2,7(3,6)-Diaryl(arylamino)-substituted Carbazoles as Components of OLEDs: A Review of the Last Decade
Abstract
:1. Introduction
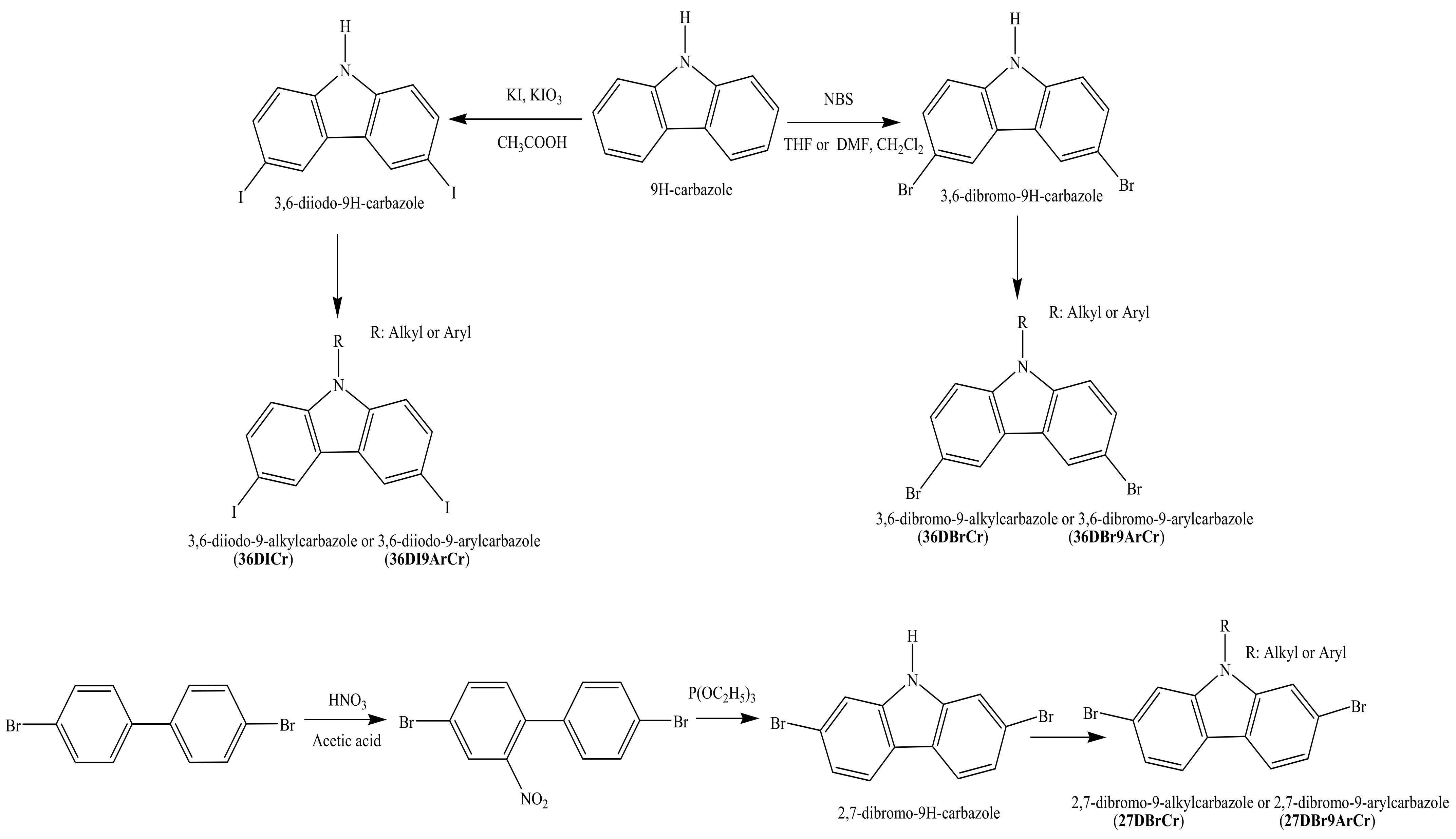
2. Synthesis of 2,7(3,6)-Diiodo(dibromo)carbazoles and 2,7(3,6)-Diaryl(diarylamino)-substituted Objective Carbazoles
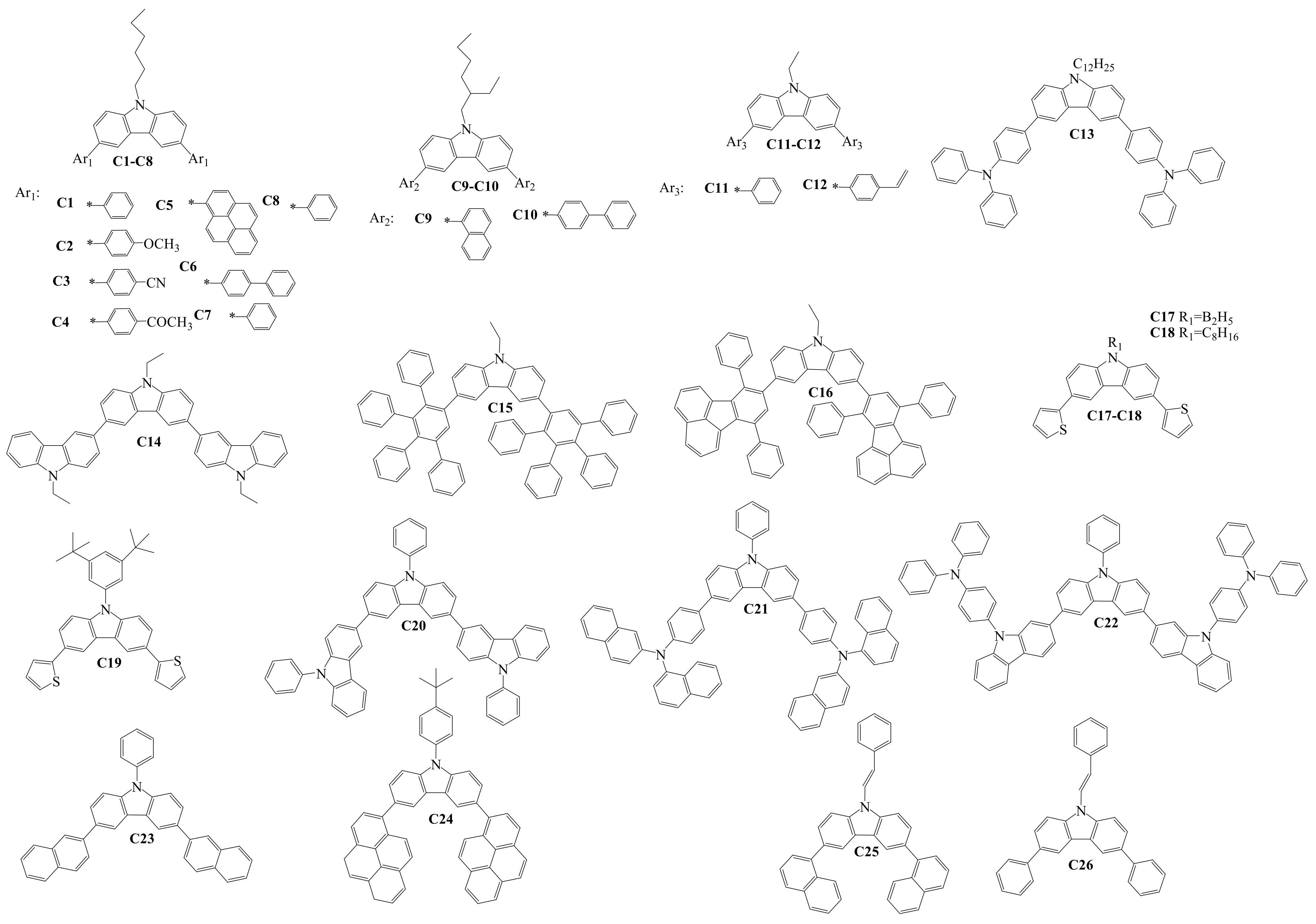
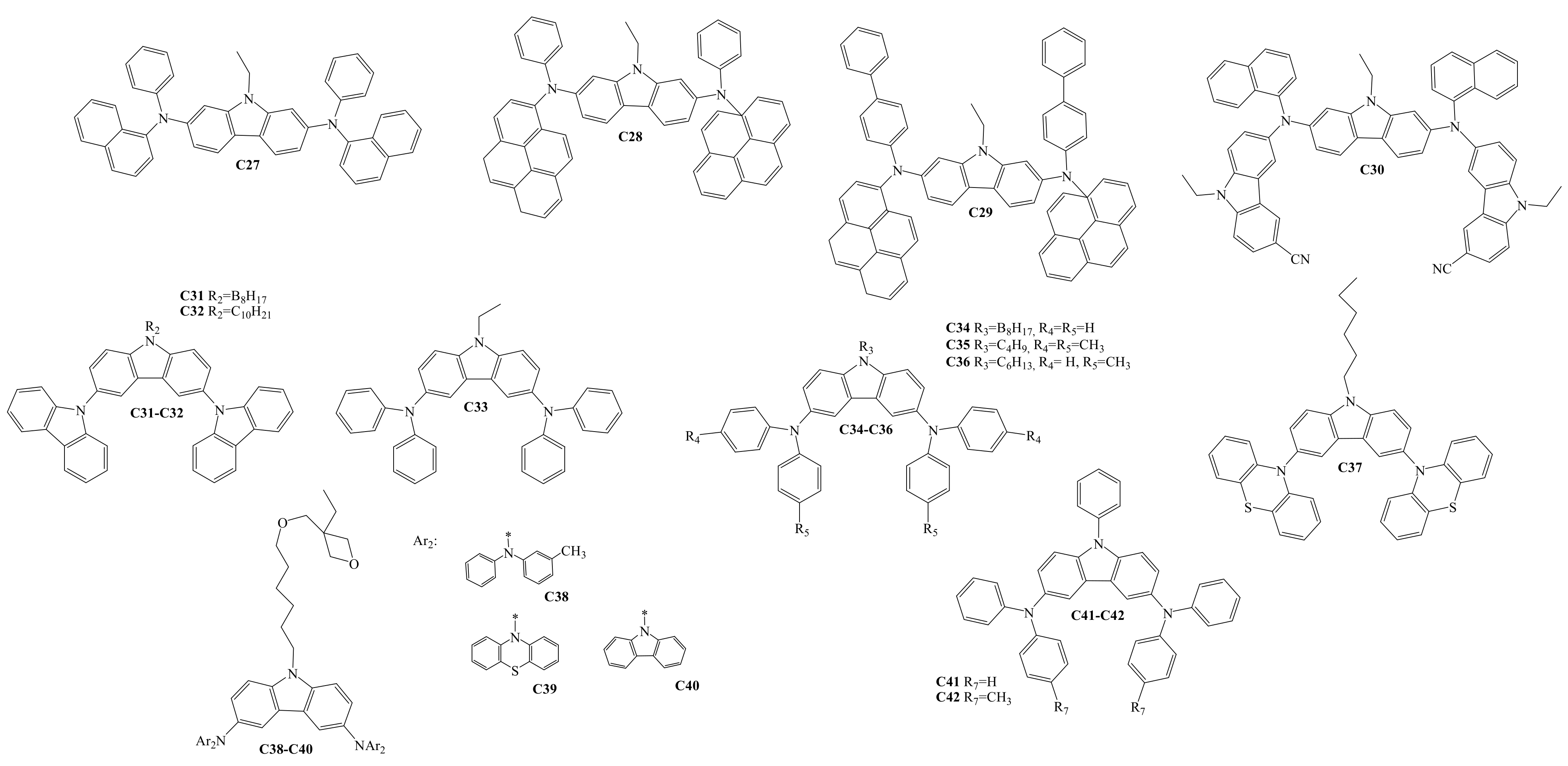
3. Diaryl(diarylamino)-substituted Carbazoles as Charge-Transporting Layer Materials for OLEDs
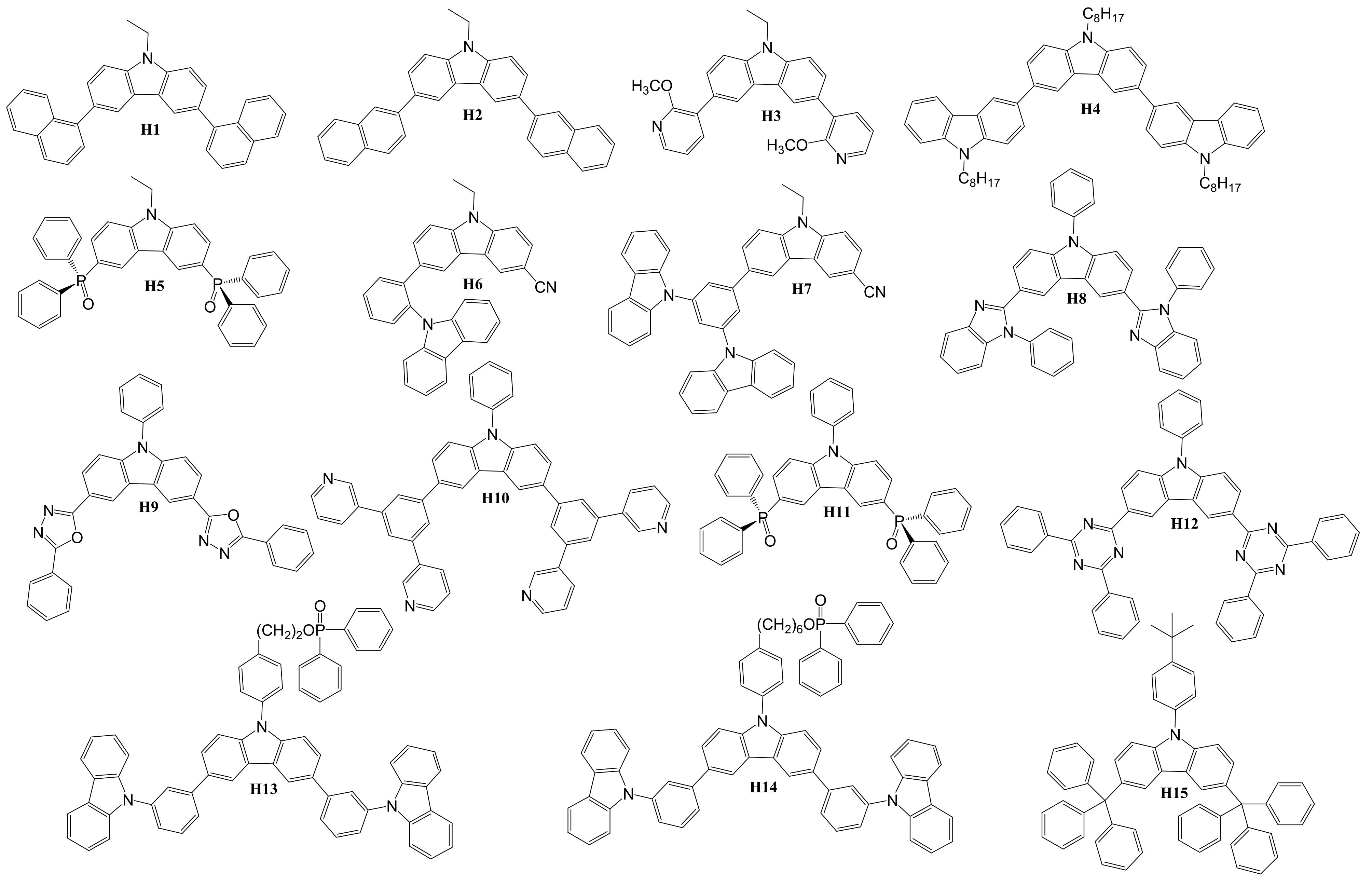
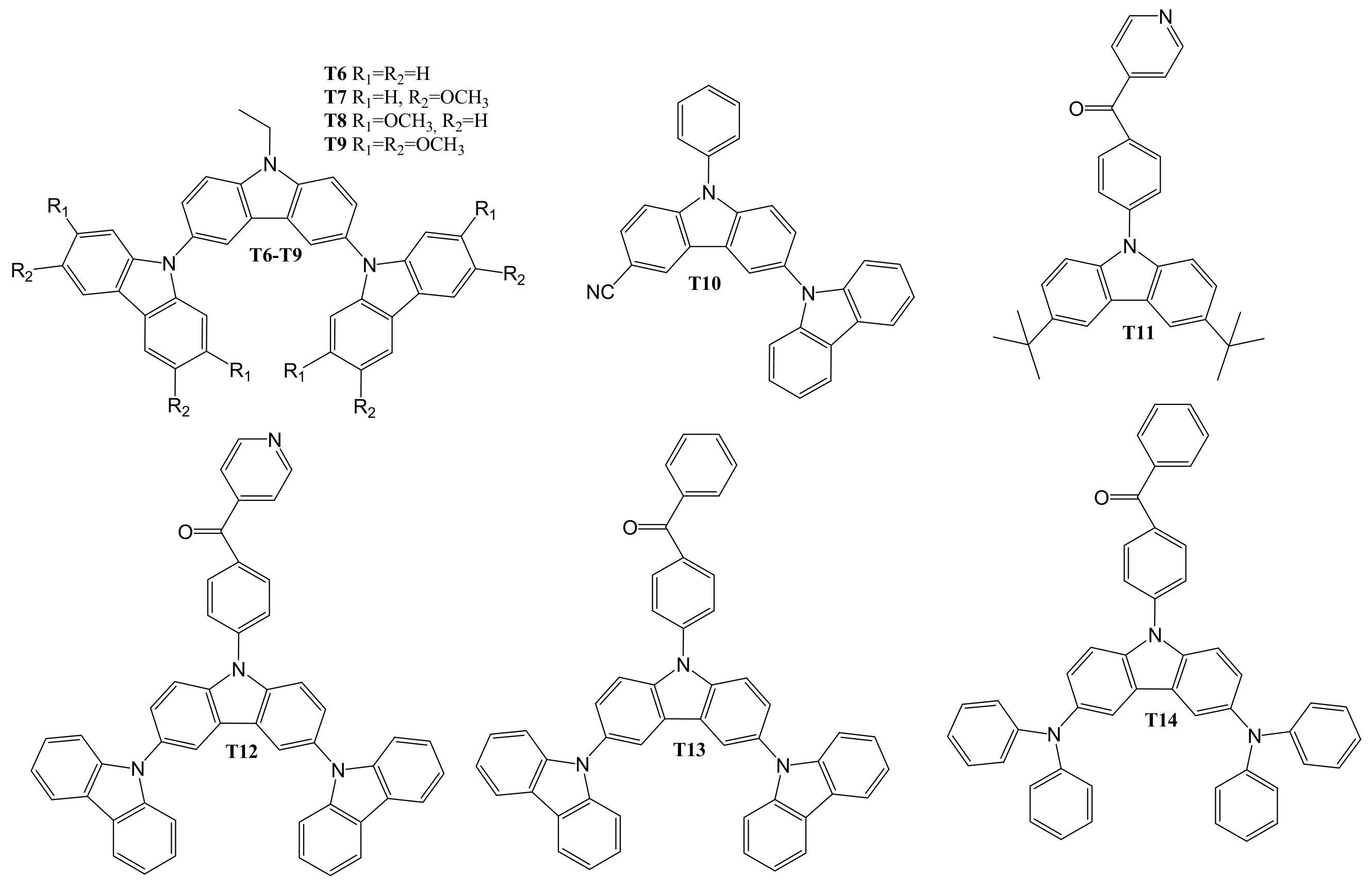
4. Diaryl(arylamino)-substituted Carbazoles as Host Materials for PhOLEDs
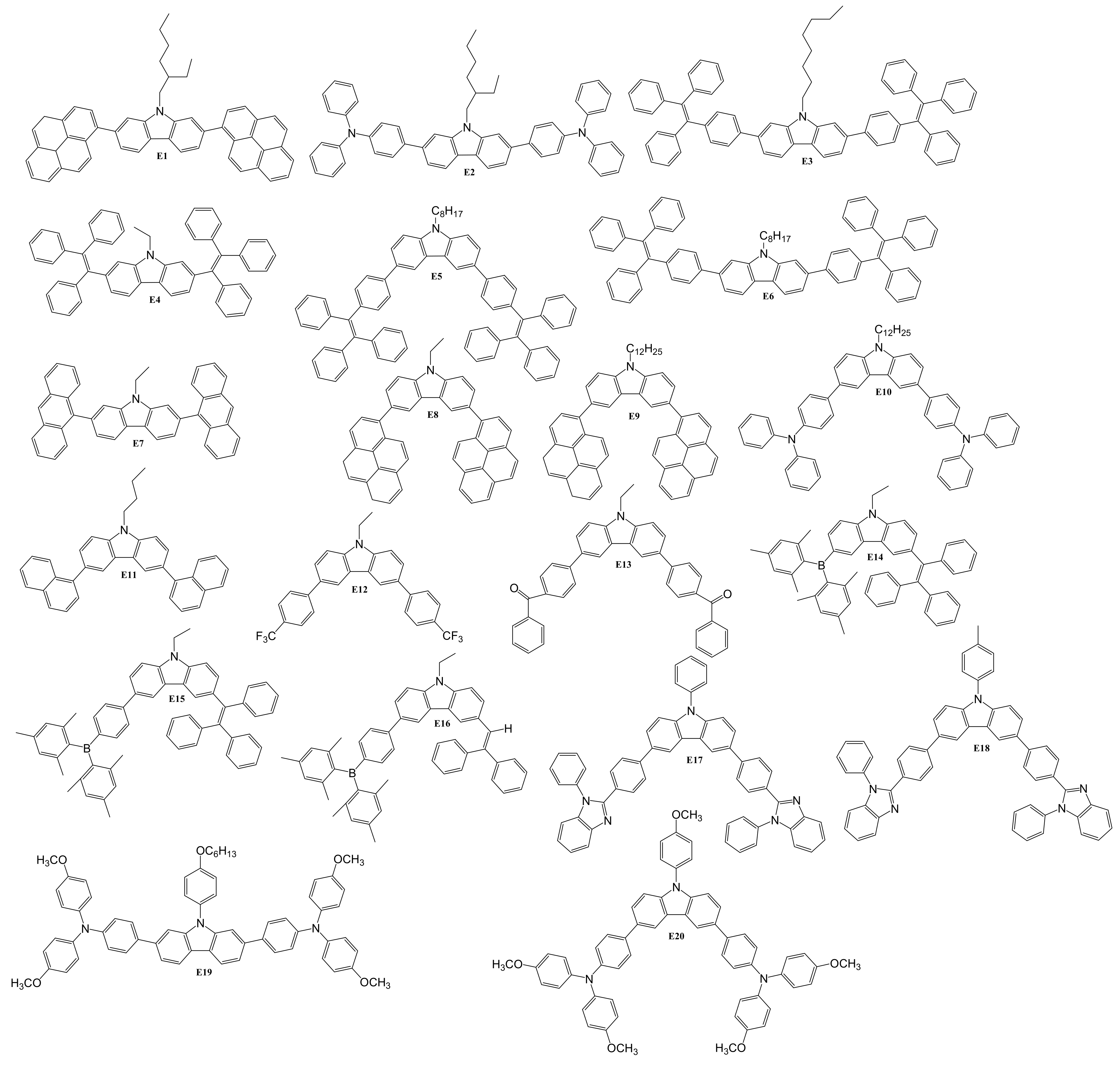
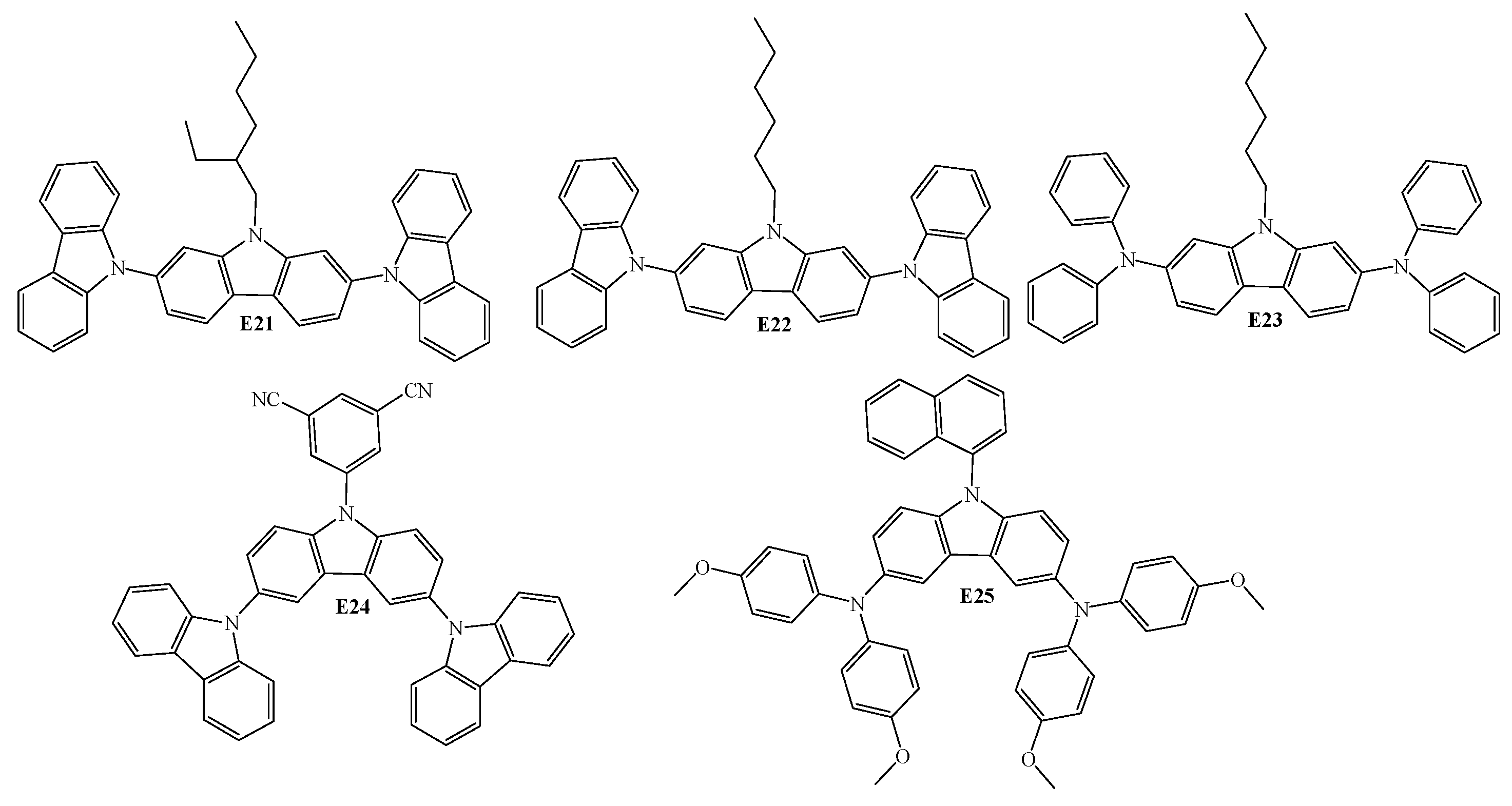
5. Diaryl(arylamino)-substituted Carbazoles as Fluorescent Emitters of OLEDs
6. Diary(arylamino)-substituted Carbazoles as TADF Emitters for OLEDs
7. Concluding Remarks
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| NBC | N-bromosuccinimide |
| 36DICr | 3,6-diiodo-9-alkylcarbazoles |
| 36DI9ArCr | 3,6-diiodo-9-arylcarbazoles |
| 36DBrCr | 3,6-dibromo-9-alkylcarbazoles |
| 36DBr9ArCr | 3,6-dibromo-9-arylcarbazoles |
| 27DBrCr | 2,7-dibromo-9-alkylcarbazoles |
| 27DBr9ArCr | 2,7-dibromo-9-arylcarbazoles |
| 27DNCr | 2,7-diarylamino-9-alkylcarbazoles |
| 27DNArCr | 2,7-diarylamino-9-arylcarbazoles |
| 36DNCr | 3,6-diarylamino-9-alkylarylcarbazoles |
| 36DNArCr | 3,6-diarylamino-9-arylcarbazoles |
| 27DCr | 2,7-diaryl-9-alkylcarbazoles |
| 27DArCr | 2,7-diaryl-9-arylcarbazoles |
| 36DCr | 3,6-diaryl-9-alkylcarbazoles |
| 36DArCr | 3,6-diaryl-9-arylcarbazoles |
| Cx | charge transporting compound |
| HTL | hole transporting layer |
| Td | thermal decomposition temperature |
| Tg | glass transition temperature |
| Ip | ionization potential |
| HOMO | the highest occupied molecular orbital |
| LUMO | the lowest unoccupied molecular orbital |
| µh | hole drift mobility |
| ITO | indium tin oxide |
| PEDOT:PSS | poly(3,4-ethylene-dioxythiophene): poly(styrene-sulfonate) |
| F4TCNQ | 2,3,5,6-tetrafluoro-7,7′,8,8′-tetracyano-p-quinodimethane |
| TPD | N,N′-bis(3-methylphenyl)-1,1′-biphenyl-4,4′-diamine |
| CBP | N,N′-dicarbazolyl-4,4′-biphenyl |
| BCP | 4,7-diphenyl-1,10-phenanthroline |
| Ir(ppy)3 | tris(2-phenylpyrydine(iridium(III) |
| TPBi | 2,2′,2″-(1,3,5-benzinetriyl)-tris(1-phenyl-1-H-benzimidazole) |
| Alq3 | tris(8-hydroxyquinolinato)aluminum |
| CuPc | copper(II)phthalocyanine |
| 2-TNATA | 4,4′,4’′-tris{N-(2-naphthyl)-N-phenylamino}-triphenylamine |
| DNTPD | 4,4′-bis[N-[4-{N,N-bis(3-methylphenyl)amino}phenyl]-N-phenylamino]biphenyl |
| Bebq2 | bis(10-hydroxybenzo[h]quinolinato)beryllium) |
| Ir(mphmq)2(tmd) | bis[2,4-dimethyl-6-(4-methyl-2-quinolinyl)phenyl](2,2,6,6-tetramethyl-3,5-heptanedionate |
| PC-Z | bisphenol Z polycarbonate |
| HATCN | 1,4,5,8,9,11-hexaazatriphenylenehexacarbonitrile |
| DCDPA | 3,5-di(carbazol-9-yl)-N,N-diphenylaniline |
| TSPO1 | diphenyl-4-triphenylsilylphenyl-phosphine oxide |
| PhOLED | Phosphorescent organic light-emitting diodes |
| TAZ | 3-(4-biphenylyl)-4-phenyl-5-(4-tert-butylphenyl)-1,2,4-triazole |
| TGA | thermogravimetric analysis |
| DSC | differential scanning calorimetry |
| Bphen | 4,7-diphenyl-1,10-phenanthroline |
| Ir(2-phq)3 | tris(2-phenylquinoline)iridium(III) |
| HATCN | dipyrazino[2,3-f:2′,3′-h]quinoxaline-2,3,6,7,10,11hexacarbonitrile |
| EQE | external quantum efficiency |
| TADF | thermally activated delayed fluorescence |
References
- Chang, J. Review—Application of Photoluminescent and Electroluminescent Metal-Organic Frameworks in White Light-Emitting Diodes. ECS J. Solid State Sci. Technol. 2021, 8, 086009. [Google Scholar] [CrossRef]
- Chitnis, D.; Kalyani, N.T.; Swart, H.; Dhoble, S. Escalating opportunities in the field of lighting. Renew. Sustain. Energy Rev. 2016, 64, 727–748. [Google Scholar] [CrossRef]
- Xu, Y.; Xu, P.; Hu, D.; Ma, Y. Recent progress in hot exciton materials for organic light-emitting diodes. Chem. Soc. Rev. 2020, 50, 1030–1069. [Google Scholar] [CrossRef] [PubMed]
- Xiao, P.; Huang, J.; Yu, Y.; Liu, B. Recent Developments in Tandem White Organic Light-Emitting Diodes. Molecules 2019, 24, 151. [Google Scholar] [CrossRef] [Green Version]
- Earmme, T. Solution-Processed Efficient Blue Phosphorescent Organic Light-Emitting Diodes (PHOLEDs) Enabled by Hole-Transport Material Incorporated Single Emission Layer. Materials 2021, 14, 554. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, Y.; Fujii, N.; Murkata, C.; Ashizawa, T.; Okabe, M.; Nakano, H. Induction of mammalian DNA topoisomerase I mediated DNA cleavage by anti-tumor indolocarbazole derivatives. Biochemistry 1992, 31, 12069–12075. [Google Scholar] [CrossRef]
- Chakraborty, D.P.; Heiz, W.; Grisebach, H.; Kirby, G.W. (Eds.) Progress in the Chemistry of Organic Natural Products; Springer: Vienna, Austria, 1977; Volume 34, pp. 1–299. [Google Scholar]
- Bashir, M.; Bano, A.; Ijaz, A.S.; Chaudhary, B.A. Recent Developments and Biological Activities of N-Substituted Carbazole Derivatives: A Review. Molecules 2015, 20, 13496–13517. [Google Scholar] [CrossRef] [Green Version]
- Yu, P.; Xiao, Y. Non-Doped Deep-Blue OLEDs Based on Carbazole-π-Imidazole Derivatives. Materials 2021, 14, 2349. [Google Scholar] [CrossRef]
- Huang, C.C.; Xue, M.M.; Wu, F.P.; Yuan, Y.; Liao, L.S.; Fung, M.K. Deep-Blue and Hybrid-White Organic Light Emitting Diodes Based on a Twisting Carbazole-Benzofuro[2,3-b]Pyrazine Fluorescent Emitter. Molecules 2019, 24, 353. [Google Scholar] [CrossRef] [Green Version]
- Dumur, F. Carbazole-based polymers as hosts for solution-processed organic light-emitting diodes: Simplicity, efficacy. Org. Electron. 2015, 25, 345–361. [Google Scholar] [CrossRef]
- Krucaite, G.; Grigalevicius, S. A review on low-molar-mass carbazole- based derivatives for organic light emitting diodes. Synth. Met. 2019, 247, 90–108. [Google Scholar]
- Shahnawaz, K.; Swayamprabha, S.S.; Nagar, M.R.; Yadav, K.R.A.; Gull, S.; Dubey, A.K.; Jou, J.H. Hole-transporting materials for organic light-emitting diodes: An overview. J. Mater. Chem. C 2019, 7, 7144–7158. [Google Scholar] [CrossRef]
- Korshunov, V.M.; Mikhailov, M.S.; Chmovzh, T.N.; Vashchenko, A.A.; Gudim, N.S.; Mikhalchenko, L.V.; Taydakov, I.V.; Rakitin, O.A. Novel D-A-D Fluorescent Dyes Based on 9-(p-Tolyl)-2,3,4,4a,9,9a-hexahydro-1H-carbazole as a Donor Unit for Solution-Processed Organic Light-Emitting-Diodes. Molecules 2021, 26, 2872. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, J.; Liu, D.; Li, D.; Dong, R. A donor design strategy for triazine-carbazole blue thermally activated delayed fluo-rescence materials. New J. Chem. 2020, 44, 9743–9754. [Google Scholar]
- Su, Y.; Lin, H.; Li, W. The Applications of Carbazole and Carbazole-Related Compounds in Blue Emitting Organic Light-Emitting Diodes. Prog. Chem. 2015, 27, 1384–1399. [Google Scholar]
- Jiang, H. Hosts for High-Performance Phosphorescent Organic Light-Emitting Diodes Based on Carbazole Derivatives. Asian J. Org. Chem. 2014, 3, 102–112. [Google Scholar] [CrossRef]
- Vaitkeviciene, V.; Kruzinauskiene, A.; Grigalevicius, S.; Grazulevicius, J.V. Well-defined [3,3′]bicarbazolyl-based electroactive compounds for optoelectronics. Electroactivity 2008, 158, 383–390. [Google Scholar] [CrossRef]
- Griniene, R.; Grazulevicius, J.V.; Tseng, K.Y.; Wang, W.B.; Jou, J.H.; Grigalevicius, S. Aryl substituted 9-(2,2-diphenylvinyl)carbazoles as efficient materials for hole transporting layers of OLEDs. Synth. Met. 2011, 161, 2466–2470. [Google Scholar] [CrossRef]
- Grigalevicius, S.; Ma, L.; Qian, G.; Xie, Z.; Forster, M.; Scherf, U. New Carbazole-Based Copolymers as Amorphous Hole-Transporting Materials for Multilayer Light-Emitting Diodes. Macro-Mol. Chem. Phys. 2007, 208, 349–355. [Google Scholar] [CrossRef]
- Tavgeniene, D.; Krucaite, G.; Baranauskyte, U.; Wu, J.; Su, H.; Huang, C.W.; Chang, C.H.; Grigalevicius, S. Phenanthro[9,10-d]imidazole based new host materials for efficient red phosphorescent OLEDs. Dye. Pigment. 2017, 137, 615–621. [Google Scholar]
- Ledwon, P. Recent advances of donor-acceptor type carbazole-based molecules for light emitting applications. Org. Electron. 2019, 75, 105422–105437. [Google Scholar] [CrossRef]
- Wex, B.; Kaafarani, B.R. Perspective on carbazole-based organic compounds as emitters and hosts in TADF applications. J. Mater. Chem. C 2017, 5, 8622–8653. [Google Scholar] [CrossRef] [Green Version]
- Smith, K.; James, D.M.; Mistry, A.G.; Bye, M.R.; Faulkner, D.J. A new method for bromination of carbazoles, β-carbolines and iminodibenzyls by use of N-bromosuccinimide and silica gel. Tetrahedron 1922, 36, 7479–7488. [Google Scholar] [CrossRef]
- Tucker, S.H. Iodination in the carbazole series. J. Chem. Soc. (Resumed) 1926, 1, 546–553. [Google Scholar] [CrossRef]
- Dierschke, F.; Grimsdale, A.C.; Mullen, K. Efficient Synthesis of 2,7-Dibromocarbazoles as Components for Electroactive Materials. Synthesis 2003, 16, 2470–2472. [Google Scholar] [CrossRef]
- Beginn, C.; Grazulevicius, J.V.; Strohriel, P.; Simmerer, J.; Haarer, D. Synthesis of poly(9-hexyl-3,6-carbazolyleneethynylene) and its model compounds. Macromol. Chem. Phys. 1944, 195, 2353–2370. [Google Scholar] [CrossRef]
- Rodriguez-Parada, J.M.; Percec, K. Interchain electron donor-acceptor complexes: A model to study polymer-polymer misci-bility? Macromolecules 1986, 19, 55–64. [Google Scholar] [CrossRef]
- Dierschke, F.; Grimsdale, A.C.; Muullen, K. A Virosome-Mimotope Approach to Synthetic Vaccine Design and Optimization: Synthesis, Conformation, and Immune Recognition of a Potential Malaria-Vaccine Candidate. Angew. Chem. Int. Ed. 2003, 115, 2470–2473. [Google Scholar]
- Wong, K.T.; Chen, Y.M.; Lin, Y.T.; Su, H.C.; Wu, C.C. Nonconjugated Hybrid of Carbazole and Fluorene: A Novel Host Material for Highly Efficient Green and Red Phosphorescent OLEDs. Org. Lett. 2005, 7, 5361–5364. [Google Scholar] [CrossRef]
- Reddy, M.A.; Thomas, A.; Mallesham, G.; Sridhar, B.; Rao, V.J.; Bhanuprakash, K. Synthesis of novel twisted carbazole–quinoxaline derivatives with 1,3,5-benzene core: Bipolar molecules as hosts for phosphorescent OLEDs. Tetrahedron Lett. 2011, 52, 6942–6947. [Google Scholar] [CrossRef]
- Gauthier, S.; Frechet, J.M.J. Phase-Transfer Catalysis in the Ullmann Synthesis of Substituted Triphenylamines. Synthesis 1987, 1987, 383–385. [Google Scholar] [CrossRef]
- Hassan, J.; Sevignon, M.; Gozzi, C.; Schulz, E.; Lemaire, M. Aryl−Aryl Bond Formation One Century after the Discovery of the Ullmann Reac-tion. Chem. Rev. 2002, 102, 1359–1470. [Google Scholar] [CrossRef] [PubMed]
- Nelson, T.D.; Crouch, R.D. Cu, Ni, and Pd Mediated Homocoupling Reactions in Biaryl Syntheses: The Ullmann Reaction. Org. React. 2004, 63, 265. [Google Scholar]
- Fihri, A.; Bouhrara, M.; Nekoueishahraki, B.; Basset, J.M.; Polshettiwar, V. Nanocatalysts for Suzuki cross-coupling reactions. Chem. Soc. Rev. 2011, 40, 5181–5203. [Google Scholar] [CrossRef]
- Suzuki, A. Recent advances in the cross-coupling reactions of organoboron derivatives with organic electrophiles, 1995–1998. J. Organomet. Chem. 1999, 576, 147–168. [Google Scholar] [CrossRef]
- Forero-Corte, P.A.; Hayd, A.M. The 25th Anniversary of the Buchwald–Hartwig Amination: Development, Applications, and Outlook. Org. Process Res. Dev. 2019, 23, 1478–1483. [Google Scholar] [CrossRef]
- Stille, J.K. The Palladium-Catalyzed Cross-Coupling Reactions of Organotin Reagents with Organic Electrophiles [New Syn-thetic Methods. Wiley Online Libr. 1986, 25, 508–524. [Google Scholar]
- Farina, V.; Krishnamurthy, V.; Scott, W.J. The Stille reactions. J. Scott. Org. React. 1998, 50, 1–565. [Google Scholar]
- Whiting, A.; Windsor, C.M. What makes a neutral imino dieneophile undergo a thermal, non-catalysed, Diels-Alder reac-tion? Tetrahedron 1998, 54, 6035–6050. [Google Scholar] [CrossRef]
- Tasdelen, M. A Diels–Alder “click” reactions: Recent applications in polymer and material science. Polym. Chem. 2011, 2, 2133–2145. [Google Scholar] [CrossRef]
- Nicolaou, K.C.; Snyder, S.A.; Montagnon, T.; Vassilikogiannakis, G. The Diels–Alder Reaction in Total Synthesis. Angew. Chem. Int. Ed. 2002, 41, 1668–1698. [Google Scholar] [CrossRef]
- Groves, J.K. The Friedel–Crafts acylation of alkenes. Chem. Soc. Rev. 1972, 1, 73. [Google Scholar] [CrossRef]
- Agarwal, N.; Nayak, P.K.; Ali, F.; Patankar, M.P.; Narasimhan, K.L.; Periasamy, N. Tuning of HOMO levels of carbazole derivatives: New molecules for blue OLED. Synth. Met. 2011, 161, 466–473. [Google Scholar] [CrossRef]
- Krucaite, G.; Tavgeniene, D.; Grazulevicius, J.V.; Wang, Y.C.; Hsieh, C.Y.; Jou, J.H.; Gasva, G.; Grigalevicius, S. 3,6-Diaryl substituted 9-alkylcarbazoles as hole transporting materials for various organic light emitting devices. Dye. Pigmens 2014, 106, 1–6. [Google Scholar] [CrossRef]
- Jou, J.H.; Li, T.H.; Kumar, S.; An, C.C.; Agrawal, A.; Chen, S.Z.; Fang, P.H.; Kručaitė, G.; Grigalevičius, S.; Gražulevičius, J.V.; et al. Enabling high-efficiency organic light-emitting diodes with a cross-linkable electron confin-ing hole transporting materials. Org. Electron. 2015, 24, 254–262. [Google Scholar] [CrossRef]
- Kochapradist, P.; Prachumrak, N.; Tarsang, R.; Keawin, T.; Jungsuttiwong, S.; Sudyoadsuk, T.; Promarak, V. Multi-triphenylamine-substituted carbazoles: Synthesis, characterization, properties, and applications as hole-transporting materials. Tetrahedron Lett. 2013, 54, 3683–3687. [Google Scholar] [CrossRef]
- Lee, Y.M.; Kim, S.K.; Lee, C.J.; Lee, J.H.; Park, J.W. Synthesis and Hole-Transporting Properties of Ethyl-Carbazyl Derivatives. Mol. Cryst. Liq. Cryst. 2009, 499, 100–111. [Google Scholar] [CrossRef]
- Reig, M.; Gozalvez, C.; Bujaldon, R.; Bagdziunas, G.; Ivaniuk, K.; Kostiv, N.; Volyniuk, D.; Grazulevicius, J.V.; Velasco, D. Easy accessible blue luminescent carbazole-based materials for organic light-emitting diodes. Dye. Pigmens 2017, 137, 24–35. [Google Scholar] [CrossRef]
- Kim, S.K.; Lee, Y.M.; Lee, C.J.; Lee, J.H.; Oh, S.Y.; Park, J.W. Synthesis and Hole-Transporting Properties of Phenyl-Carbazyl Deriva-tives. Mol. Cryst. Liq. Cryst. 2008, 491, 133–144. [Google Scholar] [CrossRef]
- Braveenth, R.; Bae, H.W.; Ko, I.J.; Qiong, W.; Nguyen, Q.P.B.; Jayashantha, P.G.S.; Kwon, J.H.; Chai, K.Y. Thermally stable efficient hole transporting materials based on carbazole and tri-phenylamine core for red phosphorescent OLEDs. Org. Electron. 2017, 51, 463–470. [Google Scholar] [CrossRef]
- Kim, K.S.; Jeong, S.; Kim, C.; Kwon, Y.; Choi, B.D.; Han, Y.S. Synthesis and electro-optical properties of carbazole derivatives with high band gap energy. Thin Solid Film. 2009, 518, 284–289. [Google Scholar] [CrossRef]
- Lai, S.L.; Tong, Q.X.; Chan, T.W.; Ng, T.W.; Lo, M.F.; Ko, C.C.; Lee, S.T.; Lee, C.S. Carbazole–pyrene derivatives for undoped organic light-emitting devices. Org. Electron. 2011, 12, 541–546. [Google Scholar] [CrossRef]
- Kumar, S.; An, C.C.; Sahoo, S.; Griniene, R.; Volyniuk, D.; Grazulevicius, J.V.; Grigalevicius, S.; Jou, J.H. Solution-processable naphthalene and phenyl substituted carbazole core based hole transporting materi-als for efficient organic light-emitting diodes. J. Mater. Chem. C 2017, 5, 9854–9864. [Google Scholar] [CrossRef]
- Park, Y.I.; Lee, S.E.; Park, J.W.; Oh, S.Y. New Multi-Phenylated Carbazole Derivatives for OLED through Diels-Alder Reaction. Mol. Cryst. Ans Liq. Cryst. 2007, 470, 223–230. [Google Scholar] [CrossRef]
- Shen, J.Y.; Yang, X.L.; Huang, T.H.; Lin, J.T.; Ke, T.H.; Chen, L.Y.; Wu, C.C.; Yeh, M.C.P. Ambipolar Conductive 2,7-Carbazole Derivatives for Electroluminescent Devices. Adv. Funct. Mater. 2007, 17, 983–995. [Google Scholar] [CrossRef]
- Grigalevicius, S.; Grazulevicius, J.; Gaidelis, V.; Jankauskas, V.; Jankauskas, V. Synthesis and properties of poly(3,9-carbazole) and low-molar-mass glass-forming carbazole compounds. Polymers 2002, 43, 2603–2608. [Google Scholar] [CrossRef]
- Grigalevicius, S.; Getautis, V.; Gražulevicius, J.V.; Gaidelis, V.; Jankauskas, V.; Montrimas, E. Hole-transporting molecular glasses based on carbazole and diphe-nylamine moieties. Mater. Chem. Phys. 2001, 72, 395–400. [Google Scholar] [CrossRef]
- Grigalevicius, S.; Buika, G.; Grazulevicius, J.V.; Gaidelis, V.; Jankauskas, V.; Montrimas, E. 3,6-Di(diphenylamino)-9-alkylcarbazoles: Novel hole-transporting molec-ular glasses. Synth. Met. 2001, 122, 311–314. [Google Scholar] [CrossRef]
- Blazys, G.; Grigalevicius, S.; Grazulevicius, J.V.; Gaidelis, V.; Jankauskas, V.; Kampars, V. Phenothiazinyl-containing aromatic amines as novel amorphous molecu-lar materials for optoelectronics. J. Photochem. Photobiol. A Chem. 2005, 174, 1–6. [Google Scholar] [CrossRef]
- Lengvinaite, S.; Grazulevicius, J.V.; Jankauskas, V.; Grigalevicius, S. Carbazole-based aromatic amines having oxetanyl groups as materials for hole transporting layers. Synth. Met. 2007, 157, 529–533. [Google Scholar] [CrossRef]
- Grigalevicius, S.; Blažys, G.; Ostrauskaitė, J.; Grazulevicius, J.V.; Gaidelis, V.; Jankauskas, V.; Montrimas, E. 3,6-Di(N-diphenylamino)-9-phenylcarbazole and its methyl-substituted de-rivative as novel hole-transporting amorphous molecular materials. Synth. Met. 2002, 128, 127–131. [Google Scholar] [CrossRef]
- Lee, W.; Kang, Y.; Lee, P.H. Synthesis and Characterization of Polyaromatic Compounds Using Tri(naphthyl)indium. J. Org. Chem. 2008, 73, 4326–4329. [Google Scholar] [CrossRef]
- Jou, J.H.; Sahoo, S.; Kumar, S.; Yu, H.H.; Fang, P.H.; Singh, M.; Krucaite, G.; Volyniuk, D.; Grazulevicius, J.V.; Grigalevicius, S. A wet- and dry-process feasible carbazole type host for highly efficient phosphorescent OLEDs. J. Mater. Chem. C 2015, 3, 12297–12307. [Google Scholar] [CrossRef]
- Gong, W.L.; Zhong, F.; Aldred, M.P.; Fu, Q.; Chen, T.; Huang, D.K.; Shen, Y.; Qiao, X.F.; Ma, D.; Zhu, M.Q. Carbazole oligomers revisited: New additions at the carbazole 1- and 8-positions. RSC Adv. 2012, 2, 10821–10828. [Google Scholar] [CrossRef]
- Deng, L.; Li, J.; Wang, G.X.; Wu, L.Z. Simple bipolar host materials incorporating CN group for highly efficient blue electrophosphorescence with slow efficiency roll-off. J. Mater. Chem. C 2013, 1, 8140–8145. [Google Scholar] [CrossRef]
- Jiang, W.; Duan, L.; Qiao, J.; Dong, G.; Zhang, D.; Wang, L.; Qiu, Y. Novel carbazole/pyridine-based host material for solution-processed blue phosphorescent organic light-emitting devices. Dye. Pigment. 2012, 92, 891–896. [Google Scholar] [CrossRef]
- Song, J.E.; Lee, S.E.; Song, Y.R.; Kim, T.W.; Lim, C.W.; Kim, Y.K. Bipolar Host Materials Based on 1,3,5-Triazine Derivative for Green Phosphorescent Organic Light-emitting Diode. Bull. Korean Chem. Soc. 2017, 38, 1003–1009. [Google Scholar] [CrossRef]
- Ye, H.; Zhou, K.; Wu, H.; Chen, K.; Xie, G.; Hu, J.; Yan, G.; Ma, S.; Su, S.J.; Cao, Y. Novel molecular host materials based on carbazole/PO hybrids with wide bandgap via unique linkages for solution-processed blue phosphorescent OLEDs. Opt. Mater. 2016, 60, 244–251. [Google Scholar] [CrossRef]
- Sapochak, L.; Padmaperuma, A.; Vecchi, P.; Cai, X.; Burrows, P. Designing organic pfoshine oxide host materials using het-eroarmatic building blocks:inductive effects on electroluminescence. Light Emit. Mater. Devices XI 2007, 6655, 665506–665517. [Google Scholar]
- Chen, H.F.; Chi, L.C.; Hung, W.Y.; Chen, W.J.; Hwa, T.Y.; Chen, Y.H.; Chou, S.H.; Mondal, E.; Liu, Y.H.; Wong, K.T. Carbazole and benzimidazole/oxadiazole hybrids as bipolar host materials for sky blue, green, and red PhOLEDs. Org. Electron. 2012, 13, 2671–2681. [Google Scholar] [CrossRef]
- Soon, J.O.; Kyoung, S.Y.; Soo, W.J.; Lee, Y.Y. Phenylcarbazole-Based Phosphine Oxide Host Materials for High Efficiency in Deep Blue Phosphorescent Organic Light-Emitting Diodes. Adv. Funct. Mater. 2019, 19, 3644–3649. [Google Scholar]
- Tsai, M.H.; Ke, T.H.; Lin, H.W.; Wu, C.C.; Chiu, S.F.; Fang, F.C.; Liao, Y.L.; Wong, K.T.; Chen, Y.H.; Wu, C.I. Triphenylsilyl- and Trityl-Substituted Carbazole-Based Host Materials for Blue Electrophosphorescence. ASC Appl. Mater. Interfaces 2009, 3, 567–574. [Google Scholar] [CrossRef]
- Kim, D.H.; Hong, C.K.; Lee, P.H.; Kang, Y. New Host Materials Containing Carbazole and Naphthyl Moieties for Green Dopant in Phosphorescence Organic Light-Emitting Diodes (OLEDs). Bull. Korean Chem. Soc. 2008, 29, 2270–2272. [Google Scholar]
- Sapochak, L.S.; Padmaperuma, A.B.; Cai, X.; Male, J.L.; Burrows, P.E. Inductive Effects of Diphenylphosphoryl Moieties on Carba-zole Host Materials: Design Rules for Blue Electrophosphorescent Organic Light-Emitting Devices. J. Phys. Chem. C 2008, 112, 7989–7996. [Google Scholar] [CrossRef]
- Ahn, D.H.; Moon, J.S.; Kim, S.W.; Lee, S.Y.; Karthik, D.; Lee, J.Y.; Kwon, J.H. Effect of various host characteristics on blue thermally activated de-layed fluorescent devices. Org. Electron. 2018, 59, 39–44. [Google Scholar] [CrossRef]
- Bagdziunas, G.; Grybauskaite, G.; Kostiv, N.; Ivaniuk, K.; Volyniuk, D.; Lazauskas, A. Green and red phosphorescent organic light-emitting diodes with am-bipolar hosts based on phenothiazine and carbazole moieties: Photoelectrical properties, morphology and efficiency. RSC. Adv. 2016, 6, 61544–61554. [Google Scholar] [CrossRef]
- Tsai, M.H.; Hong, Y.H.; Chang, C.H.; Su, H.C.; Wu, C.C.; Matoliukstyte, A.; Simokaitiene, J.; Grigalevicius, S.; Grazulevicius, J.V.; Hsu, C.P. 3-(9-Carbazolyl)carbazoles and 3,6-Di(9-carbazolyl)carbazoles as Effective Host Materials for Efficient Blue Organic Electrophosphorescence. Adv. Mater. 2007, 19, 862–866. [Google Scholar] [CrossRef]
- Zhang, Q.; Hu, Y.F.; Cheng, Y.X.; Su, G.I.; Ma, D.G.; Wang, L.X.; Jing, X.B.; Wang, F.S. Carbazole-based hole-transporting materials for electroluminescent de-vices. Synth. Met. 2003, 137, 1111–1112. [Google Scholar] [CrossRef]
- Park, M.S.; Lee, J.Y. 9-(Pyridin-3-yl)-9H-carbazole derivatives as host materials for green phosphorescent organic light-emitting diodes. Org. Electron. 2013, 14, 1291–1296. [Google Scholar] [CrossRef]
- Jiang, W.; Duan, L.; Qiao, J.; Dong, G.; Zhang, D.; Wang, L.; Qiu, Y. High-triplet-energy tri-carbazole derivatives as host materials for efficient solution-processed blue phospho-rescent devices. J. Mater. Chem. 2011, 21, 4918–4926. [Google Scholar] [CrossRef]
- Pudzs, K.; Vembris, A.; Muzikante, I.; Grzibovskis, R.; Turovska, B.; Simokaitiene, J.; Grigalevicius, S.; Grazulevicius, J.V. Energy structure and electro-optical properties of organic layers with carbazole derivative. Thin Solid Film 2014, 556, 405–409. [Google Scholar] [CrossRef]
- Xiang, C.; Fu, X.; Wei, W.; Liu, R.; Zhang, Y.; Balema, V.; Nelson, B.; So, F. Efficiency Roll-Off in Blue Emitting Phosphorescent Organic Light Emitting Diodes with Car-bazole Host Materials. Adv. Funct. Mater. 2016, 26, 1463–1469. [Google Scholar] [CrossRef]
- Orselli, E.; Maunoury, J.; Bascour, D.; Catinat, J.P. Orange phosphorescent organic light-emitting diodes with high operational stabil-ity. Org. Electron. 2012, 13, 1506–1510. [Google Scholar] [CrossRef]
- Krotkus, S.; Kazlauskas, K.; Miasojedovas, A.; Gruodis, A.; Tomkeviciene, A.; Grazulevicius, J.V.; Jursenas, S. Pyrenyl-Functionalized Fluorene and Carbazole Derivatives as Blue Light Emitters. J. Phys. Chem. C 2012, 116, 7561–7572. [Google Scholar] [CrossRef]
- Konidena, R.K.; Thomas, K.R.J.; Sahoo, S.; Dubeyb, D.K.; Jou, J.H. Multi-substituted deep-blue emitting carbazoles: A comparative study on photophysical and electroluminescence characteristics. J. Mater. Chem. C 2017, 5, 709–726. [Google Scholar] [CrossRef]
- Gong, W.L.; Wang, B.; Aldred, M.P.; Li, C.; Zhang, G.F.; Chen, T.; Wang, L.; Zhu, M.Q. Tetraphenylethene-decorated carbazoles: Synthesis, aggregation-induced emission, pho-to-oxidation and electroluminescence. J. Mater. Chem. C 2014, 2, 7001–7012. [Google Scholar] [CrossRef]
- Dang, D.; Wang, Z.; Liu, K.; Liu, Y.; Sung, H.H.Y.; Ian, D. Manipulating the Molecular Backbone to Achieve Highly Emissive Sky-Blue AIEgens and Their Applications in Nondoped Organic Light-Emitting Diodes. Adv. Electron. Mater. 2018, 4, 1800354. [Google Scholar] [CrossRef]
- Zhang, T.; Dai, H.; Li, J. Novel carbazole/anthracene hybrids for efficient blue organic light-emitting diodes. Displays 2013, 34, 447–451. [Google Scholar] [CrossRef]
- Tong, Q.X.; Lai, S.L.; Lo, M.F.; Chan, M.Y.; Ng, T.W.; Lee, S.H.; Tao, S.L.; Lee, C.S. An efficient hole-transporting blue fluorophore 3,6-dipyrenyl-9-ethylcarbazole for un-doped organic light-emitting devices. Synth. Met. 2012, 162, 415–418. [Google Scholar] [CrossRef]
- Keawin, T.; Prachumrak, N.; Namuangruk, S.; Pansay, S.; Kungwan, N.; Maensiri, S.; Jungsuttiwong, S.; Sudyoadsuk, T.; Promarak, V. Efficient bifunctional materials based on pyrene- and triphenylamine-functionalized dendrimers for electroluminescent devices. Synth. Met. 2015, 5, 73481–73489. [Google Scholar] [CrossRef]
- Feng, X.; Wei, X.; Yin, M.; Pansay, S.; Kungwan, N.; Maensiri, S.; Jungsuttiwong, S.; Promark, V. Violet/deep-blue fluorescent organic light-emitting diode based on high-efficiency novel carbazole derivative with large torsion angle. RSC Adv. 2019, 60, 151340–151344. [Google Scholar] [CrossRef]
- Krishnaa, A.; Darshana, V.; Suresha, C.H.; Miao, Y.; Tao, P. Solution processable carbazole derivatives for dopant free single molecule white electroluminescence by room temperature phosphorescence. Tetrahedron Lett. 2018, 360, 249–254. [Google Scholar] [CrossRef]
- Shi, H.; Zhang, X.; Gui, C.; Wang, S.; Fang, L.; Zhao, Z.; Chen, S.; Tang, B.Z. Synthesis, aggregation-induced emission and electroluminescence properties of three new phenylethylene derivatives comprising carbazole and (dimesitylboranyl)phenyl groups. J. Mater. Chem. C 2017, 5, 11741–11750. [Google Scholar] [CrossRef]
- Hung, W.Y.; Chi, L.C.; Chen, W.J.; Chen, Y.M.; Chou, S.H.; Wong, K.T. A new benzimidazole/carbazole hybrid bipolar material for highly efficient deep-blue electrofluo-rescence, yellow–green electrophosphorescence, and two-color-based white OLEDs. J. Mater. Chem. 2010, 20, 10113–10119. [Google Scholar] [CrossRef]
- Yang, X.; Zheng, S.; Bottger, R.; Chae, H.S.; Tanaka, T.; Li, S.; Mochizuki, A.; Jabbour, G.E. Efficient Fluorescent Deep-Blue and Hybrid White Emitting Devices Based on Carba-zole/Benzimidazole Compound. J. Phys. Chem. C 2011, 115, 14347–14352. [Google Scholar] [CrossRef]
- Skorka, L.; Kurzepa, P.; Salyga, G.W.; Luszczynska, B.; Wielgusa, I.; Wrobel, Z.; Ulanski, J.; Kulszewicz-Bajera, I. New diarylaminophenyl derivatives of carbazole: Effect of substituent position on their redox, spectroscopic and electroluminescent properties. Synth. Met. 2017, 228, 1–8. [Google Scholar] [CrossRef]
- Shi, H.; Li, M.; Xin, D.; Fang, L.; Roose, J.; Peng, H.; Chen, S.; Tang, B.Z. Two novel phenylethene-carbazole derivatives containing dimesitylboron groups: Aggrega-tion-induced emission and electroluminescence properties. Dye. Pigment. 2016, 128, 304–313. [Google Scholar] [CrossRef] [Green Version]
- Tomkeviciene, A.; Grazulevicius, J.V.; Jankauskas, V. Hole-Transporting Pyrenyl-Substituted Derivatives of Fluorene and Carbazole. Mol. Cryst. Liq. Cryst. 2014, 590, 29–34. [Google Scholar] [CrossRef]
- Tomkeviciene, A.; Grazulevicius, J.V.; Kazlauskas, K.; Gruodis, A.; Jursenas, S.; Ke, T.-H.; Wu, C.-C. Impact of Linking Topology on the Properties of Carbazole Trimers and Dimers. J. Phys. Chem. C 2011, 115, 4887–4897. [Google Scholar] [CrossRef]
- Tomkeviciene, A.; Grazulevicius, J.V.; Jankauskas, V. High Hole Mobilities in the Amorphous Films of 2,7-Di(9-carbazolyl)-9-(2-ethylhexyl)carbazole. Chem. Lett. 2008, 37, 344–345. [Google Scholar] [CrossRef]
- Li, J.; Liu, R.; Zhao, Z.; Zhou, Z. Synthesis and characterization of novel 2,7-carbazole derivatives for blue light –emitting diodes. J. Mater. Sci. Mater. Electron. 2014, 25, 1970–1975. [Google Scholar] [CrossRef]
- Park, W.J.; Leea, Y.; Kim, J.Y.; Yoon, D.W.; Kim, J.; Chae, S.H.; Kim, H.; Lee, G.; Shimc, S.; Yang, J.H.; et al. Effective thermally activated delayed fluorescence emitter and its performance in OLED device. Synth. Met. 2015, 209, 99–104. [Google Scholar] [CrossRef]
- Deksnys, T.; Simokaitiene, J.; Keruckas, J.; Volyniuk, D.; Bezvikonnyi, O.; Cherpak, V.; Stakhira, P.; Ivaniuk, K.; Helzhynskyy, I.; Baryshnikov, G. Synthesis and characterisation of a carbazole-based bipolar exciplex-forming compound for efficient and color-tunable OLEDs. J. Chem. 2017, 41, 559–568. [Google Scholar] [CrossRef]
- Luka, G.; Volyniuk, D.; Tomkeviciene, A.; Simokaitiene, J.; Grazulevicius, J.V.; Stakhira, P.; Cherpak, V.; Sybilski, P.; Witkowski, B.S.; Godlewski, M.; et al. Carbazole Derivative Based Near Ultraviolet Organic Light Emitting Diode with ZnMgO:Al Anode Layer. Solid State Phenom. 2013, 200, 45–49. [Google Scholar] [CrossRef]
- Shia, Z.; Zhangb, X.; Wang, H.; Huo, J.; Zhao, H.; Shib, H.; Tang, B.Z. The synthesis, photoluminescence and electroluminescence properties of a new emitter based on diphenylethene, carbazole and 9,9,10,10-tetraoxidethianthrene. Org. Electron. 2019, 70, 7–13. [Google Scholar] [CrossRef]
- Grybauskaite-Kaminskiene, G.; Volyniuk, D.; Mimaite, V.; Bezvikonnyi, O.; Bucinskas, A.; Bagdziunas, G.; Grazulevicius, J.V. Aggregation Enhanced Emission and Thermally Activated De-layed Fluorescence of Derivatives of 9-Phenyl-9H-Carbazole: Effects of Methoxy and tert-Butyl Substituents. Chem.-A Eur. J. 2018, 24, 9581–9591. [Google Scholar] [CrossRef]
- Liu, C.; Chen, J.; Xu, C.; Hao, H.; Xu, B.; Hu, D.; Shi, G.; Chi, Z. AIEgens with bright mechanoluminescence and thermally activated delayed fluo-rescence derived from (9H-carbazol-9-yl)(phenyl)methanone. Dye. Pigment. 2020, 174, 108093–108099. [Google Scholar] [CrossRef]
- Karthik, D.; Ahn, D.H.; Ryu, J.H.; Lee, H.; Maeng, J.H.; Lee, J.Y.; Kwon, J.H. Highly efficient blue thermally activated delayed fluorescence organic light emitting diodes based on tercarbazole donor and boron acceptor dyads. J. Mater. Chem. 2020, 8, 2272–2279. [Google Scholar] [CrossRef]
- Sallenave, X.; Bucinskas, A.; Salman, S.; Volyniuk, D.; Bezvikonnyi, O.; Mimaite, V.; Grazulevicius, J.V.; Sini, G. Sensitivity of Redox and Optical Properties of Electroactive Carbazole Deriva-tives to the Molecular Architecture and Methoxy Substitutions. J. Phys. Chem. C 2018, 122, 10138–10152. [Google Scholar] [CrossRef]
- Lin, T.C.; Sarma, M.; Chen, Y.T.; Liu, S.H.; Lin, K.T.; Chiang, P.Y.; Chuang, W.T.; Liu, Y.C.; Hsu, H.F.; Hung, W.Y.; et al. Probe exciplex structure of highly efficient thermally activated delayed fluorescence organic light emitting diodes. Nat. Commun. 2018, 9, 3111–3119. [Google Scholar] [CrossRef] [Green Version]
- Rajamalli, P.; Senthilkumar, N.; Gandeepan, P.; Ren-Wu, C.C.; Lin, H.W.; Cheng, C.H. A Method for Reducing the Singlet–Triplet Energy Gaps of TADF Materials for Improving the Blue OLED Efficiency. ACS Appl. Mater. Interfaces 2016, 8, 27026–27034. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Wang, Z.; Cai, X.; He, Z.; Liu, X.; Cao, Y.; Su, S.J. Highly efficient thermally activated delayed fluorescence materials with reduced efficiency roll-off and low on-set voltages. Mater. Chem. Front. 2017, 1, 2039–2046. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krucaite, G.; Grigalevicius, S. 2,7(3,6)-Diaryl(arylamino)-substituted Carbazoles as Components of OLEDs: A Review of the Last Decade. Materials 2021, 14, 6754. https://doi.org/10.3390/ma14226754
Krucaite G, Grigalevicius S. 2,7(3,6)-Diaryl(arylamino)-substituted Carbazoles as Components of OLEDs: A Review of the Last Decade. Materials. 2021; 14(22):6754. https://doi.org/10.3390/ma14226754
Chicago/Turabian StyleKrucaite, Gintare, and Saulius Grigalevicius. 2021. "2,7(3,6)-Diaryl(arylamino)-substituted Carbazoles as Components of OLEDs: A Review of the Last Decade" Materials 14, no. 22: 6754. https://doi.org/10.3390/ma14226754
APA StyleKrucaite, G., & Grigalevicius, S. (2021). 2,7(3,6)-Diaryl(arylamino)-substituted Carbazoles as Components of OLEDs: A Review of the Last Decade. Materials, 14(22), 6754. https://doi.org/10.3390/ma14226754






