Laser Desorption/Ionization Mass Spectrometry as a Potential Tool for Evaluation of Hydroxylation Degree of Various Types of Titanium Dioxide Materials
Abstract
1. Introduction
2. Materials and Methods
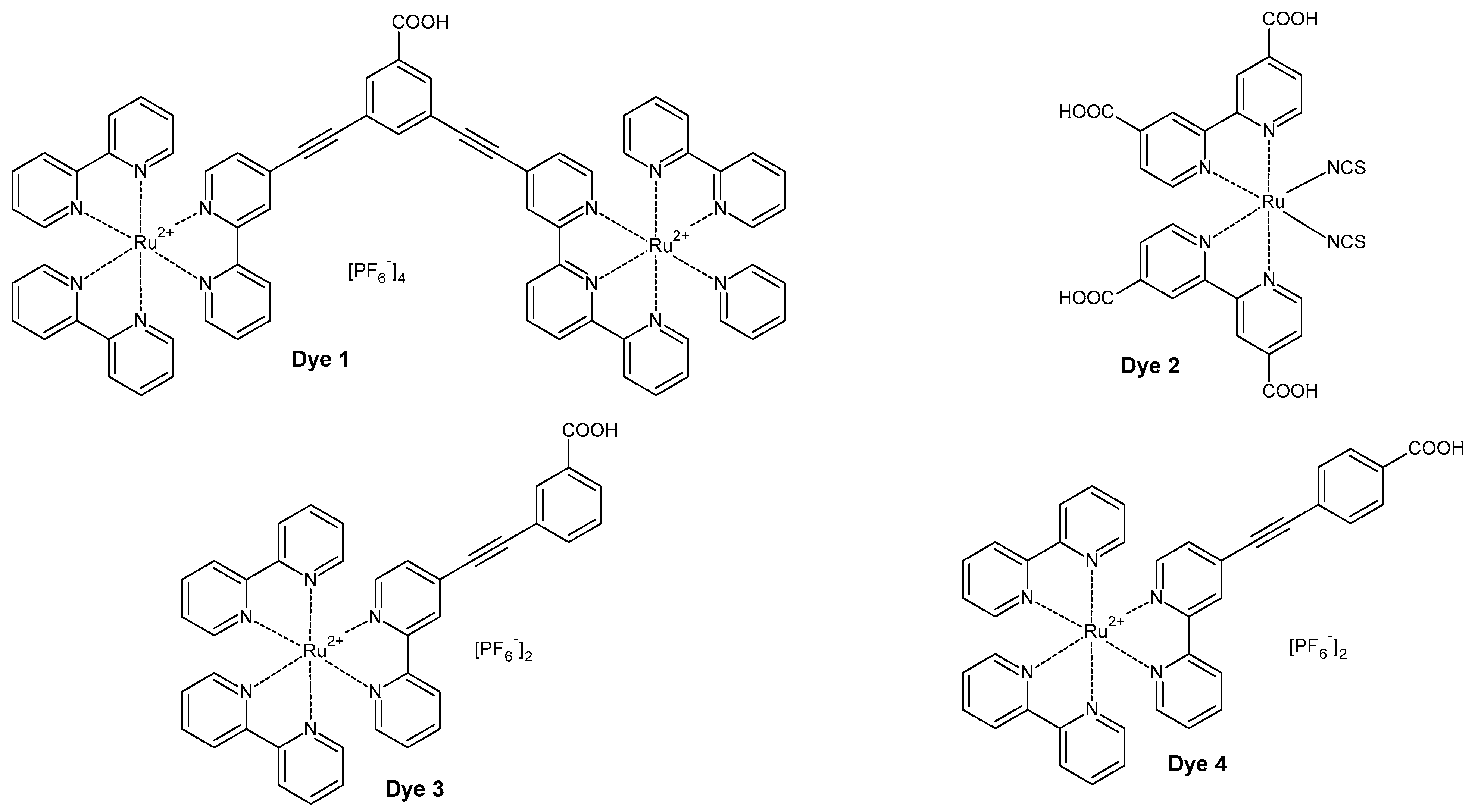
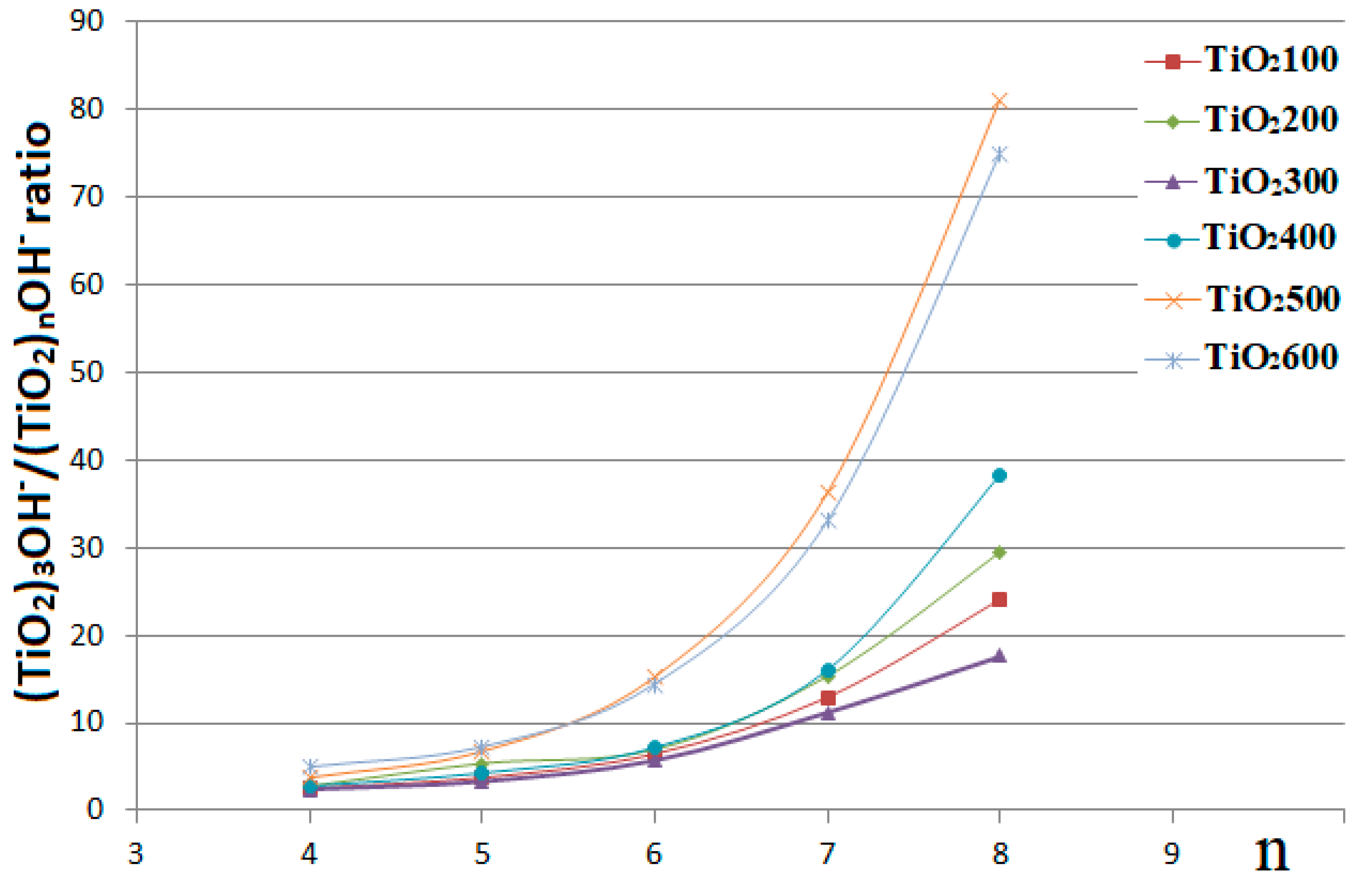
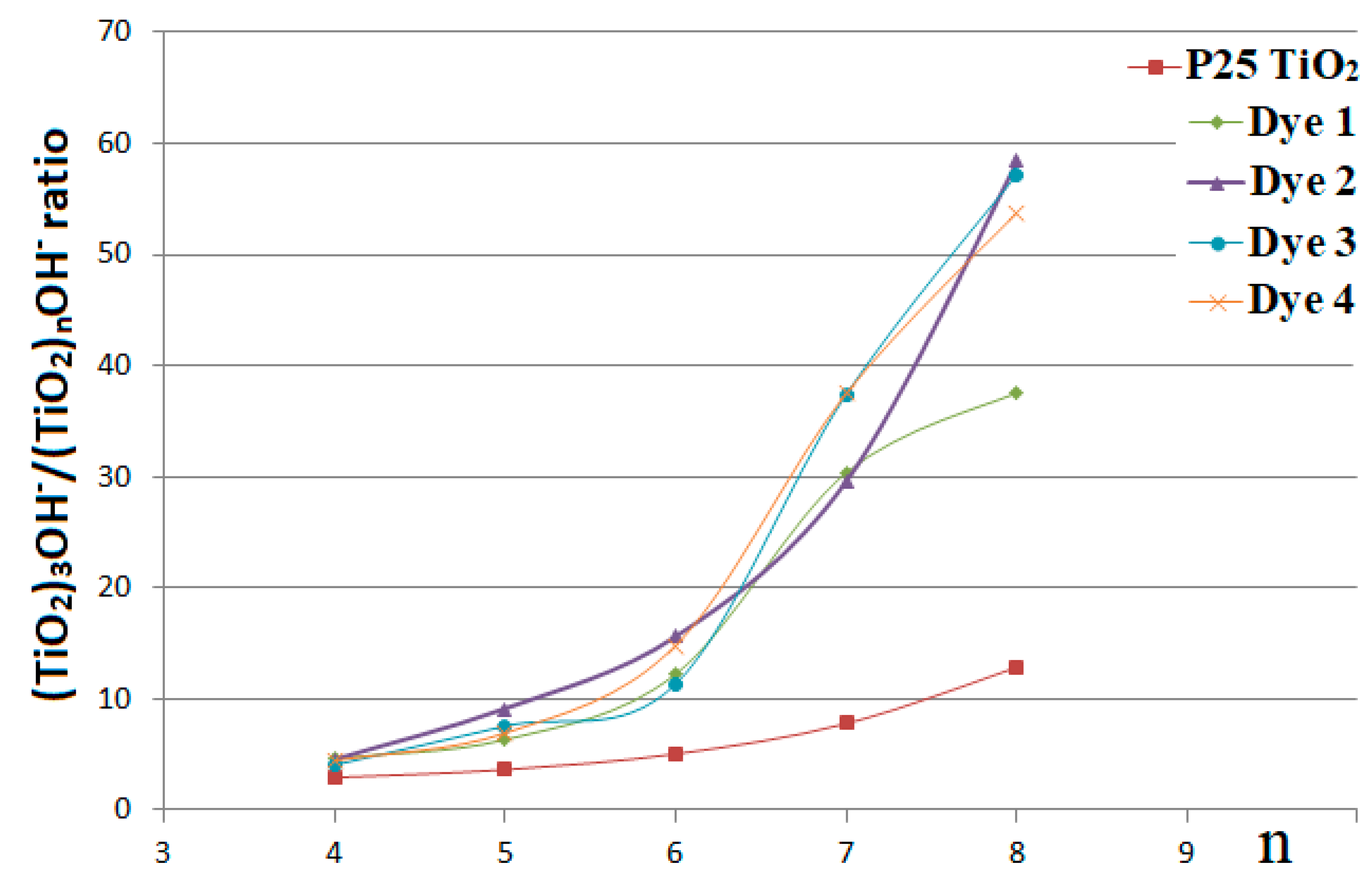
3. Results and Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Riedle, S.; Pele, L.C.; Otter, D.E.; Hewitt, R.E.; Singh, H.; Roy, N.C.; Powell, J.J. Pro-inflammatory adjuvant properties of pigment-grade titanium dioxide particles are augmented by a genotype that potentiates interleukin 1β processing. Part. Fibre Toxicol. 2017, 14, 51. [Google Scholar] [CrossRef]
- Nowotny, J. Titanium dioxide-based semiconductors for solar-driven environmentally friendly applications: Impact of point defects on performance. Energy Environ. Sci. 2008, 1, 565–572. [Google Scholar] [CrossRef]
- Carlucci, C.; Degennaro, L.; Luisi, R. Titanium dioxide as a catalyst in biodiesel production. Catalysts 2019, 9, 75. [Google Scholar] [CrossRef]
- Taha, S.; Begum, S.; Narwade, V.N.; Halge, D.; Dadge, J.W.; Mahabole, M.P.; Khairnar, R.D.; Bogle, K.A. Titanium dioxide nanostructure based alcohol vapor sensor. AIP Conf. Proc. 2020, 2220, 020195. [Google Scholar]
- Berardo, E.; Kaplan, F.; Bhaskaran-Nair, K.; Shelton, W.A.; Van Setten, M.J.; Kowalski, K.; Zwijnenburg, M.A. Benchmarking the fundamental electronic properties of small TiO2 nanoclusters by GW and coupled cluster theory calculations. J. Chem. Theory Comput. 2017, 13, 3814–3828. [Google Scholar] [CrossRef]
- Di Valentin, C.; Pacchioni, G. Electronic structure of defect states in hydroxylated and reduced rutile TiO2(110) surfaces. Phys. Rev. Lett. 2006, 97, 166803. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Sun, S.; Liao, X.; Wen, J.; Yin, G.; Pu, X.; Yao, Y.; Huang, Z. Effect of heat treatment on surface hydrophilicity-retaining ability of titanium dioxide nanotubes. Appl. Surf. Sci. 2018, 440, 440–447. [Google Scholar] [CrossRef]
- Tipawan, K.; Warawoot, T. Effects of air exposure time and annealing temperature on superhydrophobic surface of titanium dioxide films. Key Eng. Mater. 2017, 751, 137–142. [Google Scholar]
- El Seoud, O.A.; Ramadan, A.R.; Sato, B.M.; Pires, P.A.R. Surface properties of calcinated titanium dioxide probed by solvatochromic indicators: Relevance to catalytic applications. J. Phys. Chem. C 2010, 114, 10436–10443. [Google Scholar] [CrossRef]
- Li, Y.; Bian, Y.; Qin, H.; Zhang, Y.; Bian, Z. Photocatalytic reduction behavior of hexavalent chromium on hydroxyl modified titanium dioxide. Appl. Catal. B 2017, 206, 293–299. [Google Scholar] [CrossRef]
- Mamaghani, A.H.; Haghighat, F.; Lee, C.-S. Effect of titanium dioxide properties and support material on photocatalytic oxidation of indoor air pollutants. Build. Environ. 2021, 189, 107518. [Google Scholar] [CrossRef]
- Sean, N.A.; Leaw, W.L.; Nur, H. Effect of calcination temperature on the photocatalytic activity of carbon-doped titanium dioxide revealed by photoluminescence study. J. Chin. Chem. Soc. 2019, 66, 1277–1283. [Google Scholar] [CrossRef]
- Li, W.; Du, D.; Yan, T.; Kong, D.; You, J.; Li, D. Relationship between surface hydroxyl groups and liquid-phase photocatalytic activity of titanium dioxide. J. Colloid Interface Sci. 2015, 444, 42–48. [Google Scholar] [CrossRef]
- Du, J.; Wu, Q.; Zhong, S.; Gu, X.; Liu, J.; Guo, H.; Zhang, W.; Peng, H.; Zou, J. Effect of hydroxyl groups on hydrophilic and photocatalytic activities of rare earth doped titanium dioxide thin films. J. Rare Earths 2015, 33, 148–153. [Google Scholar] [CrossRef]
- Nanayakkara, C.E.; Larish, W.A.; Grassian, V.H. Titanium dioxide nanoparticle surface reactivity with atmospheric gases, CO2, SO2, and NO2: Roles of surface hydroxyl groups and adsorbed water in the formation and stability of adsorbed products. J. Phys. Chem. C 2014, 118, 23011–23021. [Google Scholar] [CrossRef]
- Nakamura, Y.; Soejima, T. TiO2 Nanocoral structures as versatile substrates for surface-assisted laser desorption/ionization mass spectrometry. ChemNanoMat 2019, 5, 447–455. [Google Scholar] [CrossRef]
- Kim, M.-J.; Yun, T.G.; Noh, J.-Y.; Song, Z.; Kim, H.-R.; Kang, M.-J.; Pyun, J.-C. Laser-induced surface reconstruction of nanoporous au-modified TiO2 nanowires for in situ performance enhancement in desorption and ionization mass spectrometry. Adv. Funct. Mater. 2021, 31, 2102475. [Google Scholar] [CrossRef]
- Piret, G.; Kim, D.; Drobecq, H.; Coffinier, Y.; Melnyk, O.; Schmuki, P.; Boukherrou, R. Surface-assisted laser desorption–ionization mass spectrometry on titanium dioxide (TiO2) nanotube layers. Analyst 2012, 137, 3058–3063. [Google Scholar] [CrossRef]
- Lee, K.-H.; Chiang, C.-K.; Lin, Z.-H.; Chang, H.-T. Determining enediol compounds in tea using surface-assisted laser desorption/ionization mass spectrometry with titanium dioxide nanoparticle matrices. Rapid Commun. Mass Spectrom. 2007, 21, 2023–2030. [Google Scholar] [CrossRef] [PubMed]
- Museur, L.; Manousaki, A.; Anglos, D.; Tsibidis, G.D.; Kanaev, A. Pathways control in modification of solid surfaces induced by temporarily separated femtosecond laser pulses. Appl. Surf. Sci. 2021, 566, 150611. [Google Scholar] [CrossRef]
- Tsibidis, G.D.; Museur, L.; Kanaev, A. The role of crystalline orientation in the formation of surface patterns on solids irradiated with femtosecond laser double pulses. Appl. Sci. 2020, 10, 8811. [Google Scholar] [CrossRef]
- Hong, R.; Shi, J.; Li, Z.; Liao, J.; Tao, C.; Wang, Q.; Lin, H.; Zhang, D. Surface enhanced raman scattering of defective TiO2 thin film decorated with silver nanoparticles by laser ablation. Opt. Mater. 2020, 109, 110338. [Google Scholar] [CrossRef]
- Bian, S.; Ma, Y.; Shi, Y.; Fan, X.; Kong, X. Superhalogen species of titanium oxide related clusters generated by laser ablation. J. Phys. Chem. A 2019, 123, 6787–6791. [Google Scholar] [CrossRef] [PubMed]
- Barthen, N.; Millon, E.; Aubriet, F. Study of cluster anions generated by laser ablation of titanium oxides: A high resolution approach based on fourier transform ion cyclotron resonance mass spectrometry. J. Am. Soc. Mass Spectrom. 2011, 22, 508–519. [Google Scholar] [CrossRef][Green Version]
- Zalas, M.; Schroeder, G. Template free synthesis of locally-ordered mesoporous titania and its application in dye-sensitized solar cells. Mater. Chem. Phys. 2012, 134, 170–176. [Google Scholar] [CrossRef]
- Zalas, M.; Gierczyk, B.; Bossic, A.; Mussini, P.R.; Kleine, M.; Pankiewicz, R.; Makowska-Janusik, M.; Popenda, Ł.; Stampor, W. The influence of anchoring group position in ruthenium dye molecule on performance of dye-sensitized solar cells. Dyes Pigm. 2018, 150, 335–346. [Google Scholar] [CrossRef]
- Longo, C.; De Paoli, M. Dye-sensitized solar cells: A successful combination of materials. J. Braz. Chem. Soc. 2003, 14, 889–901. [Google Scholar] [CrossRef]
- Zalas, M.; Gierczyk, B.; Klein, M.; Siuzdak, K.; Pędziński, T.; Łuczak, T. Synthesis of a novel dinuclear ruthenium polypyridine dye for dye-sensitized solar cells application. Polyhedron 2014, 67, 381–387. [Google Scholar] [CrossRef]
- Neubert, H.; Halket, J.M.; Ocaña, M.F.; Patel, R.K.P. MALDI post source decay and LIFT-TOF/TOF investigation of α-cyano-4-hydroxycinnamic acid cluster interferences. J. Am. Soc. Mass Spectrom. 2004, 15, 336–343. [Google Scholar] [CrossRef] [PubMed]
- Neupane, M.P.; Park, I.S.; Lee, M.H.; Bae, T.S.; Watari, F. Influence of heat treatment on morphological changes of nano-structured titanium oxide formed by anodic oxidation of titanium in acidic fluoride solution. Bio-Med. Mater. Eng. 2009, 19, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Haq, S.; Rehman, W.; Waseem, M.; Javed, R.; Rehman, M.; Shahid, M. Effect of heating on the structural and optical properties of TiO2 nanoparticles: Antibacterial activity. Appl. Nanosci. 2018, 8, 11–18. [Google Scholar] [CrossRef]
- Lee, Y.-J.; Cui, D.Z.; Jeon, H.-R.; Chung, H.J.; Park, Y.-J.; Kim, O.-S.; Kim, Y.-J. Surface characteristics of thermally treated titanium surfaces. J. Periodontal. Implant. Sci. 2012, 42, 81–87. [Google Scholar] [CrossRef]
- Uddin, M.J.; Cesano, F.; Chowdhury, A.C.; Trad, T.; Cravanzola, S.; Martra, G.; Mino, L.; Zecchina, A.; Scarano, D. Surface structure and phase composition of TiO2 P25 particles after thermal treatments and HF etching. Front. Mater. 2020, 7, 192. [Google Scholar] [CrossRef]
- Vorontsov, A.V.; Valdés, H.; Smirniotis, P.G.; Paz, Y. Recent advancements in the understanding of the surface chemistry in TiO2 photocatalysis. Surfaces 2020, 3, 72–92. [Google Scholar] [CrossRef]
- Keller, B.O.; Sui, J.; Young, A.B.; Whittal, R.M. Interferences and contaminants encountered in modern mass spectrometry. Anal. Chim. Acta 2008, 627, 71–81. [Google Scholar] [CrossRef]
- Matsuda, Y.; Bernstein, E.R. On the titanium oxide neutral cluster distribution in the gas phase: Detection through 118 nm single-photon and 193 nm multiphoton ionization. J. Phys. Chem. A 2005, 109, 314–319. [Google Scholar] [CrossRef] [PubMed]
- Garcia, J.M.; Heald, L.F.; Shaffer, R.E.; Sayres, S.G. Oscillation in excited state lifetimes with size of sub-nanometer neutral (TiO2)n clusters observed with ultrafast pump−probe spectroscopy. J. Phys. Chem. Lett. 2021, 12, 4098–4103. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Dixon, D.A. Molecular structures and energetics of the (TiO2)n (n = 1−4) clusters and their anions. J. Phys. Chem. A 2008, 112, 6646–6666. [Google Scholar] [CrossRef] [PubMed]
- Guan, B.; Lu, W.; Fang, J.; Cole, R.B. Characterization of synthesized titanium oxide nanoclusters by MALDI-TOF mass spectrometry. J. Am. Soc. Mass Spectrom. 2007, 18, 517–524. [Google Scholar] [CrossRef]
- Velegrakis, M.; Massaouti, M.; Jadraque, M. Collision-induced dissociation studies on gas-phase titanium oxide cluster cations. Appl. Phys. A 2012, 108, 127–131. [Google Scholar] [CrossRef]
- Jadraque, M.; Sierra, B.; Sfounis, A.; Velegrakis, M. Photofragmentation of mass-selected titanium oxide cluster cations. Appl. Phys. B 2010, 100, 587–590. [Google Scholar] [CrossRef]
- Velegrakis, M.; Sfounis, A. Formation and photodecomposition of cationic titanium oxide clusters. Appl. Phys. A 2009, 97, 765–770. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kasperkowiak, M.; Kurowska, M.; Zalas, M.; Frański, R. Laser Desorption/Ionization Mass Spectrometry as a Potential Tool for Evaluation of Hydroxylation Degree of Various Types of Titanium Dioxide Materials. Materials 2021, 14, 6848. https://doi.org/10.3390/ma14226848
Kasperkowiak M, Kurowska M, Zalas M, Frański R. Laser Desorption/Ionization Mass Spectrometry as a Potential Tool for Evaluation of Hydroxylation Degree of Various Types of Titanium Dioxide Materials. Materials. 2021; 14(22):6848. https://doi.org/10.3390/ma14226848
Chicago/Turabian StyleKasperkowiak, Małgorzata, Monika Kurowska, Maciej Zalas, and Rafał Frański. 2021. "Laser Desorption/Ionization Mass Spectrometry as a Potential Tool for Evaluation of Hydroxylation Degree of Various Types of Titanium Dioxide Materials" Materials 14, no. 22: 6848. https://doi.org/10.3390/ma14226848
APA StyleKasperkowiak, M., Kurowska, M., Zalas, M., & Frański, R. (2021). Laser Desorption/Ionization Mass Spectrometry as a Potential Tool for Evaluation of Hydroxylation Degree of Various Types of Titanium Dioxide Materials. Materials, 14(22), 6848. https://doi.org/10.3390/ma14226848






