Computed Tomography as a Characterization Tool for Engineered Scaffolds with Biomedical Applications
Abstract
1. Introduction
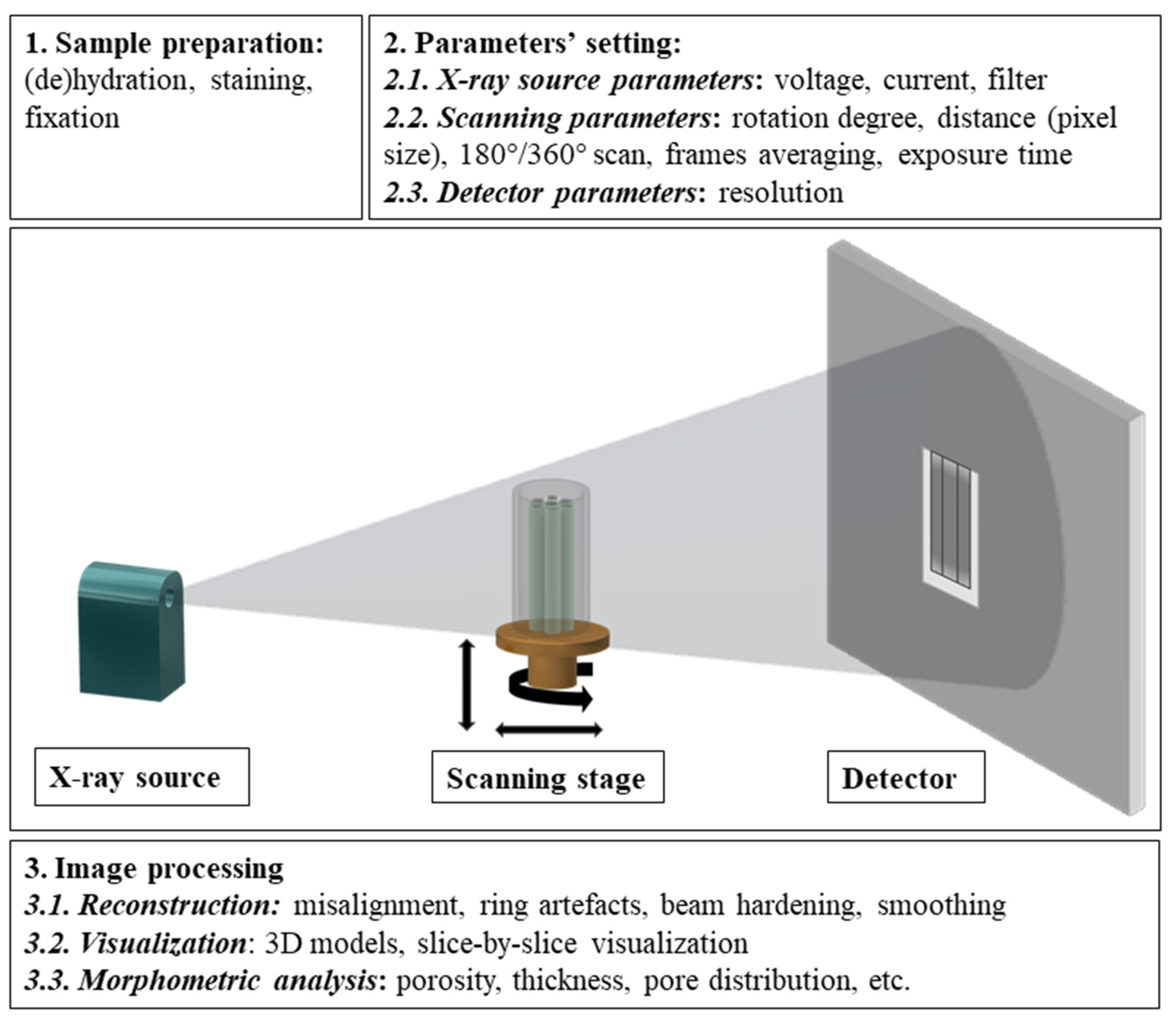
2. Operating Principle of Laboratory CT Equipment
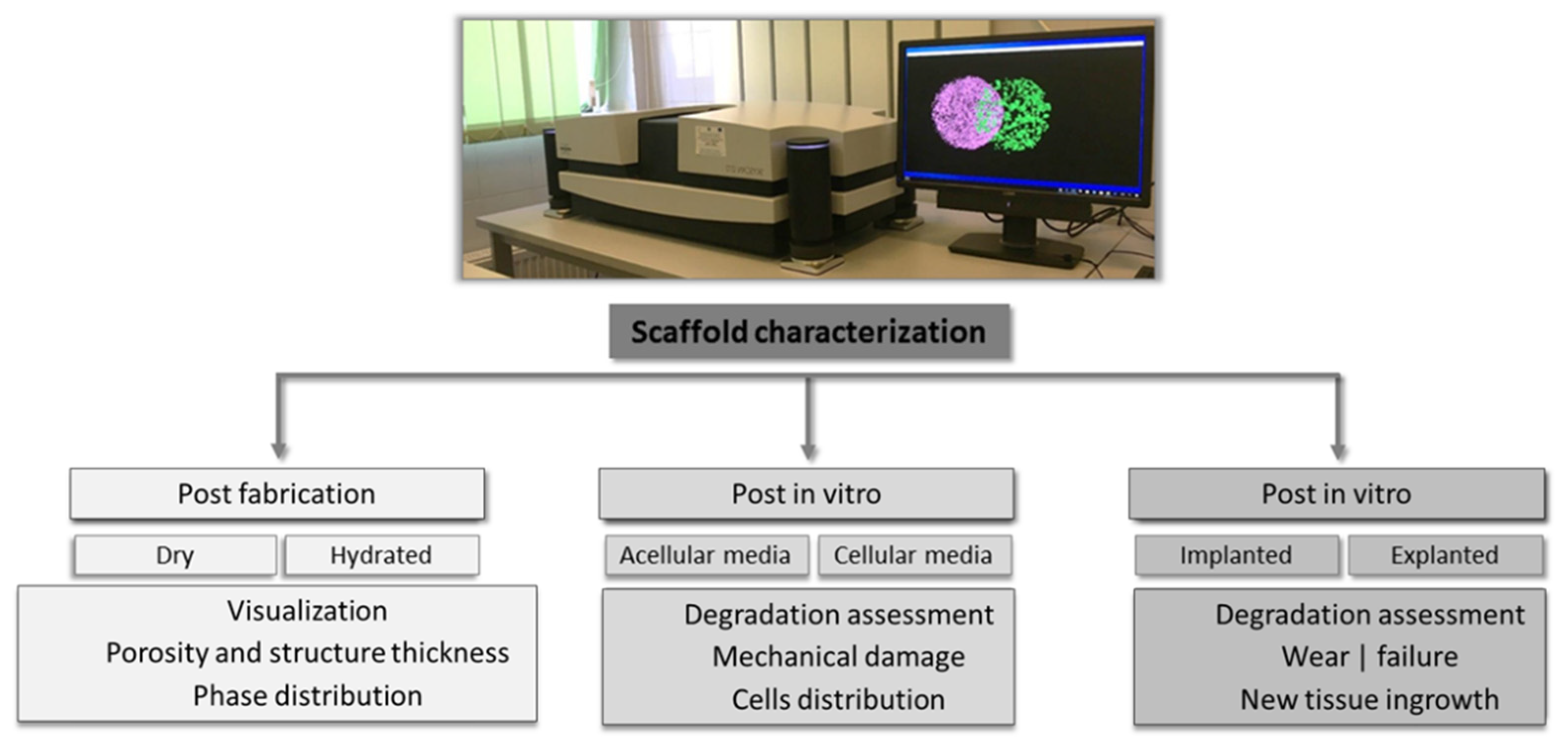
3. CT Imaging of Scaffolds with Biomedical Applications
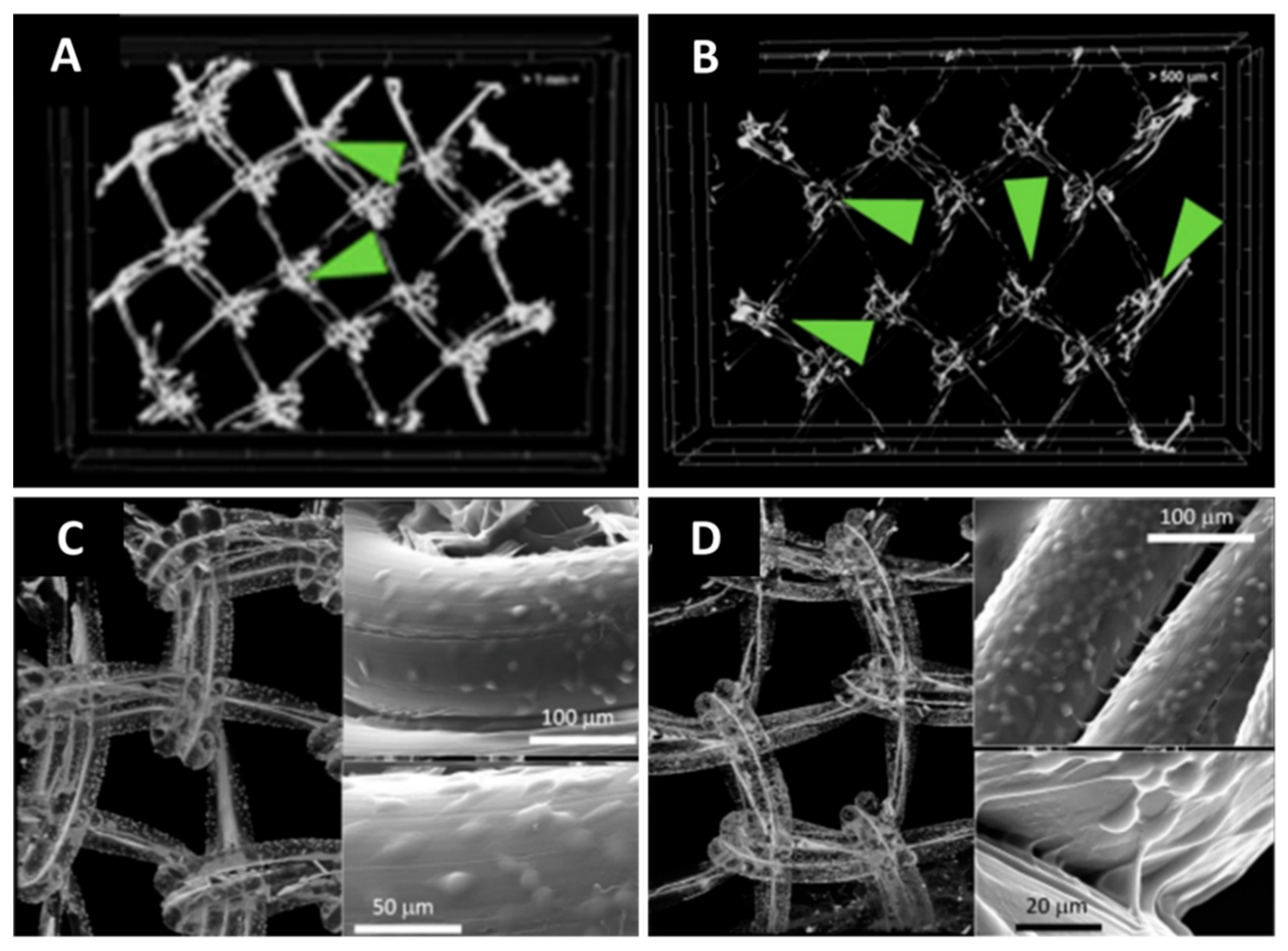
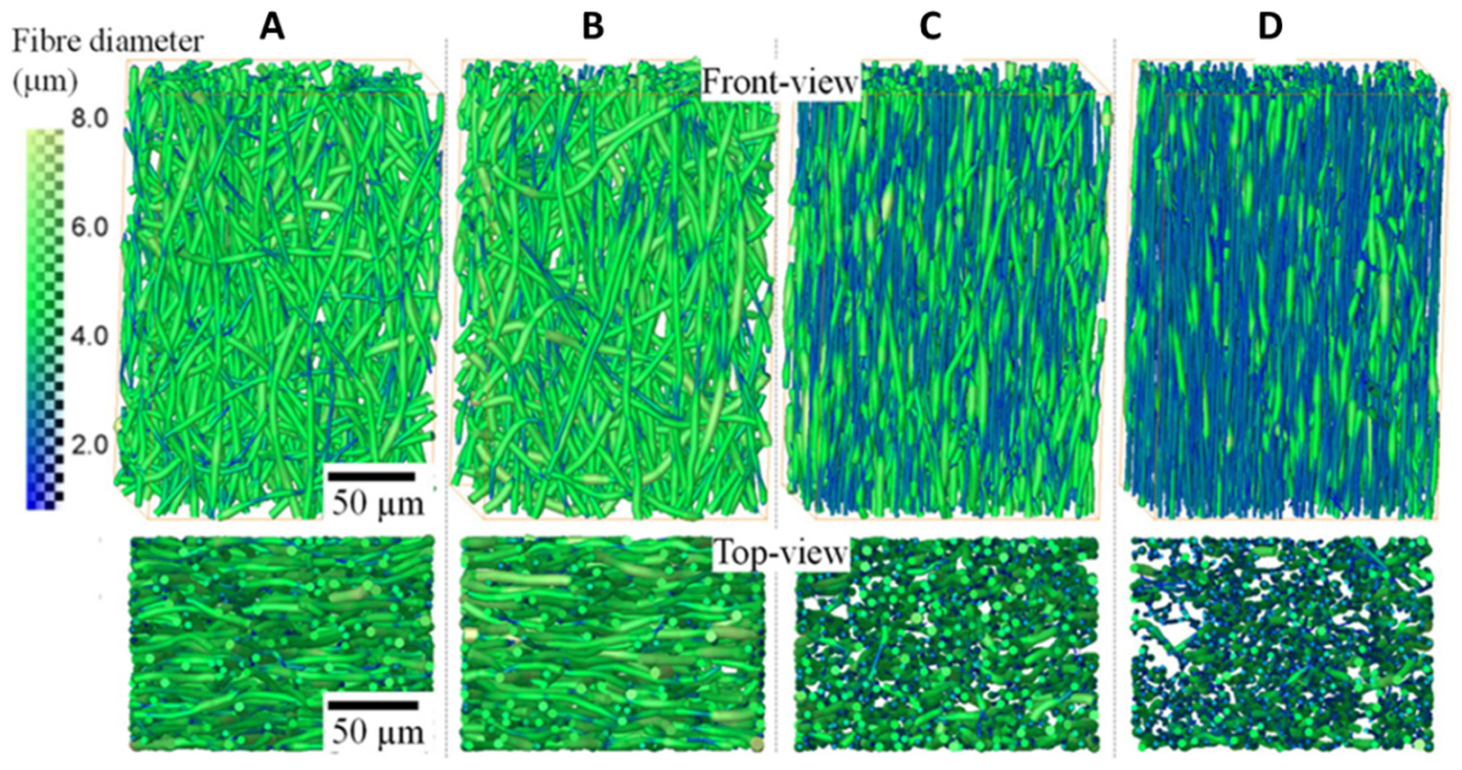
3.1. Visualization of Architectural Features through CT Imaging
3.2. Determination of 3D Morphometric Parameters
3.3. Phase Distribution
3.4. Evaluation of Architectural Features under Mechanical Load
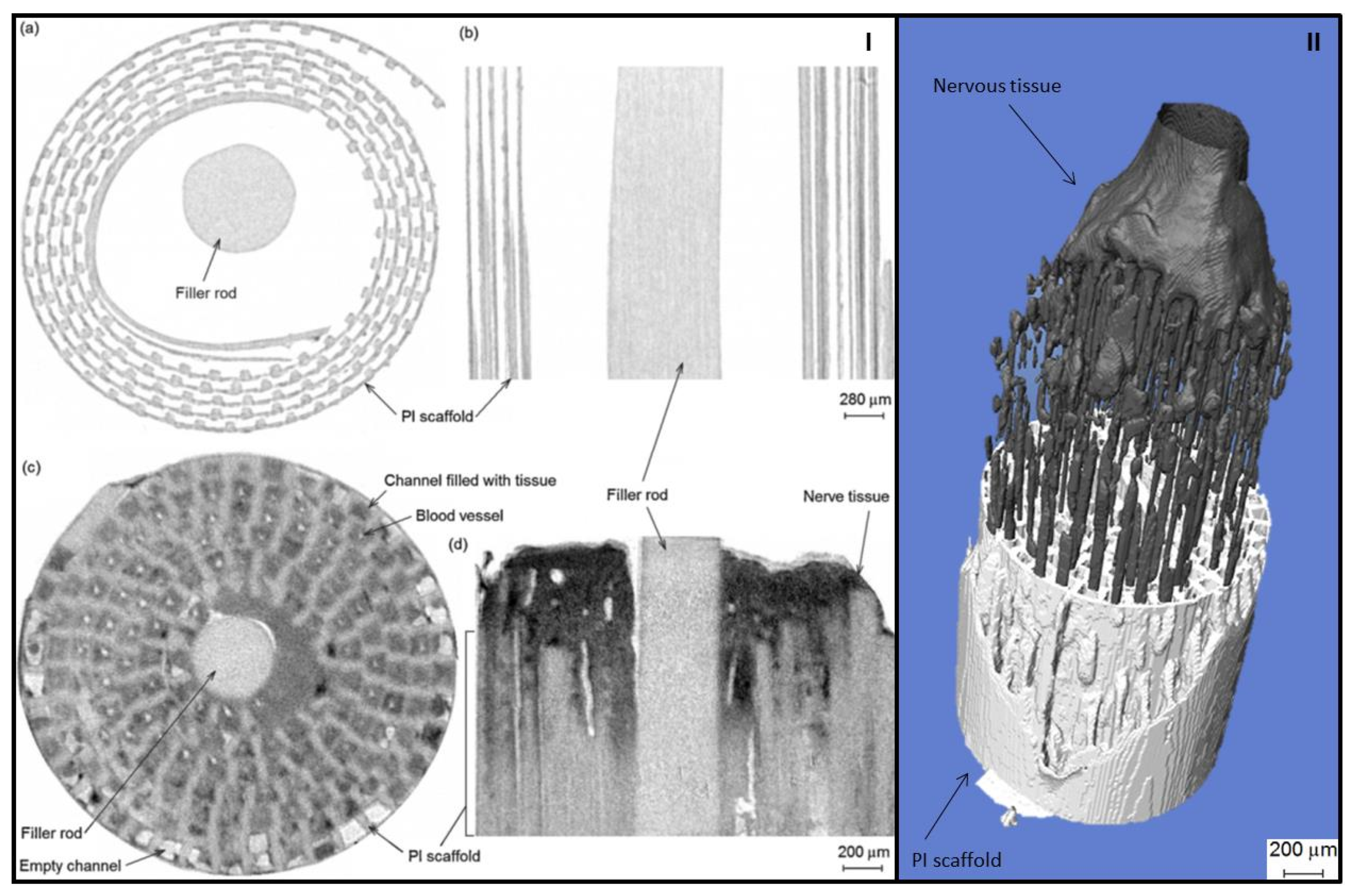
3.5. Evaluation of Degradation and Tissue Ingrowth
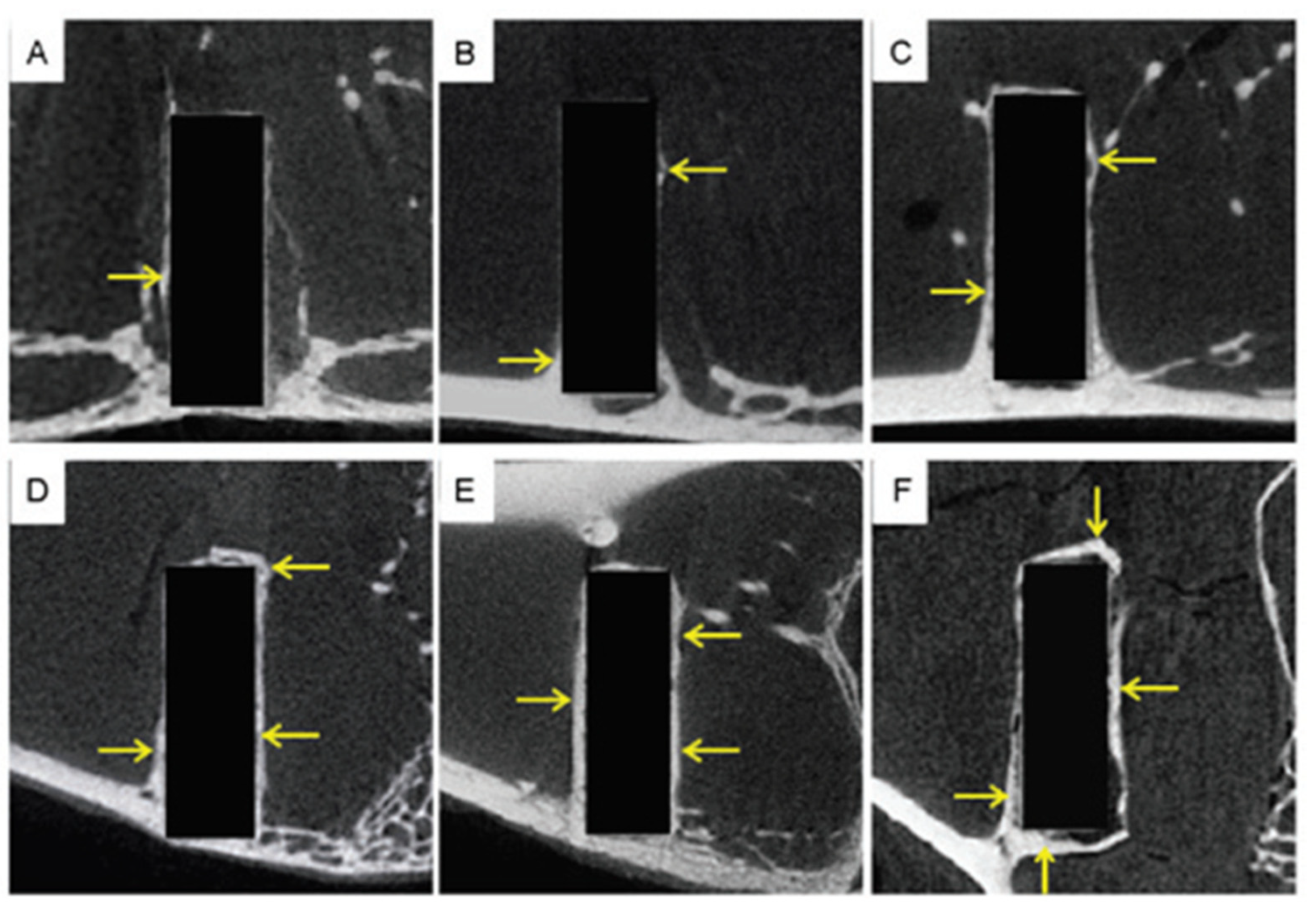
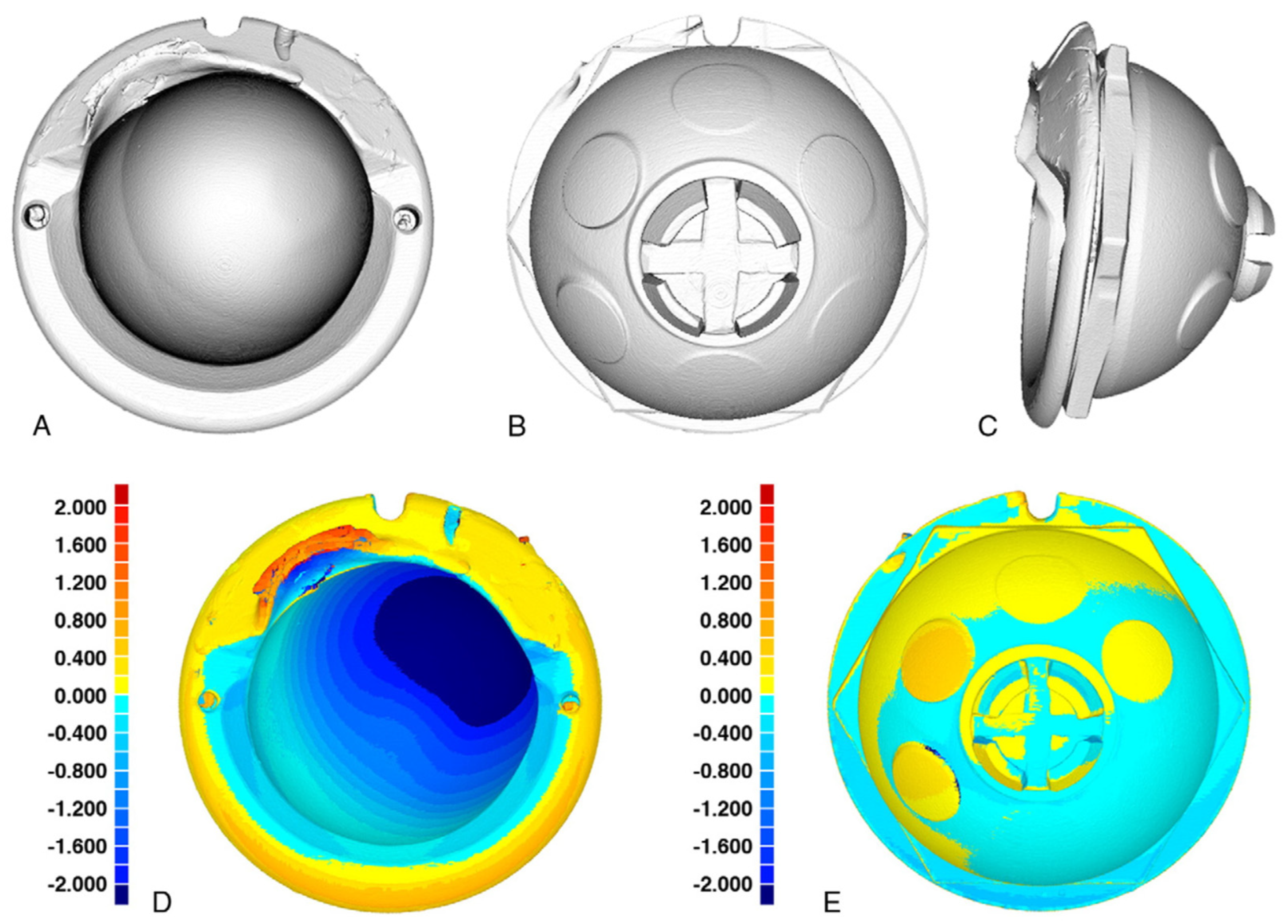
3.6. Evaluation of Implants’ Wear
| Synthetic Scaffolds | |||
|---|---|---|---|
| Imaging System | Material| Fabrication Method | Scanning Parameters | Research Objectives and Remarks|References |
| Micro-CT | PCL (freeze-dried) | 8 μm voxel size, 55 kV, 145 mA, 0.3 s integration time | Cell visualization, staining with osmium tetroxide [100] |
| PCL (selective laser sintering) | 28 μm voxel size, 75 kV, 75 mA | Bone ingrowth evaluation: comparison on compressive modulus obtained through FEA on scanned models and classical compression tests [116] | |
| PCL (electrospun) | 40 kV, power 10 W, 182° scan, 0.61 μm voxel size | Visualization of cell infiltration. Cells were stained in 1% OsO4 [105] | |
| PCL (fused deposition modeling) | Not mentioned | 3D visualization before and after in situ tensile tests; morphometric characteristics: porosity, filament thickness, surface area [167] | |
| 16b µm voxel size, 70 kV, 114 µA | Comparison between the 3D CAD model and actual printed scaffold; FE mechanical simulation using micro-CT scans [176] | ||
| Poly (L-lactic acid) and PCL | 16 μm pixel size | Scanned before and after implantation; quantification of mineral formation [158] | |
| Plasma-polymerized allyl amine deposited throughout a porous poly(D,L-lactic acid) scaffold | 8 μm voxel size, 55 kV, 145 mA, integration time of 300 ms | Osmium tetroxide-stained cell distribution within the scaffold [101] | |
| Poly(propylene fumarate) | 20.2 µm voxel size, 18 keV | 2D, 3D visualization; porosity evaluation through micro-CT and mercury porosimetry [149] | |
| Poly(propylene fumarate-co-ethylene glycol) (porous scaffold obtained using porogen agents) | 10 µm isotropic resolution, 50 Kv, 80 µA, integration time 300 ms | 3D visualization and morphometric analysis [181] | |
| Polyethylene | Resolution 74 µm, 45 kVp, 177 µA | Evaluation of wear of explanted UHMWPE acetabular components; micro-CT proved to be reliable but is limited to a single scan (ex vivo) [195] | |
| Not mentioned | The wear of an UHMWPE modular acetabular liner was evaluated. A similar unimplanted device was used as reference. The technique to create 3D deviation maps was described [200] | ||
| 80 kVp, 450 µA, 92 µm voxel resolution | Evaluation of wear of 24 retrieved tibial polyethylene inserts; imaging of components’ location, penetration, and deformation was possible [196] | ||
| 50 µm pixel size, 90 kVp, 40 mA, 0.3° increments with 10 frames averaged per view | Six unworn and six wear-simulated polyethylene tibial inserts were evaluated. No remarkable differences were registered between micro-CT results and gravimetric volume measurements [198] | ||
| Polyimide (PI) (microfabrication) | 2.7 µm pixel size, 33 kVp, 197 µA, exposure time 1904 ms, 0.5 mm Al filter | Sciatic nerve visualization within the polymeric scaffold; staining with OsO4 [112] | |
| Nano-CT | Poly(ethylene glycol)/poly(ethylene oxide) (electrospinning) | pixel size of 0.5 μm, 30 kV, 450 μA, rotation step of 0.1°, frames averaging 4, exposure time 1300 ms | 3D visualization and porosity evaluation [107] |
| Synchrotron micro-CT | Polyethylene terephthalate multifilament yarns. | 0.7 µm pixel width, f photon energy 14.5 keV | Comparison between cell visualization with SR micro-CT and confocal laser scanning microscopy. Samples were stained with gold-labeled lectin [103] |
| Poly(3-hydroxybutyrate-co-4-hydroxybutyrate) fiber mats (electrospinning) | 0.81 µm pixel size, 180° scan, step size of 0.12°, and exposure time from 0.075 to 0.15 ms | 3D visualization and morphometric characterization; in situ tensile tests [166] | |
| Natural Structures | |||
| Imaging System | Material| Fabrication Method | Scanning Parameters | Research Objectives and Remarks|References |
| Micro-CT | Collagen (freeze-dried) | 4 μm pixel size | Scanned in dry and hydrated state (0.3% PTA in 70% ethanol, 48 h) [69] |
| 2.26 μm pixel size, 47 kV, 142 mA, 1.9 s exposure time, 180° scanning with a rotation step of 0.458 | Comparison between different staining agents: osmium tetroxide, uranyl acetate, and lead citrate [99] | ||
| 1.5 μm pixel size, 25 kV | Describes the optimized workflow for data acquisition and data analysis [150] | ||
| Alginate modified with N-acetylglucosamine (freeze-dried) | 200 μA, 50 kV, 3 frame averaging 0.3°, rotation step, 665 ms exposure time | 3D visualization and porosity evaluation [125] | |
| Chitosan/fish gelatin graphene oxide loaded (freeze-dried) | Pixel size 5 μm, 45 kV, 200 µA, rotation step 0.15°, frame averaging 6 | Morphostructural characteristics and 3D visualization [151] | |
| Coral | Not mentioned | Visualization and morphometric characterization [134] | |
| Adipose and muscle tissue (bacon strips) and mice hind legs | 38–42 µm voxel size, 120 kV, 0.15–3 mm Al filter | Comparison between 28 potential staining agents [117] | |
| Fish specimens | 180° rotation, rotation step: 0.25°. Small samples: 90 keV/8 W; pixel size: <500 nm–5 μm; exposure time: 1 ms Large samples: 50 keV/40 W; pixel size: 15 μm to 75 μm; exposure time: 1.6 ms | Comparison of five staining agents (PTA, IKI, I2E, I2M, and OsO4). Scanning parameters were set for each sample [118] | |
| Chick embryo | 20–90 kV, 4–8 W, pixel size 500 μm–5 mm | Comparison of staining agents (gallocyanin-chromalum, I2, I2E, I2M, PTA, OsO4) and fixatives [119] | |
| Bio-Oss; porous HAp | 8 µm, 80 kV, 100, exposure time 5 s, Al 0.5 mm filter | 3D visualization and quantitative characterization; comparisons between blocks and granules [202] | |
| Osteopure, undecalcified bovine xenograft, Bio-Oss, CopiOs, TCP Dental, TCP Dental HP, KeraOs, TCH, Ca/P synthetic ceramics | 4.95 µm, 80 kV, 100 mA, 0.5 mm Al filter, rotation step 0.25° | Comparison between morphometric characteristics of 9 commercial bone substituents and human bone characteristics [132] | |
| Bone and coral | 20 µm voxel | Comparison between morphological characteristics [133] | |
| Porcine bone, demineralized porcine bone, and demineralized bone matrix | 10 μm voxel | 3D visualization and porosity; FEA on micro-CT models [136] | |
| Porcine bone graft, porcine type I collagen/bone graft composite, and biphasic ceramic material (MBCP™) | Not mentioned | Comparison in terms of osteoconduction capacity; quantitative evaluation of new bone formation [138] | |
| Bio-Oss Collagen, Orthogen | 19 μm3 voxel size, 50 kV, 800 μA, frame averaging of 6, angular step of 0.8°, 0.5 mm Al filter | Comparison between morphometric characteristics of both grafts; investigation of their behavior when implanted in a bone defect | |
| Fragments or granules of deproteinized bovine bone mineral | 90 kV, 200 µA, isotropic resolution of 17.2 µm, integration time of 500 ms | Comparison between morphometric characteristics of the two types of grafts; evaluation of new bone formation [140] | |
| Native glenoid and polyethylene glenoid (implant) | 150 kV, 160 µA, Isometric resolution of 35 µm; compression: 0.1 mm/min max. force: 750 N | 3D visualization and evaluation of 3D deformation of both samples [169] | |
| Synchrotron micro-CT | Gelatin (freeze-dried) | monochromatic X-ray beam at 10 keV, exposure time 0.7–1.2 s | Chondrocyte distribution after Au/Ag staining [106] |
| Synchrotron micro-CT | Femoral head | 7.50 µm voxel size, 26 keV, integration time 1800–2200 ms, | Evaluation of degree and distribution of bone mineral by micro-CT and synchrotron micro-CT [154] |
| Micro-CT | 8 µm voxel size, 0.5 mm Al filter, 70 kV, 114 µA, 250 ms exposure time | ||
| PET/CT system | Type I collagen | 20 μm voxel size | Revealed the interconnected porous architecture [96] |
| Synchrotron—phase contrast—micro-CT | Murine tibia | Photon energy of 21 keV, 18 ms exposure time, soft tissue 0.325 µm voxel size, mineralized tissue 1.3 µm voxel size | 3D visualization of intracortical blood vessels; no staining required [108] |
| Hybrid and Composite Scaffolds | |||
| Imaging System | Material| Fabrication Method | Scanning Parameters | Research Objectives and Remarks|References |
| Micro-CT | Collagen–poly(DL-lactide) (freeze-dried) | 4.5 μm pixel size | Dry state; pore evaluation in comparison with SEM [70] |
| Collagen matrix, poly(DL-lactide) nanofibers, calcium phosphate particles, and sodium hyaluronate (freeze-dried) | 4 μm pixel size, 60 kV, 166 μA, 0.25 mm Al filter, frame averaging 2, 180° rotation | Scanned in dried and in hydrated state; staining with Lugol’s solution [98] | |
| Collagen/graphene oxide (freeze-dried) | 58 kV, 385 mA | 3D visualization; quantification of apatite formation within the polymeric matrix [65] | |
| Fish skin gelatin/poly(vinyl alcohol) (freeze-dried) | 0.61 μm, 50 kV, 199 µA, 5 average frames, 0.34° rotation step | Comparison between different staining agents/protocols—iodine, barium, silver nitrate, and hexa(methyl disilazane) [97] | |
| Poly(L-lactide-co-ε-caprolactone) (PLCL) and PLCL/β-tricalcium phosphate | 5.0–6.0 µm voxel size, 50 kV, 200 µA | Cells were labeled with 50 nm USPIO nanoparticles. Porosity and cell distribution were investigated [104] | |
| Chitosan–agarose reinforced with nanohydroxyapatite | 12 µm voxel size | 3D visualization and porosity evaluation [123] | |
| Whey protein-bioactive glasses doped with Cu2+ and Co2+ | Not mentioned | 3D visualization and porosity evaluation; comparison with results obtained through mercury intrusion porosimetry [124] | |
| PCL/lactose/gelatin particulates | 15 µm voxel resolution, of 173 µA, 30 kV | 3D visualization and porosity evaluation. Samples scanned before and after dissolution of particulates—correlation with drug delivery evaluation [160] | |
| PCL and PCL/tricalcium phosphate (selective laser sintering) | 70 μm pixel size | Visualization and porosity evaluation; evaluation of new bone formation [157] | |
| Calcibon® cement/electrospun PCL fiber composites | 2.4 μm pixel size, 70 kV, and 85 μA | 3D visualization and morphometric analysis; quantification of material degradation after 4 weeks of implantation and new bone formation [182] | |
| PP meshes coated with methacryloyl derivatives of gelatin (GelMA) and mucin (MuMA) | Coating: 2.75 µm pixel size, 45 kV, 200 µA, rotation step 0.1°; cells: 1.5 µm pixel size, 70 kV, 130 µA, rotation step 0.3° | Visualization of coatings and cells. Coatings were silver-stained, and cells were stained with 0.5% uranyl acetate [102] | |
| Cellulose acetate (CA) membranes enriched with CNT and GO | Pixel size 0.5 μm, 50 kV, 200 µA, rotation step of 0.1°, exposure time 1200 ms, frame averaging 3 | 2D and 3D visualization and pore size distribution [128] | |
| CaP/silk scaffolds (freeze-drying method) | 55 kVp X-ray energy setting | Evaluation of new bone formation after 4 weeks of implantation [184] | |
| 2-hydroxyethyl methacrylate/cuttlefish bone | 7 µm pixel size, 70 kV, 175 µA, rotation step 0.4° | Visualization of new mineral formation within the polymeric matrix [156] | |
| Bioactive glass scaffolds gelatin-coated, cross-linked gelatin-coated, and poly(3-hydroxybutyrate-co-3-hydroxyvalerate) | 9 µm pixel size, 50 kVp, 200 µA, integration time of 450 ms | 3D morphostructural characterization; bone ingrowth evaluation [186] | |
| Hydroxyapatite/poly(lactic-co-glycolic acid) (3D-printed scaffolds) | Not specified | Micro-CT was influenced by the scattering effect of the metallic implant. Micro-CT images were not able to distinguish marrow space and soft tissue. SEM and histology assessments were also performed [190] | |
| Nano-CT | Chitosan–gelatin biocomposite films reinforced with graphene oxide | 3.5 µm pixel size, 5 kV, 200 μA, exposure time 300 ms, rotation step of 0.1°, frames averaging 5 | 3D visualization [126] |
| Micro-CT | Polycaprolactone/hydroxyapatite | Pixel 13.8 μm, rotation step 0.2°, exposure time 4 s, Al 0.25 mm filter | Scaffold visualization [78] |
| Synchrotron phase-contrast X-ray | White X-ray beam with 19 keV peak energy, pixel size 0.9 μm | Cell visualization [78] | |
| Ceramics | |||
| Imaging System | Material| Fabrication Method | Scanning Parameters | Research Objectives and Remarks|References |
| Micro-CT | Hydroxyapatite | 40 kV, 250 µA | 3D visualization and porosity evaluation [129] |
| Hydroxyapatite (3D-printed scaffolds) | Resolution approx. 20 µm, 3000 ms shutter speed, 1 × 1 bin size | Evaluation of the influence of scaffold design bone ingrowth through micro-CT [189] | |
| Alumina (Biolox® forte) femoral head | 17.5–35 µm pixel size, 90 kV, 278 μA, Cu filter 0.1 mm, rotation step 0.40, averaging frames 3 | Mass and volume loss were evaluated through micro-CT and gravimetrical method [201]. | |
| 13.98 μm resolution, 100 kV, 100 μA, A Cu (40 μm) + Al (0.5 mm) filter, 360° scan, rotation step of 0.7°, with an exposure time of 310 ms | Scanned in dry and hydrated state. Bone ingrowth evaluation after 6 months of implantation. Comparison with histological results [187] | ||
| Metal & Alloys | |||
| Imaging System | Material| Fabrication Method | Scanning Parameters | Research Objectives and Remarks|References |
| Micro-CT | Ti6Al4V powder (LPBF) | 2 μm voxel size, 100 keV, 100 µA | Describes the workflow powder analysis [84] |
| Ti6Al4V microlattice structures (LPBF) | 4 µm voxel size, 140 kV, 130 µA, 0.5 mm Cu filter, 500 N loading stage | 3D visualization before and after in situ compression tests; porosity evaluation [168] | |
| Ti6Al4V ELI alloy (electron beam melting) | 60 µm voxel size, 220 kV, 120 μA | Comparisons between CAD models and 3D-printed scaffolds; effect of internal porosity on mechanical properties [92] | |
| 13.98 μm resolution, 100 kV, 100 μA, A Cu (40 μm) + Al (0.5 mm) filter, 360° scan, rotation step of 0.7°, with an exposure time of 310 ms | Scanned in dry and hydrated state. Bone ingrowth evaluation after 6 months of implantation. Comparison with histological results [187] | ||
| Ti6Al4V | 18.5 µm voxel size, 135 kV, 70 μA | Visualization and porosity evaluation before and after mechanical testing | |
| Titanium | 10 µm pixel size | Porosity evaluation before and after compression tests [163] | |
| Magnesium alloy (micro-arc-oxidized AZ31) femoral condyle | 80 kV, 450 µA, 45 µm pixel size, 400 ms exposure time | Evaluation of the degradation of implants and new bone formation. Gravimetric measurements and histological analyses were also performed | |
| Zirconia foams | Pixel size of 1.5 μm (for 80 pores/inch) foam and 3 μm (for 45 and 60 pores/inch), 100 kV and 100 μA, 360° scan with a rotation step of 0.1°, 0.11 mm Cu filter | 3D visualization and morphometric analysis; compressive mechanical properties determined through FE using micro-CT scans [179] | |
| Nano-CT | Ti6Al4V (selective laser melting) | 90 kV, 170 µA, 1 mm Al filter and 1 mm Cu filter, exposure time 500 ms | ECM visualization and quantification; staining agents: Hexabrix 320 and PTA [107] |
4. Limitations and Perspectives of the CT Technique
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Röntgen, W.C. On a New Kind of Rays. Nature 1896, 53, 274–276. [Google Scholar] [CrossRef]
- Oldendorf, W.H. The quest for an image of brain: A brief historical and technical review of brain imaging techniques. Neurology 1978, 28, 517. [Google Scholar] [CrossRef]
- Hounsfield, G.N. Computerized transverse axial scanning (tomography): I. Description of system. Br. J. Radiol. 1973, 46, 1016–1022. [Google Scholar] [CrossRef] [PubMed]
- Cengiz, I.F.; Oliveira, J.M.; Reis, R.L. Micro-CT–a digital 3D microstructural voyage into scaffolds: A systematic review of the reported methods and results. Biomater. Res. 2018, 22, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Liguori, C.; Frauenfelder, G.; Massaroni, C.; Saccomandi, P.; Giurazza, F.; Pitocco, F.; Marano, R.; Schena, E. Emerging clinical applications of computed tomography. Med. Devices Evid. Res. 2015, 8, 265–278. [Google Scholar] [CrossRef]
- Cyran, C.C.; Paprottka, P.M.; Eisenblätter, M.; Clevert, D.A.; Rist, C.; Nikolaou, K.; Lauber, K.; Wenz, F.; Hausmann, D.; Reiser, M.F.; et al. Visualization, imaging and new preclinical diagnostics in radiation oncology. Radiat. Oncol. 2014, 9, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Stieb, S.; Kiser, K.; van Dijk, L.; Livingstone, N.R.; Elhalawani, H.; Elgohari, B.; McDonald, B.; Ventura, J.; Mohamed, A.S.R.; Fuller, C.D. Imaging for Response Assessment in Radiation Oncology: Current and Emerging Techniques. Hematol. Oncol. Clin. N. Am. 2020, 34, 293–306. [Google Scholar] [CrossRef] [PubMed]
- Hol, C.; Hellén-Halme, K.; Torgersen, G.; Nilsson, M.; Møystad, A. How do dentists use CBCT in dental clinics? A Norwegian nationwide survey. Acta Odontol. Scand. 2015, 73, 195–201. [Google Scholar] [CrossRef]
- Vandenberghe, B. The crucial role of imaging in digital dentistry. Dent. Mater. 2020, 36, 1–11. [Google Scholar] [CrossRef]
- Anderson, P.A.; Morgan, S.L.; Krueger, D.; Zapalowski, C.; Tanner, B.; Jeray, K.J.; Krohn, K.D.; Lane, J.P.; Yeap, S.S.; Shuhart, C.R.; et al. Use of Bone Health Evaluation in Orthopedic Surgery: 2019 ISCD Official Position. J. Clin. Densitom. 2019, 22, 517–543. [Google Scholar] [CrossRef]
- Villarraga-Gómez, H.; Herazo, E.L.; Smith, S.T. X-ray computed tomography: From medical imaging to dimensional metrology. Precis. Eng. 2019, 60, 544–569. [Google Scholar] [CrossRef]
- Ametova, E.; Probst, G.; Dewulf, W. X-ray Computed Tomography Devices and Their Components. In Industrial X-ray Computed Tomography; Carmignato, S., Dewulf, W., Leach, R., Eds.; Springer International Publishing: Cham, Switzerland, 2017; ISBN 9783319595733. [Google Scholar]
- Kruth, J.P.; Bartscher, M.; Carmignato, S.; Schmitt, R.; De Chiffre, L.; Weckenmann, A. Computed tomography for dimensional metrology. CIRP Ann.-Manuf. Technol. 2011, 60, 821–842. [Google Scholar] [CrossRef]
- Tan, Y.; Kiekens, K.; Welkenhuyzen, F.; Angel, J.; De Chiffre, L.; Kruth, J.-P.; Dewulf, W. Simulation-aided investigation of beam hardening induced errors in CT dimensional metrology. Meas. Sci. Technol. 2014, 25, 64014. [Google Scholar] [CrossRef][Green Version]
- Pejryd, L.; Beno, T.; Carmignato, S. Computed tomography as a tool for examining surface integrity in drilled holes in CFRP composites. Procedia CIRP 2014, 13, 43–48. [Google Scholar] [CrossRef]
- Kastner, J.; Plank, B.; Salaberger, D.; Sekelja, J. Defect and Porosity Determination of Fibre Reinforced Polymers by X-ray Computed Tomography. In Proceedings of the 2nd International Symposium on NDT in Aerospace, Hamburg, Germany, 22–24 November 2010; pp. 1–12. [Google Scholar]
- Cecchetto, G.; Amagliani, A.; Giraudo, C.; Fais, P.; Cavarzeran, F.; Montisci, M.; Feltrin, G.; Viel, G.; Ferrara, S.D. MicroCT detection of gunshot residue in fresh and decomposed firearm wounds. Int. J. Leg. Med. 2012, 126, 377–383. [Google Scholar] [CrossRef] [PubMed]
- Cecchetto, G.; Giraudo, C.; Amagliani, A.; Viel, G.; Fais, P.; Cavarzeran, F.; Feltrin, G.; Davide Ferrara, S.; Montisci, M.; Ferrara, S.D.; et al. Estimation of the firing distance through micro-CT analysis of gunshot wounds. Int. J. Leg. Med. 2011, 125, 245–251. [Google Scholar] [CrossRef]
- Pounder, D.J.; Sim, L.J. Virtual casting of stab wounds in cartilage using micro-computed tomography. Am. J. Forensic Med. Pathol. 2011, 32, 97–99. [Google Scholar] [CrossRef]
- Thali, M.J.; Taubenreuther, U.; Karolczak, M.; Braun, M.; Brueschweiler, W.; Kalender, W.A.; Dirnhofer, R. Forensic microradiology: Micro-computed tomography (Micro-CT) and analysis of patterned injuries inside of bone. J. Forensic Sci. 2003, 48, 1336–1342. [Google Scholar] [CrossRef]
- Sakuma, A.; Saitoh, H.; Suzuki, Y.; Makino, Y.; Inokuchi, G.; Hayakawa, M.; Yajima, D.; Iwase, H. Age estimation based on pulp cavity to tooth volume ratio using postmortem computed tomography images. J. Forensic Sci. 2013, 58, 1531–1535. [Google Scholar] [CrossRef]
- Azmi, N.A.; Heo, C.C.; Shafini, N.; Mahmud, M. Age estimation of forensically important blowfly, Chrysomya megacephala (Diptera: Calliphoridae) pupae using micro-computed tomography imaging. Trop. Biomed. 2019, 36, 640–653. [Google Scholar]
- Rahman, I.A.; Adcock, K.; Garwood, R.J. Virtual Fossils: A New Resource for Science Communication in Paleontology. Evol. Educ. Outreach 2012, 5, 635–641. [Google Scholar] [CrossRef][Green Version]
- Cunningham, J.A.; Rahman, I.A.; Lautenschlager, S.; Rayfield, E.J.; Donoghue, P.C.J. A virtual world of paleontology. Trends Ecol. Evol. 2014, 29, 347–357. [Google Scholar] [CrossRef] [PubMed]
- Keklikoglou, K.; Faulwetter, S.; Chatzinikolaou, E.; Wils, P.; Brecko, J.; Kvaček, J.; Metscher, B.; Arvanitidis, C. Micro-computed tomography for natural history specimens: A handbook of best practice protocols. Eur. J. Taxon. 2019, 522, 1–55. [Google Scholar] [CrossRef]
- Lewis, D. The fight for control over virtual fossils. Nature 2019, 567, 20–23. [Google Scholar] [CrossRef]
- Drol, C.J.; Kennedy, E.B.; Hsiung, B.K.; Swift, N.B.; Tan, K.T. Bioinspirational understanding of flexural performance in hedgehog spines. Acta Biomater. 2019, 94, 553–564. [Google Scholar] [CrossRef]
- du Plessis, A.; Broeckhoven, C.; Yadroitsev, I.; Yadroitsava, I.; le Roux, S.G. Analyzing nature’s protective design: The glyptodont body armor. J. Mech. Behav. Biomed. Mater. 2018, 82, 218–223. [Google Scholar] [CrossRef] [PubMed]
- Frank, M.B.; Naleway, S.E.; Wirth, T.S.; Jung, J.Y.; Cheung, C.L.; Loera, F.B.; Medina, S.; Sato, K.N.; Taylor, J.R.A.; McKittrick, J. A protocol for bioinspired design: A ground sampler based on sea urchin jaws. J. Vis. Exp. 2016, 2016, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Helguero, C.G.; Amaya, J.L.; Komatsu, D.E.; Pentyala, S.; Mustahsan, V.; Ramirez, E.A.; Kao, I. Trabecular Scaffolds’ Mechanical Properties of Bone Reconstruction Using Biomimetic Implants. Procedia CIRP 2017, 65, 121–126. [Google Scholar] [CrossRef]
- Rutty, G.N.; Brough, A.; Biggs, M.J.P.; Robinson, C.; Lawes, S.D.A.; Hainsworth, S.V. The role of micro-computed tomography in forensic investigations. Forensic Sci. Int. 2013, 225, 60–66. [Google Scholar] [CrossRef]
- Özge Onur, T. An application of filtered back projection method for computed tomography images. Int. Rev. Appl. Sci. Eng. 2021, 12, 194–200. [Google Scholar] [CrossRef]
- Feldkamp, L.A.; Davis, L.C.; Kress, J.W. Practical cone-beam algorithm. J. Opt. Soc. Am. A 1984, 1, 612. [Google Scholar] [CrossRef]
- Li, H.; Zhang, H.; Tang, Z.; Hu, G. Micro-computed tomography for small animal imaging: Technological details. Prog. Nat. Sci. 2008, 18, 513–521. [Google Scholar] [CrossRef]
- Mueller, K.; Xu, F.; Neophytou, N. Why do commodity graphics hardware boards (GPUs) work so well for acceleration of computed tomography. In Computational Imaging V; Bouman, C.A., Miller, E.L., Pollak, I., Eds.; International Society for Optics and Photonics: San Jose, CA, USA, 2007; p. 64980N. [Google Scholar]
- Brasse, D.; Humbert, B.; Mathelin, C.; Rio, M.-C.; Guyonnet, J.-L. Towards an inline reconstruction architecture for micro-CT systems. Phys. Med. Biol. 2005, 50, 5799–5811. [Google Scholar] [CrossRef] [PubMed]
- Geyer, L.L.; Schoepf, U.J.; Meinel, F.G.; Nance, J.W.; Bastarrika, G.; Leipsic, J.A.; Paul, N.S.; Rengo, M.; Laghi, A.; De Cecco, C.N. State of the Art: Iterative CT Reconstruction Techniques. Radiology 2015, 276, 339–357. [Google Scholar] [CrossRef]
- Ketola, J.H.; Karhula, S.S.; Finnilä, M.A.J.; Korhonen, R.K.; Herzog, W.; Siltanen, S.; Nieminen, M.T.; Saarakkala, S. Iterative and discrete reconstruction in the evaluation of the rabbit model of osteoarthritis. Sci. Rep. 2018, 8, 12051. [Google Scholar] [CrossRef]
- Gordon, R.; Bender, R.; Herman, G.T. Algebraic Reconstruction Techniques (ART) for three-dimensional electron microscopy and X-ray photography. J. Theor. Biol. 1970, 29, 471–481. [Google Scholar] [CrossRef]
- Andersen, A. Simultaneous Algebraic Reconstruction Technique (SART): A superior implementation of the ART algorithm. Ultrason. Imaging 1984, 6, 81–94. [Google Scholar] [CrossRef]
- Ji, D.; Qu, G.; Liu, B. Simultaneous algebraic reconstruction technique based on guided image filtering. Opt. Express 2016, 24, 15897. [Google Scholar] [CrossRef]
- Pang, W.-M.; Qin, J.; Lu, Y.; Xie, Y.; Chui, C.-K.; Heng, P.-A. Accelerating simultaneous algebraic reconstruction technique with motion compensation using CUDA-enabled GPU. Int. J. Comput. Assist. Radiol. Surg. 2011, 6, 187–199. [Google Scholar] [CrossRef]
- Iassonov, P.; Gebrenegus, T.; Tuller, M. Segmentation of X-ray computed tomography images of porous materials: A crucial step for characterization and quantitative analysis of pore structures. Water Resour. Res. 2009, 45, 1–12. [Google Scholar] [CrossRef]
- Zhang, L.; Luo, S. Micro Soft Tissues Visualization Based on X-ray Phase-Contrast Imaging. Open Med. Inform. J. 2012, 5, 19–25. [Google Scholar] [CrossRef]
- Yu, H.; VandeVord, P.J.; Mao, L.; Matthew, H.W.; Wooley, P.H.; Yang, S.Y. Improved tissue-engineered bone regeneration by endothelial cell mediated vascularization. Biomaterials 2009, 30, 508–517. [Google Scholar] [CrossRef]
- Jones, A.C.; Arns, C.H.; Sheppard, A.P.; Hutmacher, D.W.; Milthorpe, B.K.; Knackstedt, M.A. Assessment of bone ingrowth into porous biomaterials using MICRO-CT. Biomaterials 2007, 28, 2491–2504. [Google Scholar] [CrossRef] [PubMed]
- Neldam, C.A.; Lauridsen, T.; Rack, A.; Lefolii, T.T.; Jørgensen, N.R.; Feidenhans’L, R.; Pinholt, E.M. Application of high resolution synchrotron micro-CT radiation in dental implant osseointegration. J. Cranio-Maxillofac. Surg. 2015, 43, 682–687. [Google Scholar] [CrossRef] [PubMed]
- Pratt, I.V.; Belev, G.; Zhu, N.; Chapman, L.D.; Cooper, D.M.L. In vivo imaging of rat cortical bone porosity by synchrotron phase contrast micro computed tomography. Phys. Med. Biol. 2015, 60, 211–232. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Boughton, O.; Karunaratne, A.; Jin, A.; Cobb, J.; Hansen, U.; Abel, R. Synchrotron Imaging Assessment of Bone Quality. Clin. Rev. Bone Miner. Metab. 2016, 14, 150–160. [Google Scholar] [CrossRef]
- Matsumoto, T.; Shimizu, R.; Uesugi, K. In vivo monitoring of bone microstructure by propagation-based phase-contrast computed tomography using monochromatic synchrotron light. Lab. Investig. 2020, 100, 72–83. [Google Scholar] [CrossRef]
- Le Cann, S.; Tudisco, E.; Turunen, M.J.; Patera, A.; Mokso, R.; Tägil, M.; Belfrage, O.; Hall, S.A.; Isaksson, H. Investigating the mechanical characteristics of bone-metal implant interface using in situ synchrotron tomographic imaging. Front. Bioeng. Biotechnol. 2019, 6, 208. [Google Scholar] [CrossRef]
- Rawson, S.D.; Maksimcuka, J.; Withers, P.J.; Cartmell, S.H. X-ray computed tomography in life sciences. BMC Biol. 2020, 18, 1–15. [Google Scholar] [CrossRef]
- Endrizzi, M. X-ray phase-contrast imaging. Nucl. Instrum. Methods Phys. Res. Sect. A Accel. Spectrometers Detect. Assoc. Equip. 2018, 878, 88–98. [Google Scholar] [CrossRef]
- Gabrielson, K.; Maronpot, R.; Monette, S.; Mlynarczyk, C.; Ramot, Y.; Nyska, A.; Sysa-Shah, P. In Vivo Imaging with Confirmation by Histopathology for Increased Rigor and Reproducibility in Translational Research: A Review of Examples, Options, and Resources. ILAR J. 2018, 59, 80–98. [Google Scholar] [CrossRef]
- Vielreicher, M.; Schürmann, S.; Detsch, R.; Schmidt, M.A.; Buttgereit, A.; Boccaccini, A.; Friedrich, O. Taking a deep look: Modern microscopy technologies to optimize the design and functionality of biocompatible scaffolds for tissue engineering in regenerative medicine. J. R. Soc. Interface 2013, 10, 20130263. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Online, V.A.; Basu, B.; Swain, S.K.; Sarkar, D. Cryogenically cured hydroxyapatite–gelatin nanobiocomposite for bovine serum albumin protein adsorption and release. RSC Adv. 2013, 3, 14622–14633. [Google Scholar] [CrossRef]
- Pan, T.; Song, W.; Cao, X.; Wang, Y. 3D Bioplotting of Gelatin/Alginate Scaffolds for Tissue Engineering: Influence of Crosslinking Degree and Pore Architecture on Physicochemical Properties. J. Mater. Sci. Technol. 2016, 32, 889–900. [Google Scholar] [CrossRef]
- Liao, J.; Tian, T.; Shi, S.; Xie, X.; Ma, Q.; Li, G.; Lin, Y. The fabrication of biomimetic biphasic CAN-PAC hydrogel with a seamless interfacial layer applied in osteochondral defect repair. Bone Res. 2017, 5, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Selaru, A.; Dragusin, D.-M.; Olaret, E.; Serafim, A.; Steinmüller-Nethl, D.; Vasile, E.; Iovu, H.; Stancu, I.-C.; Dinescu, S.; Costache, M. Fabrication and Biocompatibility Evaluation of Nanodiamonds-Gelatin Electrospun Materials Designed for Prospective Tissue Regeneration Applications. Materials 2019, 12, 2933. [Google Scholar] [CrossRef] [PubMed]
- Shkarina, S.; Shkarin, R.; Weinhardt, V.; Elizaveta, M.; Kluger, P.J.; Loza, K.; Epple, M.; Ivlev, S.I.; Baumbach, T.; Surmeneva, M.A.; et al. 3D biodegradable scaffolds of polycaprolactone with silicate-containing hydroxyapatite microparticles for bone tissue engineering: High-resolution tomography and in vitro study. Sci. Rep. 2018, 8, 1–13. [Google Scholar] [CrossRef]
- Krause, M.; Hausherr, J.M.; Burgeth, B.; Herrmann, C.; Krenkel, W. Determination of the fibre orientation in composites using the structure tensor and local X-ray transform. J. Mater. Sci. 2010, 45, 888–896. [Google Scholar] [CrossRef]
- Manda, M.; Oliveira, M.B.; Mano, J.F.; Marques, A.P.; Oliveira, J.M.; Correlo, V.M.; Reis, R.L. Gellan Gum-Hydroxyapatite Composite Hydrogels for Bone Tissue Engineering Marianthi. J. Tissue Eng. Regen. Med. 2012, 6, 1–31. [Google Scholar]
- Dumont, V.C.; Mansur, H.S.; Mansur, A.A.P.; Carvalho, S.M.; Capanema, N.S.V.; Barrioni, B.R. Glycol chitosan/nanohydroxyapatite biocomposites for potential bone tissue engineering and regenerative medicine. Int. J. Biol. Macromol. 2016, 93, 1465–1478. [Google Scholar] [CrossRef]
- Gupta, D.; Kocot, M.; Tryba, A.M.; Serafim, A.; Stancu, I.C.; Jaegermann, Z.; Pamuła, E.; Reilly, G.C.; Douglas, T.E.L.L.; Maria, A.; et al. Novel naturally derived whey protein isolate and aragonite biocomposite hydrogels have potential for bone regeneration. Mater. Des. 2020, 188, 108408. [Google Scholar] [CrossRef]
- Zhou, C.; Liu, S.; Li, J.; Guo, K.; Yuan, Q.; Sun, S.J. Collagen Functionalized With Graphene Oxide Enhanced Biomimetic Mineralization and In Situ Bone Defect Repair. ACS Appl. Mater. Interfaces 2018, 10, 44080–44091. [Google Scholar] [CrossRef] [PubMed]
- Bradley, R.S.; Robinson, I.K.; Yusuf, M. 3D X-Ray Nanotomography of Cells Grown on Electrospun Scaffolds. Macromol. Biosci. 2017, 17, 1–8. [Google Scholar] [CrossRef]
- Albertini, G.; Giuliani, A.; Komlev, V.; Moroncini, F.; Pugnaloni, A.; Pennesi, G.; Belicchi, M.; Rubini, C.; Rustichelli, F.; Tasso, R.; et al. Organization of Extracellular Matrix Fibers Within Polyglycolic Acid–Polylactic Acid Scaffolds Analyzed Using X-Ray Synchrotron-Radiation Phase-Contrast Micro Computed Tomography. Tissue Eng. Part C Methods 2009, 15, 403–412. [Google Scholar] [CrossRef] [PubMed]
- Columbus, S.; Krishnan, L.K.; Krishnan, V.K. Relating pore size variation of poly (E-caprolactone) scaffolds to molecular weight of porogen and evaluation of scaffold properties after degradation. J. Biomed. Mater. Res. B Appl. Biomater. 2014, 102, 789–796. [Google Scholar] [CrossRef]
- Offeddu, G.S.; Ashworth, J.C.; Cameron, R.E.; Oyen, M.L. Structural determinants of hydration, mechanics and fluid flow in freeze-dried collagen scaffolds. Acta Biomater. 2016, 41, 193–203. [Google Scholar] [CrossRef] [PubMed]
- Bartoš, M.; Suchý, T.; Foltán, R. Note on the use of different approaches to determine the pore sizes of tissue engineering scaffolds: What do we measure? Biomed. Eng. Online 2018, 17, 1–15. [Google Scholar] [CrossRef]
- Shepherd, J.H.; Vriend, E.S.; Best, S.M.; Cameron, R.E. Analysis of structurally variable lyophilized collagen scaffolds for cell sieving using micro-CT. In Proceedings of the Micro-CT User Meeting 2018, Ghent, Belgium, 16–19 April 2018; pp. 182–186. [Google Scholar]
- Cecoltan, S.; Stancu, I.-C.; Drăguşin, D.M.; Serafim, A.; Lungu, A.; Ţucureanu, C.; Caraş, I.; Tofan, V.C.; Sălăgeanu, A.; Vasile, E.; et al. Nanocomposite particles with improved microstructure for 3D culture systems and bone regeneration. J. Mater. Sci. Mater. Med. 2017, 28, 1–12. [Google Scholar] [CrossRef]
- Ji, S.; Guvendiren, M. 3D Printed Wavy Scaffolds Enhance Mesenchymal Stem Cell Osteogenesis. Micromachines 2020, 11, 31. [Google Scholar] [CrossRef]
- Cernencu, A.I.; Lungu, A.; Stancu, I.C.; Serafim, A.; Heggset, E.; Syverud, K.; Iovu, H. Bioinspired 3D printable pectin-nanocellulose ink formulations. Carbohydr. Polym. 2019, 220, 12–21. [Google Scholar] [CrossRef]
- Curti, F.; Drăgușin, D.-M.; Serafim, A.; Iovu, H.; Stancu, I.-C. Development of thick paste-like inks based on superconcentrated gelatin/alginate for 3D printing of scaffolds with shape fidelity and stability. Mater. Sci. Eng. C 2021, 122, 111866. [Google Scholar] [CrossRef]
- Lai, Y.; Li, Y.; Cao, H.; Long, J.; Wang, X.; Li, L.; Li, C.; Jia, Q.; Teng, B.; Tang, T.; et al. Osteogenic magnesium incorporated into PLGA/TCP porous scaffold by 3D printing for repairing challenging bone defect. Biomaterials 2019, 197, 207–219. [Google Scholar] [CrossRef]
- Alexa, R.L.; Iovu, H.; Trica, B.; Zaharia, C.; Serafim, A.; Alexandrescu, E.; Radu, I.-C.; Vlasceanu, G.; Preda, S.; Ninciuleanu, C.M.; et al. Assessment of Naturally Sourced Mineral Clays for the 3D Printing of Biopolymer-Based Nanocomposite Inks. Nanomaterials 2021, 11, 703. [Google Scholar] [CrossRef]
- Gatto, M.L.; Furlani, M.; Giuliani, A.; Bloise, N.; Fassina, L.; Visai, L.; Mengucci, P. Biomechanical performances of PCL/HA micro- and macro-porous lattice scaffolds fabricated via laser powder bed fusion for bone tissue engineering. Mater. Sci. Eng. C 2021, 128, 112300. [Google Scholar] [CrossRef]
- Chen, G.; Sun, Y.; Lu, F.; Jiang, A.; Subedi, D.; Kong, P.; Wang, X.; Yu, T.; Chi, H.; Song, C.; et al. A three-dimensional (3D) printed biomimetic hierarchical scaffold with a covalent modular release system for osteogenesis. Mater. Sci. Eng. C 2019, 104, 109842. [Google Scholar] [CrossRef] [PubMed]
- Esposito Corcione, C.; Gervaso, F.; Scalera, F.; Padmanabhan, S.K.; Madaghiele, M.; Montagna, F.; Sannino, A.; Licciulli, A.; Maffezzoli, A. Highly loaded hydroxyapatite microsphere/PLA porous scaffolds obtained by fused deposition modelling. Ceram. Int. 2019, 45, 2803–2810. [Google Scholar] [CrossRef]
- Mandal, S.; Meininger, S.; Gbureck, U.; Basu, B. 3D powder printed tetracalcium phosphate scaffold with phytic acid binder: Fabrication, microstructure and in situ X-ray tomography analysis of compressive failure. J. Mater. Sci. Mater. Med. 2018, 29, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Farzadi, A.; Solati-hashjin, M.; Asadi-eydivand, M.; Azuan, N.; Osman, A. Effect of Layer Thickness and Printing Orientation on Mechanical Properties and Dimensional Accuracy of 3D Printed Porous Samples for Bone Tissue Engineering. PLoS ONE 2014, 9, e0108252. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, Y.; Xu, J.; Wang, J.; Li, C.; Wang, L. Tissue engineering using 3D printed nano-bioactive glass loaded with NELL1 gene for repairing alveolar bone defects. Regen. Biomater. 2018, 5, 213–220. [Google Scholar] [CrossRef]
- Sperling, P.; Beerlink, A.; Willie, B.; Stephan, G. Standard method for microCT-based additive manufacturing quality control 4: Metal powder analysis. MethodsX 2018, 5, 1336–1345. [Google Scholar] [CrossRef]
- Gong, H.; Nadimpalli, V.K.; Rafi, K.; Starr, T.; Stucker, B. Micro-CT Evaluation of Defects in Ti-6Al-4V Parts Fabricated by Metal Additive Manufacturing. Technologies 2019, 7, 44. [Google Scholar] [CrossRef]
- Landers, R.; Pfister, A.; Hübner, U.; John, H.; Schmelzeisen, R.; Mülhaupt, R. Fabrication of soft tissue engineering scaffolds by means of rapid prototyping techniques. J. Mater. Sci. 2002, 37, 3107–3116. [Google Scholar] [CrossRef]
- Grecchi, F.; Zecca, P.A.; Macchi, A.; Mangano, A.; Riva, F.; Grecchi, E.; Mangano, C. Full-digital workflow for fabricating a custom-made direct metal laser sintering (Dmls) mandibular implant: A case report. Int. J. Environ. Res. Public Health 2020, 17, 2693. [Google Scholar] [CrossRef]
- Gendviliene, I.; Simoliunas, E.; Rekstyte, S.; Malinauskas, M.; Zaleckas, L.; Jegelevicius, D.; Bukelskiene, V.; Rutkunas, V. Assessment of the morphology and dimensional accuracy of 3D printed PLA and PLA/HAp scaffolds. J. Mech. Behav. Biomed. Mater. 2020, 104, 103616. [Google Scholar] [CrossRef]
- Bartnikowski, M.; Vaquette, C.; Ivanovski, S. Workflow for highly porous resorbable custom 3D printed scaffolds using medical grade polymer for large volume alveolar bone regeneration. Clin. Oral Implant. Res. 2020, 31, 431–441. [Google Scholar] [CrossRef]
- Wang, L.; Kang, J.; Sun, C.; Li, D.; Cao, Y.; Jin, Z. Mapping porous microstructures to yield desired mechanical properties for application in 3D printed bone scaffolds and orthopaedic implants. Mater. Des. 2017, 133, 62–68. [Google Scholar] [CrossRef]
- Odeh, M.; Levin, D.; Inziello, J.; Lobo Fenoglietto, F.; Mathur, M.; Hermsen, J.; Stubbs, J.; Ripley, B. Methods for verification of 3D printed anatomic model accuracy using cardiac models as an example. 3D Print. Med. 2019, 5, 1–12. [Google Scholar] [CrossRef]
- Szymczyk, P.; Hoppe, V.; Ziółkowski, G.; Smolnicki, M.; Madeja, M. The effect of geometry on mechanical properties of Ti6Al4V ELI scaffolds manufactured using additive manufacturing technology. Arch. Civ. Mech. Eng. 2020, 20, 1–13. [Google Scholar] [CrossRef]
- Zheng, Y.; Zhang, T.; Wei, Q.; Fan, D.; Liu, X.; Li, W.; Song, C.; Tian, Y.; Cai, H.; Liu, Z. Improved osseointegration with rhBMP-2 intraoperatively loaded in a specifically designed 3D-printed porous Ti6Al4V vertebral implant. Biomater. Sci. 2020, 8, 1279–1289. [Google Scholar] [CrossRef] [PubMed]
- Gao, R.; Ji, W.; Xia, T.; Fan, Y.; Wei, W.; Shi, L.; Liu, J.; Zhang, C.; Xue, L.; Shen, J. Three-dimensional-printed titanium alloy porous scaffold combined with trans-cinnamaldehyde for repairing osteonecrosis of the femoral head in a dog model. Am. J. Transl. Res. 2020, 12, 1070–1079. [Google Scholar]
- Vidal, L.; Kampleitner, C.; Krissian, S.; Brennan, M.Á.; Hoffmann, O.; Raymond, Y.; Maazouz, Y.; Ginebra, M.-P.; Rosset, P.; Layrolle, P. Regeneration of segmental defects in metatarsus of sheep with vascularized and customized 3D-printed calcium phosphate scaffolds. Sci. Rep. 2020, 10, 7068. [Google Scholar] [CrossRef] [PubMed]
- Zitnay, J.L.; Reese, S.P.; Tran, G.; Farhang, N.; Bowles, R.D.; Weiss, J.A. Fabrication of dense anisotropic collagen scaffolds using biaxial compression. Acta Biomater. 2018, 65, 76–87. [Google Scholar] [CrossRef] [PubMed]
- Crica, L.E.; Wengenroth, J.; Tiainen, H.; Ionita, M.; Haugen, H.J. Enhanced X-ray absorption for micro-CT analysis of low density polymers. J. Biomater. Sci. Polym. Ed. 2016, 27, 805–823. [Google Scholar] [CrossRef] [PubMed]
- Suchý, T.; Šupová, M.; Bartoš, M.; Sedláček, R.; Piola, M.; Soncini, M.; Fiore, G.B.; Sauerová, P.; Kalbáčová, M.H.; Monika, Š.; et al. Dry versus hydrated collagen scaffolds: Are dry states representative of hydrated states? J. Mater. Sci. Mater. Med. 2018, 29, 20. [Google Scholar] [CrossRef] [PubMed]
- Faraj, K.A.; Cuijpers, V.M.J.I.J.I.; Wismans, R.G.; Walboomers, X.F.; Jansen, J.A.; Van Kuppevelt, T.H.; Daamen, W.F.; Walboomers, F.; Jansen, J.A.; van Kuppevelt, T.H.; et al. Micro-Computed Tomographical Imaging of Soft Biological Materials Using Contrast Techniques. Tissue Eng.-Part C Methods 2009, 15, 493–499. [Google Scholar] [CrossRef]
- Dorsey, S.M.; Lin-Gibson, S.; Simon, C.G. X-ray microcomputed tomography for the measurement of cell adhesionand proliferation in polymer scaffolds. Biomaterials 2009, 30, 2967–2974. [Google Scholar] [CrossRef]
- Barry, J.J.A.; Howard, D.; Shakesheff, K.M.; Howdle, S.M.; Alexander, M.R. Using a core-sheath distribution of surface chemistry through 3D tissue engineering scaffolds to control cell ingress. Adv. Mater. 2006, 18, 1406–1410. [Google Scholar] [CrossRef]
- Serafim, A.; Cecoltan, S.; Olaret, E.; Dragusin, D.-M.; Vasile, E.; Popescu, V.; Mastalier, B.S.M.; Iovu, H.; Stancu, I.-C. Bioinspired Hydrogel Coating Based on Methacryloyl Gelatin Bioactivates Polypropylene Meshes for Abdominal Wall Repair. Polymers 2020, 12, 1677. [Google Scholar] [CrossRef]
- Thurner, P.; Müller, R.; Raeber, G.; Sennhauser, U.; Hubbell, J.A. 3D morphology of cell cultures: A quantitative approach using micrometer synchrotron light tomography. Microsc. Res. Tech. 2005, 66, 289–298. [Google Scholar] [CrossRef]
- Palmroth, A.; Pitkänen, S.; Hannula, M.; Paakinaho, K.; Hyttinen, J.; Miettinen, S.; Kellomäki, M. Evaluation of scaffold microstructure and comparison of cell seeding methods using micro-computed tomography-based tools. J. R. Soc. Interface 2020, 17, 20200102. [Google Scholar] [CrossRef]
- Bosworth, L.A.; Rathbone, S.R.; Bradley, R.S.; Cartmell, S.H. Dynamic loading of electrospun yarns guides mesenchymal stem cells towards a tendon lineage. J. Mech. Behav. Biomed. Mater. 2014, 39, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Zehbe, R.; Goebbels, J.; Ibold, Y.; Gross, U.; Schubert, H. Three-dimensional visualization of in vitro cultivated chondrocytes inside porous gelatine scaffolds: A tomographic approach. Acta Biomater. 2010, 6, 2097–2107. [Google Scholar] [CrossRef]
- Papantoniou, I.; Sonnaert, M.; Geris, L.; Luyten, F.P.; Schrooten, J.; Kerckhofs, G. Three-dimensional characterization of tissue-engineered constructs by contrast-enhanced nanofocus computed tomography. Tissue Eng.-Part C Methods 2014, 20, 177–187. [Google Scholar] [CrossRef] [PubMed]
- Núñez, J.A.; Goring, A.; Hesse, E.; Thurner, P.J.; Schneider, P.; Clarkin, C.E. Simultaneous visualisation of calcified bone microstructure and intracortical vasculature using synchrotron X-ray phase contrast-enhanced tomography. Sci. Rep. 2017, 7, 1–9. [Google Scholar] [CrossRef]
- Andersson, K.M.; Nowik, P.; Persliden, J.; Thunberg, P.; Norrman, E. Metal artefact reduction in CT imaging of hip prostheses-an evaluation of commercial techniques provided by four vendors. Br. J. Radiol. 2015, 88, 20140473. [Google Scholar] [CrossRef] [PubMed]
- Ejima, K.; Omasa, S.; Motoyoshi, M.; Arai, Y.; Kai, Y.; Amemiya, T.; Yamada, H.; Honda, K.; Shimizu, N. Influence of metal artifacts on in vivo micro-CT for orthodontic mini-implants. J. Oral Sci. 2012, 54, 55–59. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Potter, K.; Sweet, D.E.; Anderson, P.; Davis, G.R.; Isogai, N.; Asamura, S.; Kusuhara, H.; Landis, W.J. Non-destructive studies of tissue-engineered phalanges by magnetic resonance microscopy and X-ray microtomography. Bone 2006, 38, 350–358. [Google Scholar] [CrossRef]
- Watling, C.P.; Lago, N.; Benmerah, S.; FitzGerald, J.J.; Tarte, E.; McMahon, S.; Lacour, S.P.; Cameron, R.E. Novel use of X-ray micro computed tomography to image rat sciatic nerve and integration into scaffold. J. Neurosci. Methods 2010, 188, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Scheller, E.L.; Troiano, N.; Vanhoutan, J.N.; Bouxsein, M.A.; Fretz, J.A.; Xi, Y.; Nelson, T.; Katz, G.; Berry, R.; Church, C.D.; et al. Use of osmium tetroxide staining with microcomputerized tomography to visualize and quantify bone marrow adipose tissue in vivo. Methods Enzymol. 2014, 537, 123–139. [Google Scholar] [CrossRef]
- Phelps, E.A.; Landázuri, N.; Thulé, P.M.; Taylor, W.R.; García, A.J. Bioartificial matrices for therapeutic vascularization. Proc. Natl. Acad. Sci. USA 2010, 107, 3323–3328. [Google Scholar] [CrossRef]
- Arkudas, A.; Beier, J.P.; Pryymachuk, G.; Hoereth, T.; Bleiziffer, O.; Polykandriotis, E.; Hess, A.; Gulle, H.; Horch, R.E.; Kneser, U. Automatic quantitative micro-computed tomography evaluation of angiogenesis in an axially vascularized tissue-engineered bone construct. Tissue Eng.-Part C Methods 2010, 16, 1503–1514. [Google Scholar] [CrossRef]
- Williams, J.M.; Adewunmi, A.; Schek, R.M.; Flanagan, C.L.; Krebsbach, P.H.; Feinberg, S.E.; Hollister, S.J.; Das, S. Bone tissue engineering using polycaprolactone scaffolds fabricated via selective laser sintering. Biomaterials 2005, 26, 4817–4827. [Google Scholar] [CrossRef] [PubMed]
- Pauwels, E.; Van Loo, D.; Cornillie, P.; Brabant, L.; Van Hoorebeke, L. An exploratory study of contrast agents for soft tissue visualization by means of high resolution X-ray computed tomography imaging. J. Microsc. 2013, 250, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Metscher, B.D. Micro CT for comparative morphology: Simple staining methods allow high-contrast 3D imaging of diverse non-mineralized animal tissues. BMC Physiol. 2009, 9, 1–14. [Google Scholar] [CrossRef]
- Metscher, B.D. MicroCT for developmental biology: A versatile tool for high-contrast 3D imaging at histological resolutions. Dev. Dyn. 2009, 238, 632–640. [Google Scholar] [CrossRef] [PubMed]
- Bružauskaitė, I.; Bironaitė, D.; Bagdonas, E.; Bernotienė, E. Scaffolds and cells for tissue regeneration: Different scaffold pore sizes—different cell effects. Cytotechnology 2016, 68, 355–369. [Google Scholar] [CrossRef] [PubMed]
- Bertoldi, S.; Farè, S.; Tanzi, M.C. Assessment of scaffold porosity: The new route of micro-CT. J. Appl. Biomater. Biomech. 2011, 9, 165–175. [Google Scholar] [CrossRef] [PubMed]
- Davidoiu, V.; Hadjilucas, L.; Teh, I.; Smith, N.P.; Schneider, J.E.; Lee, J. Evaluation of noise removal algorithms for imaging and reconstruction of vascular networks using micro-CT. Biomed. Phys. Eng. Express 2016, 2, 045015. [Google Scholar] [CrossRef]
- Kazimierczak, P.; Palka, K.; Przekora, A. Development and optimization of the novel fabrication method of highly macroporous chitosan/agarose/nanohydroxyapatite bone scaffold for potential regenerative medicine applications. Biomolecules 2019, 9, 434. [Google Scholar] [CrossRef] [PubMed]
- Dziadek, M.; Douglas, T.E.L.L.; Dziadek, K.; Zagrajczuk, B.; Serafim, A.; Stancu, I.-C.C.; Cholewa-Kowalska, K. Novel whey protein isolate-based highly porous scaffolds modified with therapeutic ion-releasing bioactive glasses. Mater. Lett. 2020, 261, 127115. [Google Scholar] [CrossRef]
- Demirbilek, M.; Türkoğlu Laçin, N.; Aktürk, S. N-acetylglucoseamine modified alginate sponges as scaffolds for skin tissue engineering. Turk. J. Biol. 2017, 41, 796–807. [Google Scholar] [CrossRef]
- Vlasceanu, G.M.; Crica, L.E.; Pandele, A.M.; Ionita, M. Graphene oxide reinforcing genipin crosslinked chitosan-gelatin blend films. Coatings 2020, 10, 189. [Google Scholar] [CrossRef]
- Zonderland, J.; Rezzola, S.; Gomes, D.; Espinosa, S.C.; Lourenço, A.H.F.; Serafim, A.; Stancu, I.C.; Koper, D.; Liu, H.; Habibovic, P.; et al. Full Cell Infiltration and Thick Tissue Formation In Vivo in Tailored Electrospun Scaffolds. Available online: https://www.biorxiv.org/content/10.1101/2020.02.19.955948v1 (accessed on 8 November 2021).
- Ignat, S.R.; Lazăr, A.D.; Şelaru, A.; Samoilă, I.; Vlăsceanu, G.M.; Ioniţă, M.; Radu, E.; Dinescu, S.; Costache, M. Versatile biomaterial platform enriched with graphene oxide and carbon nanotubes for multiple tissue engineering applications. Int. J. Mol. Sci. 2019, 20, 3868. [Google Scholar] [CrossRef]
- Tanaka, M.; Haniu, H.; Kamanaka, T.; Takizawa, T. Physico-Chemical, In Vitro, and In Vivo Evaluation of a 3D Unidirectional Porous Hydroxyapatite Scaffold for Bone Regeneration. Materials 2017, 10, 33. [Google Scholar] [CrossRef] [PubMed]
- Ndiaye, M.; Terranova, L.; Mallet, R.; Mabilleau, G.; Chappard, D. Three-dimensional arrangement of b -tricalcium phosphate granules evaluated by microcomputed tomography and fractal analysis. Acta Biomater. 2015, 11, 404–411. [Google Scholar] [CrossRef]
- Chappard, D.; Terranova, L.; Mallet, R.; Mercier, P. 3D porous architecture of stacks of β-TcP granules compared with that of trabecular bone: A micro-CT, vector analysis, and compression study. Front. Endocrinol. 2015, 6, 1–8. [Google Scholar] [CrossRef]
- Arbez, B.; Kün-Darbois, J.-D.; Convert, T.; Guillaume, B.; Mercier, P.; Hubert, L.; Chappard, D. Biomaterial granules used for filling bone defects constitute 3D scaffolds: Porosity, microarchitecture and molecular composition analyzed by microCT and Raman microspectroscopy. J. Biomed. Mater. Res. B Appl. Biomater. 2019, 107, 415–423. [Google Scholar] [CrossRef] [PubMed]
- Puvaneswary, S.; Balaji Raghavendran, H.R.; Ibrahim, N.S.; Murali, M.R.; Merican, A.M.; Kamarul, T. A comparative study on morphochemical properties and osteogenic cell differentiation within bone graft and coral graft culture systems. Int. J. Med. Sci. 2013, 10, 1608–1614. [Google Scholar] [CrossRef]
- Cui, L.; Liu, B.; Liu, G.; Zhang, W.; Cen, L.; Sun, J.; Yin, S.; Liu, W.; Cao, Y. Repair of cranial bone defects with adipose derived stem cells and coral scaffold in a canine model. Biomaterials 2007, 28, 5477–5486. [Google Scholar] [CrossRef] [PubMed]
- Matuda, Y.; Okamura, T.; Tabata, H.; Yasui, K.; Tatsumura, M.; Kobayashi, N.; Nishikawa, T.; Hashimoto, Y. Periodontal regeneration using cultured coral scaffolds in class ii furcation defects in dogs. J. Hard Tissue Biol. 2019, 28, 329–334. [Google Scholar] [CrossRef]
- Bracey, D.N.; Seyler, T.M.; Jinnah, A.H.; Lively, M.O.; Willey, J.S.; Smith, T.L.; Van Dyke, M.E.; Whitlock, P.W. A Decellularized Porcine Xenograft-Derived Bone Scaffold for Clinical Use as a Bone Graft Substitute: A Critical Evaluation of Processing and Structure. J. Funct. Biomater. 2018, 9, 45. [Google Scholar] [CrossRef] [PubMed]
- Salamanca, E.; Lee, W.-F.; Lin, C.-Y.; Huang, H.-M.; Lin, C.-T.; Feng, S.-W.; Chang, W.-J. A Novel Porcine Graft for Regeneration of Bone Defects. Materials 2015, 8, 2523–2536. [Google Scholar] [CrossRef]
- Salamanca, E.; Hsu, C.-C.; Huang, H.-M.; Teng, N.-C.; Lin, C.-T.; Pan, Y.-H.; Chang, W.-J. Bone regeneration using a porcine bone substitute collagen composite in vitro and in vivo. Sci. Rep. 2018, 8, 984. [Google Scholar] [CrossRef] [PubMed]
- Soares, M.Q.S.; Van Dessel, J.; Jacobs, R.; Yaedú, R.Y.F.; Sant’Ana, E.; da Silva Corrêa, D.; Madeira, M.F.C.; Duarte, M.A.H.; Rubira-Bullen, I.R.F. Morphometric evaluation of bone regeneration in segmental mandibular bone defects filled with bovine bone xenografts in a split-mouth rabbit model. Int. J. Implant Dent. 2019, 5, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Kuchler, U.; Heimel, P.; Stähli, A.; Strauss, F.J.; Luza, B.; Gruber, R. Impact of DBBM Fragments on the Porosity of the Calvarial Bone: A Pilot Study on Mice. Materials 2020, 13, 4748. [Google Scholar] [CrossRef]
- Haire, T.J.; Hodgskinson, R.; Ganney, P.S.; Langton, C.M. A comparison of porosity, fabric and fractal dimension as predictors of the Young’s modulus of equine cancellous bone. Med. Eng. Phys. 1998, 20, 588–593. [Google Scholar] [CrossRef]
- Sanchez-Molina, D.; Velazquez-Ameijide, J.; Quintana, V.; Arregui-Dalmases, C.; Crandall, J.R.; Subit, D.; Kerrigan, J.R. Fractal dimension and mechanical properties of human cortical bone. Med. Eng. Phys. 2013, 35, 576–582. [Google Scholar] [CrossRef]
- Velázquez-Ameijide, J.; García-Vilana, S.; Sánchez-Molina, D.; Llumà, J.; Martínez-González, E.; Rebollo-Soria, M.C.; Arregui-Dalmases, C. Prediction of mechanical properties of human rib cortical bone using fractal dimension. Comput. Methods Biomech. Biomed. Eng. 2021, 24, 506–516. [Google Scholar] [CrossRef] [PubMed]
- Odgaard, A. Three-dimensional methods for quantification of cancellous bone architecture. Bone 1997, 20, 315–328. [Google Scholar] [CrossRef]
- Chappard, D.; Legrand, E.; Haettich, B.; Chalès, G.; Auvinet, B.; Eschard, J.-P.; Hamelin, J.-P.; Baslé, M.-F.; Audran, M. Fractal dimension of trabecular bone: Comparison of three histomorphometric computed techniques for measuring the architectural two-dimensional complexity. J. Pathol. 2001, 195, 515–521. [Google Scholar] [CrossRef]
- Odgaard, A.; Kabel, J.; van Rietbergen, B.; Dalstra, M.; Huiskes, R. Fabric and elastic principal directions of cancellous bone are closely related. J. Biomech. 1997, 30, 487–495. [Google Scholar] [CrossRef]
- Chappard, C.; Peyrin, F.; Bonnassie, A.; Lemineur, G.; Brunet-Imbault, B.; Lespessailles, E.; Benhamou, C.-L. Subchondral bone micro-architectural alterations in osteoarthritis: A synchrotron micro-computed tomography study. Osteoarthr. Cartil. 2006, 14, 215–223. [Google Scholar] [CrossRef]
- Ho, S.T.; Hutmacher, D.W. A comparison of micro CT with other techniques used in the characterization of scaffolds. Biomaterials 2006, 27, 1362–1376. [Google Scholar] [CrossRef]
- Moore, M.J.; Jabbari, E.; Ritman, E.L.; Lu, L.; Currier, B.L.; Windebank, A.J.; Yaszemski, M.J. Quantitative analysis of interconnectivity of porous biodegradable scaffolds with micro-computed tomography. J. Biomed. Mater. Res.-Part A 2004, 71, 258–267. [Google Scholar] [CrossRef]
- Nair, M.; Shepherd, J.H.; Best, S.M.; Cameron, R.E. MicroCT analysis of connectivity in porous structures: Optimizing data acquisition and analytical methods in the context of tissue engineering. J. R. Soc. Interface 2020, 17, 20190833. [Google Scholar] [CrossRef] [PubMed]
- Vlasceanu, G.M.; Selaru, A.; Dinescu, S.; Balta, C.; Herman, H.; Gharbia, S.; Hermenean, A.; Ionita, M.; Costache, M. Comprehensive Appraisal of Graphene–Oxide Ratio in Porous Biopolymer Hybrids Targeting Bone-Tissue Regeneration. Nanomaterials 2020, 10, 1444. [Google Scholar] [CrossRef]
- Orhan, K.; Büyüksungur, A. Fundamentals of Micro-CT Imaging. In Micro-Computed Tomography (micro-CT) in Medicine and Engineering; Springer International Publishing: Cham, Switzerland, 2020; pp. 27–33. [Google Scholar]
- Wang, Y.; Garcea, S.C.; Withers, P.J. 7.6 Computed Tomography of Composites. In Comprehensive Composite Materials II; Beaumont, P.W.R., Zweben, C.H., Eds.; Elsevier: Oxford, UK, 2018; pp. 101–118. ISBN 978-0-08-100534-7. [Google Scholar]
- Kazakia, G.J.; Burghardt, A.J.; Cheung, S.; Majumdar, S. Assessment of bone tissue mineralization by conventional x-ray microcomputed tomography: Comparison with synchrotron radiation microcomputed tomography and ash measurements. Med. Phys. 2008, 35, 3170–3179. [Google Scholar] [CrossRef] [PubMed]
- Withers, P.J.; Grimaldi, D.; Hagen, C.K.; Maire, E.; Manley, M.; Plessis, A. Du X-ray computed tomography. Nat. Rev. Methods Prim. 2021, 1, 1–18. [Google Scholar] [CrossRef]
- Dumitrescu, G.D.; Serafim, A.; Vasile, E.; Iovu, H.; Stancu, I.C. Bioactive biogenous mineral for bone bonding applications. UPB Sci. Bull. Ser. B Chem. Mater. Sci. 2020, 82, 3–14. [Google Scholar]
- Lohfeld, S.; Cahill, S.; Barron, V.; McHugh, P.; Dürselen, L.; Kreja, L.; Bausewein, C.; Ignatius, A. Fabrication, mechanical and in vivo performance of polycaprolactone/tricalcium phosphate composite scaffolds. Acta Biomater. 2012, 8, 3446–3456. [Google Scholar] [CrossRef]
- Saito, E.; Suarez-Gonzalez, D.; Rao, R.R.; Stegemann, J.P.; Murphy, W.L.; Hollister, S.J. Use of Micro-Computed Tomography to Nondestructively Characterize Biomineral Coatings on Solid Freeform Fabricated Poly (L-Lactic Acid) and Poly (ε-caprolactone) scaffolds in vitro and in vivo. Tissue Eng. Part C Methods 2013, 19, 507–517. [Google Scholar] [CrossRef] [PubMed]
- Arnold, J.; Sarkar, K.; Smith, D. 3D printed bismuth oxide-polylactic acid composites for radio-mimetic computed tomography spine phantoms. J. Biomed. Mater. Res.-Part B Appl. Biomater. 2020, 109, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wertheim, D.F.; Jones, A.S.; Coombes, A.G.A. Micro-CT in drug delivery. Eur. J. Pharm. Biopharm. 2010, 74, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wertheim, D.F.; Jones, A.S.; Chang, H.-I.; Coombes, A.G.A. Micro-CT analysis of matrix-type drug delivery devices and correlation with protein release behaviour. J. Pharm. Sci. 2010, 99, 2854–2862. [Google Scholar] [CrossRef]
- Crean, B.; Parker, A.; Roux, D.L.; Perkins, M.; Luk, S.Y.; Banks, S.R.; Melia, C.D.; Roberts, C.J. Elucidation of the internal physical and chemical microstructure of pharmaceutical granules using X-ray micro-computed tomography, Raman microscopy and infrared spectroscopy. Eur. J. Pharm. Biopharm. 2010, 76, 498–506. [Google Scholar] [CrossRef]
- Arifvianto, B.; Leeflang, M.A.; Zhou, J. Diametral compression behavior of biomedical titanium scaffolds with open, interconnected pores prepared with the space holder method. J. Mech. Behav. Biomed. Mater. 2017, 68, 144–154. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, S. Correlation of Materials Property and Performance with Internal Structures Evolvement Revealed by Laboratory X-ray Tomography. Materials 2018, 11, 1795. [Google Scholar] [CrossRef]
- Khrapov, D.; Koptyug, A.; Manabaev, K.; Léonard, F.; Mishurova, T.; Bruno, G.; Cheneler, D.; Loza, K.; Epple, M.; Surmenev, R.; et al. The impact of post manufacturing treatment of functionally graded Ti6Al4V scaffolds on their surface morphology and mechanical strength. J. Mater. Res. Technol. 2019, 9, 1866–1881. [Google Scholar] [CrossRef]
- Maksimcuka, J.; Obata, A.; Sampson, W.W.; Blanc, R.; Gao, C.; Withers, P.J.; Tsigkou, O.; Kasuga, T.; Lee, P.D.; Poologasundarampillai, G. X-ray tomographic imaging of tensile deformation modes of electrospun biodegradable polyester fibers. Front. Mater. 2017, 4, 1–11. [Google Scholar] [CrossRef]
- van Kampen, K.A.; Olaret, E.; Stancu, I.C.; Moroni, L.; Mota, C. Controllable four axis extrusion-based additive manufacturing system for the fabrication of tubular scaffolds with tailorable mechanical properties. Mater. Sci. Eng. C 2021, 119, 111472. [Google Scholar] [CrossRef]
- Du Plessis, A.; Kouprianoff, D.P.; Yadroitsava, I.; Yadroitsev, I. Mechanical properties and in situ deformation imaging of microlattices manufactured by laser based powder bed fusion. Materials 2018, 11, 1663. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Gong, C.; Lewis, G.S.; Armstrong, A.D.; Du, J. 3D full-field biomechanical testing of a glenoid before and after implant placement. Extrem. Mech. Lett. 2020, 35, 100614. [Google Scholar] [CrossRef]
- Zhou, M.; Zhang, Y.; Zhou, R.; Hao, J.; Yang, J. Mechanical Property Measurements and Fracture Propagation Analysis of Longmaxi Shale by Micro-CT Uniaxial Compression. Energies 2018, 11, 1409. [Google Scholar] [CrossRef]
- Wang, Y.; Mikkelsen, L.P.; Pyka, G.; Withers, P.J. Time-lapse helical X-ray computed tomography (CT) study of tensile fatigue damage formation in composites for wind turbine blades. Materials 2018, 11, 2304. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Qi, D.; Hu, W.; Xi, L.; Sun, L.; Liao, B.; Berto, F.; Qian, G.; Xiao, D. Synchrotron X-ray micro-computed tomography imaging of 3D re-entrant micro lattice during in situ micro compression experimental process. Mater. Des. 2020, 192, 108743. [Google Scholar] [CrossRef]
- Loa, C.; Sanob, T.; Hogan, J.D. Deformation Mechanisms and Evolution of Mechanical Properties in Damaged Advanced Ceramics. J. Eur. Ceram. Soc. 2020, 40, 108709. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, H.; Zhou, X.; Yi, X.; Xiao, Y.; Wei, X. In situ X-ray CT investigations of Meso-damage evolution of cemented waste rock-tailings backfill (CWRTB) during triaxial deformation. Minerals 2019, 9, 52. [Google Scholar] [CrossRef]
- Kim, Y.; Yun, G.J. Effects of microstructure morphology on stress in mechanoluminescent particles: Micro CT image-based 3D finite element analyses. Compos. Part A Appl. Sci. Manuf. 2018, 114, 338–351. [Google Scholar] [CrossRef]
- Schipani, R.; Nolan, D.R.; Lally, C.; Kelly, D.J. Integrating finite element modelling and 3D printing to engineer biomimetic polymeric scaffolds for tissue engineering. Connect. Tissue Res. 2020, 61, 174–189. [Google Scholar] [CrossRef]
- Basri, H.; Prakoso, A.T.; Sulong, M.A.; Md Saad, A.P.; Ramlee, M.H.; Agustin Wahjuningrum, D.; Sipaun, S.; Öchsner, A.; Syahrom, A. Mechanical degradation model of porous magnesium scaffolds under dynamic immersion. Proc. Inst. Mech. Eng. Part L J. Mater. Des. Appl. 2020, 234, 175–185. [Google Scholar] [CrossRef]
- Biswas, P.; Guessasma, S.; Li, J. Numerical prediction of orthotropic elastic properties of 3D-printed materials using micro-CT and representative volume element. Acta Mech. 2020, 231, 503–516. [Google Scholar] [CrossRef]
- Askari, E.; Cengiz, I.F.; Alves, J.L.; Henriques, B.; Flores, P.; Fredel, M.C.; Reis, R.L.; Oliveira, J.M.; Silva, F.S.; Mesquita-Guimarães, J. Micro-CT based finite element modelling and experimental characterization of the compressive mechanical properties of 3-D zirconia scaffolds for bone tissue engineering. J. Mech. Behav. Biomed. Mater. 2020, 102, 103536. [Google Scholar] [CrossRef] [PubMed]
- Ding, W. Opportunities and challenges for the biodegradable magnesium alloys as next-generation biomaterials. Regen. Biomater. 2016, 3, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Behravesh, E.; Timmer, M.D.; Lemoine, J.J.; Liebschner, M.A.K.; Mikos, A.G. Evaluation of the in Vitro Degradation of Macroporous Hydrogels Using Gravimetry, Confined Compression Testing, and Microcomputed Tomography. Biomacromolecules 2002, 3, 1263–1270. [Google Scholar] [CrossRef]
- Yang, B.; Zuo, Y.; Zou, Q.; Li, L.; Li, J.; Man, Y.; Li, Y. Effect of ultrafine poly(ε-caprolactone) fibers on calcium phosphate cement: In vitro degradation and in vivo regeneration. Int. J. Nanomed. 2016, 11, 163–177. [Google Scholar] [CrossRef][Green Version]
- Florczyk, S.J.; Leung, M.; Li, Z.; Huang, J.I.; Hopper, R.A.; Zhang, M. Evaluation of three-dimensional porous chitosan-alginate scaffolds in rat calvarial defects for bone regeneration applications. J. Biomed. Mater. Res.-Part A 2013, 101, 2974–2983. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, C.; Friis, T.; Xiao, Y. The osteogenic properties of CaP/silk composite scaffolds. Biomaterials 2010, 31, 2848–2856. [Google Scholar] [CrossRef]
- Weiss, P.; Obadia, L.; Magne, D.; Bourges, X.; Rau, C.; Weitkamp, T.; Khairoun, I.; Bouler, J.M.; Chappard, D.; Gauthier, O.; et al. Synchrotron X-ray microtomography (on a micron scale) provides three-dimensional imaging representation of bone ingrowth in calcium phosphate biomaterials. Biomaterials 2003, 24, 4591–4601. [Google Scholar] [CrossRef]
- Westhauser, F.; Weis, C.; Prokscha, M.; Bittrich, L.A.; Li, W.; Xiao, K.; Kneser, U.; Kauczor, H.U.; Schmidmaier, G.; Boccaccini, A.R.; et al. Three-dimensional polymer coated 45S5-type bioactive glass scaffolds seeded with human mesenchymal stem cells show bone formation in vivo. J. Mater. Sci. Mater. Med. 2016, 27, 1–7. [Google Scholar] [CrossRef]
- Palmquist, A.; Shah, F.A.; Emanuelsson, L.; Omar, O.; Suska, F. A technique for evaluating bone ingrowth into 3D printed, porous Ti6Al4V implants accurately using X-ray micro-computed tomography and histomorphometry. Micron 2017, 94, 1–8. [Google Scholar] [CrossRef]
- Xu, Y.; Meng, H.; Yin, H.; Sun, Z.; Peng, J.; Xu, X.; Guo, Q.; Xu, W.; Yu, X.; Yuan, Z.; et al. Quantifying the degradation of degradable implants and bone formation in the femoral condyle using micro-CT 3D reconstruction. Exp. Ther. Med. 2018, 15, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Simon, J.L.; Rekow, E.D.; Thompson, V.P.; Beam, H.; Ricci, J.L.; Parsons, J.R.; Al, S.E.T. MicroCT analysis of hydroxyapatite bone repair scaffolds created via three-dimensional printing for evaluating the effects of scaffold architecture on bone ingrowth. J. Biomed. Mater. Res.-Part A 2008, 85, 371–377. [Google Scholar] [CrossRef] [PubMed]
- Chang, P.C.; Luo, H.T.; Lin, Z.J.; Tai, W.C.; Chang, C.H.; Chang, Y.C.; Cochran, D.L.; Chen, M.H. Preclinical evaluation of a 3D-printed hydroxyapatite/poly(lactic-co-glycolic acid) scaffold for ridge augmentation. J. Formos. Med. Assoc. 2021, 120, 1100–1107. [Google Scholar] [CrossRef]
- Chang, P.-C.C.; Luo, H.-T.T.; Lin, Z.-J.J.; Tai, W.-C.C.; Chang, C.-H.H.; Chang, Y.-C.C.; Cochran, D.L.; Chen, M.-H.H. Regeneration of critical-sized mandibular defect using a 3D-printed hydroxyapatite-based scaffold: An exploratory study. J. Periodontol. 2021, 92, 428–435. [Google Scholar] [CrossRef] [PubMed]
- Otsu, N. A Threshold Selection Method from Gray-Level Histograms. IEEE Trans. Syst. Man Cybern. 1979, 20, 62–66. [Google Scholar] [CrossRef]
- Zhang, H.; Mao, X.; Zhao, D.; Jiang, W.; Du, Z.; Li, Q.; Jiang, C.; Han, D. Three dimensional printed polylactic acid-hydroxyapatite composite scaffolds for prefabricating vascularized tissue engineered bone: An in vivo bioreactor model. Sci. Rep. 2017, 7, 15255. [Google Scholar] [CrossRef]
- Sagbas, B.; Numan Durakbasa, M. Measurement of wear in orthopedic prosthesis. Acta Phys. Pol. A 2012, 121, 131–134. [Google Scholar] [CrossRef]
- Bowden, A.E.; Kurtz, S.M.; Edidin, A.A. Validation of a micro-CT technique for measuring volumetric wear in retrieved acetabular liners. J. Biomed. Mater. Res.-Part B Appl. Biomater. 2005, 75, 205–209. [Google Scholar] [CrossRef]
- Engh, C.A.; Zimmerman, R.L.; Hopper, R.H.; Engh, G.A. Can microcomputed tomography measure retrieved polyethylene wear? Comparing fixed-bearing and rotating-platform knees. Clin. Orthop. Relat. Res. 2013, 471, 86–93. [Google Scholar] [CrossRef]
- Uddin, M.S.; Mak, C.Y.E.; Callary, S.A. Evaluating hip implant wear measurements by CMM technique. Wear 2016, 364, 193–200. [Google Scholar] [CrossRef]
- Teeter, M.G.; Naudie, D.D.R.; McErlain, D.D.; Brandt, J.M.; Yuan, X.; MacDonald, S.J.; Holdsworth, D.W. In vitro quantification of wear in tibial inserts using microcomputed tomography. Clin. Orthop. Relat. Res. 2011, 469, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.; Bradley, R.S.; Soutis, C.; Hogg, P.J.; Withers, P.J. 2D and 3D imaging of fatigue failure mechanisms of 3D woven composites. Compos. Part A Appl. Sci. Manuf. 2015, 77, 37–49. [Google Scholar] [CrossRef]
- Teeter, M.G.; Naudie, D.D.R.; Charron, K.D.; Holdsworth, D.W. Three-dimensional surface deviation maps for analysis of retrieved polyethylene acetabular liners using micro-computed tomography. J. Arthroplast. 2010, 25, 330–332. [Google Scholar] [CrossRef] [PubMed]
- Parrilli, A.; Falcioni, S.; Fini, M.; Affatato, S. Is micro-computed tomography useful for wear assessment of ceramic femoral heads? A preliminary evaluation of volume measurements. J. Appl. Biomater. Funct. Mater. 2016, 14, e483–e489. [Google Scholar] [CrossRef] [PubMed]
- Turco, G.; Porrelli, D.; Marsich, E.; Vecchies, F.; Lombardi, T.; Stacchi, C.; Di Lenarda, R. Three-Dimensional Bone Substitutes for Oral and Maxillofacial Surgery: Biological and Structural Characterization. J. Funct. Biomater. 2018, 9, 62. [Google Scholar] [CrossRef] [PubMed]
- Lin, Q.; Andrew, M.; Thompson, W.; Blunt, M.J.; Bijeljic, B. Optimization of image quality and acquisition time for lab-based X-ray microtomography using an iterative reconstruction algorithm. Adv. Water Resour. 2018, 115, 112–124. [Google Scholar] [CrossRef]
- Bartos, M.; Suchý, T.; Tonar, Z.; Foltán, R.; Kalbacova, M.H. Micro-CT in tissue engineering scaffolds for bone regeneration: Principles and application. Ceram.-Silik. 2018, 62, 194–199. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olăreț, E.; Stancu, I.-C.; Iovu, H.; Serafim, A. Computed Tomography as a Characterization Tool for Engineered Scaffolds with Biomedical Applications. Materials 2021, 14, 6763. https://doi.org/10.3390/ma14226763
Olăreț E, Stancu I-C, Iovu H, Serafim A. Computed Tomography as a Characterization Tool for Engineered Scaffolds with Biomedical Applications. Materials. 2021; 14(22):6763. https://doi.org/10.3390/ma14226763
Chicago/Turabian StyleOlăreț, Elena, Izabela-Cristina Stancu, Horia Iovu, and Andrada Serafim. 2021. "Computed Tomography as a Characterization Tool for Engineered Scaffolds with Biomedical Applications" Materials 14, no. 22: 6763. https://doi.org/10.3390/ma14226763
APA StyleOlăreț, E., Stancu, I.-C., Iovu, H., & Serafim, A. (2021). Computed Tomography as a Characterization Tool for Engineered Scaffolds with Biomedical Applications. Materials, 14(22), 6763. https://doi.org/10.3390/ma14226763









