3.1. Accelerated Corrosion Tests
The protective performance of a CIN on St3ps steel was estimated under daily moisture condensation conditions in two series of experiments. In one of them, we determined the optimal CT temperature (TCT), while in the other one, its optimal duration (τCT). In the first series of experiments, the adsorption films on the metal were created in the T range from 80–140 °C. The τCT was 1 h in these experiments.
The first corrosion damage (rust) on steel St3 that was not heat-treated was detected on the samples in 1.0 ± 0.5 h under the specified test conditions (
Table 1).
After steel chamber treatment at 80 °C, the inhibitors demonstrated almost no protective effects. An increase in TCT to 100 °C had practically no effect on the PAE of the adsorption films formed in BTA vapors. However, the protective properties of ODA and its mixture with BTA increased significantly under these conditions. The τprot of the metal was 72 h (ODA) and 120 h (ODA + BTA mixture).
Chamber treatment at 120 °C increased in the PAE of BTA adsorption films only slightly. However, the protective ability of ODA and ODA + BTA increased noticeably. After treatment with these CIN, the first indications of corrosion appeared on the samples after 120 or 168 h of steel exposure in the corrosive environment, respectively.
A further increase in
TCT (up to 140 °C) resulted in a decrease in the PAE of all the CIN studied. Chamber treatment with vapors of BTA, ODA, or their mixture provided steel protection for 2, 48, or 144 h, respectively. The possible reasons for the existence of a maximum on the temperature plot of the protective effect of CIN have been discussed elsewhere [
14,
15]. Most likely, the increase in the protective properties of the chamber inhibitors with an increase in the CT temperature results from an increase in their vapor pressure, and hence, stronger adsorption of the inhibitor on the metal. In turn, the downward branch is due to the well-known fact that adsorption decreases as the adsorbent’s temperature rises. Thus, 120 °C was found to be the optimal temperature of steel chamber treatment with the mixed CIN.
The data presented above allow us to draw some conclusions about the mutual effects of ODA and BTA. Let us consider this phenomenon in more detail.
According to a study by Ekilik and Chikov [
25] relying on the formal theory of the action of corrosion inhibitors [
26], the mutual effect of the components of an inhibitor mixture is determined by the ratio of the corrosion inhibition coefficients (γ) provided by the mixture (γ
mix) and the product of the γ values of the components. It is important for our study, so let us repeat the main provisions.
Let there be inhibitors “1” and “2” that are individually characterized by inhibition coefficients γ
1 and γ
2, respectively, in a corrosive medium of interest. Let the rate of general corrosion in this medium be K
0 in the absence of inhibitors. Inhibitor “1” provides corrosion inhibition by a factor of γ
1 to reach a value of K
1:
If γ
2 does not depend on the presence of inhibitor “1” in the system, i.e., there are no interactions between the inhibitors, the addition of inhibitor “2” to the system will further reduce the corrosion rate by a factor of γ
2. Then the corrosion rate in a system containing both inhibitors “1” and “2” (K
mix) is:
Substitution of K
1 from Equation (4) to this expression gives for the binary mixture:
It follows from Equation (6) that:
or, taking into account that K
0/K
mix = γ
mix:
Hence, if the addition of inhibitor “1” to the system slows down corrosion, for example, by a factor of 2, while the addition of inhibitor “2” slows it down additionally by a factor of 3, then their mixture will slow down corrosion by a factor of 6 if no mutual strengthening or weakening of the protective action occurs.
Equation (8) is a criterion for the independence of the protective action of inhibitors of general corrosion of metals in binary mixtures. It was derived for acid corrosion but is also useful for neutral environments [
27]. If γ
mix > γ
1γ
2, synergism is observed, i.e., the ability of inhibitors “1” and “2” to enhance the protective effects of each other. In contrast, if γ
mix < γ
1γ
2, this indicates that antagonism is observed, and the protective effect decreases.
Let us develop the concept mentioned above [
26] by adapting it to systems where the protective properties of inhibitors are estimated through
τprot. In such a case, Equation (8) takes the form:
Let us consider a system where
τprot, i.e., the full protection period, is the criterion of inhibitor efficiency. Let us analyze the mutual effect of the components of a binary mixture of inhibitors “1” and “2” for this system. Let the first corrosion damage on the metal become visually noticeable upon transition of a metal with mass
M on area
s to an oxide-hydroxide form. Then the rate of the corrosion process that determines the formation of the first corrosion damage on the metal in the absence of inhibitors can be expressed as:
It should be noted that both general and local corrosion may be concerned.
Similarly, for inhibitor “1” that provides metal protection for a period of
τprot,1, inhibitor “2” whose metal protection period is
τprot,2, and a mixture of inhibitors “1” and “2” (
τprot,mix), the following expressions are valid:
and
By definition, the coefficient of metal corrosion inhibition by inhibitor “1” can be expressed as:
By substituting the expressions for γ into Equation (8) we obtain the following expression for a mixture of inhibitors “1” and “2” that do not interact with each other:
This expression can be transformed to Equation (8).
Thus, expression (9) determines the lack of a mutual effect of the protective properties of inhibitors “1” and “2” expressed through the full metal protection time. The situation where τprot,mix > τprot,1τprot,2/τprot,0 indicates that synergy exists, i.e., components “1” and “2” amplify the protective effects of each other, whereas if τprot,mix < τprot,1τprot,2/τprot,0, antagonism exists, i.e., the components mutually weaken the protective effects of each other.
To estimate the mutual effects of components in mixed inhibitors more convenient, let us introduce a coefficient:
where “meas” refers to a value measured experimentally, while “calc” refers to the result of a calculation by Formula (6). The value of α exceeding one indicates a synergism of protective effects. Moreover, the higher α, the more pronounced this interaction.
A simple calculation shows that the coefficient α of the mutual effect of BTA and ODA in the tests described above was 0.23 and indicated an antagonism of the components. This antagonism may result from a decrease in the vapor pressure of the mixture due to the formation of a salt between a weak acid, BTA, and a base, ODA.
At first glance, the antagonism of the protective action of ODA and BTA contradicts the fact that the efficiency of the mixed CIN is higher than that of its components. However, an analysis of the situation shows that there is no contradiction. This is very important for the creation of efficient CIN. Let us consider it in more detail in order to solve the following question: is synergism a prerequisite for creating formulations with a protective effect exceeding those of its components?
It is easy to show that in the absence of any interactions, the protective properties of a mixture of inhibitors “1” and “2” are always higher than those of its components. Since compounds “1” and “2” are inhibitors, i.e., γ
1 > 1 and γ
2 > 1, the following expressions are valid:
and
Similarly, in the systems where the efficiency of inhibitors is expressed via
τprot, the following is true:
τ1/τ0 > 1 and
τ2/τ0 > 1. Then, for a binary mixture:
and
This means that the addition of two non-interacting inhibitors to a system is always accompanied by an increase in the protective ability in comparison with the individual components. This conclusion is quite obvious and not particularly useful in the practice of creating inhibitors for bulk solutions. In this case, the mixing of components is always accompanied by an increase in their total concentration in the electrolyte. In fact, inequalities (21) and (22) only indicate that as the total concentration of the inhibitor in a bulk electrolyte increases, the protective effect of the mixture increases. For example, if 5 g of inhibitor “1” and 5 g of inhibitor “2” are mixed together in a unit volume of a solution, then the total amount, i.e., 10 g of the mixture, will inhibit corrosion more efficiently than 5 g/l of any single inhibitor.
The situation is different in the case of the chamber protection of metals where the protective properties of a CIN depend on the atmosphere composition. The content of chamber inhibitors in the atmosphere and their efficiency in a wide range of conditions is not dependent on their amount introduced into the chamber but is determined by their saturated vapor pressures in that chamber. Mixing of non-interacting CIN “1” and “2” increases the total vapor pressure of inhibitors in the system and the protection efficiency.
One more example: let us introduce 5 g of inhibitor “1” and 5 g of inhibitor “2” into a chamber. The mixture (10 g) will provide metal protection for a longer time than its components. However, the same protection will be provided by 5 g of the mixture, or 1 g of the mixture, etc.
This is an answer to the question raised above. Synergism is desirable but not mandatory for the creation of mixed CIN whose efficiency exceeds that of the components. If there are no interactions between the components, their mixture will provide complete protection of a metal for a longer period than its components. Moreover, as shown by the data presented above, this is possible even if antagonism between the components is displayed. In fact, the calculated coefficient of the mutual effect of BTA and ODA (α = 0.23) indicates antagonism between the components. The reasons of this phenomenon are not entirely clear; however, it may be assumed that this antagonism is due to a decrease in the vapor pressure of the mixture due to the formation of a salt between a weak acid, BTA, and a base, ODA.
The above results were obtained after steel chamber treatment for 1 h. According to the data in
Table 2, this CT duration is sufficient to form adsorption films with the maximum PAE. Anyway, a 2- or 4-times longer CT of steel in vapors of ODA, BTA, or their mixture at the optimum temperature did not improve the corrosion protection of the samples. On the other hand, shortening the CT time to 20 min did decrease the protection efficiency. Surface layers that are in equilibrium at 120 °C cannot be formed on the metal in such a short time. Thus, the optimal
τCT of steel by the mixed CIN or by its components is 1 h.
The specific features of steel protection by the CIN in question were studied at TCT = 120 °C and τCT = 1 h.
3.2. Voltammetric Experiments
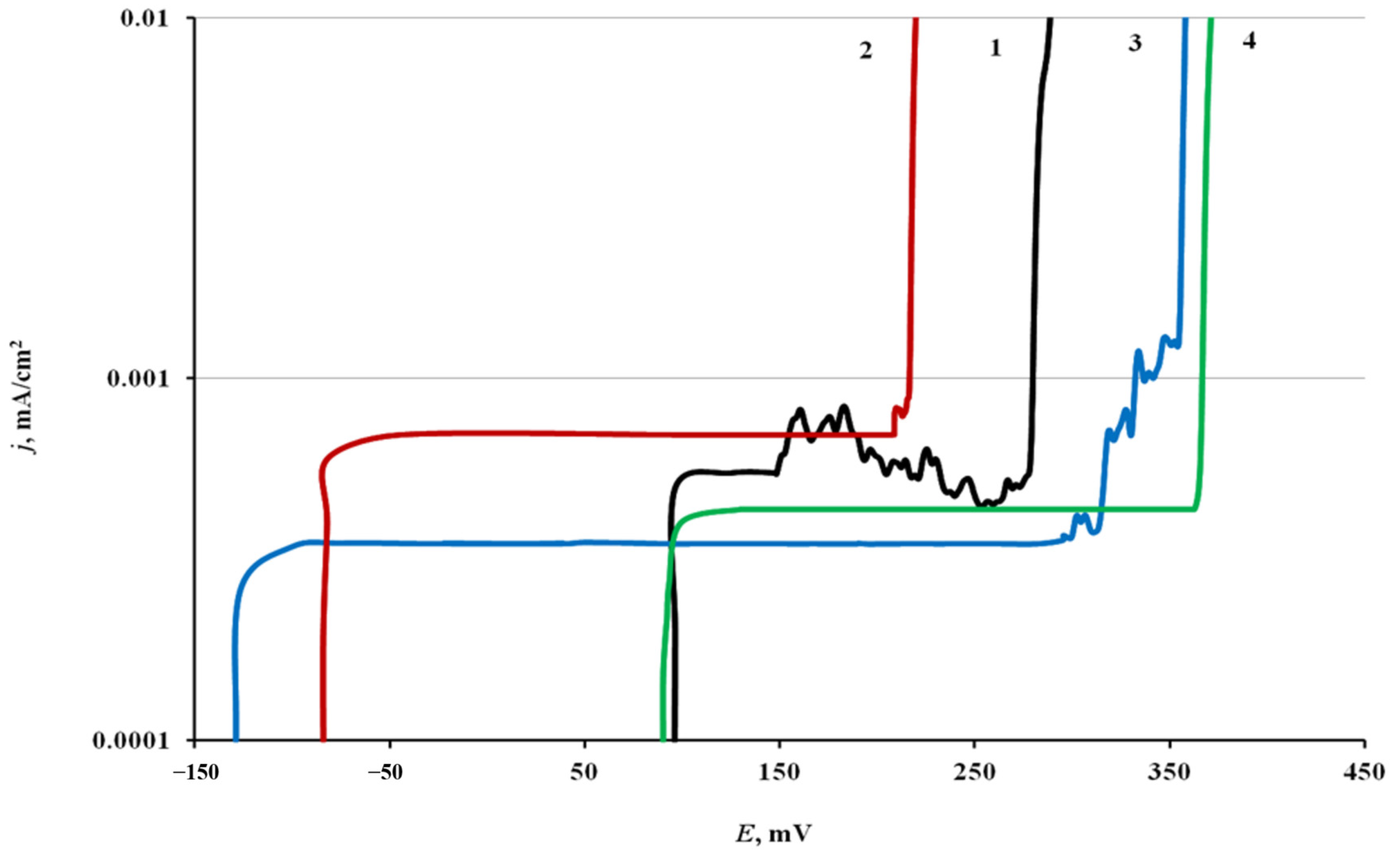
The anodic potentiodynamic polarization curves of steel electrodes, both treated in CIN vapors and not subjected to CT, were typical of passive steel in a chloride-containing borate buffer solution (
Figure 2 and
Table 3). They have a wide passivity region, current oscillations associated with the initiation and repassivation of pits, and a sharp increase in the current density when
Ebr is reached. In some cases, there are no current oscillations on the polarization curves.
The mean value of Ecor of a chamber-treated steel electrode in borate buffer was 0.095 V. Its anodic polarization did not result in noticeable changes in current up to E = 0.15 V. On reaching this potential, the oscillations that appeared on the polarization curves were due to pitting. This was confirmed by a visual inspection of the electrodes withdrawn from the electrolyte. Small pits on the electrode surface were visible through a magnifying glass. The further polarization of steel resulted in the breakdown of the passive film. The j values sharply increased at Ebr = 0.27 V. In this case, dark dots with “scorch marks” on the metal were visible with a naked eye.
Chamber treatment of steel in BTA vapors shifted Ecor cathodically to −0.085 V. Anodic polarization of electrodes treated in this way resulted in passive film breakdown at Ebr = 0.210 V. In this case, no current oscillations preceding the breakdown were observed on the polarization curves.
After steel chamber treatment in ODA vapors, the stationary potential shifted to more negative values, Ecor = −0.130 V, than after treatment in BTA vapors. At E = 0.295 V, insignificant current oscillations appeared on the polarization curves, followed by passive film breakdown at Ebr = 0.345 V.
Unlike BTA and ODA, the mixed CIN almost did not affect Ecor, but it ennobled Epit more strongly than the components (up to 0.36 V). Breakdown of the passive film occurred immediately, without current oscillations.
Thus, the adsorption films of all the CIN in question stabilized the passive state of steel by increasing its resistance to local anionic depassivation. The ennoblement of Epit caused by the mixed CIN exceeded the protective action of its components taken alone.
3.4. Electrochemical Impedance Spectroscopy
Additional information on the protective effect of the mixed CIN and its components on steel is provided by electrochemical impedance spectroscopy (EIS). The Nyquist diagrams of steel samples after heat treatment without an inhibitor and after treatment in CIN vapors are shown in
Figure 3.
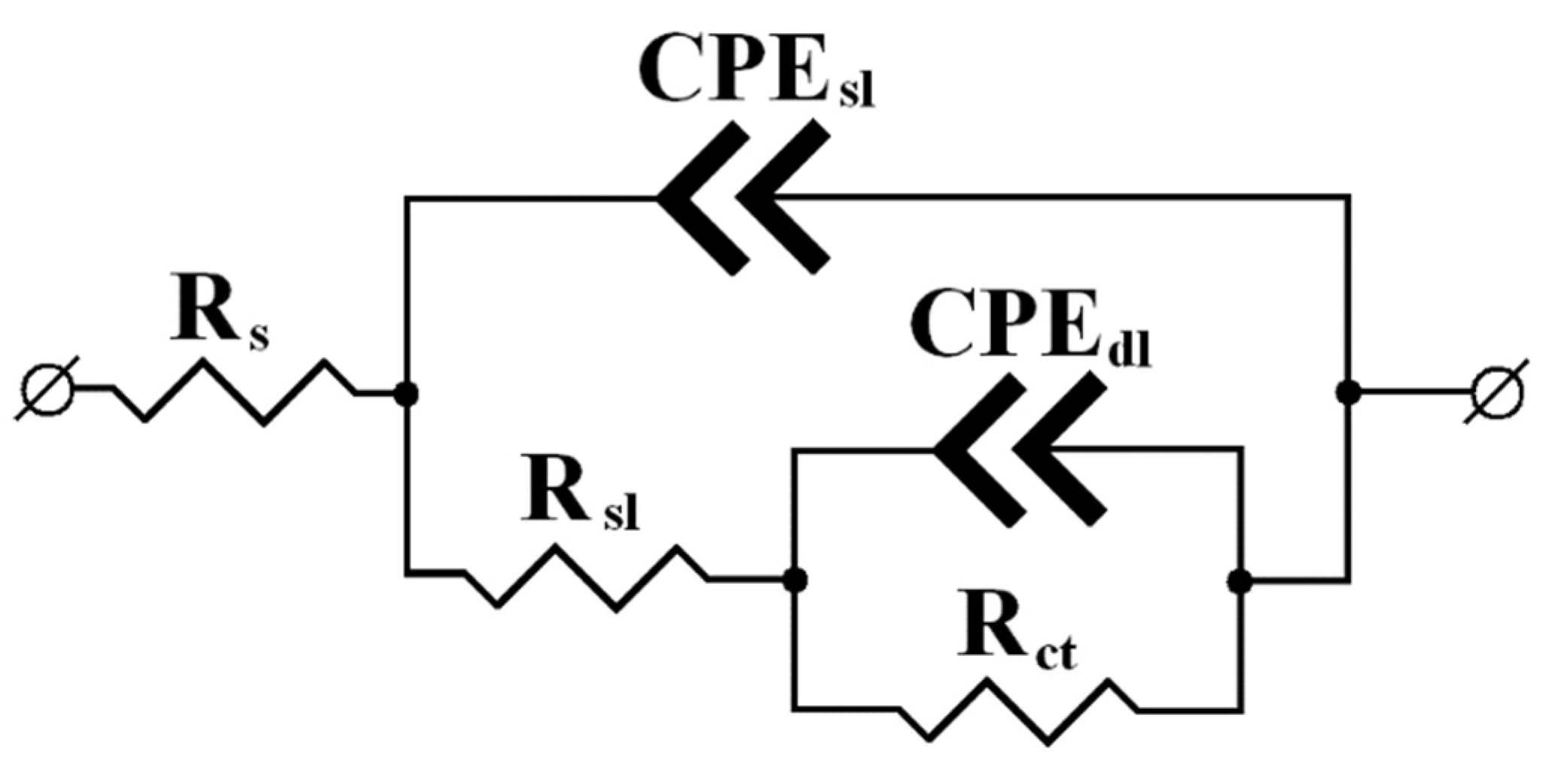
All the Nyquist plots manifest two more or less distinct semicircular arcs, which makes it possible to apply a common equivalent circuit with two time constants in all the cases. The high-frequency (small) semicircle on the Nyquist plot in the selected model corresponds to the time constant mainly associated with the Rsl/CPEsl circuit, i.e., it depends on the surface layer conductivity. The low-frequency (large) arc on the Nyquist plot is associated with the kinetics of the Faraday reaction on the metal, which actually determines the corrosion behavior of steel. The parameters of this semicircle are described by the Rct/CPEdl circuit as part of the overall equivalent circuit.
It is evident from the figure that the radii of the arcs on the Nyquist plots of samples treated in CIN vapors are considerably larger than those obtained on steel heat-treated without a CIN. This qualitatively indicates a strong inhibitory effect of the CIN studied. The values of elements obtained by calculations are presented in
Table 5.
These results imply that after treatment with a CIN, the surface layer resistance Rsl increases by more than an order of magnitude. The resistance of oxide and oxide-inhibitor surface layers is determined by the transfer of anions and cations involved in the corrosion process. Taking the results of the ellipsometric determination of the thicknesses of these layers into account, it is obvious that the layer thickness itself does not correlate with the Rsl values obtained. What is more, the highest resistance is obtained for the thinnest layer after treatment with ODA + BTA. Hence, it can be concluded that all the CIN studied drastically hinder ion transport to the active metal surface.
This is also indicated by a sharp decrease in the capacitance of samples (by modulo A of the CPEsl element (Judging by the values of phase factor n, all the CPE elements are of a capacitive nature. Although the modulo A formally has the dimension S·sn/cm2, if phase factor n is close to 1, then the parameters of this circuit element can be numerically estimated in capacitance units, F/cm2.)). There is no correlation between the thicknesses of oxide and oxide-inhibitor layers and the capacitance of samples. This is probably due to a change in the surface structure and dielectric properties upon treatment with a CIN. It should also be noted that the results of electrochemical impedance spectroscopy, unlike ellipsometry, do not allow one to separate the effect of the oxide layer and the CIN deposited on top of it.
The phase factor nsl lies in the range from 0.85–0.97, which characterizes these systems as rather homogeneous in structure. The closest approximation to an ideal capacitor is obtained upon steel treatment in ODA + BTA vapors.
To complete the discussion of the results concerning the surface layer, let us note that the efficiency of blocking the ion transfer to the active metal increases in the following series: BTA < ODA < ODA + BTA.
Concerning the elements responsible for the low-frequency process that directly affects the corrosion kinetics, it should be noted that after treatment with a CIN, the charge transfer resistance Rct changed most considerably (by a factor of 40–50), whereas the capacitance of the Faraday reaction decreased by only one order of magnitude. Interestingly, the comparison of inhibited samples with each other shows that the charge transfer resistance Rct changes much less strongly (1.36-fold) than the Rsl values (3.28-fold). At the same time, the inhibition of the Faraday corrosion process by the CIN changes in the same series: BTA < ODA < ODA + BTA.
The capacitance of the double electric layer also decreases in this order. It is worth noting that upon the treatment of steel with BTA, the phase factor is ndl = 0.64 (in contrast to the other inhibitors where n = 1). This indicates a significant inhomogeneity and/or diffuseness of the capacitor plates. Recalculation to the parameters of an equivalent ideal capacitor in this chain for comparison with the other results gives 11 × 10−6 F/cm2.
Thus, BTA has the smallest inhibitory effect among the CIN studied, both in terms of surface blocking and in its effect on the electrochemical process itself. ODA is in second place in this CIN rating based on both parameters. CT of steel with the ODA + BTA formulation manifests the highest inhibiting properties in terms of all parameters.
The results of experimental data simulation based on an equivalent circuit make it possible to quantitatively estimate the contribution of various mechanisms that provide the inhibiting effect of the CIN and to determine the partial corrosion inhibition coefficients.
Two principal mechanisms of action of adsorption-type corrosion inhibitors are known, i.e., the blocking and activation mechanisms [
26]. In the former case, an inhibitor adsorbs and blocks a fraction of the metal surface, thus, reducing the corrosion rate, but does not alter the kinetics of electrochemical processes on the remaining unblocked surface. In contrast, the activation mechanism implies that corrosion inhibition occurs due to changes in the activation energy of corrosion processes, and hence, their kinetics. Both mechanisms usually operate simultaneously, but their contributions to the inhibitory effect may differ.
The R
sl value in the equivalent circuit that we use reflects the effect of the surface layer and can, therefore, serve as a criterion for estimating the blocking effect of an inhibitor. The coefficient of corrosion inhibition due to surface blocking (γ
sl) equals the ratio of R
sl values of samples after CT and after TT without a CIN:
Using a similar approach, the R
ct value can be used to estimate the effect of a CIN on the Faraday corrosion process. Hence, the coefficient of electrochemical reaction inhibition by a CIN (γ
ct) can be determined as the ratio of the charge transfer resistances R
ct for inhibited and non-inhibited samples:
The degrees of protection by CIN provided by the blocking and activation mechanisms for different variants of steel St3 chamber treatment are shown in
Table 6.
The comparison of γsl and γct values implies a mixed blocking activation mechanism of action of the CIN studied. However, in all the cases, the γsl < γct inequality is observed, which indicates that the activation mechanism is predominant, especially in the case of BTA.
Thus, on the one hand, the EIS results confirm the conclusions made by the comparison of the CIN efficiency determined in corrosion experiments, and on the other hand, they indicate a mixed blocking activation mechanism of their action.