Hybrid Materials Based on Fly Ash, Metakaolin, and Cement for 3D Printing
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Materials
2.2. Characterization of Raw Materials
2.3. Preparation of Geopolymer Specimens
2.4. Characterization of Geopolymers
2.5. Hybrid Preparation
2.6. Statistical Analysis
3. Results and Discussion
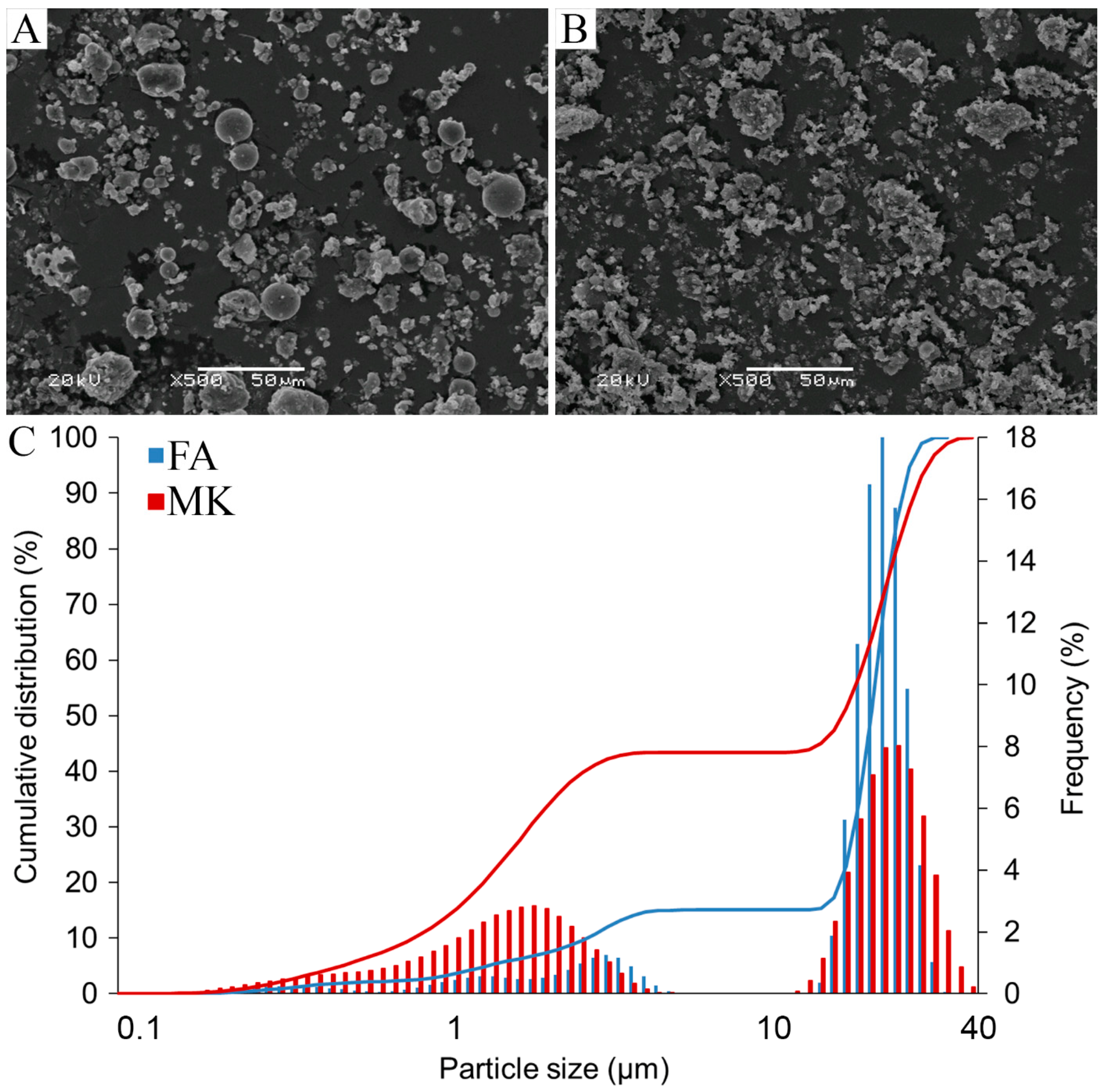
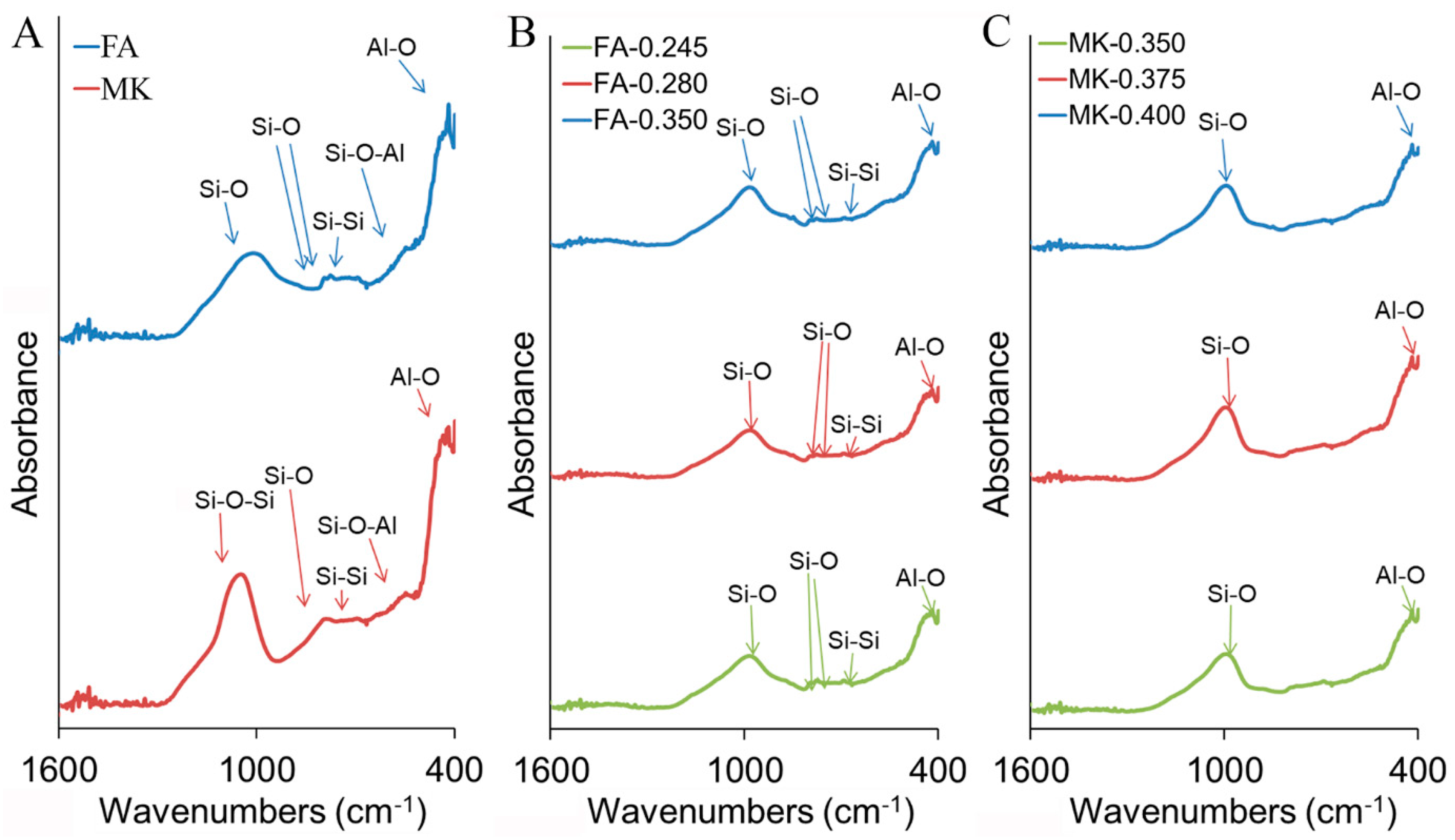
3.1. Characterization of Raw Materials
3.2. Properties of Concrete and Geopolymers
3.3. Concrete and Geopolymer Hybrid Materials and Their 3D Printing
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ferreira, E.G.A.; Yokaichiya, F.; Rodrigues, M.S.; Beraldo, A.L.; Isaac, A.; Kardjilov, N.; Franco, M.K.K.D. Assessment of Greener Cement by employing thermally treated sugarcane straw ashes. Constr. Build. Mater 2017, 141, 343–352. [Google Scholar] [CrossRef]
- Abrão, P.C.R.A.; Cardoso, F.A.; John, V.M. Efficiency of Portland-pozzolana cements: Water demand, chemical reactivity and environmental impact. Constr. Build. Mater. 2020, 247, 118546. [Google Scholar] [CrossRef]
- Wu, Y.; Lu, B.; Bai, T.; Wang, H.; Du, F.; Zhang, Y.; Cai, L.; Jiang, C.; Wang, W. Geopolymer, green alkali activated cementitious material: Synthesis, applications and challenges. Constr. Build. Mater. 2019, 224, 930–949. [Google Scholar] [CrossRef]
- Schneider, M.; Romer, M.; Tschudin, M.; Bolio, H. Sustainable cement production-present and future. Cem. Concr. Res. 2011, 41, 642–650. [Google Scholar] [CrossRef]
- Yan, B.; Duan, P.; Ren, D. Mechanical strength, surface abrasion resistance and microstructure of fly ash-metakaolin-sepiolite geopolymer composites. Ceram. Int. 2017, 43, 1052–1060. [Google Scholar] [CrossRef]
- Celik, A.; Yilmaz, K.; Canpolat, O.; Al-mashhadani, M.M.; Aygörmez, Y.; Uysal, M. High-temperature behavior and mechanical characteristics of boron waste additive metakaolin based geopolymer composites reinforced with synthetic fibers. Constr. Build. Mater. 2018, 187, 1190–1203. [Google Scholar] [CrossRef]
- Lahoti, M.; Wijaya, S.F.; Tan, K.H.; Yang, E.H. Tailoring sodium-based fly ash geopolymers with variegated thermal performance. Cem. Concr. Compos. 2020, 107, 103507. [Google Scholar] [CrossRef]
- Singh, N.B.; Middendorf, B. Geopolymers as an alternative to Portland cement: An overview. Constr. Build. Mater. 2020, 237, 117455. [Google Scholar] [CrossRef]
- Lahoti, M.; Tan, K.H.; Yang, E.H. A critical review of geopolymer properties for structural fire-resistance applications. Constr. Build. Mater. 2019, 221, 514–526. [Google Scholar] [CrossRef]
- Ling, Y.; Wang, K.; Wang, X.; Hua, S. Effects of mix design parameters on heat of geopolymerization, set time, and compressive strength of high calcium fly ash geopolymer. Constr. Build. Mater. 2019, 228, 116763. [Google Scholar] [CrossRef]
- Zhang, H.Y.; Kodur, V.; Qi, S.L.; Cao, L.; Wu, B. Development of metakaolin-fly ash based geopolymers for fire resistance applications. Constr. Build. Mater. 2014, 55, 38–45. [Google Scholar] [CrossRef]
- Ji, Z.; Pei, Y. Bibliographic and visualized analysis of geopolymer research and its application in heavy metal immobilization: A review. J. Environ. Manag. 2019, 231, 256–267. [Google Scholar] [CrossRef]
- Lahoti, M.; Wong, K.K.; Yang, E.H.; Tan, K.H. Effects of Si/Al molar ratio on strength endurance and volume stability of metakaolin geopolymers subject to elevated temperature. Ceram. Int. 2018, 44, 5726–5734. [Google Scholar] [CrossRef]
- Cheng, T.W.; Lee, M.L.; Ko, M.S.; Ueng, T.H.; Yang, S.F. The heavy metal adsorption characteristics on metakaolin-based geopolymer. Appl. Clay Sci. 2012, 56, 90–96. [Google Scholar] [CrossRef]
- Mohseni, E. Assessment of Na2SiO3 to NaOH ratio impact on the performance of polypropylene fiber-reinforced geopolymer composites. Constr. Build. Mater. 2018, 186, 904–911. [Google Scholar] [CrossRef]
- Wongpa, J.; Kiattikomol, K.; Jaturapitakkul, C.; Chindaprasirt, P. Compressive strength, modulus of elasticity, and water permeability of inorganic polymer concrete. Mater. Des. 2010, 31, 4748–4754. [Google Scholar] [CrossRef]
- Belmokhtar, N.; Ammari, M.; Brigui, J.; Allal, L.B. Comparison of the microstructure and the compressive strength of two geopolymers derived from Metakaolin and an industrial sludge. Constr. Build. Mater. 2017, 146, 621–629. [Google Scholar] [CrossRef]
- Akono, A.T.; Koric, S.; Kriven, W.M. Influence of pore structure on the strength behavior of particle- and fiber-reinforced metakaolin-based geopolymer composites. Cem. Concr. Compos. 2019, 104, 103361. [Google Scholar] [CrossRef]
- Komnitsas, K.; Zaharaki, D. Geopolymerisation: A review and prospects for the minerals industry. Miner. Eng. 2007, 20, 1261–1277. [Google Scholar] [CrossRef]
- Abdel-Gawwad, H.A.; Abo-El-Enein, S.A. A novel method to produce dry geopolymer cement powder. HBRC J. 2016, 12, 13–24. [Google Scholar] [CrossRef]
- Wang, A.; Liu, H.; Hao, X.; Wang, Y.; Liu, X.; Li, Z. Geopolymer Synthesis Using Garnet Tailings from Molybdenum Mines. Minerals 2019, 9, 48. [Google Scholar] [CrossRef]
- Lin, W.T.; Korniejenko, K.; Hebda, M.; Łach, M.; Mikuła, J. Engineering properties of ternary cementless blended materials. Int. J. Eng. Technol. Innov. 2020, 10, 191–199. [Google Scholar] [CrossRef]
- Grela, A.; Łach, M.; Bajda, T.; Mikuła, J.; Hebda, M. Characterization of the products obtained from alkaline conversion of tuff and metakaolin. J. Therm. Anal. Calorim. 2018, 133, 217–226. [Google Scholar] [CrossRef]
- Selmani, S.; Sdiri, A.; Bouaziz, S.; Joussein, E.; Rossignol, S. Effects of metakaolin addition on geopolymer prepared from natural kaolinitic clay. Appl. Clay Sci. 2017, 146, 457–467. [Google Scholar] [CrossRef]
- Prasanphan, S.; Wannagon, A.; Kobayashi, T.; Jiemsirilers, S. Reaction mechanisms of calcined kaolin processing waste-based geopolymers in the presence of low alkali activator solution. Constr. Build. Mater. 2019, 221, 409–420. [Google Scholar] [CrossRef]
- Gado, R.A.; Hebda, M.; Łach, M.; Mikuła, J. Alkali Activation of Waste Clay Bricks: Influence of The Silica Modulus, SiO2/Na2O, H2O/Na2O Molar Ratio, and Liquid/Solid Ratio. Materials 2020, 13, 383. [Google Scholar] [CrossRef]
- Huang, X.; Huang, T.; Li, S.; Muhammad, F.; Xu, G.; Zhao, Z.; Yu, L.; Yan, Y.; Li, D.; Jiao, B. Immobilization of chromite ore processing residue with alkali-activated blast furnace slag-based geopolymer. Ceram. Int. 2016, 42, 9538–9549. [Google Scholar] [CrossRef]
- Toniolo, N.; Boccaccini, A.R. Fly ash-based geopolymers containing added silicate waste. A review. Ceram. Int. 2017, 43, 14545–14551. [Google Scholar] [CrossRef]
- Bohra, V.K.J.; Nerella, R.; Madduru, S.R.C. Material properties, processing & characterization of fly ash based geopolymer. Mater. Today Proc. 2019, 19, 2617–2621. [Google Scholar] [CrossRef]
- Doğan-Sağlamtimur, N.; Bilgil, A.; Szechyńska-Hebda, M.; Parzych, S.; Hebda, M. Eco-Friendly Fired Brick Produced from Industrial Ash and Natural Clay: A Study of Waste Reuse. Materials 2021, 14, 877. [Google Scholar] [CrossRef]
- Lin, W.T.; Lin, K.L.; Chen, K.; Korniejenko, K.; Hebda, M.; Łach, M. Circulation Fluidized Bed Combustion Fly Ash as Partial Replacement of Fine Aggregates in Roller Compacted Concrete. Materials 2019, 12, 4204. [Google Scholar] [CrossRef]
- Grela, A.; Łach, M.; Mikuła, J.; Hebda, M. Thermal analysis of the products of alkali activation of fly ash from CFB boilers. J. Therm. Anal. Calorim. 2016, 124, 1609–1621. [Google Scholar] [CrossRef]
- Kaur, K.; Singh, J.; Kaur, M. Compressive strength of rice husk ash based geopolymer: The effect of alkaline activator. Constr. Build. Mater. 2018, 168, 188–192. [Google Scholar] [CrossRef]
- Burciaga-Díaz, O.; Durón-Sifuentes, M.; Díaz-Guillén, J.A.; Escalante-García, J.I. Effect of waste glass incorporation on the properties of geopolymers formulated with low purity metakaolin. Cem. Concr. Compos. 2020, 107, 103492. [Google Scholar] [CrossRef]
- Toniolo, N.; Rincón, A.; Avadhut, Y.S.; Hartmann, M.; Bernardo, E.; Boccaccini, A.R. Novel geopolymers incorporating red mud and waste glass cullet. Mater. Lett. 2018, 219, 152–154. [Google Scholar] [CrossRef]
- Koshy, N.; Dondrob, K.; Hu, L.; Wen, Q.; Meegoda, J.N. Synthesis and characterization of geopolymers derived from coal gangue, fly ash and red mud. Constr. Build. Mater. 2019, 206, 287–296. [Google Scholar] [CrossRef]
- Nath, P.; Sarker, P.K. Flexural strength and elastic modulus of ambient-cured blended low-calcium fly ash geopolymer concrete. Constr. Build. Mater. 2017, 130, 22–31. [Google Scholar] [CrossRef]
- Juenger, M.C.G.; Winnefeld, F.; Provis, J.L.; Ideker, J.H. Advances in alternative cementitious binders. Cem. Concr. Res. 2011, 41, 1232–1243. [Google Scholar] [CrossRef]
- Nguyen, K.T.; Ahn, N.; Le, T.A.; Lee, K. Theoretical and experimental study on mechanical properties and flexural strength of fly ash-geopolymer concrete. Constr. Build. Mater. 2016, 106, 65–77. [Google Scholar] [CrossRef]
- Wang, Y.C.; Zhao, J.P. Facile preparation of slag or fly ash geopolymer composite coatings with flame resistance. Constr. Build. Mater. 2019, 203, 655–661. [Google Scholar] [CrossRef]
- Zhang, Z.; Provis, J.L.; Reid, A.; Wang, H. Geopolymer foam concrete: An emerging material for sustainable construction. Constr. Build. Mater. 2014, 56, 113–127. [Google Scholar] [CrossRef]
- Zhang, H.Y.; Kodur, V.; Wu, B.; Cao, L.; Wang, F. Thermal behavior and mechanical properties of geopolymer mortar after exposure to elevated temperatures. Constr. Build. Mater. 2016, 109, 17–24. [Google Scholar] [CrossRef]
- Ng, C.; Alengaram, U.J.; Wong, L.S.; Mo, K.H.; Jumaat, M.Z.; Ramesh, S. A review on microstructural study and compressive strength of geopolymer mortar, paste and concrete. Constr. Build. Mater. 2018, 186, 550–576. [Google Scholar] [CrossRef]
- Shuai, Q.; Xu, Z.; Yao, Z.; Chen, X.; Jiang, Z.; Peng, Z.; An, R.; Li, Y.; Jiang, X.; Li, H. Fire resistance of phosphoric acid-based geopolymer foams fabricated from metakaolin and hydrogen peroxide. Mater. Lett. 2020, 263, 1–4. [Google Scholar] [CrossRef]
- Zhuang, H.J.; Zhang, H.Y.; Xu, H. Resistance of geopolymer mortar to acid and chloride attacks. Procedia Eng. 2017, 210, 126–131. [Google Scholar] [CrossRef]
- Sanjayan, J.G.; Nazari, A.; Pouraliakbar, H. FEA modelling of fracture toughness of steel fibre-reinforced geopolymer composites. Mater. Des. 2015, 76, 215–222. [Google Scholar] [CrossRef]
- Ling, Y.; Wang, K.; Fu, C. Shrinkage behavior of fly ash based geopolymer pastes with and without shrinkage reducing admixture. Cem. Concr. Compos. 2019, 98, 74–82. [Google Scholar] [CrossRef]
- Zhao, R.; Yuan, Y.; Cheng, Z.; Wen, T.; Li, J.; Li, F.; Ma, Z.J. Freeze-thaw resistance of Class F fly ash-based geopolymer concrete. Constr. Build. Mater. 2019, 222, 474–483. [Google Scholar] [CrossRef]
- Zhang, Y.J.; Wang, Y.C.; Xu, D.L.; Li, S. Mechanical performance and hydration mechanism of geopolymer composite reinforced by resin. Mater. Sci. Eng. A 2010, 527, 6574–6580. [Google Scholar] [CrossRef]
- Wijaya, M.F.; Olivia, M.; Wibisono, G.; Saputra, E.; Wang, S. Characteristics of geopolymer hybrid concrete in peat water. IOP Conf. Ser. Mater. Sci. Eng. 2019, 615, 012120. [Google Scholar] [CrossRef]
- Barboza-Chavez, A.C.; Gómez-Zamorano, L.Y.; Acevedo-Dávila, J.L. Synthesis and Characterization of a Hybrid Cement Based on Fly Ash, Metakaolin and Portland Cement Clinker. Materials 2020, 13, 1084. [Google Scholar] [CrossRef]
- Rojas-Duque, O.; Espinosa, L.M.; Robayo-Salazar, R.A.; Mejía de Gutiérrez, R. Alkali-Activated Hybrid Concrete Based on Fly Ash and Its Application in the Production of High-Class Structural Blocks. Crystals 2020, 10, 946. [Google Scholar] [CrossRef]
- Marczyk, J.; Ziejewska, C.; Łach, M.; Korniejenko, K.; Lin, W.T.; Hebda, M. Possibilities of using the 3D printing process in the concrete and geopolymers application. IOP Conf. Ser. Mater. Sci. Eng. 2019, 706, 012019. [Google Scholar] [CrossRef]
- Xia, M.; Sanjayan, J. Method of formulating geopolymer for 3D printing for construction applications. Mater. Des. 2016, 110, 382–390. [Google Scholar] [CrossRef]
- Siddika, A.; Mamun, A.A.A.; Ferdous, W.; Saha, A.K. 3D-printed concrete: Applications, performance, and challenges. J. Sustain. Cement-Based Mater. 2020, 9, 127–164. [Google Scholar] [CrossRef]
- Bagheri, A.; Cremona, C. Formulation of mix design for 3D printing of geopolymers: A machine learning approach. Mater. Adv. 2020, 1, 720. [Google Scholar] [CrossRef]
- PN-EN 197-1. Cement—Part 1: Composition, Specification and Conformity Criteria for Common Cements. 2014. Available online: https://standards.iteh.ai/catalog/standards/cen/64f4e2ca-0c2e-4f68-8c50-16f9f69bc572/pren-197-1 (accessed on 12 November 2021).
- Szechyńska-Hebda, M.; Marczyk, J.; Ziejewska, C.; Hordyńska, N.; Mikuła, J.; Hebda, M. Neutral geopolymer foams reinforced with cellulose studied with the FT-Raman spectroscopy. IOP Conf. Ser. Mater. Sci. Eng. 2019, 706, 012017. [Google Scholar] [CrossRef]
- Szechyńska-Hebda, M.; Marczyk, J.; Ziejewska, C.; Hordyńska, N.; Mikuła, J.; Hebda, M. Optimal Design of pH-neutral Geopolymer Foams for Their Use in Ecological Plant Cultivation Systems. Materials 2019, 12, 2999. [Google Scholar] [CrossRef]
- Mierzwiński, D.; Łach, M.; Hebda, M.; Walter, J.; Szechyńska-Hebda, M.; Mikuła, J. Thermal phenomena of alkali-activated metakaolin studied with a negative temperature coefficient system. J. Therm. Anal. Calorim. 2019, 138, 4167–4175. [Google Scholar] [CrossRef]
- PN-EN 1744-1:2010+A1:2013 Tests for Chemical Properties of Aggregates. Chemical Analysis. Available online: https://standards.iteh.ai/catalog/standards/sist/b1e5043b-1edc-49e8-9409-ed59a5808203/sist-en-1744-1-2010a1-2013 (accessed on 9 January 2013).
- PN-EN 15934:2012 Sludge, Treated Biowaste, Soil and Waste—Calculation of Dry Matter Fraction after Determination of Dry Residue or Water Content. Available online: https://standards.iteh.ai/catalog/standards/cen/c7e440f9-c400-4318-8ee5-637e7f127c5d/en-15934-2012 (accessed on 22 August 2012).
- PN-EN ISO 10523:2012 Water Quality—Determination of pH. Available online: https://standards.iteh.ai/catalog/standards/cen/8e85ce30-e43b-4af1-a586-386403da6b56/en-iso-10523-2012 (accessed on 15 February 2012).
- PN-EN 12457-2:2002 Characterisation of Waste—Leaching-Compliance Test for Leaching of Granular Waste Materials and Sludges—Part 2: One Stage Batch Test at a Liquid to Solid Ratio of 10 L/kg for Materials with Particle Size below 4 mm (without or with Size Reduction). Available online: https://standards.iteh.ai/catalog/standards/cen/db6fbdf3-1de7-457c-a506-46c4898e3f09/en-12457-2-2002 (accessed on 18 September 2002).
- Łach, M.; Hebdowska-Krupa, M.; Stefańska, A.; Stefanek, J.; Stanek, A.; Mikuła, J.; Hebda, M. Characterisation of post-production raw material from the Raciszyn II deposit as a material suitable for the production of alkaline-activated materials. J. Therm. Anal. Calorim. 2019, 138, 4551–4559. [Google Scholar] [CrossRef]
- Kalak, T.; Kłopotek, A.; Cierpiszewski, R. Effective adsorption of lead ions using fly ash obtained in the novel circulating fluidized bed combustion technology. Microchem. J. 2019, 145, 1011–1025. [Google Scholar] [CrossRef]
- Hebda, M.; Laska, M.; Szechyńska-Hebda, M. Application of a device used for observation of controlled thermal processes in a furnace: Examples of delubrication, oxidation, melting, pyrolysis, and combustion. J. Therm. Anal. Calorim. 2013, 114, 1099–1109. [Google Scholar] [CrossRef][Green Version]
- PN-EN 12390:2019 Testing Hardened Concrete. Available online: https://standards.iteh.ai/catalog/standards/cen/ae7e6a86-1cbc-455e-8b2a-8964be9087f9/en-12390-2-2019 (accessed on 26 June 2019).
- PN-EN 13892-3:2014 Methods of Test for Screed Materials—Part 3: Determination of Wear Resistance—Böhme. Available online: https://standards.iteh.ai/catalog/standards/cen/e92c20b0-6afa-4ab6-a2dd-9240e13a7ba4/en-13892-3-2014 (accessed on 17 December 2014).
- Akgün, Y.; Yazıcıoğlu, O.F. The Abrasion Resistance of Mortars Containing Natural Zeolite Analcime. Eur. J. Eng. Nat. Sci. 2019, 3, 6–11. [Google Scholar]
- PN-EN ISO 1182:2020 Reaction to Fire Tests for Products—Non-Combustibility Test. Available online: https://standards.iteh.ai/catalog/standards/sist/36888eed-5e43-41c3-ad45-9078a16a66ba/sist-en-iso-1182-2020 (accessed on 16 July 2020).
- Aboulayt, A.; Jaafri, R.; Samouh, H.; Idrissi, A.C.E.; Roziere, E.; Moussa, R.; Loukili, A. Stability of a new geopolymer grout: Rheological and mechanical performances of metakaolin-fly ash binary mixtures. Constr. Build. Mater. 2018, 181, 420–436. [Google Scholar] [CrossRef]
- Korniejenko, K.; Łach, M.; Marczyk, J.; Ziejewska, C.; Halyag, N.P.; Mucsi, G. Fly ash as a raw material for geopolymerisation-mineralogical composition and morphology. IOP Conf. Ser. Mater. Sci. Eng. 2019, 706, 1–8. [Google Scholar] [CrossRef]
- Sujjavanich, S.; Suwanvitaya, P.; Chaysuwan, D.; Heness, G. Synergistic effect of metakaolin and fly ash on properties of concrete. Constr. Build. Mater. 2017, 155, 830–837. [Google Scholar] [CrossRef]
- Assi, L.N.; Deaver, E.E.; Ziehl, P. Effect of source and particle size distribution on the mechanical and microstructural properties of fly Ash-Based geopolymer concrete. Constr. Build. Mater. 2018, 167, 372–380. [Google Scholar] [CrossRef]
- Li, Z.; Gao, Y.; Zhang, J.; Zhang, C.; Chen, J.; Liu, C. Effect of particle size and thermal activation on the coal gangue based geopolymer. Mater. Chem. Phys. 2021, 267, 124657. [Google Scholar] [CrossRef]
- Almutairi, A.L.; Tayeh, B.A.; Adesina, A.; Isleem, H.F.; Zeyad, A.M. Potential applications of geopolymer concrete in construction: A review. Case Stud. Constr. Mater. 2021, 15, e00733. [Google Scholar] [CrossRef]
- Pawluczuk, E.; Kalinowska-Wichrowska, K.; Jiménez, J.R.; Fernández-Rodríguez, J.M.; Suescum-Morales, D. Geopolymer concrete with treated recycled aggregates: Macro and microstructural behavior. J. Build. Eng. 2021, 44, 103317. [Google Scholar] [CrossRef]
- Traven, K.; Češnovar, M.; Ducman, V. Particle size manipulation as an influential parameter in the development of mechanical properties in electric arc furnace slag-based AAM. Ceram. Int. 2019, 45, 22632–22641. [Google Scholar] [CrossRef]
- Łach, M.; Gado, R.A.; Marczyk, J.; Ziejewska, C.; Dogan-Saglamtimur, N.; Mikuła, J.; Szechyńska-Hebda, M.; Hebda, M. Process design for a production of sustainable materials from post-production clay. Materials 2021, 14, 953. [Google Scholar] [CrossRef]
- Horvat, B.; Ducman, V. Influence of Particle Size on Compressive Strength of Alkali Activated Refractory Materials. Materials 2020, 13, 2227. [Google Scholar] [CrossRef]
- Kaminska, K.; Dzierwa, P. The influence of compaction and saturation on the compressibility of colliery waste. Therm. Sci. 2019, 23, 1345–1355. [Google Scholar] [CrossRef]
- Górski, M.; Wielgus, N.; Loska, K.; Kozioł, M.; Landrat, M.; Ścierski, W.; Pikoń, K. Characteristics of Metakaolin-Based Geopolymer with Cathode Ray Tube Glass. Polymers 2021, 13, 1149. [Google Scholar] [CrossRef]
- Sing, K. Reporting Physisorption Data for Gas/Solid Systems with Special Reference to the Determination of Surface Area and Porosity. Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- El Alouani, M.; Alehyen, S.; El Achouri, M.; Taibi, M. Comparative study of the adsorption of micropollutant contained in aqueous phase using coal fly ash and activated coal fly ash: Kinetic and isotherm studies. Chem. Data Collect. 2019, 23, 1–8. [Google Scholar] [CrossRef]
- Liang, G.; Zhu, H.; Zhang, Z.; Wu, Q. Effect of rice husk ash addition on the compressive strength and thermal stability of metakaolin based geopolymer. Constr. Build. Mater. 2019, 222, 872–881. [Google Scholar] [CrossRef]
- Nežerka, V.; Bílý, P.; Hrbek, V.; Fládr, J. Impact of silica fume, fly ash, and metakaolin on the thickness and strength of the ITZ in concrete. Cem. Concr. Compos. 2019, 103, 252–262. [Google Scholar] [CrossRef]
- Luo, H.; Law, W.W.; Wu, Y.; Zhu, W.; Yang, E.H. Hydrothermal synthesis of needle-like nanocrystalline zeolites from metakaolin and their applications for efficient removal of organic pollutants and heavy metals. Microporous Mesoporous Mater. 2018, 272, 8–15. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Chen, F.; Zhang, Y.; Liu, J.; Wang, X.; Chu, P.K.; Chu, B.; Zhang, N. Fly ash based lightweight wall materials incorporating expanded perlite/SiO2 aerogel composite: Towards low thermal conductivity. Constr. Build. Mater. 2020, 249, 118728. [Google Scholar] [CrossRef]
- Guo, X.; Sji, H. Metakaolin-, fly ash- and calcium hydroxide-based geopolymers: Effects of calcium on performance. Adv. Cem. Res. 2015, 27, 559–566. [Google Scholar] [CrossRef]
- Pantazopoulou, E.; Zouboulis, A. Chemical toxicity and ecotoxicity evaluation of tannery sludge stabilized with ladle furnace slag. J. Environ. Manag. 2018, 216, 257–262. [Google Scholar] [CrossRef]
- Council of the European Union and 2003/33/EC. Council Decision Establishing Criteria and Procedures for the Acceptance of Waste at Landfills Pursuant to Article 16 of and Annex II to Directive 1999/31/EC; EUR-lex, European Union: Luxembourg, 2003; Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=uriserv%3AOJ.L.2003.011.01.0027.01.ENG&toc=OJ%3AL%3A2003%3A011%3ATOC (accessed on 16 January 2003).
- Wei, Y.; Wang, J.; Wang, J.; Zhan, L.; Ye, X.; Tan, H. Hydrothermal processing, characterization and leaching toxicity of Cr-added ‘fly ash-metakaolin’ based geopolymer. Constr. Build. Mater. 2020, 251, 118931. [Google Scholar] [CrossRef]
- Kaiser, S. Radiological protection principles concerning the natural radioactivity of building materials. Radiat. Prot. 1999, 112, 1–16. [Google Scholar]
- Gupta, M.; Mahur, A.K.; Varshney, R.; Sonkawade, R.G.; Verma, K.G.; Prasad, R. Measurement of natural radioactivity and radon exhalation rate in fly ash samples from a thermal power plant and estimation of radiation doses. Radiat. Meas. 2013, 50, 160–165. [Google Scholar] [CrossRef]
- Chen, H.-J.; Shih, N.-H.; Wu, C.-H.; Lin, S.-K. Effects of the Loss on Ignition of Fly Ash on the Properties of High-Volume Fly Ash Concrete. Sustainability 2019, 11, 2704. [Google Scholar] [CrossRef]
- Sun, Z.; Vollpracht, A.; van der Sloot, H.A. pH dependent leaching characterization of major and trace elements from fly ash and metakaolin geopolymers. Cem. Concr. Res. 2019, 125, 105889. [Google Scholar] [CrossRef]
- Osholana, T.S.; Dludlu, M.K.; Oboirien, B.; Sadiku, R. Enhanced reactivity of geopolymers produced from fluidized bed combustion bottom ash. S. Afr. J. Chem. Eng. 2020, 34, 72–77. [Google Scholar] [CrossRef]
- Zenabou, N.N.M.; Benoit-Ali, N.; Zekeng, S.; Rossignol, S.; Melo, U.C.; Tchamba, A.B.; Kamseu, E.; Leonelli, C. Improving insulation in metakaolin based geopolymer: Effects of metabauxite and metatalc. J. Build. Eng. 2019, 23, 403–415. [Google Scholar] [CrossRef]
- Kocak, Y. Effects of metakaolin on the hydration development of Portland–composite cement. J. Build. Eng. 2020, 31, 101419. [Google Scholar] [CrossRef]
- Xie, J.; Kayali, O. Effect of initial water content and curing moisture conditions on the development of fly ash-based geopolymers in heat and ambient temperature. Constr. Build. Mater. 2014, 67, 20–28. [Google Scholar] [CrossRef]
- Xu, H.; van Deventer, J.S.J. The geopolymerisation of alumino-silicate minerals. Int. J. Miner. Process. 2000, 59, 247–266. [Google Scholar] [CrossRef]
- Clausi, M.; Fernández-Jiménez, A.M.; Palomo, A.; Tarantino, S.C.; Zema, M. Reuse of waste sandstone sludge via alkali activation in matrices of fly ash and metakaolin. Constr. Build. Mater. 2018, 172, 212–223. [Google Scholar] [CrossRef]
- Ismail, I.; Bernal, S.A.; Provis, J.L.; San Nicolas, R.; Hamdan, S.; van Deventer, J.S.J. Modification of phase evolution in alkali-activated blast furnace slag by the incorporation of fly ash. Cem. Concr. Compos. 2014, 45, 125–135. [Google Scholar] [CrossRef]
- Carabba, L.; Moricone, R.; Scarponi, G.E.; Tugnoli, A.; Bignozzi, M.C. Alkali activated lightweight mortars for passive fire protection: A preliminary study. Constr. Build. Mater. 2019, 195, 75–84. [Google Scholar] [CrossRef]
- Silva, G.; Kim, S.; Aguilar, R.; Nakamatsu, J. Natural fibers as reinforcement additives for geopolymers—A review of potential eco-friendly applications to the construction industry. Sustain. Mater. Technol. 2020, 23, e00132. [Google Scholar] [CrossRef]
- Panda, B.; Tan, M.J. Experimental study on mix proportion and fresh properties of fly ash based geopolymer for 3D concrete printing. Ceram. Int. 2018, 44, 10258–10265. [Google Scholar] [CrossRef]
- Cui, X.M.; Liu, L.P.; He, Y.; Chen, J.Y.; Zhou, J. A novel aluminosilicate geopolymer material with low dielectric loss. Mater. Chem. Phys. 2011, 130, 1–4. [Google Scholar] [CrossRef]
- Pangdaeng, S.; Sata, V.; Aguiar, J.B.; Pacheco-Torgal, F.; Chindaprasirt, P. Apatite formation on calcined kaolin-white Portland cement geopolymer. Mater. Sci. Eng. C 2015, 51, 1–6. [Google Scholar] [CrossRef]
- Chen, L.; Wang, Z.; Wang, Y.; Feng, J. Preparation and Properties of Alkali Activated Metakaolin-Based Geopolymer. Materials 2016, 9, 767. [Google Scholar] [CrossRef]
- Barbosa, V.F.F.; MacKenzie, K.J.D.; Thaumaturgo, C. Synthesis and characterisation of materials based on inorganic polymers of alumina and silica: Sodium polysialate polymers. Int. J. Inorg. Mater. 2000, 2, 309–317. [Google Scholar] [CrossRef]
- Jonbi, J.; Fulazzaky, M.A. Modeling the water absorption and compressive strength of geopolymer paving block: An empirical approach. Meas. J. Int. Meas. Confed. 2020, 158, 107695. [Google Scholar] [CrossRef]
- Elyamany, H.E.; Abd Elmoaty, A.E.M.; Elshaboury, A.M. Setting time and 7-day strength of geopolymer mortar with various binders. Constr. Build. Mater. 2018, 187, 974–983. [Google Scholar] [CrossRef]
- Ramujee, K.; Potharaju, M. Permeability and abrasion resistance of geopolymer concrete. Indian Concr. J. 2014, 88, 34–43. [Google Scholar] [CrossRef]
- Kim, H.K.; Hwang, E.A.; Lee, H.K. Impacts of metakaolin on lightweight concrete by type of fine aggregate. Constr. Build. Mater. 2012, 36, 719–726. [Google Scholar] [CrossRef]
- Singh, D.B.; Kumar, N.; Kaushal, D.R.; Sharma, A.K.; Yadav, J.K. Effect of solid concentration and grain size on the rheology of fly ash slurries. Mater. Today-Proc. 2021, 46, 10904–10908. [Google Scholar] [CrossRef]
- Chen, Y.; Veer, F.; Copuroglu, O.A. Critical Review of 3D Concrete Printing as a Low CO2 Concrete Approach. Heron 2017, 62, 167–194. [Google Scholar] [CrossRef]
- Salam, N.M.; Ma, G.; Ijaz, N.; Wang, L. Importance and potential of cellulosic materials and derivatives in extrusion-based 3D concrete printing (3DCP): Prospects and challenges. Constr. Build. Mater. 2021, 291, 123281. [Google Scholar] [CrossRef]
- Łach, M.; Mierzwiński, D.; Korniejenko, K.; Mikuła, J.; Hebda, M. Geopolymers as a material suitable for immobilization of fly ash from municipal waste incineration plants. J. Air Waste Manag. 2018, 68, 1190–1197. [Google Scholar] [CrossRef] [PubMed]
- Korniejenko, K.; Figiela, B.; Miernik, K.; Ziejewska, C.; Marczyk, J.; Hebda, M.; Cheng, A.; Lin, W.T. Mechanical and Fracture Properties of Long Fiber Reinforced Geopolymer Composites. Materials 2021, 14, 5183. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.A.; Zafar, A.; Farooq, F.; Javed, M.F.; Alyousef, R.; Alabduljabbar, H.; Khan, M.I. Geopolymer Concrete Compressive Strength via Artificial Neural Network, Adaptive Neuro Fuzzy Interface System, and Gene Expression Programming with K-Fold Cross Validation. Front. Mater. 2021, 8, 621163. [Google Scholar] [CrossRef]
- Dao, D.V.; Ly, H.B.; Trinh, S.H.; Le, T.T.; Pham, B.T. Artificial Intelligence Approaches for Prediction of Compressive Strength of Geopolymer Concrete. Materials 2019, 12, 983. [Google Scholar] [CrossRef]

| Sample | Composition, wt. % | Liquid/Solid Ratio | ||
|---|---|---|---|---|
| FA: Sand 1:1 | MK: Sand 1:1 | 10M NaOH: Water Glass 1:2.5 | ||
| FA-0.245 | 80.32 | - | 19.68 | 0.25 |
| FA-0.280 | 78.12 | - | 21.88 | 0.28 |
| FA-0.350 | 74.07 | - | 25.93 | 0.35 |
| MK-0.350 | - | 74.07 | 25.93 | 0.35 |
| MK-0.375 | - | 72.73 | 27.27 | 0.38 |
| MK-0.400 | - | 71.43 | 28.57 | 0.40 |
| Parameter | FA | MK |
|---|---|---|
| Single-Point BET (m2 g−1) | 10.431 | 12.999 |
| Multi-Point BET (m2 g−1) | 12.760 | 15.315 |
| Surface Area BET (m2 g−1) | 14.616 | 21.415 |
| Total pore volume BJH (cm3 g−1) | 0.026 | 0.140 |
| Pore volume BJH (cm3 g−1) | 0.027 | 0.142 |
| Average pore diameter BJH (nm) | 2.134 | 2.975 |
| Main Minerals, % | Accessory Minerals, ppm | Mineral Phases, % | ||||||
|---|---|---|---|---|---|---|---|---|
| FA | MK | FA | MK | FA | MK | |||
| SiO2 | 48.220 | 52.430 | BaO | 800.0 | 99.7 | Quartz | 42.0 | 9.4 |
| TiO2 | 1.110 | 0.310 | SrO | 600.0 | 103.5 | Mullite | 52.5 | 5.8 |
| Al2O3 | 26.130 | 42.750 | Zn | 199.7 | 37.1 | Hematite | 2.6 | - |
| Fe2O3 | 7.010 | 1.200 | Pb | 146.4 | 151.7 | Magnetite | 1.0 | - |
| MnO | 0.090 | 0.012 | V | 274.2 | 34.6 | Anhydrite | 1.2 | - |
| MgO | 1.720 | 0.175 | Cr | 171.4 | - | Rutile | 0.7 | - |
| CaO | 5.120 | 0.490 | Cu | 129.8 | 15.3 | Illite-2M1 | - | 43.4 |
| Na2O | 1.615 | 0.000 | Ni | 109.6 | - | Kaolinite-1A | - | 41.4 |
| K2O | 3.480 | 1.300 | Rb | 184.0 | 156.6 | |||
| P2O5 | 0.700 | 0.440 | Ga | 31.6 | 57.6 | |||
| SO3 | 1.110 | 0.030 | Zr | 209.0 | 84.0 | |||
| Cl | 0.090 | 0.060 | Te | 40.1 | 22.5 | |||
| LOI | 3.284 | 0.722 | As | - | 20.4 | |||
| Sb | 20.6 | - | ||||||
| Sn | 45.2 | 37.0 | ||||||
| Y | 49.1 | 17.7 | ||||||
| Sample | Compressive Strength | Flexural Strength | Abrasion Resistance | ||
|---|---|---|---|---|---|
| 1 Day | 28 Days | 28 Days | 28 Days | 28 Days | |
| FA-0.245 | 44.73 ± 8.05 | 39.55 ± 3.29 | 7.58 ± 0.54 | 26.27 ± 9.67 | 7.20 ± 2.64 |
| FA-0.280 | 41.71 ± 11.27 | 47.47 ± 1.12 | 9.38 ± 0.36 | 36.07 ± 2.21 | 9.81 ± 0.67 |
| FA-0.350 | 25.45 ± 2.75 | 40.43 ± 7.20 | 5.68 ± 0.33 | 14.22 ± 1.72 | 3.82 ± 0.46 |
| MK-0.350 | 68.34 ± 2.64 | 53.24 ± 3.78 | 6.25 ± 0.56 | 12.40 ± 0.04 | 3.34 ± 0.04 |
| MK-0.375 | 61.40 ± 7.90 | 34.65 ± 4.69 | 4.50 ± 0.02 | 30.17 ± 13.24 | 8.50 ± 3.96 |
| MK-0.400 | 24.62 ± 0.52 | 34.23 ± 3.26 | 4.29 ± 0.67 | 18.24 ± 4.29 | 4.70 ± 1.14 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marczyk, J.; Ziejewska, C.; Gądek, S.; Korniejenko, K.; Łach, M.; Góra, M.; Kurek, I.; Doğan-Sağlamtimur, N.; Hebda, M.; Szechyńska-Hebda, M. Hybrid Materials Based on Fly Ash, Metakaolin, and Cement for 3D Printing. Materials 2021, 14, 6874. https://doi.org/10.3390/ma14226874
Marczyk J, Ziejewska C, Gądek S, Korniejenko K, Łach M, Góra M, Kurek I, Doğan-Sağlamtimur N, Hebda M, Szechyńska-Hebda M. Hybrid Materials Based on Fly Ash, Metakaolin, and Cement for 3D Printing. Materials. 2021; 14(22):6874. https://doi.org/10.3390/ma14226874
Chicago/Turabian StyleMarczyk, Joanna, Celina Ziejewska, Szymon Gądek, Kinga Korniejenko, Michał Łach, Mateusz Góra, Izabela Kurek, Neslihan Doğan-Sağlamtimur, Marek Hebda, and Magdalena Szechyńska-Hebda. 2021. "Hybrid Materials Based on Fly Ash, Metakaolin, and Cement for 3D Printing" Materials 14, no. 22: 6874. https://doi.org/10.3390/ma14226874
APA StyleMarczyk, J., Ziejewska, C., Gądek, S., Korniejenko, K., Łach, M., Góra, M., Kurek, I., Doğan-Sağlamtimur, N., Hebda, M., & Szechyńska-Hebda, M. (2021). Hybrid Materials Based on Fly Ash, Metakaolin, and Cement for 3D Printing. Materials, 14(22), 6874. https://doi.org/10.3390/ma14226874









