3. Results
The total content of the elements, such as carbon, hydrogen, and sulfur, is one of the main factors that determines the possibility of using solid fuel to supply DC-SOFCs.
Table 1 shows the results of the elemental analysis of carbon, hydrogen, and sulfur for raw, ground pistachio shells (P0), torrefied pistachio shells (P200, P300, and P400), and charred samples (P600 and P850) and the calculated amount of oxygen as the sum of oxygen and nitrogen. Additionally, the data for the content of ash and moisture that was determined for these samples was added.
The data in
Table 1 demonstrates that increasing the temperature of the thermal treatment of ground pistachio shells from 200 °C to 850 °C results in a gradual increase of the total carbon content in all the solid carbonaceous fuels. Increasing the pyrolysis temperature from 600 °C to 850 °C does not cause significant changes to the total carbon content in the solid fuel samples. Regarding the P600 and P850 samples, the carbon content remains around 87–88%. Increasing the pyrolysis temperature of the pistachio shells leads to a gradual decrease of the hydrogen, oxygen and sulfur content. The hydrogen and oxygen are mainly removed as H
2O from the samples. Moreover, a variation in the humidity content for the series of samples from P0 to P850 is observed. Although the applied thermal treatment of pistachio shells in higher temperatures led to decreased moisture, it did not completely remove it. This is probably due to the adsorption of moisture during the cooling samples when it was flowing through quartz reactor nitrogen as a shielding gas. Regarding combustion technology, a higher content of moisture decreases the calorific values of solid fuels. However, when solid carbon fuels in DC-SOFCs technology are applied, the phenomena can be different. A small amount of moisture can induce an additional gasification process in a solid carbon bed that is placed in DC-SOFC [
1,
35]. The increased temperature during the thermal treatment of pistachio shells caused the ash content to increase. When applying carbonaceous-based materials as solid carbon fuels in DC-SOFCs, a high content of carbon and low content of mineral matters in such fuels are desired. The main drawback of DC-SOFCs is the limited reaction zone of electrochemical oxidation of carbon particles, which corresponds to the direct contact with the surface of anode materials. The increased contamination of solid carbon fuels that are used in these types of fuel cells can reduce the reaction zone of the anodic oxidation of carbon particles [
1,
2]. Previous data that were reported in the literature and our previous works [
1,
35,
36] showed that solid fuels involving more than 70% elemental carbon mass should lead to a good performance of the DC-SOFC. The presence of mineral content in solid carbon fuels is lower than 2–3% and does not cause the internal electrical resistance of fuel cells to increase significantly. The electrochemical performance of the DC-SOFC is expected to improve when inorganic elements that are included in mineral matter act as natural catalysts in the Boudouard reaction and improve production of CO in the anode chamber of DC-SOFC. Additionally, a low content of sulfur is required due to its negative impact on the Ni-GDC or Ni-YSZ anode structure [
1,
5,
37]
The data obtained from the ultimate and technical analyses for the investigated samples from series P0 to P850 is sufficient to select these samples for further investigation as fuel to power the SOFCs.
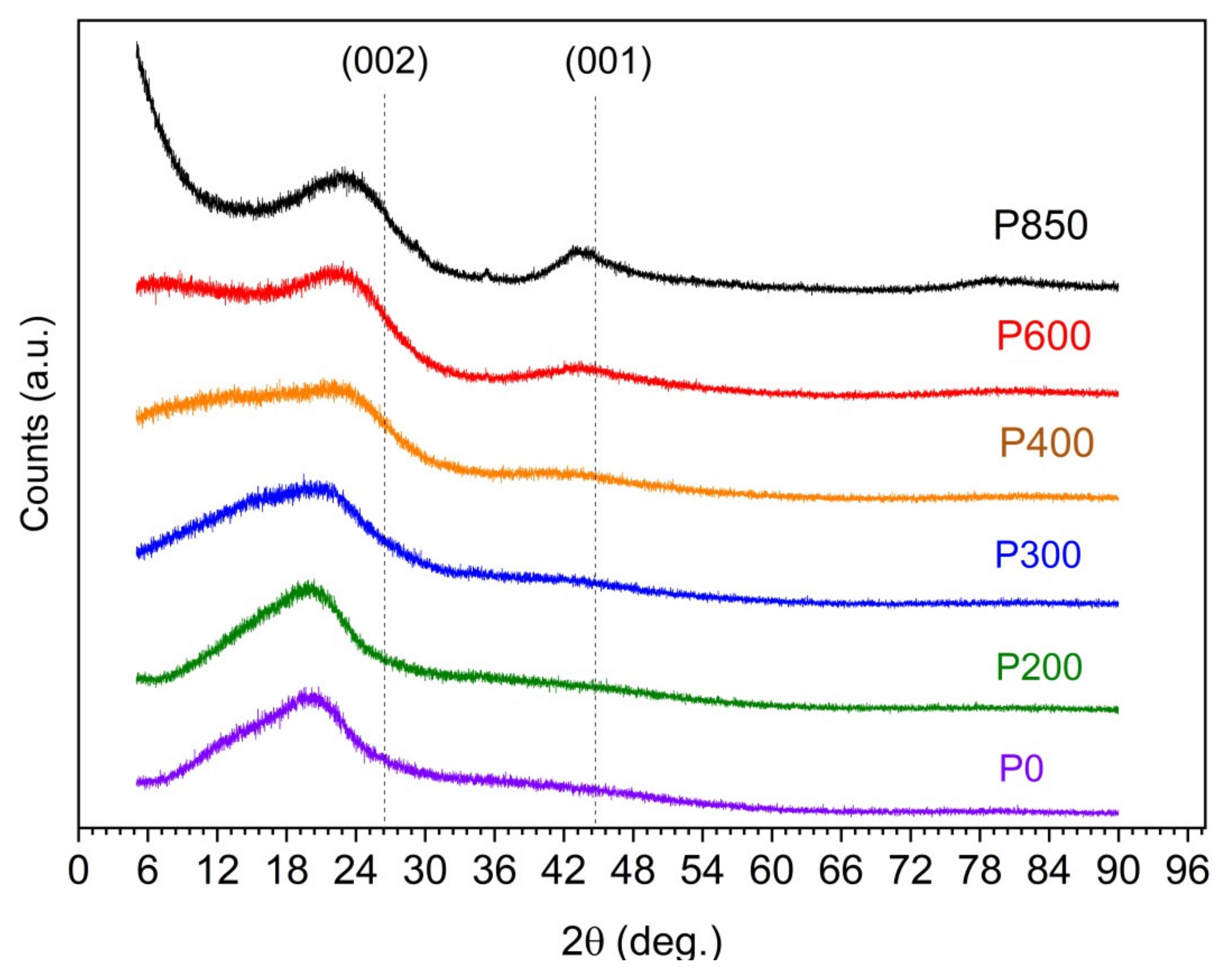
Figure 1 illustrates the evolution of the XRD patterns that were recorded for the serial samples from P0 to P850 and reflects the variation in the structural ordering of the carbon particles of the solid fuels as a function of the applied temperature of the thermal treatment.
The diffraction patterns only show the weakly broadened peaks (002) and (001) that correspond to the partial graphitization of the samples. It should be emphasized that obtaining carbon samples with a disordered structure is a desirable feature due to their use as fuel for the utilization of DC-SOFCs.
As shown by the recorded XRD patterns, the initial P0 sample is characterized by a high background level with a strongly broadened peak (002) at 20–24°. A direct comparison of the recorded XRD patterns for the investigated sample series shows that the main peak in these patterns is mostly (002), which is typical for graphite-based carbon materials. The variation of the temperature preparation samples leads to differences regarding the intensity, width, and position of the (002) peak. The maximum diffraction reflection (002) shifts to higher angles than the typical graphite (002) position (~26.5 degrees). Additionally, the charred samples (P400, P600, and P850) that were prepared at the highest temperature showed a second peak (001), which is characteristic of the carbon-graphite structure. These results agree with the XRD studies that were carried out for other biomass types [
17,
23,
26].
An XRD analysis was used to investigate the variation of the phase composition of the mineral residue (ash). The ash samples that were investigated came from the combustion of the P0–P850 origin series. The results revealed mineral residue, which might have undergone some variations in its chemical composition depending on the history of the previous thermal treatment in the temperature range of 200–850 °C and the subsequent combustion process at a temperature of 1000 °C.
Nevertheless, these experiments and results are useful to determine the influence of the chemical composition of inorganic compounds (especially alkaline oxides or iron-based oxides) on the gasification process of the carbon fixed solid bed, which was formed in the anode chamber of the DC-SOFC operation.
As shown in
Figure 2, the XRD pattern reflects the variation of the phase composition of the ash samples, which depends on the thermal history of the original samples (P0, P400, P600, and P850). The analyses that were performed on the variation of the composition phase of the ashes that were obtained after burning the samples showed that the main components of the ash that was obtained from the pistachio shells and burnt samples were as follows: Periclase (MgO), Calcite (CaCO
3), and Magnesite (MgCO
3). On the one hand, the individual inorganic phases that comprise the mineral residue can influence the operating parameters of the DC-SOFC. Within the operating temperatures (800–850 °C) of the DC-SOFC, decomposition of the alkali carbonates CaCO
3 or MgCO
3 to MgO and CO
2 may occur [
38,
39]. The presence of CO
2, MgO, and CaO can facilitate the solid fuel gasification process in the anode chamber in the DC-SOFC [
40]. On the other hand, the presence of Ca
2SiO
4 and 4CaO·Al
2O
3·Fe
2O
3 oxides in the solid fuel can lead to an increase of the electric ohmic resistance of the fuel cell. Additionally, they can block the electrochemical reaction zone between the carbon particles and the surface of the cermet anode material where the electrochemical oxidation process takes place [
41,
42]. Additionally, an MIR was applied to study the chemical composition of the biomass of the original ashes.
Figure 3 presents the collection of the MIR spectra that were recorded for the selected ashes that were obtained after combustion of the P0, P400, P600, and P850 samples.
All spectra presented in
Figure 3 demonstrate a band at 3643 cm
−1. This is characteristic of OH- groups (O-H stretching vibration) and occurred in the Portlandite (Ca[OH]
2) [
43]. Another peculiarity is the presence of bands at approximately 1410 cm
−1, 875 cm
−1, and 712 cm
−1, which corresponds to the presence of CO
32− groups. The location of the band at 712 cm
−1 indicates the presence of calcite in the investigated ash samples [
44]. In turn, the bands at approximately 940 cm
−1 and 520 cm
−1 (internal vibrations in [SiO4]
4−) confirm the presence of dicalcium silicate in the samples. In addition to the bands mentioned above, all spectra show band(s) at approximately 1050 cm
−1, which is typical for polymerized silicates [
45]. An analysis of the MIR spectra presented in
Figure 3 shows that as the temperature increases, the decomposition of portlandite progresses, which is indicated by a systematic decrease in the band intensity at 3643 cm
−1. At 850 °C, a sharp decrease in the intensity of the bands that are associated with carbonate groups is characteristic, which indicates the decomposition of carbonates [
46,
47].
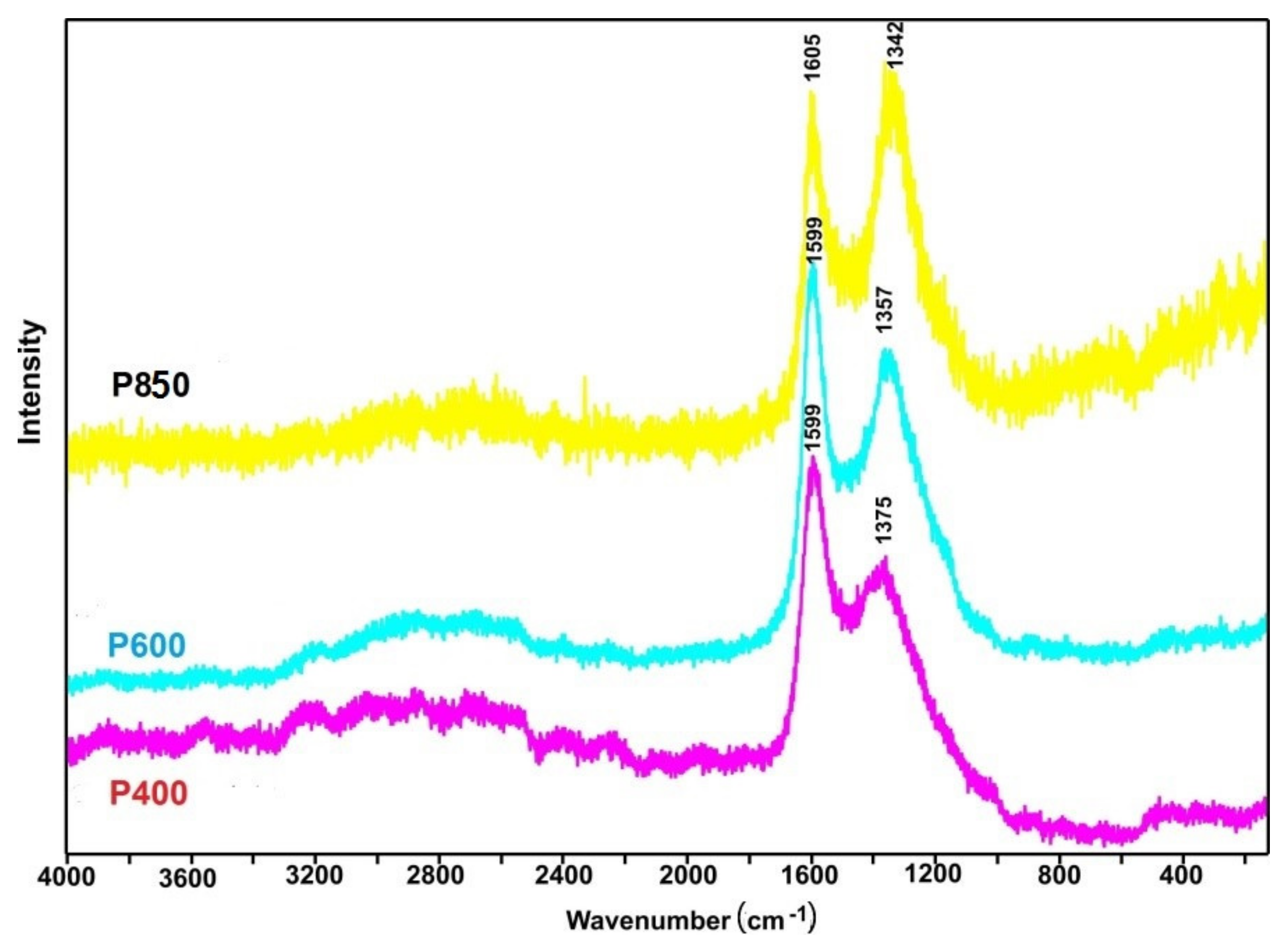
The Raman spectroscopy techniques determine the degree of disorder in the crystallographic structure of the tested series of samples in relation to traditional carbon materials (graphite, amorphous carbon, such as carbon black or other solid fuels made from waste biomass).
Figure 4 shows the Raman spectra for charred pistachio shells and samples P400–P850. Based on Raman research, it is relatively easy to determine the form of carbon that is present in the tested materials.
An analysis of the Raman spectra showed clearly visible G bands at approximately 1600 cm
−1, which indicates the presence of carbon in the sp
2 hybridization. The presence of D bands at the range of 1340–1375 cm
−1 indicates the presence of numerous structural defects in the carbon phase [
48]. The ratio of intensities of the G and D bands can be attributed to the degree of disorder of the carbon structure [
49]. Additionally, the evolution of the D band with the increasing temperature of pyrolysis was observed from 1375 to 1342 cm
−1.
It was previously determined that the presence of highly disordered carbon particles in solid fuel enhances the electrochemical oxidation of carbon particles in DC-SOFCs [
24,
26,
50]. One key factor of diffusion and convection in the small solid carbon bed is the structure (i.e., the distribution of particle sizes, their compaction, and the surface area that is available for the medium to flow between and within the carbon particles)
Skrzypkiewicz and Antunes [
51] examined the mechanism pathway in the direct carbon bed SOFC and its implications for the electrochemistry of such fuel cells. It was determined that the reaction mechanism at the anode is rate-limited by the electro-oxidation of the CO (anode), and the gas transport is mainly driven by natural convection (anode). The dominant process for the transport of CO, which can be electrochemically oxidized, is convection through the solid carbon bed. Knowledge of the evolution of the morphological structure of solid fuels produced from pistachio shells is necessary to explain the influence of the physicochemical properties of applied solid fuels on the performance of DC-SOFCs.
Figure 5a–c present the SEM images of carbonaceous materials prepared from pistachio shells.
The analysis of the SEM images from
Figure 5a–c shows that increasing the temperature during the thermal treatment of pistachio shells results in a slight increase in the average grain size. Observing the morphology of grains shows that they are more isometric in shape. Desalux et al. [
49] investigated the impact of the morphology of carbon particles on the anodic oxidation of solid fuels in DC-SOFC. They found that irregular shapes of carbon particles in solid fuels led to “only point contact” with surface anode electrodes. This phenomenon reduced the limited electrochemical oxidation of the carbon particles’ reaction zone. Additionally, a higher temperature of above 400 °C increased porosity in the grains of prepared solid fuels. During the SEM observation, a qualitative analysis of the samples was performed. Although the grains of solid carbon fuels derived from pistachio shells mainly consisted of carbon, small amounts of Al, Fe, Cr were detected [
21,
52]. Wavelength Dispersive X-ray Fluorescence (WDXRF) was used as a second method for a rapid qualitative chemical analysis of solid carbonaceous samples [
53,
54]. The main metallic elements that were detected were K, Mg, Ca, Zn, Sr, and Fe, and some traces of P and Cl was visible. Inorganic content can vary depending on the biomass types, as thermal treatment affects the variation of concentrations of elements and compounds. This study focused on the semi-quantitative comparative analysis of the main elements, such as K, Mg, Ca, Sr and Fe, which can vary depending on the temperature of the prepared samples. Data in review papers that analyzed the influence of factors on the efficiency of solid carbon fuels gasified with CO
2 as medium indicated that these elements improve the kinetics of the Boudouard reaction during the carbon gasification process, especially in the temperature range of 700–850 °C [
55]. The comparative qualitative analysis that was performed for the solid fuel samples P300, P400, P600, and P850, which are derived from pistachio shells, is presented in
Figure 6.
The analysis of the comparative variation between the mass content of Fe or alkaline elements, focusing on K, Na, Ca, and Mg for carbonaceous-based materials from the series P300 to P850, found no considerable differences in the chemical composition due to the applied thermal treatment of pistachio shells from 300 °C to 850 °C. The difference in the content of K and Na is obvious in the case of sample P400, probably because of the inhomogeneity of P400 samples, which can happen when applying thermal treatment at 400 °C for 2 h. At this temperature, a high number of gaseous products and liquid compounds are removed. This statement agrees with the DTA curve recorded for the P400 sample. A small exothermic effect at ~415 °C is visible, which may correlate with the decomposition of potassium salts. The data that were obtained using the WDXRF method are in good agreement with the observed changes of the phase composition recorded using the XRD method for the ashes (
Figure 2) and the MIR investigations for the solid carbonaceous samples (P400 to P850) (
Figure 3). Fe
2O
3 catalyzes the reverse Boudouard reaction according to chemical Equations (5) and (6) as follows [
55]:
Tan and You [
56] confirmed the usefulness of the Fe-load charcoal samples as fuels for DC-SOFCs. The power output of the investigated DC-SOFC was significantly improved compared to that of the same DC-SOFC that was supplied by charcoal without the addition of the Fe
2O
3 catalyst. Skrzypkiewicz et al. [
57] investigated the impact of Fe
2O
3-loaded charcoal on the performance of DC-SOFC. The reverse Boudouard reaction (C + CO
2→2CO) is a limiting process for the fuel cell performance. The in-situ-generated CO improved the performance of DC-SOFC compared to that of the same cells that were supplied charcoal without the Fe
2O
3 catalyst.
Wu et al. [
19] investigated the performance of DC-SOFC when involving the internal catalytic gasification of carbon to gaseous carbon monoxide via the reverse Boudouard reaction (C[s] + CO2[g]→2CO[g]). The carbon gasification reaction rate was greatly enhanced when Fe
mO
n–M
xO (M = Li, K, Ca) was introduced as a catalyst for solid carbon fuels. They proposed that the following reactions are responsible for improving the gasification and performance of the DC-SOFC:
The above inorganic compounds are natural components of the series of biomass-derived solid fuels (P300 to P850).
Using the obtained carbonaceous-based materials as a solid fuel to power SOFCs requires an understanding of the thermal effects that can occur in powdered solid fuels that are placed in an anode chamber of the DC-SOFC. The temperature range in which a DC-SOFC operates is large. It includes the following stages: (i) turning on the DC-SOFC at RT temperature; (ii) heating it up to the operating temperature of the cell (700–850 °C); and (iii) targeting the operation under stabilized conditions (700–850 °C). Changes in the physicochemical properties of solid fuels with temperature variations influences the DC-SOFC operation, as this can affect the durability of the anode and electrolyte materials and the achieved operating parameters (i.e., the current and power densities derived from the DC-SOFC).
Pistachio shells are assumed to be composed of hemicellulose, cellulose, and lignin, and products of thermal decomposition can also influence the performance of DC-SOFC.
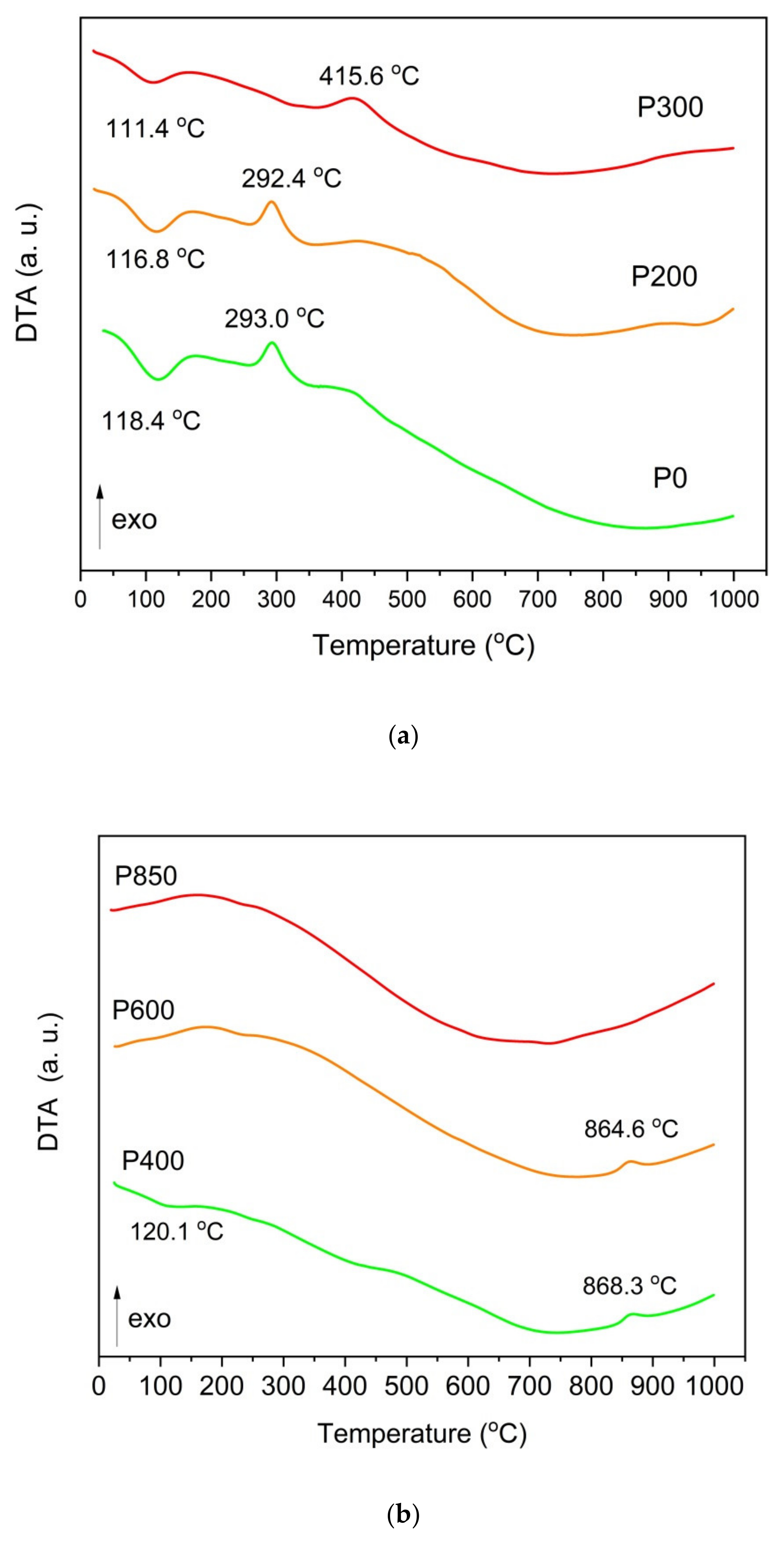
Figure 7a,b record the DTA curves for ground raw pistachio shells, torrefied shells, (
Figure 7a) and chars that are prepared at temperatures of 25–1000 °C (
Figure 7b).
As shown in
Figure 7a, gradually heating these samples resulted in an initial slight thermal effect at a temperature of approximately 100 °C, which is a result of the water evaporating from the sample. Heating the tested samples between the temperature range of 100–350 °C initiates the pyrolysis processes related to the first decomposition of organic matter, which occurs between 150–350 °C and 275–350 °C, respectively, for hemicellulose and cellulose. The lignin decomposition occurred at higher temperatures between 275–500 °C. In the DTA curves for the solid fuel samples (P400–P850, which are derived from pistachio shells) (
Figure 7b), some thermal effects corresponded to the decomposition of inorganic compounds at 840–850 °C. The wide exothermic peak that is visible on the DTA curve is likely connected to the decomposition of calcium carbonate (CaCO
3).
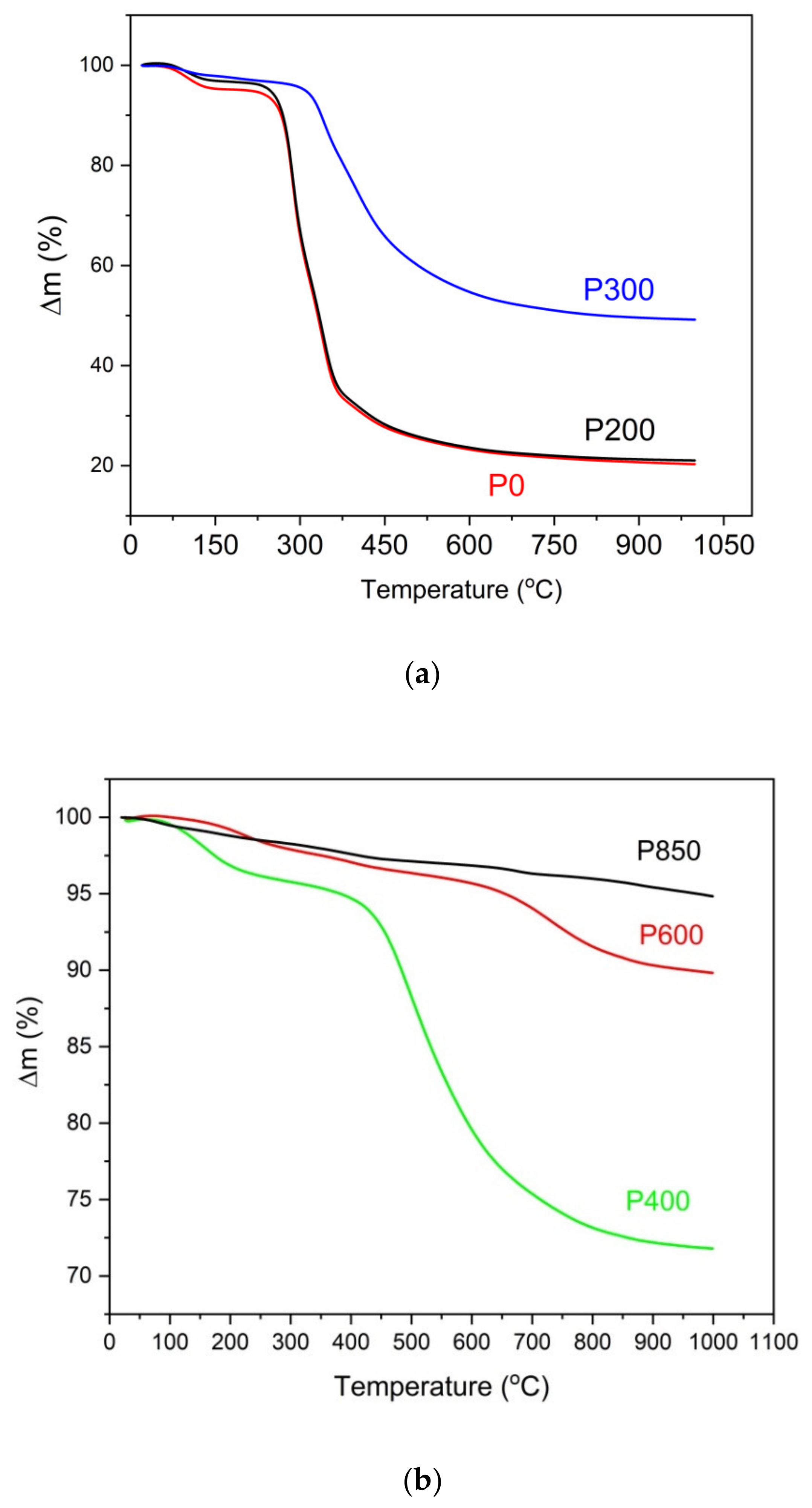
Figure 8a,b show the registered TG curves vs. the temperature that was recorded for the raw samples (P0), torrefied pistachio samples (P200 and P300) (
Figure 8a) and charred samples (P400, P600, and P850) (
Figure 8b).
The analysis of the TG curves that were recorded for the studied pistachio shells show that the increased temperature of the thermal treatment of the pistachio shells led to decreased mass losses in the N2 gas atmosphere. The biochar is formed from pistachio shells at approximately 600 °C. When the temperature of the thermal treatment of pistachio shells is higher than 600 °C, it decreases the sample mass due to possible surface oxidation of carbon or gasification carbon samples. The observed mass losses for samples P600 and P850 are considerably lower than for sample P400.
The conditions of the solid fuel preparation reflect the efficiency of the gasification process of solid carbon via a CO
2 gas medium, which is introduced through an external source. Knowledge about the progress of the gasification reaction of solid carbon to carbon monoxide via CO
2 as a gasification agent reaction (3) is important for the practical application of prepared solid fuels in DC-SOFC technology [
58]. In this study, the TG curves using a thermobalance registered the variation of mass losses vs. the temperature in nonisothermal and isothermal conditions at a temperature of 850 °C vs. time. The registered mass losses vs. the temperature and time are shown in
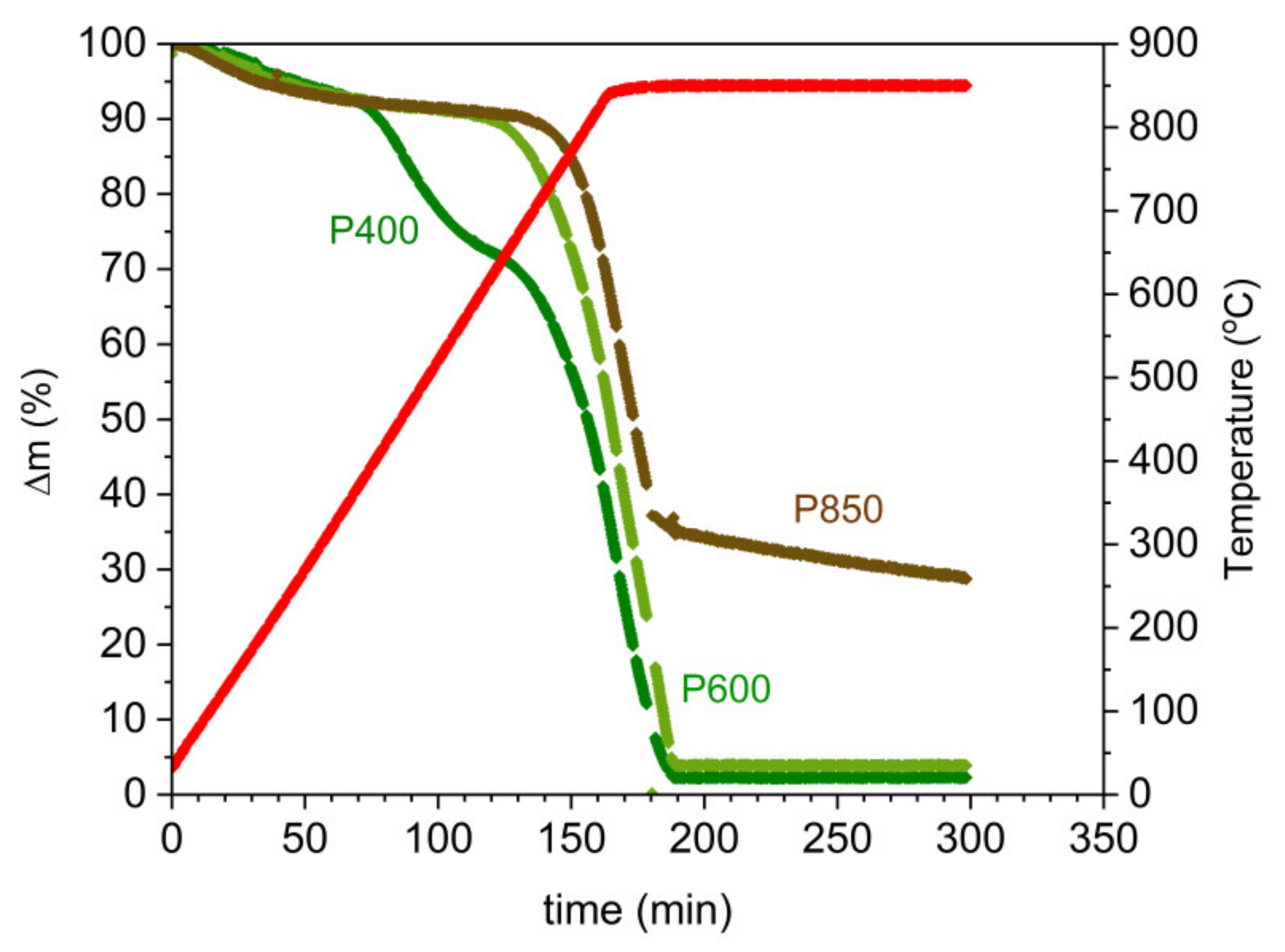
Figure 9.
Based on the plotted dependence of the thermogravimetric curves Δm = f (T) for the samples P400, P600, and P850, the following two characteristic stages can be distinguished: (i) the heating of the sample from 20 °C to 850 °C with a temperature increase of 5 °C/min, where the duration of the first stage is approximately 200 min; and (ii) isothermal, i.e., heating the tested materials at 850 °C until a constant mass is reached.
The analysis of the dependence Δm = f (T) for the samples P400, P600, and P850 determined two temperature ranges, in which the pyrolysis and gasification processes occurred.
Table 2 presents the data of the estimated temperature ranges for the first and second stages and the loss of mass Δm of the samples P400 to P850 during the pyrolysis and gasification processes.
The data in
Table 2 show that the greatest weight loss in the gas atmosphere of CO
2 in the first temperature stage (200–640 °C) is observed for the P400 sample, and the lowest is observed for the P850 sample. One of the reasons for increasing the gasification temperature of the P850 char sample compared to the P600 sample is the higher degree of carbon graphitization. The order of the carbon structure in sample P850 led to a decrease of many surface defects and active centers, which are desired for carbon reactivity towards CO
2. The SEM observations demonstrated that the grain size of the solid fuel increases along with the increase in the preparation temperature of the chars. The increased grain sizes of the solid carbon fuels can postpone the temperature of the gasification process to a higher temperature range. Changes in the dispersion area of inorganic compounds, which are considered natural catalysts, also exhibited a limited catalytic effect compared to the prepared samples in lower temperatures. The analysis of the variation of mass losses Δm vs. temperature or time shows that the obtained solid fuels exhibited high reactivity in the CO
2 gas atmosphere. Based on this data, it is expected that the CO that is produced from the gasification reaction C + CO
2→2CO can improve the performance of the DC-SOFC at a temperature range of 750–850 °C.
Additionally, the possible production of carbon according to the reaction C + CO
2→2CO in the anode chamber of DC-SOFC is considered. In
Figure 10a, the variation of the main evolved gases as CO and CO
2 from the anode chamber of DC-SOFC is presented compared to the temperature that was established in a solid carbon bed.
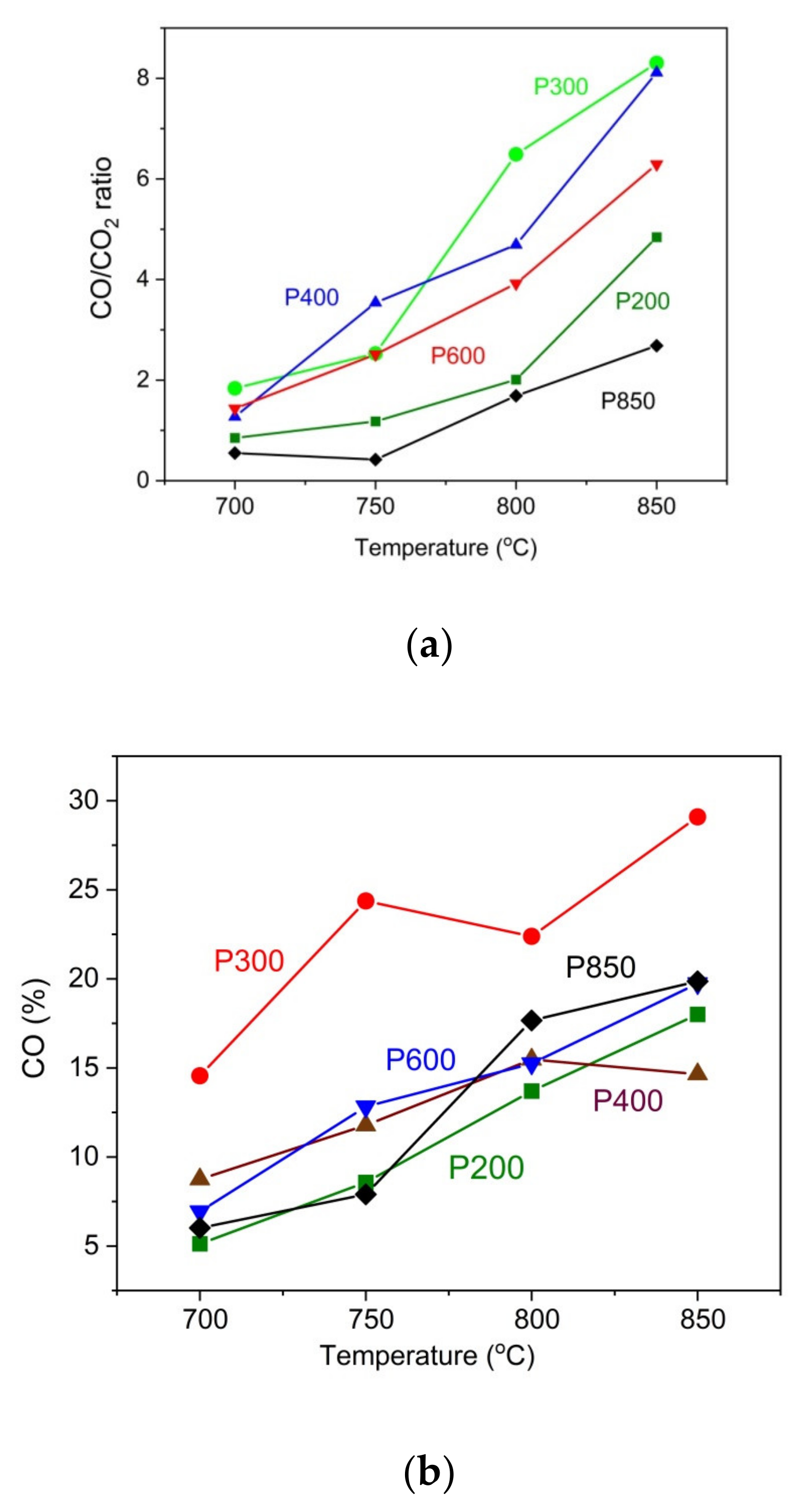
The data in
Figure 10a show that the ratio of CO to CO
2 increases as the temperature rises. In general, the increase in temperature improves the chemical kinetics of the Boudouard reaction, and a larger amount CO is produced. Moreover, the influence of the thermal treatment of pistachio shells on the CO/CO
2 ratio variation is considerable. The highest CO/CO
2 ratio in these experimental conditions was recorded for the P300 and P400 samples. The lowest CO/CO
2 ratio was observed for the P850 biochar and the P200 sample. Regarding the pistachio samples that were prepared at a temperature range of 300–400 °C, the observed increase of evolved gas products as CO and CO
2 is a direct consequence of the thermal decomposition of residual organic carbon compounds, which originated from gradually degraded cellulose, hemicellulose and lignin in an inert gas atmosphere. The lowest concentration of CO/CO
2 in the evolved gases was found in the biochar P850 sample, which was fuel-constituted solid carbon fuel. Therefore, the production of CO and CO
2 is possible due to the partial oxidation of carbon particles.
Figure 10b presents a positive correlation between the amount of CO in the gas outlet of the anode chamber from DC-SOFC (I) and the temperature of the fixed carbon solid bed. This is a direct consequence of the Boudouard reaction C+CO
2→2CO (4). In these experimental conditions, the CO
2 from an external source is supplied to solid carbon powders and placed on the anode surface of the DC-SOFC. One result of this reaction is an increase in the amount of CO in the anode chamber. The highest reactivity was observed in the P300 sample, whereas the lowest reactivity was observed in the P200 sample. Regarding the charred samples (P850 and P600), a similar range of evolved CO from the anode chamber was observed at temperatures of 800 °C and 850 °C.
The pistachio shells that were charred at a temperature of 850 °C were selected for gasification investigations in a steam gas atmosphere. Knowledge of producing CO, H
2, and CH
4 is important for practical applications of such carbonaceous-based materials in the DC-SOFC [
58,
59,
60]. The gasification reaction of carbon particles with water vapor as a medium may result in the formation of further gaseous products, such as H
2, CO, and CH
4, which are valuable fuels for the DC-SOFC. Knowledge of the formation of such fuels in the anode chamber during the reactivity of H
2O with carbon particles will be helpful for understanding that the power output of DC-SOFCs is supplied by different solid carbon fuels and the performance of DC-SOFCs in different experimental conditions. This includes when the humidified gas medium as N
2 or CO
2 is introduced to the anode chamber of the DC-SOFC to induce the gasification process and improve the performance of the DC-SOFC.
As the data in the literature that corresponds to an analysis of solid carbon fuels’ reactivity with H2O are limited, it is difficult to analyze how humidifying gases impacts the DC-SOFC’s performance.
The efficiency of the chemical reactivity of solid carbon particles under the gasification process can be expressed using the following main Equation (9):
where dVi/dt equals the rate of formation of a given product in cm
3/min∙g, and t equals time in minutes.
These dependencies (dV/dt) were determined by measuring the concentration of gaseous products in the post-reaction gas, the flow of which was maintained at a constant level during the entire measurement. The release rate of a given product over time can be calculated using the following formula (10):
where V̇ equals the volume flow of the reaction gas in cm
3/min and c
i(t) equals the concentration of a given product in the postreaction gas at time (t) in the percentage of volume. Based on the composition of the resulting gas, the curves of the generation rates of CO, CO
2, H
2 and CH
4 vs. time are presented for the charred pistachio sample (P850) and commercial charcoal, which was utilized in the study as the reference material. During the first stage of the process, relatively high rates of gas generation were observed in pyrolysis using charcoal. During the second stage, the proper gasification of the resulting char was characterized by lower generation rates of the examined gases. Regarding the P850 sample, the shape of the curve differed to the curve of the commercial charcoal. During the initial stage of the process, relatively lower peaks were observed.
Later, a continuous increase in the gaseous generation rates of H2, CO, and CO2 was observed, which slowly decreased after reaching a peak. H2 is characterized as having the highest generation rate in both investigated samples. The generation curves of CO and CO2 are diverse and depend on the sample type.
Figure 11a illustrates the main product of the gasification reaction of the analyzed biochar P850. The highest generation rate was H
2, followed by CO
2 and CO, respectively. Methane was only generated during the first few minutes of the gasification of the samples due to the simultaneously occurring residual pyrolysis reaction from the biochar. During the gasification of the char samples, the formation of methane was not observed. The generation rates of H
2, CO
2, and CO increased rapidly during the first few minutes of the gasification process. This was followed by a slight increase in the generation rates of both H
2 and CO
2 (up to 40 min) and then a decrease. The generation rate of CO decreased steadily after reaching a peak during the first few minutes of the process. At approximately 90 min, the CO generation reaction was negligible. From that moment, the only observed gaseous reactions were H
2 and CO
2, which were generated until the end of the process, i.e., up to the 180th min. Based on the analysis of gasification efficiency, the sample P850 was more reactive in the H
2O gas atmosphere. Moreover, a higher generation rate of gaseous products, such as H
2 and CO, was observed with the P850 sample. However, regarding methane, a slightly higher rate of CO
2 generation was noticed in the commercial charcoal sample CH-M. Contrary to the previous samples, the Carbon Black (CB-221) exhibited very low reactivity when steam was used as a gasification agent.
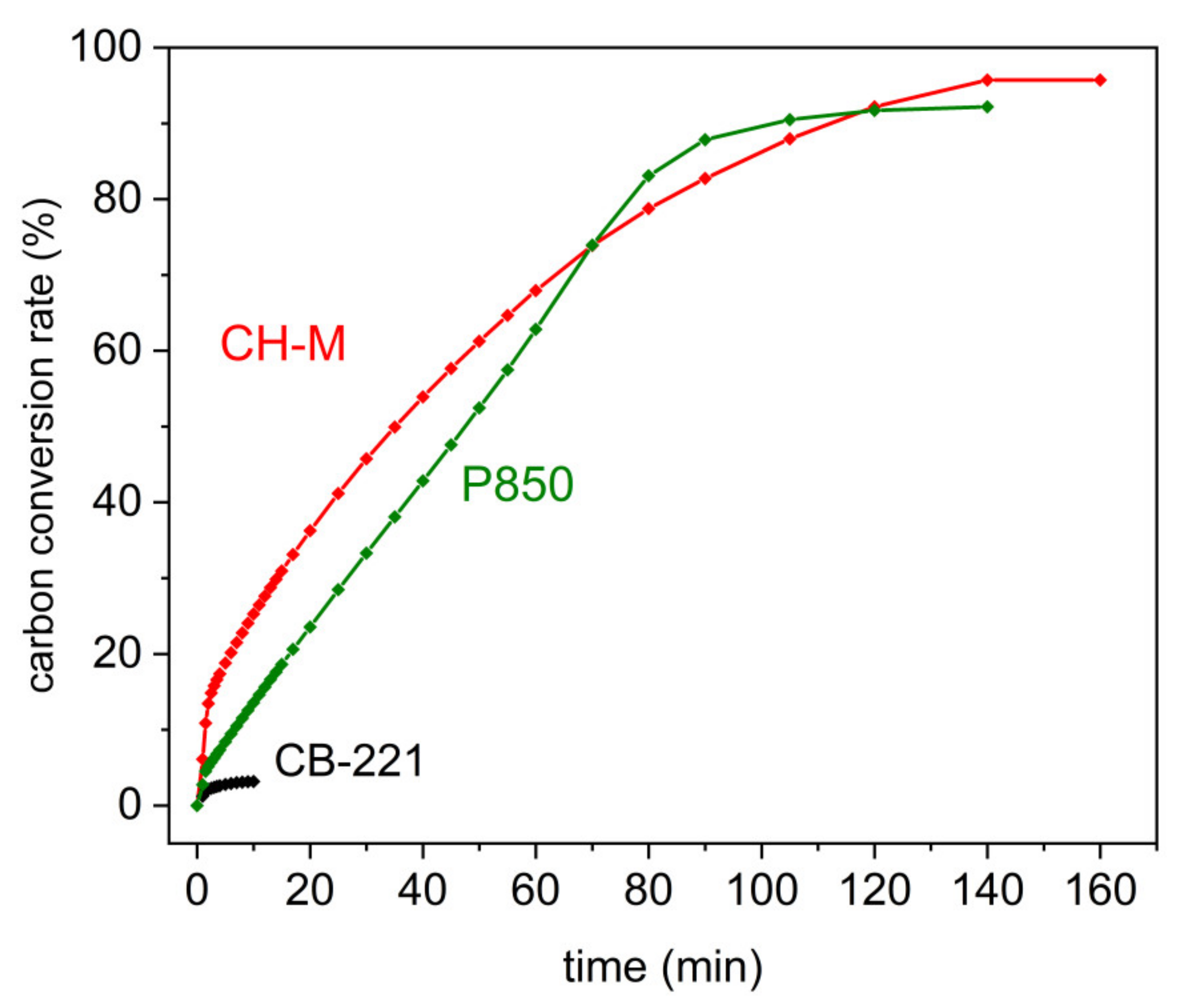
The effectiveness of the carbon conversion rate from the P850 charred sample; charcoal CH-M or carbon black CB-221 to the gaseous products (CO, CO
2, CH
4, and H
2) in the presence of a gasification agent (water vapor) is determined based on previous data presented in
Figure 11a–c.
Figure 12 indicates that the conversion of the solid fuels P850 or charcoal CH-M to gaseous products is comparable and more than 90%. The lowest total conversion rate was noticed for the Carbon Black CB-221 sample. Based on the research, it can be concluded that biomass-derived solid fuels can be easily converted to gaseous products in a humidified gas atmosphere. However, regarding carbon black sample, this amount is minimal. It also confirms that regarding biomass-derived solid fuels, the impact of gaseous fuel, such as CO, H
2, and CH
4, is more predictable than when applying carbon black as a solid fuel to supply the DC-SOFC.
3.1. Chemical Stability of Ni-YSZ or Ni-GDC Anode Materials in a Fixed Carbon Bed
Cermet composite materials containing Ni-YSZ and Ni-GDC are anode materials that are commonly used to construct a DC-SOFC. The anode material should exhibit significant chemical durability against the solid fuels that are used. In a DC-SOFC anode chamber, chemical reactions occur between the components of the anode material, carbonaceous-based materials, and a chemical gas atmosphere [
60,
61,
62]. Issues related to the possible destructive effect of biomass-based fuels used in the DC-SOFC cell and the analysis of the problem of carbide formation from Ni-C and related binary systems were presented and discussed in a previous article [
60]. Despite numerous attempts, this problem is not yet fully investigated and explained.
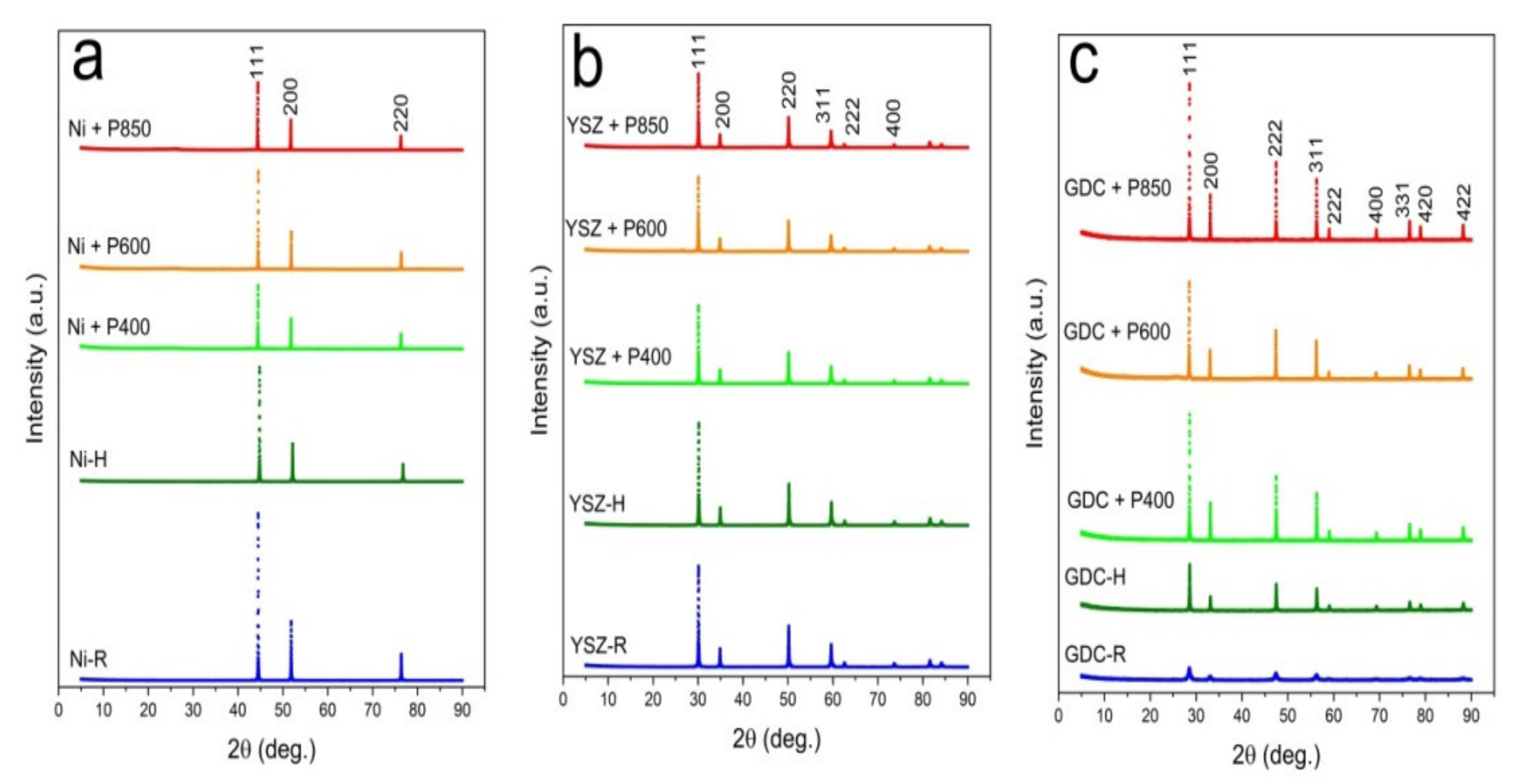
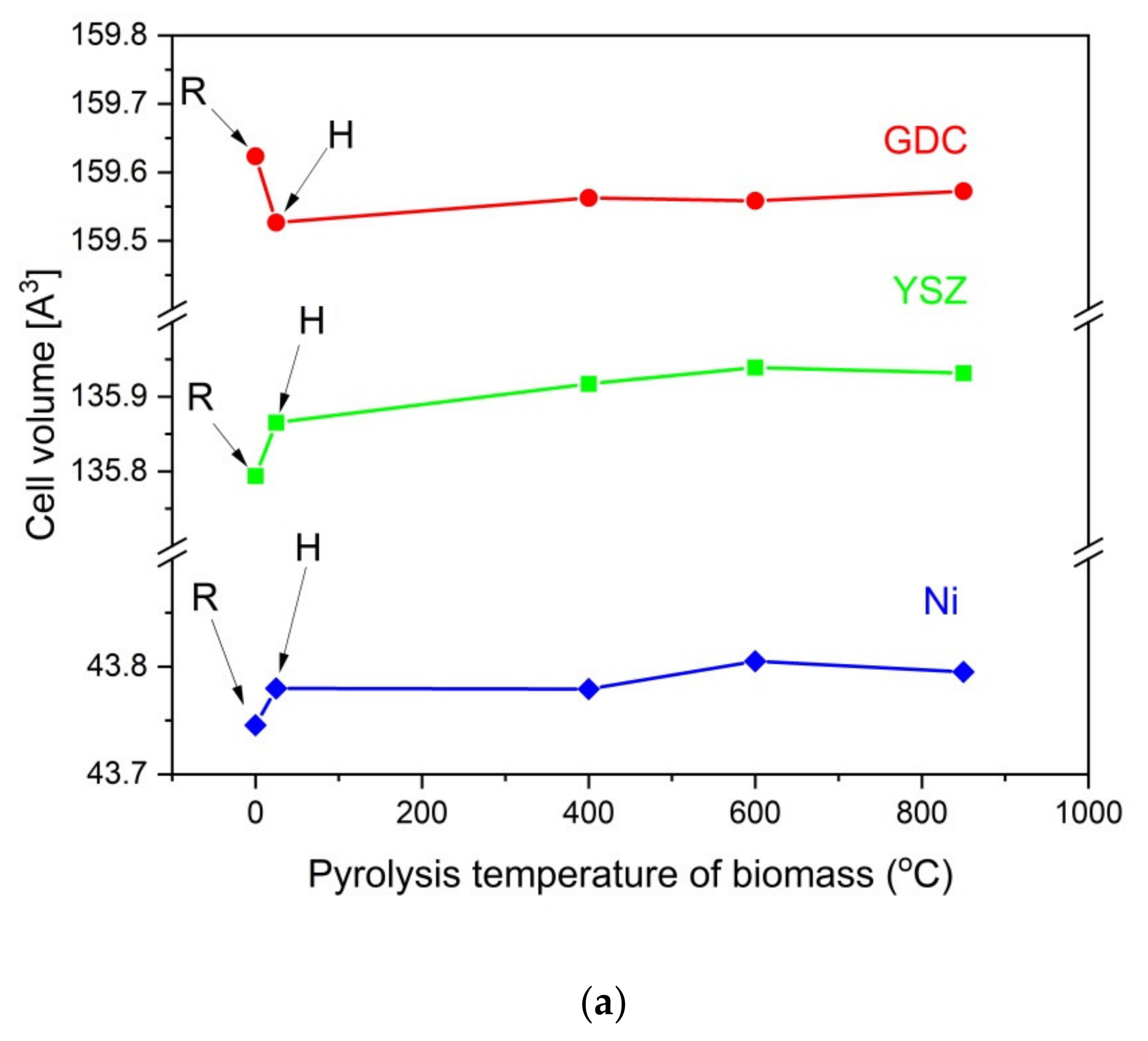
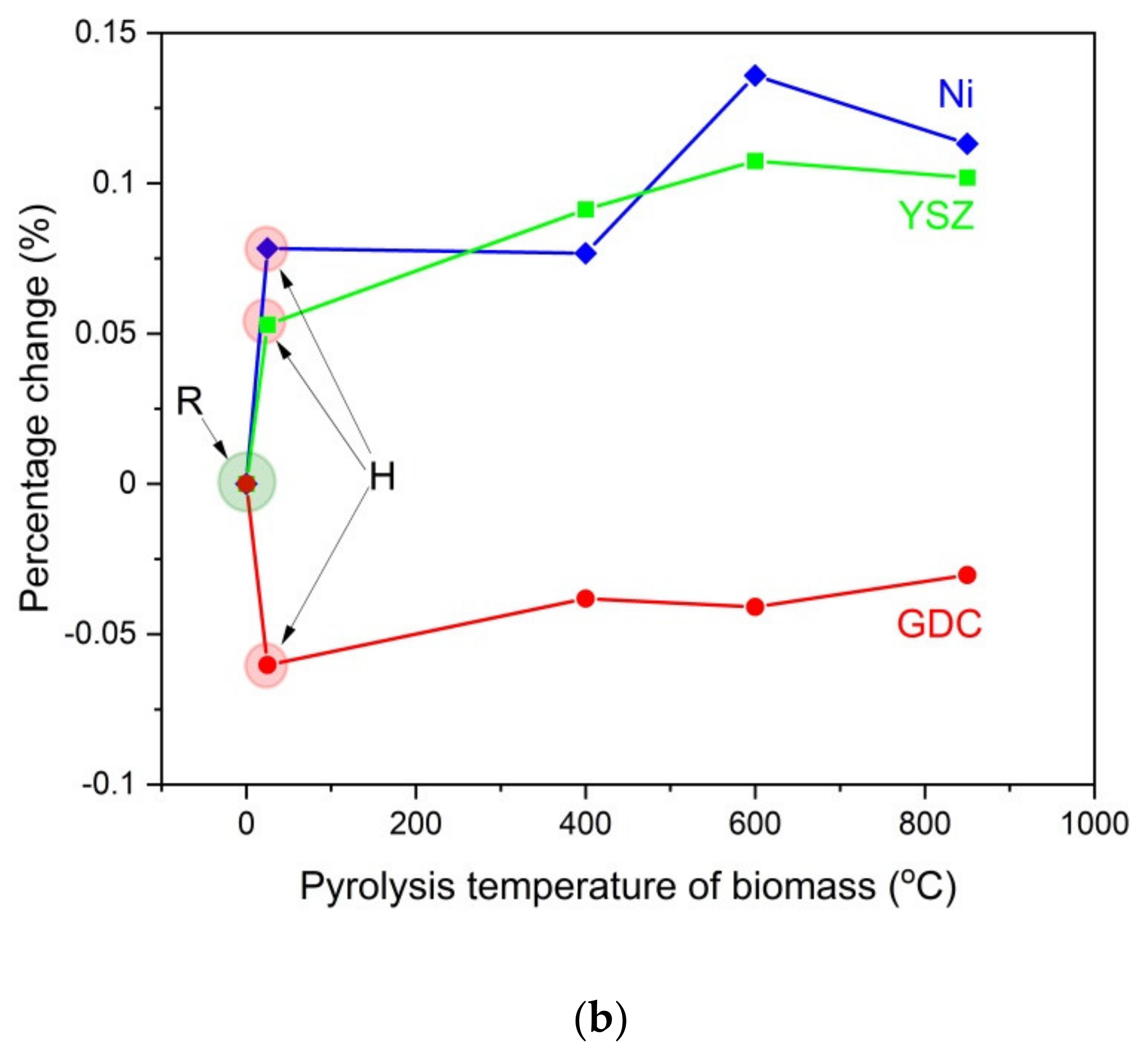
Figure 13a–c present the recorded diffraction patterns for the initial powder samples of Ni-R (a), YSZ-R (b), and GDC-R (c), powders Ni-H, YSZ-H, and GDC-H, when they were heated at 850 °C for 100 h without contact with solid carbon fuels, as were the mixtures of YSZ, GDC, and Ni samples with the appropriate carbonized products from samples P-400, P-600 and P-850. The initial samples of Ni, GDC, and YSZ in the XRD patterns are marked as R, and the same samples were additionally subjected to the heating process at temperatures of 400 °C, 600 °C, and 850 °C for 100 h, respectively, without contact with solid carbon fuels, which are marked as H.
Based on the recorded XRD patterns, variations in the unit cell volume were calculated for the base Ni, YSZ, and GDC samples before after being heated at 850 °C for 100 h without contact with solid fuels, as were a series of mixtures with carbonaceous materials that were obtained from pistachio shells. These data are presented in
Figure 14a,b.
Individual analyses of cell volume variations for the base Ni, GDC, and YSZ samples (R) after the heat treatment without adding solid carbon fuels (H) were completed first. It was found that additional heating of each component led to a minimal increase (<0.05%) in the cell volume of Ni or YSZ. In the GDC sample, a decrease in cell volume was observed. Additional heating of the YSZ and GDC samples or allowing Ni to have direct contact with the carbon particles did not lead to significant changes in the cell volume of these materials. The greatest change in the cell volume was visible in the metallic Ni, which is possibly due to the diffusion of carbon into Ni particles. This phenomenon can be attributed to the dissolution of carbon particles in the metallic nickel structure. These results agree with the data that was analyzed for the chemical stability of Ni-YSZ or Ni-GDC anode materials with commercial charcoals, for instance Charcoal CH-M, which is described in this paper as reference material for charred pistachio shells (P850) [
60].
3.2. Electrochemical Performance of SOFCs Powered by Solid Fuels from Pistachio Shells
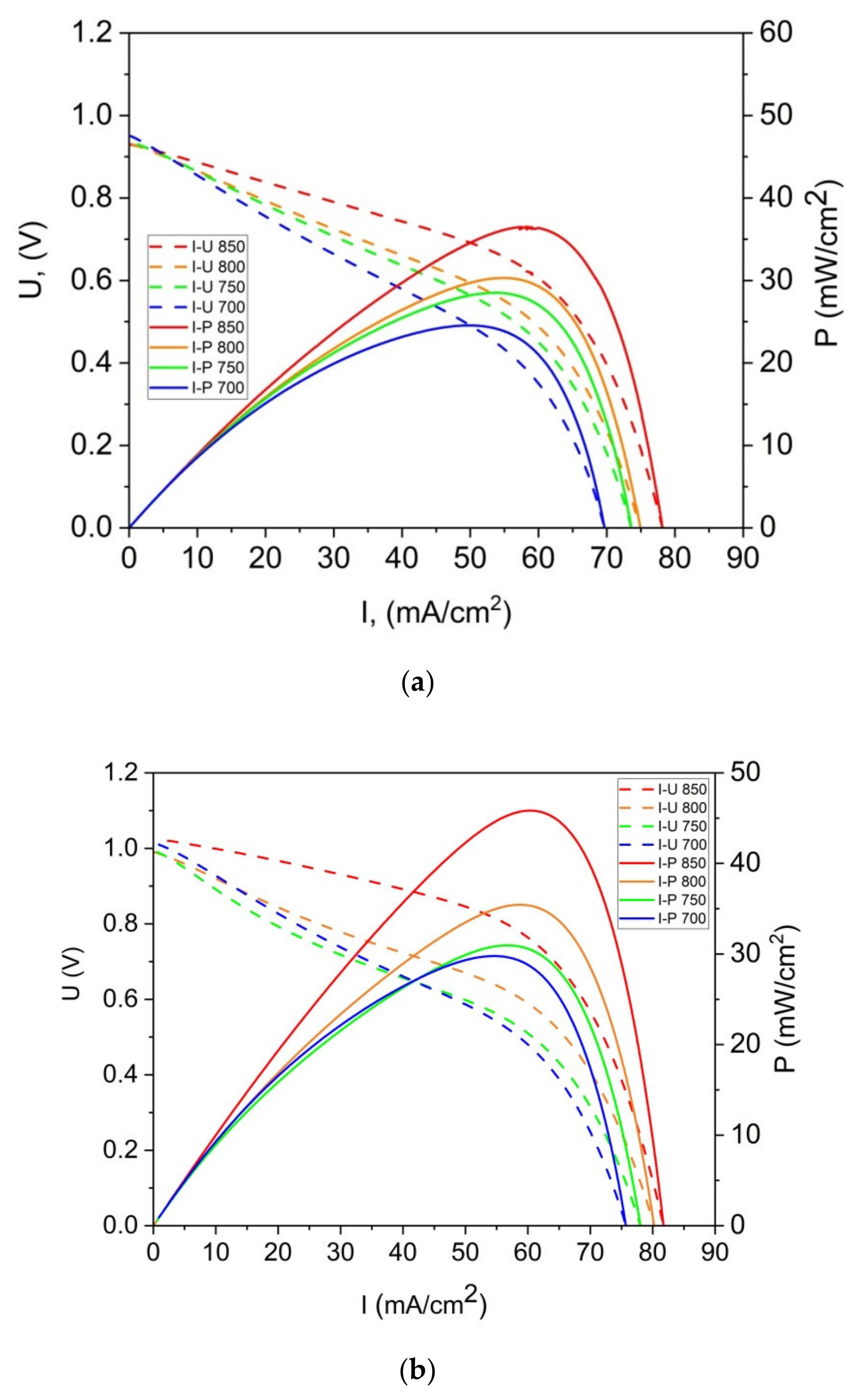
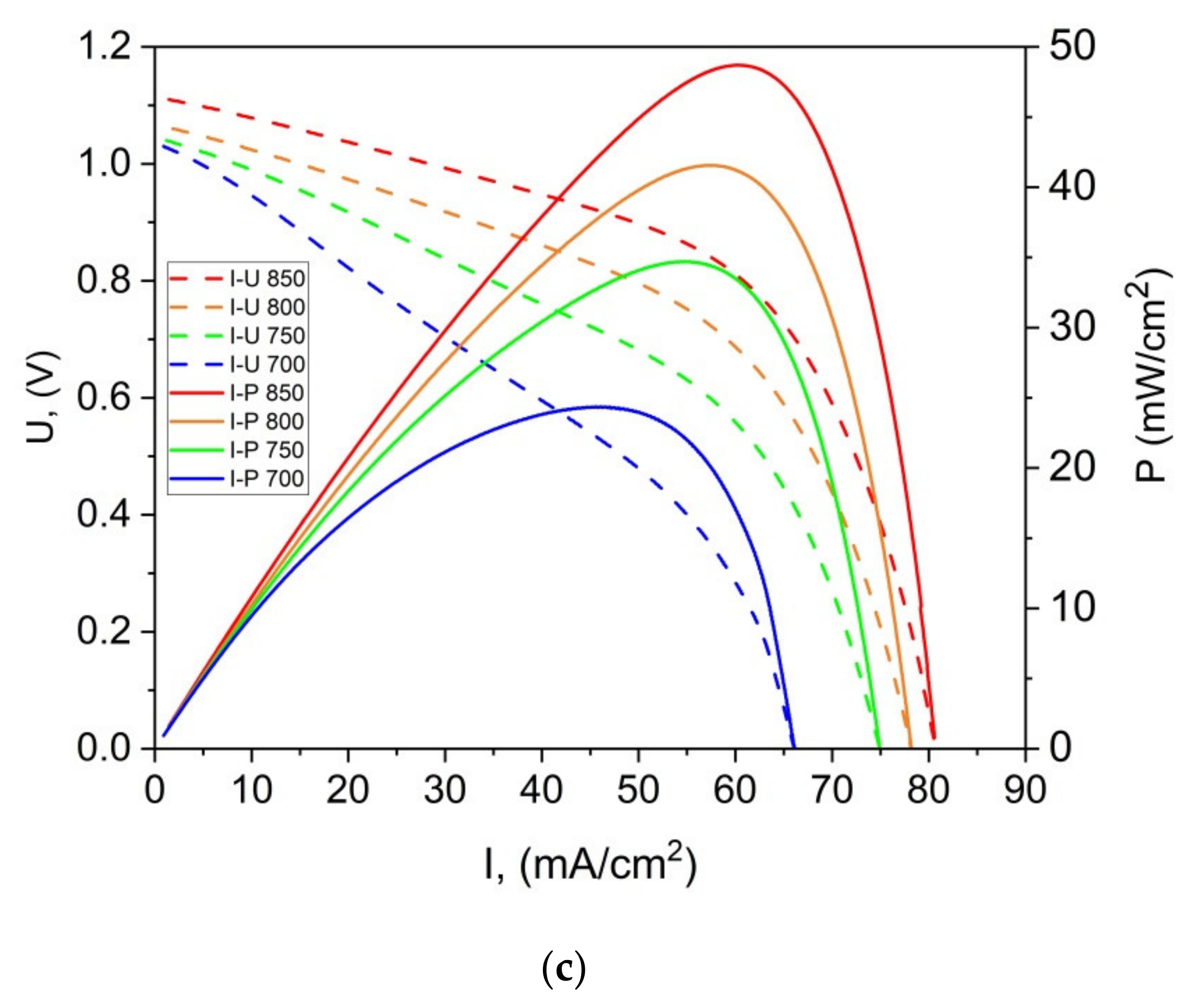
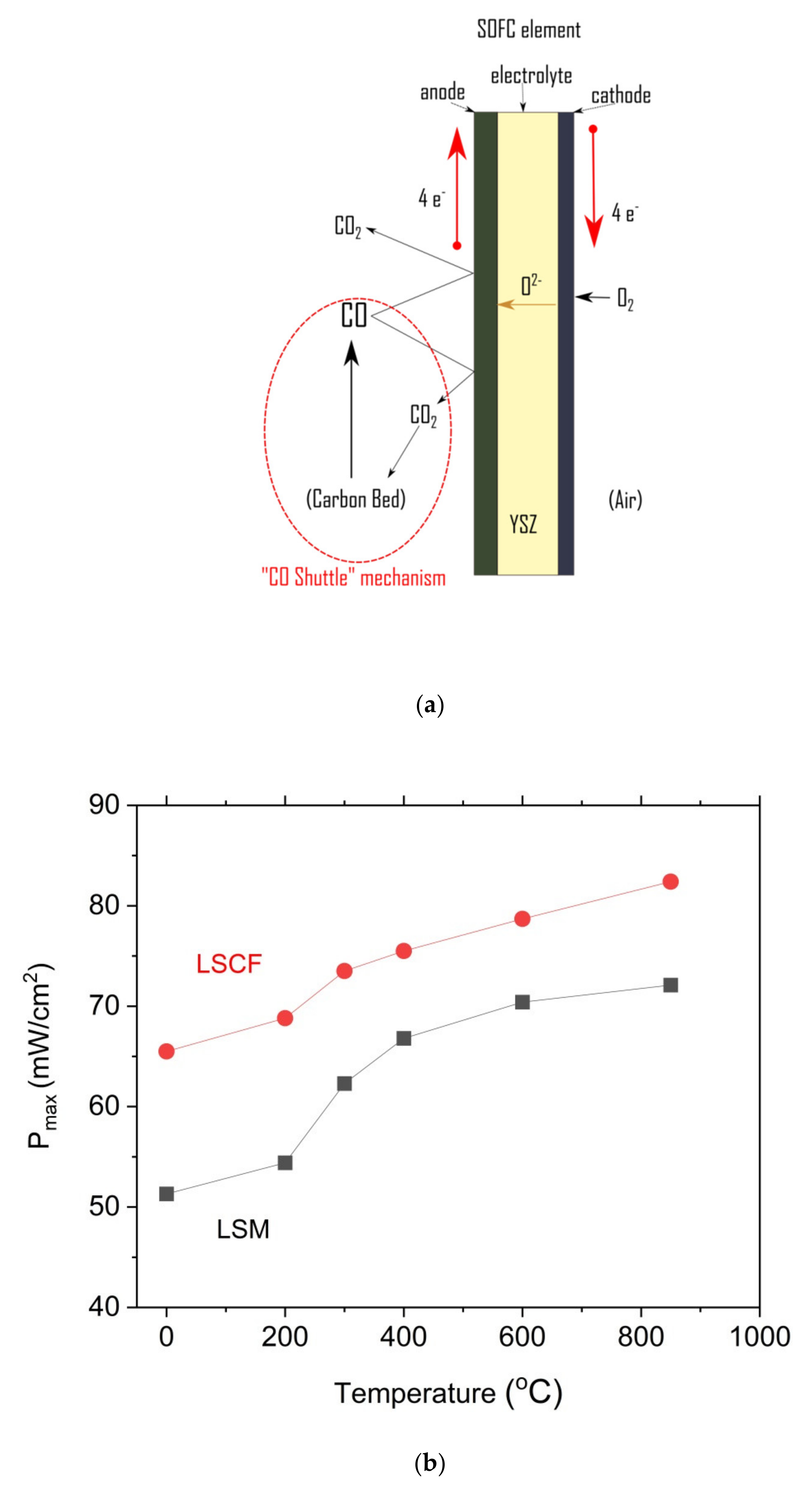
Figure 15a,b present the representative U-I and P-I curves that were recorded for a DC-SOFC that was fueled with ground raw pistachio (P0), the torrefied sample (P300) or charred pistachio shells (P850). The data were recorded for the DC-SOFC (I). Nitrogen was used as a shielding gas in these experimental investigations of DC-SOFC (I).
As shown in
Figure 15a–c, the power output (P
max) and current density gradually increase with the increase in temperature of the DC-SOFC (I). The effects of the physicochemical properties of the solid fuels that were used with the investigated pistachio shells from P0 to P850 on the performance of the direct carbon fuel cells, varying only the cathode materials used, are shown in
Figure 16. The data refer to a temperature of 850 °C
A direct comparison of the results of the power output for the DC-SOFCs (I) and (II) indicated that the higher values of the power output P
max were obtained for DC-SOFC (II) with a LSCF cathode compared to DC-SOFC (I), where the LSM cathode was used. These results are directly related to their greater electrochemical activity, which reduces oxygen at the LSCF cathode at lower temperatures than the LSM cathode [
63,
64]. The kinetics of the oxygen reduction process in direct carbon fuel cells has a significant impact on the performance of the DC-SOFC. These results agree with the observations that were described in a previous article [
26].
The main factors that influence the values of the maximum power density Pmax of DC-SOFCs are the physicochemical properties of the raw ground, torrefied, or charred pistachio shells and the chemical gas composition that formed in the anode chamber. Analyzing the preparation conditions’ influence on the pistachio shells charging the DC-SOFC cells revealed that the lowest values of the power density Pmax were obtained for solid oxide fuels that were only fueled with ground pistachio shells.
A pyrolysis of the ground pistachio shells in an anaerobic atmosphere led to the formation of torrefied biomass or charred samples. The utilization of such prepared waste-biomass solid fuels in the DC-SOFC led to a gradual improvement of the DC-SOFC’s performance. The increased elemental carbon content and the surface area of the carbon particles are advantageous for the direct electrochemical oxidation of the carbon particles in the anode material Ni-YSZ. Additionally, the indirect gasification of carbon to CO in the anode chamber may affect the electrochemical pathways of anodic oxidation of fuel in the DC-SOFC. A chemical analysis confirmed the presence of CO in the anode chamber in the DC-SOFC and was the only result of the chemical processes, which took place under indirect gasification. This affected the obtained values of the power density P
max from the DC-SOFC (I) or (II) cells. These data correspond with the previously observed variations of CO/CO
2 in a solid carbon bed (
Figure 11a) for investigations into carbon solid fuels (P00–P850).
Figure 16 shows that the power output P
max of the DC-SOFCs (I) and (II) gradually increased alongside the applied thermal treatment of the samples P00 to P850.
The increased thermal treatment of the ground pistachio shells up to 850 °C increased the specific surface area to Sw ~ 377 m2/g compared to that of the P600 sample, which obtained the lower value of Sw ~ 180 m2/g. The increased carbon content surface area of solid fuels enables a better contact to be obtained between solid carbon grains with an Ni-GDC or Ni-YSZ anode surface and extends the reaction zone of electrochemical oxidation of carbon according to reaction (1) C + 2O2−→CO2 + 4e−. Additionally, it improves the performance of the DC-SOFC. These facts indicate that increasing the surface area and carbon content of solid fuels also benefits the performance of the DC-SOFC.
The most common way to explain the performance of the DC-SOFC that is supplied by different carbonaceous-materials is to analyze the electrochemical oxidation mechanism of solid carbons, which is called the “CO shuttle mechanism”. This was previously proposed by Gür [
65]. The CO shuttle mechanism (
Figure 17a) illustrates the impact of CO, which was first oxidized in the anode of the DC-SOFC to form CO
2.
The CO
2, which is a product of the electrochemical oxidation of carbon or CO in the anode, reacts with the carbon bed to produce more CO.
Figure 17b suggests that the performance of DC-SOFC can be improved of CO production in solid carbon fuels. There are two main methods of improving the performance of DC-SOFC according to the CO shuttle mechanism idea. The first is adding a catalyst to carbon-based solid fuels, which improves the kinetics of the CO production, according to the Boudouard reaction C + CO
2→2CO. The second is the introduction of CO
2 to the anode chamber of the DC-SOFC, where solid carbon fuel is placed. In this way, the generation process is supported.
The effect of the CO
2 gas atmosphere on solid fuels in the DC-SOFC (I) with LSM or DC-SOFC (II) with LSCF cathodes is presented in
Figure 17b.
Introducing CO
2 as a shielding gas in the DC-SOFC anode chamber leads to much higher power density P
max values than using N
2 as a shielding gas (
Figure 17b). One of the reasons for this phenomenon is the possible formation of CO as a product of the Boudouard reaction after the C + CO
2→2CO reaction. The product of the electrochemical oxidation process is CO
2 and it has a significant influence on the maximum power density due to the contribution of CO to the electrochemical oxidation of carbon, which proceeds according to the CO shuttle mechanism. The presence of inorganic substances based on alkali oxides and iron promotes the coal gasification reaction in the CO
2 atmosphere [
7,
56,
57,
58]. In our experiments, the addition of CO
2 to the anode chamber increased the formation of CO and the maximum power density (from approximately 65 mW/cm
2 to ~83 mW/cm
2) that was obtained for the DC-SOFC (II) when it was fueled by charred pistachio shells (P850). In this situation, the Boudouard reaction (CO
2 + C→2CO) that is associated with the electrochemical oxidation of CO on the DC-SOFC results in increased performance. These observations confirmed that the responsible mechanism for the DC-SOFC operation is a shuttle mechanism.
Table 3 data show that the DC-SOFC (I) is supplied by other types of waste-biomass solid fuels.
Table 3 shows that using torrefied or carbonized pistachio shell samples as fuel for the DC-SOFC (I) provides comparable power densities P
max in comparison with that of commercially available charcoal and woody biomasses, such as acacia chips. However, they are slightly lower than the power densities when walnut shell-derived samples were used. The results that were obtained for the carbonized samples in hydrothermal conditions were due to the lower temperature that is required when preparing solid fuels [
66].
The results of the electrochemical studies on the influence of the physicochemical properties of pistachio shell-based solid fuels on the operating parameters of the DC-SOFC cells do not reflect the full work at this stage, which aims to achieve the highest possible electrochemical parameters of the operation of solid cells. Further directions for research that aims to achieve a P
max in the range of 100–400 mW/cm
2 should focus on selecting catalysts for the electrochemical oxidation of biomass fuels and new groups of anode and cathode materials; optimizing the construction of a single DC-SOFC fuel cell; and expanding the fuel cell stack [
67,
68,
69,
70].
The investigation into the impact of humidity on the solid carbon gasification process according to the following chemical reactions (11) and (12) is analyzed:
The gas humidification process was carried out at room temperature by passing a gas stream through the scrubber, which was then directed to the anode space. The U-I and P-I curves were recorded for the DC-SOFC (I) during a period of 70 min.
Figure 18 presents the variation of the P
max values that were recorded for the DC-SOFC (I), which was fueled with pistachio char P850, a sample of charcoal CH-M or carbon black CB-221, respectively. The data refers to a temperature of 850 °C. The first power output P
max of the DC-SOFC (I) was registered immediately following the introduction of humidified nitrogen to the anode chamber of the DC-SOFC (I), and they were further recorded after breaks of less than 10 min, with 15 min for whole duration of the experiment (70 min).
As shown in
Figure 18, the P
max of the DC-SOFC (I) is observed to gradually increase over a longer period after injecting the humidified nitrogen into it. When applying biochar P850 as a solid fuel to DC-SOFC (I), the gradual increase of power output in P
max vs. time may be indicative of the possible gasification of solid fuels over time by steam. The source of the steam is the humified nitrogen, which was introduced to the anode chamber of DC-SOFC (I), where the investigated solid fuel is placed. According to the results presented in
Figure 11a, the possible products of gasification for the steamed pistachio P850 sample are H
2, CH
4 and CO, which are well-known gaseous fuels for SOFCs. The increased concentration of these gases, especially H
2 and CO, in the anodic chamber of DC-SOFC (I) is one of the possible reasons for the observed increase in electrical performance of the investigated DC-SOFC (I) supplied by the biochar P850 sample. On the other hand, the observed gradual improvement of power output in Pmax vs. time for DC-SOFC (I), which was supplied by charcoal CH-M, could also correspond to an increase in H
2 and CO content in the anode chamber of DC-SOFC (I). These results correspond to results presented in
Figure 11b. There was no considerable variation of P
max vs. time observed in the case of DC-SOFC (I), which utilized Carbon Black 221 as a solid fuel. This type of carbon black sample exhibited minimal reactivity due to H
2O gasification (
Figure 11c).
3.3. Postmortem SEM Observation of the Anode Materials after the Electrochemical Test of the DC-SOFC (I)
A cross-section analysis of the anode-electrolyte interface and surface using SEM observations (
Figure 19a,b) took place after the DC-SOFC tests. An EDS analysis was also performed.
No significant changes were observed in either the structure of the surface of the Ni-GDC anode (
Figure 19a,b) or its chemical composition compared to that of the starting samples. No areas of corrosive degradation were found on the surface of the Ni-GDC or Ni-GDC|Ni-YSZ|8YSZ anode. These results are in accordance with the X-ray investigations that were performed for the anode materials.