Fabrication of Graphene/Zinc Oxide Nano-Heterostructure for Hydrogen Sensing
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation and Transfer of Graphene
2.2. Preparation of Zinc Oxide Nanostructure
2.3. Measurement Method of Hydrogen Sensing
3. Results and Discussion
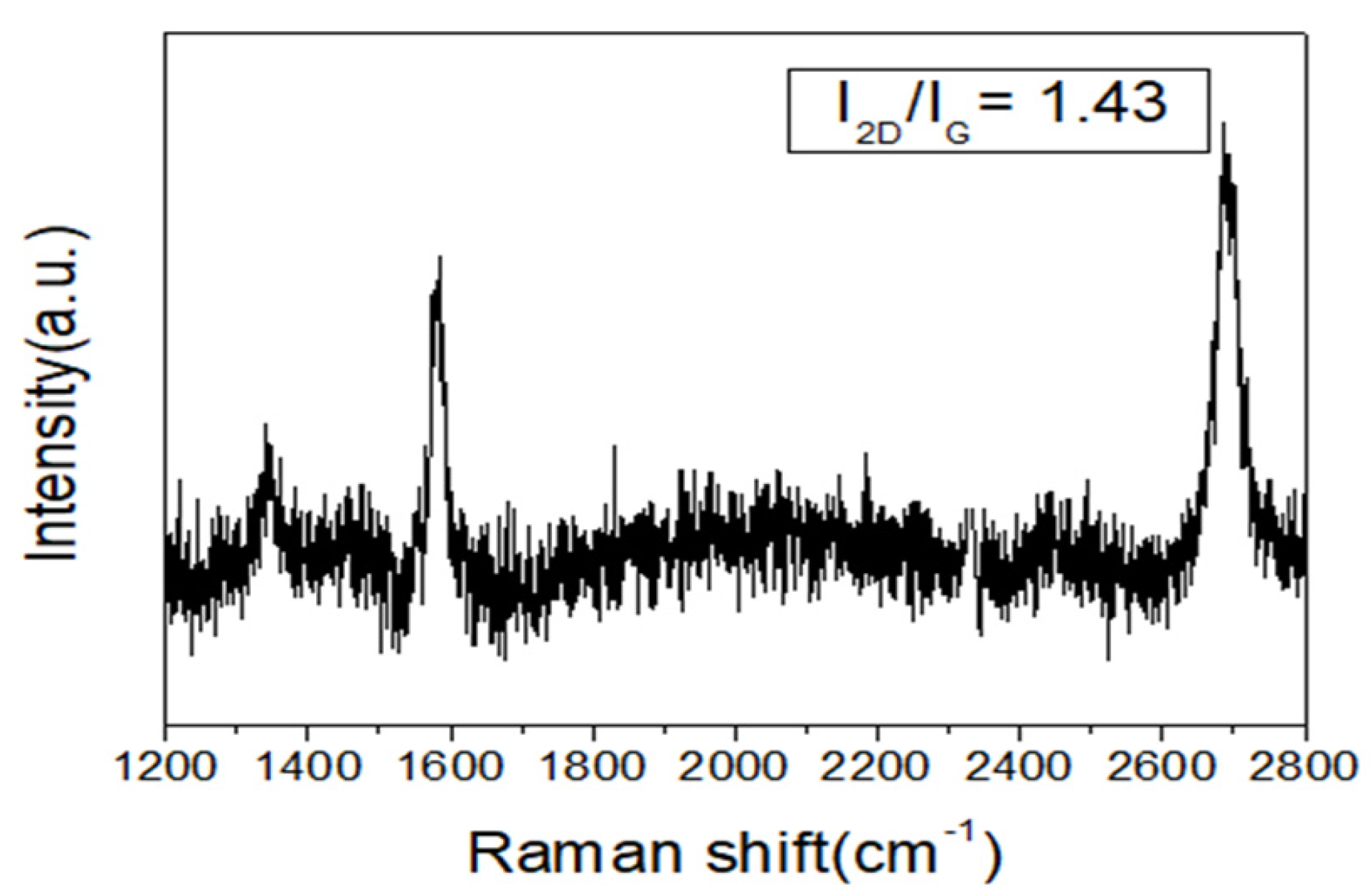
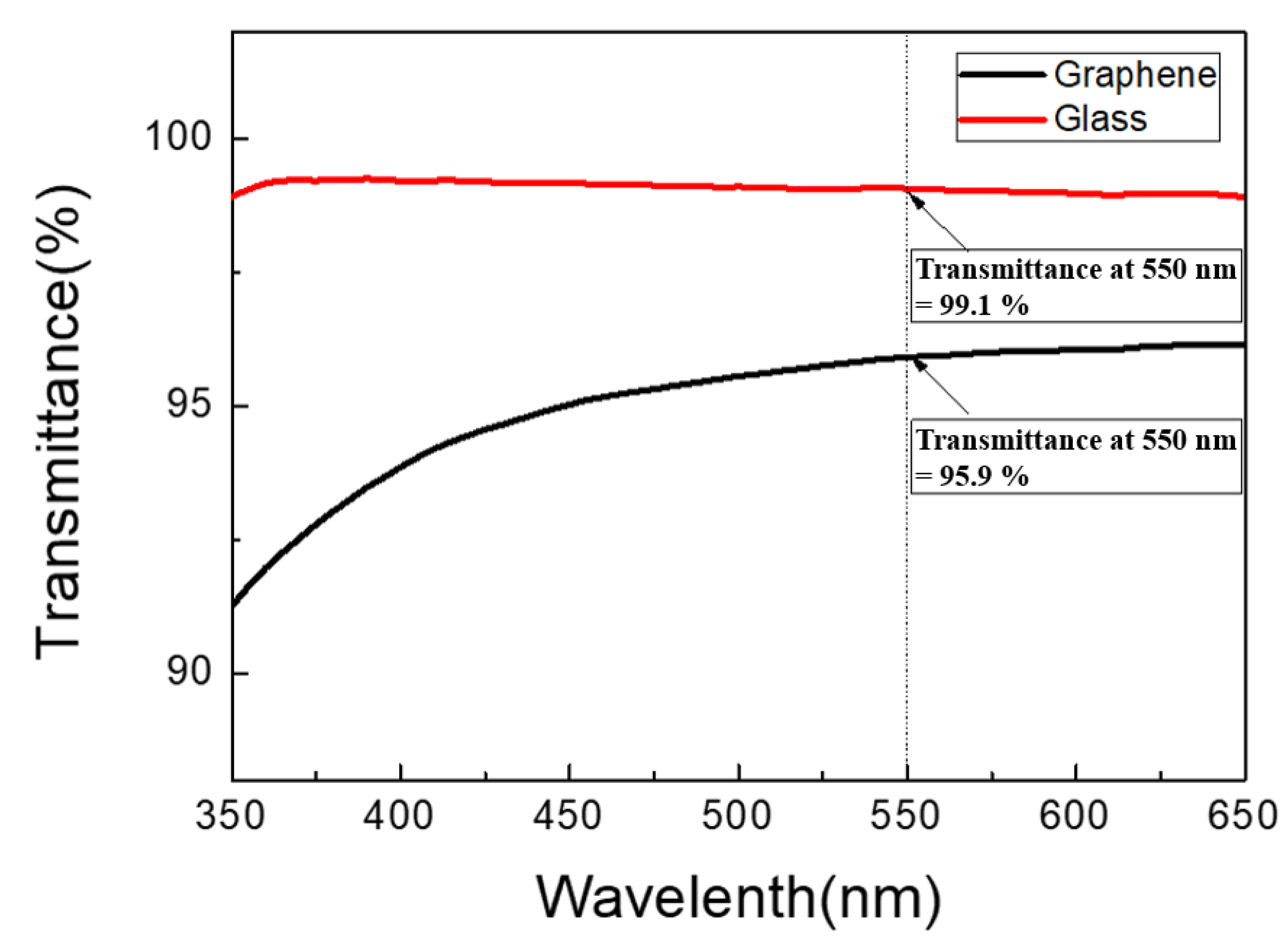
3.1. Graphene
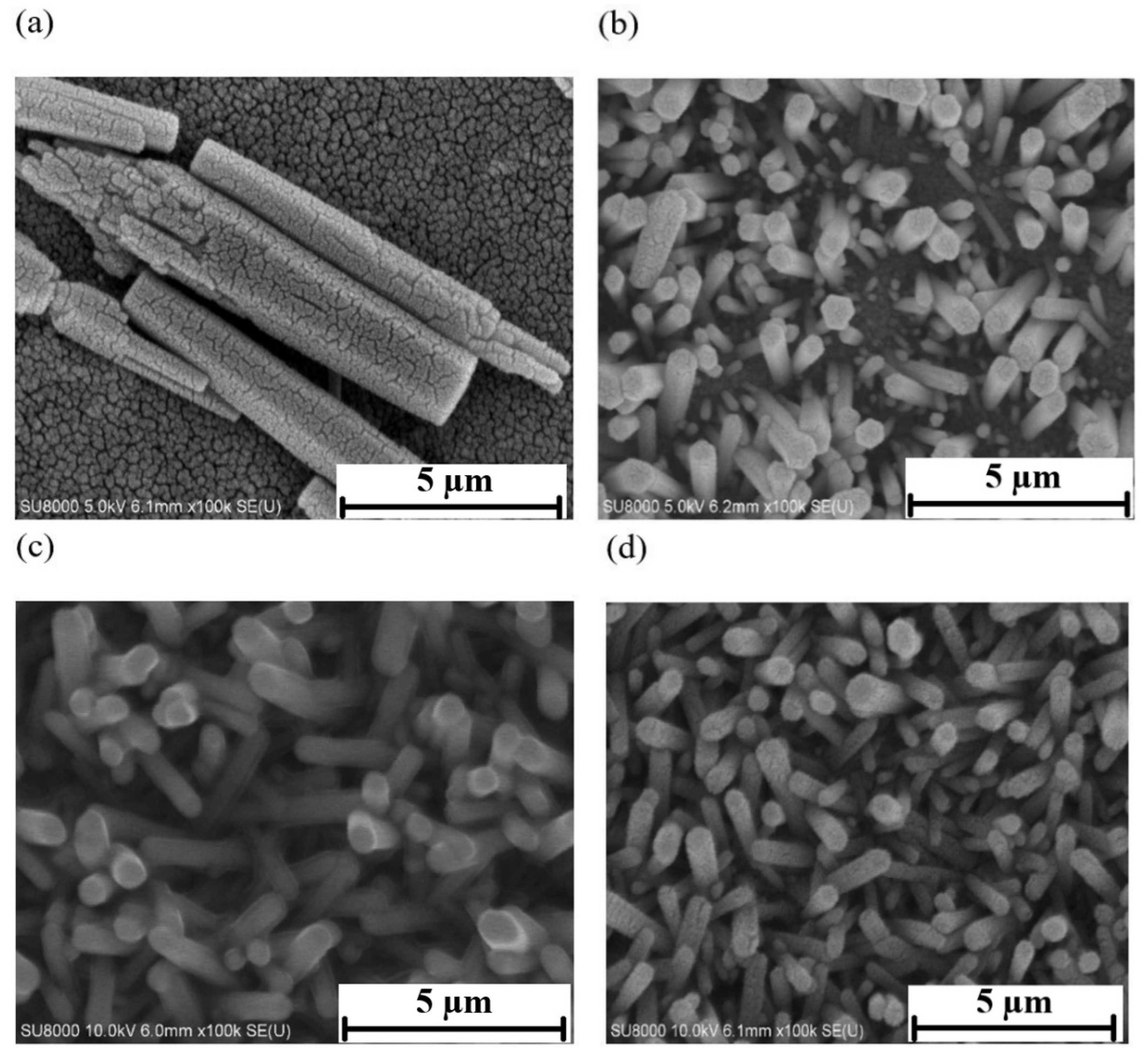
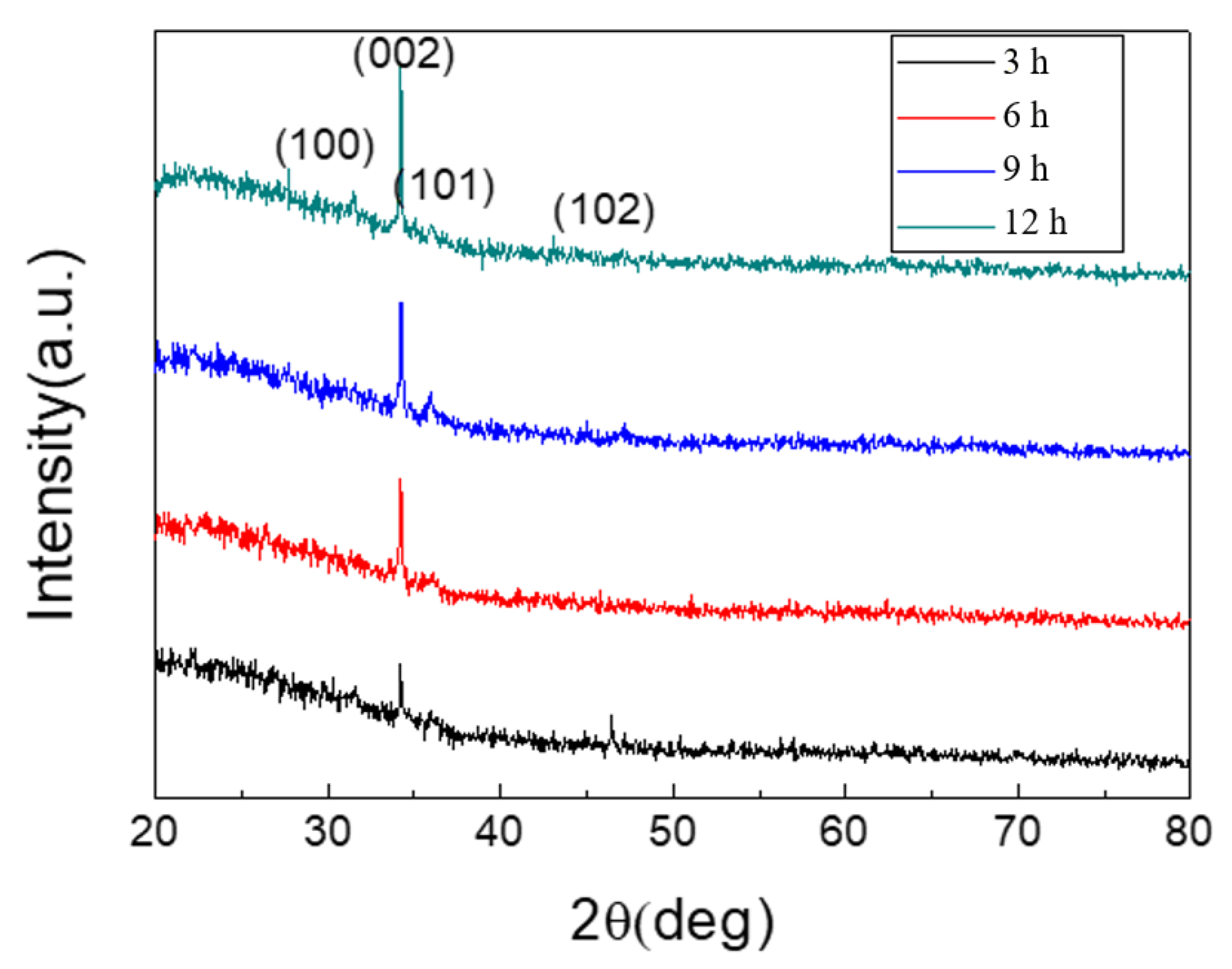
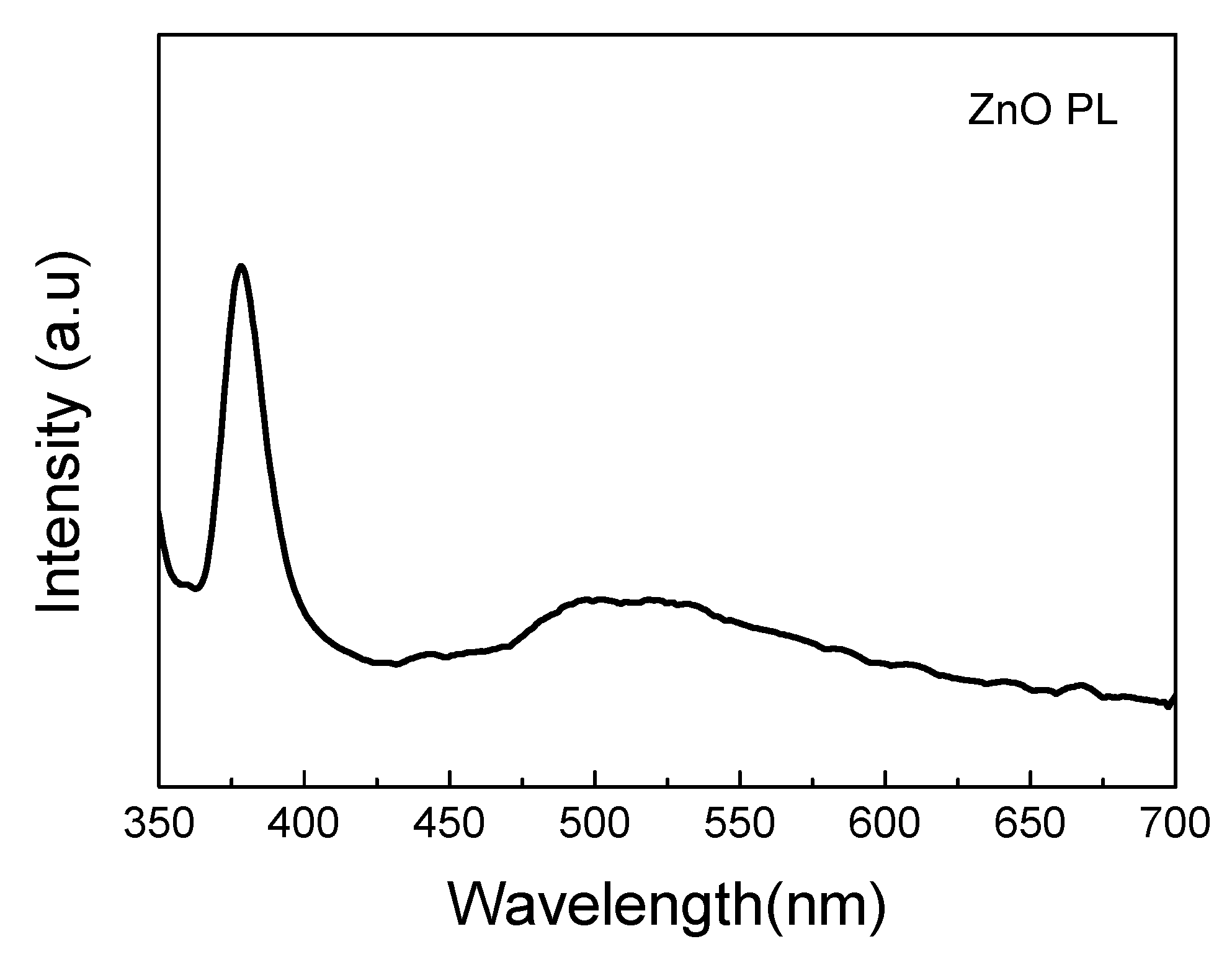
3.2. Zinc Oxide Nanostructure
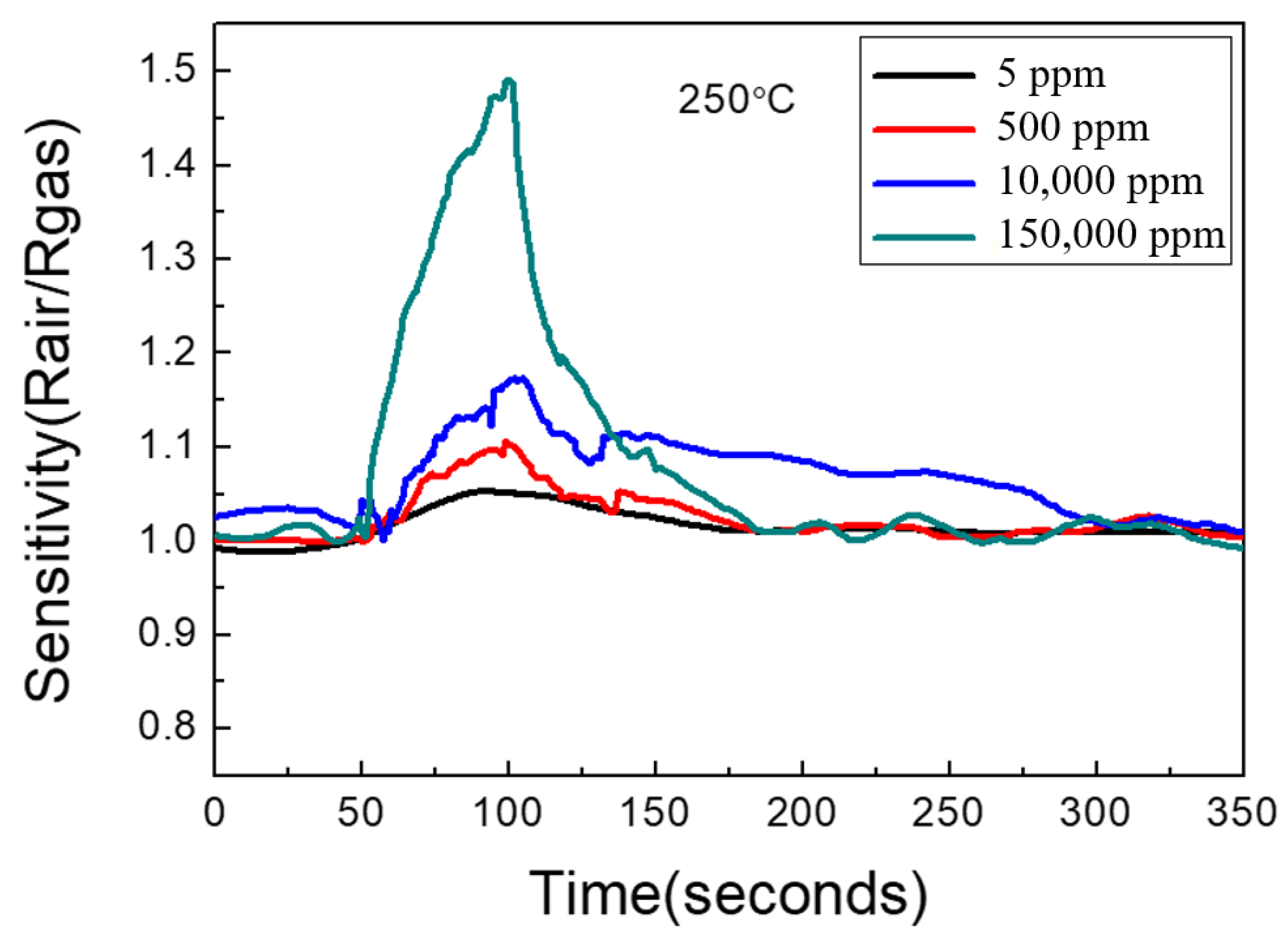
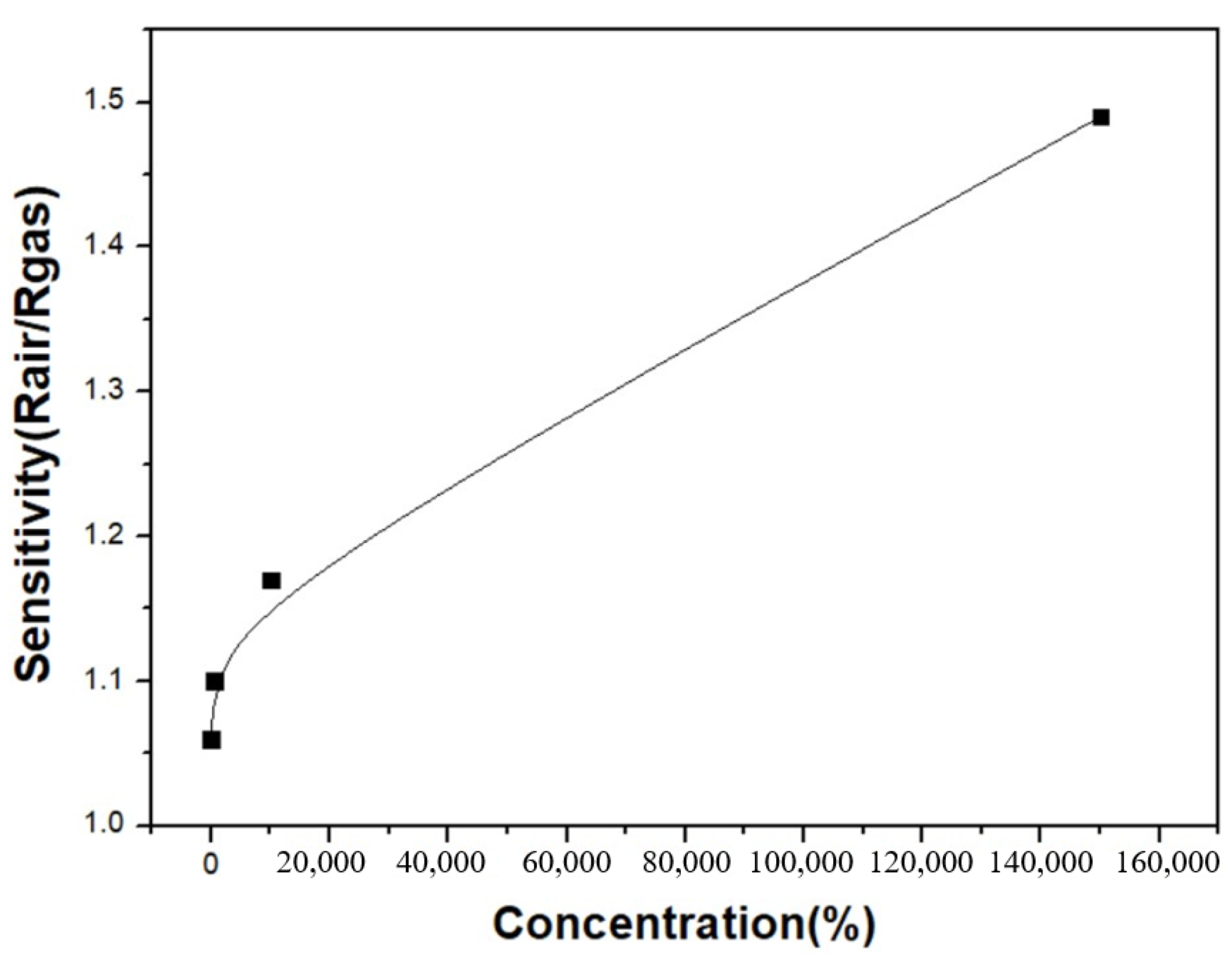
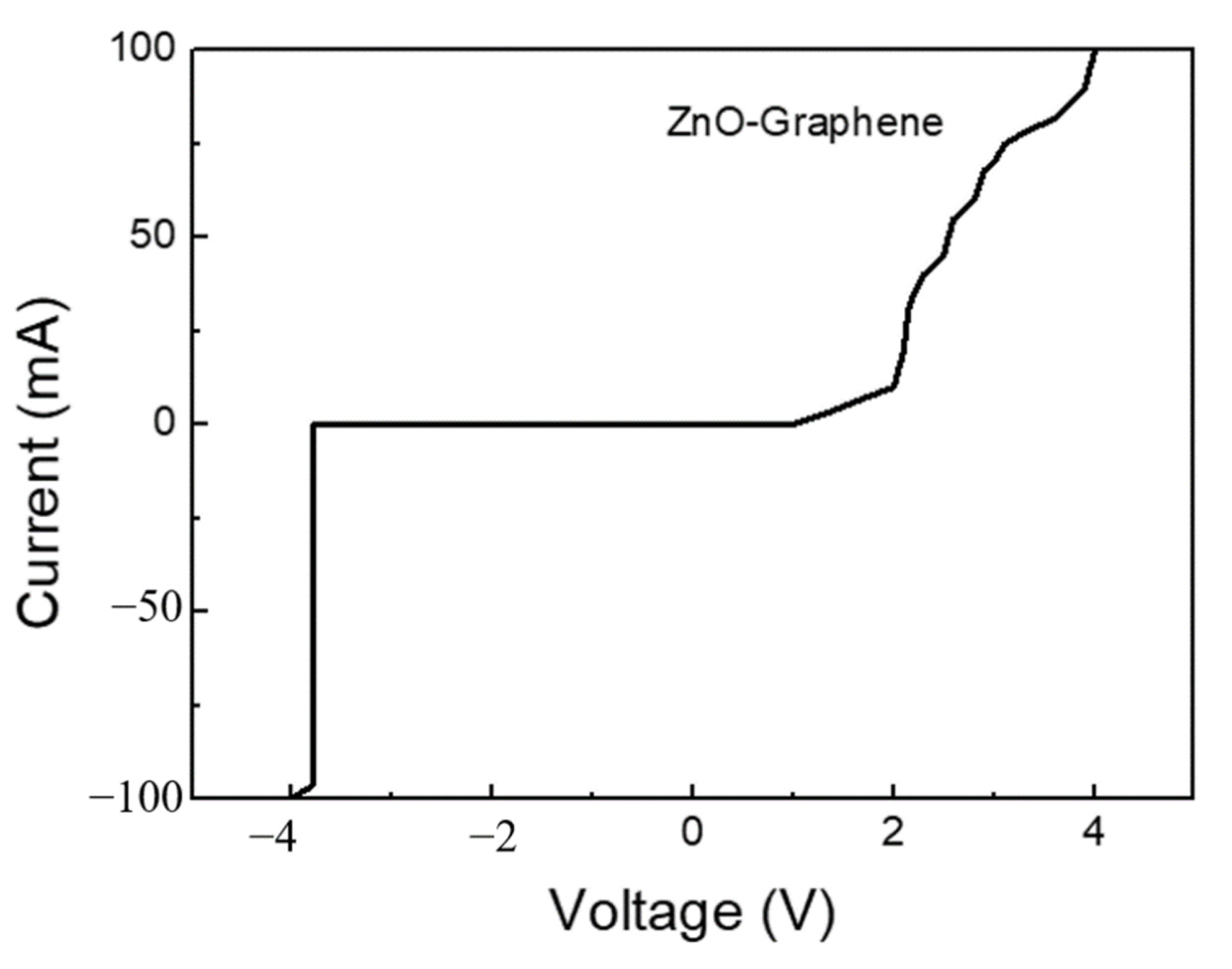
3.3. Graphene/Zinc Oxide Nano-Heterostructure
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Eichert, H.; Fischer, M. Combustion-related safety aspects of hydrogen in energy applications. Int. J. Hydrogen Energy 1986, 11, 117–124. [Google Scholar] [CrossRef]
- Wan, Q.; Li, Q.H.; Chen, Y.J.; Wang, T.H.; He, X.L.; Li, J.P.; Lin, C.L. Fabrication and ethanol sensing characteristics of ZnO nanowire gas sensors. Appl. Phys. Lett. 2004, 84, 3654–3656. [Google Scholar] [CrossRef] [Green Version]
- Xie, G.; Yu, J.; Chen, X.; Jiang, Y. Gas sensing characteristics of WO3 vacuum deposited thin films. Sens. Actuators B 2007, 123, 909–914. [Google Scholar] [CrossRef]
- Zappa, D.; Galstyan, V.; Kaur, N.; Arachchige, H.M.M.; Sisman, O.; Comini, E. Metal oxide-based heterostructures for gas sensors—A review. Anal. Chim. Acta 2018, 1039, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Liu, Z.; Jauregui, L.A.; Yu, Q.; Pillai, R.; Cao, H.; Pei, S.S. Wafer-scale synthesis of graphene by chemical vapor deposition and its application in hydrogen sensing. Sens. Actuators 2010, 150, 296–300. [Google Scholar] [CrossRef]
- Geim, A.K.; Novoselov, K.S. The rise of graphene. Nat. Mater. 2007, 6, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Hsieh, C.; Su, G. The Role of ALD-ZnO Seed Layers in the Growth of ZnO Nanorods for Hydrogen Sensing. Micromachines 2019, 10, 491. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ullah, S.; Yang, X.; Ta, H.Q.; Hasan, M.; Bachmatiuk, A.; Tokarska, K.; Trzebicka, B.; Fu, L.; Rummeli, M.H. Graphene transfer methods: A review. Nano Res. 2021, 14, 3576–3772. [Google Scholar] [CrossRef]
- Qing, F.; Zhang, Y.; Niu, Y.; Stehle, R.; Chen, Y.; Li, X. Toward large scale graphene transfer. Nanoscale 2020, 1220, 10890–10911. [Google Scholar] [CrossRef] [PubMed]
- Bonaccorso, F.; Sun, Z.; Hasan, T.; Ferrari, A.C. Graphene photonics and optoelectronics. Nat. Photonics 2010, 4, 611–622. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.; Riley, D.J.; Ashfold, M.N.R. Mechanism of ZnO nanotube growth by hydrothermal methods on ZnO film-coated si substrates. J. Phys. Chem. B 2006, 110, 15186–15192. [Google Scholar] [CrossRef] [PubMed]
- Vanheusden, K.; Seager, C.; Warren, W.; Tallant, D.; Voigt, J. Correlation between photoluminescence and oxygen vacancies in ZnO phosphors. Appl. Phys. Lett. 1996, 68, 403. [Google Scholar] [CrossRef]
- Greene, L.E.; Law, M.; Goldberger, J.; Kim, F.; Johnson, J.C.; Zhang, Y.; Saykally, R.J.; Yang, P. Low-temperature wafer-scale production of ZnO nanowire arrays. Angew. Chem. Int. Ed. 2003, 42, 3031–3034. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.; Diao, P.; Cai, S.M. Hydrothermal growth of well-aligned ZnO nanorodarrays: Dependence of morphology and alignment ordering upon preparing conditions. J. Solid State Chem. 2005, 178, 1864–1873. [Google Scholar] [CrossRef]
- Ahn, M.W.; Park, K.S.; Heo, J.H.; Park, J.G.; Kim, D.W.; Choi, K.J.; Lee, J.H.; Hong, S.H. Gas sensing properties of defect-controlled ZnO-nanowire gas sensor. Appl. Phys. Lett. 2008, 93, 263103–263105. [Google Scholar] [CrossRef]
- Lupan, O.; Ursaki, V.V.; Chai, G.; Chow, L.; Emelchenko, G.A.; Tiginyanu, I.M.; Gruzintsev, A.N.; Redkin, A.N. Selective hydrogen gas nanosensor using individualZnO nanowire with fast response at room temperature. Sens. Actuators B Chem. 2010, 144, 56–66. [Google Scholar] [CrossRef]
- An, W.; Wu, X.; Zeng, X.C. Adsorption of O2, H2, CO, NH3, and NO2 on ZnO Nanotube: A density functional theory study. J. Phys. Chem. C 2008, 112, 5747–5755. [Google Scholar] [CrossRef]
- Dong, X.; Fu, D.; Fang, W.; Shi, Y.; Chen, P.; Li, L.J. Doping single-layer graphene with aromatic molecules. Small 2009, 5, 1422–1426. [Google Scholar] [CrossRef] [PubMed]
- Norton, D.P.; Heo, Y.W.; Ivill, M.P.; Ip, K.; Pearton, S.J.; Chisholm, M.F.; Steiner, T. ZnO: Growth, doping & processing. Mater. Today 2004, 7, 34–40. [Google Scholar]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, Y.-M.; Tseng, C.-F.; Lan, B.-Y.; Hsieh, C.-F. Fabrication of Graphene/Zinc Oxide Nano-Heterostructure for Hydrogen Sensing. Materials 2021, 14, 6943. https://doi.org/10.3390/ma14226943
Lu Y-M, Tseng C-F, Lan B-Y, Hsieh C-F. Fabrication of Graphene/Zinc Oxide Nano-Heterostructure for Hydrogen Sensing. Materials. 2021; 14(22):6943. https://doi.org/10.3390/ma14226943
Chicago/Turabian StyleLu, Yang-Ming, Chi-Feng Tseng, Bing-Yi Lan, and Chia-Fen Hsieh. 2021. "Fabrication of Graphene/Zinc Oxide Nano-Heterostructure for Hydrogen Sensing" Materials 14, no. 22: 6943. https://doi.org/10.3390/ma14226943
APA StyleLu, Y.-M., Tseng, C.-F., Lan, B.-Y., & Hsieh, C.-F. (2021). Fabrication of Graphene/Zinc Oxide Nano-Heterostructure for Hydrogen Sensing. Materials, 14(22), 6943. https://doi.org/10.3390/ma14226943





