Perinone—New Life of an Old Molecule
Abstract
1. Introduction
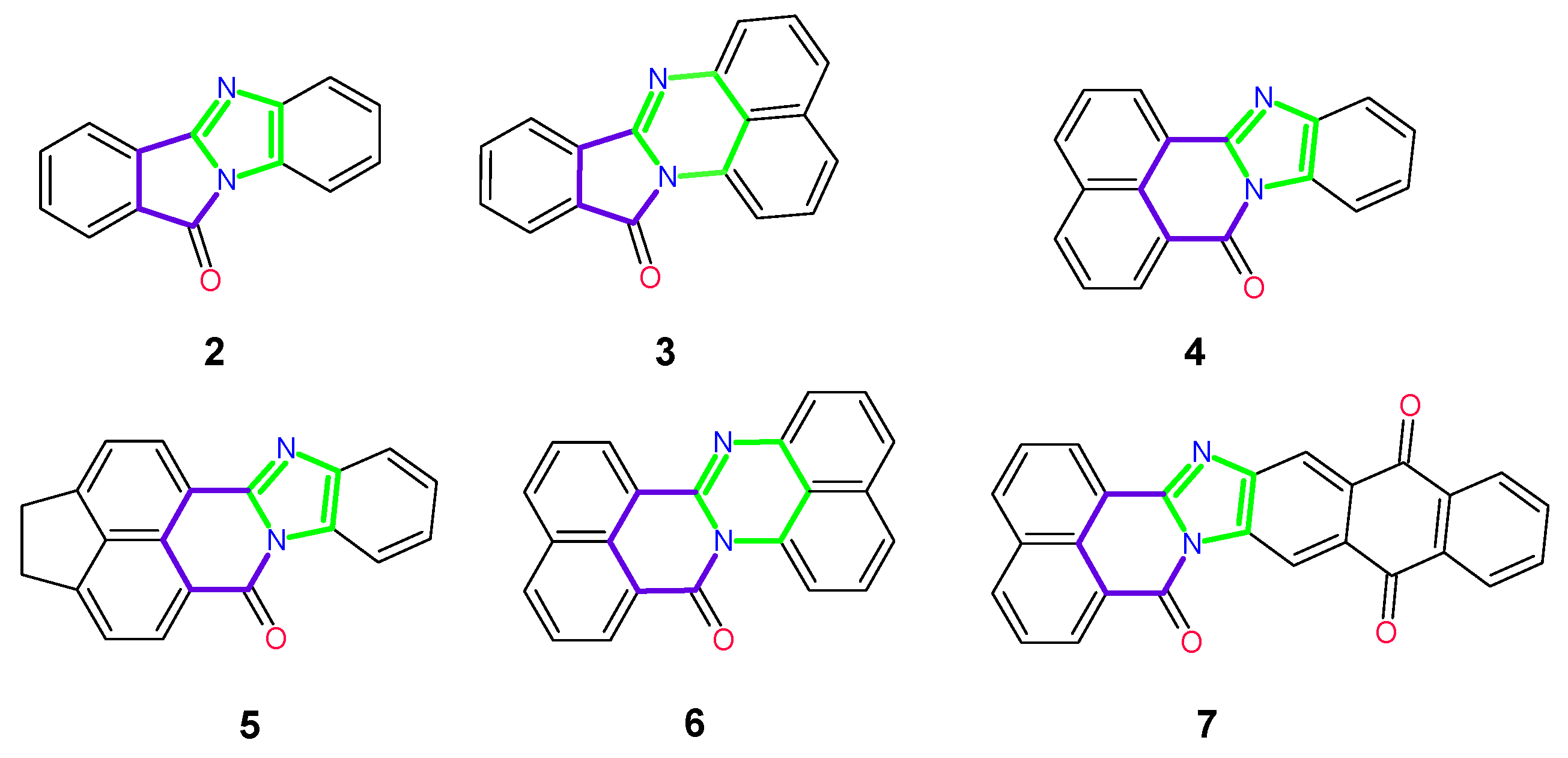
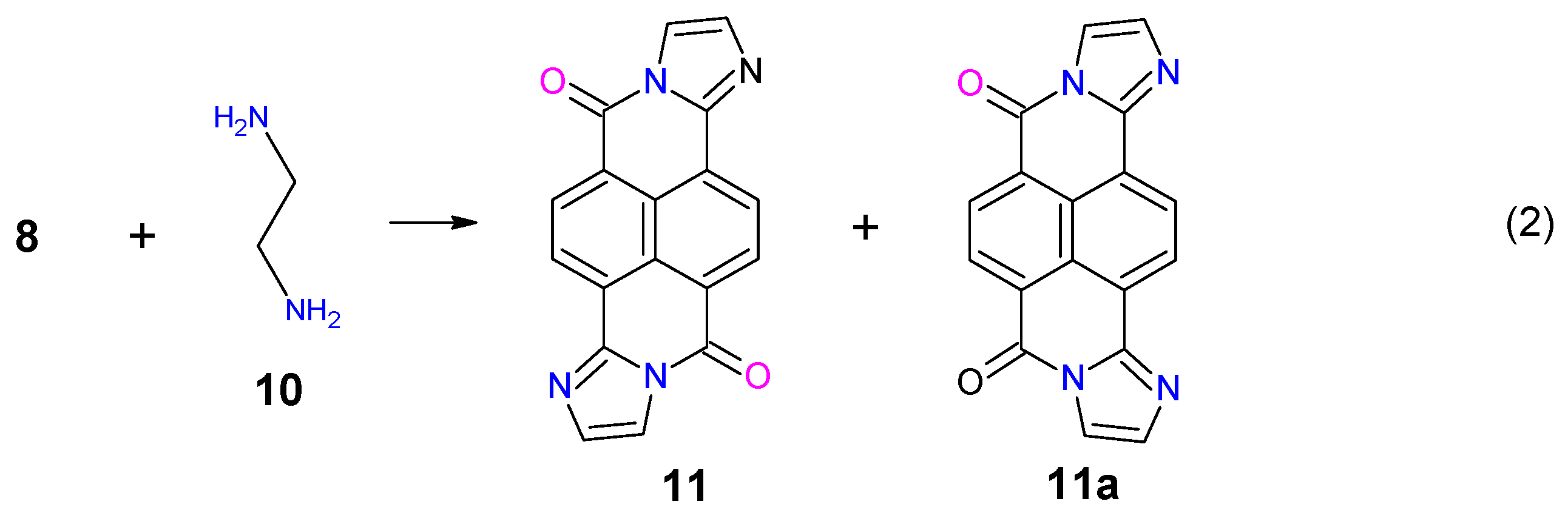
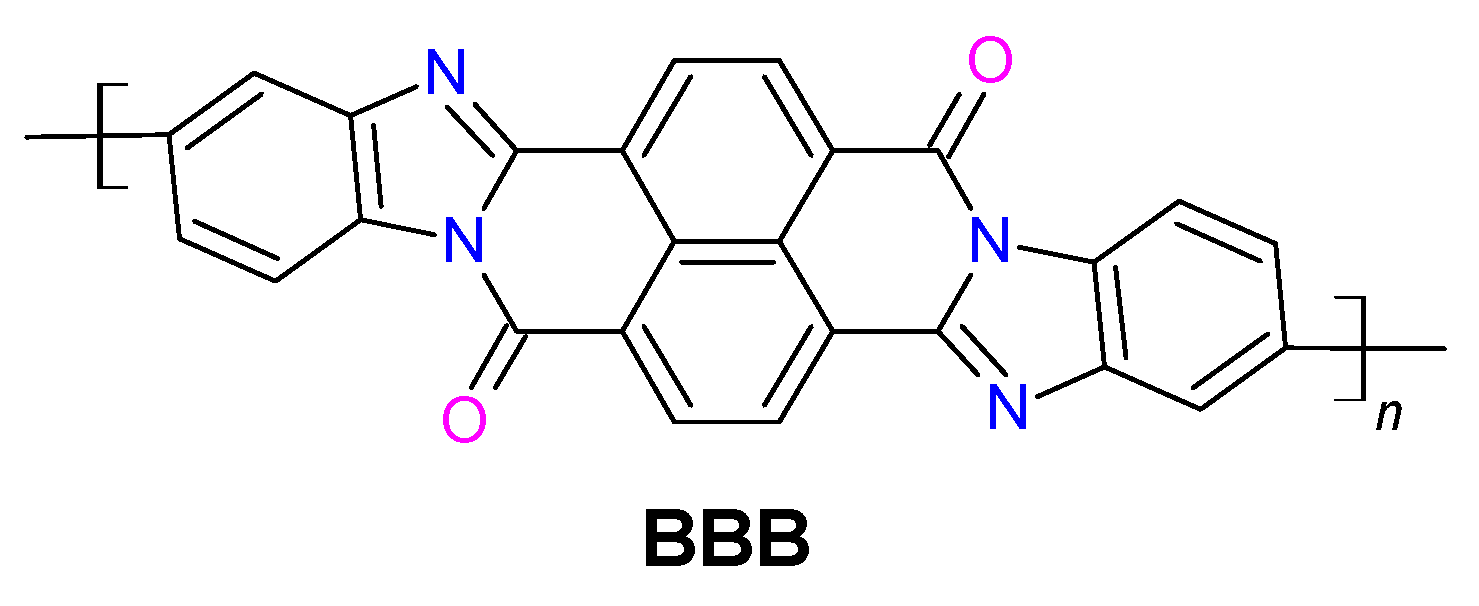
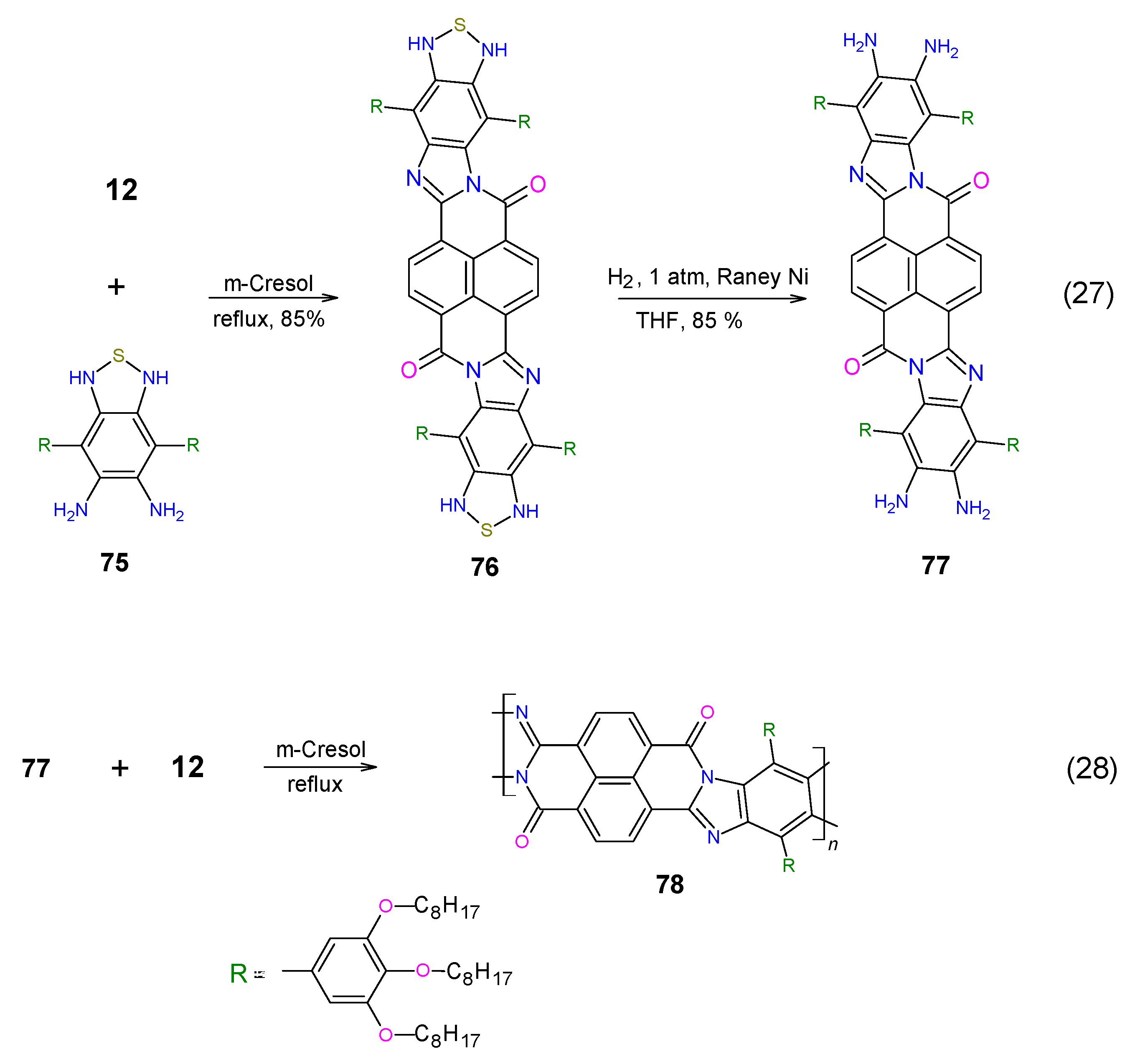
2. Synthesis
3. Properties
3.1. Solubility
3.2. Spectroscopic Properties
3.3. Conductivity
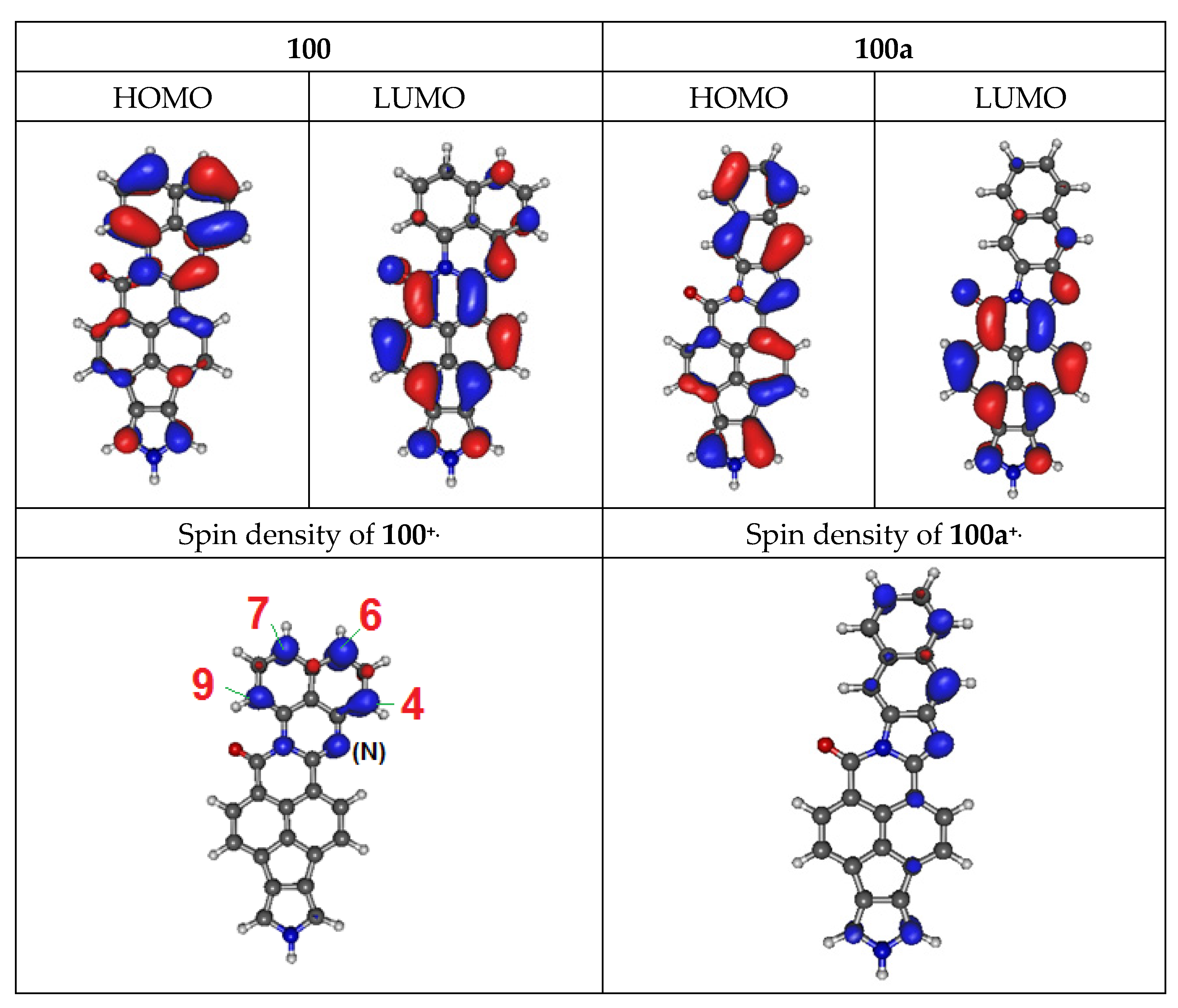
3.4. Electrochemical Properties
- BBL(I, insulator) + 0.25e− ⇄ BBL0.25− (II, conductor) (30)
- BBL0.25− (II, conductor) + 0.25e− ⇄ BBL0.50− (III insulator) (31)
- BBL0.50− (III, insulator) + 0.35e− ⇄ BBL0.85− (IV, conductor) (32)
- BBL0.85− (IV, conductor) + 0.15e− ⇄ BBL− (V, insulator) (33)
3.5. Organic Electronic Applications
3.6. Photovoltaic Applications
4. Summary
5. Patents
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hasegawa, T. Advances in Device Fabrication Scale-Up Methods, 2nd ed.; Elsevier Ltd.: Amsterdam, The Netherlands, 2019. [Google Scholar] [CrossRef]
- Reynolds, J.R.; Thompson, B.C.; Skotheim, T.A. Handbook of Conducting Polymers, 4th ed.; CRC Press: Boca Raton, FL, USA, 2019. [Google Scholar]
- Cirera, B.; Sánchez-Grande, A.; De La Torre, B.; Santos, J.; Edalatmanesh, S.; Rodríguez-Sánchez, E.; Lauwaet, K.; Mallada, B.; Zbořil, R.; Miranda, R.; et al. Tailoring topological order and π-conjugation to engineer quasi-metallic polymers. Nat. Nanotechnol. 2020, 15, 437–443. [Google Scholar] [CrossRef]
- Peng, H.; Sun, X.; Weng, W.; Fang, X. Polymer Materials for Energy and Electronic Applications; Elsevier Inc.: Amsterdam, The Netherlands, 2017. [Google Scholar] [CrossRef]
- Holten, D.; Bocian, D.F.; Lindsey, J.S. Probing Electronic Communication in Covalently Linked Multiporphyrin Arrays. A Guide to the Rational Design of Molecular Photonic Devices. Acc. Chem. Res. 2002, 35, 57–69. [Google Scholar] [CrossRef] [PubMed]
- Szyszko, B.; Latos-Grażyński, L. Core chemistry and skeletal rearrangements of porphyrinoids and metalloporphyrinoids. Chem. Soc. Rev. 2015, 44, 3588–3616. [Google Scholar] [CrossRef]
- Parsa, Z.; Naghavi, S.S.; Safari, N. Designing Push–Pull Porphyrins for Efficient Dye-Sensitized Solar Cells. J. Phys. Chem. A 2018, 122, 5870–5877. [Google Scholar] [CrossRef] [PubMed]
- Curutchet, C.; Mennucci, B. Quantum chemical studies of light harvesting. Chem. Rev. 2017, 117, 294–343. [Google Scholar] [CrossRef] [PubMed]
- Steingruber, E. Indigo and Indigo Colorants. In Ullmann’s Encyclopedia of Industrial Chemystry; Wiley: Hoboken, NJ, USA, 2000; pp. 264–322. [Google Scholar] [CrossRef]
- Irimia-Vladu, M.; Głowacki, E.D.; Troshin, P.; Schwabegger, G.; Leonat, L.; Susarova, D.K.; Krystal, O.; Ullah, M.; Kanbur, Y.; Bodea, M.A.; et al. Sariciftci, Indigo—A natural pigment for high performance ambipolar organic field effect transistors and circuits. Adv. Mater. 2012, 24, 375–380. [Google Scholar] [CrossRef]
- Splitstoser, J.C.; Dillehay, T.D.; Wouters, J.; Claro, A. Early pre-Hispanic use of indigo blue in Peru. Sci. Adv. 2016, 2, 1–5. [Google Scholar] [CrossRef]
- Bottari, G.; de Torre, G.; Guldi, D.M.; Torres, T. Covalent and Noncovalent Phthalocyanine-Carbon Nanostructure Systems Solar cell. Chem. Rev. 2010, 110, 6768–6816. [Google Scholar] [CrossRef]
- Jiang, J. Functional Phthalocyanine Molecular Materials; Structure and Bonding, Series No 135; Springer: Berlin/Heidelberg, Germany, 2015. [Google Scholar]
- Lei, T.; Wang, J.; Pei, J. Design, Synthesis, and Structure−Property Relationships of Isoindigo-Based Conjugated Polymers. Acc. Chem. Res. 2014, 47, 1117–1126. [Google Scholar] [CrossRef]
- Gaboriaud-Kolar, N.; Nam, S.; Skaltsounis, A.-L. A Colorful History: The Evolution of Indigoids; Springer: Cham, Switzerland, 2014. [Google Scholar] [CrossRef]
- Chen, L.; Li, C.; Klaus, M. Beyond perylene diimides: Synthesis, assembly and function of higher rylene chromophores. J. Mater. Chem. C 2014, 117, 1938–1956. [Google Scholar] [CrossRef]
- Zhan, X.; Facchetti, A.; Barlow, S.; Marks, T.J.; Ratner, M.A.; Wasielewski, M.R.; Marder, S.R. Rylene and related diimides for organic electronics. Adv. Mater. 2011, 23, 268–284. [Google Scholar] [CrossRef] [PubMed]
- Nagao, Y. Synthesis and properties of perylene pigments. Prog. Org. Coat. 1997, 31, 43–49. [Google Scholar] [CrossRef]
- Greene, M. Perylene Pigments. In High Performance Pigments; Edwin, B., Russell, F., Schwartz, J., Eds.; WILEY-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 1913; pp. 261–274. [Google Scholar] [CrossRef]
- Shirota, Y.; Kageyama, H. Organic Materials for Optoelectronic Applications: Overview, 2nd ed.; Elsevier Ltd.: Amsterdam, The Netherlands, 2019. [Google Scholar] [CrossRef]
- Gsänger, M.; Bialas, D.; Huang, L.; Stolte, M.; Würthner, F. Organic Semiconductors based on Dyes and Color Pigments. Adv. Mater. 2016, 28, 3615–3645. [Google Scholar] [CrossRef]
- Sun, Z.; Wu, J. 7,14-Diaryl-Substituted Zethrene Diimides as Stable Far-Red Dyes with Tunable Photophysical Properties. J. Org. Chem. 2013, 78, 9032–9040. [Google Scholar] [CrossRef]
- Pozharskii, A.F.; Dal’nikovskaya, V.V. Perimidines. Russ. Chem. Rev. 1981, 50, 816–835. [Google Scholar] [CrossRef]
- Burdeska, K. Naphthoylene-Benzimidazole and Naphthaloperinone. U.S. Patent 3,819,632, 30 November 1971. [Google Scholar] [CrossRef]
- Rappoport, Z. (Ed.) The Chemistry of Anilines; John Wiley & Sons Ltd.: Chichester, UK, 2007. [Google Scholar]
- Eckert, W.; Greune, H. Derivatives of 1,4,5,8-Naphthalene Tetracarboxylic Acid and Process of Preparing It. U.S. Patent 1,765,662, 1 May 1994. [Google Scholar]
- Eckert, W.B.O. Naphthalene-Aryl-Imidazol-peri-Dicarboxylic Acid, a Process of Preparation It and Intermediate Product Obtained During this Process. U.S. Patent 1,910,465, 24 June 1930. [Google Scholar]
- Eckert, W.; Braunsdorf, O. Mono-Molecular Condensation Product of 1,4,5,8-Naphthalene-Tetracarboxylic Acid and a Process of Prepring It. U.S. Patent 1,924,090, 5 September 1930. [Google Scholar]
- Rogovik, V.I.; Tikhonov, V.I. Investigation of Perinone Compounds III. Effect of the Electronic Nature of the Susbstituents in Naphthalic Anhydride on the Formation of isomeric Naphthaloperinones. Chem. Heterocycl. Compd. 1970, 6, 842. [Google Scholar]
- Tikhonov, V.I.; Rogovik, V.I. Research on perinone compounds. Chem. Heterocycl. Compd. 1973, 9, 1257–1259. [Google Scholar] [CrossRef]
- Tikhonov, V.I.; Rogovik, V.I. Research on perinone compounds. Chem. Heterocycl. Compd. 1974, 10, 1450–1454. [Google Scholar] [CrossRef]
- Hunger, K. The effect of crystal structure on colour application properties of organic pigments. Rev. Prog. Color. 1999, 29, 71–84. [Google Scholar] [CrossRef]
- Langhals, H.; Sprenger, S.; Brandherm, M.-T. Perylenamidine-imide dyes. Liebigs Ann. 1995, 1995, 481–486. [Google Scholar] [CrossRef]
- Tapmeyer, L.; Bolte, M.; Chierotti, M.R.; Schmidt, M.U. Structure of the intermediates in the industrial separation of perinone isomers. Dye Pigment. 2020, 181, 108442. [Google Scholar] [CrossRef]
- Sachs, F. Ueber Ringschlüsse in Peristellung der Naphtalinreihe. Justus Liebigs Ann. Chem. 1909, 365, 53–133. [Google Scholar] [CrossRef]
- Sachs, F.; Mosebach, G. Zur Kenntnis des Acenapthens II. Ber. Dtsch. Chem. Gesellschaft. 1911, 43, 2852–2862. [Google Scholar] [CrossRef][Green Version]
- Palmer, J.R.; Wells, K.A.; Yarnell, J.E.; Favale, J.M.; Castellano, F.N. Visible-Light-Driven Triplet Sensitization of Polycyclic Aromatic Hydrocarbons Using Thionated Perinones. J. Phys. Chem. Lett. 2020, 11, 5092–5099. [Google Scholar] [CrossRef] [PubMed]
- Dietz, E.; Kapaun, G.; Schiessler, S. Preparation of Vat Dyes and Pigments of the Perinone Series. E.U. Patent 0367067, 24 October 1989. [Google Scholar]
- Taublaender, M.J.; Glöcklhofer, F.; Marchetti-Deschmann, M.; Unterlass, M.M. Green and Rapid Hydrothermal Crystallization and Synthesis of Fully Conjugated Aromatic Compounds. Angew. Chem. Int. Ed. 2018, 57, 12270–12274. [Google Scholar] [CrossRef]
- Luo, X.; Yang, Y.; Qian, X. Recent progresses on the development of thioxo-naphthalimides. Chin. Chem. Lett. 2020, 31, 2877–2883. [Google Scholar] [CrossRef]
- Huang, T.-B.; Zhang, J.; Zhu, D.; Yao, W.; Qian, X. General Synthesis of Thioxo-1,8-naphthalimides via Thioxo-1,8-naphthalic Anhydrides. Synthesis 1999, 1999, 1109–1111. [Google Scholar] [CrossRef]
- Langhals, H.; Jaschke, H. Naphthalene Amidine Imide Dyes by Transamination of Naphthalene Bisimides. Chem. A Eur. J. 2006, 12, 2815–2824. [Google Scholar] [CrossRef]
- Nagao, Y.; Ishikawa, N.; Tanabe, Y.; Misono, T. Synthesis of Unsymmetrical Perylenebis(dicarboximide) Derivatives. Chem. Lett. 1979, 8, 151–154. [Google Scholar] [CrossRef]
- Aksakal, N.E.; Bayar, M.; Dumrul, H.; Atilla, D.; Chumakov, Y.; Yuksel, F. Structural and Optical Properties of New Naphthalene and Perylene Imide Imidazoles. Polycycl. Aromat. Compd. 2017, 39, 363–373. [Google Scholar] [CrossRef]
- Herzog, H.; Wunderlich, K.; Hohmann, W. Küpenfarbstoffgemische. EP Patent 0,104,530, 4 April.
- Kobrakov, K.I.; Zubkova, N.S.; Stankevich, G.S.; Shestakova, Y.S.; Stroganov, V.S.; Adrov, O.I. New aroyleneimidazoles as dyes for thermoplastic polymeric materials. Fibre Chem. 2006, 38, 183–187. [Google Scholar] [CrossRef]
- Meng, Y.; Abu-Yousef, I.A.; Hlil, A.A.R.; Hay, A.S. Colored Poly(arylene ether)s Containing Benzoylenebenzimidazole, Phthaloperinone, and Phthalocyanine Moieties. Macromolecules 2000, 33, 9185–9191. [Google Scholar] [CrossRef]
- Zhang, Y.; Hanifi, D.; Alvarez, S.; Antonio, F.; Pun, A.; Klivansky, L.M.; Hexemer, A.; Ma, B.; Liu, Y. Charge Transport Anisotropy inn-Type Disk-Shaped Triphenylene-Tris(aroyleneimidazole)s. Org. Lett. 2011, 13, 6528–6531. [Google Scholar] [CrossRef]
- Zhang, Y.; Hanifi, D.A.; Fernández-Liencres, M.P.; Klivansky, L.M.; Ma, B.; Navarro, A.; Liu, Y. Understanding Electron Transport in Disk-Shaped Triphenylene-Tris(naphthaleneimidazole)s through Structural Modification and Theoretical Investigation. ACS Appl. Mater. Interfaces 2017, 9, 20010–20019. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, L.; Zhang, K.; Wang, H.; Xiao, Y. A Soluble Ladder-Conjugated Star-Shaped Oligomer Composed of Four Perylene Diimide Branches and a Fluorene Core: Synthesis and Properties. Chem. Eur. J. 2014, 20, 10170–10178. [Google Scholar] [CrossRef]
- Lemaur, V.; Filho, D.D.S.; Coropceanu, V.; Lehmann, M.; Geerts, Y.; Piris, J.; Debije, M.G.; van de Craats, A.M.; Senthilkumar, K.; Siebbeles, L.D.A.; et al. Charge Transport Properties in Discotic Liquid Crystals: A Quantum-Chemical Insight into Structure−Property Relationships. J. Am. Chem. Soc. 2004, 126, 3271–3279. [Google Scholar] [CrossRef]
- Pisula, W.; Feng, X.; Müllen, K. Tuning the Columnar Organization of Discotic Polycyclic Aromatic Hydrocarbons. Adv. Mater. 2010, 22, 3634–3649. [Google Scholar] [CrossRef]
- Hanifi, D.; Cao, D.; Klivansky, L.M.; Liu, Y. Novel C3-symmetric n-type tris(aroyleneimidazole) and its analogs: Synthesis, physical properties and self-assembly. Chem. Commun. 2011, 47, 3454–3456. [Google Scholar] [CrossRef]
- Menke, E.H.; Leibold, D.; Lami, V.; Hofstetter, Y.J.; Mastalerz, M.; Vaynzof, Y. Triptycene-trisaroyleneimidazoles as non-fullerene acceptors—Influence of side-chains on solubility, device morphology and performance. Org. Electron. 2017, 47, 211–219. [Google Scholar] [CrossRef]
- Menke, E.H.; Leibold, D.; Berger, F.J.; Rominger, F.; Vaynzof, Y.; Mastalerz, M. Triptycene-Bis(aroyleneimidazole)s as Non-Fullerene Acceptors: The Missing Links. ChemPlusChem 2017, 82, 1390–1395. [Google Scholar] [CrossRef]
- Li, X.; Xiao, Y.; Qian, X. 2,3,6,7-Tetraamino-9,9-bis(2-ethylhexyl)fluorene: New Multifunctional Monomer for Soluble Ladder-Conjugated Molecules and Polymers. Org. Lett. 2008, 10, 2885–2888. [Google Scholar] [CrossRef]
- Xiong, K.; Xiao, Y. Synthesis of tetraalkyl naphthalene bisanhydride and its model condensations with amines. Tetrahedron Lett. 2013, 54, 3171–3175. [Google Scholar] [CrossRef]
- Maki, T.; Hashimoto, H. Vat Dyes of Acenaphthene Series. Acid IV. Condensation Anhydride with o-Phenylenediamine. Bull. Chem. Soc. Jpn. 1952, 25, 411–413. [Google Scholar] [CrossRef]
- Quante, H.; Geerts, Y.; Müllen, K. Synthesis of Soluble Perylenebisamidine Derivatives. Novel Long-Wavelength Absorbing and Fluorescent Dyes. Chem. Mater. 1997, 9, 495–500. [Google Scholar] [CrossRef]
- Wicklein, A.; Kohn, P.; Ghazaryan, L.; Thurn-Albrecht, T.; Thelakkat, M. Synthesis and structure elucidation of discotic liquid crystalline perylene imide benzimidazole. Chem. Commun. 2010, 46, 2328–2330. [Google Scholar] [CrossRef]
- Maki, T.; Hashimoto, H. Vat Dyes of Acenaphthene Series. VI. Derivatives of Acenaphthene Violet. Bull. Chem. Soc. Jpn. 1954, 27, 602–605. [Google Scholar] [CrossRef]
- Perrin, L.; Hudhomme, P. Synthesis, Electrochemical and Optical Absorption Properties of New Perylene-3,4:9,10-bis(dicarboximide) and Perylene-3,4:9,10-bis(benzimidazole) Derivatives. Eur. J. Org. Chem. 2011, 2011, 5427–5440. [Google Scholar] [CrossRef]
- Nagao, Y.; Misono, T. Synthesis and properties of N-alkyl-N′-aryl-3,4:9,10-perylenebis(dicarboximide). Dye. Pigment. 1984, 5, 171–188. [Google Scholar] [CrossRef]
- Blanco, R.; Gómez, R.; Seoane, C.; Segura, J.L.; Mena-Osteritz, E.; Bäuerle, P. An Ambipolar Peryleneamidine Monoimide-Fused Polythiophene with Narrow Band Gap. Org. Lett. 2007, 9, 2171–2174. [Google Scholar] [CrossRef] [PubMed]
- Schönamsgruber, J.; Maid, H.; Bauer, W.; Hirsch, A. Fused Perylene-Phthalocyanine Macrocycles: A New Family of NIR-Dyes with Pronounced Basicity. Chem. A Eur. J. 2014, 20, 16969–16979. [Google Scholar] [CrossRef]
- Kimura, M.; Nomoto, H.; Suzuki, H.; Ikeuchi, T.; Matsuzaki, H.; Murakami, T.N.; Furube, A.; Masaki, N.; Griffith, M.J.; Mori, S. Molecular Design Rule of Phthalocyanine Dyes for Highly Efficient Near-IR Performance in Dye-Sensitized Solar Cells. Chem. A Eur. J. 2013, 19, 7496–7502. [Google Scholar] [CrossRef] [PubMed]
- Schönamsgruber, J.; Hirsch, A. Benz-Bisimidazole-Bridged Perylenes—Linearly Expanded Chromophores. Eur. J. Org. Chem. 2015, 2015, 2167–2174. [Google Scholar] [CrossRef]
- Sun, P.; Li, L.; Guang, S.; Xu, H. The investigation of the dipole-dipole action direction and molecular space configuration effect during the dipole–dipole induced azobenzene supramolecular self-assembly. Colloids Surf. A Physicochem. Eng. Asp. 2019, 580, 123742. [Google Scholar] [CrossRef]
- Czichy, M.; Motyka, R.; Zassowski, P.; Grabiec, E.; Janasik, P.; Brzeczek-Szafran, A.; Laba, K.; Wolinska-Grabczyk, A.; Lapkowski, M. Effects of solution-phase ordering on the spectroscopic properties and electrooxidative reactivity of isomeric mixtures and isolated isomers of synthesized amidine derivatives. Dye Pigment. 2020, 178, 108309. [Google Scholar] [CrossRef]
- Czichy, M.; Janasik, P.; Motyka, R.; Zassowski, P.; Grabiec, E.; Wolinska-Grabczyk, A.; Lapkowski, M. Influence of isomeric phthaloperinone monomers on the formation of π-dimers and σ-bonded segments in electrochemically-crosslinked products. Electrochim. Acta 2021, 370, 137669. [Google Scholar] [CrossRef]
- Metanomski, R.; Bareiss, J.; Kahovec, K.; Loening, L.; Shi, V.; Shibaev, W. Nomenclature of Regular Double-strand (Ladder and Spiro) Organic Polymers. Pure Appl. Chem. 1993, 65, 1561–1580. [Google Scholar] [CrossRef]
- Van Deusen, R.L. Benzimidazo-benzophenanthroline polymers. Polym. Lett. 1966, 4, 211–214. [Google Scholar] [CrossRef]
- Van Deusen, R.L.; Goins, O.K.; Sicree, A.J. Thermally stable polymers from 1,4,5,8-naphtha-lenetetracarboxylic acid and aromatic tetraamines. J. Polym. Sci. Part A-1 Polym. Chem. 1969, 7, 2467. [Google Scholar] [CrossRef]
- Babel, A.; Jenekhe, S.A. High Electron Mobility in Ladder Polymer Field-Effect Transistors. J. Am. Chem. Soc. 2003, 125, 13656–13657. [Google Scholar] [CrossRef]
- Belaish, I.; Rettori, C.; Davidov, D.; Mclean, M.; Dalton, L. Electric Properties of Ladder Type Polymers BBB and BBL. MRS Online Proc. Libr. Arch. 2011, 134, 689–695. [Google Scholar] [CrossRef]
- Choi, J.; Song, H.; Kim, N.; Kim, F.S. Development of n-type polymer semiconductors for organic field-effect transistors. Semicond. Sci. Technol. 2015, 30, 1–16. [Google Scholar] [CrossRef]
- Nartsissov, B. Surveys on Heat-Resistant Polymers. I. Pyrrones. J. Macromol. Sci. Part C Polym. Rev. 1974, 11, 143–176. [Google Scholar] [CrossRef]
- Van Deusen, R.L.; Goins, O.K.; Sicree, A.J. The Formation and Properties of a Class of Highly Condensed Aromatic-Heterocyclic Polymers; Technical Report AFML-TR; The Defense Technical Information Center: Fort Belvoir, VA, USA, 1967; pp. 66–373. [Google Scholar]
- Berlin, A.A.; Liogon’Kii, B.I.; Shamraev, G.M. Thermostable Polymers from Dianhydrides of Aromatic Tetracarboxylic Acids and Tetra-amines. Russ. Chem. Rev. 1971, 40, 284–300. [Google Scholar] [CrossRef]
- Korshak, V. Functionality of monomers and structure of polymers obtained by polycondensation. Review. Polym. Sci. U.S.S.R. 1982, 24, 1783–1797. [Google Scholar] [CrossRef]
- Kimmel, B.G.; Karre, L.E. Preparation and Characterization of the Pyrrones as Thermal Structural Materials; NASA Rep. 1791; The Defense Technical Information Center: Fort Belvoir, VA, USA, 1971. [Google Scholar]
- Rusanov, A.L. Ladder Polyheteroarylenes—Progress and Problems. Russ. Chem. Rev. 1979, 48, 62–78. [Google Scholar] [CrossRef]
- Korshak, V.; Rusanov, A. Some trends in the development of the chemistry of polyheteroarylenes. Review. Polym. Sci. U.S.S.R. 1984, 26, 1–16. [Google Scholar] [CrossRef]
- Korshak, V.; Berestneva, G.; Petrovskii, P.; Ormotsadze, P.; Rusanov, A.; Berlin, A.; Adyrkhayeva, F. The stepwise synthesis of polynaphthoylenebenzimidazoles. Polym. Sci. U.S.S.R. 1981, 23, 1902–1908. [Google Scholar] [CrossRef]
- Dawans, F.; Marvel, C.S. Polymers from ortho Aromatic Tetraamines and Aromatic Dianhydrides. J. Polym. Sci. Part A 1965, 3, 3549–3571. [Google Scholar] [CrossRef]
- Bell, V.L.; Pezdirtz, G.F. Polyimidazopyrrolones: A new route to ladder polymer. J. Polym. Sci. Part B Polym. Lett. 1965, 3, 977–984. [Google Scholar] [CrossRef]
- Korshak, Y.E.; Doroshenko, M.M.; Tepl’akov, R.D.; Fedorova, B.W.; Volkov, V.V. Effect of Synthesis Conditions on Properties of Polyaminoamidoacids. Vysok. Soied. 1970, 12, 677–680. [Google Scholar]
- Korshak, V.V.; Rusanov, A.L.; Katsarava, R.D. Synthesis and investigation of new polybenzimidazoles. Dokl. Akad. Nauk SSSR 1968, 182, 1327. [Google Scholar] [CrossRef]
- Arnold, F.E. Ladder polymers from tetraaminodiquinoxalpyrene. J. Polym. Sci. Part A-1 Polym. Chem. 1970, 8, 2079–2089. [Google Scholar] [CrossRef]
- Wang, J.; Qi, W.; Qiao, Z.Y.; Wang, Q. Soluble ladder conjugated polypyrrones: Synthesis, characterization and application in photodetectors. Dye. Pigment. 2015, 113, 160–164. [Google Scholar] [CrossRef]
- Loutfy, A.M.; Hor, P.M.; Kazmaier, R.A.; Burt, G.K.; Hamer, R.O. Organic photoconductive (OPC) devices incorporating bisarylimidazole perinone pigments. Dye. Pigment. 1991, 15, 139–156. [Google Scholar] [CrossRef]
- Belykh, S.I.; Gushchina, Y.A.; Liogon’kii, B.I.; Berlin, A.A. Synthesis and study of polymers prepared from dianhydrides of 3,4,9,10-perylene-1,4,5,8-naphthalene tetracarboxylic acids and aromatic tetra-amines. Polym. Sci. U.S.S.R. 1974, 16, 2199–2205. [Google Scholar] [CrossRef]
- Korshak, V.; Rusanov, A.; Berlin, A.; Fidler, S.; Livshits, B.; Dymshits, T.; Silyutina, L.; Blinov, V. Synthesis and study of polynaphthoylene benzimidazoles soluble in organic solvents. Polym. Sci. U.S.S.R. 1979, 21, 719–725. [Google Scholar] [CrossRef]
- Stenger-Smith, J.D.; Lai, W.W.; Irvin, D.J.; Yandek, G.R.; Irvin, J.A. Electroactive polymer-based electrochemical capacitors using poly(benzimidazo-benzophenanthroline) and its pyridine derivative poly(4-aza-benzimidazo-benzophenanthroline) as cathode materials with ionic liq-uid electrolyte. J. Power Sources 2012, 220, 236–242. [Google Scholar] [CrossRef]
- Facchetti, T.J.; Marks, Y.; Zheng, A. Organic n-Channel Field-Effect Transistors Based on Arylenediimide-Thiophene Derivatives. J. Am. Chem. Soc. 2010, 132, 8440–8452. [Google Scholar]
- González, S.R.; Casado, J.; Navarrete, J.T.L.; Blanco, R.; Segura, J.L. A β-Naphthaleneimide-Modified Terthiophene Exhibiting Charge Transfer and Polarization through the Short Molecular Axis. Joint Spectroscopic and Theoretical Study. J. Phys. Chem. A 2008, 112, 6732–6740. [Google Scholar] [CrossRef]
- Berlin, A.; Liogon’Kii, B.; Shamrayev, G.; Belova, G. Poly-(naphthoylene-bis-benzimidazoles). Polym. Sci. U.S.S.R. 1968, 9, 2184–2195. [Google Scholar] [CrossRef]
- Jedlinski, Z.J.; Kowalski, B.; Gaik, U. New poly[bis(benzimidazobenzisoquinolinones)]. Macromolecules 1983, 16, 522–526. [Google Scholar] [CrossRef]
- Korshak, V.V.; Rusanov, A. Sintez i issledovanie niekotorykh polibezoilenbenzimidazolov. Vysok. Soied. 1972, 14, 186–201. [Google Scholar]
- Arnold, C. Stability of high-temperature polymers. J. Polym. Sci. Macromol. Rev. 1979, 14, 265–378. [Google Scholar] [CrossRef]
- Smigasiewicz, S.; Kowalski, B. The Dielectric B-Relaxation of Poly (bis-benzimidazobenzisoquinolinones). J. Polym. Sci. Part B Polym. Physics 1986, 24, 1961–1970. [Google Scholar] [CrossRef]
- Mezhikovskii, S.; Belykh, S.; Liogon’Kii, B.; Berlin, A. Thermo-oxidative degradation of pol-ynaphthylene-bis-benzimidazoles and of some of their analogues. Polym. Sci. U.S.S.R. 1974, 16, 2650–2658. [Google Scholar] [CrossRef]
- Coleman, J.F.; Van Deusen, R.L. Synthesis and Properties of Some Benzimidazobenzophenanthroline Polymers Possessing Both Non-Ladder and Ladder Structures; Techical Report AFML-TR; Air Force Materiel Command: Dayton, OH, USA, 1970; pp. 69–289. [Google Scholar]
- Alam, M.M.; Jenekhe, S.A. Conducting Ladder Polymers: Insulator-to-Metal Transition and Evolution of Electronic Structure upon Protonation by Poly(styrenesulfonic Acid). J. Phys. Chem. B 2002, 106, 11172–11177. [Google Scholar] [CrossRef]
- Karre, L.E.; Keller, L.B.; Miller, L.J. Development and Processing of Pyrrone Polymers; Annu. Rep. NASA,1-6287; The Defense Technical Information Center: Fort Belvoir, VA, USA, 1967; pp. 68–148. [Google Scholar]
- Batz, P.; Schmeisser, D.; Beliash, I.; Davidov, D.; Gopel, W. Semiconductor-to-metal Transition in the Ladder Polymer BBB. Synth. Met. 1991, 41–43, 1609–1613. [Google Scholar] [CrossRef]
- Lee, J.F.; Hsu, S.L.C.; Lee, P.I.; Chuang, H.Y.; Yang, M.L.; Chen, J.S.; Chou, W.Y. A new intramolecular donor–acceptor polyfluorene copolymer for bulk heterojunction solar cells. Sol. Energy Mater. Sol. Cells 2010, 94, 1166–1172. [Google Scholar] [CrossRef]
- Lee, J.F.; Hsu, S.L.C.; Lee, P.I.; Chuang, H.Y.; Yang, M.L.; Chen, J.S.; Chou, W.Y. Low bandgap carbazole copolymers containing an electron-withdrawing side chain for solar cell applications. Sol. Energy Mater. Sol. Cells 2011, 95, 2795–2804. [Google Scholar] [CrossRef]
- Teteruk, J.L.; Glinnemann, J.; Heyse, W.; Johansson, K.E.; van de Streek, J.; Schmidt, M.U. Local structure in the disordered solid solution ofcis- andtrans-perinones. Acta Crystallogr. Sect. B Struct. Sci. Cryst. Eng. Mater. 2016, 72, 416–433. [Google Scholar] [CrossRef]
- Scherf, U. Ladder-type materials. J. Mater. Chem. 1999, 9, 1853–1864. [Google Scholar] [CrossRef]
- Jenekhe, S.A.; Johnson, P.O. Complexation-mediated solubilization and processing of rigid-chain and ladder polymers in aprotic organic solvents. Macromolecules 1990, 23, 4419–4429. [Google Scholar] [CrossRef]
- Berry, G.C. Dilute and concentrated solutions of a heterocyclic polymer (BBB). Discuss. Faraday Soc. 1970, 49, 121–136. [Google Scholar] [CrossRef]
- Wang, Q.; Qiao, W.; Wang, Z.Y. Facile synthesis and characterization of well-defined soluble poly(benzimidazobenzophenanthroline)-like derivatives. RSC Adv. 2014, 4, 9967–9970. [Google Scholar] [CrossRef]
- Briseno, A.L.; Mannsfeld, S.C.B.; Shamberger, P.; Ohuchi, F.S.; Bao, Z.; Jenekhe, S.A.; Xia, Y. Self-Assembly, Molecular Packing, and Electron Transport in n-Type Polymer Semiconductor Nanobelts. Chem. Mater. 2008, 20, 4712–4719. [Google Scholar] [CrossRef]
- Na, J.Y.; Kang, B.; Park, Y.D. Influence of Molecular Weight on the Solidification of a Semiconducting Polymer during Time-Controlled Spin-Coating. J. Phys. Chem. C 2019, 123, 17102–17111. [Google Scholar] [CrossRef]
- Matthews, R.; Swisher, J.; Hutchins, K.M.; Pentzer, E.B. Perylene Diimide Bearing Different Trialkyl Silyl Ethers: Impact of Asymmetric Functionalization on Self-Assembly into Nanostructures. Chem. Mater. 2018, 30, 3571–3577. [Google Scholar] [CrossRef]
- Seymour, R.B.; Stahl, G.A. Solvent-Property Relationships in Polymers. In Macromolecular Solutions; Pergamon Press: New York, NY, USA, 1969. [Google Scholar]
- Roberts, M.F.; Jenekhe, S.A. Lewis acid coordination complexes of polymers: Poly(benzobisimidazobenzophenanthroline) ladder and semiladder polymers. Polymer 1994, 35, 4313–4325. [Google Scholar] [CrossRef]
- Janietz, S.; Sainova, D. Significant Improvement of the Processability of Ladder-Type Polymers by Using Aqueous Colloidal Dispersions. Macromol. Rapid Commun. 2006, 27, 943–947. [Google Scholar] [CrossRef]
- Hirvonen, S.-P.; Mänttäri, M.; Wigren, V.; Salomäki, M.; Kvarnström, C.; Tenhu, H. A novel method to prepare water dispersible poly(benzimidazobenzophenanthroline) (BBL) by partial substitution of chain ends with poly(ethylene oxide). Colloid Polym. Sci. 2011, 289, 1065–1072. [Google Scholar] [CrossRef]
- Hirvonen, S.-P.; Karesoja, M.; Karjalainen, E.; Hietala, S.; Laurinmäki, P.; Vesanen, E.; Butcher, S.J.; Tenhu, H. Colloidal properties and gelation of aqueous dispersions of conductive poly(benzimidazobenzophenanthroline) derivatives. Polymer 2013, 54, 694–701. [Google Scholar] [CrossRef]
- Hirvonen, S. Enhancing the Processability of Poly(Benzimidazobenzophenanthroline) Through Chemical Modification. Ph.D. Thesis, University of Helsinki, Helsinki, Finland, 30 October 2015. [Google Scholar]
- Herbst, K.; Hunger, G.; Wilker, H.; Ohleier, R.; Winter, W. Industrial Organic Pigments: Production, Properties, Applications; John Wiley & Sons: Hoboken, NJ, USA, 2004. [Google Scholar] [CrossRef]
- Mizuguchi, J. Crystal Structure and Electronic Characterization of trans- and cis-Perinone Pigments. J. Phys. Chem. B 2004, 108, 8926–8930. [Google Scholar] [CrossRef]
- Mizuguchi, J. Dibenzimidazo[2,1-a:2′,1′-a′]anthra[2,1,9-def:6,5,10-d′e′f′]diisoquinoline-10,21-dione: Trans form (I). Acta Crystallogr. Sect. E Struct. Rep. Online 2005, 61, 1064–1065. [Google Scholar] [CrossRef]
- Zherebtsov, M.U.; Schmidt, R.; Niewa, C.P.; Sakthidharan, F.V.; Podgornov, Y.V.; Matveychuk, S.A.; Nayfert, M.A.; Polozov, S.N.; Ivashevskaya, A.I.; Stash, Y.S.; et al. Two new polymorphs of cis-perinone: Crystal structures, physical and electric properties, Acta Crystallogr. Sect. B Struct. Sci. Cryst. Eng. Mater. 2019, 75, 384–392. [Google Scholar] [CrossRef] [PubMed]
- Fabian, J.; Hartmann, H. Light Absorption of Organic Colorants: Theoretical Treatment and Empirical Rules; Springer: Berlin/Heidelberg, Germany, 1980. [Google Scholar] [CrossRef]
- Christie, R.M. Colour Chemistry; The Royal Society of Chemistry: Cambridge, UK, 2001. [Google Scholar] [CrossRef]
- Mamada, M.; Pérez-Bolívar, C.; Kumaki, D.; Esipenko, N.A.; Tokito, S.; Anzenbacher, P. Benzimidazole Derivatives: Synthesis, Physical Properties, and n-Type Semiconducting Properties. Chem. A Eur. J. 2014, 20, 11835–11846. [Google Scholar] [CrossRef] [PubMed]
- Jenekhe, S.A.; Tibbetts, S.J. Ion implantation doping and electrical properties of high-temperature ladder polymers. J. Polym. Sci. Part B Polym. Phys. 1988, 26, 201–209. [Google Scholar] [CrossRef]
- Berry, G.C.; Fox, T.G. Properties of Heterocyclic Condensation Polymers. J. Macromol. Sci. Part A Chem. 1969, 3, 1125–1146. [Google Scholar] [CrossRef]
- Berry, G.C. Thermal–mechanical studies on a heterocyclic polymer (BBB). I. Tensile creep and recovery. J. Polym. Sci. Part A-2 Polym. Phys. 1976, 14, 451–478. [Google Scholar] [CrossRef]
- Kim, O.-K. Ladder Polymers as New Polymeric Conductors. Mol. Cryst. Liq. Cryst. 1984, 105, 161–173. [Google Scholar] [CrossRef]
- Kim, O.-H. Electrical Conductivity of Heteroaromatic Ladder Polymers. J. Polym. Sci. Polym. Lett. 1982, 20, 663–666. [Google Scholar] [CrossRef]
- Kitamura, C.; Tanaka, S.; Yamashita, Y. Design of Narrow-Bandgap Polymers. Syntheses and Properties of Monomers and Polymers Containing Aromatic-Donor and o-Quinoid-Acceptor Units. Chem. Mater. 1996, 8, 570–578. [Google Scholar] [CrossRef]
- Zheng, T.; Badrun, F.; Brown, I.; Leopold, D.; Sandreczki, T. Correlation of electron spin concentration and conductivity in the ladder polymer BBL as a function of electrochemical potential. Synth. Met. 1999, 107, 39–45. [Google Scholar] [CrossRef]
- Antoniadis, H.; Abkowitz, M.A.; Osaheni, J.A.; Jenekhe, S.A.; Stolka, M. Effects of humidity on the dark conductivity and dielectric properties of poly(benzimidazobenzophenanthroline) thin films. Chem. Mater. 1994, 6, 63–66. [Google Scholar] [CrossRef]
- Leopold, D.; Brown, I.; Sandreczki, T. Electronic states induced by ion irradiation in a conjugated ladder polymer. Synth. Met. 1996, 78, 67–71. [Google Scholar] [CrossRef]
- Wang, S.; Sun, H.; Mikhail, V.; Vagin, M.; Persson, P.O.Å.; Andreasen, J.W.; Thiel, W.; Berggren, M.; Crispin, X.; Fazzi, D.; et al. Thermoelectric Properties of Solution-Processed n-Doped Ladder-Type Conducting Polymers. Adv. Mater. 2016, 28, 10764–10771. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, A.I.; Kroon, R.; Müller, C. Doping and processing of organic semiconductors for plastic thermoelectrics. In Handbook of Organic Materials for Electronic and Photonic Devices; Elsevier: Amsterdam, The Netherlands, 2019; pp. 429–449. [Google Scholar] [CrossRef]
- Sworakowski, J.; Janus, K. On the reliability of determination of energies of HOMO levels in organic semiconducting polymers from electrochemical measurements. Org. Electron. 2017, 48, 46–52. [Google Scholar] [CrossRef]
- Sworakowski, J. How accurate are energies of HOMO and LUMO levels in small-molecule organic semiconductors determined from cyclic voltammetry or optical spectroscopy? Synth. Met. 2018, 235, 125–130. [Google Scholar] [CrossRef]
- Menke, E.H.; Leibold, D.; Ullrich, A.P.; Vaynzof, Y.; Mastalerz, M. Planar versus triptycenylene end-capped aroyleneimidazoles as electron acceptors in organic photovoltaics. Org. Chem. Front. 2017, 4, 834–838. [Google Scholar] [CrossRef]
- Shao, J.; Chang, J.; Chi, C. Solution-Processable n-Type Semiconductors Based on Unsymmetrical Naphthalene Imides: Synthesis, Characterization, and Applications in Field-Effect Transistors. Chem. Asian J. 2014, 9, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Zhang, J.; Yu, G.; Chen, H.; Huang, J.; Liu, Y. Dialkyl-14H-benzo[4,5]isoquino[2,3-a]perimidin-14-one-3,4,10,11-tetracarboxylic diimides: A New Family ofn-Type Organic Semiconductors. Chem. Asian J. 2012, 7, 2208–2212. [Google Scholar] [CrossRef]
- Wilbourn, K.; Murray, R.W. The electrochemical doping reactions of the conducting ladder polymer benzimidazobenzophenanthroline (BBL). Macromolecules 1988, 21, 89–96. [Google Scholar] [CrossRef]
- Wilbourn, K.; Murray, R.W. The d.c. redox versus electronic conductivity of the ladder polymer poly(benzimidazobenzophenanthroline). J. Phys. Chem. 1988, 92, 3642–3648. [Google Scholar] [CrossRef]
- Yohannes, T.; Neugebauer, H.; Luzzati, S.; Catellani, M.; Jenekhe, S.A.; Sariciftci, N.S. Multiple Electrochemical Doping-Induced Insulator-to-Conductor Transitions Observed in the Conjugated Ladder Polymer Polybenzimidazobenzophenanthroline (BBL). J. Phys. Chem. B 2000, 104, 9430–9437. [Google Scholar] [CrossRef]
- Quinto, M.; Jenekhe, S.A.; Bard, A.J. Polymer Films on Electrodes. Electrochemistry and Scanning Electrochemical Microscopy Characterization of Benzimidazolebenzophenanthroline-Type Ladder (BBL) and Semiladder (BBB) Polymer Films. Chem. Mater. 2001, 13, 2824–2832. [Google Scholar] [CrossRef]
- Irvin, D.; Stenger-Smith, J.D.; Yandek, G.R.; Carberry, J.R.; Currie, D.A.; Theodoropoulou, N.; Irvin, J.A. Enhanced electrochemical response of solution-deposited n-doping polymer via cocasting with ionic liquid. J. Polym. Sci. Part B Polym. Phys. 2012, 50, 1145–1150. [Google Scholar] [CrossRef]
- Zhylitskaya, H.; Cybińska, J.; Chmielewski, P.; Lis, T.; Stepien, M. Bandgap Engineering in π-Extended Pyrroles. A Modular Approach to Electron-Deficient Chromophores with Multi-Redox Activity. J. Am. Chem. Soc. 2016, 138, 11390–11398. [Google Scholar] [CrossRef]
- Tang, C.W. Two-layer organic photovoltaic cell. Appl. Phys. Lett. 1986, 48, 183–185. [Google Scholar] [CrossRef]
- Peumans, V.; Bulović, S.R.; Forrest, P. Efficient photon harvesting at high optical intensities in ultrathin organic double-heterostructure photovoltaic diodes. Appl. Phys. Lett. 2000, 76, 2650–2652. [Google Scholar] [CrossRef]
- Troshin, H.; Hoppe, J.; Renz, M.; Egginger, J.Y.; Mayorova, A.E.; Goryachev, A.S.; Peregudov, R.N.; Lyubovskaya, G.; Gobsch, N.S.; Sariciftci, V.F.; et al. Material solubility-photovoltaic performance relationship in the design of novel fullerene derivatives for bulk heterojunction solar cells. Adv. Funct. Mater. 2009, 19, 779–788. [Google Scholar] [CrossRef]
- Guo, M.; Zhang, J.; Tan, S.; Zhang, L.; Huo, W.; Hu, Y.; Li, J.; Hou, X. Influence of D/A ratio on photovoltaic performance of a highly efficient polymer solar cell system. Adv. Mater. 2012, 24, 6536–6541. [Google Scholar] [CrossRef]
- Bertho, G.; Janssen, T.J.; Cleij, B.; Conings, W.; Moons, A.; Gadisa, J.; D’Haen, E.; Goovaerts, L.; Lutsen, J.; Manca, D.; et al. Effect of temperature on the morphological and photovoltaic stability of bulk heterojunction polymer:fullerene solar cells. Sol. Energy Mater. Sol. Cells. 2008, 92, 753–760. [Google Scholar] [CrossRef]
- Savoie, A.; Rao, A.A.; Bakulin, S.; Gelinas, B.; Movaghar, R.H.; Friend, T.J.; Marks, M.A.; Ratner, B.M. Unequal partnership: Asymmetric roles of polymeric donor and fullerene acceptor in generating free charge. J. Am. Chem. Soc. 2014, 136, 2876–2884. [Google Scholar] [CrossRef]
- Liu, D.B.; Shaikh, P.S.; Rao, R.S.; Bhosale, A.A.; Said, A.M.; Mak, Z.; Wang, M.; Zhao, W.; Gao, B.; Chen, Y.M.; et al. Molecular Aggregation of Naphthalene Diimide(NDI) Derivatives in Electron Transport Layers of Inverted Perovskite Solar Cells and Their Influence on the Device Performance. Chem. Asian J. 2020, 15, 112–121. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Chen, H.; Hu, L.; Zhang, T.; Ma, J.Y.L.; Lai, Z.; Li, A.; Qin, X.; Huang, B.; Tang, H.; et al. Reduced Intramolecular Twisting Improves the Performance of 3D Molecular Acceptors in Non-Fullerene Organic Solar Cells. Adv. Mater. 2016, 28, 8546–8551. [Google Scholar] [CrossRef] [PubMed]
- Liao, C.C.; Ho, C.Y.; Chang, M.H.; Jao, S.B.; Darling, W.F.; Su, H.C. Additives for morphology control in high-efficiency organic solar cells. Mater. Today 2013, 16, 326–336. [Google Scholar] [CrossRef]
- Zhan, C.; Yao, J. More than Conformational “Twisting” or “Coplanarity”: Molecular Strategies for Designing High-Efficiency Nonfullerene Organic Solar Cells. Chem. Mater. 2016, 28, 1948–1964. [Google Scholar] [CrossRef]
- Yan, Y.; Zhou, Y.Q.; Zheng, J.; Pei, D.; Zhao, Q. Towards rational design of organic electron acceptors for photovoltaics: A study based on perylenediimide derivatives. Chem. Sci. 2013, 4, 4389–4394. [Google Scholar] [CrossRef]
- Debije, P.P.C.; Verbunt, P.J.; Nadkarni, S.; Velate, K.; Bhaumik, S.; Nedumbamana, B.C.; Rowan, B.S.; Richards, T.L.; Hoeks, M.G. Promising fluorescent dye for solar energy conversion based on a perylene perinone. Appl. Opt. 2011, 50, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Dhagat, H.M.; Haverinen, R.J.; Kline, Y.; Jung, D.A.; Fischer, D.M.; DeLongchamp, G.E.; Jabbour, P. Influence of dielectric surface chemistry on the microstructure and carrier mobility of an n-type organic semiconductor. Adv. Funct. Mater. 2009, 19, 2365–2372. [Google Scholar] [CrossRef]
- Jenekhe, M.; Roberts, A.K.; Agrawal, J.S.; Meth, H.; Vanherzeele, S.A. Nonlinear Optical Properties of Ladder Polymers and Their Model Compound. MRS Proc. 1990, 214, 55–59. [Google Scholar] [CrossRef]
- Yi, S.; Jenekhe, S.A. Nanocomposites of metallophthalocyanines and conjugated Polymers. MRS Proc. 1997, 488, 1274–1278. [Google Scholar] [CrossRef]
- Jenekhe, S.A.; Yi, S. Efficient photovoltaic cells from semiconducting polymer heterojunctions. Appl. Phys. Lett. 2000, 77, 2635–2637. [Google Scholar] [CrossRef]
- Popovic, Z.D.; Loutfy, R.O.; Hor, A.-M. Photoconductivity studies of perylene tetracarboxyl-diimides. Can. J. Chem. 1985, 63, 134–139. [Google Scholar] [CrossRef]
- Babel, A.; Jenekhe, S.A. n-Channel Field-Effect Transistors from Blends of Conjugated Polymers. J. Phys. Chem. B 2002, 106, 6129–6132. [Google Scholar] [CrossRef]
- Briseno, A.L.; Kim, F.S.; Babel, A.; Xia, Y.; Jenekhe, S.A. n-Channel polymer thin film transistors with long-term air-stability and durability and their use in complementary inverters. J. Mater. Chem. 2011, 21, 16461–16466. [Google Scholar] [CrossRef]
- Babel, A.; Jenekhe, S. Electron Transport in Thin-Film Transistors from an n-Type Conjugated Polymer. Adv. Mater. 2002, 14, 371–374. [Google Scholar] [CrossRef]
- Chen, X.L.; Bao, Z.; Schön, J.H.; Lovinger, A.J.; Lin, Y.-Y.; Crone, B.; Dodabalapur, A.; Batlogg, B. Ion-modulated ambipolar electrical conduction in thin-film transistors based on amorphous conjugated polymers. Appl. Phys. Lett. 2001, 78, 228–230. [Google Scholar] [CrossRef]
- Babel, A.; Zhu, Y.; Cheng, K.-F.; Chen, W.-C.; Jenekhe, S.A. High Electron Mobility and Ambipolar Charge Transport in Binary Blends of Donor and Acceptor Conjugated Polymers. Adv. Funct. Mater. 2007, 17, 2542–2549. [Google Scholar] [CrossRef]
- Kim, F.S.; Park, C.H.; Na, Y.; Jenekhe, S.A. Effects of ladder structure on the electronic properties and field-effect transistor performance of Poly(benzobisimidazobenzophenanthroline). Org. Electron. 2019, 69, 301–307. [Google Scholar] [CrossRef]
- Kim, F.S.; Hwang, D.-K.; Kippelen, B.; Jenekhe, S.A. Enhanced carrier mobility and electrical stability of n-channel polymer thin film transistors by use of low-k dielectric buffer layer. Appl. Phys. Lett. 2011, 99, 173303. [Google Scholar] [CrossRef]
- Kim, F.S.; Ahmed, E.; Subramaniyan, S.; Jenekhe, S.A. Air-Stable Ambipolar Field-Effect Transistors and Complementary Logic Circuits from Solution-Processed n/p Polymer Heterojunctions. ACS Appl. Mater. Interfaces 2010, 2, 2974–2977. [Google Scholar] [CrossRef] [PubMed]
- Munz, A.W.; Schmeisser, D.; Goepel, W. Structural Precursors and Electronic Structure of the Ladder Type Polymer Poly(bis(benzimidazo)benzophenanthroline) (BBB): A Combined UPS/XPS and STM Study. Chem. Mater. 1994, 6, 2288–2302. [Google Scholar] [CrossRef]
- Bornoz, P.; Prévot, M.; Yu, X.; Guijarro, N.; Sivula, K. Direct Light-Driven Water Oxidation by a Ladder-Type Conjugated Polymer Photoanode. J. Am. Chem. Soc. 2015, 137, 15338–15341. [Google Scholar] [CrossRef] [PubMed]
- Erten, S.; Icli, S. Bilayer heterojunction solar cell based on naphthalene bis-benzimidazole. Inorg. Chim. Acta 2008, 361, 595–600. [Google Scholar] [CrossRef]
- Rim, S.-B.; Fink, R.F.; Schöneboom, J.C.; Erk, P.; Peumans, P. Effect of molecular packing on the exciton diffusion length in organic solar cells. Appl. Phys. Lett. 2007, 91, 173504. [Google Scholar] [CrossRef]
- Erten, S.; Meghdadi, F.; Gunes, S.; Koeppe, R.; Sariciftci, N.S.; Icli, S. Donor-acceptor heterojunction solar cells based on perylene dimide and perylene bisbenzimidazole. Eur. Phys. J. Appl. Phys. 2007, 36, 225–229. [Google Scholar] [CrossRef]
- Kukhta, N.A.; Zeika, O.; Widmer, J.; Koerner, C.; Meerheim, R.; Petrich, A.; Behrnd, N.-R.; Leo, K.; Grazulevicius, J.V. New Electron Transport Materials for High Performance Organic Solar Cells: Synthesis and Properties of Symmetrical and Asymmetrical 1,4,5,8-Naphthalenetetracarboxylic Dianhydride Derivatives. Adv. Electron. Mater. 2016, 2, 1600047. [Google Scholar] [CrossRef]
- Alam, M.M.; Jenekhe, S.A. Efficient Solar Cells from Layered Nanostructures of Donor and Acceptor Conjugated Polymers. Chem. Mater. 2004, 16, 4647–4656. [Google Scholar] [CrossRef]
- Manoj, A.; Narayan, K. Photovoltaic properties of polymer p–n junctions made with P3OT/BBL bilayers. Opt. Mater. 2003, 21, 417–420. [Google Scholar] [CrossRef]
- Menke, E.H.; Lami, V.; Vaynzof, Y.; Mastalerz, M. π-Extended rigid triptycene-trisaroylenimidazoles as electron acceptors. Chem. Commun. 2016, 52, 1048–1051. [Google Scholar] [CrossRef]
- Roberts, M.F.; Jenekhe, S.A. Lewis acid coordination complexes of polymers. 1. Boron chloride, aluminum chloride and gallium chloride complexes of poly(p-phenylenebenzobisthiazole). Chem. Mater. 1993, 5, 1744–1754. [Google Scholar] [CrossRef]
- Roberts, M.F.; Jenekhe, S.A.; Cameron, A.; McMillan, M.; Perlstein, J. Lewis Acid Coordination Complexes of Polymers. 2. Computational Modeling of Single-Chain and Aggregate Structures of Rigid-Rod Poly(p-phenylenebenzobisthiazole). Chem. Mater. 1994, 6, 658–670. [Google Scholar] [CrossRef]



























| Dianhydride | Tetramines | Yeld, % | Tdec., °C | Ref. |
|---|---|---|---|---|
 | 53 | 86–88 | 450 | [85,86] |
 | 60–90 | 450 | [85] | |
 | 90 | 440 | [87] | |
 | 62–95 | 450 | [86,88] | |
 | - | 450 | [89] | |
| 8 |  | - | 300 | [90] |
| 15a; R = H | 42.5 | - | [91] | |
| 58 | 82 | 400 | [85] | |
| 53 | 80 | 540 | [72] | |
| 59 | 95 | 365 | [92] | |
| 31 | - | - | [56] | |
| 53 | ≈100 | 470 | [93] | |
 | - | - | [94] | |
| 61 | - | 500 | [89] | |
 | - | - | [95,96] | |
| 12 |  | 20 | - | [64,96] |
| 53a | - | 300 | [90] | |
| 40 | 53 | 85 | >400 | [97] |
| 59 | 91 | >400 | [92] | |
| 60 | 85.5 | >400 | [92] | |
| 64 | 20 | - | [96] | |
 | 60 | 90 | 404 | [98] |
 | 59 | 85 | 380 | [98] |
 | - | >400 | [99] | |
 | - | >400 | [99] | |
| 53 | ~80 | >400 | [100,101] | |
 | 60 | ~80 | >400 | [100,101] |
| 53 | ~80 | >400 | [101] | |
 | 53 | ~80 | >400 | [101] |
| Implant | Saturation Conductivity (Ω−1cm−1) | |
|---|---|---|
| Jb = 1.0 μA/cm2 | Jb = 2.0 μA/cm2 | |
| 200 keV 11B+ | 26 | 56 |
| 200 keV 40Ar+ | 74 | 224 |
| 200 keV 84Kr+ | 50 | 136 |
| Compound | Eox (V) | Ered (V) | HOMO (eV) | LUMO (eV) | Ref. |
|---|---|---|---|---|---|
| 1 | −1.03 | 1.40 | −6.20 a −5.79 b −6.40 c | −3.78 d −2.71 c | [129] |
| 2 | −1.80 | 1.44 | −6.24 a −5.84 b −6.47 c | −2.98 d −2.73 c | [129] |
| 4 | −1.72 | 1.32 | −6.02 a −5.86 b −6.14 c | −3.06 d −2.73 c | [129] |
| 6 | −1.61 | 1.62 | −6.06 | −3.16 | [129] |
| 13 | − | −0.9 | −6.3 | −3.9 | [143] |
| 23 | −1.1 | − | 2.0 | 1.6 × 10−5 | [48] |
| 25 | −1.2 | − | −5.8 | −3.7 | [53] |
| 26 | −1.1 | − | −5.6 | −3.6 | [53] |
| 27 | −1.0 | − | −6.1 | −3.8 | [53] |
| 34 | −1.40 | − | − | − | [57] |
| 74 | −1.1 | 0.9 | −5.21 | −3.32 | [107] |
| 80 | −1.13 | 0.66 | −5.32 a −5.17 b −5.89 c | −3.81 d −3.63 c | [129] |
| 82 | −1.18 | 1.59 | −6.39 a −5.81 b −6.51 c | −3.62 d −2.55 c | [129] |
 | −1.02 | − | −6.05 a | −3.82 d | [129] |
| 83 | −1.24 | 1.13 | −5.31 | −3.51 | [129] |
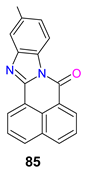 | −1.73 | 1.15 | −5.76 | −3.06 | [129] |
 | −1.71 | 0.96 | −5.69 | −3.07 | [129] |
 | −1.62 | 1.59 | −6.01 | −3.16 | [129] |
 | −1.74 | 1.01 | −5.86 | −3.04 | [129] |
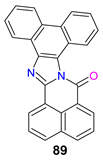 | −1.71 | 0.94 | −5.51 | −3.08 | [129] |
 | −1.67 | 0.76 | −5.86 | −3.11 | [129] |
 | −0.95 | − | −6.68 b | −4.00 | [144] |
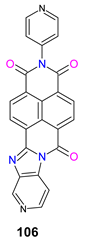 | −0.88 | − | −6.58 b | −3.90 | [144] |
 | −0.95 | − | −5.83 b | −3.79 | [144] |
 | −0.87 | − | −6.01 a | −3.92 d | [129] |
 | −0.65 | 1.39 | −5.83 | −3.79 | [95] |
 | −0.57 | 1.11 | −5.55 | −3.87 | [95] |
 | −0.56 | −1.32 | −5.7 | −3.88 | [95] |
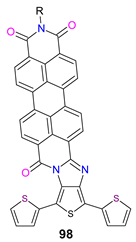 | −1.07 | 0.54 | −5.57 | −3.85 | [64] |
| −0.55 | 1.06 | −5.50 | −3.89 | [95] | |
 | −0.83 | − | −5.78 | −3.85 | [145] |
| Molecule | ΦF | τ (ms) | Eg (eV) | Mobilitiy (cm2V−1s−1) | Ref. |
|---|---|---|---|---|---|
| 1 | 0.02 | 4.9 | 2.01 | 0.005 a | [129] |
| 2 | 0.04 | 5.1 | 2.86 | - | [129] |
| 3 | - | - | 1.61 | 0.0013 | [95] |
| - | - | 1.56 | - | [64] | |
| 4 | 0.71 | 11.1 | 2.8 | - | [129] |
| 23 | - | - | 2.0 | 1.6 × 10−5 | [48] |
| - | - | 2.0 | 0.0013 c | [49] | |
| 26 | - | - | 2.1 | 2.3 × 10−5 | [54] |
| 34a R = nonylphenol | 0.9 | [163] | |||
| 27 | - | - | 2.3 | 2.3 × 10−5 | [54] |
| 80 | - b | - b | 1.51 | - | [129] |
| 0.05 | [164] | ||||
| 82 | 0.02 | 2.7 | 2.19 | - | [129] |
 | 0.11 | 3.6 | 2.23 | 0.027 d | [129] |
| 91 | 0.65 | −3.90 | 2.53 | 0.0021 | [144] |
| 92 | 0.81 | −4.00 | 2.68 | 0.0016 | [144] |
| 93 | 0.45 | −3.90 | 2.68 | 0.0016 | [144] |
| 94 | 0.11 | 4.0 | 2.09 | 0.0013 d | [129] |
| 95 | - | - | 2.04 | 0.35 | [95] |
| 96 | - | - | 1.68 | 3.5 × 10−4 | [95] |
| 97 | - | - | 1.88 | 0.15 | [95] |
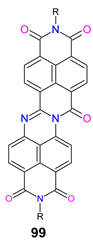
| 99 | −5.57 | −3.85 | 1.72 | 0.05 | [145] |
 | 0.58 | 19.4 | 3.05 | - | [129] |
 | 0.51 | 3.5 | 2.10 | 0.0013 d | [129] |
| Polymer | Thin Film Processing | Buffer Layers | Mobility, μe, (cm2V−1s−1) | Ion/Iof | Treshold Voltage, (V) | Ref. |
|---|---|---|---|---|---|---|
| BBL | MSA (0.8 wt. %) | - | 1.4–5.0 × 10−4 | 30–150 | −5 | [171] |
| BBL | MSA, FA a (5:1 v/v), (0.7 wt.%) | - | 2.4 × 10−5 | 8–30 | −20 | [171] |
| BBL | FeCl3,NM b (0.1 wt.%) | - | 9 × 10−6 | <10 | −5 | [171] |
| BBL | AlCl3,NM b (n/a) | - | 4 × 10−6 | 2–50 | n/a | [172] |
| BBL | GaCl3,NM b (n/a) | - | 1 × 10−6 | 2–30 | n/a | [172] |
| BBL | MSA | - | 6.2 × 10−4 | 103 | 36.5 | [170] |
| BBL | MSA | - | 8.0 × 10−4 | 104 | [173] | |
| BBL | Nanobelt network | - | 5–7 × 10−3 | 1 × 104 | 11.9 | [114] |
| BBL | MSA, (0.8 wt.%) | Polystyrene | 2.2 × 10−2 | >104 | 10.93 | [174] |
| BBL | MSA | - | 3.6 × 10−4 | >103 | ~35 | [175] |
| BBL | MSA | Polystyrene | 1.8 × 10−2 | >103 | ~35 | [175] |
| BBL | MSA | Benzocyclobutene | 1.6 × 10−2 | >103 | ~35 | [175] |
| BBL | MSA | Ethylene-norborenene copolymer | 2.0 × 10−2 | >103 | ~35 | [175] |
| BBL | MSA | Poly(vinylchloride) | 5.2–5.8 × 10−4 | >103 | ~35 | [175] |
| BBL | MSA | Poly(vinylcarbazole) | 5.2–5.8 × 10−4 | >103 | ~35 | [175] |
| BBL | MSA | Poly(thiazolothiazole) | 7.8 × 10−3 | 103 | 10.1 | [176] |
| BBL | MSA | poly(benzobisthiazole-alt-3-octylquarterthiophene) | 5.1 × 10−3 | 103 | 4.3 | [176] |
| BBL | MSA c | 1,1,1,3,3,3-hexamethyldisila-zane | 0.1 | 2 × 103 | 10 | [74] |
| BBB | 1,1,1,3,3,3-hexamethyldisi-lazane | 10−6 | >104 | - | [74] | |
| BBB | MSA, (0.8 wt.%) | Polystyrene | 1.75 × 10−3 | >104 | 14.21 | [174] |
| BBL/BBB blend | MSA | 1,1,1,3,3,3-hexamethyldisi-lazane | 10−5 | >104 | - | [74] |
| BBL/PTQ d blend | MSA | - | 1.0 × 10−3 | 200 | - | [173] |
| BBL/POT e blend | - | 8.0 × 10−4 | 200 | - | [173] |
| Perinone Acceptor | Donor | JSC [mA/cm2] | VOC [V] | FF | PCE [%] | Ref. |
|---|---|---|---|---|---|---|
| 1 | CuPc | 2.3 | 0.45 | 0.65 | 1 | [152] |
| 1 | ZnPc a | 2.11 | 0.5 | 0.51 | 0.54 | [179] |
| 13 | PTB7 b | 3.6 | 0.82 | 0.4 | 1.2 | [143] |
| 30 | PTB7 | 4.47 | 0.90 | 0.45 | 1.8 | [55] |
| 30a | PTB7 | 5.3 | 0.96 | 0.39 | 2.0 | [55] |
| 37 | CuPc c | 2.3 | 0.45 | 0.65 | 0.95 | [152] |
| 4.18 | 0.4 | - | 1.1 | [180] | ||
| 0.54 | 0.57 | 2.4 d | [153] | |||
| 38a | ZnPc | 4.9 | 0.48 | 0.56 | 1.3 | [181] |
| CuPc | 3.66 | 0.4 | - | 0.93 | [180] | |
| 74 | PCBM, 1:1 | 0.60 | 0.82 | 0.253 | 0.125 | [107] |
| PCBM, 1:2 | 0.55 | 1.21 | 0.267 | 0.178 | ||
| PCBM, 1:3 | 0.60 | 4.00 | 0.254 | 0.612 | ||
| PCBM, 1:4 | 0.55 | 4.46 | 0.271 | 0.666 | ||
 | C60/DCV5T e -MeC60 | 12.5 | 0.96 | 0.657 | 7.9 | [182] |
| BBL | PPV | 1.2 | 1.2 | 0.41–0.43 | 0.7–1.2 | [167] |
| 0.182 | 0.54 | 0.47 | 4.6 f | [183] | ||
| BBL | MEH-PPV | 0.144 | 0.62 | 0.41 | 3.7 c | [183] |
| BBL | P3OT | - | 0.9 | 0.023 | 0.2 | [184] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Łapkowski, M. Perinone—New Life of an Old Molecule. Materials 2021, 14, 6880. https://doi.org/10.3390/ma14226880
Łapkowski M. Perinone—New Life of an Old Molecule. Materials. 2021; 14(22):6880. https://doi.org/10.3390/ma14226880
Chicago/Turabian StyleŁapkowski, Mieczysław. 2021. "Perinone—New Life of an Old Molecule" Materials 14, no. 22: 6880. https://doi.org/10.3390/ma14226880
APA StyleŁapkowski, M. (2021). Perinone—New Life of an Old Molecule. Materials, 14(22), 6880. https://doi.org/10.3390/ma14226880







