Mesoporous Silica and Titania-Based Materials for Stability Enhancement of Polyphenols
Abstract
1. Introduction
2. Results and Discussion
2.1. Characterization of Common Sage Polyphenolic Extracts by Spectrometric Measurements
2.2. Radical Scavenger Activity of Polyphenolic Extracts
2.3. Chemical Profile of Common Sage Extracts
2.4. Antibacterial Activity Assessment of Polyphenolic Extracts
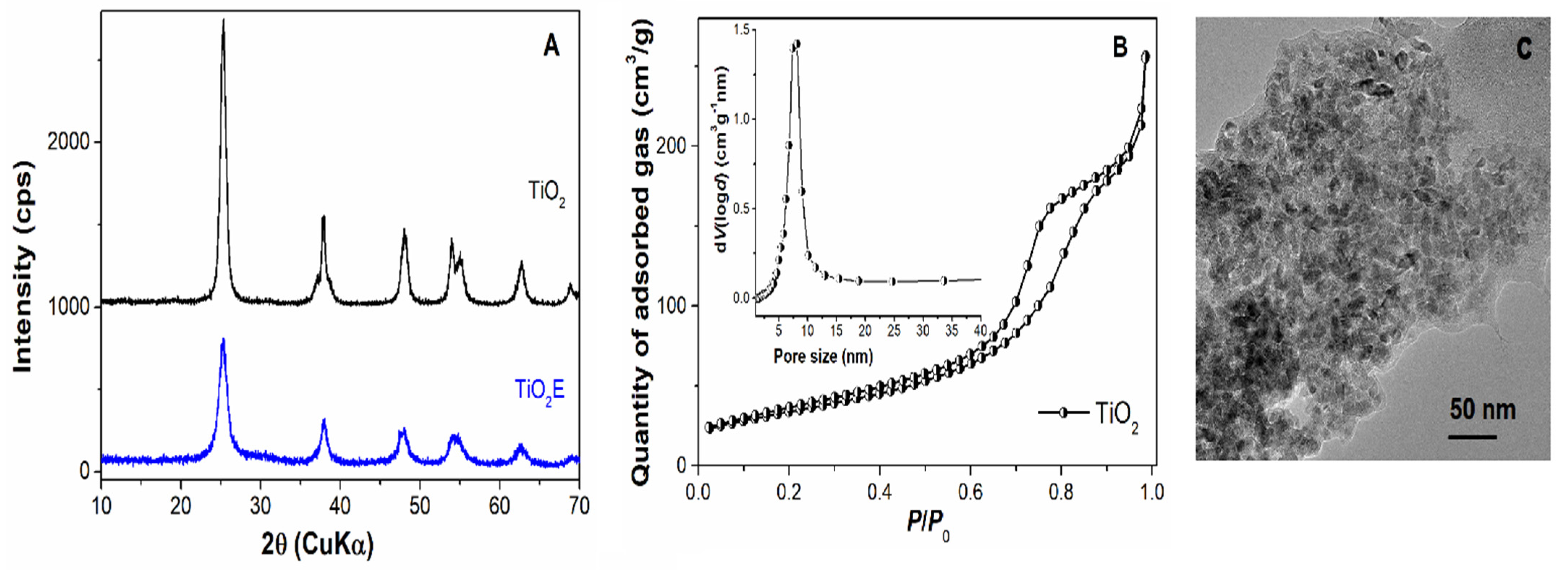
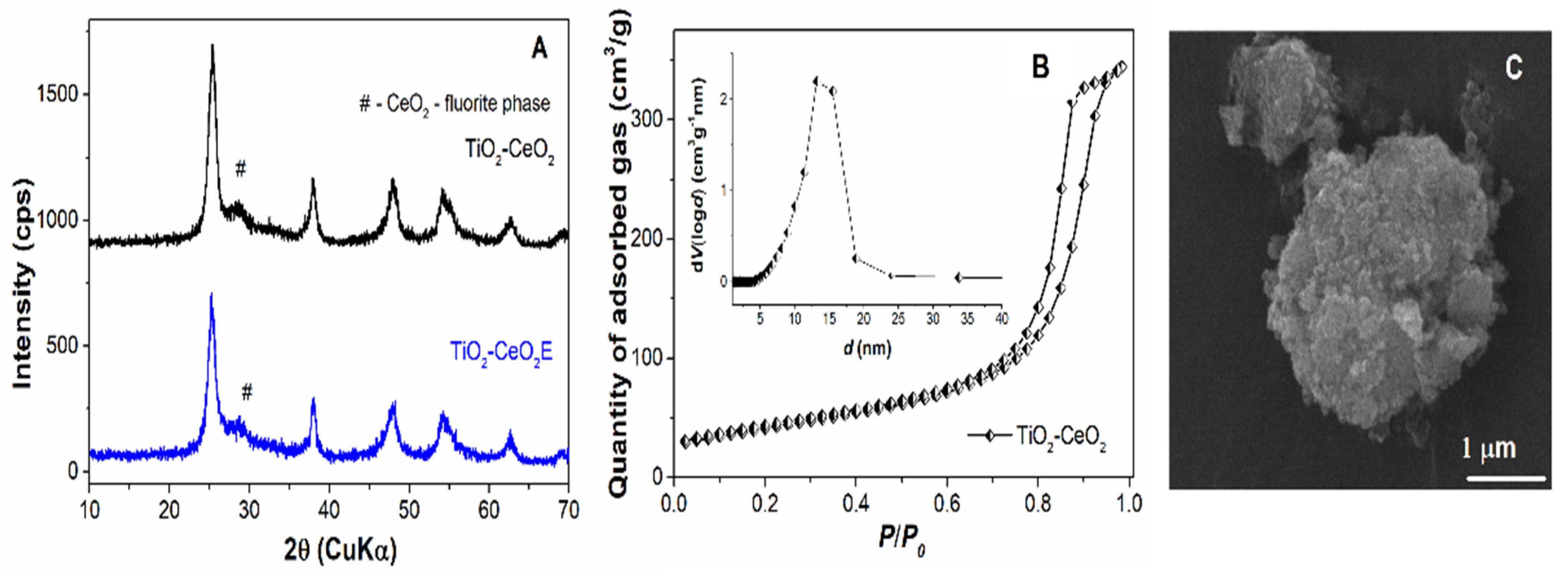
2.5. Characterisation of Mesoporous Supports
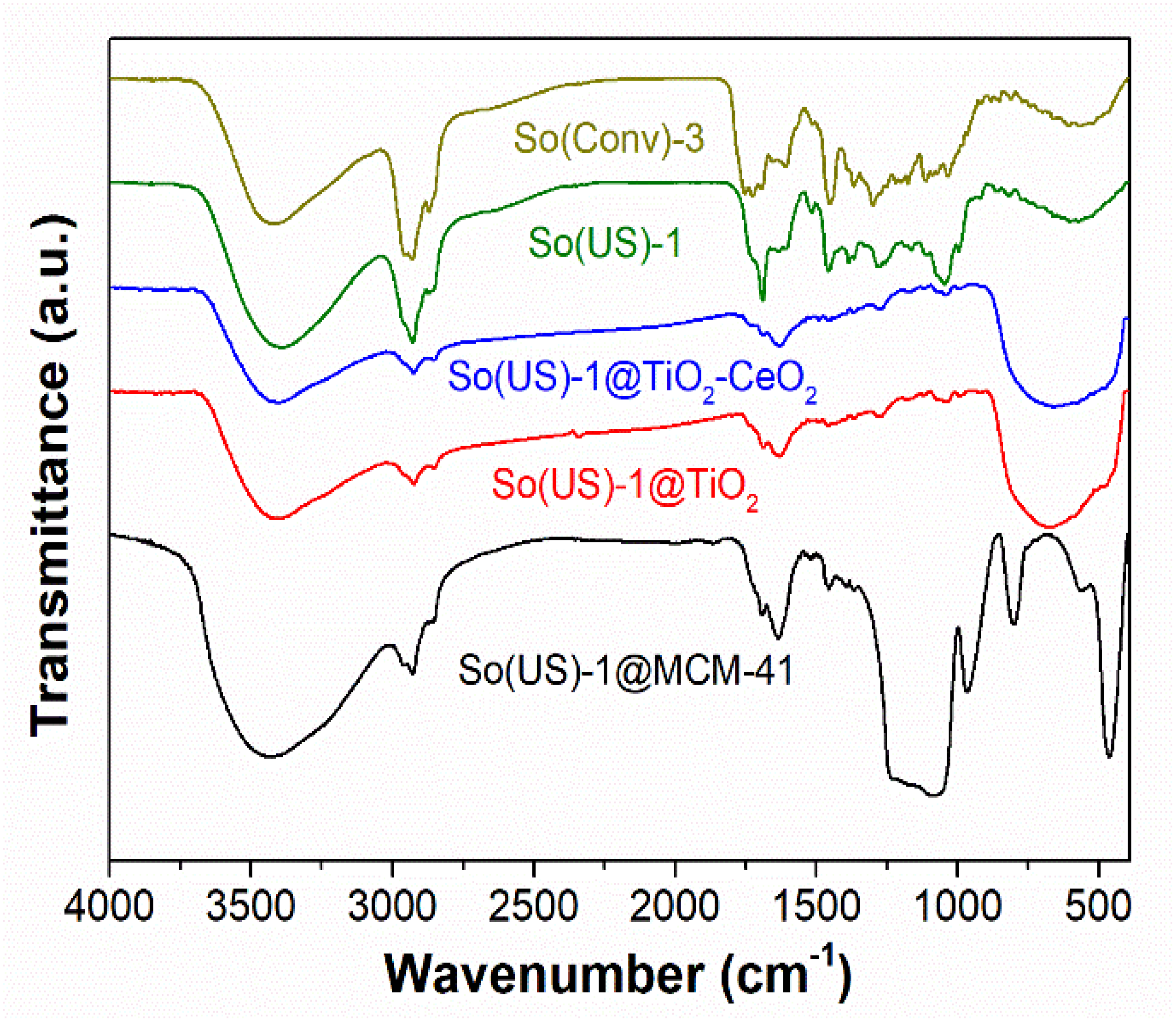
2.6. Characterization of Extract-Loaded Materials
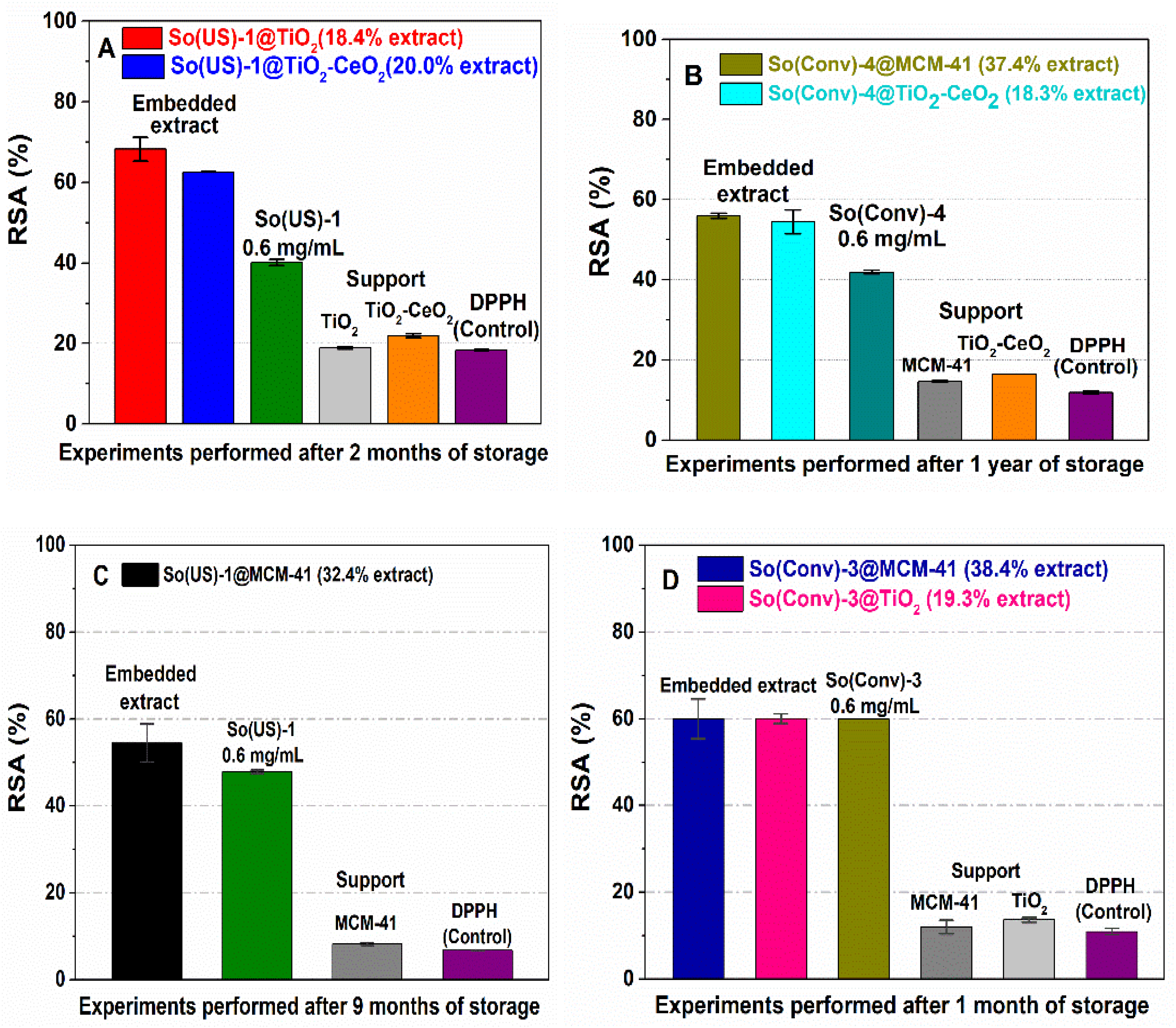
2.7. Radical Scavenging Activity of Extract-Loaded Materials
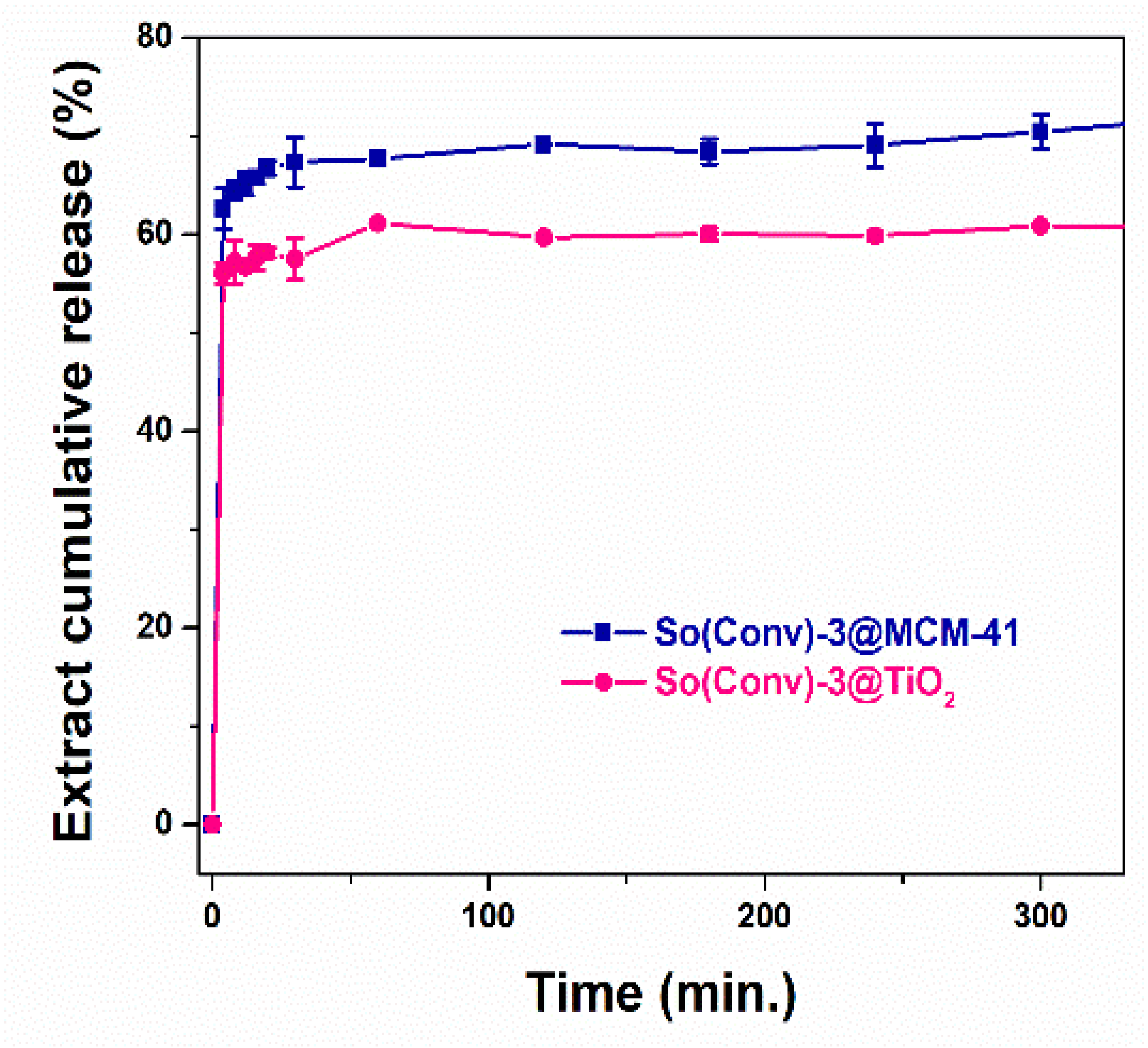
2.8. Polyphenols Release Profiles from Mesoporous Titania and Silica Supports
2.9. Bactericidal Activity of Extract-Loaded Materials
3. Materials and Methods
3.1. Materials
3.2. Preparation and Characterisation of Common Sage Extracts
3.3. Obtaining Mesoporous Titania and Titania-Ceria Composite Supports
3.4. Loading of Salvia Officinalis Extracts into Mesoporous Inorganic Matrices
3.5. Characterization of Inorganic Matrices and Materials Containing Extract
3.6. Antibacterial Activity Assessment of S. officinalis Extracts
3.6.1. Minimum Inhibitory Concentration (MIC)
3.6.2. Minimum Biofilm Eradication Concentration (MBEC)
3.7. Antibacterial Activity of Materials Containing Extract through Disk Diffusion Method
3.8. Determination of Polyphenols Release Profiles from Mesoporous Supports
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Quideau, S.; Deffieux, D.; Douat-Casassus, C.; Pouysgu, L. Plant Polyphenols: Chemical Properties, Biological Activities, and Synthesis. Angew. Chem. Int. Ed. 2011, 50, 586–621. [Google Scholar] [CrossRef]
- Dai, J.; Mumper, R.J. Plant Phenolics: Extraction, Analysis and Their Antioxidant and Anticancer Properties. Molecules 2010, 15, 7313–7352. [Google Scholar] [CrossRef] [PubMed]
- Hajimehdipoor, H.; Gohari, A.R.; Ajani, Y.; Saeidnia, S. Comparative study of the total phenol content and antioxidant activity of some medicinal herbal extracts. Res. J. Pharmacogn. 2014, 1, 21–25. [Google Scholar]
- Poulios, E.; Giaginis, C.; Vasios, G.K. Current Advances on the Extraction and Identification of Bioactive Components of Sage (Salvia spp.). Curr. Pharm. Biotechnol. 2019, 20, 845–857. [Google Scholar] [CrossRef] [PubMed]
- Afonso, A.F.; Pereira, O.R.; Fernandes, A.; Calhelha, R.C.; Silva, A.M.S.; Ferreira, I.C.; Cardoso, S.M. Phytochemical Composition and Bioactive Effects of Salvia africana, Salvia officinalis ‘Icterina’ and Salvia mexicana Aqueous Extracts. Molecules 2019, 24, 4327. [Google Scholar] [CrossRef]
- Grzegorczyk-Karolak, I.; Kiss, A.K. Determination of the Phenolic Profile and Antioxidant Properties of Salvia viridis L. Shoots: A Comparison of Aqueous and Hydroethanolic Extracts. Molecules 2018, 23, 1468. [Google Scholar] [CrossRef]
- Brezoiu, A.-M.; Prundeanu, M.; Berger, D.; Deaconu, M.; Matei, C.; Oprea, O.; Vasile, E.; Negreanu-Pîrjol, T.; Muntean, D.; Danciu, C. Properties of Salvia officinalis L. and Thymus serpyllum L. Extracts Free and Embedded into Mesopores of Silica and Titania Nanomaterials. Nanomaterials 2020, 10, 820–841. [Google Scholar] [CrossRef]
- Nicolai, M.; Pereira, P.; Vitor, R.F.; Reis, C.P.; Roberto, A.; Rijo, P. Antioxidant activity and rosmarinic acid content of ultrasound-assisted ethanolic extracts of medicinal plants. Measurement 2016, 89, 328–332. [Google Scholar] [CrossRef]
- Nutrizio, M.; Kljusurić, J.G.; Sabolović, M.B.; Kovačević, D.B.; Šupljika, F.; Putnik, P.; Čakić, M.S.; Dubrovic, I.; Vrsaljko, D.; Maltar-Strmečki, N.; et al. Valorization of sage extracts (Salvia officinalis L.) obtained by high voltage electrical discharges: Process control and antioxidant properties. Innov. Food Sci. Emerg. Technol. 2019, 60, 102284. [Google Scholar] [CrossRef]
- Tušek, A.J.; Benković, M.; Valinger, D.; Jurina, T.; Belščak-Cvitanović, A.; Kljusurić, J.G. Optimizing bioactive compounds extraction from different medicinal plants and prediction through nonlinear and linear models. Ind. Crop. Prod. 2018, 126, 449–458. [Google Scholar] [CrossRef]
- Giacometti, J.; Kovačević, D.B.; Putnik, P.; Gabrić, D.; Bilušić, T.; Krešić, G.; Stulić, V.; Barba, F.J.; Chemat, F.; Barbosa-Cánovas, G.; et al. Extraction of bioactive compounds and essential oils from mediterranean herbs by conventional and green innovative techniques: A review. Food Res. Int. 2018, 113, 245–262. [Google Scholar] [CrossRef]
- Durling, N.E.; Catchpole, O.; Grey, J.B.; Webby, R.F.; Mitchell, K.A.; Foo, L.Y.; Perry, N. Extraction of phenolics and essential oil from dried sage (Salvia officinalis) using ethanol–water mixtures. Food Chem. 2007, 101, 1417–1424. [Google Scholar] [CrossRef]
- Jakovljević, M.; Jokić, S.; Molnar, M.; Jašić, M.; Babić, J.; Jukić, H.; Banjari, I. Bioactive Profile of Various Salvia officinalis L. Preparations. Plants 2019, 8, 55. [Google Scholar] [CrossRef]
- Vergara-Salinas, J.R.; Perez-Jimenez, J.; Torres, J.L.; Agosin, E.; Perez-Correa, J.R. Effects of Temperature and Time on Polyphenolic Content and Antioxidant Activity in the Pressurized HotWater Extraction of Deodorized Thyme (Thymus vulgaris). J. Agric. Food Chem. 2012, 60, 10920–10929. [Google Scholar] [CrossRef] [PubMed]
- Sulaiman, S.F.; Sajak, A.A.B.; Ooi, K.L.; Seow, E.M. Effect of solvents in extracting polyphenols and antioxidants of selected raw vegetables. J. Food Compos. Anal. 2011, 24, 506–515. [Google Scholar] [CrossRef]
- Jafri, L.; Saleem, S.; Ullah, N.; Mirza, B. In vitro assessment of antioxidant potential and determination of polyphenolic compounds of Hedera nepalensis K. Koch. Arab. J. Chem. 2017, 10, S3699–S3706. [Google Scholar] [CrossRef]
- Koffi, E.; Sea, T.; Dodehe, Y.; Soro, S. Effect of solvent type on extraction of polyphenols from twenty-three Ivorian plants. J. Anim. Plant Sci. 2010, 5, 550–558. [Google Scholar]
- Anokwuru, C.P.; Anyasor, G.N.; Ajibaye, O.; Fakoya, O.; Okebugwu, P. Effect of extraction solvents on phenolic, flavonoid and antioxidant activities of three nigerian medicinal plants. Nat. Sci. 2011, 9, 53–61. [Google Scholar]
- Dent, M.; Dragovic-Uzelac, V.; Penic, M.; Brncic, M.; Bosiljkov, T.; Levaj, B. The Effect of Extraction Solvents, Temperature and Time on the Composition and Mass Fraction of Polyphenols in Dalmatian Wild Sage (Salvia officinalis L.) Extracts. Food Technol. Biotechnol. 2013, 51, 84–91. [Google Scholar]
- Ramić, M.; Vidović, S.; Zeković, Z.; Vladić, J.; Cvejin, A.; Pavlić, B. Modeling and optimization of ultrasound-assisted extraction of polyphenolic compounds from Aronia melanocarpa by-products from filter-tea factory. Ultrason. Sonochem. 2015, 23, 360–368. [Google Scholar] [CrossRef]
- Martins, N.; Barros, L.; Santos-Buelga, C.; Henriques, M.; Silva, S.; Ferreira, I.C. Evaluation of bioactive properties and phenolic compounds in different extracts prepared from Salvia officinalis L. Food Chem. 2015, 170, 378–385. [Google Scholar] [CrossRef] [PubMed]
- Vieira, S.F.; Ferreira, H.; Neves, N.M. Antioxidant and Anti-Inflammatory Activities of Cytocompatible Salvia officinalis Extracts: A Comparison between Traditional and Soxhlet Extraction. Antioxidants 2020, 9, 1157. [Google Scholar] [CrossRef]
- Tohma, H.; Köksal, E.; Kılıç, Ö.; Alan, Y.; Yılmaz, M.A.; Gülçin, I.; Bursal, E.; Alwasel, S.H. RP-HPLC/MS/MS Analysis of the Phenolic Compounds, Antioxidant and Antimicrobial Activities of Salvia L. Species. Antioxidants 2016, 5, 38. [Google Scholar] [CrossRef] [PubMed]
- Dal Prá, V.; Bisol, L.B.; Detoni, S.; Denti, M.; Grando, J.; Pollo, C.; Pasquali, T.R.; Hofmann Júnio, A.E.; Mazzuti, M.A.; Macedo, S.M. Anti-inflammatory activity of fractionated extracts of Salvia officinalis L. J. Appl. Pharm. Sci. 2011, 7, 67–71. [Google Scholar]
- Jiang, R.-W.; Lau, K.-M.; Hon, P.-M.; Mak, T.C.W.; Woo, K.-S.; Fung, K.P. Chemistry and Biological Activities of Caffeic Acid Derivatives from Salvia miltiorrhiza. Curr. Med. Chem. 2005, 12, 237–246. [Google Scholar] [CrossRef] [PubMed]
- Lopresti, A.L. Salvia (Sage): A Review of its Potential Cognitive-Enhancing and Protective Effects. Drugs R D 2016, 17, 53–64. [Google Scholar] [CrossRef]
- Ahmed, G.H.G.; Fernández-González, A.; García, M.E.D. Nano-encapsulation of grape and apple pomace phenolic extract in chitosan and soy protein via nanoemulsification. Food Hydrocoll. 2020, 108, 105806. [Google Scholar] [CrossRef]
- Abdin, M.; Salama, M.A.; Riaz, A.; Akhtar, H.M.S.; Elsanat, S.Y. Enhanced the entrapment and controlled release of Syzygium cumini seeds polyphenols by modifying the surface and internal organization of Alginate-based microcapsules. J. Food Process. Preserv. 2020, 45, e15100. [Google Scholar] [CrossRef]
- Păvăloiu, R.-D.; Sha’At, F.; Bubueanu, C.; Deaconu, M.; Neagu, G.; Sha’At, M.; Anastasescu, M.; Mihailescu, M.; Matei, C.; Nechifor, G.; et al. Polyphenolic Extract from Sambucus ebulus L. Leaves Free and Loaded into Lipid Vesicles. Nanomaterials 2019, 10, 56. [Google Scholar] [CrossRef]
- Brezoiu, A.M.; Matei, C.; Deaconu, M.; Stanciuc, A.-M.; Trifan, A.; Gaspar, A.; Berger, D. Polyphenols extract from grape pomace. Characterization and valorisation through encapsulation into mesoporous silica-type matrices. Food Chem. Toxicol. 2019, 133, 110787. [Google Scholar] [CrossRef] [PubMed]
- Brezoiu, A.-M.; Bajenaru, L.; Berger, D.; Mitran, R.-A.; Deaconu, M.; Lincu, D.; Guzun, A.S.; Matei, C.; Moisescu, M.G.; Negreanu-Pirjol, T. Effect of Nanoconfinement of Polyphenolic Extract from Grape Pomace into Functionalized Mesoporous Silica on Its Biocompatibility and Radical Scavenging Activity. Antioxidants 2020, 9, 696. [Google Scholar] [CrossRef]
- Li, L.; Liu, T.; Fu, C.; Tan, L.; Meng, X.; Liu, H. Biodistribution, excretion, and toxicity of mesoporous silica nanoparticles after oral administration depend on their shape. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 1915–1924. [Google Scholar] [CrossRef]
- Huang, X.; Teng, X.; Chen, D.; Tang, F.; He, J. The effect of the shape of mesoporous silica nanoparticles on cellular uptake and cell function. Biomaterials 2010, 31, 438–448. [Google Scholar] [CrossRef]
- Doadrio, J.C.; Salinas, A.; Sánchez-Montero, J.; Vallet-Regí, M. Drug release from ordered mesoporous silicas. Curr. Pharm. Des. 2015, 21, 6213–6819. [Google Scholar] [CrossRef]
- Rowe, R.C.; Sheskey, P.J.; Weller, P.J. Handbook of Pharmaceutical Excipients, 4th ed.; Pharmaceutical Press: London, UK; American Pharmaceutical Association: Washington, DC, USA, 2003. [Google Scholar]
- Croissant, J.G.; Butler, K.S.; Zink, J.I.; Brinker, C.J. Synthetic amorphous silica nanoparticles: Toxicity, biomedical and environmental implications. Nat. Rev. Mater. 2020, 5, 886–909. [Google Scholar] [CrossRef]
- Bogdan, J.; Zarzyńska, J.; Pławińska-Czarnak, J. Comparison of Infectious Agents Susceptibility to Photocatalytic Effects of Nanosized Titanium and Zinc Oxides: A Practical Approach. Nanoscale Res. Lett. 2015, 10, 1–15. [Google Scholar] [CrossRef]
- Liao, C.; Li, Y.; Tjong, S.C. Visible-Light Active Titanium Dioxide Nanomaterials with Bactericidal Properties. Nanomaterials 2020, 10, 124. [Google Scholar] [CrossRef] [PubMed]
- Kasinathan, K.; Kennedy, J.V.; Elayaperumal, M.; Henini, M.; Malik, M. Photodegradation of organic pollutants RhB dye using UV simulated sunlight on ceria based TiO2 nanomaterials for antibacterial applications. Sci. Rep. 2016, 6, 38064. [Google Scholar] [CrossRef]
- Herrero, M.; Plaza, M.; Cifuentes, A.; Ibáñez, E. Extraction Techniques for the Determination of Phenolic Compounds in Food, Comprehensive Sampling and Sample Preparation. Analytical Techniques for Scientists. Anal. Tech. Sci. 2012, 159–180. [Google Scholar] [CrossRef]
- Veličković, D.T.; Karabegović, I.T.; Stojičević, S.S.; Lazić, M.L.; Marinković, V.D.; Veljković, V.B. Comparison of antioxidant and antimicrobial activities of extracts obtained from Salvia glutinosa L. and Salvia officinalis L. Chem. Ind. 2011, 65, 599–605. [Google Scholar] [CrossRef]
- Atanassova, M.; Georgieva, S.; Ivancheva, K. Total phenolic and total flavonoid contents, antioxidant capacity and biological contaminants in medicinal herbs. Chem. Technol. Metall. 2011, 46, 81–88. [Google Scholar]
- Jasicka-Misiak, I.; Poliwoda, A.; Petecka, M.; Buslovych, O.; Shlyapnikov, V.A.; Wieczorek, P.P. Antioxidant Phenolic Compounds in Salvia officinalis L. and Salvia sclarea L. Ecol. Chem. Eng. S 2018, 25, 133–142. [Google Scholar] [CrossRef]
- Duletic-Lausevic, S.; Alimpic, A.; Pavlovic, D.; Marin, P.D.; Lakusic, D. Salvia officinalis of different origins. Antioxidant activity, phenolic and flavonoid content of extracts. Agro. Food Ind. Hi Tech. 2016, 27, 52–55. [Google Scholar]
- Prundeanu, M.; Brezoiu, A.M.; Deaconu, M.; Berger, D. Chemical Profiling of Polyphenols from Salvia officinalis and Thymus serpyllum Extracts during a Three-Stage Extraction Process. U.P.B. Sci. Bull. Ser. B 2021, 83, 1–16. [Google Scholar]
- Sotiropoulou, N.S.; Megremi, S.F.; Tarantilis, P. Evaluation of Antioxidant Activity, Toxicity, and Phenolic Profile of Aqueous Extracts of Chamomile (Matricaria chamomilla L.) and Sage (Salvia officinalis L.) Prepared at Different Temperatures. Appl. Sci. 2020, 10, 2270. [Google Scholar] [CrossRef]
- Torres-Romero, A.; Cajero-Juárez, M.; Nuñez-Anita, R.E.; Garcia, M.E.C. Ceria-Doped Titania Nanoparticles as Drug Delivery System. J. Nanosci. Nanotechnol. 2020, 20, 3971–3980. [Google Scholar] [CrossRef]
- Luo, L.-J.; Nguyen, D.D.; Lai, J.-Y. Dually functional hollow ceria nanoparticle platform for intraocular drug delivery: A push beyond the limits of static and dynamic ocular barriers toward glaucoma therapy. Biomaterials 2020, 243, 119961. [Google Scholar] [CrossRef]
- Yu, D.; Ma, M.; Liu, Z.; Pi, Z.; Du, X.; Ren, J.; Qu, X. MOF-encapsulated nanozyme enhanced siRNA combo: Control neural stem cell differentiation and ameliorate cognitive impairments in Alzheimer’s disease model. Biomaterials 2020, 255, 120160. [Google Scholar] [CrossRef]
- Ghedini, E.; Nichele, V.; Signoretto, M.; Cerrato, G. Structure-Directing Agents for the Synthesis of TiO2-Based Drug-Delivery Systems. Chem. Eur. J. 2012, 18, 10653–10660. [Google Scholar] [CrossRef]
- Socrates, G. Infrared and Raman Characteristic Group Frequencies, 3rd ed.; John Wiley & Sons Ltd.: Chichester, UK, 2001. [Google Scholar]
- Haładyn, K.; Tkacz, K.; Wojdyło, A.; Nowicka, P. The Types of Polysaccharide Coatings and Their Mixtures as a Factor Affecting the Stability of Bioactive Compounds and Health-Promoting Properties Expressed as the Ability to Inhibit the α-Amylase and α-Glucosidase of Chokeberry Extracts in the Microencapsulation Process. Foods 2021, 10, 1994. [Google Scholar] [CrossRef]
- Khalil, W.K.B.; Abdu, F. Effects of Salvia officinalis Extract and its Nano-Encapsulated Form on Methylmercury Induced Neurotoxic-Stress in Male Rats. World Appl. Sci. J. 2013, 24, 826–837. [Google Scholar]
- Benković, M.; Sarić, I.; Tušek, A.J.; Jurina, T.; Kljusurić, J.G.; Valinger, D. Analysis of the Adsorption and Release Processes of Bioactives from Lamiaceae Plant Extracts on Alginate Microbeads. Food Bioprocess Technol. 2021, 14, 1216–1230. [Google Scholar] [CrossRef]
- Matouskova, P.; Marova, I.; Bokrova, J.; Benesova, P. Effect of Encapsulation on Antimicrobial Activity of Herbal Extracts with Lysozyme. Food Technol. Biotechnol. 2016, 54, 304–316. [Google Scholar] [CrossRef] [PubMed]
- Stecoza, C.E.; Draghici, C.; Caproiu, M.T.; Gradisteanu Pircalabioru, G.; Marutescu, L. Synthesis and evaluation of the antimicrobial and antibiofilm activity of novel dibenzothiepines. Farmacia 2020, 68, 1091–1098. [Google Scholar] [CrossRef]

| Extract | Solvent, T (°C), Plant/Solvent (w/v) | E (%wt) | TPC (mgGAE/ gextract) | TFC (mgQE/ gextract) | TChC (mgCh/ gextract) | RSAABTS (mg TE/ gextract) | RSADPPH (mg TE/ gextract) | IC50% (mg/mL) |
|---|---|---|---|---|---|---|---|---|
| So(US)-1 | ethanol/50 °C; 1/30 | 13.0 | 192.81 ± 5.43 | 24.35 ± 0.20 | 12.86 ± 0.61 | 245.68 ± 6.28 | 201.29 ± 16.36 | 1.35 |
| So(MW)-2 | 50% ethanol/80 °C; 1/50 | 19.9 | 168.97 ± 1.57 | 26.52 ± 0.20 | 0.53 ± 0.01 | 232.32 ± 0.73 | 211.86 ± 4.45 | 1.28 |
| So(Conv)-3 | 50% ethanol/50 °C; 1/30 | 31.7 | 145.40 ± 2.31 | 25.11 ± 0.49 | 0.57 ± 0.08 | 215.20 ± 4.22 | 298.34 ± 10.42 | 0.91 |
| So(Conv)-4 | ethanol/ 80 °C; 1/18 | 14.0 | 129.20 ± 5.59 | 36.98 ± 1.22 | 4.40 ± 0.40 | 128.89 ± 4.80 | 249.44 ± 11.55 | 1.09 |
| So(Conv)-5 | 50% ethanol/80 °C; 1/18 | 24.5 | 165.52 ± 2.99 | 23.62 ± 0.06 | 4.19 ± 0.15 | 249.07 ± 6.93 | 268.11 ± 11.22 | 1.01 |
| So(Conv)-6 | ethanol/50 °C; 1/30 | 8.2 | 138.11 ± 2.45 | 15.42 ± 0.11 | 3.56 ± 0.12 | 113.36 ± 2.40 | 98.22 ± 8.72 | 2.76 |
| Concentration in Extract (mg/g Extract) | ||||||
|---|---|---|---|---|---|---|
| Compound | So(US)-1 | So(MW)-2 | So(Conv)-3 | So(Conv)-4 | So(Conv)-5 | So(Conv)-6 |
| protocatechuic acid | nd | 0.571 ± 0.003 | 0.235 ± 0.007 | nd | 0.569 ± 0.014 | nd |
| caftaric acid | 0.760 ± 0.000 | 0.587 ± 0.001 | nd | nd | 0.746 ± 0.003 | nd |
| chlorogenic acid | 0.330 ± 0.000 | 0.675 ± 0.000 | 0.828 ± 0.004 | 1.028 ± 0001 | 0.753 ± 0.005 | 0.094 ± 0.000 |
| caffeic acid | 0.552 ± 0.000 | 2.494 ± 0.019 | 2.175 ± 0.000 | 0.874 ± 0.003 | 2.632 ± 0.000 | 0.174 ± 0.001 |
| rosmarinic acid | 35.335 ± 0.000 | 14.861 ± 0.008 | 22.877 ± 0.004 | 26.618 ± 0.063 | 20.542 ± 0.009 | 5.673 ± 0.025 |
| Extract | MIC (µg/mL) | MBEC (µg/mL) | |||
|---|---|---|---|---|---|
| P. aeruginosa | E. coli | S. aureus | P. aeruginosa | E. coli | |
| So(US)-1 | 875 | 1750 | 437.5 | 437.5 | 437.5 |
| So(Conv)-6 | 875 | 1750 | 875 | 437.5 | 437.5 |
| Support | dBJH (nm) | SBET (m2/g) | Vp (cm3/g) | Embedded Extract | Extract (% wt) |
|---|---|---|---|---|---|
| TiO2 | 7.43 | 124 | 0.26 | So(US)-1@TiO2 | 18.4 |
| So(Conv)-3@TiO2 | 19.3 | ||||
| TiO2-CeO2 | 13.18 | 150 | 0.54 | So(US)-1@TiO2-CeO2 | 20.0 |
| So(Conv)-4@TiO2-CeO2 | 18.3 | ||||
| MCM-41 | 2.67 | 976 | 0.88 | So(US)-1@MCM-41 | 32.4 |
| So(Conv)-4@MCM-41 | 37.4 | ||||
| So(Conv)-3@MCM-41 | 38.4 |
| Φ, mm | ||||||
|---|---|---|---|---|---|---|
| Sample | P. aeruginosa ATCC 27853 | S. aureus ATCC 25923 | ||||
| E | EM | S | E | EM | S | |
| So(US)-1@TiO2 | 14 | 15 | 14 | 16 | 17 | 11 |
| So(US)-1@MCM-41 | 14 | 14 | 13 | 16 | 16 | 10 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prundeanu, M.; Brezoiu, A.-M.; Deaconu, M.; Gradisteanu Pircalabioru, G.; Lincu, D.; Matei, C.; Berger, D. Mesoporous Silica and Titania-Based Materials for Stability Enhancement of Polyphenols. Materials 2021, 14, 6457. https://doi.org/10.3390/ma14216457
Prundeanu M, Brezoiu A-M, Deaconu M, Gradisteanu Pircalabioru G, Lincu D, Matei C, Berger D. Mesoporous Silica and Titania-Based Materials for Stability Enhancement of Polyphenols. Materials. 2021; 14(21):6457. https://doi.org/10.3390/ma14216457
Chicago/Turabian StylePrundeanu, Mioara, Ana-Maria Brezoiu, Mihaela Deaconu, Gratiela Gradisteanu Pircalabioru, Daniel Lincu, Cristian Matei, and Daniela Berger. 2021. "Mesoporous Silica and Titania-Based Materials for Stability Enhancement of Polyphenols" Materials 14, no. 21: 6457. https://doi.org/10.3390/ma14216457
APA StylePrundeanu, M., Brezoiu, A.-M., Deaconu, M., Gradisteanu Pircalabioru, G., Lincu, D., Matei, C., & Berger, D. (2021). Mesoporous Silica and Titania-Based Materials for Stability Enhancement of Polyphenols. Materials, 14(21), 6457. https://doi.org/10.3390/ma14216457