Synthesis of High-Performance CSA Cements as Low Carbon OPC Alternative
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
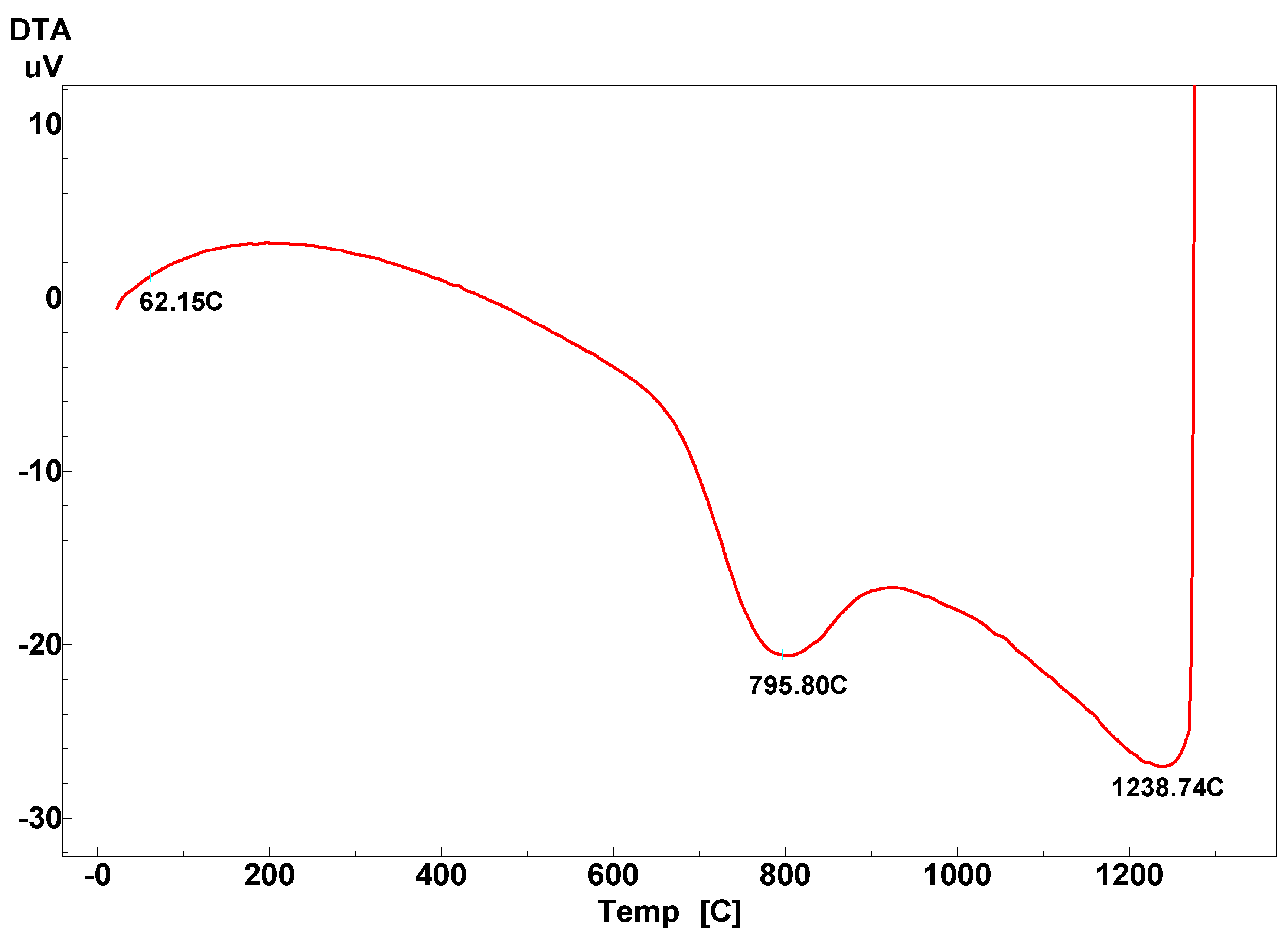
3.1. Clinker Characterization
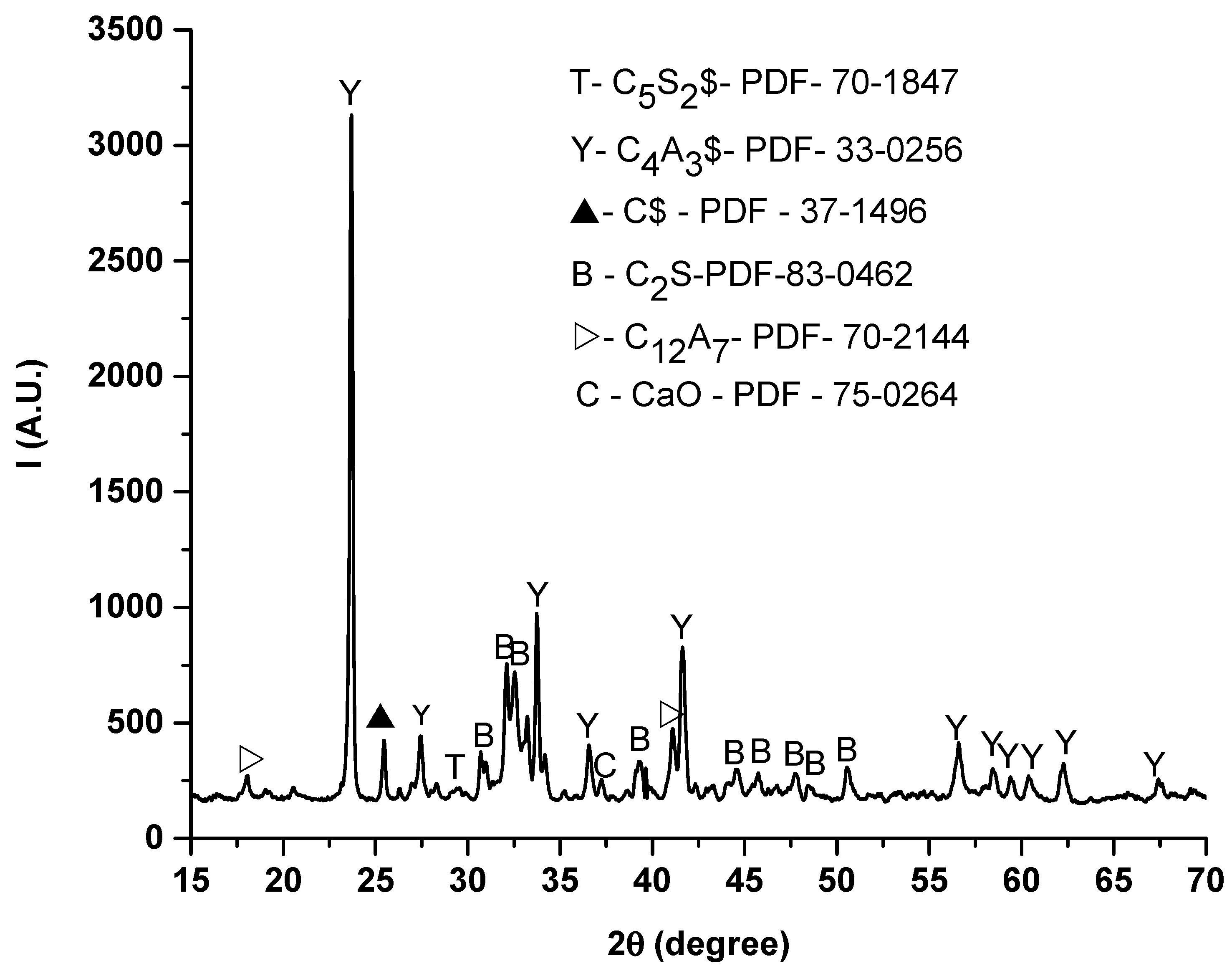
3.1.1. XRD Analysis
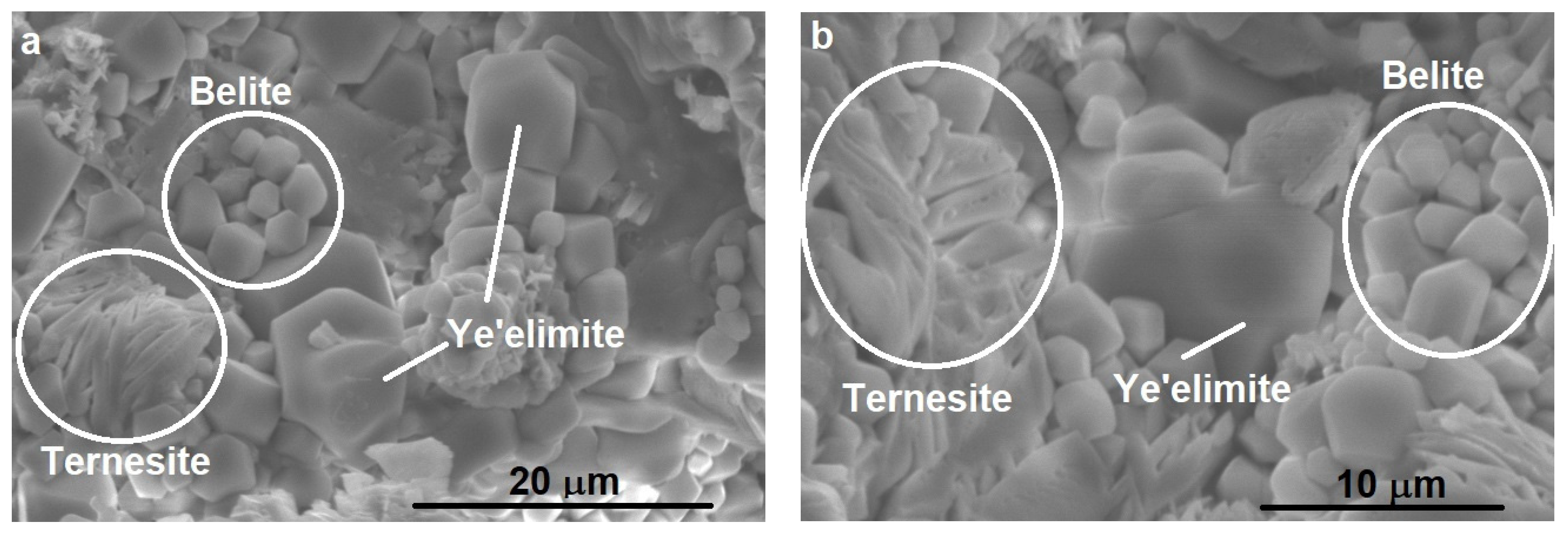
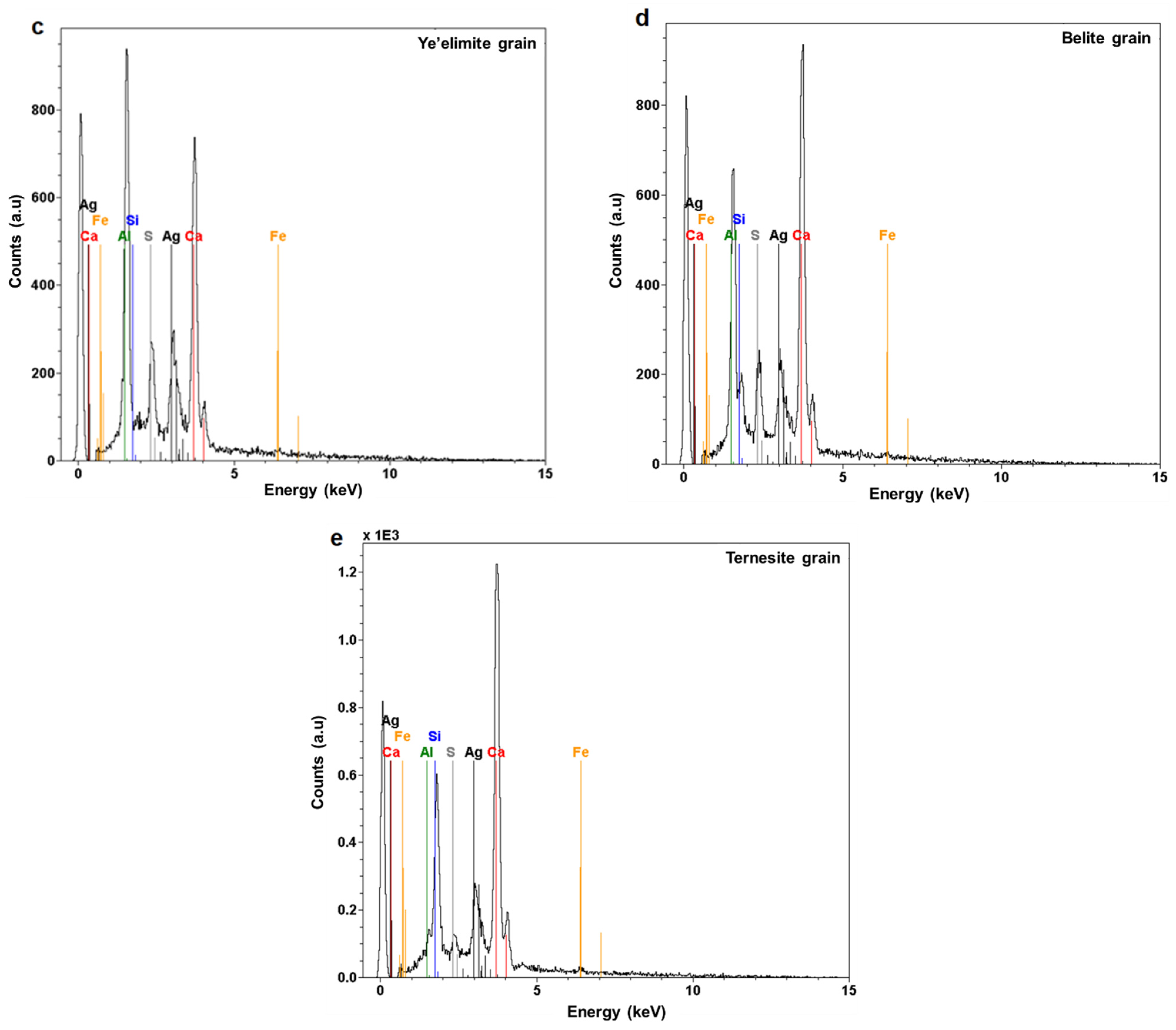
3.1.2. SEM-EDS Analysis
3.2. Cements Characterization
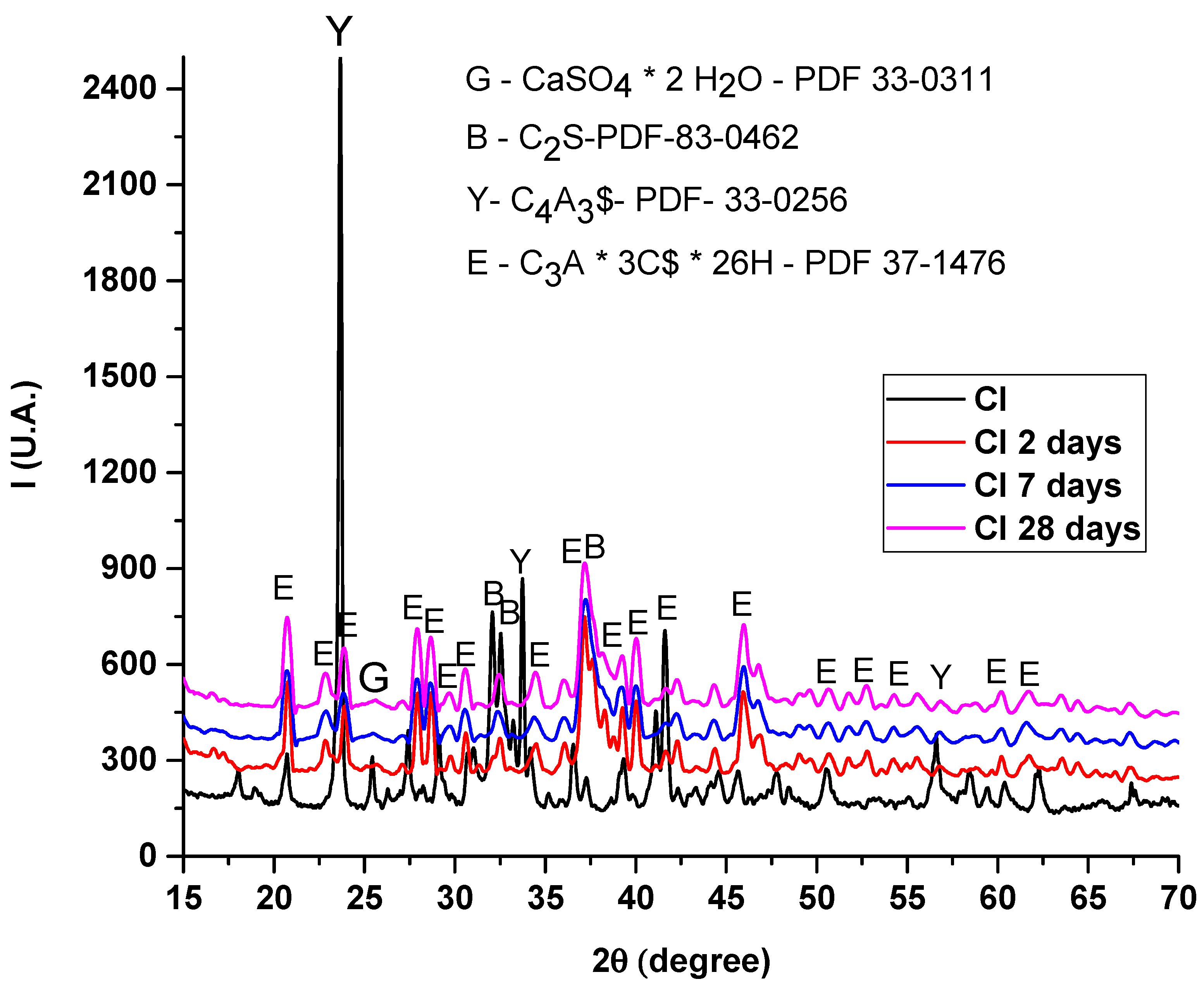
3.2.1. XRD Analysis
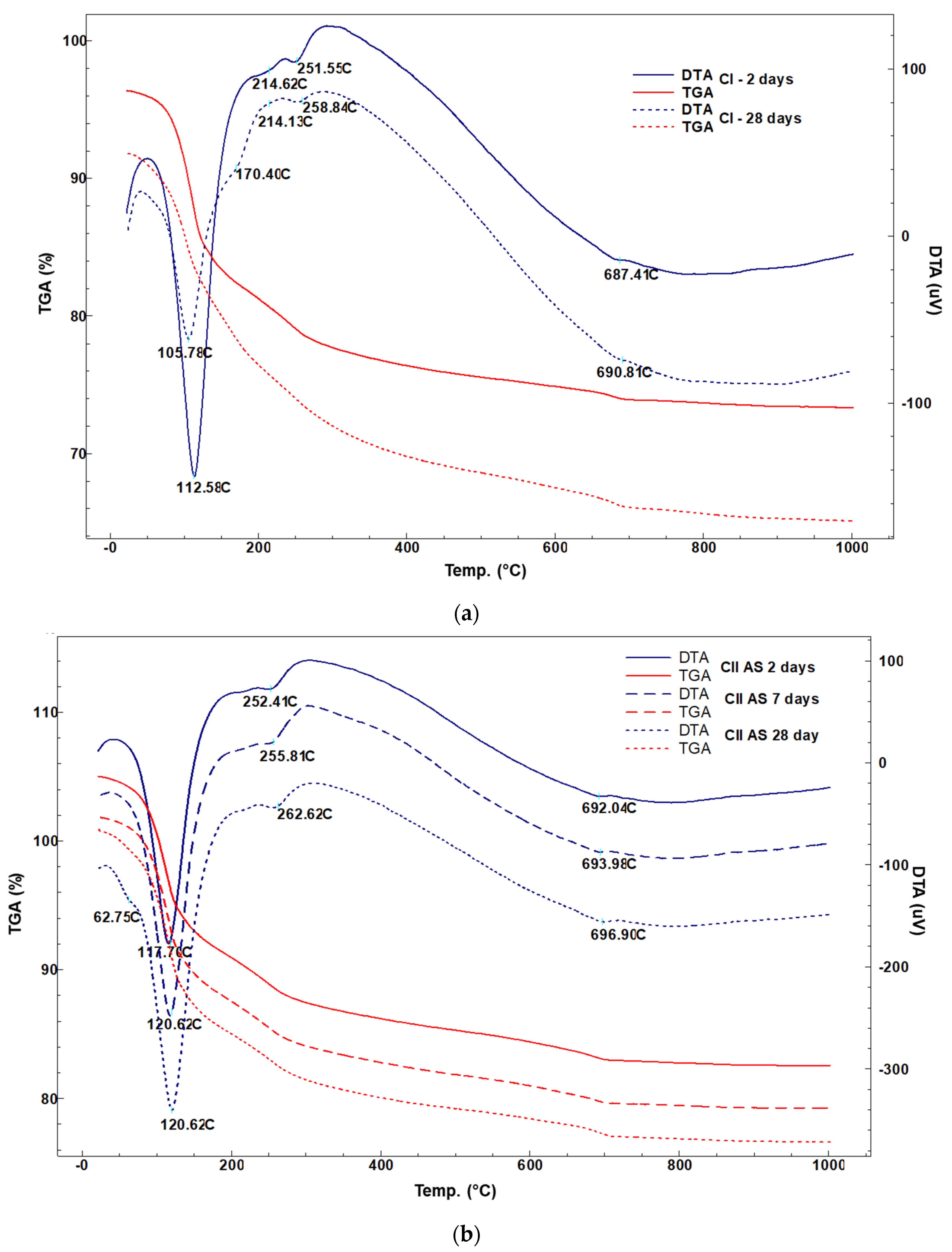
3.2.2. TG-DTG Analysis
3.2.3. Mechanical Strength
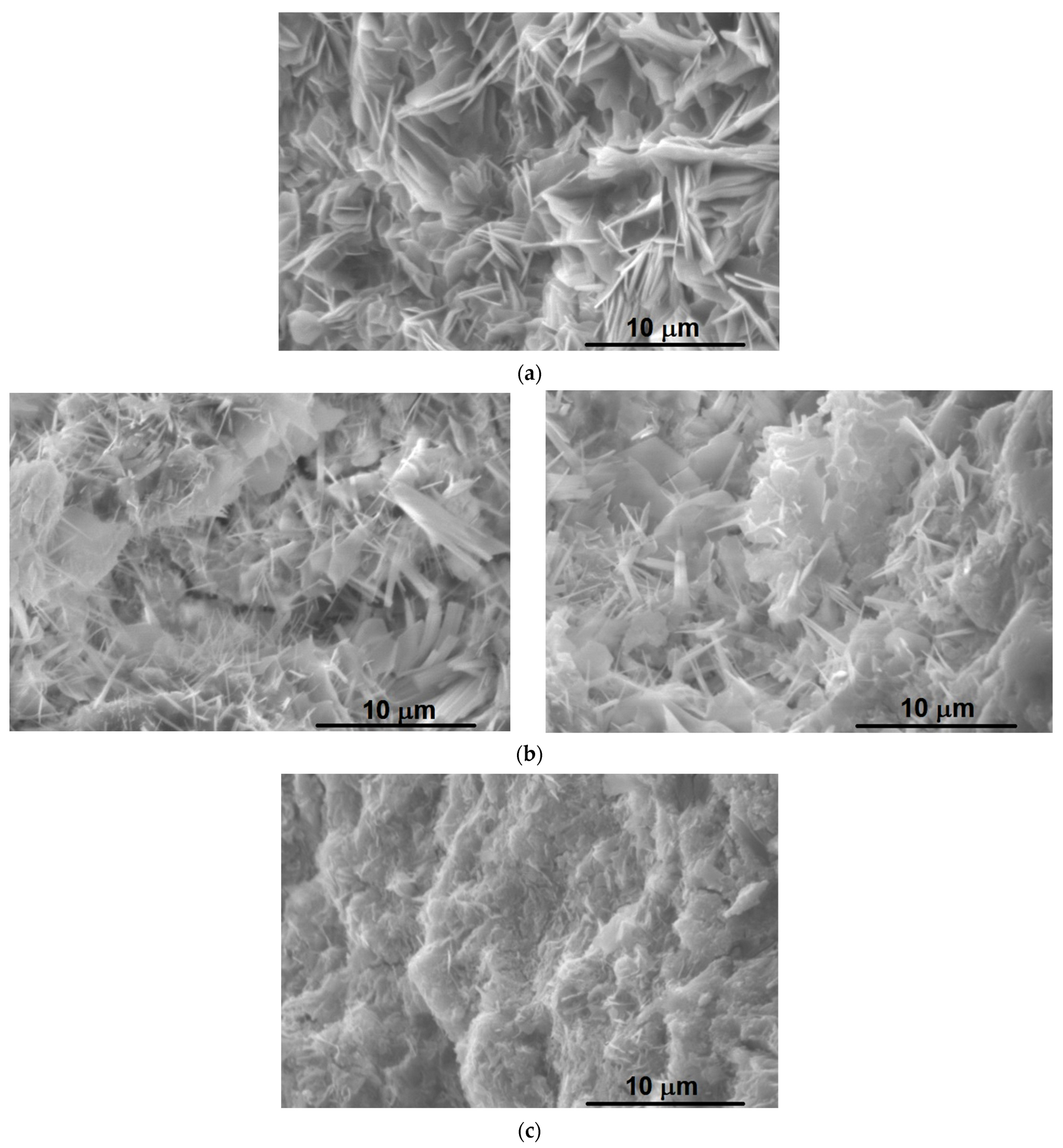
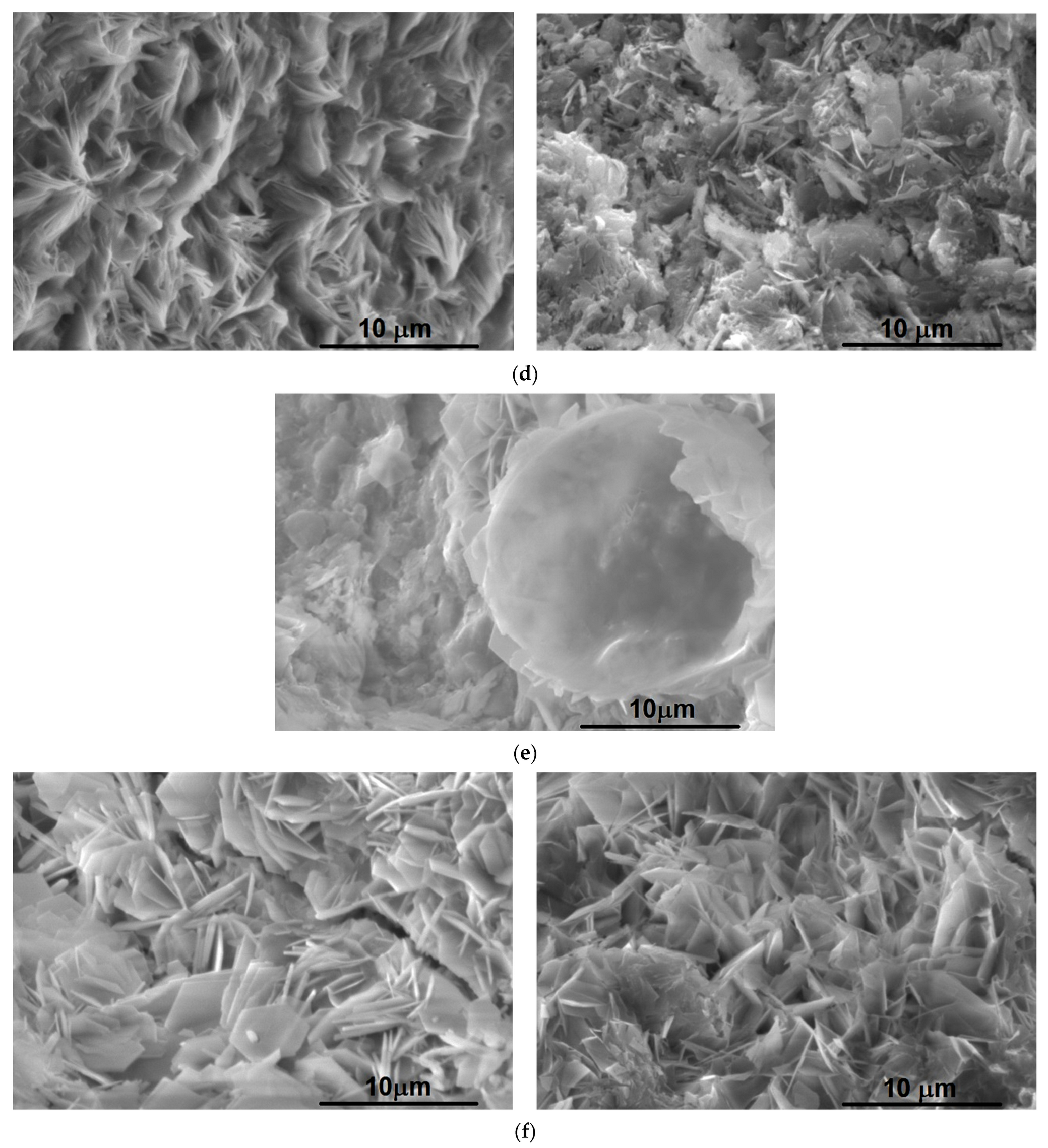
3.2.4. SEM-EDS Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zea-Garcia, J.D.; Santacruz, I.; Aranda, M.A.G.; De la Torre, A.G. Alite-belite-ye’elimite cements: Effect of dopants on the clinker phase composition and properties. Cem. Concr. Res. 2019, 115, 192–202. [Google Scholar] [CrossRef]
- Zea-Garcia, J.D.; De la Torre, A.G.; Aranda, M.A.G.; Santacruz, I. Processing and characterisation of standard and doped alite-belite-ye’elimite ecocement pastes and mortars. Cem. Concr. Res. 2020, 127, 105911. [Google Scholar] [CrossRef]
- Huang, Y.; Pei, Y.; Qian, J.; Gao, X.; Liang, J.; Duan, G.; Zhao, P.; Lu, L.; Cheng, X. Bauxite free iron rich calcium sulfoaluminate cement: Preparation, hydration and properties, Constr. Build. Mater. 2020, 249, 118774. [Google Scholar] [CrossRef]
- Gastaldi, D.; Paul, G.; Marchese, L.; Irico, S.; Boccaleri, E.; Mutke, S.; Buzzi, L.; Canonico, F. Hydration products in sulfoaluminate cements: Evaluation of amorphous phases by XRD/solid-state NMR. Cem. Concr. Res. 2016, 90, 162–173. [Google Scholar] [CrossRef]
- Alvarez-Pinazo, G.; Santacruz, I.; Leon-Reina, L.; Aranda, M.A.G.; De la Torre, A.G. Hydration reactions and mechanical strength developments of iron-rich sulfobelite eco-cements. Ind. Eng. Chem. Res. 2013, 52, 16606–16614. [Google Scholar] [CrossRef]
- Cuesta, A.; Alvarez-Pinazo, G.; Sanfelix, S.G.; Peral, I.; Aranda, M.A.G.; De la Torre, A.G. Hydration mechanisms of two polymorphs of synthetic ye’elimite. Cem. Concr. Res. 2014, 63, 127–136. [Google Scholar] [CrossRef] [Green Version]
- Winnefeld, F.; Barlag, S. Influence of calcium sulfate and calcium hydroxide on the hydration of calcium sulfoaluminate clinker. ZKG Int. 2009, 62, 42–53. [Google Scholar]
- Hargis, C.W.; Telesca, A.; Monteiro, P.J.M. Calcium sulfoaluminate (Ye’elimite) hydration in the presence of gypsum, calcite, and vaterite. Cem. Concr. Res. 2014, 65, 15–20. [Google Scholar] [CrossRef]
- Hargis, C.W.; Kirchheim, A.P.; Monteiro, P.J.M.; Gartner, E.M. Early age hydration of calcium sulfoaluminate (synthetic ye’elimite, C4A3S) in the presence of gypsum and varying amounts of calcium hydroxide. Cem. Concr. Res. 2013, 48, 105–115. [Google Scholar] [CrossRef]
- Pelletier-Chaignat, L.; Winnefeld, F.; Lothenbach, B.; Muller, C.J. Beneficial use of limestone filler with calcium sulphoaluminate cement. Constr. Build. Mater. 2012, 26, 619–627. [Google Scholar] [CrossRef]
- Glasser, F.P.; Zhang, L. Calculation of chemical water demand for hydration of calcium sulfoaluminate cement. In Proceedings of the 4th International Symposium on Cement and Concrete, Shanghai, China, 26–29 October 1998; pp. 38–44. [Google Scholar]
- Damtoft, J.S.; Lukasik, J.; Herfort, D.; Sorrentino, D.; Gartner, E.M. Sustainable development and climate change initiatives. Cem. Concr. Res. 2008, 38, 115–127. [Google Scholar] [CrossRef]
- Lin, R.S.; Wang, X.Y.; Lee, H.S.; Cho, H.K. Hydration and microstructure of cement pastes with calcined Hwangtoh clay. Materials 2019, 12, 458. [Google Scholar] [CrossRef] [Green Version]
- Gartner, E. Industrially interesting approaches to “low-CO2” cements. Cem. Concr. Res. 2004, 34, 1489–1498. [Google Scholar] [CrossRef]
- Berrio, A.; Rodriguez, C.; Tobón, J.I. Effect of Al2O3/SiO2 ratio on ye’elimite production on CSA cement. Constr. Build. Mater. 2018, 168, 512–521. [Google Scholar] [CrossRef]
- Glasser, F.P.; Zhang, L. High-performance cement matrices based on calcium sulfoaluminate–belite compositions. Cem. Concr. Res. 2001, 31, 1881–1886. [Google Scholar] [CrossRef]
- Galluccio, S.; Beirau, T.; Pöllmann, H. Maximization of the reuse of industrial residues for the production of eco-friendly CSA-belite clinker. Constr. Build. Mater. 2019, 208, 250–257. [Google Scholar] [CrossRef]
- EN 197-1:2011. Cement, Part 1: Composition, Specifications and Conformity Criteria for Common Cements; EN: Brussels, Belgium, 2011. [Google Scholar]
- Bullerjahn, F.; Zajac, M.; Haha, M.B. CSA raw mix design: Effect on clinker formation and reactivity. Mater. Struct. 2015, 48, 3895–3911. [Google Scholar] [CrossRef]
- Perez-Bravo, R.; Alvarez-Pinazo, G.; Compana, J.M.; Santacruz, I.; Losilla, E.R.; Bruque, S.; De la Torre, A.G. Alite sulfoaluminate clinker: Rietveld mineralogical and SEM-EDX analysis. Adv. Cem. Res. 2014, 26, 10–20. [Google Scholar] [CrossRef]
- Ma, S.H.; Snellings, R.; Li, X.R.; Shen, X.D.; Scrivener, K.L. Alite-ye’elimite cement: Synthesis and mineralogical analysis. Cem. Concr. Res. 2013, 45, 15–20. [Google Scholar] [CrossRef]
- Bescher, E.; Kim, J. Belitic Calcium Sulfoaluminate Cement: History, Chemistry, Performance, and Use in the United States. In Proceedings of the 1st International Conference on Innovation in Low-Carbon Cement and Concrete Technology, London, UK, 23–26 June 2019. [Google Scholar]
- Shen, Y.; Li, X.; Chen, X.; Zhang, W.; Yang, D. Synthesis and calorimetric study of hydration behavior of sulfate-rich belite sulfoaluminate cements with different phase compositions. J. Therm. Anal. Calorim. 2018, 133, 1281–1289. [Google Scholar] [CrossRef]
- Winnefeld, F.; Martin, L.H.J.; Muller, C.J.; Lothenbach, B. Using gypsum to control hydration kinetics of CSA cements. Constr. Build. Mater. 2017, 155, 154–163. [Google Scholar] [CrossRef]
- Cody, A.M.; Lee, H.; Cody, R.D.; Spry, P.G. The effects of chemical environment on the nucleation, growth, and stability of ettringite Ca3Al(OH)6.2(SO4)3·26H2O. Cem. Concr. Res. 2004, 34, 869–881. [Google Scholar] [CrossRef]
| Chemical | Raw Materials | Clinker | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Limestone [wt %] | Gypsum [wt %] | Clay [wt %] | Slag [wt %] | Fly ash [wt %] | Bauxite [wt %] | [wt %] | |||||
| CaCO3 | 99.5 | CaSO4۔2H2O | 83.53 | Chemical | Mineralogical | ||||||
| CaO | 97.2 | 34.5 | 3.33 | 3.8 | 41.8 | 4.5 | 0.3 | C4A3$ | 45 | ||
| Na2O | 0.0 | 0.0 | 0.9 | 1.0 | 0.6 | 0.5 | 0.1 | C5S2$ | 20 | ||
| MgO | 0.2 | 0.4 | 2 | 2.3 | 7.1 | 2.0 | 0.4 | C2S | 20 | ||
| Al2O3 | 0.9 | 0.5 | 18 | 20.6 | 7.7 | 25.5 | 89.0 | C4AF | 8 | ||
| SiO2 | 1.6 | 2.1 | 53 | 60.5 | 41.4 | 55.7 | 5.7 | C$ | 7 | ||
| SO3 | 0.0 | 62.1 | 0.2 | 0.2 | 0.6 | 0.9 | 1.2 | ||||
| K2O | 0.1 | 0.1 | 3.13 | 3.6 | 0.6 | 2.6 | 0.8 | ||||
| Fe2O3 | 0.0 | 0.3 | 7 | 8.0 | 0.2 | 8.2 | 2.5 | ||||
| Sample | CI | CIIAS | CIIBM | CP | |
|---|---|---|---|---|---|
| SSP Blaine (cm2/g) | 2580 | 3055 | 2676 | 4600 | |
| Compressive strength (MPa) | 2 days | 31.6 | 33 | 22.9 | 37.2 |
| 7 days | 31.5 | 39.5 | 27.2 | - | |
| 28 days | 60.9 | 42 | 43 | 65 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marin, B.-C.; Voicu, G.; Stoleriu, S. Synthesis of High-Performance CSA Cements as Low Carbon OPC Alternative. Materials 2021, 14, 7057. https://doi.org/10.3390/ma14227057
Marin B-C, Voicu G, Stoleriu S. Synthesis of High-Performance CSA Cements as Low Carbon OPC Alternative. Materials. 2021; 14(22):7057. https://doi.org/10.3390/ma14227057
Chicago/Turabian StyleMarin, Bogdan-Catalin, Georgeta Voicu, and Stefania Stoleriu. 2021. "Synthesis of High-Performance CSA Cements as Low Carbon OPC Alternative" Materials 14, no. 22: 7057. https://doi.org/10.3390/ma14227057
APA StyleMarin, B.-C., Voicu, G., & Stoleriu, S. (2021). Synthesis of High-Performance CSA Cements as Low Carbon OPC Alternative. Materials, 14(22), 7057. https://doi.org/10.3390/ma14227057





