Effect of CeO2 Size on Microstructure, Synthesis Mechanism and Refining Performance of Al-Ti-C Alloy
Abstract
:1. Introduction
2. Experiment
2.1. Quenching Experiments
2.2. Grain Refinement Experiments
3. Results and Discussion
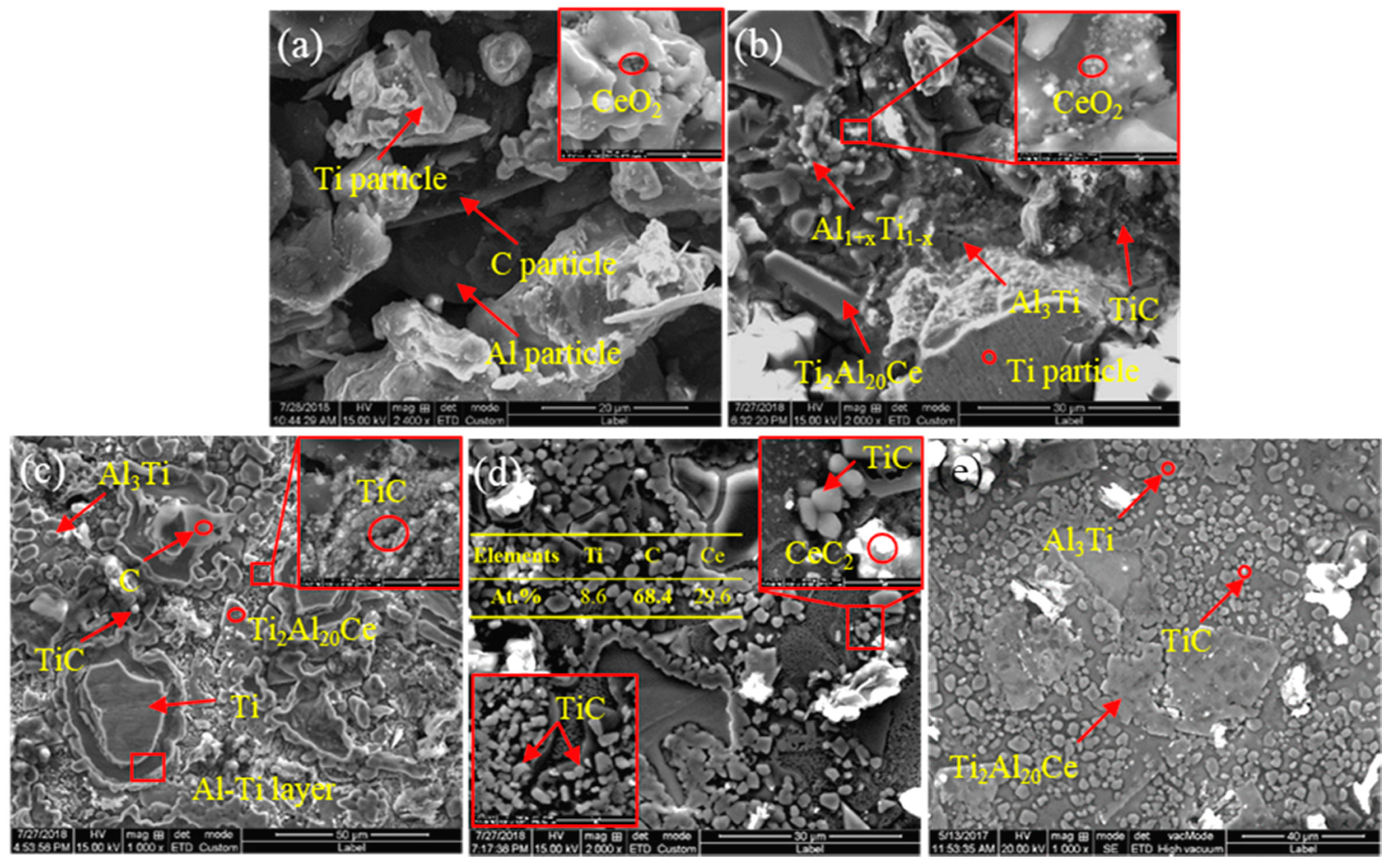
3.1. The Raw Materials of CeO2 and the Mixed Particles
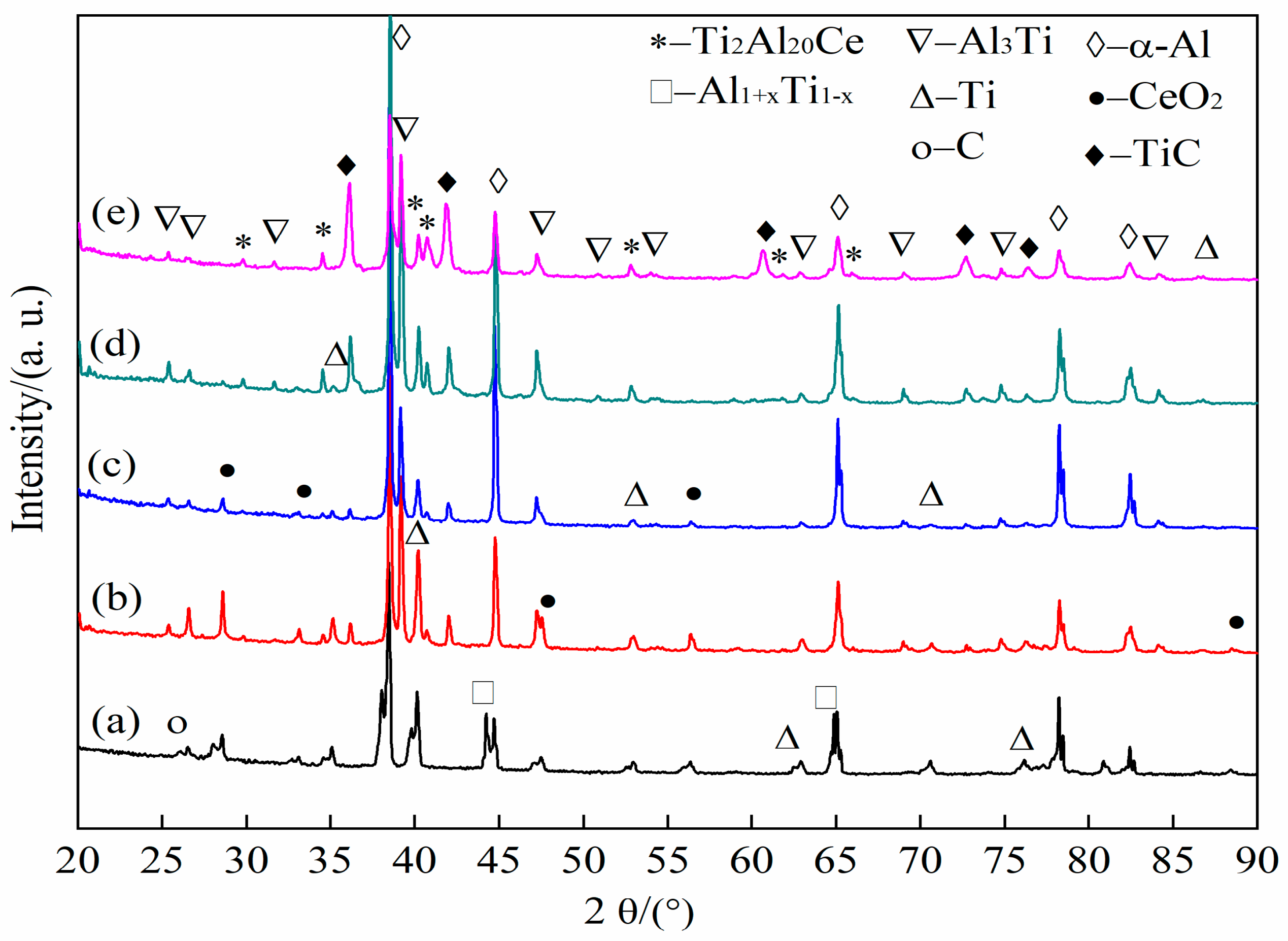
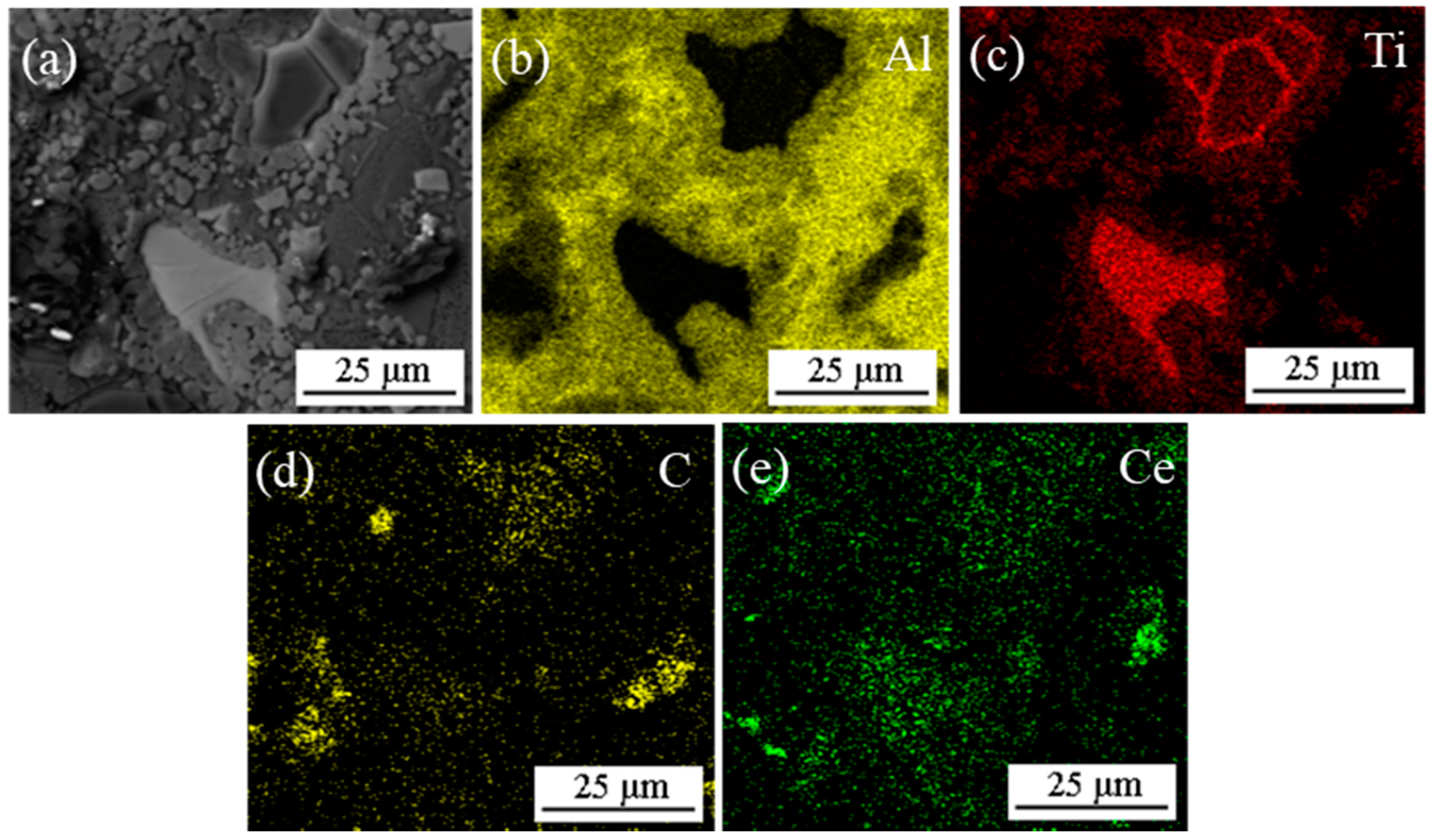
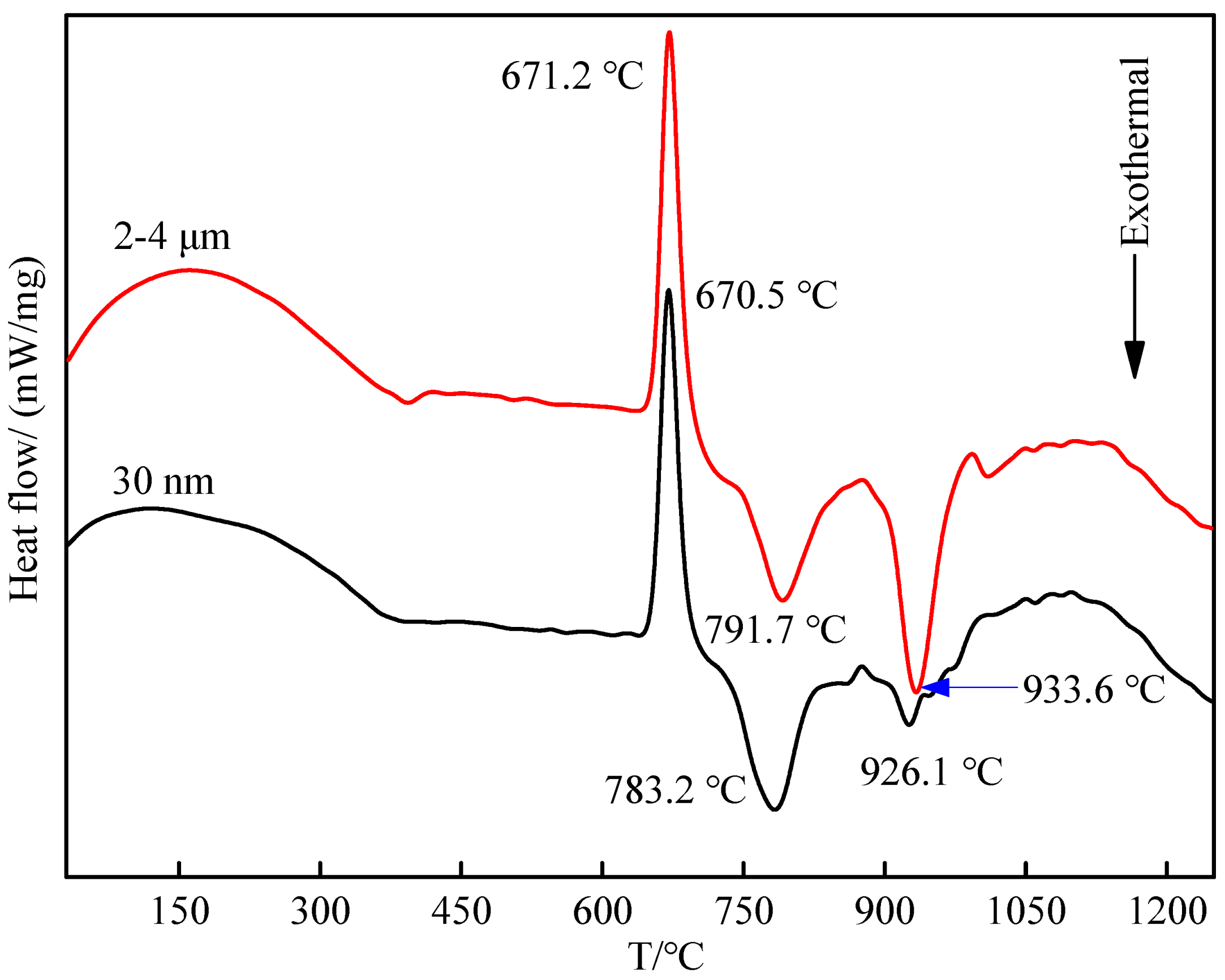
3.2. Effect of CeO2 Particle Size on Phase Transformation and Microstructure Transformation of Al-Ti-C Master Alloy during Preparation
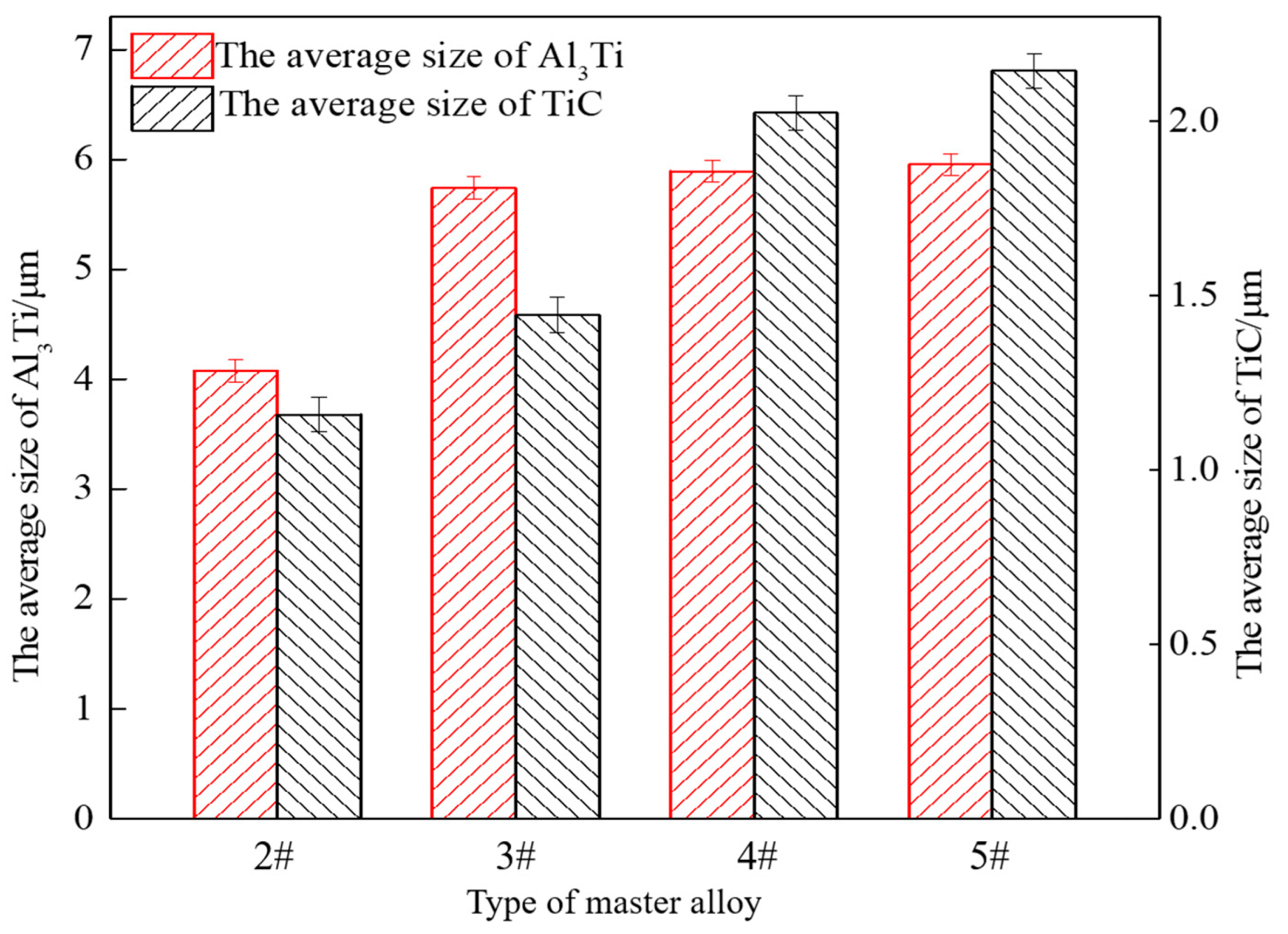
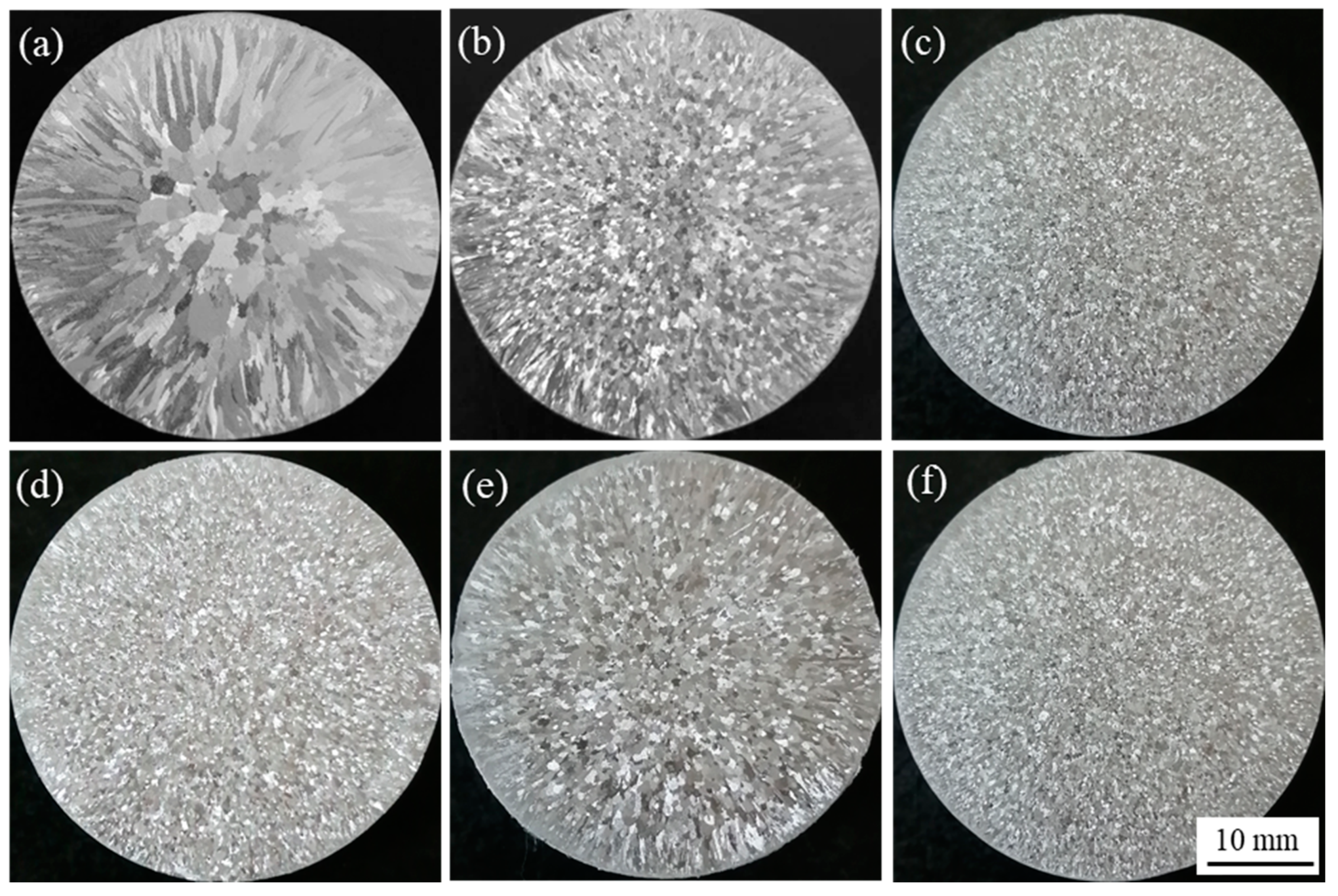
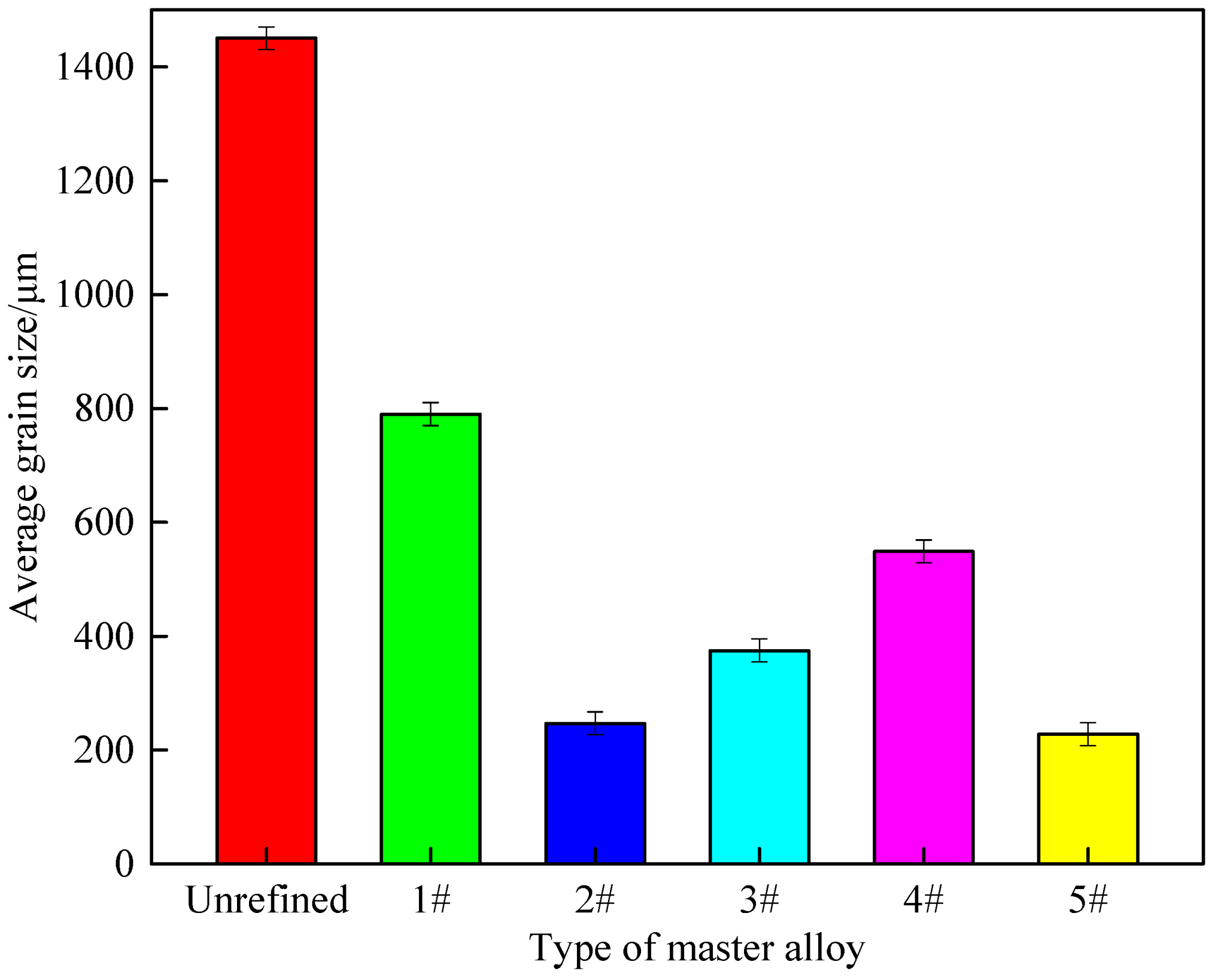
3.3. Refinement Performance Evaluation
4. Conclusions
- (1)
- The Al-Ti-C-Ce system is mainly composed of α-Al, Al3Ti, TiC and Ti2Al20Ce. The addition of CeO2 can obviously speed up the progress of the reaction, and the promotion effect of CeO2 size is 2–4 microns.
- (2)
- The addition of CeO2 can promote the uniform distribution of Al3Ti and TiC, reduce the size of Al3Ti and TiC, and the smaller the size of CeO2, the smaller the size of Al3Ti and TiC synthesized in the system.
- (3)
- The addition of CeO2 reduces the latent heat of melting of aluminum, accelerates the melting of aluminum, promotes the reaction process and lowers the formation temperature of second-phase particles. The smaller the size of CeO2, the lower the formation temperature of Al3Ti and TiC, and the smaller the heat released by the synthesis of TiC.
- (4)
- Al-Ti-C-Ce master alloy has a significant refinement effect on A 99.7. With the increase in CeO2 size, the refinement effect tends to increase first and then decrease. When the CeO2 size is 2–4 μm, Al-Ti-C-Ce master alloy has the best refining effect.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Birol, Y. Grain refining efficiency of Al-Ti-C alloys. J. Alloys Compd. 2006, 422, 128–131. [Google Scholar] [CrossRef]
- Kumar, G.; Murty, B.S.; Chakraborty, M. Development of A-Ti-C grain refiners and study of their grain refining efficiency on Al and Al-7Si alloy. J. Alloys Compd. 2005, 396, 143–150. [Google Scholar] [CrossRef]
- Xxa, B.; Yfa, B.; Hui, F.C.; Qwa, B.; Gda, B.; Gla, B. The grain refinement of 1070 alloy by different Al-Ti-B mater alloys and its influence on the electrical conductivity. Results Phys. 2019, 14, 102482. [Google Scholar]
- Wang, Y.; Fang, C.M.; Zhou, L. Mechanism for Zr poisoning of Al-Ti-B based grain refiners. Acta Mater. 2018, 164, 428–439. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Hu, B.; Liu, B. Insight into Si poisoning on grain refinement of Al-Si/Al-5Ti-B system. Acta Mater. 2020, 187, 51–65. [Google Scholar] [CrossRef]
- Peng, T.; Li, W.F.; Wang, K. Effect of Al-Ti-C master alloy addition on microstructures and mechanical properties of cast eutectic Al-Si-Fe-Cu alloy. Mater. Des. 2017, 115, 147–157. [Google Scholar]
- Li, P.T.; Liu, S.D.; Zhang, L.L. Grain refinement of A356 alloy by Al-Ti-B-C master alloy and its effect on mechanical properties. Mater. Des. 2013, 47, 522–528. [Google Scholar] [CrossRef]
- Wang, H.J.; Xu, J.; Kang, Y.L. Effect of Al-5Ti-1B-1Re on the microstructure and hot crack of as-cast Al-Zn-Mg-Cu alloy. J. Mater. Eng. Perform. 2014, 23, 1165–1172. [Google Scholar] [CrossRef]
- Zhou, X.F.; He, Y.D.; Zhang, Y.Q. The Microstructure and Solidification Mechanism of Al-Ti-C-Ce Prepared by Petroleum Coke. Mater. Sci. Forum 2019, 960, 14–20. [Google Scholar] [CrossRef]
- Ding, H.M.; Liu, X.F.; Yu, L. Influence of zirconium on grain refining efficiency of Al-Ti-C master alloys. J. Mater. Sci. 2007, 42, 9817–9821. [Google Scholar] [CrossRef]
- Doheim, M.A.; Omran, A.M.; Abdel, G.A. Evaluation of Al-Ti-C master alloys as grain refiner for aluminum and its alloys. Metall. Mater. Trans. A 2011, 42, 2862–2867. [Google Scholar] [CrossRef]
- Jiang, H.; Sun, Q.; Zhang, L. Al-Ti-C master alloy with nano-sized TiC particles dispersed in the matrix prepared by using carbon nanotubes as C source. J. Alloys Compd. 2018, 748, 774–782. [Google Scholar] [CrossRef]
- Ao, M.; Liu, H.; Dong, C. The effect of La2O3 addition on intermetallic-free aluminium matrix composites reinforced with TiC and Al2O3 ceramic particles. Ceram. Int. 2019, 45, 12001–12009. [Google Scholar] [CrossRef]
- Wang, L.D.; Wei, Z.L.; Yang, X.B. Thermodynamics analysis of Al-Ti-C-RE prepared by rare earth oxide Ce2O3. Chin. J. Nonferrous Met. 2013, 23, 2928–2935. [Google Scholar]
- Korotcenkov, G.; Al-Douri, Y. (Eds.) Metal Oxides: Powder Technologies; Elsevier: Cambridge, MA, USA, 2020. [Google Scholar]
- Auffan, M.; Rose, J.; Bottero, J.Y. Towards a definition of inorganic nanoparticles from an environmental, health and safety perspective. Nat. Nanotechnol. 2009, 4, 634. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Xing, X.; Rao, L. Interface relation between CeO2/TiC in hypereutectic Fe-Cr-C-Ti-CeO2 hardfacing alloy. Mater. Chem. Phys. 2019, 222, 181–192. [Google Scholar] [CrossRef]
- Ding, W.W.; Chen, T.L.; Zhao, X.Y. Effect of CeO2 on Microstructure and Synthesis Mechanism of Al-Ti-C Alloy. Materials 2018, 11, 2508. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.F.; Bian, X.F. Master Alloys for the Structure Refinement of Aluminium Alloys, 1st ed.; Central South University: Changsha, China, 2012; pp. 7–10, 57–64. ISBN 978-7-5487-0450-8. [Google Scholar]
- Liu, X.F.; Wang, Z.Q.; Zhang, Z.G. The relationship between microstructures and refining performances of Al-Ti-C master alloys. Mater. Sci. Eng. A 2002, 332, 70–74. [Google Scholar]
- Xu, C.; Xiao, W.L.; Zhao, W.T. Microstructure and formation mechanism of grain-refining particles in Al-Ti-C-RE grain refiners. J. Rare Earths 2015, 33, 553–560. [Google Scholar] [CrossRef]
- Wang, K.; Cui, C.X.; Wang, Q. The microstructure and formation mechanism of core-shell-like TiAl3/Ti2Al20Ce in melt-spun Al-Ti-B-Re grain refiner. Mater. Lett. 2012, 85, 153–156. [Google Scholar] [CrossRef]
- Jarfors, A.; Fredriksson, H.; Froyen, L. On the thermodynamics and kinetics of carbides in the aluminum-rich corner of the Al-Ti-C system. Mater. Sci. Eng. A 1991, 135, 119–123. [Google Scholar] [CrossRef]
- Ding, W.W.; Chen, T.L.; Zhao, X.Y. Investigation of Microstructure of Al-5Ti-0.62C System and Synthesis Mechanism of TiC. Materials 2020, 13, 310. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nie, B.X.; Liu, H.M.; Qu, Y.; H, Q.L. Effect of La2O3 on the in-situ reaction products in Al-CuO-Ti-C system. Mater. Res. Express 2019, 6, 046555. [Google Scholar] [CrossRef]
- Dong, K. (Al-) Study on Reaction Mechanism and Mechanical Properties of Copper Matrix Composites Synthesized by In-Situ Reaction of Ti-C-Cu System. Master’s Thesis, Nanjing University of Technology, Nanjing, China, 2016. [Google Scholar]
- Wang, P. Thermodynamic Analysis and Kinetic Mechanism of Ti-Al-C System. Ph.D. Thesis, Wuhan University of Technology, Wuhan, China, 2008. [Google Scholar]


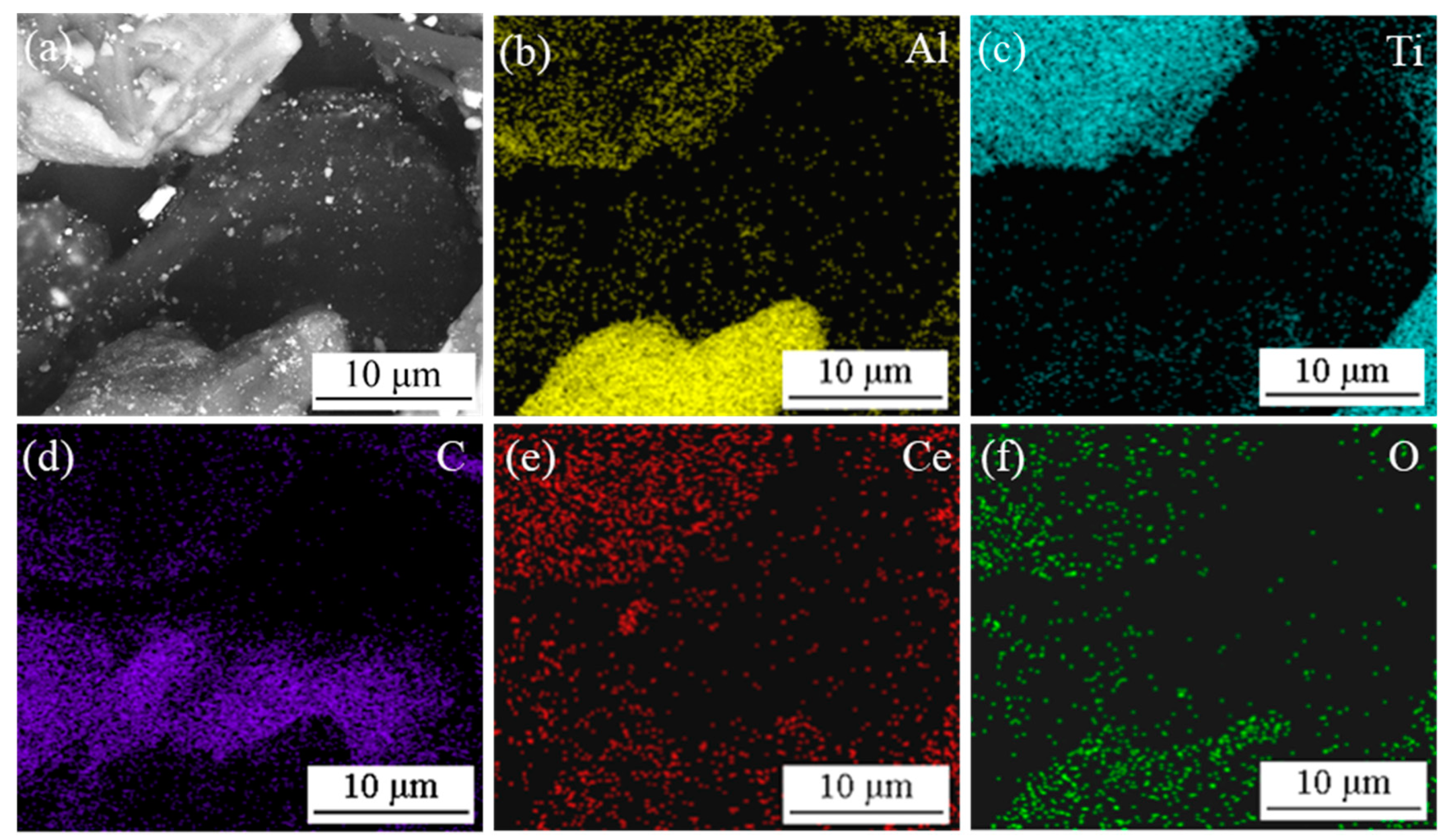
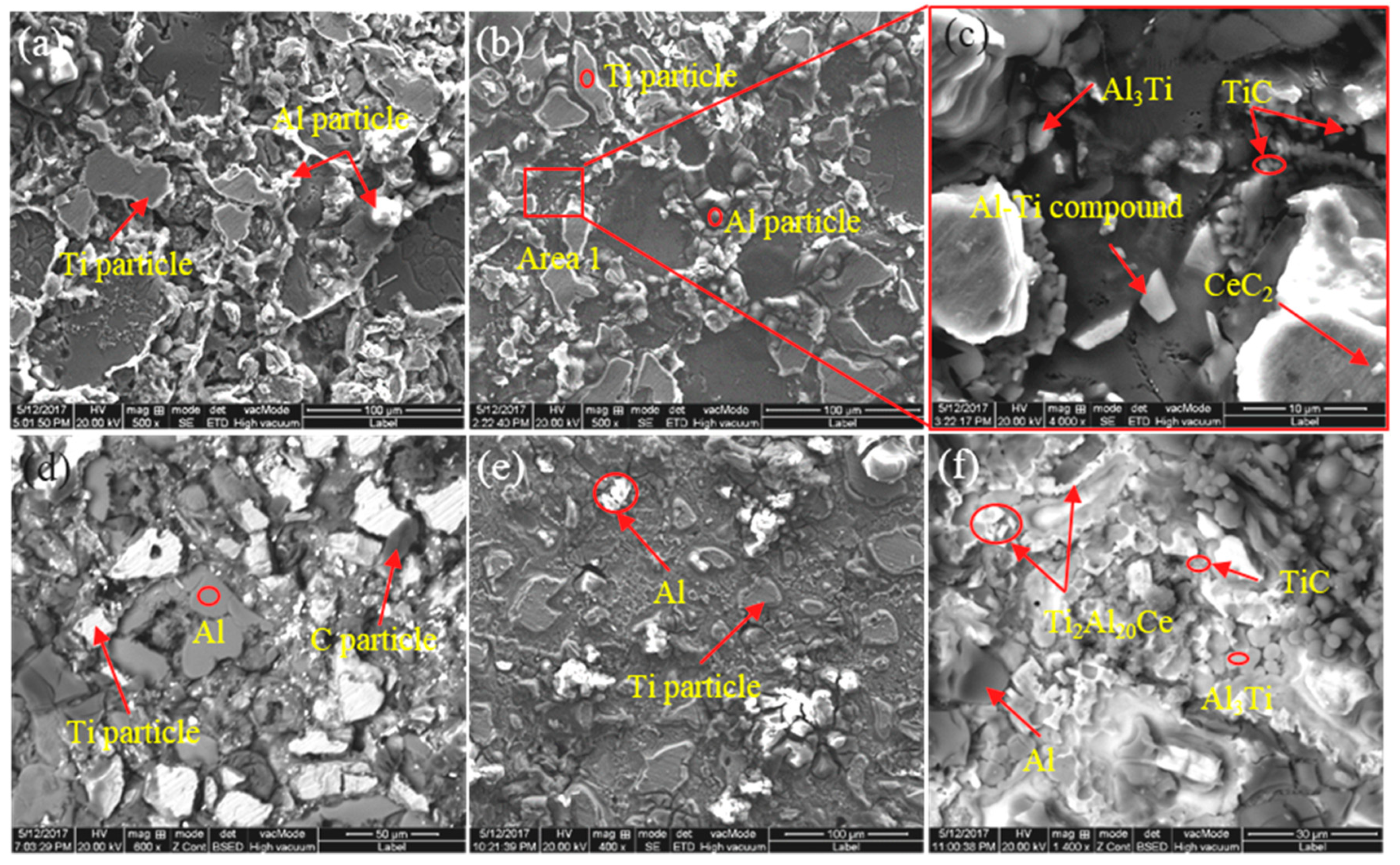
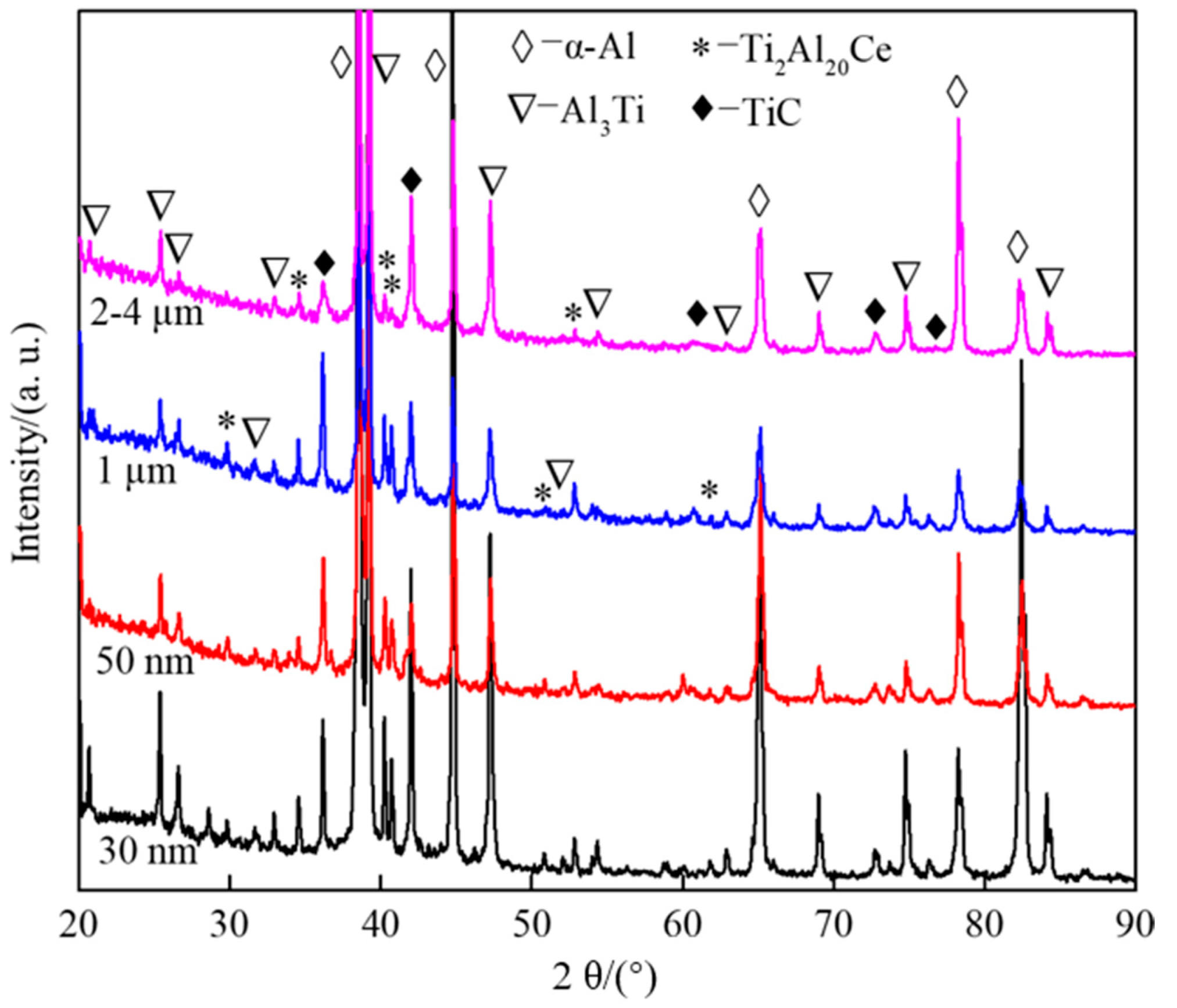
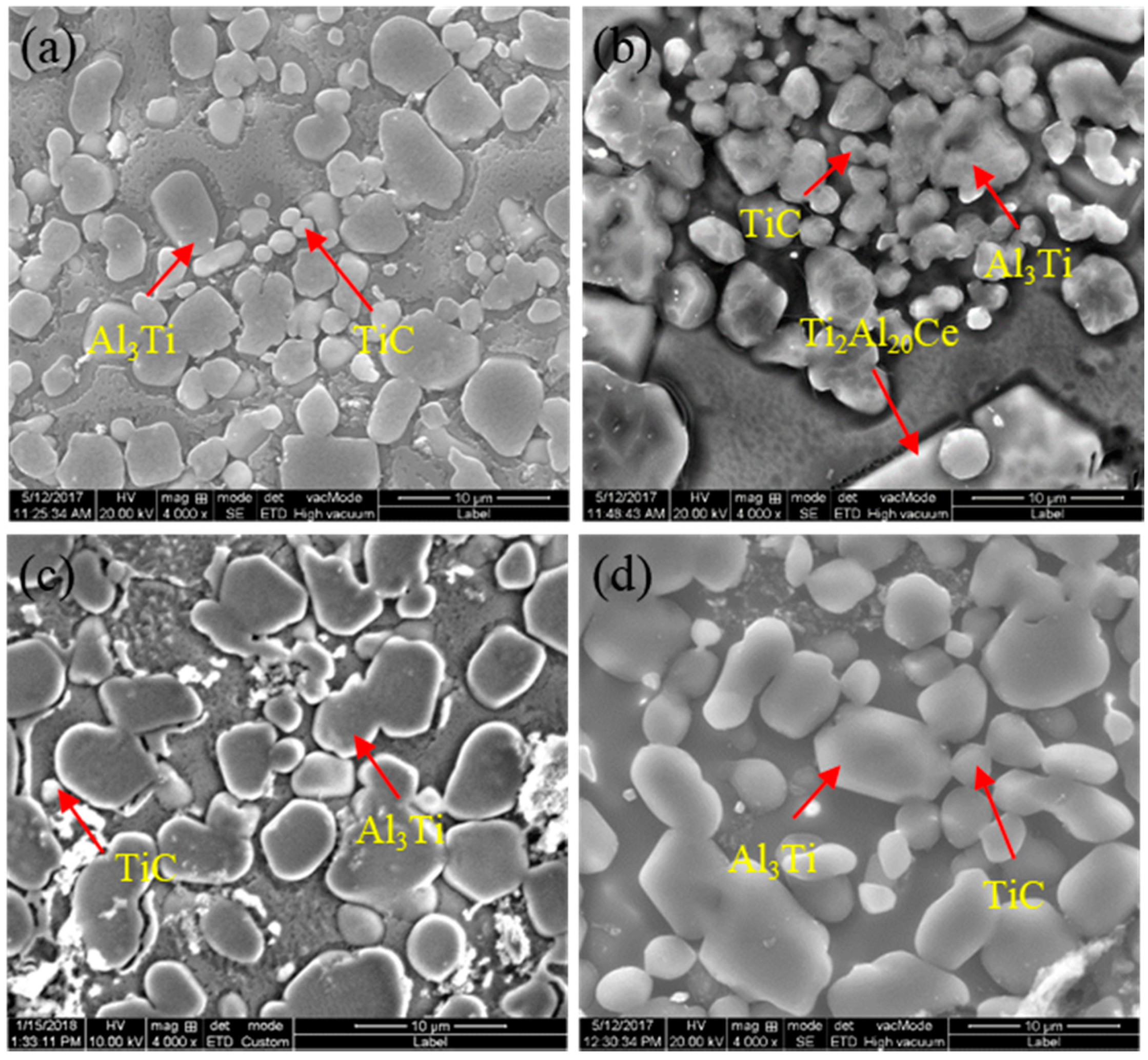
| Sample No. | Composite of Master Alloy | Size of CeO2 | Preparation Temperature/°C | Holding Time/Min |
|---|---|---|---|---|
| #1 | Al-Ti-C | None | 800 | 15 |
| #2 | Al-Ti-C-Ce | 30 nm | 800 | 15 |
| #3 | Al-Ti-C-Ce | 50 nm | 800 | 15 |
| #4 | Al-Ti-C-Ce | 1 μm | 800 | 15 |
| #5 | Al-Ti-C-Ce | 2–4 μm | 800 | 15 |
| Type of Alloy | △HAl/(J/g) | △HAl3Ti/(J/g) | △HTiC/(J/g) |
|---|---|---|---|
| Al-Ti-C | 214.7 | −163.4 | −838.8 |
| Al-Ti-C-Ce (30 nm) | 173.5 | −168.8 | −92.99 |
| Al-Ti-C-Ce (2–4 μm) | 195.5 | −137.7 | −198.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, Y.; Chen, T.; Gou, L.; Ding, W. Effect of CeO2 Size on Microstructure, Synthesis Mechanism and Refining Performance of Al-Ti-C Alloy. Materials 2021, 14, 6739. https://doi.org/10.3390/ma14226739
Ma Y, Chen T, Gou L, Ding W. Effect of CeO2 Size on Microstructure, Synthesis Mechanism and Refining Performance of Al-Ti-C Alloy. Materials. 2021; 14(22):6739. https://doi.org/10.3390/ma14226739
Chicago/Turabian StyleMa, Yanli, Taili Chen, Lumin Gou, and Wanwu Ding. 2021. "Effect of CeO2 Size on Microstructure, Synthesis Mechanism and Refining Performance of Al-Ti-C Alloy" Materials 14, no. 22: 6739. https://doi.org/10.3390/ma14226739
APA StyleMa, Y., Chen, T., Gou, L., & Ding, W. (2021). Effect of CeO2 Size on Microstructure, Synthesis Mechanism and Refining Performance of Al-Ti-C Alloy. Materials, 14(22), 6739. https://doi.org/10.3390/ma14226739






