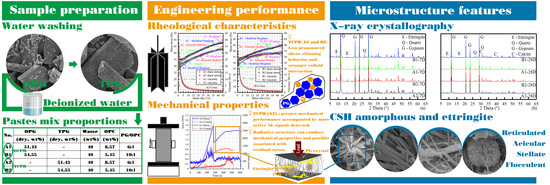
In Situ Remediation of Phosphogypsum with Water-Washing Pre-Treatment Using Cemented Paste Backfill: Rheology Behavior and Damage Evolution
Abstract
:1. Introduction
2. Sample Preparation and Test Methods
2.1. Materials
2.2. Mix Proportions
2.3. Test Methods
2.3.1. Characterization Experiments
2.3.2. Experiments of Rheological Characteristics
2.3.3. Experiments of Mechanical Properties and AE
3. Results and Discussion
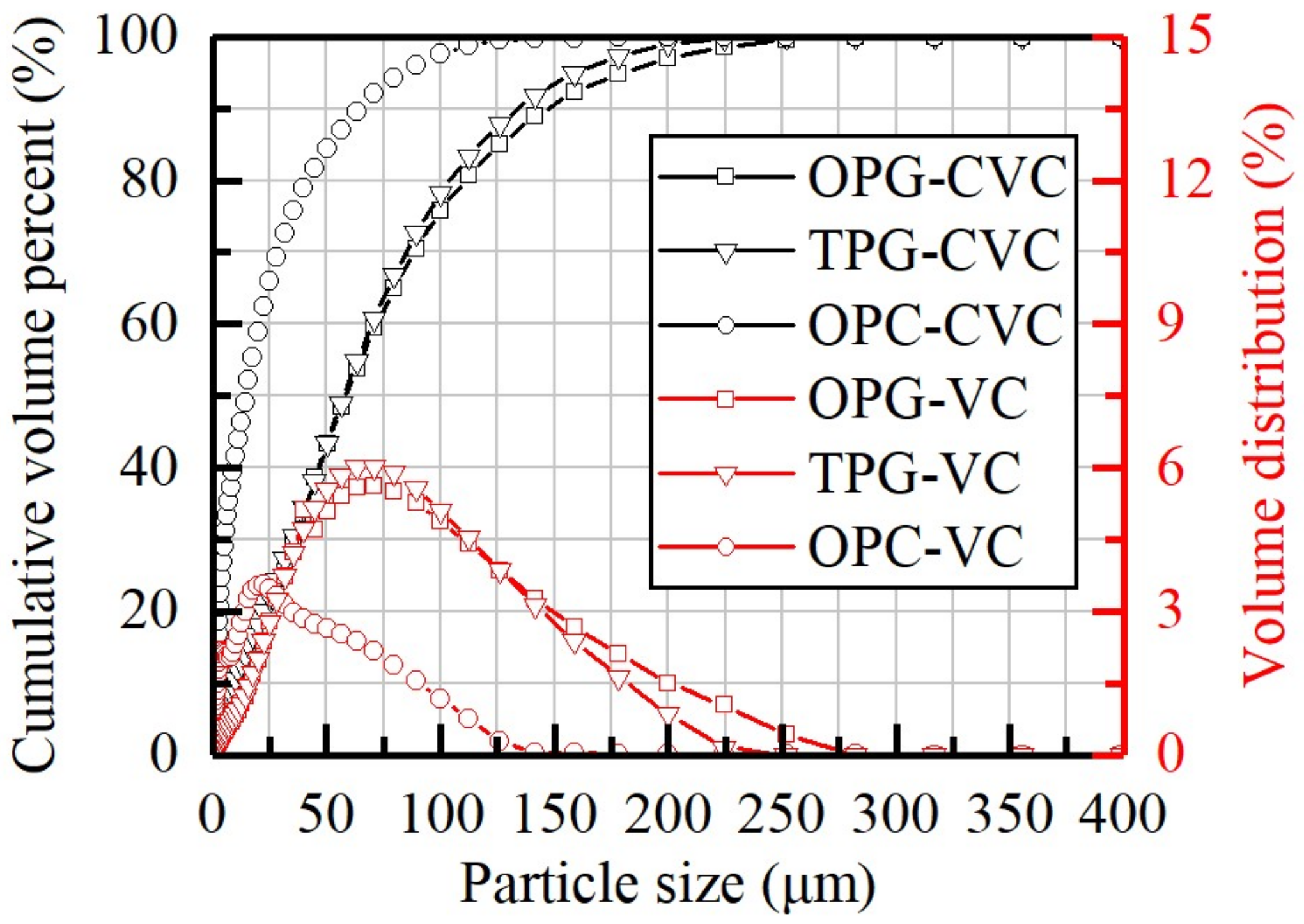
3.1. Raw Materials Characterization
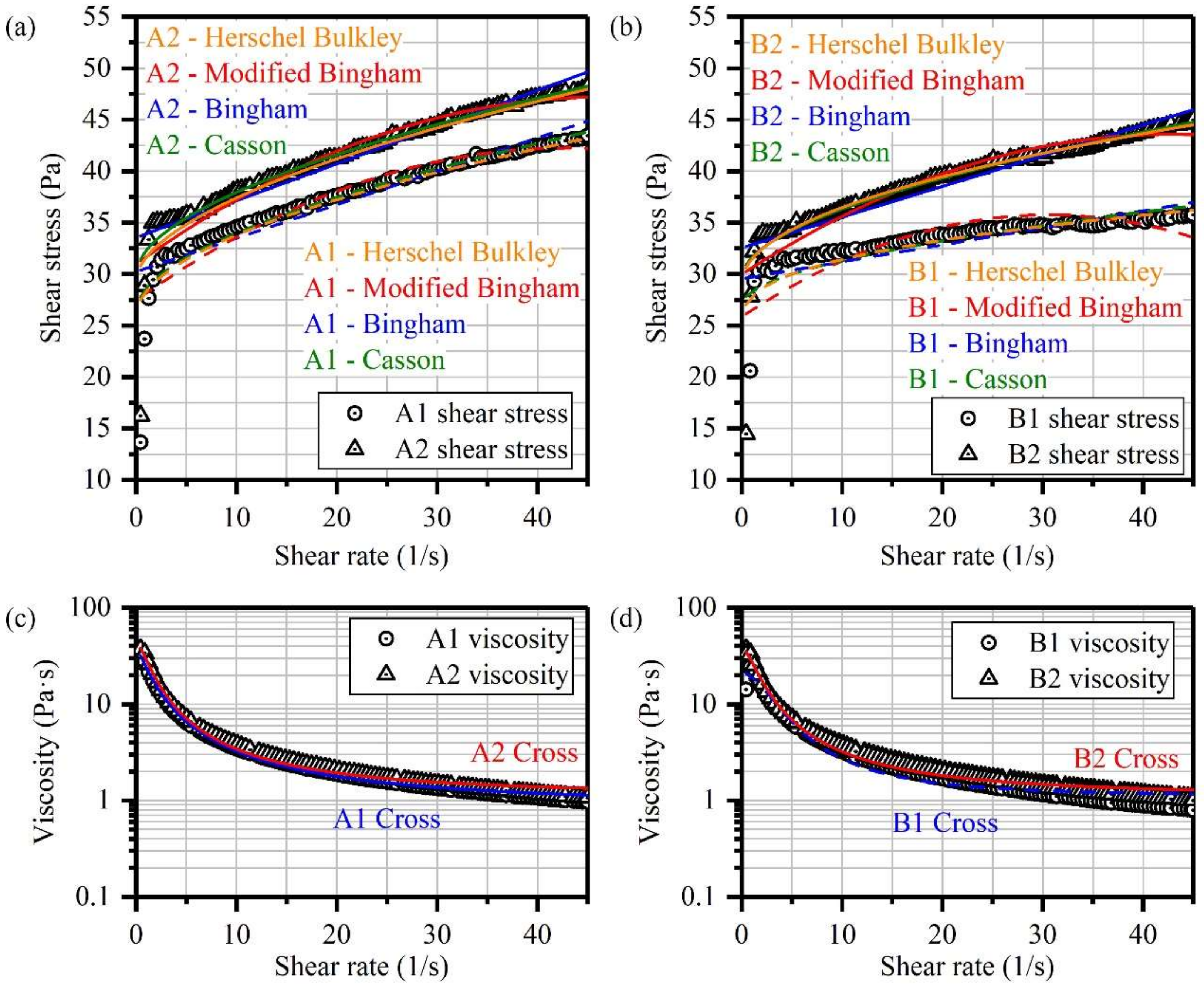
3.2. Rheological Characterization of OCPB and TCPB
3.3. Mechanical Performance of OCPB and TCPB
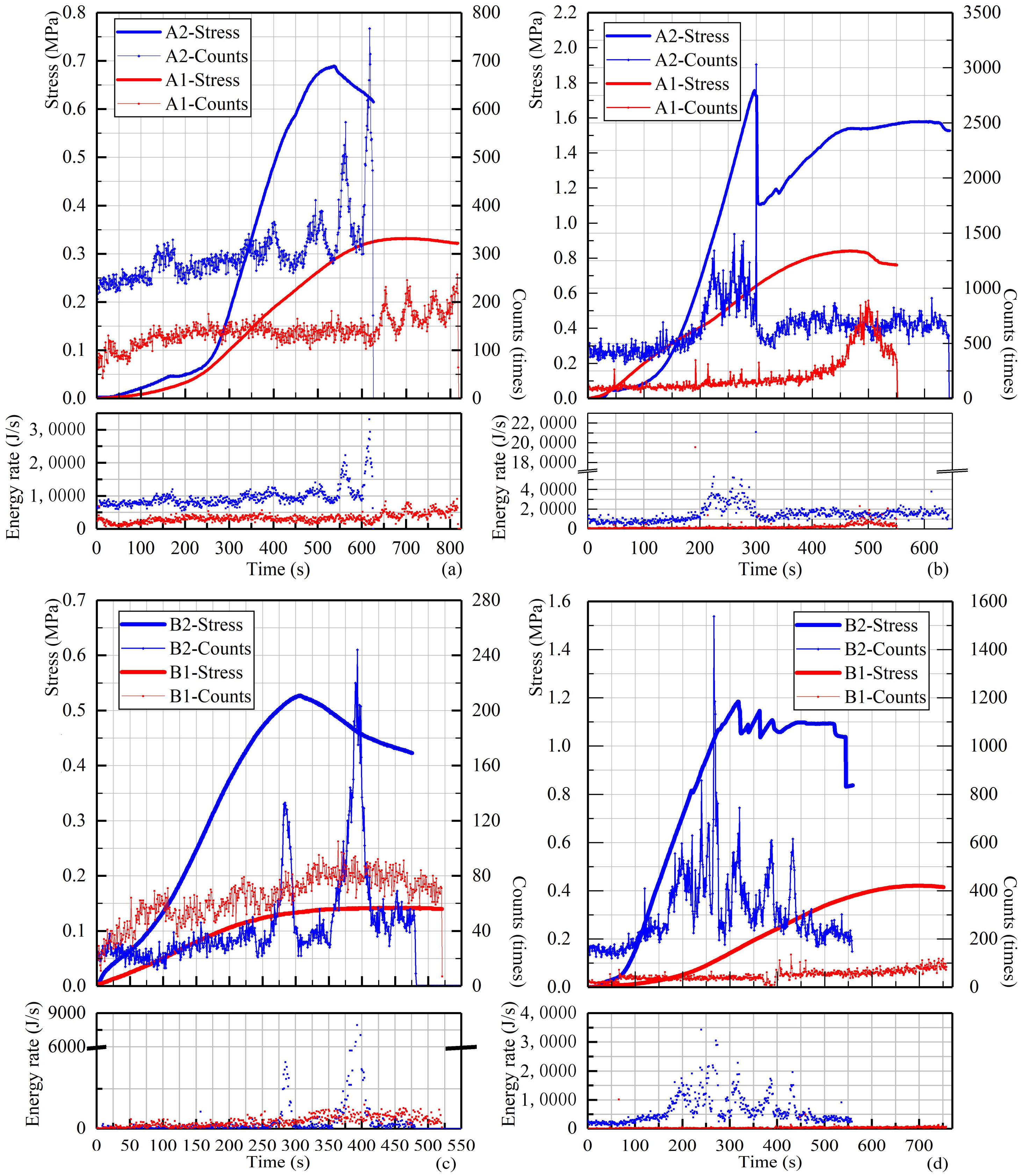
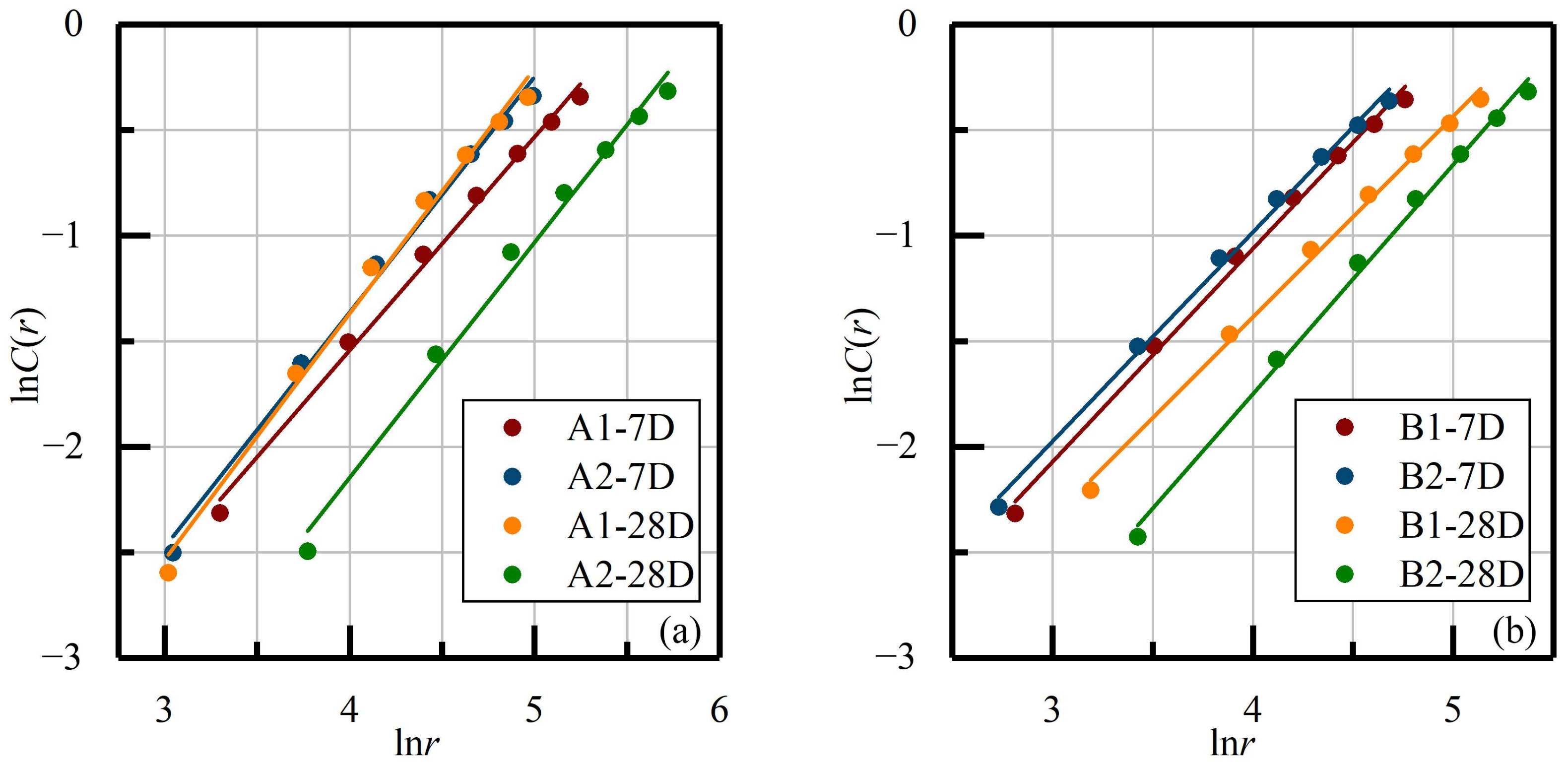
3.3.1. Internal Damage Fractal Dimension (D) Characteristics
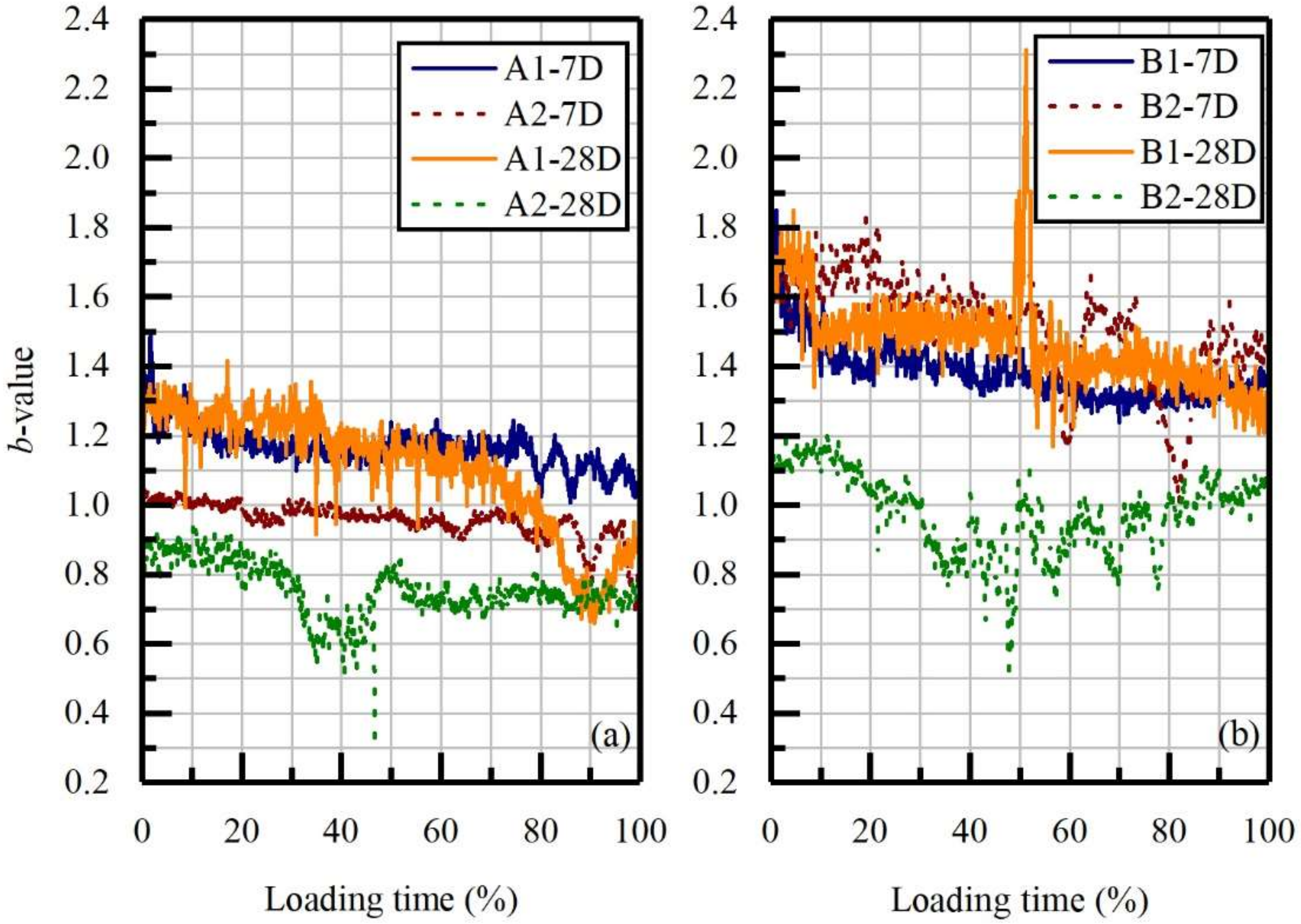
3.3.2. Loading Time Sequence Investigation: DN-Values and b-Values Analyses
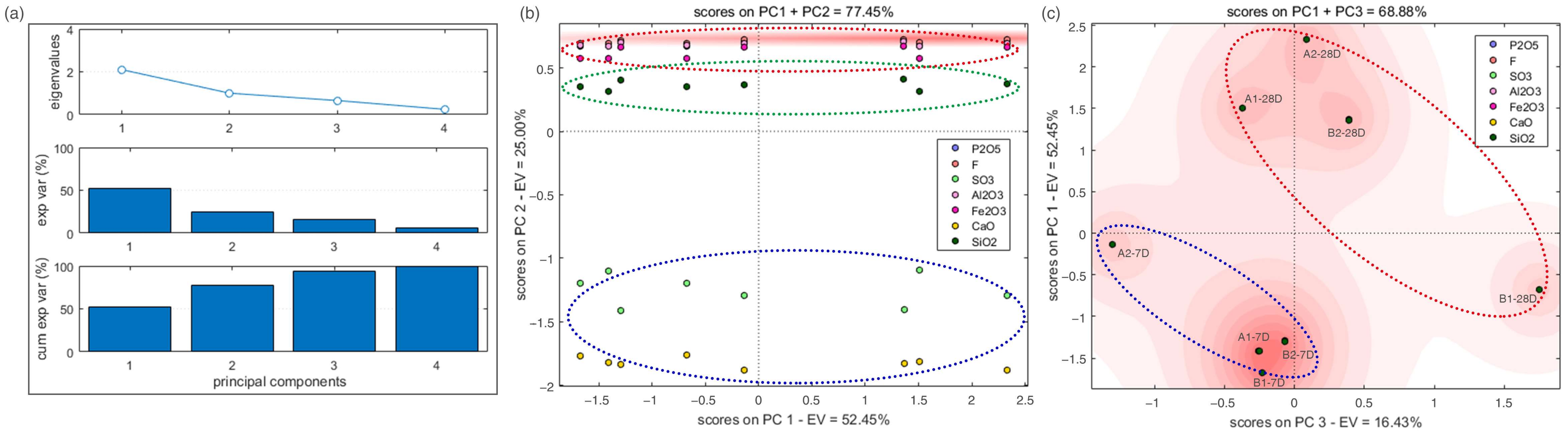
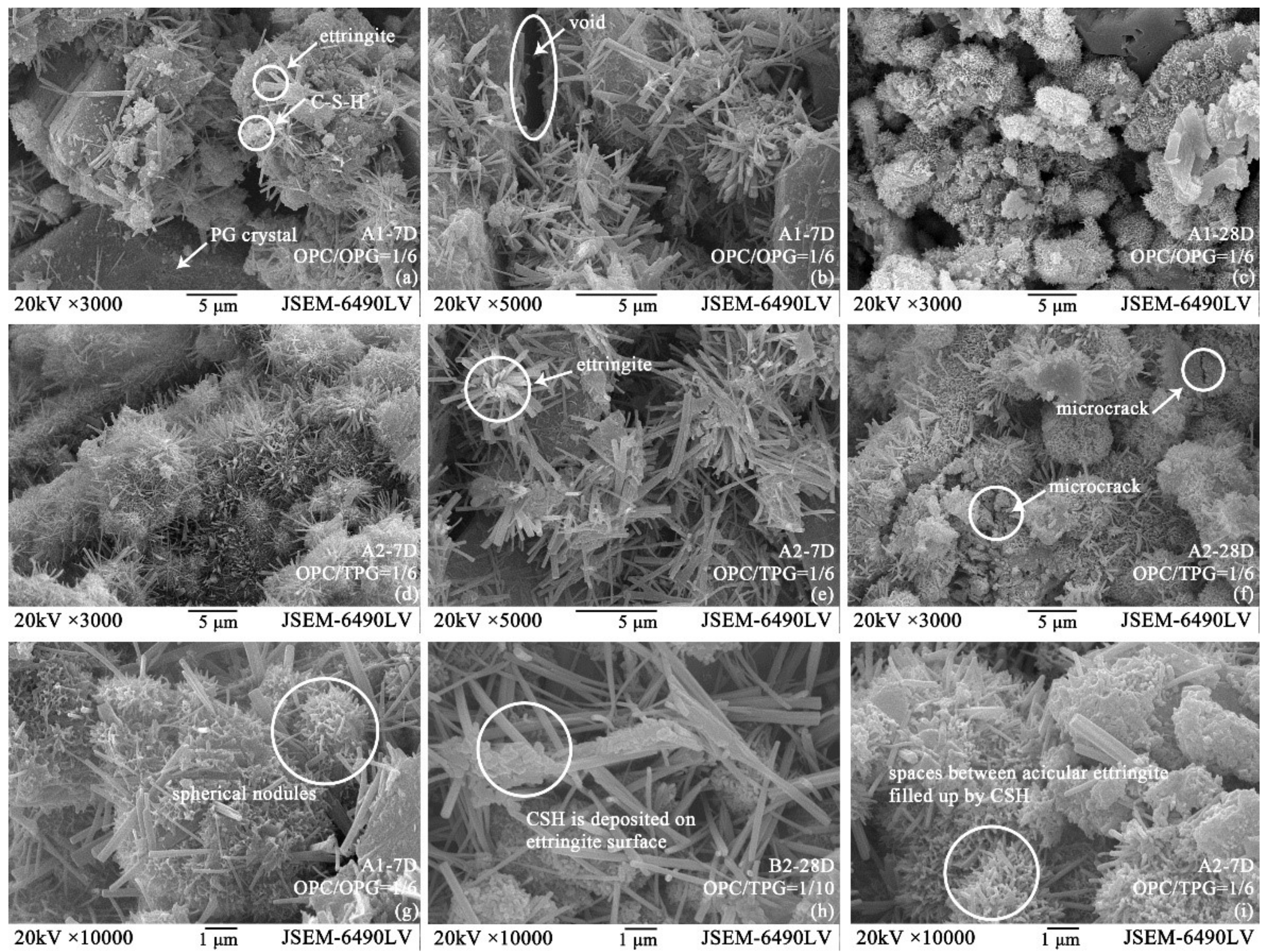
3.4. Microstructure Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AE | Acoustic emission |
| CPB | Cemented paste backfill |
| CSH | Calcium silicate hydrates |
| DW | Deionized water |
| OCPB | Ordinary phosphogypsum based cemented paste backfill |
| OPC | Ordinary Portland cement |
| OPG | Ordinary phosphogypsum |
| PCA | Principal component analysis |
| PSD | Particle size distribution |
| SEM | Scanning electron microscopy |
| TCPB | Water-washing treated phosphogypsum based cemented paste backfill |
| TPG | Water-washing pre-treated phosphogypsum |
| UCS | Uniaxial compression strength |
| XRD | X-ray powder diffraction |
| XRF | X-ray fluorescence |
References
- Chen, Q.; Zhang, Q.; Qi, C.; Fourie, A.; Xiao, C. Recycling phosphogypsum and construction demolition waste for cemented paste backfill and its environmental impact. J. Clean. Prod. 2018, 186, 418–429. [Google Scholar] [CrossRef]
- Fatila, E.M.; Pink, M.; Twum, E.B.; Karty, J.A.; Flood, A.H. Phosphate-phosphate oligomerization drives higher order co-assemblies with stacks of cyanostar macrocycles. Chem. Sci. 2018, 9, 2863–2872. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geissler, B.; Mew, M.C.; Weber, O.; Steiner, G. Efficiency performance of the world’s leading corporations in phosphate rock mining. Resour. Conserv. Recycl. 2015, 105, 246–258. [Google Scholar] [CrossRef]
- Dong, L.; Shu, W.; Li, X.; Zhou, Z.; Gong, F.; Liu, X. Quantitative Evaluation and Case Study of Risk Degree for Underground Goafs with Multiple Indexes considering Uncertain Factors in Mines. Geofluids 2017, 2017, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Zhao, B.; Guo, G.; Zha, J.; Bi, J. The influence of an abandoned goaf on surface subsidence in an adjacent working coal face: A prediction method. Bull. Eng. Geol. Environ. 2018, 77, 305–315. [Google Scholar] [CrossRef]
- Baryakh, A.A.; Telegina, E.A.; Samodelkina, N.A.; Devyatkov, S.Y. Prediction of the intensive surface subsidences in mining potash series. J. Min. Sci. 2005. [Google Scholar] [CrossRef]
- Gorman, M.R.; Dzombak, D.A. A review of sustainable mining and resource management: Transitioning from the life cycle of the mine to the life cycle of the mineral. Resour. Conserv. Recycl. 2018, 137, 281–291. [Google Scholar] [CrossRef]
- Geissler, B.; Hermann, L.; Mew, M.C.; Steiner, G. Striving toward a circular economy for phosphorus: The role of phosphate rock mining. Minerals 2018, 8, 395. [Google Scholar] [CrossRef] [Green Version]
- Pinto, P.X.; Al-Abed, S.R.; Holder, C.; Reisman, D.J. Evaluation of metal partitioning and mobility in a sulfidic mine tailing pile under oxic and anoxic conditions. J. Environ. Manag. 2014, 140, 135–144. [Google Scholar] [CrossRef]
- Chen, X.; Shi, X.; Zhou, J.; Chen, Q.; Yang, C. Feasibility of recycling ultrafine leaching residue by backfill: Experimental and CFD approaches. Minerals 2017, 7, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Rashad, A.M. Phosphogypsum as a construction material. J. Clean. Prod. 2017, 166, 732–743. [Google Scholar] [CrossRef]
- Borges, R.C.; Ribeiro, F.C.A.; Da Costa Lauria, D.; Bernedo, A.V.B. Influence of phosphogypsum stacks on the distribution of natural radionuclides in surface and subsurface waters in the city of Imbituba, SC, Brazil. Water. Air. Soil Pollut. 2013, 224. [Google Scholar] [CrossRef]
- Xu, J.; Fan, L.; Xie, Y.; Wu, G. Recycling-equilibrium strategy for phosphogypsum pollution control in phosphate fertilizer plants. J. Clean. Prod. 2019, 215, 175–197. [Google Scholar] [CrossRef]
- Abril, J.M.; García-Tenorio, R.; Periáñez, R.; Enamorado, S.M.; Andreu, L.; Delgado, A. Occupational dosimetric assessment (inhalation pathway) from the application of phosphogypsum in agriculture in South West Spain. J. Environ. Radioact. 2009, 100, 29–34. [Google Scholar] [CrossRef]
- Soares, J.R.; Cantarella, H.; de Campos Menegale, M.L. Ammonia volatilization losses from surface-applied urea with urease and nitrification inhibitors. Soil Biol. Biochem. 2012, 52, 82–89. [Google Scholar] [CrossRef]
- Cánovas, C.R.; Chapron, S.; Arrachart, G.; Pellet-Rostaing, S. Leaching of rare earth elements (REEs) and impurities from phosphogypsum: A preliminary insight for further recovery of critical raw materials. J. Clean. Prod. 2019, 219, 225–235. [Google Scholar] [CrossRef]
- Deǧirmenci, N. Utilization of phosphogypsum as raw and calcined material in manufacturing of building products. Constr. Build. Mater. 2008, 22, 1857–1862. [Google Scholar] [CrossRef]
- Yang, L.; Zhang, Y.; Yan, Y. Utilization of original phosphogypsum as raw material for the preparation of self-leveling mortar. J. Clean. Prod. 2016, 127, 204–213. [Google Scholar] [CrossRef]
- Bagade, M.A.; Satone, S.R. An experimental investigation of partial replacement of cement by various percentage of Phosphogypsum in cement concrete. Int. J. Eng. Res. Appl. 2012, 2, 785–787. [Google Scholar]
- Liu, S.; Wang, L.; Yu, B. Effect of modified phosphogypsum on the hydration properties of the phosphogypsum-based supersulfated cement. Constr. Build. Mater. 2019, 214, 9–16. [Google Scholar] [CrossRef]
- Tian, T.; Yan, Y.; Hu, Z.; Xu, Y.; Chen, Y.; Shi, J. Utilization of original phosphogypsum for the preparation of foam concrete. Constr. Build. Mater. 2016, 115, 143–152. [Google Scholar] [CrossRef]
- Li, X.; Du, J.; Gao, L.; He, S.; Gan, L.; Sun, C.; Shi, Y. Immobilization of phosphogypsum for cemented paste backfill and its environmental effect. J. Clean. Prod. 2017, 156, 137–146. [Google Scholar] [CrossRef]
- Zhang, Q.L.; Li, Y.T.; Chen, Q.S.; Liu, Y.K.; Feng, Y.; Wang, D. Effects of temperatures and pH values on rheological properties of cemented paste backfill. J. Cent. South Univ. 2021, 28, 1707–1723. [Google Scholar] [CrossRef]
- Fall, M.; Adrien, D.; Célestin, J.C.; Pokharel, M.; Touré, M. Saturated hydraulic conductivity of cemented paste backfill. Miner. Eng. 2009, 22, 1307–1317. [Google Scholar] [CrossRef]
- Jiang, H.; Fall, M.; Cui, L. Freezing behaviour of cemented paste backfill material in column experiments. Constr. Build. Mater. 2017, 147, 837–846. [Google Scholar] [CrossRef]
- Li, X.; Zhou, S.; Zhou, Y.; Min, C.; Cao, Z.; Du, J.; Luo, L.; Shi, Y. Durability Evaluation of Phosphogypsum-Based Cemented Backfill Through Drying-Wetting Cycles. Minerals 2019, 9, 321. [Google Scholar] [CrossRef] [Green Version]
- Nizevičienė, D.; Vaičiukynienė, D.; Vaitkevičius, V.; Rudžionis, Ž. Effects of waste fluid catalytic cracking on the properties of semi-hydrate phosphogypsum. J. Clean. Prod. 2016, 137, 150–156. [Google Scholar] [CrossRef]
- Potgieter, J.H.; Potgieter, S.S.; McCrindle, R.I.; Strydom, C.A. An investigation into the effect of various chemical and physical treatments of a South African phosphogypsum to render it suitable as a set retarder for cement. Cem. Concr. Res. 2003, 33, 1223–1227. [Google Scholar] [CrossRef]
- Huang, Y.; Qian, J.; Liang, J.; Liu, N.; Li, F.; Shen, Y. Characterization and calorimetric study of early-age hydration behaviors of synthetic ye’elimite doped with the impurities in phosphogypsum. J. Therm. Anal. Calorim. 2016, 123, 1545–1553. [Google Scholar] [CrossRef]
- Jiang, G.; Wu, A.; Wang, Y.; Lan, W. Low cost and high efficiency utilization of hemihydrate phosphogypsum: Used as binder to prepare filling material. Constr. Build. Mater. 2018, 167, 263–270. [Google Scholar] [CrossRef]
- Liu, S.; Ouyang, J.; Ren, J. Mechanism of calcination modification of phosphogypsum and its effect on the hydration properties of phosphogypsum-based supersulfated cement. Constr. Build. Mater. 2020, 243, 118226. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, Q.; Chen, Q.; Qi, C.; Su, Z.; Huang, Z. Utilisation of water-washing pre-treated phosphogypsum for cemented paste backfill. Minerals 2019, 9, 175. [Google Scholar] [CrossRef] [Green Version]
- Monat, L.; Chaudhury, S.; Nir, O. Enhancing the Sustainability of Phosphogypsum Recycling by Integrating Electrodialysis with Bipolar Membranes. ACS Sustain. Chem. Eng. 2020, 8, 2490–2497. [Google Scholar] [CrossRef]
- Yan, D.; Peng, Z.; Yu, L.; Sun, Y.; Yong, R.; Karstensen, K.H. Characterization of heavy metals and PCDD/Fs from water-washing pretreatment and a cement kiln co-processing municipal solid waste incinerator fly ash. Waste Manag. 2018, 76, 106–116. [Google Scholar] [CrossRef] [PubMed]
- Mao, Y.; Wu, H.; Wang, W.; Jia, M.; Che, X. Pretreatment of municipal solid waste incineration fly ash and preparation of solid waste source sulphoaluminate cementitious material. J. Hazard. Mater. 2020, 385, 121580. [Google Scholar] [CrossRef]
- Rosales, J.; Pérez, S.M.; Cabrera, M.; Gázquez, M.J.; Bolivar, J.P.; de Brito, J.; Agrela, F. Treated phosphogypsum as an alternative set regulator and mineral addition in cement production. J. Clean. Prod. 2020, 244, 118752. [Google Scholar] [CrossRef]
- Ohno, K.; Ohtsu, M. Crack classification in concrete based on acoustic emission. Constr. Build. Mater. 2010, 24, 2339–2346. [Google Scholar] [CrossRef]
- Jiang, Y.; Xu, F.; Xu, B.; Jia, M.; Hu, J.; Gallego, A. Simulation and experimental investigation on the AE tomography to improve AE source location in the concrete structure. Math. Probl. Eng. 2014, 2014, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Alver, N.; Tanarslan, H.M.; Sülün, Ö.Y.; Ercan, E.; Karcili, M.; Selman, E.; Ohno, K. Effect of CFRP-spacing on fracture mechanism of CFRP-strengthened reinforced concrete beam identified by AE-SiGMA. Constr. Build. Mater. 2014, 67, 146–156. [Google Scholar] [CrossRef]
- Ingraham, M.D.; Issen, K.A.; Holcomb, D.J. Use of acoustic emissions to investigate localization in high-porosity sandstone subjected to true triaxial stresses. Acta Geotech. 2013, 8, 645–663. [Google Scholar] [CrossRef]
- Dong, L.; Shu, W.; Sun, D.; Li, X.; Zhang, L. Pre-Alarm System Based on Real-Time Monitoring and Numerical Simulation Using Internet of Things and Cloud Computing for Tailings Dam in Mines. IEEE Access 2017, 5, 21080–21089. [Google Scholar] [CrossRef]
- Li, J.; Huang, Y.; Qi, W.; Kong, G.; Song, T. Loose gangues backfill body’s acoustic emissions rules during compaction test: Based on solid backfill mining. C.-Comput. Model. Eng. Sci. 2018, 115, 85–103. [Google Scholar] [CrossRef]
- Qi, T.; Feng, G. Resistivity and AE response characteristics in the failure process of CGB under uniaxial loading. Adv. Mater. Sci. Eng. 2017, 2017, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, A.; Sluiter, J.; Templeton, D. Preparation of Samples for Compositional Analysis. Lab. Anal. Proced. (LAP) 2008, 1617, 65–71. [Google Scholar]
- Zhou, S.; Li, X.; Zhou, Y.; Min, C.; Shi, Y. Effect of phosphorus on the properties of phosphogypsum-based cemented backfill. J. Hazard. Mater. 2020, 399, 122993. [Google Scholar] [CrossRef]
- Liu, G.; Li, L.; Yao, M.; Landry, D.; Malek, F.; Yang, X.; Guo, L. An investigation of the uniaxial compressive strength of a cemented hydraulic backfill made of alluvial sand. Minerals 2017, 7, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Ke, X.; Hou, H.; Zhou, M.; Wang, Y.; Zhou, X. Effect of particle gradation on properties of fresh and hardened cemented paste backfill. Constr. Build. Mater. 2015, 96, 378–382. [Google Scholar] [CrossRef]
- Gong, C.; Li, C.H.; Zhao, K. Study on fractal dimension of acoustic emission during cemented backfill micro crack evolution process. Appl. Mech. Mater. 2014, 556–562, 236–240. [Google Scholar] [CrossRef]
- Hot, J.; Bessaies-Bey, H.; Brumaud, C.; Duc, M.; Castella, C.; Roussel, N. Adsorbing polymers and viscosity of cement pastes. Cem. Concr. Res. 2014, 63, 12–19. [Google Scholar] [CrossRef]
- Yahia, A.; Khayat, K.H. Applicability of rheological models to high-performance grouts containing supplementary cementitious materials and viscosity enhancing admixture. Mater. Struct. Constr. 2003, 36, 402–412. [Google Scholar] [CrossRef]
- Wang, Y.; Gao, B.; Yue, Q.; Wang, Y. Effect of viscosity, basicity and organic content of composite flocculant on the decolorization performance and mechanism for reactive dyeing wastewater. J. Environ. Sci. 2011, 23, 1626–1633. [Google Scholar] [CrossRef]
- Bouras, R.; Kaci, A.; Chaouche, M. Influence of viscosity modifying admixtures on the rheological behavior of cement and mortar pastes. Korea Aust. Rheol. J. 2012, 24, 35–44. [Google Scholar] [CrossRef]
- Nehdi, M.; Rahman, M.A. Estimating rheological properties of cement pastes using various rheological models for different test geometry, gap and surface friction. Cem. Concr. Res. 2004, 34, 1993–2007. [Google Scholar] [CrossRef]
- Benyounes, K. Rheological behavior of cement-based grout with Algerian bentonite. SN Appl. Sci. 2019, 1, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Roussel, N.; Lemaître, A.; Flatt, R.J.; Coussot, P. Steady state flow of cement suspensions: A micromechanical state of the art. Cem. Concr. Res. 2010, 40, 77–84. [Google Scholar] [CrossRef]
- Chen, Q.; Tao, Y.; Zhang, Q.; Qi, C. The rheological, mechanical and heavy metal leaching properties of cemented paste backfill under the influence of anionic polyacrylamide. Chemosphere 2022. [Google Scholar] [CrossRef] [PubMed]
- Bender, J.W.; Wagner, N.J. Optical measurement of the contributions of colloidal forces to the rheology of concentrated suspensions. J. Colloid Interface Sci. 1995. [Google Scholar] [CrossRef]
- Wu, J.; Jing, H.; Yin, Q.; Yu, L.; Meng, B.; Li, S. Strength prediction model considering material, ultrasonic and stress of cemented waste rock backfill for recycling gangue. J. Clean. Prod. 2020, 276, 123189. [Google Scholar] [CrossRef]
- Zhao, K.; Yu, X.; Zhu, S.; Zhou, Y.; Wang, Q.; Wang, J. Acoustic emission investigation of cemented paste backfill prepared with tantalum–niobium tailings. Constr. Build. Mater. 2020, 237, 117523. [Google Scholar] [CrossRef]
- Xu, W.; Cao, P.; Tian, M. Strength development and microstructure evolution of cemented tailings backfill containing different binder types and contents. Minerals 2018, 8, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Carpinteri, A.; Corrado, M.; Lacidogna, G. Three different approaches for damage domain characterization in disordered materials: Fractal energy density, b-value statistics, renormalization group theory. Mech. Mater. 2012, 53, 15–28. [Google Scholar] [CrossRef]
- Carpinteri, A.; Lacidogna, G.; Pugno, N. Structural damage diagnosis and life-time assessment by acoustic emission monitoring. Eng. Fract. Mech. 2007, 74, 273–289. [Google Scholar] [CrossRef]
- Carpinteri, A.; Cornetti, P. A fractional calculus approach to the description of stress and strain localization in fractal media. Chaos, Solitons and Fractals 2002, 13, 85–94. [Google Scholar] [CrossRef]
- Zhao, Y.; Qiu, J.; Zhang, S.; Guo, Z.; Ma, Z.; Sun, X.; Xing, J. Effect of sodium sulfate on the hydration and mechanical properties of lime-slag based eco-friendly binders. Constr. Build. Mater. 2020, 250, 118603. [Google Scholar] [CrossRef]
- Albano, A.M.; Muench, J.; Schwartz, C.; Mees, A.I.; Rapp, P.E. Singular-value decomposition and the Grassberger-Procaccia algorithm. Phys. Rev. A 1988. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dexing, L.; Enyuan, W.; Xiangguo, K.; Haishan, J.; Dongming, W.; Muhammad, A. Damage precursor of construction rocks under uniaxial cyclic loading tests analyzed by acoustic emission. Constr. Build. Mater. 2019, 206, 169–178. [Google Scholar] [CrossRef]
- Patil, H.; Dwivedi, A. Prediction of properties of the cement incorporated with nanoparticles by principal component analysis (PCA) and response surface regression (RSR). Mater. Today Proc. 2021, 43, 1358–1367. [Google Scholar] [CrossRef]
- Ballabio, D. A MATLAB toolbox for Principal Component Analysis and unsupervised exploration of data structure. Chemom. Intell. Lab. Syst. 2015. [Google Scholar] [CrossRef]
- van Mier, J.G.M. Failure of concrete under uniaxial compression: An overview. Fract. Mech. Concr. Struct. 1998, 2, 1169–1182. [Google Scholar]
- Rostami, V.; Shao, Y.; Boyd, A.J. Durability of concrete pipes subjected to combined steam and carbonation curing. Constr. Build. Mater. 2011, 25, 3345–3355. [Google Scholar] [CrossRef]
- Sideris, K.K.; Savva, A.E.; Papayianni, J. Sulfate resistance and carbonation of plain and blended cements. Cem. Concr. Compos. 2006, 28, 47–56. [Google Scholar] [CrossRef]
- Grounds, T.; Midgley, H.G.; Novell, D.V. Carbonation of ettringite by atmospheric carbon dioxide. Thermochim. Acta 1988, 135, 347–352. [Google Scholar] [CrossRef]
- Sharma, D.; Goyal, S. Effect of accelerated carbonation curing on near surface properties of concrete. Eur. J. Environ. Civ. Eng. 2020, 1–22. [Google Scholar] [CrossRef]
- Pelletier-Chaignat, L.; Winnefeld, F.; Lothenbach, B.; Le Saout, G.; Müller, C.J.; Famy, C. Influence of the calcium sulphate source on the hydration mechanism of Portland cement-calcium sulphoaluminate clinker-calcium sulphate binders. Cem. Concr. Compos. 2011, 33, 551–561. [Google Scholar] [CrossRef]
- Hargis, C.W.; Kirchheim, A.P.; Monteiro, P.J.M.; Gartner, E.M. Early age hydration of calcium sulfoaluminate (synthetic ye’elimite, C4A3S) in the presence of gypsum and varying amounts of calcium hydroxide. Cem. Concr. Res. 2013, 48, 105–115. [Google Scholar] [CrossRef]
- Scherer, G.W. Stress from crystallization of salt. Cem. Concr. Res. 2004, 34, 1613–1624. [Google Scholar] [CrossRef]
- Telesca, A.; Marroccoli, M.; Pace, M.L.; Tomasulo, M.; Valenti, G.L.; Monteiro, P.J.M. A hydration study of various calcium sulfoaluminate cements. Cem. Concr. Compos. 2014, 53, 224–232. [Google Scholar] [CrossRef]
- Komatsu, R.; Mizukoshi, N.; Makida, K.; Tsukamoto, K. In-situ observation of ettringite crystals. J. Cryst. Growth 2009, 311, 1005–1008. [Google Scholar] [CrossRef]





| No. | OPG (Dry, wt%) | TPG (Dry, wt%) | Water (wt%) | OPC (wt%) | OPC/Water | OPG (TPG)/OPC |
|---|---|---|---|---|---|---|
| A1 | 51.43 | - | 40.00 | 8.57 | 0.214 | 6:1 |
| B1 | 54.55 | - | 40.00 | 5.45 | 0.136 | 10:1 |
| A2 | - | 51.43 | 40.00 | 8.57 | 0.214 | 6:1 |
| B2 | - | 54.55 | 40.00 | 5.45 | 0.136 | 10:1 |
| Chemical Composition (%) | OPC | OPG | TPG |
|---|---|---|---|
| Na2O | 0.10 | 0.14 | 0.06 |
| MgO | 1.82 | 0.11 | 0.01 |
| Al2O3 | 4.32 | 0.80 | 0.29 |
| SiO2 | 17.85 | 6.24 | 5.01 |
| P2O5 | 0.21 | 1.45 | 0.57 |
| SO3 | 3.49 | 40.02 | 44.43 |
| Cl | 0.02 | - | - |
| K2O | 0.66 | 0.62 | 0.31 |
| CaO | 61.02 | 45.80 | 47.15 |
| TiO2 | 0.30 | 0.69 | 0.55 |
| V2O5 | 0.04 | - | - |
| Cr2O3 | 0.03 | - | - |
| MnO | 0.27 | - | - |
| Fe2O3 | 3.80 | 3.01 | 1.08 |
| CuO | 0.01 | - | - |
| ZnO | 0.04 | - | - |
| SrO | 0.07 | 0.10 | 0.06 |
| ZrO2 | 0.01 | - | - |
| BaO | 0.04 | 0.08 | 0.07 |
| F | - | 0.93 | 0.42 |
| Loss | 5.91 | - | - |
| Samples | D | d | R2 | Samples | D | d | R2 |
|---|---|---|---|---|---|---|---|
| A1-7D | 1.011 | −5.589 | 0.995 | B1-7D | 1.009 | −5.100 | 0.996 |
| A2-7D | 1.115 | −5.821 | 0.994 | B2-7D | 0.993 | −4.956 | 0.997 |
| A1-28D | 1.162 | −6.019 | 0.992 | B1-28D | 0.953 | −5.199 | 0.996 |
| A2-28D | 1.116 | −6.611 | 0.990 | B2-28D | 1.087 | −6.097 | 0.996 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Chen, Q.; Wang, Y.; Zhang, Q.; Li, H.; Jiang, C.; Qi, C. In Situ Remediation of Phosphogypsum with Water-Washing Pre-Treatment Using Cemented Paste Backfill: Rheology Behavior and Damage Evolution. Materials 2021, 14, 6993. https://doi.org/10.3390/ma14226993
Liu Y, Chen Q, Wang Y, Zhang Q, Li H, Jiang C, Qi C. In Situ Remediation of Phosphogypsum with Water-Washing Pre-Treatment Using Cemented Paste Backfill: Rheology Behavior and Damage Evolution. Materials. 2021; 14(22):6993. https://doi.org/10.3390/ma14226993
Chicago/Turabian StyleLiu, Yikai, Qiusong Chen, Yunmin Wang, Qinli Zhang, Hongpeng Li, Chaoyu Jiang, and Chongchong Qi. 2021. "In Situ Remediation of Phosphogypsum with Water-Washing Pre-Treatment Using Cemented Paste Backfill: Rheology Behavior and Damage Evolution" Materials 14, no. 22: 6993. https://doi.org/10.3390/ma14226993
APA StyleLiu, Y., Chen, Q., Wang, Y., Zhang, Q., Li, H., Jiang, C., & Qi, C. (2021). In Situ Remediation of Phosphogypsum with Water-Washing Pre-Treatment Using Cemented Paste Backfill: Rheology Behavior and Damage Evolution. Materials, 14(22), 6993. https://doi.org/10.3390/ma14226993