Effect of the Structural and Morphological Properties of Surfactant-Assisted Hydroxyapatite on Dermal Irritation and Antibacterial Activity
Abstract
1. Introduction
2. Materials and Methods
2.1. Hydroxyapatite Synthesis
2.2. Characterization Techniques
2.3. Antibacterial Activity
2.4. Dermal Irritation Procedure
3. Results
3.1. Structural and Morphological Characterization
3.2. Morphological Studies
3.3. Antibacterial Activity
3.4. Dermal Irritation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, Y.; Zhang, S.; Wei, K.; Zhao, N.; Chen, J.; Wang, X. Hydrothermal synthesis of hydroxyapatite nanopowders using cationic surfactant as a template. Mater. Lett. 2006, 60, 1484–1487. [Google Scholar] [CrossRef]
- Zhao, X.Y.; Zhu, Y.J.; Chen, F.; Lu, B.Q.; Qi, C.; Zhao, J.; Wu, J. Hydrothermal synthesis of hydroxyapatite nanorods and nanowires using riboflavin-5′-phosphate monosodium salt as a new phosphorus source and their application in protein adsorption. CrystEngComm 2013, 15, 7926–7935. [Google Scholar] [CrossRef]
- Curtin, C.M.; Cunniffe, G.M.; Lyons, F.G.; Bessho, K.; Dickson, G.R.; Duffy, G.P.; O'Brien, F.J. Innovative Collagen Nano-Hydroxyapatite Scaffolds Offer a Highly Efficient Non-Viral Gene Delivery Platform for Stem Cell-Mediated Bone Formation. Adv. Mater. 2012, 24, 749–754. [Google Scholar] [CrossRef]
- Matsumoto, T.; Okazaki, M.; Inoue, M.; Yamaguchi, S.; Kusunose, T.; Toyonaga, T.; Hamada, Y.; Takahashi, J. Hydroxyapatite particles as a controlled release carrier of protein. Biomaterials 2004, 25, 3807–3812. [Google Scholar] [CrossRef]
- Yang, P.; Quan, Z.; Li, C.; Kang, X.; Lian, H.; Lin, J. Bioactive, luminescent and mesoporous europium-doped hydroxyapatite as a drug carrier. Biomaterials 2008, 29, 4341–4347. [Google Scholar] [CrossRef]
- Kalita, S.J.; Bhardwaj, A.; Bhatt, H.A. Nanocrystalline calcium phosphate ceramics in biomedical engineering. Mater. Sci. Eng. C 2007, 27, 441–449. [Google Scholar] [CrossRef]
- Iqbal, N.; Kadir, M.R.A.; Mahmood, N.H.; Salim, N.; Froemming, G.; Balaji, H.; Kamarul, T. Characterization, antibacterial and in vitro compatibility of zinc–silver doped hydroxyapatite nanoparticles prepared through microwave synthesis. Ceram. Int. 2014, 40, 4507–4513. [Google Scholar] [CrossRef]
- Lamkhao, S.; Phaya, M.; Jansakun, C.; Chandet, N.; Thongkorn, K.; Rujijanagul, G.; Bangrak, P.; Randorn, C. Synthesis of Hydroxyapatite with Antibacterial Properties Using a Microwave-Assisted Combustion Method. Sci. Rep. 2019, 9, 4015. [Google Scholar] [CrossRef]
- Sims, C.R.; Paetznick, V.L.; Rodriguez, J.R.; Chen, E.; Ostrosky-Zeichner, L. Correlation between microdilution, E-test, and disk diffusion methods for antifungal susceptibility testing of posaconazole against Candida spp. J. Clin. Microbiol. 2006, 44, 2105–2108. [Google Scholar] [CrossRef]
- Qi, M.L.; Xiao, G.Y.; Lu, Y.P. Rapid hydrothermal synthesis of submillimeter ultralong flexible hydroxyapatite fiber using different pH regulators. Acta Metall. Sin. Engl. Lett. 2016, 29, 609–613. [Google Scholar] [CrossRef]
- Ashok, M.; Kalkura, S.N.; Sundaram, N.M.; Arivuoli, D. Growth and characterization of hydroxyapatite crystals by hydrothermal method. J. Mater. Sci. Mater. Med. 2007, 18, 895–898. [Google Scholar] [CrossRef]
- López-Ortiz, S.; Mendoza-Anaya, D.; Sánchez-Campos, D.; Fernandez-García, M.E.; Salinas-Rodríguez, E.; Reyes-Valderrama, M.I.; Rodríguez-Lugo, V. The pH Effect on the Growth of Hexagonal and Monoclinic Hydroxyapatite Synthesized by the Hydrothermal Method. J. Nanomater. 2020, 2020, 5912592. [Google Scholar] [CrossRef]
- Singh, G.; Singh, R.P.; Jolly, S.S. Customized hydroxyapatites for bone-tissue engineering and drug delivery applications: A review. J. Sol-Gel Sci. Technol. 2020, 94, 505–530. [Google Scholar] [CrossRef]
- Bricha, M.; Belmamouni, Y.; Essassi, E.M.; Ferreira, J.M.; Mabrouk, K.E. Surfactant-Assisted Hydrothermal Synthesis of Hydroxyapatite Nanopowders. J. Nanosci. Nanotechnol. 2012, 12, 8042–8049. [Google Scholar] [CrossRef]
- Gopi, D.; Indira, J.; Nithiya, S.; Kavitha, L.; Mudali, U.K.; Kanimozhi, K. Influence of surfactant concentration on nanohydroxyapatite growth. Bull. Mater. Sci. 2013, 36, 799–805. [Google Scholar] [CrossRef]
- Chow, L.C. Next generation calcium phosphate-based biomaterials. Dent. Mater. J. 2009, 28, 1–10. [Google Scholar] [CrossRef]
- Londoño-restrepo, S.M.; Jeronimo-cruz, R.; Millán-malo, B.M.; Rivera-muñoz, E.M.; Rodriguez-garcía, M.E. Effect of the Nano Crystal Size on the X-ray Diffraction Patterns of Biogenic Hydroxyapatite from Human, Bovine, and Porcine Bones. Sci. Rep. 2019, 9, 5915. [Google Scholar] [CrossRef]
- Motskin, M.; Wright, D.; Muller, K.; Kyle, N.; Gard, T.; Porter, A.; Skepper, J. Hydroxyapatite nano and microparticles: Correlation of particle properties with cytotoxicity and biostability. Biomaterials 2009, 30, 3307–3317. [Google Scholar] [CrossRef]
- Eilbagi, M.; Emadi, R.; Raeissi, K.; Kharaziha, M.; Valiani, A. Mechanical and cytotoxicity evaluation of nanostructured hydroxyapatite-bredigite scaffolds for bone regeneration. Mater. Sci. Eng. C 2016, 68, 603–612. [Google Scholar] [CrossRef]
- Rabiei, M.; Palevicius, A.; Monshi, A.; Nasiri, S.; Vilkauskas, A.; Janusas, G. Comparing Methods for Calculating Nano Crystal Size of Natural Hydroxyapatite Using X-Ray Diffraction. Nanomaterials 2020, 10, 1627. [Google Scholar] [CrossRef]
- Campbell, B. Technical section. Ann. R. Coll. Surg. Engl. 2013, 95, 532. [Google Scholar] [CrossRef]
- Phatai, P.; Futalan, C.M.; Utara, S.; Khemthong, P.; Kamonwannasit, S. Structural characterization of cerium-doped hydroxyapatite nanoparticles synthesized by an ultrasonic-assisted sol-gel technique. Results Phys. 2018, 10, 956–963. [Google Scholar] [CrossRef]
- Sillen, A.; Sealy, J.C. Diagenesis of Strontium in Fossil Bone: A Reconsideration of Nelson et al. (1986). J. Archaeol. Sci. 1995, 22, 313–320. [Google Scholar] [CrossRef]
- In, Y.; Amornkitbamrung, U.; Hong, M.; Shin, H. On the Crystallization of Hydroxyapatite under Hydrothermal Conditions: Role of Sebacic Acid as an Additive. ACS Omega 2020, 5, 27204–27210. [Google Scholar] [CrossRef]
- Scherrer, P. Bestimmung der inneren Struktur und der Größe von Kolloidteilchen mittels Röntgenstrahlen. Kolloidchem. Ein Lehrb. 1912, 277, 387–409. [Google Scholar] [CrossRef]
- Djuriši, A.B.; Leung, Y.H.; Tam, K.H.; Ding, L.; Ge, K.; Chen, H.; Gwo, S. Green, yellow, and orange defect emission from ZnO nanostructures: Influence of excitation wavelength. Appl. Phys. Lett. 2006, 88, 103107. [Google Scholar] [CrossRef]
- Irfan, H.; Racik K, M.; Anand, S. Microstructural evaluation of CoAl2O4 nanoparticles by Williamson–Hall and size–strain plot methods. J. Asian Ceram. Soc. 2018, 6, 54–62. [Google Scholar] [CrossRef]
- Devesa, S.; Rooney, A.P.; Graça, M.P.; Cooper, D.; Costa, L.C. Williamson-hall analysis in estimation of crystallite size and lattice strain in Bi1.34Fe0.66Nb1.34O6.35 prepared by the sol-gel method. Mater. Sci. Eng. B 2021, 263, 114830. [Google Scholar] [CrossRef]
- Liao, J.G.; Li, Y.Q.; Duan, X.Z.; Liu, Q. Synthesis and Characterization of CO32− Doping Nano Hydroxyapatite. Spectrosc. Spectr. Anal. 2014, 34, 3011–3014. [Google Scholar]
- Okada, M.; Fujiwara, K.; Uehira, M.; Matsumoto, N.; Takeda, S. Expansion of nanosized pores in low-crystallinity nanoparticle-assembled plates via a thermally induced increase in solid-state density. J. Colloid Interface Sci. 2013, 405, 58–63. [Google Scholar] [CrossRef]
- Yang, Y.; Li, X.; Feng, W.; Yang, W.; Li, W.; Tao, C. Effect of surfactants on morphology and luminescent properties of CaMoO4: Eu3+ red phosphors. J. Alloys Compd. 2011, 509, 845–848. [Google Scholar] [CrossRef]
- Kunzelmann, U.; Schumacher, H.; Künzelmann, U.; Schumacher, H. Characterization of surface processes during oxide CMP by in situ FTIR spectroscopy. In Advances in Chemical Mechanical Planarization (CMP); Woodhead Publishing: Sawston, UK, 2016; pp. 359–396. ISBN 9780081001653. [Google Scholar]
- Okabayashi, R.; Nakamura, M.; Okabayashi, T.; Tanaka, Y.; Nagai, A.; Yamashita, K. Efficacy of polarized hydroxyapatite and silk fibroin composite dressing gel on epidermal recovery from full-thickness skin wounds. J. Biomed. Mater. Res. B Appl. Biomater. 2009, 90, 641–646. [Google Scholar] [CrossRef]
- Habraken, W.; Habibovic, P.; Epple, M.; Bohner, M. Calcium phosphates in biomedical applications: Materials for the future? Mater. Today 2016, 16, 69–87. [Google Scholar] [CrossRef]
- Ishikawa, T.; Wakamura, M.; Kondo, S. Surface Characterization of Calcium Hydroxylapatite by Fourier Transform Infrared Spectroscopy. Langmuir 1989, 5, 140–144. [Google Scholar] [CrossRef]
- Ślósarczyk, A.; Paszkiewicz, Z.; Paluszkiewicz, C. FTIR and XRD evaluation of carbonated hydroxyapatite powders synthesized by wet methods. J. Mol. Struct. 2005, 744, 657–661. [Google Scholar] [CrossRef]
- Gautam, C.; Yadav, A.K.; Singh, A.K. A Review on Infrared Spectroscopy of Borate Glasses with Effects of Different Additives. ISRN Ceram. 2012, 2012, 428497. [Google Scholar] [CrossRef]
- Zhao, Z.; Zhang, L.; Dai, H.; Du, Y.; Meng, X.; Zhang, R.; Liu, Y.; Deng, J. Microporous and Mesoporous Materials Surfactant-assisted solvo- or hydrothermal fabrication and characterization of high-surface-area porous calcium carbonate with multiple morphologies. Microporous Mesoporous Mater. 2011, 138, 191–199. [Google Scholar] [CrossRef]
- Sanosh, K.P.; Chu, M.C.; Balakrishnan, A.; Lee, Y.J.; Kim, T.N.; Cho, S.J. Synthesis of nano hydroxyapatite powder that simulate teeth particle morphology and composition. Curr. Appl. Phys. 2009, 9, 1459–1462. [Google Scholar] [CrossRef]
- Hajimirzaee, S.; Chansai, S.; Hardacre, C.; Banks, C.E.; Doyle, A.M. Effects of surfactant on morphology, chemical properties and catalytic activity of hydroxyapatite. J. Solid State Chem. 2019, 276, 345–351. [Google Scholar] [CrossRef]
- Cao, X.L.; Cheng, C.; Ma, Y.L.; Zhao, C.S. Preparation of silver nanoparticles with antimicrobial activities and the researches of their biocompatibilities. J. Mater. Sci. Mater. Med. 2010, 21, 2861–2868. [Google Scholar] [CrossRef]
- Ruparelia, J.P.; Chatterjee, A.K.; Duttagupta, S.P.; Mukherji, S. Strain specificity in antimicrobial activity of silver and copper nanoparticles. Acta Biomater. 2008, 4, 707–716. [Google Scholar] [CrossRef]
- Spencer, R.C. Predominant Pathogens Found in the European Prevalence of Infection in Intensive Care Study. Eur. J. Clin. Microbiol. Infect. Dis. 1996, 15, 281–285. [Google Scholar] [CrossRef]
- Chen, L.; Mccrate, J.M.; Lee, J.C.M.; Li, H. The role of surface charge on the uptake and biocompatibility of hydroxyapatite nanoparticles with osteoblast cells. Nanotechnology 2011, 22, 105708. [Google Scholar] [CrossRef] [PubMed]
- Guzman, M.; Dille, J.; Godet, S. Synthesis and antibacterial activity of silver nanoparticles against gram-positive and gram-negative bacteria. Nanomed. Nanotechnol. Biol. Med. 2012, 8, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Cristine, K.; Pontes, S.; Duarte, T.S.; Correna, E. Avaliação Do Efeito Osteoindutor Da Hidroxiapatita E Do Biovidro Implantados Em Tecido Subcutâneo De Cão. Rev. Ceres 2007, 54, 492–500. [Google Scholar]
- Kwon, B.-J.; Kim, J.; Kim, Y.H.; Lee, M.H.; Baek, H.S.; Lee, D.H.; Kim, H.-L.; Seo, H.J.; Lee, M.H.; Kwon, S.-Y.; et al. Biological Advantages of Porous Hydroxyapatite Scaffold Made by Solid Freeform Fabrication for Bone Tissue Regeneration. Artif. Organs 2013, 37, 663–670. [Google Scholar] [CrossRef] [PubMed]
- Sepúlveda, R.V.; Pacheco, A.; Gonçalves, L.; Luciani, F.; Carlo, E.C.; Bitencourt, I. Composite synthetic hydroxyapatite 30%, in two physical states, as dermal filler. Rev. Ceres 2013, 60, 458–464. [Google Scholar] [CrossRef]
- Stewart, M.; Webster, J.R.; Schaefer, A.L.; Cook, N.J.; Scott, S.L. Infrared thermography as a non-invasive tool to study animal welfare. Anim. Welf. 2005, 14, 319–325. [Google Scholar]
- Sinha, R.; Bhakat, M.; Mohanty, T.K.; Ranjan, A.; Kumar, R.; Lone, S.A. Infrared thermography as non-invasive technique for early detection of mastitis in dairy animals-A review. Asian J. Dairy Food Res. 2018, 37, 1–6. [Google Scholar] [CrossRef]
- Frothingham, C., Jr.; Minot, G.R. Normal temperature of rabbits. Am. J. Physiol. -Leg. Content 1912, 30, 430–435. [Google Scholar] [CrossRef]
- Ludwig, N.; Gargano, M.; Luzi, F.; Carenzi, C.; Verga, M. Technical note: Applicability of infrared thermography as a non invasive measurement of stress in rabbit. World Rabbit Sci. 2007, 15, 199–205. [Google Scholar] [CrossRef]
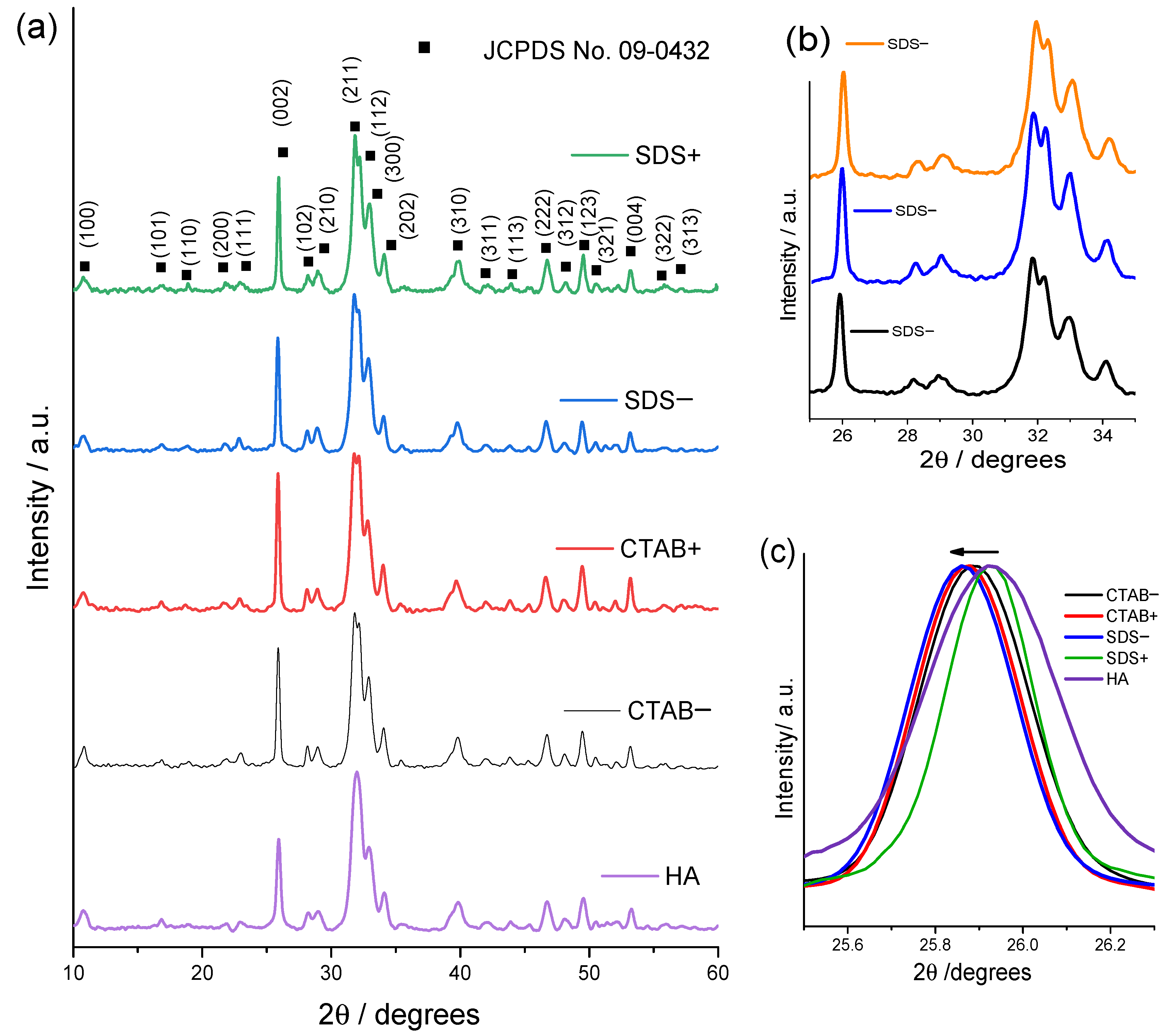



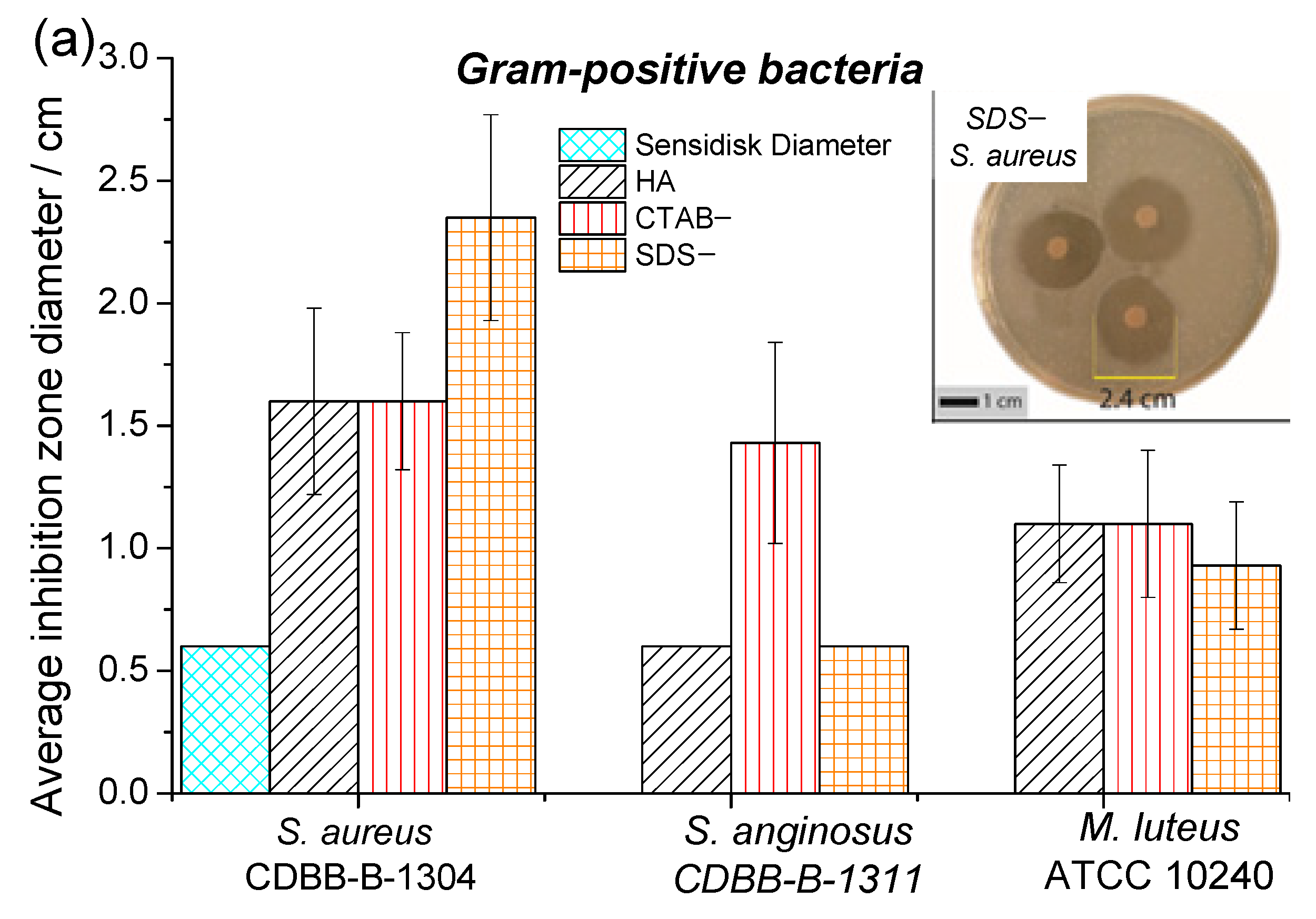
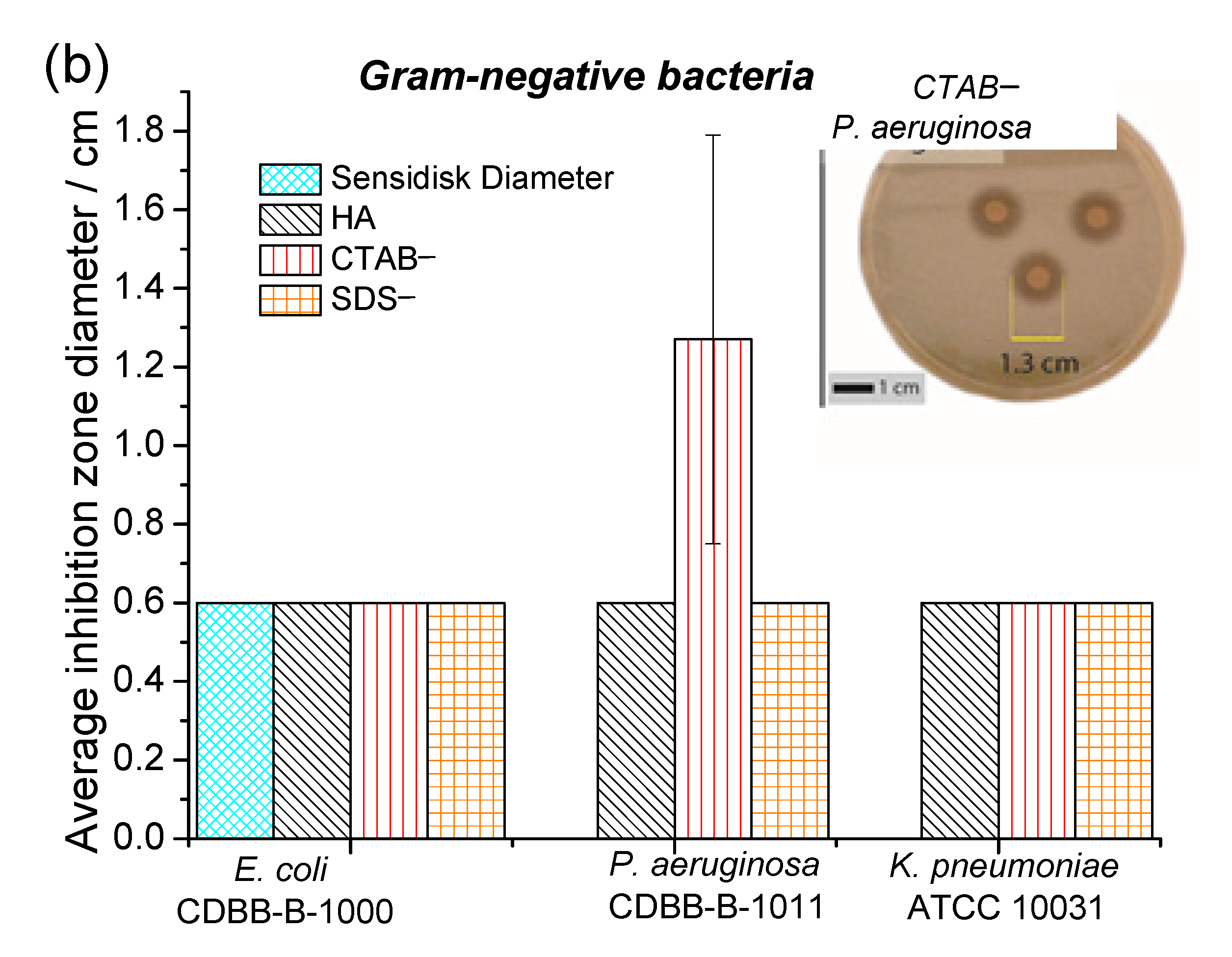

| Samples | Lattice Parameter (nm) | FWHM of (002) (Grades) | Crystallite Size (nm) | I002/I211 | |
|---|---|---|---|---|---|
| a = b | c | ||||
| HA | 9.42 ± 0.009 | 6.842 ± 0.041 | 0.371 | 23.827 ± 1.65 | 0.639 ± 0.073 |
| CTAB+ | 9.424 ± 0.034 | 6.835 ± 0.048 | 0.272 | 34.027 ± 1.308 | 0.823 ± 0.048 |
| CTAB− | 9.447 ± 0.012 | 6.846 ± 0.011 | 0.301 | 29.717 ± 1.854 | 0.763 ± 0.024 |
| SDS+ | 9.421 ± 0.026 | 6.854 ± 0.021 | 0.203 | 47.3 ± 1.188 | 0.8 ± 0.062 |
| SDS− | 9.43 ± 0.022 | 6.838 ± 0.076 | 0.293 | 30.347 ± 1.227 | 0.739 ± 0.025 |
| Sample | Rabbit | 1 h | 24 h | 48 h | 72 h | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C+ | T | C− | C+ | T | C− | C+ | T | C− | C+ | T | C− | ||
| CTAB− | 24 | ++++p | 0 | 0 | ++p | +e | 0 | +p | +e | 0 | 0 | +n | 0 |
| 25 | ++++p | 0 | 0 | ++p | ++e | 0 | 0 | +e | 0 | 0 | 0 | 0 | |
| HA | 23 | ++++p | 0 | 0 | ++p | ++e | 0 | +p | 0 | 0 | 0 | 0 | 0 |
| 26 | ++++p | 0 | 0 | ++p | ++e | 0 | ++p | +e | 0 | 0 | +n | 0 | |
| SDS− | 27 | ++++p | 0 | 0 | ++p | ++++e | 0 | 0 | +e | 0 | 0 | ++n | 0 |
| 28 | ++++p | 0 | 0 | ++p | +e | 0 | 0 | +e | 0 | 0 | +n | 0 | |
| Time (h) | Group | ||||
|---|---|---|---|---|---|
| C (+) | HA | C (−) | CTAB− | SDS− | |
| 0 | 39.6 ± 0.6 | 39.2 ± 0.8 | 39.3 ± 0.8 | 39.7 ± 0.7 | 39.1 ± 0.6 |
| 1 | 41.2 ± 0.2 | 41.7 ± 0.2 | 41.2 ± 0.1 | 40.9 ± 0.2 | 41 ± 0.3 |
| 24 | 37.3 ± 0.4 | 37.2 ± 0.4 | 37.05 ± 0.6 | 37.4 ± 0.5 | 37.1 ± 0.6 |
| 72 | 37.9 ± 0.5 | 37.4 ± 0.6 | 38.2 ± 0.4 | 38.3 ± 0.3 | 37.5 ± 0.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
García Domínguez, G.; Diaz De La Torre, S.; Chávez Güitrón, L.; Vergara Hernández, E.; Reyes Miranda, J.; Quezada Cruz, M.; Garrido Hernández, A. Effect of the Structural and Morphological Properties of Surfactant-Assisted Hydroxyapatite on Dermal Irritation and Antibacterial Activity. Materials 2021, 14, 6522. https://doi.org/10.3390/ma14216522
García Domínguez G, Diaz De La Torre S, Chávez Güitrón L, Vergara Hernández E, Reyes Miranda J, Quezada Cruz M, Garrido Hernández A. Effect of the Structural and Morphological Properties of Surfactant-Assisted Hydroxyapatite on Dermal Irritation and Antibacterial Activity. Materials. 2021; 14(21):6522. https://doi.org/10.3390/ma14216522
Chicago/Turabian StyleGarcía Domínguez, Giovanni, Sebastián Diaz De La Torre, Lorena Chávez Güitrón, Erasto Vergara Hernández, Joan Reyes Miranda, Maribel Quezada Cruz, and Aristeo Garrido Hernández. 2021. "Effect of the Structural and Morphological Properties of Surfactant-Assisted Hydroxyapatite on Dermal Irritation and Antibacterial Activity" Materials 14, no. 21: 6522. https://doi.org/10.3390/ma14216522
APA StyleGarcía Domínguez, G., Diaz De La Torre, S., Chávez Güitrón, L., Vergara Hernández, E., Reyes Miranda, J., Quezada Cruz, M., & Garrido Hernández, A. (2021). Effect of the Structural and Morphological Properties of Surfactant-Assisted Hydroxyapatite on Dermal Irritation and Antibacterial Activity. Materials, 14(21), 6522. https://doi.org/10.3390/ma14216522








