Lateral Ridge Augmentation with Guided Bone Regeneration Using Particulate Bone Substitutes and Injectable Platelet-Rich Fibrin in a Digital Workflow: 6 Month Results of a Prospective Cohort Study Based on Cone-Beam Computed Tomography Data
Abstract
:1. Introduction
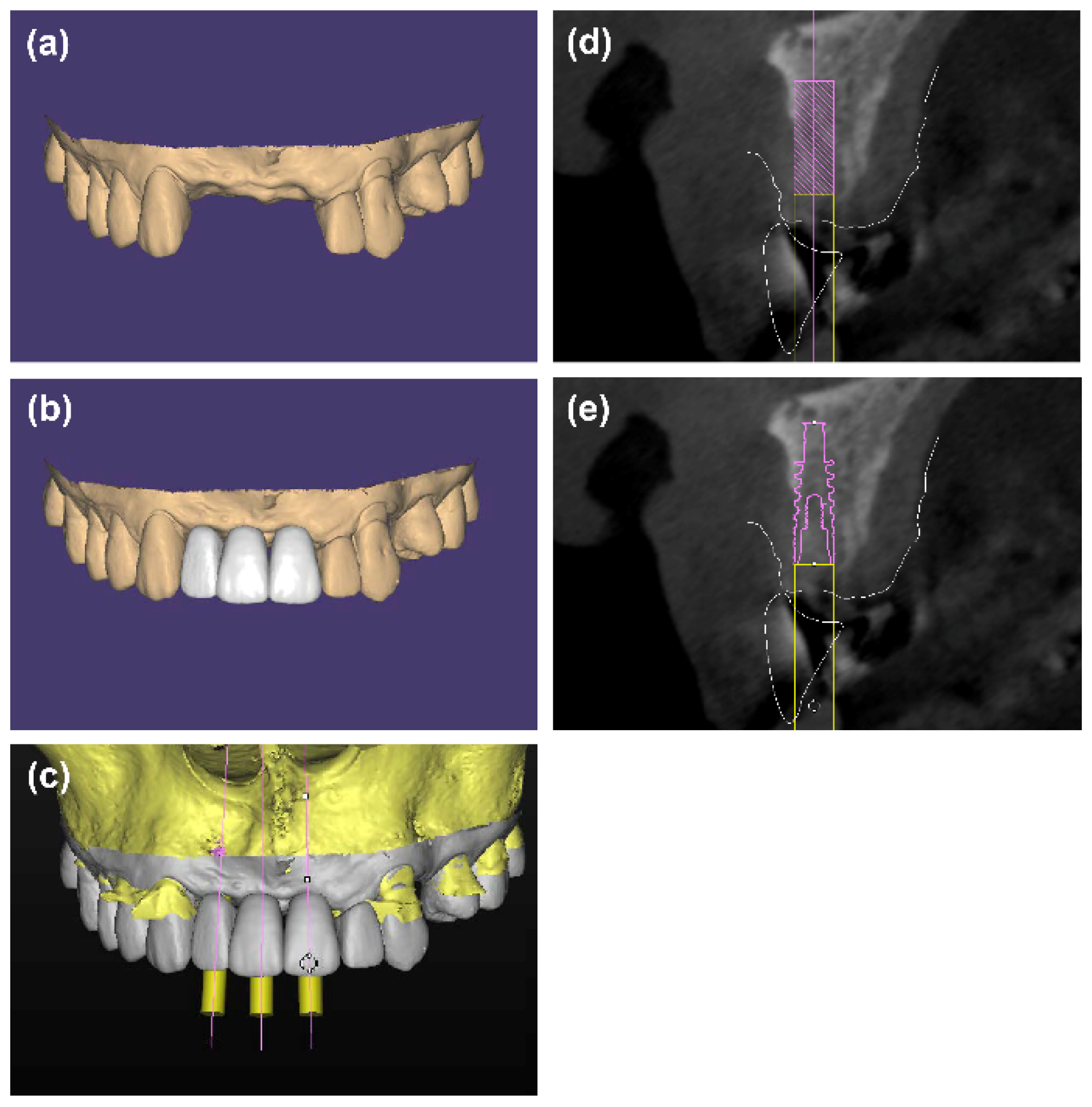
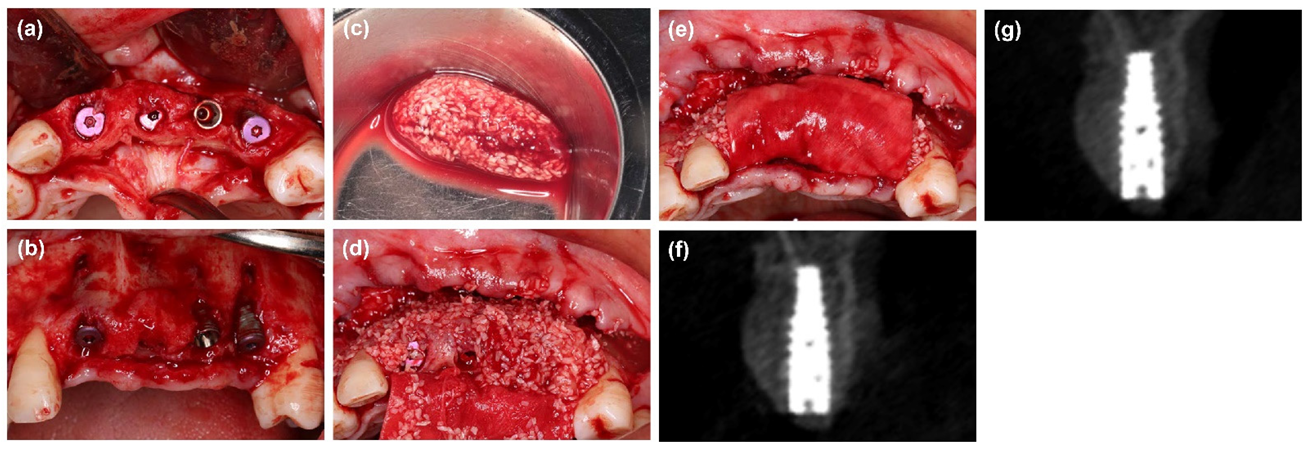
2. Materials and Methods
2.1. Study Population
2.1.1. Inclusion Criteria
- Male or female patients aged 18 to 60 years (including 18 and 60 years).
- Presence of a three-wall or two-wall horizontal bone defect in the anterior region.
- Bone augmentation was applied >3 months after tooth extraction.
- Good general health.
- Patients were willing to participate in this study and signed the informed consent form.
2.1.2. Exclusion Criteria
- Uncontrolled systemic diseases.
- Presence of acute infection.
- Uncontrolled periodontal disease.
- Heavy smokers (>20 cigarettes per day).
- Females in pregnancy or lactation.
2.2. Surgical Procedures
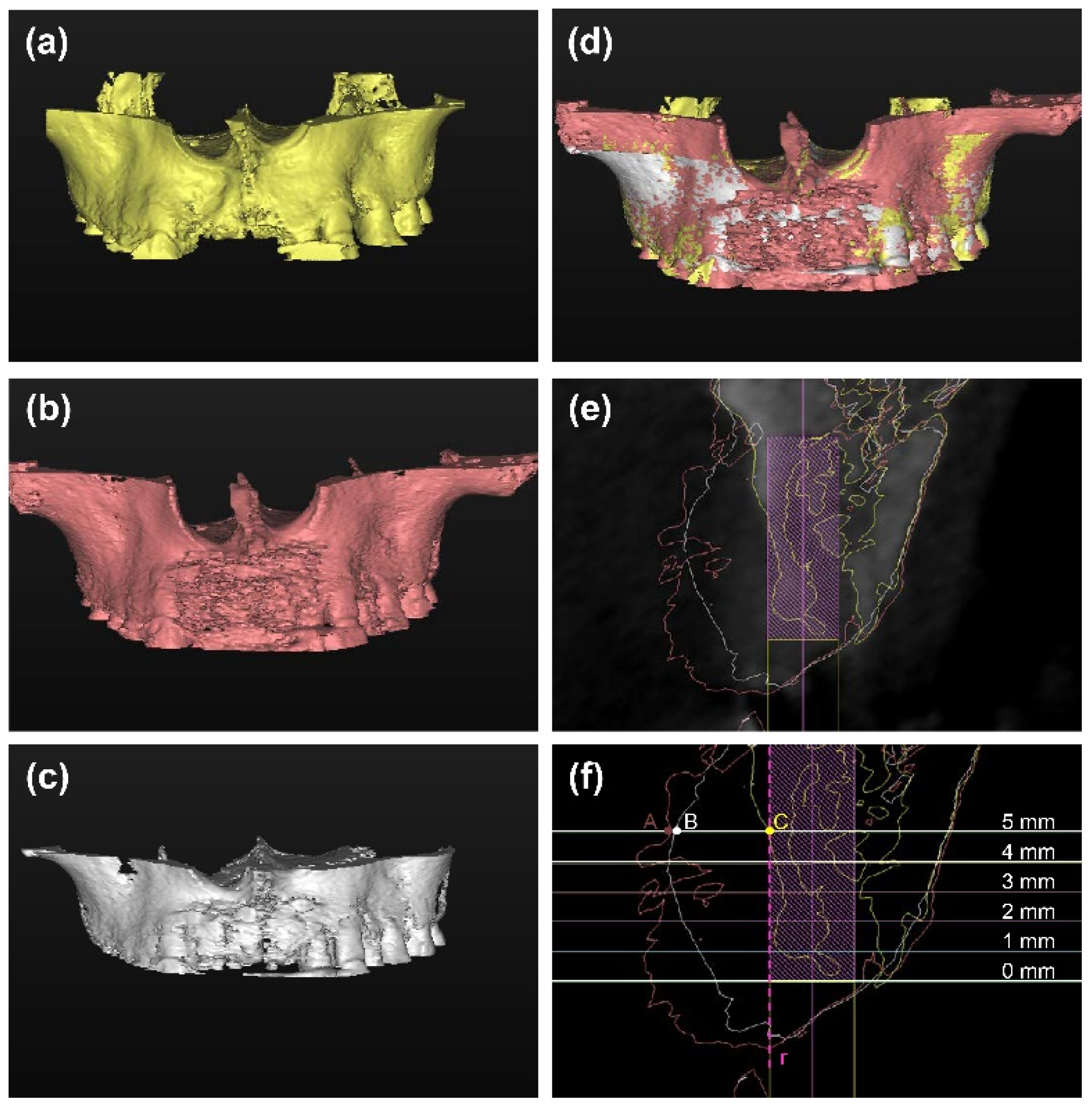
2.3. Radiographic Evaluation
2.4. Sample Size Calculation
2.5. Statistical Analysis
3. Results
3.1. Patients
3.2. Soft Tissue Condition
3.3. Radiographic Outcomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tan, W.L.; Wong, T.L.; Wong, M.C.; Lang, N.P. A systematic review of post-extractional alveolar hard and soft tissue dimensional changes in humans. Clin. Oral Implant. Res. 2012, 23 (Suppl. 5), 1–21. [Google Scholar] [CrossRef]
- Chiapasco, M.; Casentini, P. Horizontal bone-augmentation procedures in implant dentistry: Prosthetically guided regeneration. Periodontology 2000 2018, 77, 213–240. [Google Scholar] [CrossRef] [PubMed]
- Chiapasco, M.; Zaniboni, M.; Boisco, M. Augmentation procedures for the rehabilitation of deficient edentulous ridges with oral implants. Clin. Oral Implant. Res. 2006, 17 (Suppl. 2), 136–159. [Google Scholar] [CrossRef] [PubMed]
- Bassetti, M.A.; Bassetti, R.G.; Bosshardt, D.D. The alveolar ridge splitting/expansion technique: A systematic review. Clin. Oral Implant. Res. 2016, 27, 310–324. [Google Scholar] [CrossRef] [PubMed]
- Retzepi, M.; Donos, N. Guided Bone Regeneration: Biological principle and therapeutic applications. Clin. Oral Implant. Res. 2010, 21, 567–576. [Google Scholar] [CrossRef] [PubMed]
- Dahlin, C.; Sennerby, L.; Lekholm, U.; Linde, A.; Nyman, S. Generation of new bone around titanium implants using a membrane technique: An experimental study in rabbits. Int. J. Oral Maxillofac. Implant. 1989, 4, 19–25. [Google Scholar]
- Jung, R.E.; Fenner, N.; Hämmerle, C.H.; Zitzmann, N.U. Long-term outcome of implants placed with guided bone regeneration (GBR) using resorbable and non-resorbable membranes after 12–14 years. Clin. Oral Implant. Res. 2013, 24, 1065–1073. [Google Scholar] [CrossRef] [Green Version]
- Urban, I.A.; Monje, A.; Lozada, J.L.; Wang, H.L. Long-term Evaluation of Peri-implant Bone Level after Reconstruction of Severely Atrophic Edentulous Maxilla via Vertical and Horizontal Guided Bone Regeneration in Combination with Sinus Augmentation: A Case Series with 1 to 15 Years of Loading. Clin. Implant Dent. Relat. Res. 2017, 19, 46–55. [Google Scholar] [CrossRef] [Green Version]
- Sanz-Sanchez, I.; Ortiz-Vigon, A.; Sanz-Martin, I.; Figuero, E.; Sanz, M. Effectiveness of Lateral Bone Augmentation on the Alveolar Crest Dimension: A Systematic Review and Meta-analysis. J. Dent. Res. 2015, 94, 128S–142S. [Google Scholar] [CrossRef]
- Benic, G.I.; Hammerle, C.H. Horizontal bone augmentation by means of guided bone regeneration. Periodontology 2000 2014, 66, 13–40. [Google Scholar] [CrossRef] [PubMed]
- Troeltzsch, M.; Troeltzsch, M.; Kauffmann, P.; Gruber, R.; Brockmeyer, P.; Moser, N.; Rau, A.; Schliephake, H. Clinical efficacy of grafting materials in alveolar ridge augmentation: A systematic review. J. Craniomaxillofac. Surg. 2016, 44, 1618–1629. [Google Scholar] [CrossRef]
- Mir-Mari, J.; Wui, H.; Jung, R.E.; Hammerle, C.H.; Benic, G.I. Influence of blinded wound closure on the volume stability of different GBR materials: An in vitro cone-beam computed tomographic examination. Clin. Oral Implant. Res. 2016, 27, 258–265. [Google Scholar] [CrossRef]
- Benic, G.I.; Thoma, D.S.; Jung, R.E.; Sanz-Martin, I.; Unger, S.; Cantalapiedra, A.; Hämmerle, C. Guided bone regeneration with particulate vs. block xenogenic bone substitutes: A pilot cone beam computed tomographic investigation. Clin. Oral Implant. Res. 2017, 28, e262–e270. [Google Scholar] [CrossRef]
- Benic, G.I.; Eisner, B.M.; Jung, R.E.; Basler, T.; Schneider, D.; Hammerle, C. Hard tissue changes after guided bone regeneration of peri-implant defects comparing block versus particulate bone substitutes: 6-month results of a randomized controlled clinical trial. Clin. Oral Implant. Res. 2019, 30, 1016–1026. [Google Scholar] [CrossRef]
- Jung, U.W.; Lee, J.S.; Lee, G.; Lee, I.K.; Hwang, J.W.; Kim, M.S.; Choi, S.H.; Chai, J.K. Role of collagen membrane in lateral onlay grafting with bovine hydroxyapatite incorporated with collagen matrix in dogs. J. Periodontal. Implant Sci. 2013, 43, 64–71. [Google Scholar] [CrossRef]
- Mertens, C.; Braun, S.; Krisam, J.; Hoffmann, J. The influence of wound closure on graft stability: An in vitro comparison of different bone grafting techniques for the treatment of one-wall horizontal bone defects. Clin. Implant Dent. Relat. Res. 2019, 21, 284–291. [Google Scholar] [CrossRef] [PubMed]
- Mir-Mari, J.; Benic, G.I.; Valmaseda-Castellon, E.; Hammerle, C.; Jung, R.E. Influence of wound closure on the volume stability of particulate and non-particulate GBR materials: An in vitro cone-beam computed tomographic examination. Part II. Clin. Oral Implant. Res. 2017, 28, 631–639. [Google Scholar] [CrossRef] [Green Version]
- Chenchev, I.L.; Ivanova, V.V.; Neychev, D.Z.; Cholakova, R.B. Application of Platelet-Rich Fibrin and Injectable Platelet-Rich Fibrin in Combination of Bone Substitute Material for Alveolar Ridge Augmentation—A Case Report. Folia Med. 2017, 59, 362–366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shah, R.; Gowda, T.M.; Thomas, R.; Kumar, T.; Mehta, D.S. Biological activation of bone grafts using injectable platelet-rich fibrin. J. Prosthet. Dent. 2019, 121, 391–393. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Y.; Choukroun, J.; Ghanaati, S.; Miron, R.J. Effects of an injectable platelet-rich fibrin on osteoblast behavior and bone tissue formation in comparison to platelet-rich plasma. Platelets 2018, 29, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Zhang, X.; Li, Y.; Mo, A. The Influence of Different Guided Bone Regeneration Procedures on the Contour of Bone Graft after Wound Closure: A Retrospective Cohort Study. Materials 2021, 14, 583. [Google Scholar] [CrossRef]
- Buser, D.; Martin, W.; Belser, U.C. Optimizing esthetics for implant restorations in the anterior maxilla: Anatomic and surgical considerations. Int. J. Oral Maxillofac. Implant. 2004, 19, 43–61. [Google Scholar]
- Rojas-Vizcaya, F. Biological aspects as a rule for single implant placement. The 3A-2B rule: A clinical report. J. Prosthodont. 2013, 22, 575–580. [Google Scholar] [CrossRef]
- Miron, R.J.; Fujioka-Kobayashi, M.; Hernandez, M.; Kandalam, U.; Zhang, Y.; Ghanaati, S.; Choukroun, J. Injectable platelet rich fibrin (i-PRF): Opportunities in regenerative dentistry? Clin. Oral Investig. 2017, 21, 2619–2627. [Google Scholar] [CrossRef]
- Jiang, X.; Zhang, Y.; Di, P.; Lin, Y. Hard tissue volume stability of guided bone regeneration during the healing stage in the anterior maxilla: A clinical and radiographic study. Clin. Implant Dent. Relat. Res. 2018, 20, 68–75. [Google Scholar] [CrossRef]
- César, N.J.; Cavalcanti, M.C.; Sapata, V.M.; Pannuti, C.M.; Hämmerle, C.; Naenni, N.; Thoma, D.S.; Romito, G.A. The positive effect of tenting screws for primary horizontal guided bone regeneration: A retrospective study based on cone-beam computed tomography data. Clin. Oral Implant. Res. 2020, 31, 846–855. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Boyapati, L. “PASS” principles for predictable bone regeneration. Implant Dent. 2006, 15, 8–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chaushu, G.; Mardinger, O.; Peleg, M.; Ghelfan, O.; Nissan, J. Analysis of complications following augmentation with cancellous block allografts. J. Periodontol. 2010, 81, 1759–1764. [Google Scholar] [CrossRef]
- Amorfini, L.; Migliorati, M.; Signori, A.; Silvestrini-Biavati, A.; Benedicenti, S. Block allograft technique versus standard guided bone regeneration: A randomized clinical trial. Clin. Implant Dent. Relat. Res. 2014, 16, 655–667. [Google Scholar] [CrossRef]
- Soni, R.; Priya, A.; Yadav, H.; Mishra, N.; Kumar, L. Bone augmentation with sticky bone and platelet-rich fibrin by ridge-split technique and nasal floor engagement for immediate loading of dental implant after extracting impacted canine. Natl. J. Maxillofac. Surg. 2019, 10, 98–101. [Google Scholar] [CrossRef]
- Scarano, A.; Inchingolo, F.; Murmura, G.; Traini, T.; Piattelli, A.; Lorusso, F. Three-Dimensional Architecture and Mechanical Properties of Bovine Bone Mixed with Autologous Platelet Liquid, Blood, or Physiological Water: An In Vitro Study. Int. J. Mol. Sci. 2018, 19, 1230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choukroun, J.; Ghanaati, S. Reduction of relative centrifugation force within injectable platelet-rich-fibrin (PRF) concentrates advances patients’ own inflammatory cells, platelets and growth factors: The first introduction to the low speed centrifugation concept. Eur. J. Trauma Emerg. Surg. 2018, 44, 87–95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mu, Z.; He, Q.; Xin, L.; Li, Y.; Yuan, S.; Zou, H.; Shu, L.; Song, J.; Huang, Y.; Chen, T. Effects of injectable platelet rich fibrin on bone remodeling in combination with DBBM in maxillary sinus elevation: A randomized preclinical study. Am. J. Transl. Res. 2020, 12, 7312–7325. [Google Scholar] [PubMed]
- Nasirzade, J.; Kargarpour, Z.; Hasannia, S.; Strauss, F.J.; Gruber, R. Platelet-rich fibrin elicits an anti-inflammatory response in macrophages in vitro. J. Periodontol. 2020, 91, 244–252. [Google Scholar] [CrossRef] [Green Version]
- Miron, R.J.; Bosshardt, D.D. OsteoMacs: Key players around bone biomaterials. Biomaterials 2016, 82, 1–19. [Google Scholar] [CrossRef]

| Test | Control | p-Value | |
|---|---|---|---|
| Patient demographics | |||
| Male/Female | 6/6 | 7/7 | 1.000 |
| Smoking/No smoking | 2/10 | 1/13 | 0.580 |
| Mean age ± SD | 38.92 ± 14.12 | 41.71 ± 13.24 | 0.607 |
| Information about augmented sites | |||
| Location CI/LI | 6/6 | 6/8 | 1.000 |
| Jaw Maxilla/Mandible | 11/1 | 12/2 | 1.000 |
| Three-wall defect/Two-wall defect | 8/4 | 11/3 | 0.665 |
| Implant | 0.583 | ||
| NobelActive | 5 | 8 | |
| Bone Level Titanium SLA | 2 | 3 | |
| Staged implant placement | 5 | 3 | |
| Bone substitutes | 0.713 | ||
| Bio-Oss (xenograft) | 5 | 7 | |
| Bio-Gene (allograft) | 7 | 7 |
| Level | Before Surgery (T0) | Immediately after Surgery (T1) | 6 Months after Surgery(T2) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Test | Control | p-Value | Test | Control | p-Value | Test | Control | p-Value | |
| Mean ± SD | |||||||||
| LT0 | 0.03 ± 0.11 | 0.14 ± 0.36 | 0.316 | 4.31 ± 0.73 | 2.99 ± 1.02 | 0.000 * | 1.88 ± 0.57 | 1.08 ± 0.60 | 0.001 * |
| LT1 | 0.15 ± 0.51 | 0.38 ± 0.58 | 0.256 | 4.55 ± 0.69 | 3.60 ± 0.96 | 0.008 * | 2.36 ± 0.66 | 1.69 ± 0.58 | 0.009 * |
| LT2 | 0.20 ± 0.48 | 0.40 ± 0.55 | 0.320 | 4.76 ± 0.54 | 4.05 ± 1.01 | 0.034 * | 2.62 ± 0.81 | 2.10 ± 0.55 | 0.069 |
| LT3 | 0.09 ± 0.27 | 0.33 ± 0.51 | 0.144 | 5.01 ± 0.65 | 4.42 ± 1.06 | 0.072 | 2.97 ± 0.77 | 2.49 ± 0.73 | 0.150 |
| LT4 | 0.17 ± 0.32 | 0.40 ± 0.60 | 0.254 | 5.03 ± 0.67 | 4.67 ± 1.07 | 0.288 | 3.12 ± 0.88 | 2.65 ± 0.78 | 0.143 |
| LT5 | 0.16 ± 0.34 | 0.47 ± 0.68 | 0.160 | 5.13 ± 0.63 | 4.94 ± 1.15 | 0.594 | 3.26 ± 0.86 | 2.67 ± 0.81 | 0.090 |
| Level | Graft Gain (T1–T0) | Bone Gain (T2–T0) | Bone Resorption(T1–T2) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Test | Control | p-Value | Test | Control | p-Value | Test | Control | p-Value | |
| Mean ± SD | |||||||||
| LT0 | 4.28 ± 0.75 | 2.85 ± 0.96 | 0.000 * | 1.85 ± 0.56 | 0.94 ± 0.53 | 0.000 * | 2.43 ± 0.98 | 1.91 ± 1.21 | 0.243 |
| LT1 | 4.41 ± 0.85 | 3.22 ± 0.89 | 0.002 * | 2.21 ± 0.60 | 1.31 ± 0.67 | 0.002 * | 2.19 ± 0.95 | 1.91 ± 1.15 | 0.506 |
| LT2 | 4.55 ± 0.61 | 3.65 ± 0.90 | 0.007 * | 2.42 ± 0.86 | 1.70 ± 0.53 | 0.017 * | 2.13 ± 0.94 | 1.95 ± 1.14 | 0.742 |
| LT3 | 4.92 ± 0.69 | 4.09 ± 0.78 | 0.009 * | 2.87 ± 0.90 | 2.15 ± 0.84 | 0.009 * | 2.04 ± 0.96 | 1.93 ± 1.15 | 1.000 |
| LT4 | 4.85 ± 0.60 | 4.27 ± 0.90 | 0.070 | 2.95 ± 1.15 | 2.25 ± 0.98 | 0.085 | 1.90 ± 1.10 | 2.02 ± 1.10 | 0.462 |
| LT5 | 4.97 ± 0.53 | 4.47 ± 1.05 | 0.150 | 3.10 ± 1.09 | 2.20 ± 1.07 | 0.045 | 1.87 ± 1.04 | 2.27 ± 1.24 | 0.274 |
| Factors | p-Value |
|---|---|
| Time | 0.000 |
| Group ∗ Time | 0.000 * |
| Gender ∗ Time | 0.208 |
| Age ∗ Time | 0.991 |
| Location ∗ Time | 0.435 |
| Jaw ∗ Time | 0.210 |
| Defect type ∗ Time | 0.089 |
| Implant type ∗ Time | 0.806 |
| Bone substitutes ∗ Time | 0.067 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, M.; Zhang, X.; Li, Y.; Mo, A. Lateral Ridge Augmentation with Guided Bone Regeneration Using Particulate Bone Substitutes and Injectable Platelet-Rich Fibrin in a Digital Workflow: 6 Month Results of a Prospective Cohort Study Based on Cone-Beam Computed Tomography Data. Materials 2021, 14, 6430. https://doi.org/10.3390/ma14216430
Wang M, Zhang X, Li Y, Mo A. Lateral Ridge Augmentation with Guided Bone Regeneration Using Particulate Bone Substitutes and Injectable Platelet-Rich Fibrin in a Digital Workflow: 6 Month Results of a Prospective Cohort Study Based on Cone-Beam Computed Tomography Data. Materials. 2021; 14(21):6430. https://doi.org/10.3390/ma14216430
Chicago/Turabian StyleWang, Maoxia, Xiaoqing Zhang, Yazhen Li, and Anchun Mo. 2021. "Lateral Ridge Augmentation with Guided Bone Regeneration Using Particulate Bone Substitutes and Injectable Platelet-Rich Fibrin in a Digital Workflow: 6 Month Results of a Prospective Cohort Study Based on Cone-Beam Computed Tomography Data" Materials 14, no. 21: 6430. https://doi.org/10.3390/ma14216430
APA StyleWang, M., Zhang, X., Li, Y., & Mo, A. (2021). Lateral Ridge Augmentation with Guided Bone Regeneration Using Particulate Bone Substitutes and Injectable Platelet-Rich Fibrin in a Digital Workflow: 6 Month Results of a Prospective Cohort Study Based on Cone-Beam Computed Tomography Data. Materials, 14(21), 6430. https://doi.org/10.3390/ma14216430





