In-Silico Conceptualisation of Continuous Millifluidic Separators for Magnetic Nanoparticles
Abstract
:1. Introduction
2. Concept and Methodology
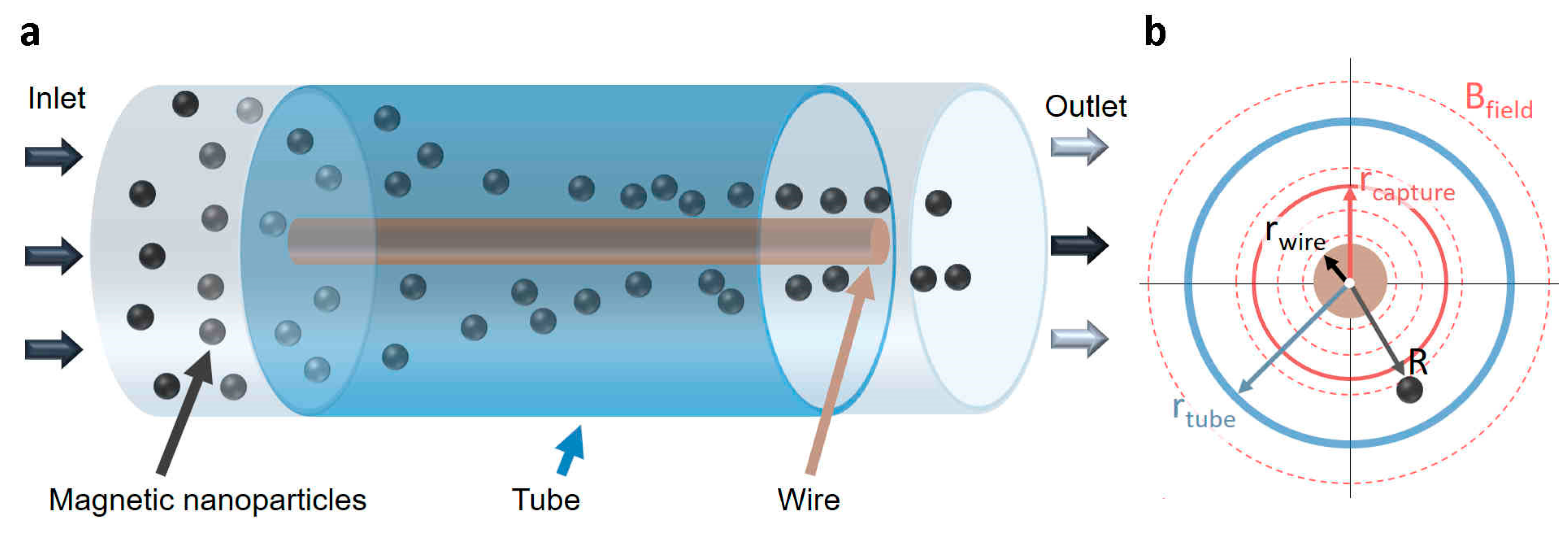
2.1. Electromagnetic Separator Designs
2.2. Modeling Magnetic Particle Transport
2.2.1. Magnetophoretic Forces
2.2.2. Drag Forces
2.2.3. Particle Tracking Algorithm
- (1)
- The particle tracking time was a tenfold of the average residence time (referring to the liquid phase) in the separator, which was determined by the flow rate and the separator channel cross section).
- (2)
- The updated axial position exceeded the separator length , i.e., the particle exited the separator.
- (3)
- The particle collided with the wire more than 1000 times, which was determined by the collision frequency counter.
2.2.4. Time-Step
2.2.5. Separation Efficiency Definition
2.2.6. Computation
3. Results
3.1. Effect of Design and Operating Parameters on Separator Efficiency
3.2. Optimum Separation Conditions for 250 nm MNPs
3.3. Optimum Separation Conditions for 500 nm MNPs
4. Conclusions and Perspective
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ayansiji, A.O.; Dighe, A.V.; Linninger, A.A.; Singh, M.R. Constitutive relationship and governing physical properties for magnetophoresis. Proc. Natl. Acad. Sci. USA 2020, 117, 30208–30214. [Google Scholar] [CrossRef]
- Gijs, M.A.M.; Lacharme, F.; Lehmann, U. Microfluidic Applications of Magnetic Particles for Biological Analysis and Catalysis. Chem. Rev. 2010, 110, 1518–1563. [Google Scholar] [CrossRef]
- Lim, B.; Vavassori, P.; Sooryakumar, R.; Kim, C. Nano/micro-scale magnetophoretic devices for biomedical applications. J. Phys. D Appl. Phys. 2016, 50, 033002. [Google Scholar] [CrossRef]
- Zaidi, N.S.; Sohaili, J.; Muda, K.; Sillanpää, M. Magnetic Field Application and its Potential in Water and Wastewater Treatment Systems. Sep. Purif. Rev. 2014, 43, 206–240. [Google Scholar] [CrossRef]
- Castelo-Grande, T.; Augusto, P.A.; Rico, J.; Marcos, J.; Iglesias, R.; Hernández, L.; Barbosa, D. Magnetic water treatment in a wastewater treatment plant: Part I sorption and magnetic particles. J. Environ. Manag. 2021, 281, 111872. [Google Scholar] [CrossRef]
- Solsona, M.; Nieuwelink, A.-E.; Meirer, F.; Abelmann, L.; Odijk, M.; Olthuis, W.; Weckhuysen, B.M.; Berg, A.V.D. Magnetophoretic Sorting of Single Catalyst Particles. Angew. Chem. Int. Ed. 2018, 57, 10589–10594. [Google Scholar] [CrossRef]
- Rossi, L.M.; Costa, N.J.S.; Silva, F.P.; Wojcieszak, R. Magnetic nanomaterials in catalysis: Advanced catalysts for magnetic separation and beyond. Green Chem. 2014, 16, 2906–2933. [Google Scholar] [CrossRef]
- Munaz, A.; Shiddiky, M.; Nguyen, N.-T. Recent advances and current challenges in magnetophoresis based micro magnetofluidics. Biomicrofluidics 2018, 12, 031501. [Google Scholar] [CrossRef]
- Song, K.; Li, G.; Zu, X.; Du, Z.; Liu, L.; Hu, Z. The Fabrication and Application Mechanism of Microfluidic Systems for High Throughput Biomedical Screening: A Review. Micromachines 2020, 11, 297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xi, H.-D.; Zheng, H.; Guo, W.; Gañán-Calvo, A.M.; Ai, Y.; Tsao, C.-W.; Zhou, J.; Li, W.; Huang, Y.; Nguyen, N.-T.; et al. Active droplet sorting in microfluidics: A review. Lab Chip 2017, 17, 751–771. [Google Scholar] [CrossRef]
- Banerjee, U.; Mandal, C.; Jain, S.K.; Sen, A.K. Cross-stream migration and coalescence of droplets in a microchannel co-flow using magnetophoresis. Phys. Fluids 2019, 31, 112003. [Google Scholar] [CrossRef]
- Zhou, R.; Bai, F.; Wang, C. Magnetic separation of microparticles by shape. Lab Chip 2017, 17, 401–406. [Google Scholar] [CrossRef] [PubMed]
- Lin, G.; Makarov, D.; Schmidt, O.G. Magnetic sensing platform technologies for biomedical applications. Lab Chip 2017, 17, 1884–1912. [Google Scholar] [CrossRef]
- Chircov, C.; Grumezescu, A.M.; Holban, A.M. Magnetic Particles for Advanced Molecular Diagnosis. Materials 2019, 12, 2158. [Google Scholar] [CrossRef] [Green Version]
- Hejazian, M.; Nguyen, N.-T. Negative magnetophoresis in diluted ferrofluid flow. Lab Chip 2015, 15, 2998–3005. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Munaz, A.; Kamble, H.; Shiddiky, M.J.A.; Nguyen, N.-T. Magnetofluidic micromixer based on a complex rotating magnetic field. RSC Adv. 2017, 7, 52465–52474. [Google Scholar] [CrossRef] [Green Version]
- Pamme, N.; Wilhelm, C. Continuous sorting of magnetic cells via on-chip free-flow magnetophoresis. Lab Chip 2006, 6, 974–980. [Google Scholar] [CrossRef] [PubMed]
- Hejazian, M.; Li, W.; Nguyen, N.-T. Lab on a chip for continuous-flow magnetic cell separation. Lab Chip 2015, 15, 959–970. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Q.; Yin, T.; Xu, R.; Gao, W.; Zhao, H.; Shapter, J.G.; Wang, K.; Shen, Y.; Huang, P.; Gao, G.; et al. Large-scale immuno-magnetic cell sorting of T cells based on a self-designed high-throughput system for potential clinical application. Nanoscale 2017, 9, 13592–13599. [Google Scholar] [CrossRef] [Green Version]
- Ngamsom, B.; Esfahani, M.M.N.; Phurimsak, C.; Lopez-Martinez, M.J.; Raymond, J.-C.; Broyer, P.; Patel, P.; Pamme, N. Multiplex sorting of foodborne pathogens by on-chip free-flow magnetophoresis. Anal. Chim. Acta 2016, 918, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Myklatun, A.; Cappetta, M.; Winklhofer, M.; Ntziachristos, V.; Westmeyer, G.G. Microfluidic sorting of intrinsically magnetic cells under visual control. Sci. Rep. 2017, 7, 6942. [Google Scholar] [CrossRef] [Green Version]
- Ge, W.; Encinas, A.; Araujo, E.; Song, S. Magnetic matrices used in high gradient magnetic separation (HGMS): A review. Results Phys. 2017, 7, 4278–4286. [Google Scholar] [CrossRef]
- Lim, J.; Yeap, S.P.; Low, S.C. Challenges associated to magnetic separation of nanomaterials at low field gradient. Sep. Purif. Technol. 2014, 123, 171–174. [Google Scholar] [CrossRef]
- Leong, S.S.; Yeap, S.P.; Lim, J. Working principle and application of magnetic separation for biomedical diagnostic at high- and low-field gradients. Interface Focus 2016, 6, 20160048. [Google Scholar] [CrossRef] [Green Version]
- Zeng, L.; Chen, X.; Du, J.; Yu, Z.; Zhang, R.; Zhang, Y.; Yang, H. Label-free separation of nanoscale particles by an ultrahigh gradient magnetic field in a microfluidic device. Nanoscale 2021, 13, 4029–4037. [Google Scholar] [CrossRef]
- Chen, Q.; Li, D.; Lin, J.; Wang, M.; Xuan, X. Simultaneous Separation and Washing of Nonmagnetic Particles in an Inertial Ferrofluid/Water Coflow. Anal. Chem. 2017, 89, 6915–6920. [Google Scholar] [CrossRef] [PubMed]
- Eskandarpour, A.; Iwai, K.; Asai, S. Superconducting Magnetic Filter: Performance, Recovery, and Design. IEEE Trans. Appl. Supercond. 2009, 19, 84–95. [Google Scholar] [CrossRef]
- Moeser, G.D.; Roach, K.A.; Green, W.H.; Hatton, T.A.; Laibinis, P.E. High-gradient magnetic separation of coated magnetic nanoparticles. AIChE J. 2004, 50, 2835–2848. [Google Scholar] [CrossRef]
- Toh, P.Y.; Yeap, S.P.; Kong, L.P.; Ng, B.W.; Chan, D.J.C.; Ahmad, A.L.; Lim, J. Magnetophoretic removal of microalgae from fishpond water: Feasibility of high gradient and low gradient magnetic separation. Chem. Eng. J. 2012, 211–212, 22–30. [Google Scholar] [CrossRef]
- Mayo, J.T.; Yavuz, C.T.; Yean, S.; Cong, L.; Shipley, H.; Yu, W.; Falkner, J.; Kan, A.; Tomson, M.; Colvin, V.L. The effect of nanocrystalline magnetite size on arsenic removal. Sci. Technol. Adv. Mater. 2007, 8, 71–75. [Google Scholar] [CrossRef] [Green Version]
- Surenjav, E.; Priest, C.; Herminghaus, S.; Seemann, R. Manipulation of gel emulsions by variable microchannel geometry. Lab Chip 2009, 9, 325–330. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Kilinc, D.; Ran, Y.-F.; Lee, G.U. Flow enhanced non-linear magnetophoretic separation of beads based on magnetic susceptibility. Lab Chip 2013, 13, 4400. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Zhu, Z.; Xiang, N.; Long, F.; Ni, Z. Automated Microfluidic Instrument for Label-Free and High-Throughput Cell Separation. Anal. Chem. 2018, 90, 4212–4220. [Google Scholar] [CrossRef] [PubMed]
- Banis, G.; Tyrovolas, K.; Angelopoulos, S.; Ferraro, A.; Hristoforou, E. Pushing of Magnetic Microdroplet Using Electromagnetic Actuation System. Nanomaterials 2020, 10, 371. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, T.; Li, J.; Morozov, K.I.; Wu, Z.; Xu, T.; Rozen, I.; Leshansky, A.M.; Li, L.; Wang, J. Highly Efficient Freestyle Magnetic Nanoswimmer. Nano Lett. 2017, 17, 5092–5098. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Tang, W.; Mu, G.; Wang, H.; Chang, X.; Dong, H.; Qi, L.; Zhang, G.; Li, T. Micro-/Nanorobots Propelled by Oscillating Magnetic Fields. Micromachines 2018, 9, 540. [Google Scholar] [CrossRef] [Green Version]
- TSSF005.00 Wire Size & Current Rating Guide. Available online: www.jst.fr/doc/jst/pdf/current_rating.pdf (accessed on 31 October 2021).
- Jackson, J.D. Classical Electrodynamics, 3rd ed.; Wiley: Hoboken, NJ, USA, 1998; Available online: https://www.wiley.com/en-gb/Classical+Electrodynamics%2C+3rd+Edition-p-9780471309321 (accessed on 22 September 2021).
- Natukunda, F.; Twongyirwe, T.M.; Schiff, S.J.; Obungoloch, J. Approaches in cooling of resistive coil-based low-field Magnetic Resonance Imaging (MRI) systems for application in low resource settings. BMC Biomed. Eng. 2021, 3, 3. [Google Scholar] [CrossRef] [PubMed]
- Ansorge, R. Magnetic Field Generation. Phys. Math. MRI 2016. [CrossRef]
- Heider, F.; Zitzelsberger, A.; Fabian, K. Magnetic susceptibility and remanent coercive force in grown magnetite crystals from 0.1 μm to 6 mm. Phys. Earth Planet. Inter. 1996, 93, 239–256. [Google Scholar] [CrossRef]
- Sparrow, E.; Chen, T.; Jónsson, V. Laminar flow and pressure drop in internally finned annular ducts. Int. J. Heat Mass Transf. 1964, 7, 583–585. [Google Scholar] [CrossRef]
- Schaller, V.; Kräling, U.; Rusu, C.; Petersson, K.; Wipenmyr, J.; Krozer, A.; Wahnström, G.; Sanz-Velasco, A.; Enoksson, P.; Johansson, C. Motion of nanometer sized magnetic particles in a magnetic field gradient. J. Appl. Phys. 2008, 104, 093918. [Google Scholar] [CrossRef]
- Sinha, A.; Ganguly, R.; Puri, I.K. Magnetic separation from superparamagnetic particle suspensions. J. Magn. Magn. Mater. 2009, 321, 2251–2256. [Google Scholar] [CrossRef]
- Orenstein, W.A.; Bernier, R.H.; Dondero, T.J.; Hinman, A.R.; Marks, J.S.; Bart, K.J.; Sirotkin, B. Field evaluation of vaccine effi-cacy. Bull. World Health Organ. 1985, 63, 1055–1068. [Google Scholar]
- Leong, S.S.; Ahmad, Z.; Low, S.C.; Camacho, J.; Faraudo, J.; Lim, J. Unified View of Magnetic Nanoparticle Separation under Magnetophoresis. Langmuir 2020, 36, 8033–8055. [Google Scholar] [CrossRef] [PubMed]
- Leong, S.S.; Ahmad, Z.; Lim, J. Magnetophoresis of superparamagnetic nanoparticles at low field gradient: Hydrodynamic effect. Soft Matter 2015, 11, 6968–6980. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wen, Y.; Jiang, D.; Gavriilidis, A.; Besenhard, M.O. In-Silico Conceptualisation of Continuous Millifluidic Separators for Magnetic Nanoparticles. Materials 2021, 14, 6635. https://doi.org/10.3390/ma14216635
Wen Y, Jiang D, Gavriilidis A, Besenhard MO. In-Silico Conceptualisation of Continuous Millifluidic Separators for Magnetic Nanoparticles. Materials. 2021; 14(21):6635. https://doi.org/10.3390/ma14216635
Chicago/Turabian StyleWen, Yanzhe, Dai Jiang, Asterios Gavriilidis, and Maximilian O. Besenhard. 2021. "In-Silico Conceptualisation of Continuous Millifluidic Separators for Magnetic Nanoparticles" Materials 14, no. 21: 6635. https://doi.org/10.3390/ma14216635
APA StyleWen, Y., Jiang, D., Gavriilidis, A., & Besenhard, M. O. (2021). In-Silico Conceptualisation of Continuous Millifluidic Separators for Magnetic Nanoparticles. Materials, 14(21), 6635. https://doi.org/10.3390/ma14216635








