Hierarchical Interfaces as Fracture Propagation Traps in Natural Layered Composites
Abstract
:1. Introduction
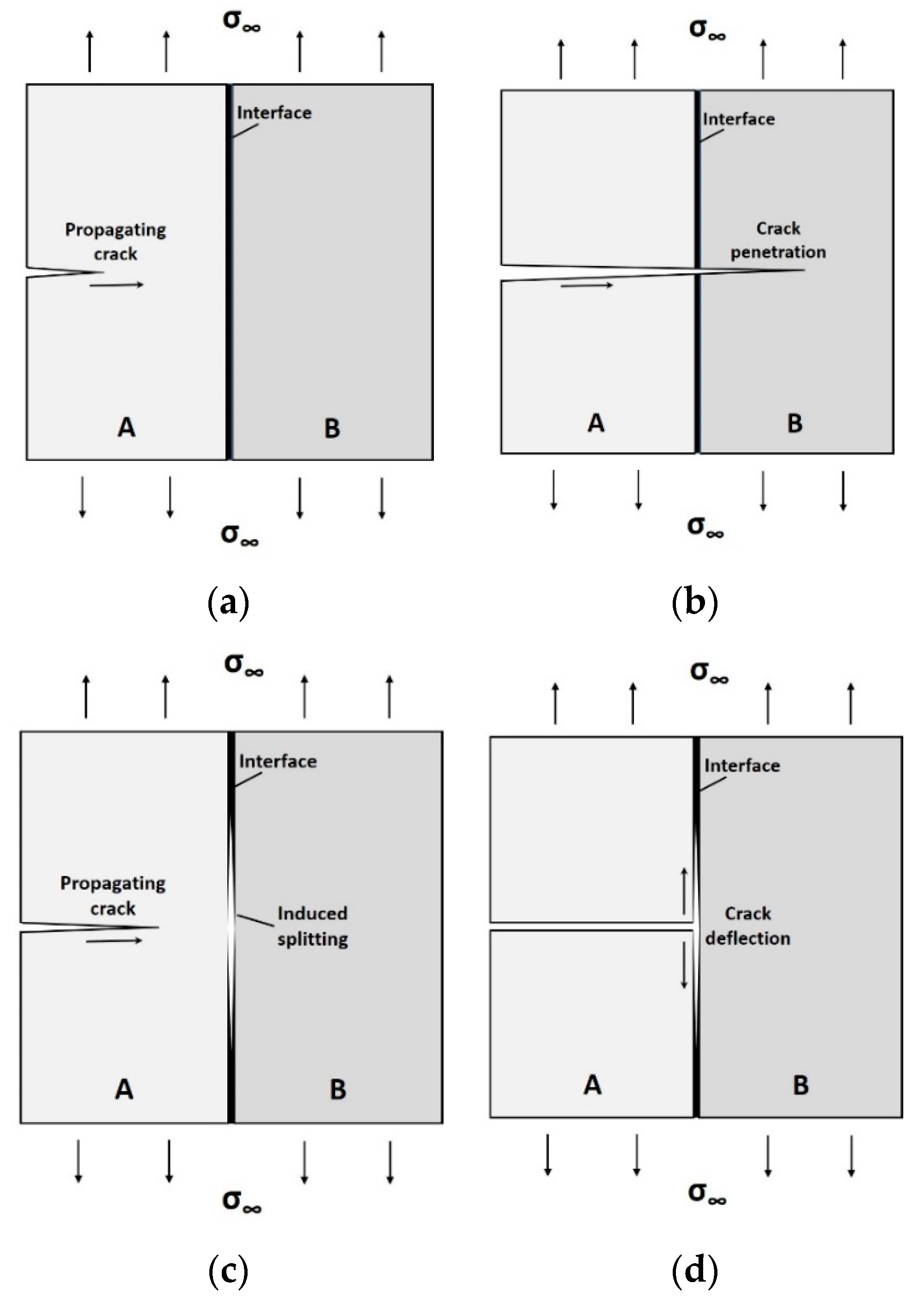
2. Interfaces in Engineering Materials
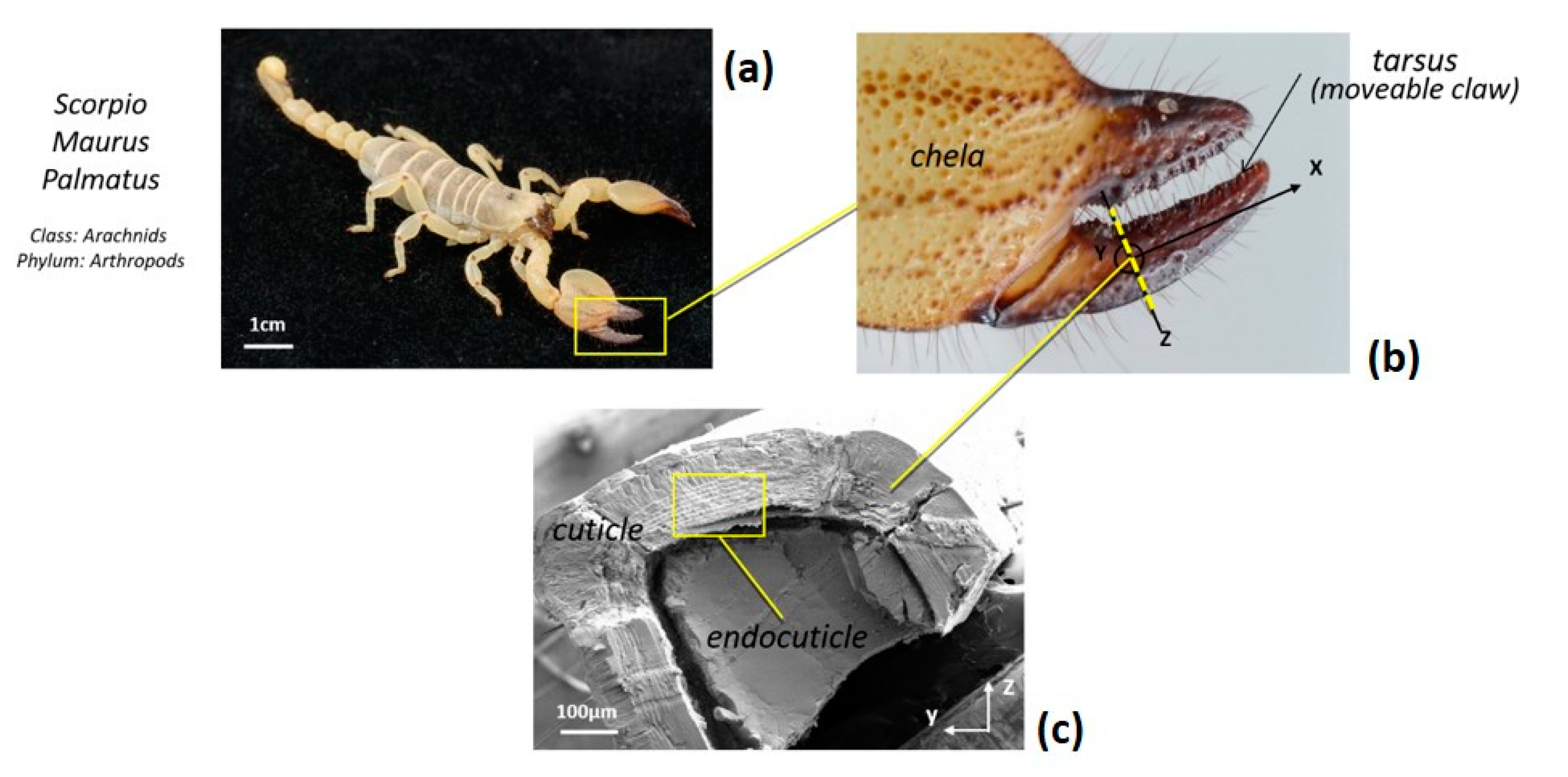
3. Interfaces in Natural Materials
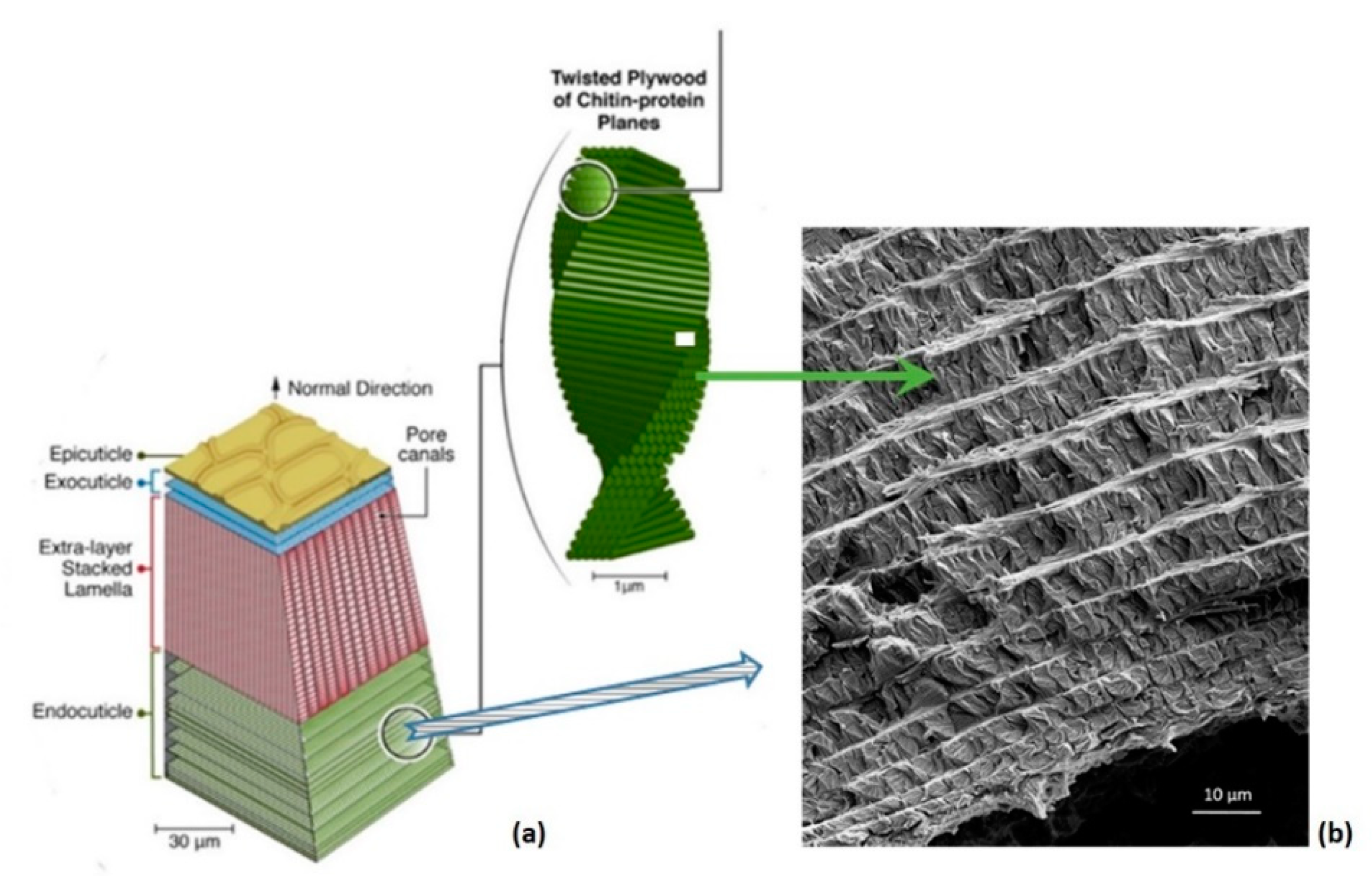
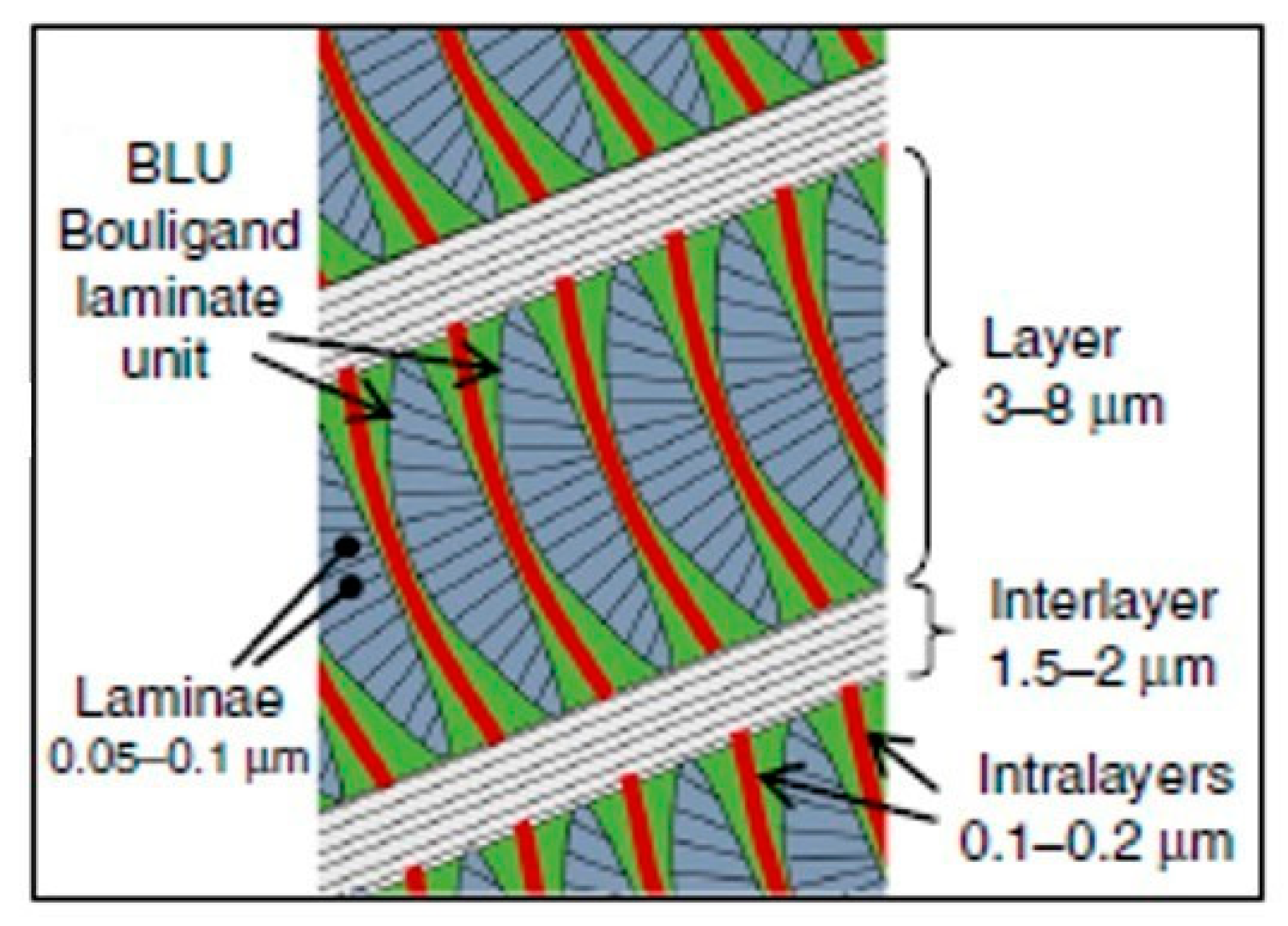
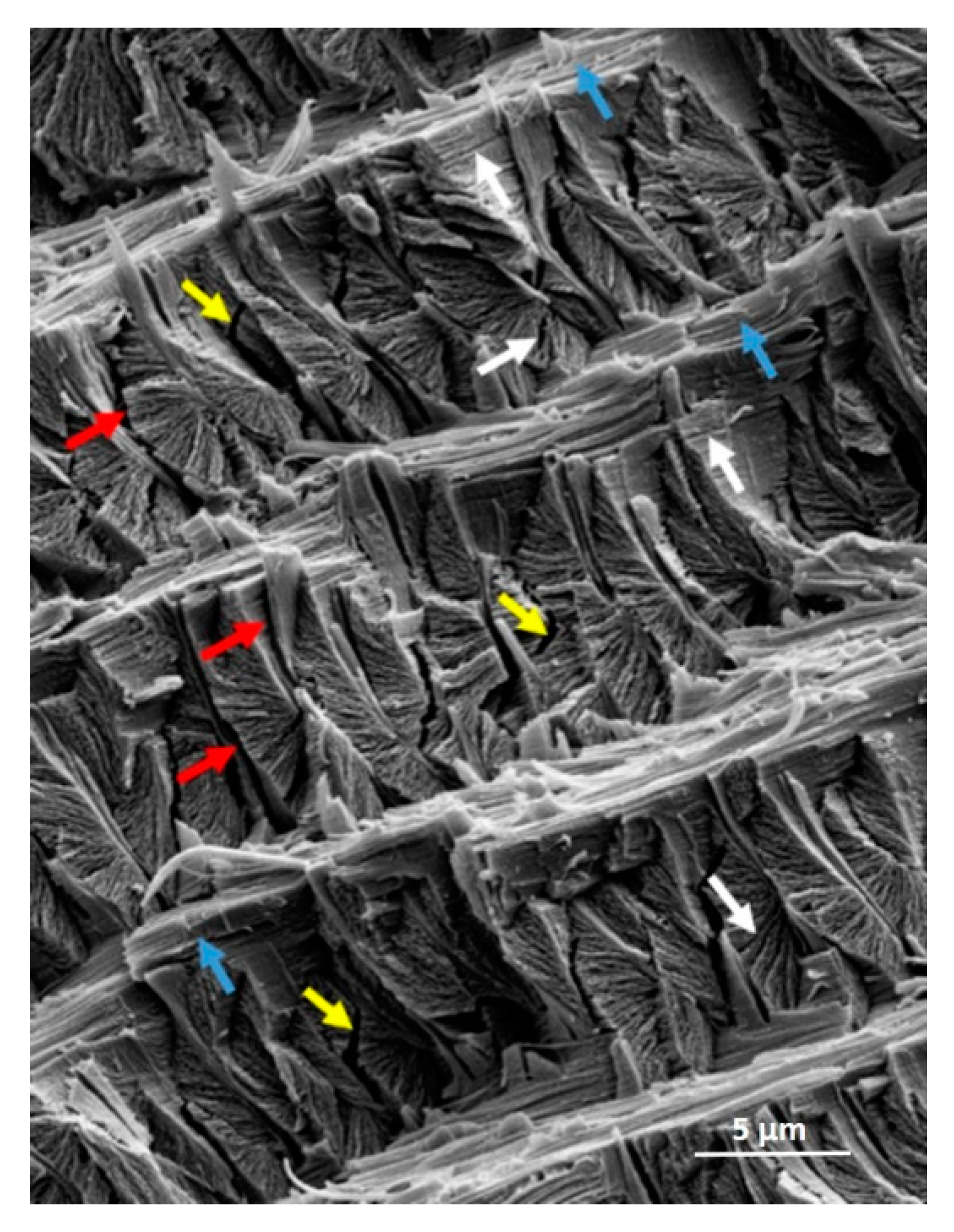
3.1. Hierarchical Interfaces
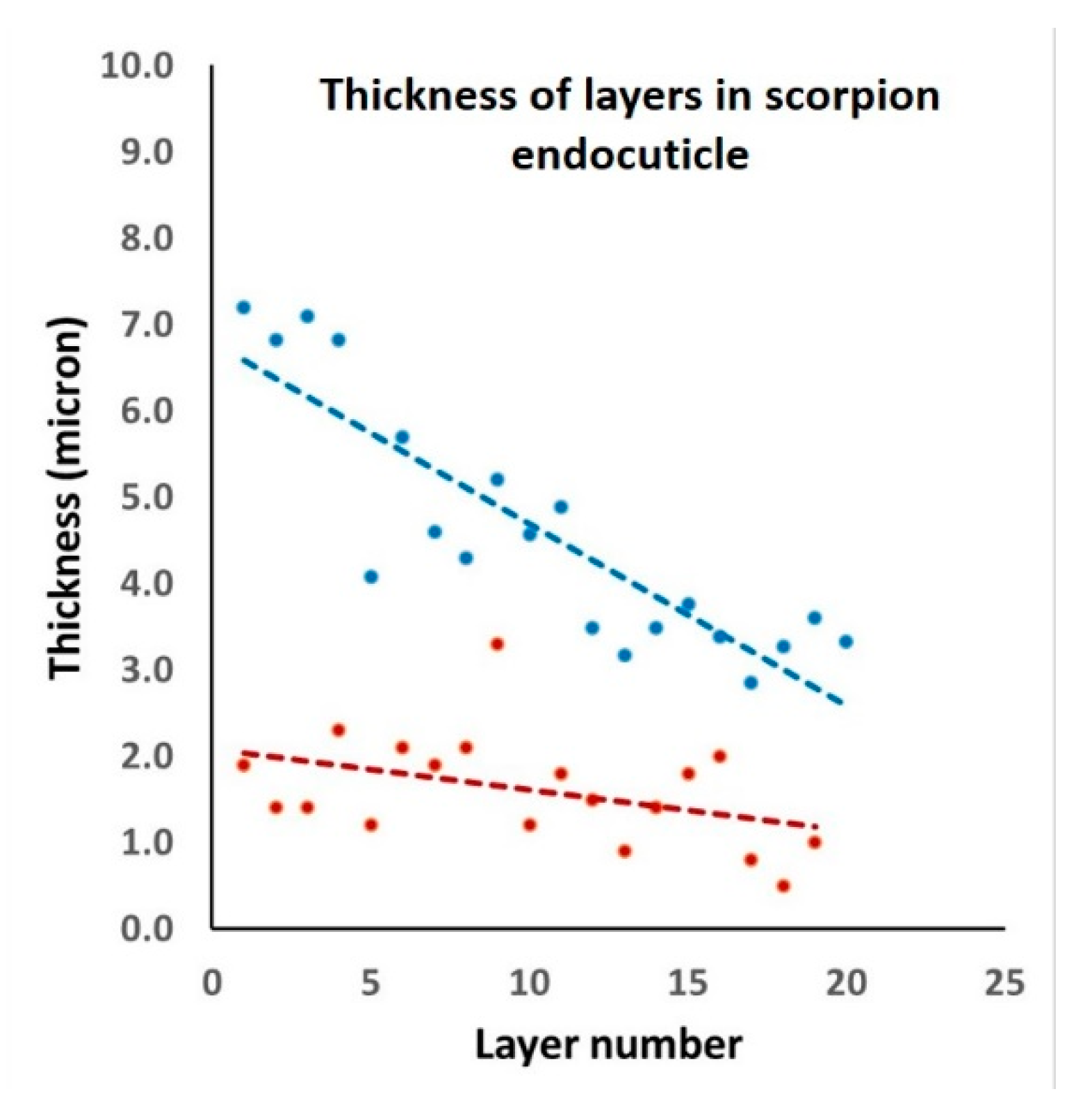
3.2. Layer Thickness Variability in Cylindrical Structures
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Launey, M.E.; Ritchie, R.O. On the Fracture Toughness of Advanced Materials. Adv. Mater. 2009, 21, 2103–2110. [Google Scholar] [CrossRef]
- Cook, J.; Gordon, J.E. A mechanism for the control of crack propagation in all-brittle systems. Proc. R. Soc. Lond. A 1964, 282, 508–520. [Google Scholar]
- Liu, Z.; Zhang, Z.; Ritchie, R.O. Structural Orientation and Anisotropy in Biological Materials: Functional Designs and Mechanics. Adv. Funct. Mater. 2020, 30, 1908121. [Google Scholar] [CrossRef]
- Kendall, K. Transition between Cohesive and Interfacial Failure in a Laminate. Proc. Roy. Soc. Lond. A 1975, 344, 287–302. [Google Scholar]
- Thouless, M.D.; Cao, H.C.; Mataga, P.A. Delamination from surface cracks in composite materials. J. Mater. Sci. 1989, 24, 1406–1412. [Google Scholar] [CrossRef]
- Bermejo, R.; Danzer, R. High failure resistance layered ceramics using crack bifurcation and interface delamination as reinforcement mechanisms. Eng. Fract. Mech. 2010, 77, 2126–2135. [Google Scholar] [CrossRef]
- He, M.Y.; Hutchinson, J.W. Crack deflection at an interface between dissimilar elastic materials. Int. J. Solids Struct. 1989, 25, 1053–1067. [Google Scholar]
- Evans, A.G.; Rühle, M.; Dalgleish, B.J.; Charalambides, P.G. The Fracture Energy of Bimaterial Interfaces. Metall. Trans. A 1990, 21, 2419–2429. [Google Scholar] [CrossRef]
- Wagner, H.D.; Marom, G. Delamination failure in hybrid composites: The effect of the stacking sequence. In Proceedings of the Reinforced Plastics/Composites Institute, Annual Conference, Houston, TX, USA, 7–11 February 1983. [Google Scholar]
- Evans, A.G. Perspective on the development of high-toughness ceramics. J. Am. Ceram Soc. 1990, 73, 187–206. [Google Scholar] [CrossRef]
- Clegg, W.J.; Kendall, K.; Alford, N.N.; Button, T.W.; Birchall, J.D. A simple way to make tough ceramics. Nature 1990, 347, 455–457. [Google Scholar] [CrossRef]
- Mayer, G. New classes of tough composite materials—Lessons from natural rigid biological systems. Mater. Sci. Eng. C 2006, 26, 1261–1268. [Google Scholar] [CrossRef]
- Livanov, K.; Jelitto, H.; Bar-On, B.; Schulte, K.; Schneider, G.A.; Wagner, H.D. Tough alumina/polymer layered composites with high ceramic content. J. Am. Ceram. Soc. 2015, 98, 1285–1291. [Google Scholar] [CrossRef]
- Livanov, K.; Yang, L.; Nissenbaum, A.; Wagner, H.D. Interphase tuning for stronger and tougher composites. Nature Sc. Rep. 2016, 6, 26305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dunlop, J.W.C.; Weinkamer, R.; Fratzl, P. Artful interfaces within biological materials. Materials Today 2011, 14, 70–78. [Google Scholar] [CrossRef]
- Barthelat, F.; Yin, Z.; Buehler, M.J. Structure and mechanics of interfaces in biological materials. Nat. Rev. Mater. 2016, 1, 16007. [Google Scholar] [CrossRef]
- Ritchie, R.O.; Buehler, M.J.; Hansma, P. Plasticity and toughness in bone. Physics Today 2009, 62, 41–47. [Google Scholar] [CrossRef]
- Dastjerdi, A.K.; Rabiei, R.; Barthelat, F. The weak interfaces within tough natural composites: Experiments on three types of nacre. J. Mech. Behav. Biomed. Mater. 2013, 19, 50–60. [Google Scholar] [CrossRef]
- Weiner, S.; Wagner, H.D. The material bone: Structure mechanical function relations. Ann. Rev. Mater. Sci. 1998, 28, 271–298. [Google Scholar] [CrossRef]
- Rho, J.Y.; Kuhn-Spearing, L.; Zioupos, P. Mechanical properties and the hierarchical structure of bone. Med. Eng. Phys. 1998, 20, 92–102. [Google Scholar] [CrossRef]
- Giesa, T.; Spivak, D.I.; Buehler, M.J. Category Theory Based Solution for the Building Block Replacement Problem in Materials Design. Adv. Eng. Mater. 2012, 14, 810–817. [Google Scholar] [CrossRef] [Green Version]
- Kushner, A.M.; Gabuchian, V.; Johnson, E.G.; Guan, Z. Biomimetic Design of Reversibly Unfolding Cross-Linker to Enhance Mechanical Properties of 3D Network Polymers. J. Amer. Chem. Soc. 2007, 129, 14110–14111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Studart, A.R. Towards High-Performance Bioinspired Composites. Adv. Mater. 2012, 24, 5024–5044. [Google Scholar] [CrossRef] [PubMed]
- Kellersztein, I.; Cohen, S.R.; Bar-On, B.; Wagner, H.D. The Exoskeleton of scorpion’s pincers: Structure and micro-mechanical properties. Acta Biomater. 2019, 94, 565–573. [Google Scholar] [CrossRef]
- Greenfeld, I.; Kellersztein, I.; Wagner, H.D. Nested helicoids in biological microstructures. Nat. Commun. 2020, 11, 224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kellersztein, I.; Greenfeld, I.; Wagner, H.D. Structural analysis across length scales of the scorpion pincer cuticle. Bioinsp. Biomim. 2021, 16, 026013. [Google Scholar] [CrossRef] [PubMed]
- Neville, A.C. The physics of helicoids. Phys. Bull. 1986, 37, 74–76. [Google Scholar] [CrossRef]
- Bouligand, Y. Sur une architecture torsadée répandue dans de nombreuses cuticules d’arthropodes. C. R. Acad. Sci. 1965, 261, 3665–3668. [Google Scholar]
- Ouyang, W.; Gong, B.; Wang, H.; Scarpa, F.; Su, B.; Peng, H.-X. Identifying optimal rotating pitch angles in composites with Bouligand structure. Compos. Commun. 2021, 23, 100602. [Google Scholar] [CrossRef]
- Suksangpanya, N.; Yaraghib, N.A.; Kisailus, D.; Zavattieri, P. Twisting cracks in Bouligand structures. J. Mech. Behav. Biomed. Mater. 2017, 76, 38–57. [Google Scholar] [CrossRef]
- Aizenberg, J.; Weaver, J.C.; Thanawala, M.S.; Sundar, V.C.; Morse, D.E.; Fratzl, P. Skeleton of Euplectella sp.: Structural Hierarchy from the Nanoscale to the Macroscale. Science 2005, 309, 275–278. [Google Scholar] [CrossRef] [Green Version]
- Mayer, G. Rigid biological systems as models for synthetic composites. Science 2005, 310, 1144–1147. [Google Scholar] [CrossRef]
- Miserez, A.; Weaver, J.C.; Thurner, J.; Aizenberg, J.; Dauphin, Y.; Fratzl, P.; Morse, D.E.; Zok, F.W. Effects of laminate architecture on fracture resistance of sponge biosilica: Lessons from nature. Adv. Funct. Mater. 2008, 18, 1241–1248. [Google Scholar] [CrossRef]
- Monn, M.A.; Weaver, J.C.; Zhanga, T.; Aizenberg, J.; Kesaria, H. New functional insights into the internal architecture of the laminated anchor spicules of Euplectella aspergillum. Proc. Natl. Acad. Sci. USA 2015, 112, 4976–4981. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weaver, J.C.; Aizenberg, J.; Fantner, G.E.; Kisailus, D.; Woesz, A.; Allen, P.; Fields, K.; Porter, M.J.; Zok, F.W.; Hansma, P.K.; et al. Hierarchical assembly of the siliceous skeletal lattice of the hexactinellid sponge Euplectella aspergillum. J. Struct. Biol. 2007, 158, 93–106. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Ji, B.; Jäger, I.L.; Arzt, E.; Fratzl, P. Materials become insensitive to flaws at nanoscale: Lessons from nature. Proc. Natl. Acad. Sci. USA 2003, 100, 5597–5600. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brach, S.; Hossain, M.Z.; Bourdin, B.; Bhattacharya, K. Anisotropy of the effective toughness of layered media. J. Mech. Phys. Sol. 2019, 131, 96–111. [Google Scholar] [CrossRef]
- Alessi, R.; Freddi, F. Failure and complex crack patterns in hybrid laminates: A phase-field approach. Compos. Part B 2019, 179, 107256. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wagner, H.D. Hierarchical Interfaces as Fracture Propagation Traps in Natural Layered Composites. Materials 2021, 14, 6855. https://doi.org/10.3390/ma14226855
Wagner HD. Hierarchical Interfaces as Fracture Propagation Traps in Natural Layered Composites. Materials. 2021; 14(22):6855. https://doi.org/10.3390/ma14226855
Chicago/Turabian StyleWagner, Hanoch Daniel. 2021. "Hierarchical Interfaces as Fracture Propagation Traps in Natural Layered Composites" Materials 14, no. 22: 6855. https://doi.org/10.3390/ma14226855
APA StyleWagner, H. D. (2021). Hierarchical Interfaces as Fracture Propagation Traps in Natural Layered Composites. Materials, 14(22), 6855. https://doi.org/10.3390/ma14226855




