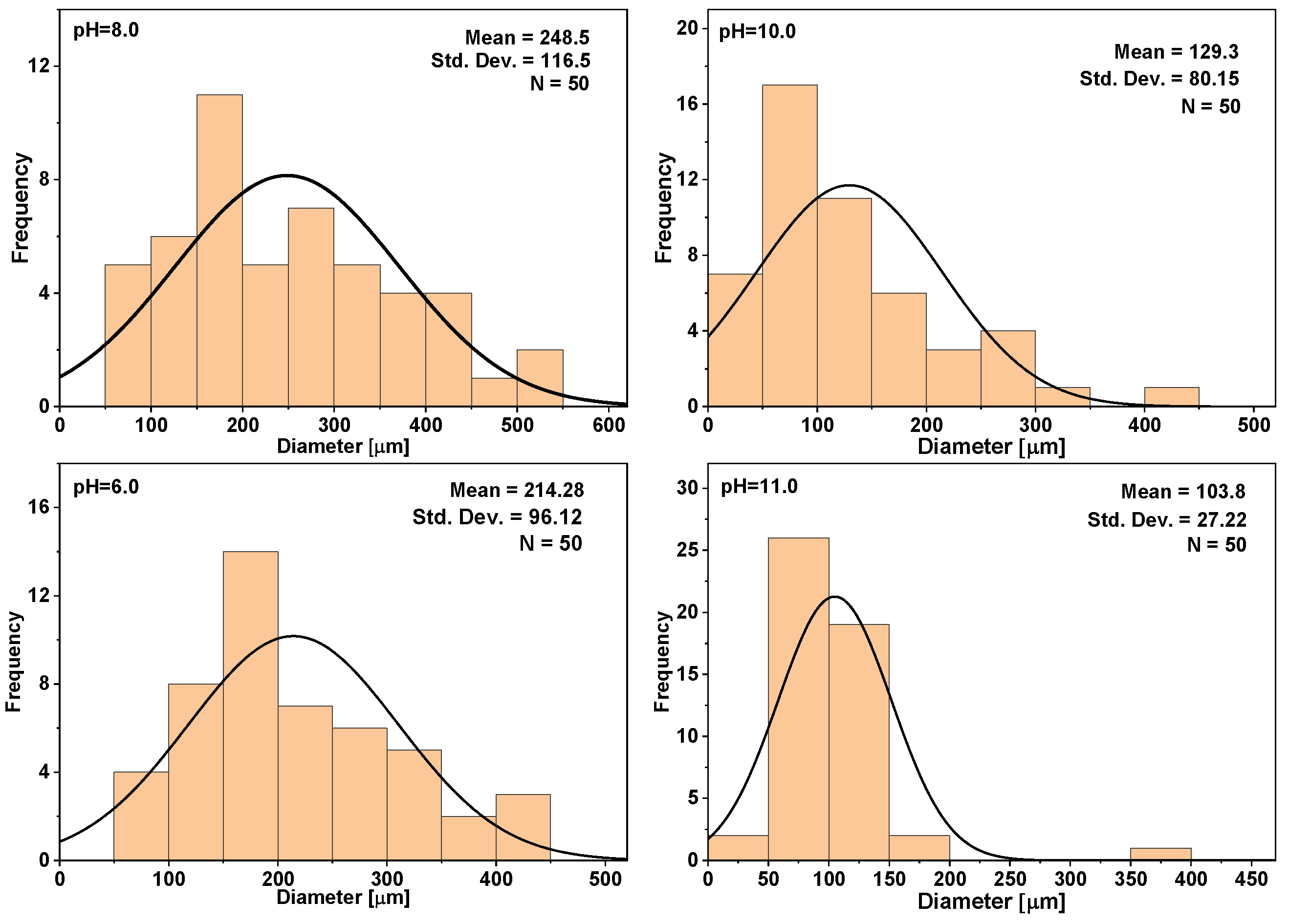
3.2. SF/CaP Composite Characterization
3.3. SF/G/CaP Hydrogel Properties
3.3.1. Gel fraction
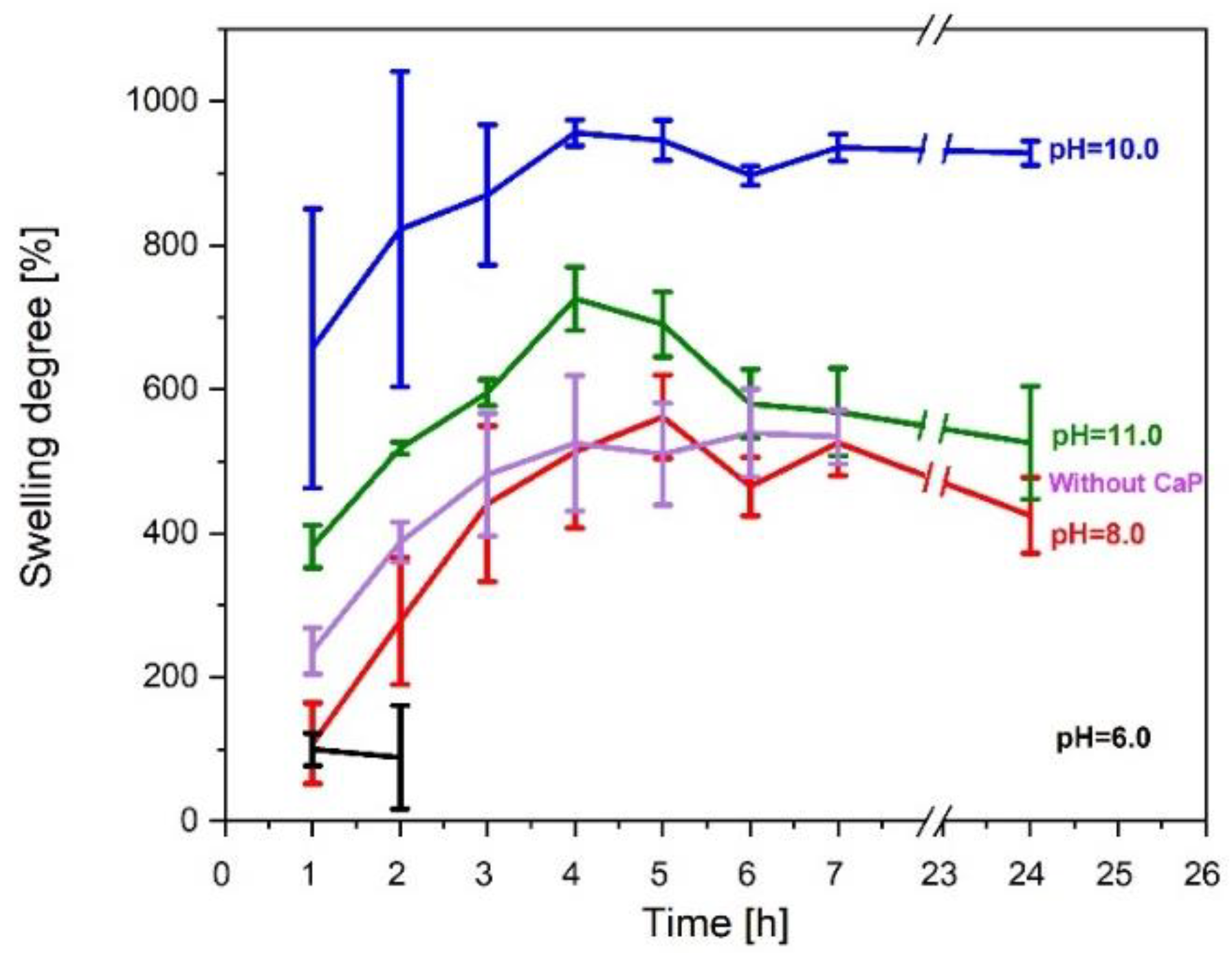
3.3.2. Swelling Degree
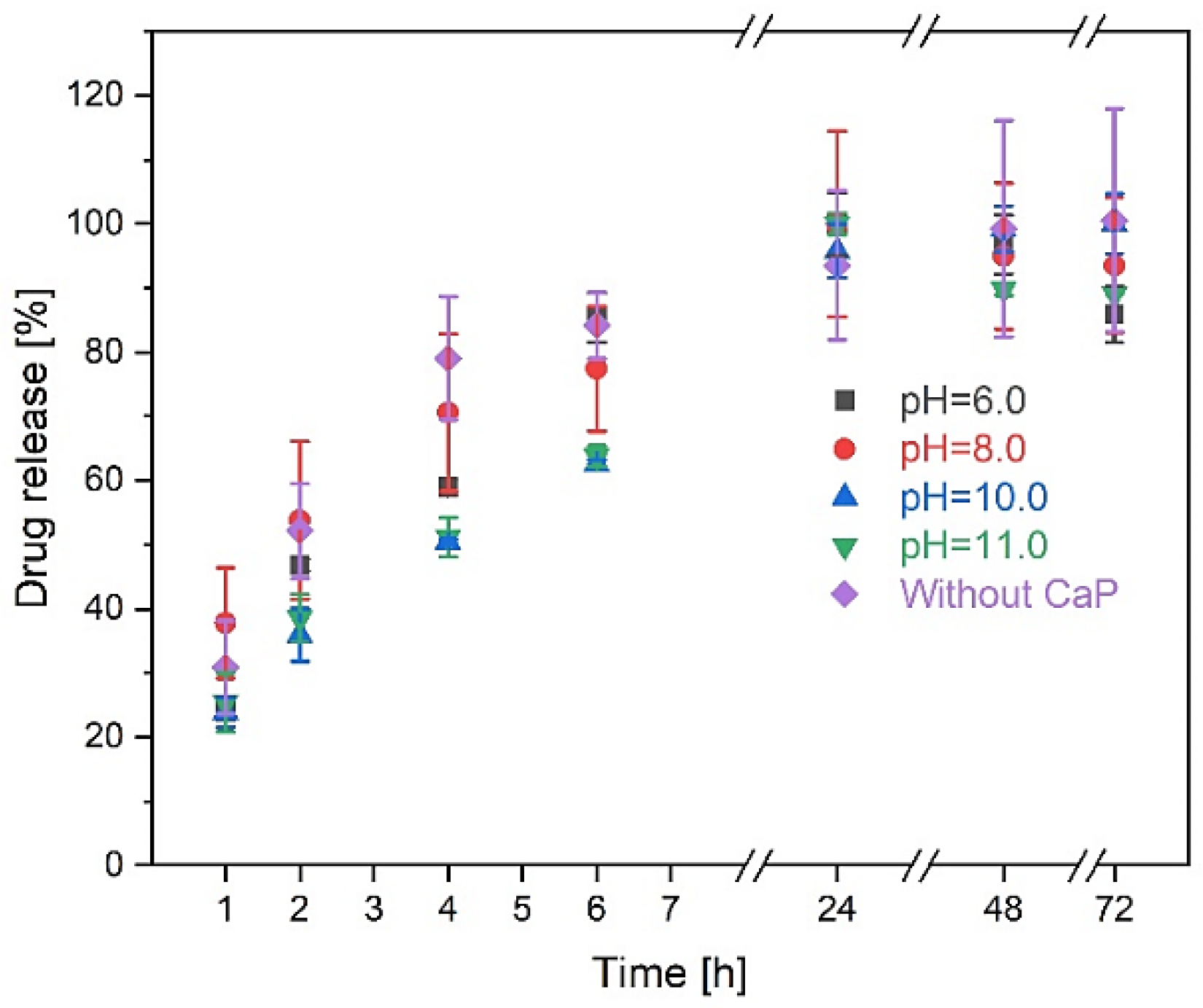
3.3.3. Drug Release Kinetics
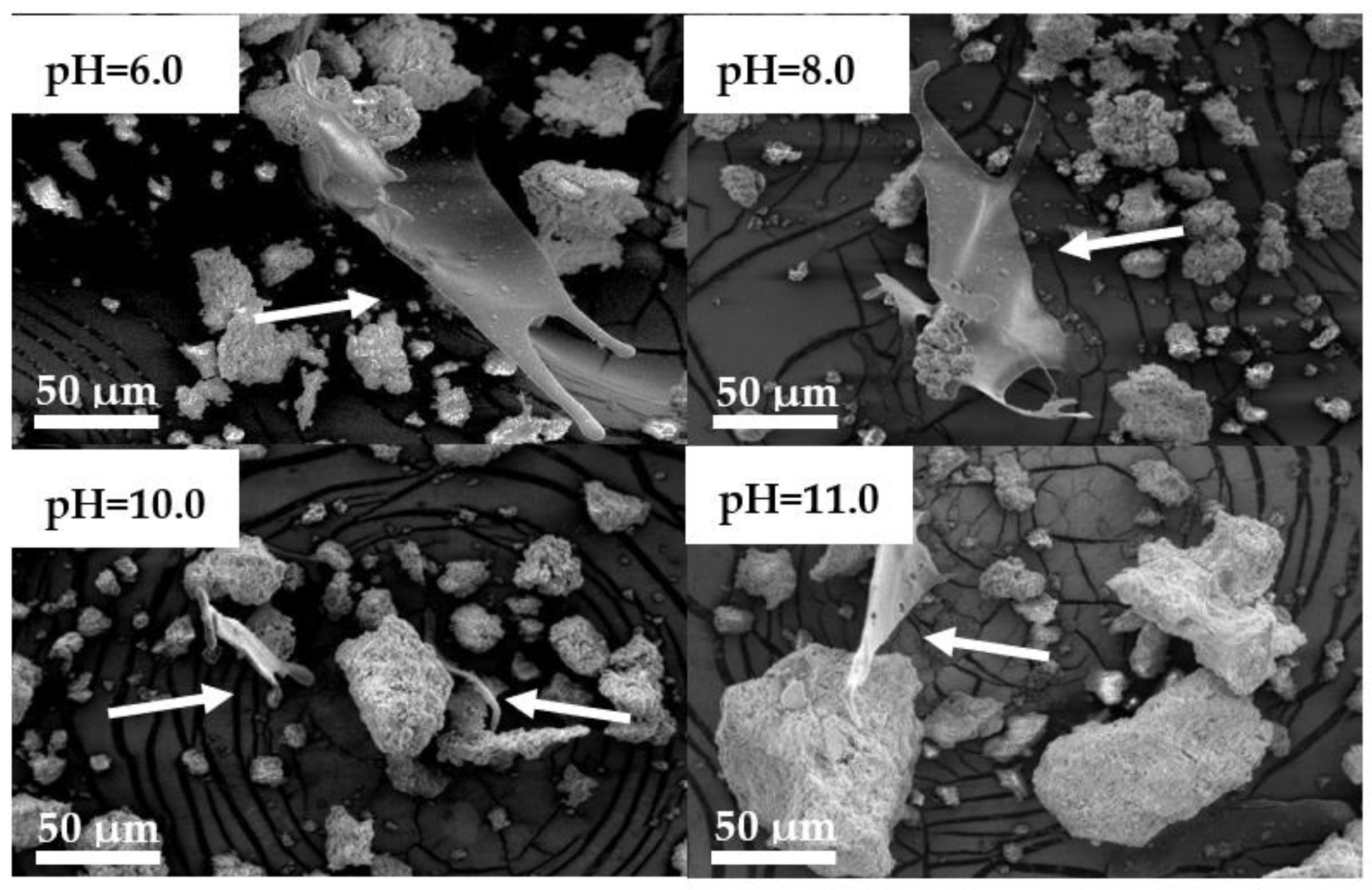
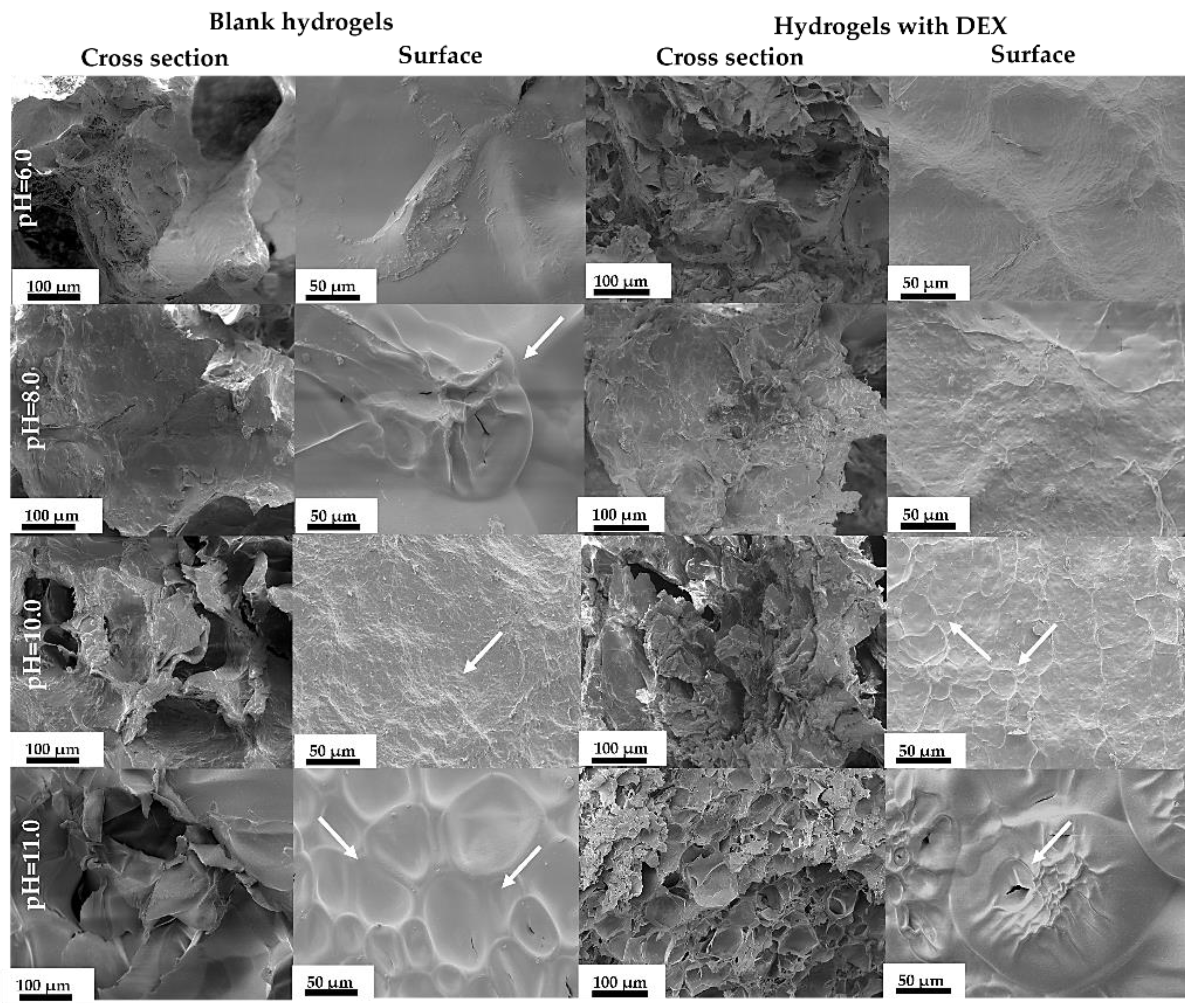
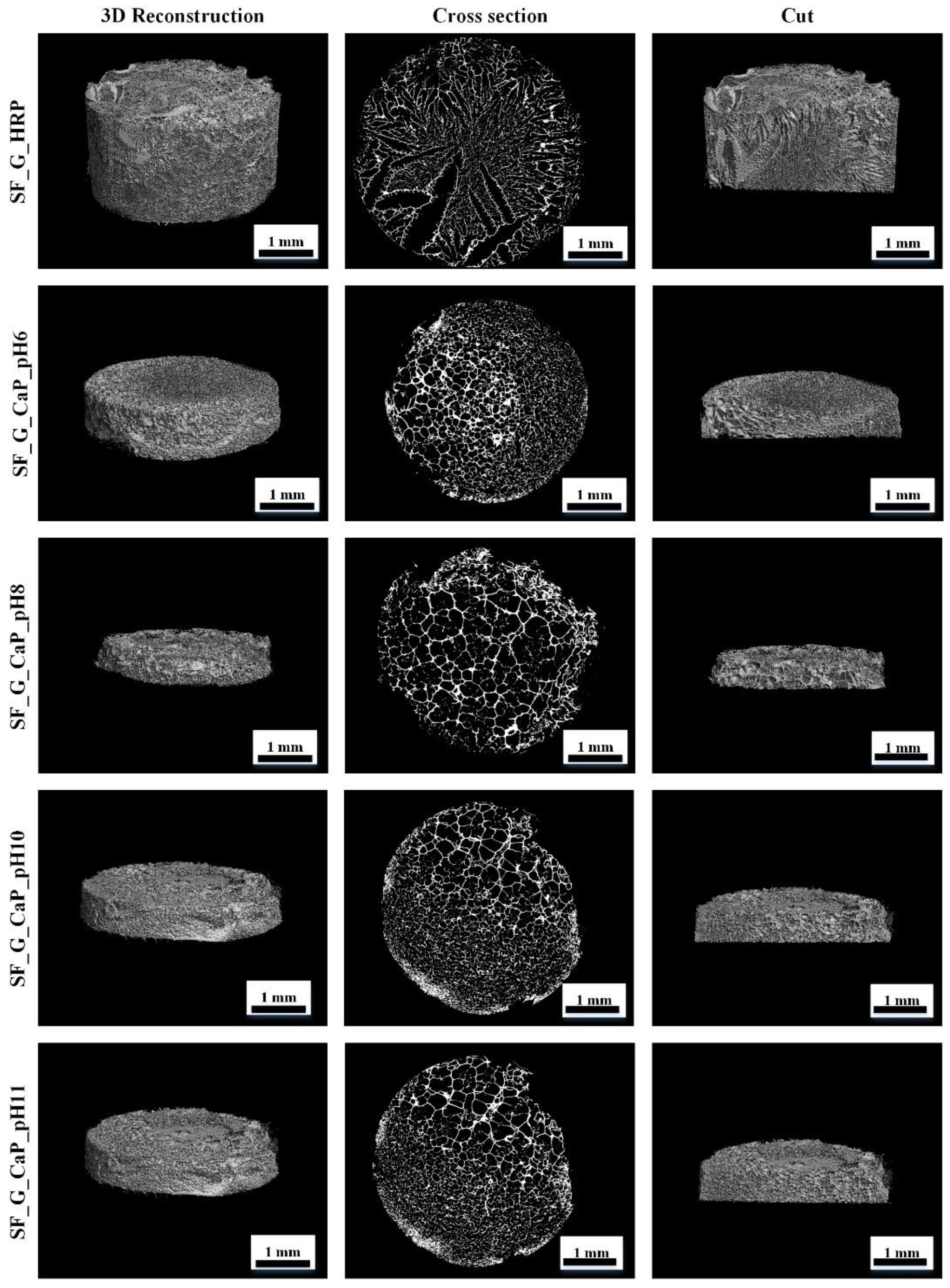
3.3.4. SF/G/CaP Hydrogel Morphology
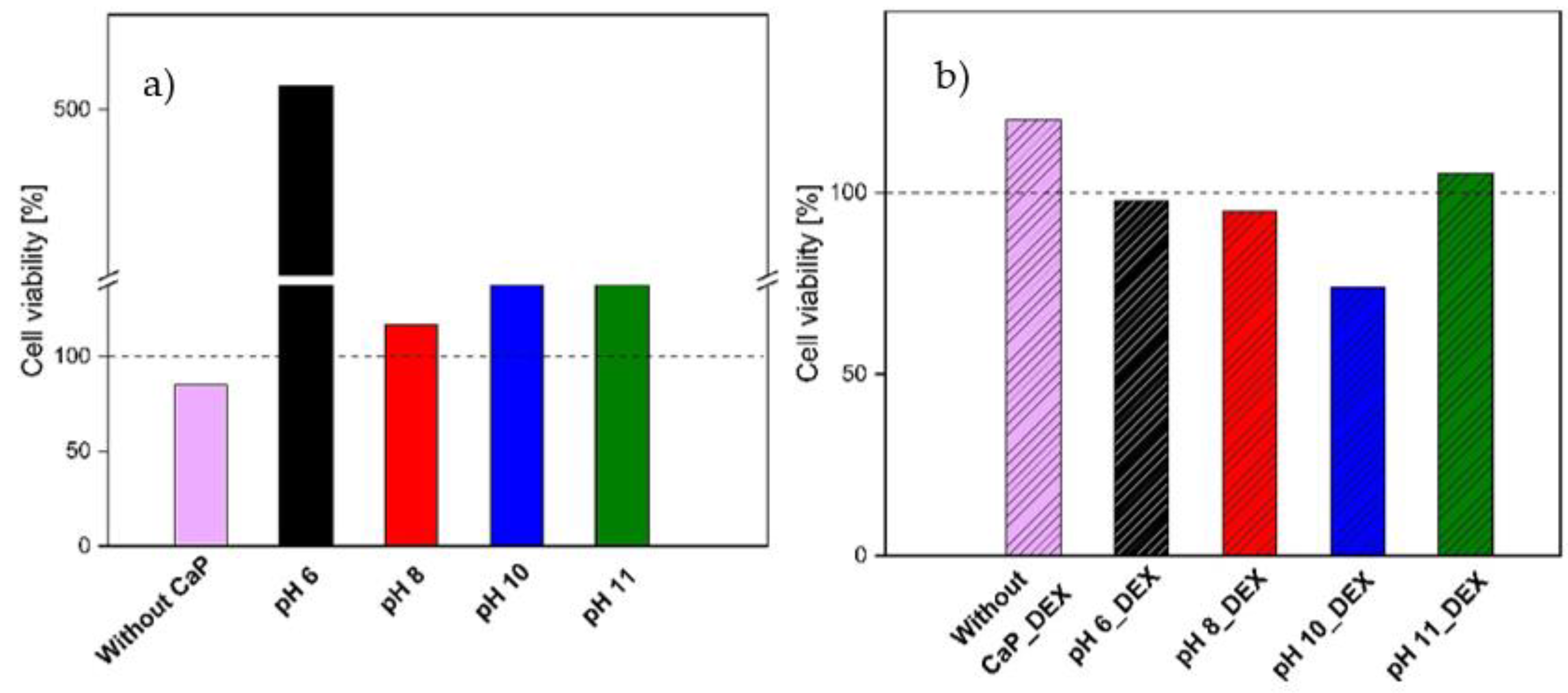
3.3.5. Cell Toxicity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lin, X.-L.; Gao, L.-L.; Li, R.; Cheng, W.; Zhang, C.-Q.; Zhang, X. Mechanical Property and Biocompatibility of Silk Fibroin–Collagen Type II Composite Membrane. Mater. Sci. Eng. C 2019, 105, 110018. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). World Report of Ageing and Health; WHO Library Catalog Data; WHO: Geneva, Switzerland, 2015; 270p. [Google Scholar]
- Rajak, D.K.; Pagar, D.D.; Kumar, R.; Pruncu, C.I. Recent Progress of Reinforcement Materials: A Comprehensive Overview of Composite Materials. J. Mater. Res. Technol. 2019, 8, 6354–6374. [Google Scholar] [CrossRef]
- Ude, A.U.; Eshkoor, R.A.; Zulkifili, R.; Ariffin, A.K.; Dzuraidah, A.W.; Azhari, C.H. Bombyx Mori Silk Fibre and Its Composite: A Review of Contemporary Developments. Mater. Des. 2014, 57, 298–305. [Google Scholar] [CrossRef]
- Cao, T.T.; Zhang, Y.Q. Processing and Characterization of Silk Sericin from Bombyx Mori and Its Application in Biomaterials and Biomedicines. Mater. Sci. Eng. C 2016, 61, 940–952. [Google Scholar] [CrossRef]
- Ma, D.; Wang, Y.; Dai, W. Silk Fibroin-Based Biomaterials for Musculoskeletal Tissue Engineering. Mater. Sci. Eng. C 2018, 89, 456–469. [Google Scholar] [CrossRef]
- Hardy, J.G.; Scheibel, T.R. Composite Materials Based on Silk Proteins. Prog. Polym. Sci. 2010, 35, 1093–1115. [Google Scholar] [CrossRef] [Green Version]
- Johari, N.; Moroni, L.; Samadikuchaksaraei, A. Tuning the Conformation and Mechanical Properties of Silk Fibroin Hydrogels. Eur. Polym. J. 2020, 134, 109842. [Google Scholar] [CrossRef]
- Galateanu, B.; Hudita, A.; Zaharia, C.; Bunea, M.-C.; Vasile, E.; Buga, M.-R.; Costache, M. Silk-Based Hydrogels for Biomedical Applications. Polym. Polym. Compos. A Ref. Ser. 2019, 1791–1817. [Google Scholar] [CrossRef]
- Xu, Z.; Shi, L.; Yang, M.; Zhu, L. Preparation and Biomedical Applications of Silk Fibroin-Nanoparticles Composites with Enhanced Properties—A Review. Mater. Sci. Eng. C 2019, 95, 302–311. [Google Scholar] [CrossRef]
- Patil, P.P.; Reagan, M.R.; Bohara, R.A. Silk Fibroin and Silk-Based Biomaterial Derivatives for Ideal Wound Dressings. Int. J. Biol. Macromol. 2020, 164, 4613–4627. [Google Scholar] [CrossRef]
- Wani, S.U.D.; Gautam, S.P.; Qadrie, Z.L.; Gangadharappa, H.V. Silk Fibroin as a Natural Polymeric Based Bio-Material for Tissue Engineering and Drug Delivery Systems-A Review. Int. J. Biol. Macromol. 2020, 163, 2145–2161. [Google Scholar] [CrossRef]
- Ribeiro, M.; De Moraes, M.A.; Beppu, M.M.; Garcia, M.P.; Fernandes, M.H.; Monteiro, F.J.; Ferraz, M.P. Development of Silk Fibroin/Nanohydroxyapatite Composite Hydrogels for Bone Tissue Engineering. Eur. Polym. J. 2015, 67, 66–77. [Google Scholar] [CrossRef]
- Tas, A.C. The Use of Physiological Solutions or Media in Calcium Phosphate Synthesis and Processing. Acta Biomater. 2014, 10, 1771–1792. [Google Scholar] [CrossRef]
- Hakimimehr, D.; Liu, D.; Troczynski, T. In-Situ Preparation of Poly (Propylene Fumarate)—Hydroxyapatite Composite. Biomaterials 2005, 26, 7297–7303. [Google Scholar] [CrossRef]
- Mobika, J.; Rajkumar, M.; Nithya Priya, V.; Linto Sibi, S.P. Substantial Effect of Silk Fibroin Reinforcement on Properties of Hydroxyapatite/Silk Fibroin Nanocomposite for Bone Tissue Engineering Application. J. Mol. Struct. 2020, 1206, 127739. [Google Scholar] [CrossRef]
- Timofejeva, A.; D’Este, M.; Loca, D. Calcium Phosphate/Polyvinyl Alcohol Composite Hydrogels: A Review on the Freeze-Thawing Synthesis Approach and Applications in Regenerative Medicine. Eur. Polym. J. 2017, 95, 547–565. [Google Scholar] [CrossRef]
- Lawson, A.C.; Czernuszka, J.T. Collagen-Calcium Phosphate Composites. J. Eng. Med. 1998, 212, 413–425. [Google Scholar] [CrossRef]
- Lin, X.; Li, X.; Fan, H.; Wen, X.; Lu, J.; Zhang, X. In Situ Synthesis of Bone-like Apatite / Collagen Nano-Composite at Low Temperature. Mater. Lett. 2004, 58, 3569–3572. [Google Scholar] [CrossRef]
- Jaipan, P.; Nguyen, A.; Narayan, R.J. Gelatin-Based Hydrogels for Biomedical Applications. MRS Commun. 2017, 7, 416–426. [Google Scholar] [CrossRef]
- Kengla, C.; Lee, S.J.; Yoo, J.J.; Atala, A. 3-D Bioprinting Technologies for Tissue Engineering Applications. Rapid Prototypign Biomater. 2019, 269–288. [Google Scholar] [CrossRef]
- Zhou, B.; Wang, P.; Cui, L.; Yu, Y.; Deng, C.; Wang, Q.; Fan, X. Self-Crosslinking of Silk Fibroin Using H2O2-Horseradish Peroxidase System and the Characteristics of the Resulting Fibroin Membranes. Appl. Biochem. Biotechnol. 2017, 182, 1548–1563. [Google Scholar] [CrossRef] [PubMed]
- Salma, K.; Berzina-Cimdina, L.; Borodajenko, N. Calcium Phosphate Bioceramics Prepared from Wet Chemically Precipitated Powders. Process. Appl. Ceram. 2010, 4, 45–51. [Google Scholar] [CrossRef]
- Daculsi, G.; Legeros, R.Z.; Nery, E.; Lynch, K.; Kerebel, B. Transformation of Biphasic Calcium Phosphate Ceramics In Vivo: Ultrastructural and Physicochemical Characterization. J. Biomed. Mater. Res. 1989, 23, 883–894. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.H.; Tabata, Y. Dual-Controlled Release System of Drugs for Bone Regeneration. Adv. Drug Deliv. Rev. 2015, 94, 28–40. [Google Scholar] [CrossRef]
- Wu, C.; Miron, R.; Sculean, A.; Kaskel, S.; Doert, T.; Schulze, R.; Zhang, Y. Proliferation, Differentiation and Gene Expression of Osteoblasts in Boron-Containing Associated with Dexamethasone Deliver from Mesoporous Bioactive Glass Scaffolds. Biomaterials 2011, 32, 7068–7078. [Google Scholar] [CrossRef]
- Egle, K.; Dubnika, A. Development of Bioadhesive Biomaterials Based on Silk and Hyaluronic Acid. Key Eng. Mater. 2020, 850, 236–241. [Google Scholar] [CrossRef]
- Tsihlis, N.D.; Murar, J.; Kapadia, M.R.; Ahanchi, S.S.; Oustwani, C.S.; Saavedra, J.E.; Keefer, L.K.; Kibbe, M.R. Hydrogels: Methods of Preparation. J. Vasc. Surg. 2010, 51, 1248–1259. [Google Scholar] [CrossRef] [Green Version]
- Qu, X.; Yan, L.; Liu, S.; Tan, Y.; Xiao, J.; Cao, Y.; Chen, K.; Xiao, W.; Li, B.; Liao, X. Preparation of Silk Fibroin/Hyaluronic Acid Hydrogels with Enhanced Mechanical Performance by a Combination of Physical and Enzymatic Crosslinking. J. Biomater. Sci. Polym. Ed. 2021, 32, 1635–1653. [Google Scholar] [CrossRef]
- Friedrich, R.B.; Ravanello, A.; Cichota, L.C.; Rolim, C.M.B.; Beck, R.C.R. Validation of a Simple and Rapid UV Spectrophotometric Method for Dexamethasone Assay in Tablets. Quim. Nova 2009, 32, 1052–1054. [Google Scholar] [CrossRef]
- Stefan, N.; Miroiu, F.M.; Socol, G. Degradable Silk Fibroin—Poly (Sebacic Acid)Diacetoxy Terminated, (SF-PSADT)Polymeric Composite Coatings for Biodegradable Medical Applications Deposited by Laser Technology. Prog. Org. Coatings 2019, 134, 11–21. [Google Scholar] [CrossRef]
- Boulet-Audet, M.; Vollrath, F.; Holland, C. Identification and Classification of Silks Using Infrared Spectroscopy. J. Exp. Biol. 2015, 218, 3138–3149. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.M.; Wyeth, P. Using FTIR Spectroscopy to Detect Sericin on Historic Silk. Sci. China Chem. 2010, 53, 626–631. [Google Scholar] [CrossRef]
- Chen, X.; Knight, D.P.; Shao, Z.; Vollrath, F. Regenerated Bombyx Silk Solutions Studied with Rheometry and FTIR. Polymer 2001, 42, 09969–09974. [Google Scholar] [CrossRef]
- Nemoto, R.; Nakamura, S.; Isobe, T.; Senna, M. Direct Synthesis of Hydroxyapatite-Silk Fibroin Nano-Composite Sol via a Mechanochemical Route. J. Sol. Gel Sci. Technol. 2001, 21, 7–12. [Google Scholar] [CrossRef]
- Landi, E.; Celotti, G.; Logroscino, G.; Tampieri, A. Carbonated Hydroxyapatite as Bone Substitute. J. Eur. Ceram. Soc. 2003, 23, 2931–2937. [Google Scholar] [CrossRef]
- Stipniece, L.; Salma-ancane, K.; Borodajenko, N.; Sokolova, M. Characterization of Mg-Substituted Hydroxyapatite Synthesized by Wet Chemical Method. Ceram. Int. 2014, 40, 3261–3267. [Google Scholar] [CrossRef]
- Weska, R.F.; Vieira, W.C.; Nogueira, G.M.; Beppu, M.M. Effect of Freezing Methods on the Properties of Lyophilized Porous Silk Fibroin Membranes. Mater. Res. 2009, 12, 233–237. [Google Scholar] [CrossRef]
- Jeong, J.; Kim, J.H.; Shim, J.H.; Hwang, N.S.; Heo, C.Y. Bioactive Calcium Phosphate Materials and Applications in Bone Regeneration. Biomater. Res. 2019, 23, 9. [Google Scholar] [CrossRef] [Green Version]
- Huang, J.; Best, S.M.; Brooks, R.A.; Rushton, N.; Bonfield, W. In Vitro Evaluation of Nanosized Carbonate-Substituted Hydroxyapatite and Its Polyhydroxyethylmethacrylate Nanocomposite. J. Biomed. Mater. Res. Part A 2008, 87, 598–607. [Google Scholar] [CrossRef]
- Moreira Teixeira, L.S.; Feijen, J.; van Blitterswijk, C.A.; Dijkstra, P.J.; Karperien, M. Enzyme-Catalyzed Crosslinkable Hydrogels: Emerging Strategies for Tissue Engineering. Biomaterials 2012, 33, 1281–1290. [Google Scholar] [CrossRef] [PubMed]
- Thakre, A.A.; Singh, A.K. Determination of Work of Adhesion of Gelatin Hydrogels on a Glass Substrate. Mater. Res. Express 2018, 5, 045302. [Google Scholar] [CrossRef]
- Zakharov, N.A.; Demina, L.I.; Aliev, A.D.; Kiselev, M.R.; Matveev, V.V.; Orlov, M.A.; Zakharova, T.V.; Kuznetsov, N.T. Synthesis and Properties of Calcium Hydroxyapatite/Silk Fibroin Organomineral Composites. Inorg. Mater. 2017, 53, 333–342. [Google Scholar] [CrossRef]
- Hickey, T.; Kreutzer, D.; Burgess, D.J.; Moussy, F. Dexamethasone/PLGA Microspheres for Continuous Delivery of an Anti-Inflammatory Drug for Implantable Medical Devices. Biomaterials 2002, 23, 1649–1656. [Google Scholar] [CrossRef]
- Long, J.; Nand, A.V.; Bunt, C.; Seyfoddin, A. Controlled Release of Dexamethasone from Poly(Vinyl Alcohol) Hydrogel. Pharm. Dev. Technol. 2019, 24, 839–848. [Google Scholar] [CrossRef]
- Khodaverdi, E.; Kheirandish, F.; Mirzazadeh Tekie, F.S.; Khashyarmanesh, B.Z.; Hadizadeh, F.; Moallemzadeh Haghighi, H. Preparation of a Sustained Release Drug Delivery System for Dexamethasone by a Thermosensitive, In Situ Forming Hydrogel for Use in Differentiation of Dental Pulp. ISRN Pharm. 2013, 2013, 983053. [Google Scholar] [CrossRef]
- Petta, D.; Fussell, G.; Hughes, L.; Buechter, D.D.; Sprecher, C.M.; Alini, M.; Eglin, D.; D’Este, M. Calcium Phosphate/Thermoresponsive Hyaluronan Hydrogel Composite Delivering Hydrophilic and Hydrophobic Drugs. J. Orthop. Transl. 2016, 5, 57–68. [Google Scholar] [CrossRef] [Green Version]
- Sceglovs, A.; Salma-Ancane, K. Novel Hydrogels and Composite Hydrogels Based on Ԑ-Polylysine, Hyaluronic Acid, and Hydroxyapatite. Key Eng. Mater. 2020, 850, 242–248. [Google Scholar] [CrossRef]
- Kim, M.H.; Kim, B.S.; Lee, J.; Cho, D.; Kwon, O.H.; Park, W.H. Silk Fibroin/Hydroxyapatite Composite Hydrogel Induced by Gamma-Ray Irradiation for Bone Tissue Engineering. Biomater. Res. 2017, 21, 12. [Google Scholar] [CrossRef] [Green Version]
- Annabi, N.; Nichol, J.W.; Zhong, X.; Ji, C.; Koshy, S.; Khademhosseini, A.; Dehghani, F. Controlling the Porosity and Microarchitecture of Hydrogels for Tissue Engineering. Tissue Eng. Part. B Rev. 2010, 16, 371–383. [Google Scholar] [CrossRef]
- Wu, W.; Zhang, Z.; Xiong, T.; Zhao, W.; Jiang, R.; Chen, H.; Li, X. Calcium Ion Coordinated Dexamethasone Supramolecular Hydrogel as Therapeutic Alternative for Control of Non-Infectious Uveitis. Acta Biomater. 2017, 61, 157–168. [Google Scholar] [CrossRef]
- Aramwit, P.; Kanokpanont, S.; Nakpheng, T.; Srichana, T. The Effect of Sericin from Various Extraction Methods on Cell Viability and Collagen Production. Int. J. Mol. Sci. 2010, 11, 2200–2211. [Google Scholar] [CrossRef] [Green Version]
- Liu, T.; Miao, J.; Sheng, W.; Xie, Y. Cytocompatibility of Regenerated Silk Fibroin Film: A Medical Biomaterial Applicable to Wound Healing. J. Zhejiang Univ. B Biomed. Biotechnol. 2010, 11, 10–16. [Google Scholar] [CrossRef] [Green Version]
- Tremble, L.F.; Heffron, C.C.B.B.; Forde, P.F. The Effect of Calcium Electroporation on Viability, Phenotype and Function of Melanoma Conditioned Macrophages. Sci. Rep. 2020, 10, 20645. [Google Scholar] [CrossRef]
- Vaz, C.V.; Rodrigues, D.B.; Socorro, S.; Maia, C.J. Effect of Extracellular Calcium on Regucalcin Expression and Cell Viability in Neoplastic and Non-Neoplastic Human Prostate Cells. Biochim. Biophys. Acta Mol. Cell Res. 2015, 1853, 2621–2628. [Google Scholar] [CrossRef] [Green Version]
- Velard, F.; Braux, J.; Amedee, J.; Laquerriere, P. Acta Biomaterialia Inflammatory Cell Response to Calcium Phosphate Biomaterial Particles: An Overview. Acta Biomater. 2013, 9, 4956–4963. [Google Scholar] [CrossRef]
- Olkowski, R.; Kaszczewski, P.; Czechowska, J. Cytocompatibility of the Selected Calcium Phosphate Based Bone Cements: Comparative Study in Human Cell Culture. J. Mater. Sci. Mater. Med. 2015, 26, 270. [Google Scholar] [CrossRef] [Green Version]
- Klammert, U.; Reuther, T.; Jahn, C.; Kraski, B.; Ku, A.C. Cytocompatibility of Brushite and Monetite Cell Culture Scaffolds Made by Three-Dimensional Powder Printing. Acta Biomater. 2009, 5, 727–734. [Google Scholar] [CrossRef]
- Shi, Z.; Huang, X.; Cai, Y.; Tang, R.; Yang, D. Size Effect of Hydroxyapatite Nanoparticles on Proliferation and Apoptosis of Osteoblast-like Cells. Acta Biomater. 2009, 5, 338–345. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grava, A.; Egle, K.; Dubnika, A. Enzymatically Crosslinked In Situ Synthesized Silk/Gelatin/Calcium Phosphate Hydrogels for Drug Delivery. Materials 2021, 14, 7191. https://doi.org/10.3390/ma14237191
Grava A, Egle K, Dubnika A. Enzymatically Crosslinked In Situ Synthesized Silk/Gelatin/Calcium Phosphate Hydrogels for Drug Delivery. Materials. 2021; 14(23):7191. https://doi.org/10.3390/ma14237191
Chicago/Turabian StyleGrava, Andra, Karina Egle, and Arita Dubnika. 2021. "Enzymatically Crosslinked In Situ Synthesized Silk/Gelatin/Calcium Phosphate Hydrogels for Drug Delivery" Materials 14, no. 23: 7191. https://doi.org/10.3390/ma14237191
APA StyleGrava, A., Egle, K., & Dubnika, A. (2021). Enzymatically Crosslinked In Situ Synthesized Silk/Gelatin/Calcium Phosphate Hydrogels for Drug Delivery. Materials, 14(23), 7191. https://doi.org/10.3390/ma14237191